Abstract
The autoimmune regulator (Aire) mediates central tolerance for many autoantigens, and autoimmunity occurs spontaneously in Aire-deficient humans and mice. Using a mouse model of Graves’ disease, we investigated the role of Aire in tolerance to the TSH receptor (TSHR) in Aire-deficient and wild-type mice (hyperthyroid-susceptible BALB/c background). Mice were immunized three times with TSHR A-subunit expressing adenovirus. The lack of Aire did not influence T-cell responses to TSHR protein or TSHR peptides. However, antibody levels were higher in Aire-deficient than wild-type mice after the second (but not the third) immunization. After the third immunization, hyperthyroidism persisted in a higher proportion of Aire-deficient than wild-type mice. Aire-deficient mice were crossed with transgenic strains expressing high or low-intrathyroidal levels of human TSHR A subunits. In the low-expressor transgenics, Aire deficiency had the same effect on the pattern of the TSHR antibody response to immunization as in nontransgenics, although the amplitude of the response was lower in the transgenics. High-expressor A-subunit transgenics were unresponsive to immunization. We examined intrathymic expression of murine TSHR, thyroglobulin, and thyroid peroxidase (TPO), the latter two being the dominant autoantigens in Hashimoto’s thyroiditis (particularly TPO). Expression of the TSHR and thyroglobulin were reduced in the absence of Aire. Dramatically, thymic expression of TPO was nearly abolished. In contrast, the human A-subunit transgene, lacking a potential Aire-binding motif, was unaffected. Our findings provide insight into how varying intrathymic autoantigen expression may modulate thyroid autoimmunity and suggest that Aire deficiency may contribute more to developing Hashimoto’s thyroiditis than Graves’ disease.
Using mice deficient for the Autoimmune Regulator (Aire) and/or transgenic for the human TSH receptor A-subunit, it is shown that Aire modulates intrathymic expression of thyroid autoantigens and that Aire deficiency likely contributes more to developing Hashimoto’s thyroiditis than Graves’ disease.
Graves’ hyperthyroidism occurs after the loss of tolerance to the TSH receptor (TSHR) and the generation of thyroid stimulatory antibodies (TSAbs) that mimic the action of TSH (reviewed in Ref. 1). There are no spontaneous animals models of Graves’ disease. However, in vivo expression of the TSHR cDNA induces TSAb and hyperthyroidism in susceptible mouse strains (reviewed in Ref. 2). At present, the most effective and reproducible method uses an adenovirus (Ad) vector (3). There is evidence that the autoantigen in Graves’ disease is not the membrane-bound receptor but its shed A-subunit component (4) generated by intramolecular cleavage of the TSHR expressed on the cell surface (5,6,7). Indeed, immunization with vectors expressing the A subunit alone induces hyperthyroidism more efficiently than the uncleaved or membrane-associated holoreceptor (8,9,10,11).
Because of the prominent role of the A subunit in TSAb generation, as in TSHR autoantibody binding (12,13), we generated transgenic mice with the human TSHR A subunit targeted to the thyroid (14). One transgenic line expresses low amounts of A-subunit protein and responds to A-subunit Ad immunization, albeit at reduced levels compared with nontransgenic littermates (15). A second line expresses high levels of intrathyroidal A subunits and exhibits strong tolerance to the autoantigen as reflected by unresponsiveness to A-subunit Ad immunization.
Central tolerance is based on negative selection of autoreactive T cells in the thymus (16). In the last 10 yr, significant progress has been made in understanding the mechanisms involved in this process. Stromal medullary thymic epithelial cells (mTECs) express a spectrum of self-proteins (17) and, in cooperation with dendritic cells, present them to immature T cells (reviewed in Ref. 18). T cells that recognize self-peptides with high affinity undergo negative selection (16). Insight into the factor(s) controlling thymic self-protein expression came from studies of autoimmune polyendocrinopathy candidiasis-ectodermal dystrophy (APECED) or autoimmune polyendocrine syndrome-type 1 (APS-1), which was genetically linked to defects in the autoimmune regulator (Aire) gene (19,20). Aire is a transcription factor that regulates the expression of numerous self-proteins in mTECs. Mice genetically engineered to be Aire deficient have decreased self-protein levels in mTECs (21,22) and display characteristics of APS-1 patients, including self-reactive T cells and autoantibodies. Importantly, the spectrum of autoimmunity that develops depends on the genetic background of the Aire-deficient mice (23).
In the absence of Aire, expression of many self-proteins (including insulin) is reduced in the thymus, but others (such as glutamic acid decarboxylase 65, and a-fodrin) are unaffected (21,24). Consequently, this mechanism cannot be responsible for central tolerance to all autoantigens. Thyroglobulin (Tg) and thyroid peroxidase (TPO) mRNA transcripts are present in human thymus (25), and truncated Tg isoforms are detected in murine thymus (26). The TSHR is also present in human and rat thymic tissue (27,28,29). However, of potential relevance to the pathogenesis of Graves’ disease, no data have been reported on the role of Aire in tolerance to the TSHR.
We have examined this relationship by applying the TSHR A-subunit Ad model of Graves’ disease to Aire-deficient mice on a BALB/c background, a strain susceptible to induced hyperthyroidism (3,30). In addition, because regulatory T-cell (Treg) defects have been observed in APECED/APS-1 patients (31), we repeated these experiments in Treg-depleted mice. Finally, we explored the hypothesis that crossing TSHR A-subunit transgenic and Aire-null mice would reduce central tolerance to the TSHR, thereby permitting or enhancing a TSHR antibody response to TSHR-Ad immunization in A-subunit transgenic mice.
Materials and Methods
Aire-deficient mice
Breeding pairs of Aire+/− BALB/c mice were provided by Dr. C. Benoist (Joslin Diabetes Center, Boston, MA) (23). Because homozygous Aire−/− mice have decreased fertility (21,22), heterozygotes were bred, and Aire +/+, Aire+/−, and Aire−/− mice were identified by genotyping. Aire+/− mice were bred to two transgenic BALB/c lines (maintained as heterozygotes) in which the human TSHR A subunit was targeted to the thyroid by the bovine Tg promoter (14): Lo-expressor (51.9) and Hi-expressor (50.6) mice (15). A-subunit expression was determined by PCR (14). Mice of the 51.9 line are cryopreserved [C.Cg-Tg(TG-TSHR) 51.9Smcl, no. 014125; Mutant Mouse Regional Resource Center; University of California, Davis, CA]. Animal studies were approved by the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center and performed with the highest standards of care in a pathogen-free facility.
TSHR-Ad immunization
Adenovirus encoding the human TSHR A subunit in the pAdHM4 vector (A-subunit Ad) was propagated in human embryonic kidney 293 cells, purified by CsCl density gradient centrifugation and viral particle concentration determined by absorbance at 260 nm, as described (8). Control immunizations used Ad lacking an insert (Con-Ad) (32). Mice (6–7 wk old) were immunized three times at three weekly intervals with TSHR Ad or Con-Ad (∼1010 particles per injection). In some studies, Tregs were depleted. Blood was drawn 1 wk after the second injection, and animals were euthanized 1 month after the third injection to harvest blood, spleens, and thyroids.
Treg depletion
Rat hybridomas PC61 (anti-CD25) and TMβ-1 (anti-CD122, from Drs. K. Yui, Nagasaki University, Nagasaki and T. Tanaka, Osaka University, Osaka, Japan) were used to generate ascites in nude mice, as described (33,34). After protein G purification, the antibody efficacy was tested in BALB/c mice (Charles River Laboratory Inc., Yokohama, Japan). Splenocytes from untreated and injected mice were compared by FACScan flow cytometry (CellQuest software; BD Biosciences, Mountain View, CA) using the following antibodies: fluorescein (fluorescein isothiocyanate)-anti-CD4 (H129.19) and phyco (PE)-anti-CD25 (7D4) (BD Biosciences, San Jose, CA); or fluorescein isothiocyanate-conjugated anti-CD122 (5H4; eBioscience, San Diego, CA) and PE-conjugated anti-CD8 (53–6.7; BD Biosciences, San Jose CA). Four days after ip injection of 500 μg PC61, CD25+ CD24+ T cells were reduced from 8–2.1%; after injecting 250 μg TMβ-1,CD122+, CD8+ T cells were reduced from 17.5–2.9%, similar to previous findings (33,34). These studies followed the Guideline for the Care and Use of Laboratory Animals, Nagasaki University. From these data, Treg depletion in Los Angeles was performed by injecting 500 μg/mouse anti-CD25 or 250 μg/mouse anti-CD122 ip 4 d before each Ad immunization.
Serum T4 and thyroid histology
Total T4 was measured in mouse serum (25 μl) by RIA (Diagnostic Products Corp., Los Angeles, CA). Mice were considered hyperthyroid if their T4 levels exceeded the mean + 2 sd values of Con-Ad immunized animals. Thyroids were fixed in buffered formaldehyde (pH 7.4), paraffin embedded, and serial sections were stained with hematoxylin and eosin (Research Animal Diagnostic Laboratory, University of Missouri, Columbia, MO).
TSHR A-subunit protein and splenocyte responses to TSHR protein and peptides
TSHR-289 is a recombinant A subunit expressed in Chinese hamster ovary (CHO) cells, purified by affinity chromatography (35) and dialyzed against 10 mm Tris (pH 7.4) and 50 mm NaCl. A total of 26 peptides (A to Z, each 20 amino acids long) corresponding to amino acids in the human TSHR extracellular domain and three extracellular loop peptides (36) were provided by Dr. John Morris (Mayo Clinic, Rochester, MN). The amino acid sequences for these peptides were reported previously (37).
Splenocytes (200 ml aliquots, ∼5 × 105 cells) were incubated in round-bottomed 96-well plates with or without TSHR A-subunit protein (10 μg/ml), TSHR synthetic peptides (10 μg/ml), or concanavalin A (5 μg/ml; Sigma-Aldrich Corp., St. Louis, MO). Culture medium was RPMI 1640, 10% heat-inactivated fetal bovine serum, 2 mm glutamine, 50 mm β-mercaptoethanol, 50 μg/ml gentamycin, and 100 U/ml penicillin (Life Technologies, Inc., Gaithersburg, MD). After 5–6 d (37 C, 5% CO2), the plates were centrifuged to remove cell debris. Interferon (IFN)-γ was measured by ELISA using capture and biotinylated-detection antibodies (BD PharMingen, San Diego, CA).
TSHR antibodies
TSHR antibodies were determined by TSH binding inhibition (TBI) and TSAb activity. TBI was measured using a kit (Kronus, Boise, ID): mouse sera (25 μl) were incubated with detergent solubilized TSHR; 125I-TSH was added; and TSHR-antibody complexes were precipitated with polyethylene glycol. Sera were tested undiluted or, when specified in the text, after dilution (1:5 in normal human serum). TBI values were calculated from the formula: [1 − (TSH binding in test serum − nonspecific binding/TSH binding in control serum − nonspecific binding) × 100. TSAb activity was measured by cAMP generation in CHO cells expressing the human TSHR as reported previously (38) and using recently generated CHO cells expressing the mouse TSHR (mTSHR) (39). TSAb activity was expressed as a percentage of cAMP values attained with sera from control, unimmunized mice.
Thymic mRNA isolation and real-time PCR for thyroid autoantigens
Whole thymuses (30 d old mice) were diced and stored in RNAlater (QIAGEN, Inc., Valencia, CA). Material from four thymuses (∼25 mg each) was pooled; total RNA and mRNA were prepared using RNeasy and Oligotex mRNA kits (QIAGEN), respectively. The mRNA samples were treated with TURBO deoxyribonuclease (Applied Biosystems, Foster City, CA/Ambion, Inc., Austin, TX) to remove genomic DNA. Reverse transcription was performed (AffinityScript QPCR cDNA Synthesis Kit; Stratagene, La Jolla, CA) using oligo(deoxythymidine) primers. Real-time PCR was performed using the FastStart SYBR Green Master mix (Roche Diagnostics Corporation, Indianapolis, IN) with 5% of cDNA (25 μl final volume). Reactions were run on an iCycler Thermal Cycler with iQ5 Real-Time PCR Detection System module (Bio-Rad Laboratories, Inc., Hercules, CA). An initial denaturation step at 95 C (10 min) was followed by denaturation at 95 C (30 sec), and annealing and extension at 55 C (30 sec) for 40 cycles. Relative gene expression levels were calculated using the comparative Ct method (ΔΔ Ct), according to the Pfaffl model (40), using Bio-Rad iQ5 2.0 software. Samples were tested in triplicate; parallel controls lacked reverse transcriptase. At least three independent sets of animals were used for each mouse strain. Data were normalized to keratin-8, which is specifically expressed in the epithelial cell fraction and is unaffected by Aire expression (17,41).
Real-time PCR used the following primers: keratin 8, K2–8 sense 5′-aggagctcattccgtagctg-3′ and K2–8 antisense 5′-tctgggatgcagaacatgag-3′ (41); mTSHR sense 5′-ctgcttgttctgctgctc-3′ and mTSHR antisense 5′-ctctgaagtcgtcctcctg-3′; human TSHR A-subunit, A-subunit sense 5′-ggactcttaagaaacttccactttcc-3′ and A-subunit antisense 5′-gtgatggtggtggtgatggctagt-3′; Tg sense 5′-agggtaccctgctggaccaagtg-3′ and Tg antisense 5′-tgtctagatgtcacacgctgagg-3′ (26); TPO sense 5′-ggtcctcctgtgcgaatag-3′ and TPO antisense 5′-ctggtgtcctcaagtctctg-3′; and insulin, Ins2 sense 5′-gacccacaagtggcacaac-3′ and Ins2 antisense 5′-tctacaatgccacgcttctg-3′ (41). Note that primers for the mTSHR were designed to avoid overlap with A subunit of human TSHR in transgenic animals.
Statistical analyses
The statistical significance of differences between responses in multiple groups was determined by ANOVA or Student’s t tests when normally distributed or by rank sum tests. For relative expression measured by real-time PCR, the mean + 2 sd values was used as the cutoff for significant difference between groups.
Results
In the mice used for this study, the Aire gene was inactivated by deletion of exon 2 and portions of adjacent introns (21). Because it was not known if the mutated Aire allele impacts the function of the normal allele, we studied Aire heterozygotes (+/−) in addition to Aire knockouts (−/−) and Aire wild types (+/+).
TSHR antibodies (TBI) and hyperthyroidism
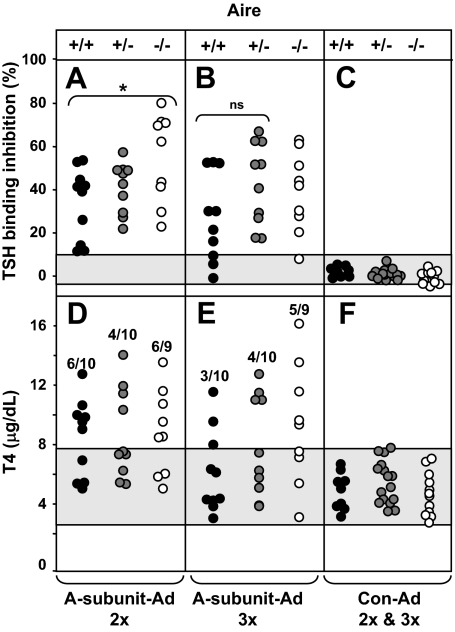
Sera from immunized animals were tested for TSHR antibodies (measured by TBI) and T4. Because A-subunit Ad immunized mice developed high (near maximal) TBI levels, the assay was repeated on diluted sera (1:5). One week after the second immunization, TSHR antibody levels were significantly higher in Aire−/− than Aire+/+ or Aire +/− mice (Fig. 1A), but this difference was lost after the third immunization (Fig. 1B). Comparable numbers of Aire-defective and wild-type mice had elevated T4 levels after two immunizations (Fig. 1D). However, after the third immunization, hyperthyroidism persisted in more Aire−/− mice (five of nine mice, 55%) than in Aire +/+ mice (three of 10 mice, 30%) (Fig. 1E). Although differences in these absolute numbers are not statistically significant, the data are considered in more detail below.
Figure 1.
TSHR antibodies and serum T4 in Aire−/−, +/−, and +/+ mice. Mice were immunized three times with A-subunit Ad. Sera were tested 1 wk after two immunizations (2×, A and B) and 4 wk after the third injection (3×, D and E). Data are shown for individual mice. TBI and T4 values for Con-Ad immunized mice immunized twice and three times are combined (C and D). A–C, TSHR antibodies measured by TBI (%) in sera diluted 1:5 (Materials and Methods). The shaded area represents the mean ± 2 sd values for Con-Ad immunized mice. Values in Aire −/− mice were significantly greater than in Aire +/+ mice: *, P = 0.025 (t test). D–F, Serum T4 (mg/dl). The shaded area represents the mean ± 2 sd values for T4 levels in Con-Ad immunized mice. The number of hyperthyroid vs. the total number of animals in each group is indicated. ns, Not significant.
Treg depletion, TBI, and hyperthyroidism
Because Treg defects have been observed in Aire-defective patients (31), we repeated A-subunit Ad immunization after Treg depletion before A-subunit Ad immunization. The data obtained using anti-CD25 and anti-CD122 pretreatment were similar (supplemental Table SI, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org); therefore, we pooled data for both Treg depletion treatments. All groups of Treg-depleted mice developed high TSHR antibody levels, but, unlike untreated animals, there was no difference between Aire+/+ and Aire−/− mice after the second A-subunit Ad immunization. Many mice developed elevated T4 levels, but there were no differences between mice of the three genotypes (supplemental Table SI).
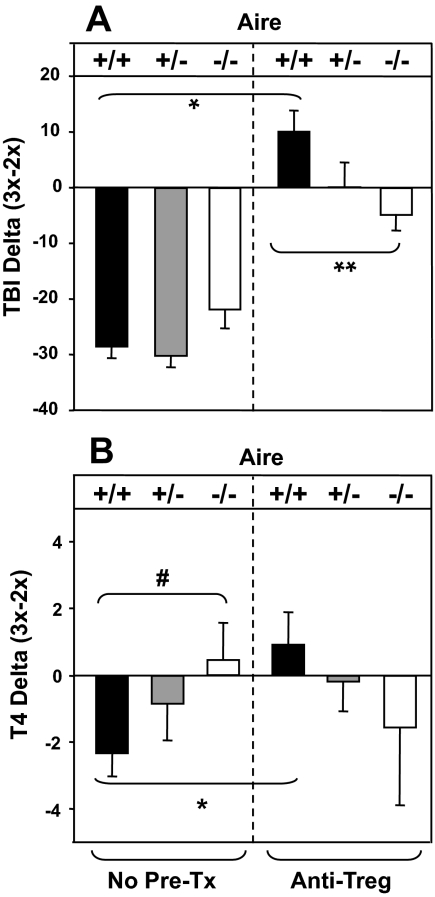
However, an effect of Treg depletion was revealed by analyzing the difference (delta) in individual mice between the levels of TSHR antibody or T4 after the second and third A-subunit immunizations. This analysis was performed on data for untreated mice (Fig. 1) and Treg depleted mice (supplemental Table SI). Without Treg depletion, the delta-TBI values reflect decreased TSHR antibody levels in all Aire genotypes after the third immunization (Fig. 2A, left panel). In contrast, TBI values were significantly increased in Treg-depleted vs. untreated Aire +/+ mice (Fig. 2A, right vs. left panels). However, the levels were significantly lower in Treg-depleted Aire −/− vs. Aire +/+ mice (Fig. 2A, right vs. left panels).
Figure 2.
Changes in TBI (A) and T4 (B) levels after three vs. two immunizations. Aire −/−, +/+, and +/+ mice were untreated (no Pre-Tx) before A-subunit Ad immunization (A and B, left section) or depleted of Treg using antibodies (see Materials and Methods) before immunization (A and B, right section). The change in TBI or T4 (delta) was calculated as the difference between the values for each individual animal after three immunizations minus the value after two immunizations. Values are presented as the mean + sem. The number of mice in each group is: untreated Aire+/+ (n = 9), Aire +/+ (n = 10), and Aire −/− (n = 10); and Treg-depleted Aire+/+ (n = 14), Aire+/− (n = 18), and Aire −/− (n = 11). Values are significantly different: *, P < 0.001 (rank sum) (A); **, P = 0.006 (t test) (A); #, P = 0.046 (B); *, P = 0.019 (B) (t tests).
For thyroid function, in untreated mice, delta-T4 levels were significantly lower in Aire+/+ vs. Aire−/− mice (Fig. 2B, left panel). These data are consistent with hyperthyroidism persisting in a greater proportion of Aire knockout than Aire wild-type mice after the third immunization (Fig. 1D). Treg depletion before A-subunit Ad immunization reversed this relationship: in Aire+/+ (but not Aire−/−) mice, delta-T4 values were significantly increased after Treg depletion (Fig. 2B, right vs. left panel).
Thyroid histology in Aire-defective mice
A-subunit Ad immunized mice with elevated T4 levels, unlike euthyroid animals, had markedly enlarged and hyperemic thyroid glands. Histologically, in Aire-sufficient or deficient mice, these glands had hypertrophic thyrocytes and signs of colloid resorption but without evidence of lymphocytic infiltration, as reported previously (3,8). There were no differences between mice that were, or were not, Treg depleted before A-subunit Ad immunization.
Recognition of A-subunit protein and TSHR synthetic peptides in Aire-defective mice
T-cell responses were tested in mice of three Aire genotypes immunized three times with A-subunit Ad by challenging their splenocytes in vitro with A-subunit protein or TSHR peptides and measuring IFN-γ generation. Splenocytes from A-subunit Ad, but not Con-Ad, immunized mice generated IFN-γ in response to A-subunit protein (supplemental Fig. S1A). There were no differences between splenocyte responses of the three Aire genotypes to A-subunit protein or concanavalin A (supplemental Fig. S1B). In terms of TSHR peptides, splenocytes from Aire+/+, Aire+/−, and Aire−/− mice recognized peptides C and J (TSHR amino acids 52-71 and 157-176, respectively) (supplemental Fig. S2). Overall, there were no differences between Aire+/+, Aire+/−, and Aire−/− mice for recognition of TSHR protein or peptides.
TSHR antibodies in A-subunit transgenics crossed with Aire−/− mice
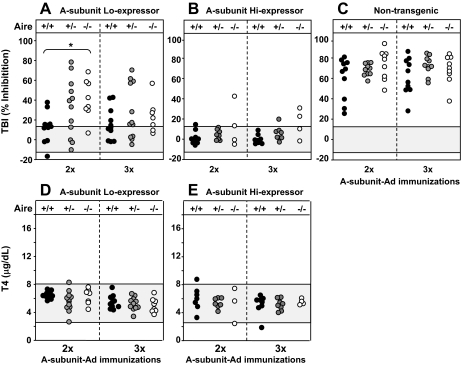
Transgenic BALB/c mice with the human TSHR A subunit targeted to the thyroid (“Lo” and “Hi” expressors) were bred to heterozygous Aire-defective mice (+/−) to generate the following groups: Lo expressor transgenics that were Aire −/−, Aire−/+, and Aire+/+; and Hi expressor transgenics that were Aire −/−, Aire −/+, and Aire+/+. One week after the second A-subunit Ad immunization, TBI levels were significantly higher in Aire−/− Lo expressor transgenic (Tg-ic) than Aire+/+ Lo expressor Tg-ic mice, but this difference was no longer apparent after the third immunization (Fig. 3A, left vs. right panel). Although low-expressor transgenics developed TBI activity, these levels were considerably lower than those for nontransgenic mice (three Aire genotypes) analyzed in undiluted sera (Fig. 3C). Unlike the Lo-expressor transgenics, few high-expressor transgenics developed detectable TBI activity, whether Aire −/−, +/−, or +/+ (Fig. 3B). Neither low-expressor nor high-expressor transgenics became hyperthyroid regardless of Aire genotype (Fig. 3, D and E).
Figure 3.
TSHR antibodies and serum T4 in transgenic mice expressing the human A subunit at low levels (Lo-expressors, A and D) or high levels (Hi- expressors, B and E) or in nontransgenics (C) of the following Aire genotypes: +/+, +/− and −/−. Mice were immunized three times with A-subunit Ad; blood was drawn 1 wk after two immunizations and 4 wk after the third injection. Data are shown for individual mice. A–C, TSHR antibodies measured by TBI (%) in undiluted sera (see Materials and Methods) in Lo and Hi A-subunit transgenics (A and B, respectively) and nontransgenic mice (C). The shaded area represents the mean ± 2 sd values for sera from nontransgenic mice immunized with Con-Ad. Values in Aire −/− Lo-expressor transgenic mice were significantly greater than in Aire +/+Lo expressor transgenics: *, P = 0.002 (t test). Note that because of the near-maximal TBI values for undiluted sera in nontransgenic mice (C), the difference between TBI levels for Aire −/− and +/+ mice after two immunizations was not significantly different. D and E, Serum T4 (μg/dl) in Lo (D) and Hi (E) expressors transgenic for the A subunit of the following Aire genotypes: Aire −/−, Aire +/−, and +/+. T4 values for nontransgenic mice are shown in Fig. 1, D and E. The shaded area represents the mean ± 2 sem values in nontransgenic mice immunized with Con-Ad.
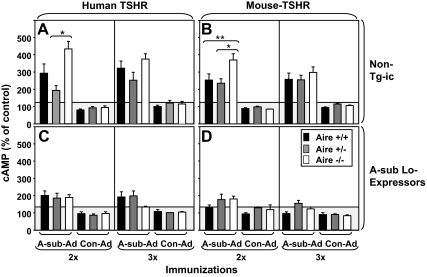
Development of hyperthyroidism depends on TSAb activity, which does not always correlate with TBI values. Moreover, the mice were immunized with the A subunit of the human TSHR, but development of hyperthyroidism requires antibody recognition of the mTSHR. Therefore, we measured TSAb activity in separate assays using eukaryotic cells expressing the human or mTSHR. Sera from Aire +/+, Aire +/−, and Aire −/− mice had previously been tested for TBI (Fig. 1, A and B). After the second immunization, Aire −/− animals developed higher TSAb levels than Aire +/− mice, measured using human- or mTSHR-expressing cells (Fig. 4, A and B, left panels). After the third immunization, there were no significant TSAb differences (Fig. 4, A and B, right panels).
Figure 4.
TSAb specific for the human TSHR and the mTSHR. Sera were from nontransgenic mice and Lo-expressor transgenic mice of three Aire genotypes (+/+, +/−, and −/−) tested for TBI and T4 (Figs. 1 and 3). TSAb activity was measured as cAMP generated in CHO cells expressing the human TSHR (A and C) or the mTSHR (B and D). Data are shown as mean + sem. The number of mice in each group was as follows: A, A-subunit Ad immunized nontransgenic mice: Aire +/+ (n = 9), Aire +/− (n = 10), Aire −/− (n = 9); B, Con-Ad immunized nontransgenics: Aire +/+ (n = 3), Aire +/− (n = 4), Aire −/− (n = 4); C, A-subunit Ad immunized Lo-expressor transgenics: Aire +/+ (n = 8), Aire +/− (n = 10), Aire −/− (n = 10). Values in Aire−/− mice were significantly greater than in Aire +/− mice for both human and mouse TSAb: *, P < 0.05 (ANOVA). Values in Aire−/− mice were significantly greater than in Aire +/− mice for mouse TSAb: **, P < 0.05 (ANOVA).
In A-subunit low-expressor transgenics immunized with A-subunit Ad, TSAb levels were very low, barely exceeding the stimulation observed for Con-Ad immunized mouse sera. TSAb values did not differ between Lo-expressor Tg-ic mice of the three Aire genotypes (Fig. 4, C and D). These sera were previously assayed for TBI activity (Fig. 3A).
Intrathymic expression of TSHR, Tg, TPO, and the transgenic human A subunit
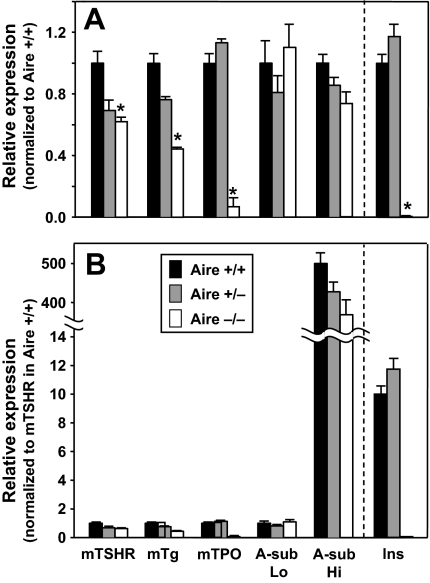
To determine whether thyroid autoantigen expression is Aire dependent, mRNA transcripts for mTSHR, Tg, and TPO, and for the transgenic human TSHR A subunit were compared by real-time PCR. Because insulin mRNA levels are almost undetectable in Aire−/− mice (17,41), insulin was included to control for Aire deficiency. Gene expression levels are shown relative to a value of 1.0 in Aire +/+ animals.
The greatest effect of Aire deficiency was on TPO, with expression levels 25–30 times lower in Aire −/− than Aire +/+ mice (Fig. 5A). To a lesser extent, intrathymic TSHR and Tg expression were also Aire dependent: mTSHR and Tg were 50–70% of the levels in Aire −/− vs. Aire +/+ mice. As expected, insulin expression was dramatically decreased (greater than 90%) by Aire deficiency. Turning to the transgenic autoantigen, there was no difference in intrathymic TSHR A-subunit expression in Aire −/− vs. +/+ mice. Intrathymic expression of thyroid autoantigens and insulin did not differ between Aire +/− and Aire +/+ mice (Fig. 5A).
Figure 5.
Intrathymic expression of mouse thyroid autoantigens (TSHR, Tg, TPO), the transgenic human TSHR A subunit, and insulin. Real-time PCR was used to measure expression of these autoantigens in nontransgenic Aire−/−, +/−. and +/+ mice, and in Hi- and Lo-expressor transgenics with the same Aire genotypes. A, Relative expression normalized to Aire +/+ mice for the mTSHR, mouse Tg (mTg), mouse TPO (mTPO), transgenic human A subunit (A-sub Lo or A-sub-Hi), and insulin (Ins). The data are shown as the mean + sem of triplicate measurements from one of three or more representative experiments. *, Values significantly lower for Aire −/− vs. Aire +/+ mice (<2 sd values for +/+ animals; see Materials and Methods). B, Comparison of intrathymic expression levels of different self-antigens. Data for the autoantigens shown in A were normalized to expression of the mTSHR in Aire+/+ mice. The data are shown as the mean + sem of triplicate measurements.
In addition to studying differences of the same autoantigen between mice of three Aire genotypes, we compared the relative expression of different thyroid antigens in the same tissue (Fig. 5B). Transcript levels of mouse Tg, TPO, and the human TSHR A subunit in low-expressor transgenics were all comparable with those of the mTSHR in wild-type animals (used as a standard adjusted to 1.0). Insulin expression was 10 times higher than the other thyroid autoantigens. However, in Aire −/− mice, the reduced levels of insulin and TPO were almost the same, emphasizing the Aire dependency of TPO. Finally, most striking was the expression of the human TSHR A subunit, which was 500 times higher in high-expressor than Lo-expressor transgenics.
Discussion
Little is known about factors controlling immunological tolerance to the TSHR. Because recent studies highlighted the role of Aire in regulating intrathymic expression of numerous autoantigens (reviewed in Ref. 42), we investigated a role for Aire in TSHR tolerance. We used a model in which TSHR antibodies and hyperthyroidism are induced by immunizing susceptible BALB/c mice with adenovirus encoding the human TSHR A subunit. Despite 87% homology (43), the human and mTSHRs are not identical, and these studies involve immunization with a cross-reacting A subunit, rather than a self-antigen. However, we generated transgenic BALB/c mice in which the human A subunit is targeted to the thyroid (14). Two transgenic lines, low expressors and high expressors, are susceptible or resistant, respectively, to immunization with Adenosine encoding the human A subunit (15). Consequently, our transgenic mice provide the opportunity for investigating self-tolerance to a genuine thyroid autoantigen.
The outcome of A-subunit Ad immunization was different in Aire −/− vs. Aire +/+ or Aire +/− mice in terms of TSHR antibodies and hyperthyroidism. TSHR antibodies were measured by TBI and a functional bioassay (TSAb). Although there is a good correlation between these two assays, not all competitive inhibitors of TSH binding have thyroid stimulatory activity. TBI levels were significantly higher in Aire −/− than wild-type mice after two Ad immunizations. However, the TBI difference was not sustained after the third immunization. TSAb levels followed the same pattern. In terms of thyroid function, about 50% of all three Aire genotypes had elevated serum T4 after two immunizations. In contrast, after the third immunization, a higher proportion of Aire−/− than Aire +/+ mice remained hyperthyroid.
We tested the effect of Treg depletion on the outcome of A-subunit immunization in Aire-deficient and wild-type mice. Aire deficiency does not affect selection and maturation of CD4+CD25+ Tregs (reviewed in Ref. 42), but mice deficient for Aire and Foxp3 (the later required for Treg generation) develop exacerbated inflammatory damage (44). Moreover, some APECED patients have Treg defects (31). Without Treg depletion, in our paired analysis of the data for individual mice, all three Aire genotypes had lower TBI levels after three vs. two immunizations, a pattern that was reversed by Treg depletion in Aire +/+ mice.
In terms of thyroid function, the difference (delta) in T4 levels after three vs. two immunizations was significantly less in untreated Aire −/− vs. Aire +/+ mice, consistent with more hyperthyroid Aire knockout than wild-type animals after the third immunization. In Aire +/+ (not Aire −/−) mice, Treg depletion before immunization eliminated the reduction in delta-T4 values. Although analyzed in a different way, this outcome for Treg depletion is reminiscent of the enhanced hyperthyroidism previously documented by one of us (Y.N.) for Treg depletion in BALB/c mice (33,34). [Incidentally, our data for delta-TBI and delta-T4 suggest that Aire−/− mice have a Treg defect, at least on the BALB/c background]. Overall, these observations support our conclusion of persistent (albeit moderate) hyperthyroidism in Aire−/− vs. Aire+/+ mice.
In Aire−/− mice, TBI and TSAb levels were elevated after the second immunization, whereas hyperthyroidism persisted after the third immunization. The reason for the time course difference is not fully understood. However, we suggest that the higher TSHR antibody levels (particularly mouse TSAb) after the second immunization in Aire knockout animals had long-term effects on the thyroid. Regardless of the explanation, these findings highlight how altered timing of immune responses changes the pathophysiological outcome. In contrast to TSHR antibodies, we observed no difference between Aire genotypes for T-cell responses to TSHR protein or peptides. A possible reason for this discrepancy is that splenocytes were obtained at euthanasia after the third immunization, at which time antibody differences were no longer evident.
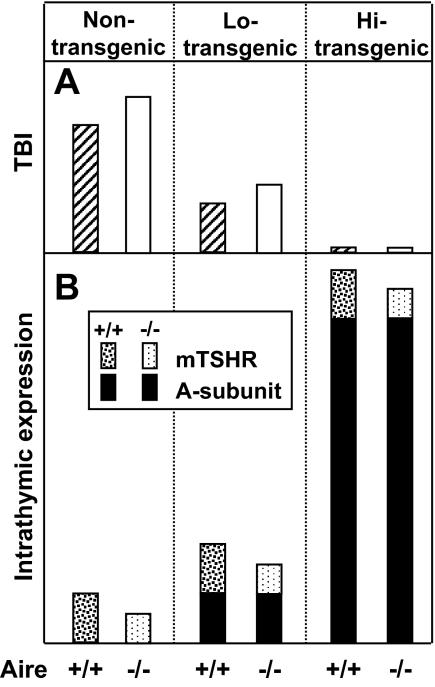
TSHR antibody levels and thyroid function for the low expressor A-subunit transgenic mice crossed with Aire-defective mice presented another interesting finding. Because the human A subunit is a self-antigen, TBI levels after human A-subunit Ad immunization were (as expected) far lower than in nontransgenic mice. Nevertheless, the pattern of TBI responses in Aire-defective transgenic mice was similar to that in the nontransgenics: higher TBI levels after two immunizations in Aire−/− Lo-expressor Tg-ic than Aire+/+ Hi-expressor Tg-ic mice (shown schematically in Fig. 6A), but no difference after the third immunization. None of the high expressor A-subunit transgenics developed TBI after immunization, and none became hyperthyroid. Surprisingly, despite the presence of TBI, hyperthyroidism did not develop in the A-subunit low expressor transgenics. The explanation for this discrepancy was revealed when TSHR antibodies were measured in a functional bioassay: TSAb activity was barely detectable and probably insufficient to induce hyperthyroidism.
Figure 6.
Schematic relationship between intrathymic expression of the endogenous TSHR or the transgenic human TSHR A subunit and induced TSHR antibody responses in mice that are deficient or sufficient for Aire. A, TBI levels after the second immunization in Aire +/+ and Aire −/− mice that are nontransgenic or transgenic mice that express low (Lo) or high (Hi) intrathyroidal levels of the human A subunit. B, Intrathymic expression of the mTSHR and human A subunit in mouse strains indicated in A.
Unlike extensive studies on spontaneous autoimmunity, there is little information on the response of Aire-deficient mice to immunization. One immunization with hen egg lysozyme (HEL) in complete Freund’s adjuvant induced higher T-cell proliferation (three to five times) in Aire−/− than wild-type mice (22). These findings are consistent with our observations of higher TSHR antibody levels after two A-subunit Ad immunizations in Aire−/− than in wild-type mice. A second issue concerns the outcome in Aire +/− (heterozygotes) vs. Aire+/+ mice. Several Aire-defective mouse strains have been generated that differ in the nature of the mutant allele. Some Aire mutations have a dominant negative effect on the normal allele that decreases intrathymic self-antigen expression in heterozygous Aire-defective mice (22,41). Little information was available on the model used in the present study (21). Our present findings showing no difference between the wild-type and Aire+/− mice are consistent with very recent data showing no effect of the mutated allele on the normal allele (45).
Negative selection of autoreactive T cells in the thymus (16) is dependent on intrathymic expression of self-antigens, with weak intrathymic expression increasing the risk of organ-specific autoimmunity. Indeed, even small changes in intrathymic self-antigen expression affect negative selection and increase the predisposition to autoimmunity (46,47). Thymic expression of thyroid autoantigens in human or rodent thymic tissue has been observed (27,28,29), but the effect of Aire was not investigated. Using microarrays, no difference was detected for intrathymic expression of mTSHR, Tg, or TPO in Aire −/− vs. +/+ mice (21) (www.ncbi.nlm.nih.gov/geo, accession no. GSE85). However, Aire dependency has been demonstrated for Tg. First, thymic deletion of self-reactive T cells was abolished in Aire-deficient mice that were transgenic for a HEL-specific T-cell receptor and for membrane-bound HEL controlled by the rat Tg promoter (48). Second, Ruan et al. (49) showed that Aire binds to the mouse Tg promoter and that intrathymic expression of Tg in Aire-deficient mice is 50% lower than in wild-type C57BL/6 mice.
Our studies provide insight into the relationship between Aire expression and central tolerance to murine thyroid autoantigens: the TSHR, Tg, TPO, and the transgenic human TSHR A subunit. Intrathymic expression of the endogenous TSHR is significantly reduced in Aire −/− vs. Aire +/+ mice (assessed by real-time PCR), and we confirmed the Aire dependency of Tg. However, our most striking finding was the 20- to 30-fold reduction of intrathymic TPO in the absence of Aire, far greater than the reduction for the TSHR or Tg. The potential clinical significance of these observations deserves consideration. Aire mutations do not appear to be susceptibility genes for autoimmune thyroid disease (e.g. Refs. 50 and 51). However, a novel Aire mutation (G228W) in a family with APECED cosegregated with autoimmune thyroiditis and hypothyroidism (52). Moreover, a high prevalence (50%) of APECED patients in southern Italy had antibodies to Tg, and particularly to TPO, and hypothyroidism in some patients (53). These clinical data are consistent with the marked reduction we observed for intrathymic TPO expression in Aire −/− mice. It is tempting to speculate that genetic variants of the TPO promoter region may affect intrathymic expression in humans and, in concert with other genes, contribute to the predisposition to developing Hashimoto’s thyroiditis.
Despite the Aire dependency for intrathymic TSHR expression we observed in mice, there are no reported cases of Graves’ disease in APECED patients. Moreover, the Italian APECED cohort lacked detectable TSHR antibodies (53). However, because APECED is rare and Graves’ disease is less common than Hashimoto’s thyroiditis, their coincidence may not be readily recognized. In addition, even if APECED/APS-1 patients have lower intrathymic TSHR levels, the magnitude of the decrease may be insufficient to permit the breakdown of tolerance to this autoantigen. However, Aire deficiency in mice accelerated the timing of the antibody response to A-subunit Ad immunization. These observations are reminiscent of myasthenia gravis, in which decreased intrathymic expression of the acetylcholine receptor α subunit was associated with early disease onset (47). It is conceivable that Aire mutations might play a role in a subset of patients with autoimmune hyperthyroidism, namely those that develop juvenile Graves’ disease.
Unlike the decreases for murine thyroid autoantigens, intrathymic expression of the human TSHR A-subunit transgene was not reduced in Aire −/− mice (Fig. 6B). We used the bovine Tg promoter to target the A subunit to the thyroid gland (14), whereas the model antigen HEL was targeted using the rat Tg promoter (48). A recent study provided information on an Aire-binding motif in the mouse Tg promoter (and likely in the rat Tg promoter) (49). This motif is absent from the bovine Tg promoter (GenBank accession no. M35823). Although a consensus Aire binding site is controversial (42), it is tempting to speculate that the absence of the putative Aire motif in the bovine Tg promoter explains the lack of Aire dependence of the A-subunit transgene.
How can we interpret the responses in A-subunit transgenic mice? Lo-expressor Aire +/+ transgenics have a partial response to human A-subunit immunization, and their intrathymic human A-subunit expression is similar to that of the endogenous mTSHR. Reduced TSHR antibody responses in Aire +/+ mice are likely explained by high homology between mouse and human A-subunit sequences (∼87%), which increases total intrathymic TSHR expression compared with nontransgenic mice (Fig. 6B, middle vs. left panels). However, Lo-expressor Aire −/− mice have the same intrathymic A-subunit levels (its expression is independent of Aire) but decreased mTSHR compared with that in their Aire +/+ counterparts. The small decrease in the latter is consistent with induction of higher TSHR antibody levels in Aire −/− Lo-expressors than in Aire +/+ Lo-expressor animals. Finally, in the high-expressor transgenics, intrathymic A-subunit expression is approximately 500 times greater than in the low expressors, completely masking the contribution of the mTSHR and making the mice totally unresponsive to A-subunit immunization (Fig. 6A, right panel).
In conclusion, our study provides insight into the role of Aire-mediated central tolerance in the development of thyroid autoimmunity. First, intrathymic expression of Tg and especially of TPO is decreased in Aire −/− mice, consistent with the high prevalence of destructive thyroid autoimmunity in some APECED/APS-1 populations. Second, moderate Aire dependence of thymic mTSHR expression influences the time course and magnitude of the antibody response to A-subunit Ad immunization. These features are associated with prolonged thyroid stimulation and persistent hyperthyroidism in Aire −/− mice, factors with potential clinical impact. Finally, thymic overexpression of the human A-subunit transgene demonstrates the critical role of intrathymic expression for breaking tolerance to a self-antigen.
Supplementary Material
Acknowledgments
We thank our colleagues for generously providing us with reagents: Dr. C. Benoist, Joslin diabetes Center, Boston, MA, for breeding pairs of Aire knockout heterozygotes on the BALB/c background; Dr. K. Yui, Nagasaki University, Nagasaki, Japan, for anti-CD25 hybridoma PC61; Dr. T. Tanaka, Osaka University, Osaka, Japan, for anti-CD122 hybridoma TMβ1; and Dr. John C. Morris, III, Mayo Clinic, Rochester, Minnesota, for the panel of synthetic TSH receptor synthetic peptides. We are also grateful for contributions by Dr. Boris Catz, Los Angeles, California.
Footnotes
This work was supported by National Institutes of Health Grants DK54684 (to S.M.M.) and DK19289 (to B.R.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 5, 2009
Abbreviations: Ad, Adenovirus; Aire, autoimmune regulator; APECED, autoimmune polyendocrinopathy candidiasis-ectodermal dystrophy; APS-1, autoimmune polyendocrine syndrome-type 1; CHO, Chinese hamster ovary; Con-Ad, control adenovirus lacking an insert; HEL, hen egg lysozyme; IFN, interferon; mTEC, medullary thymic epithelial cell; mTSHR, mouse TSH receptor; TBI, TSH binding inhibition; Tg, thyroglobulin; Tg-ic, transgenic; TPO, thyroid peroxidase; Treg, regulatory T cell; TSAb, thyroid stimulatory antibody; TSHR, TSH receptor.
References
- Rapoport B, McLachlan SM 2007 The thyrotropin receptor in Graves’ disease. Thyroid 17:911–922 [DOI] [PubMed] [Google Scholar]
- Nagayama Y 2007 Graves’ animal models of Graves’ hyperthyroidism. Thyroid 17:981–988 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Kita-Furuyama M, Ando T, Nakao K, Mizuguchi H, Hayakawa T, Eguchi K, Niwa M 2002 A novel murine model of Graves’ hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol 168:2789–2794 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Pichurin P, Chen CR, Latrofa F, Johnstone AP, McLachlan SM, Rapoport B 2002 Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor. J Clin Invest 110:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland PR, Rickards CR, Howells RD, Jones ED, Rees Smith B 1982 Photo-affinity labelling of the thyrotropin receptor. FEBS Lett 145:245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo D, Nagayama Y, Chazenbalk GD, Wadsworth HL, Rapoport B 1992 Role of amino acids 261–418 in proteolytic cleavage of the extracellular region of the human thyrotropin receptor. Endocrinology 130:2135–2138 [DOI] [PubMed] [Google Scholar]
- Loosfelt H, Pichon C, Jolivet A, Misrahi M, Caillou B, Jamous M, Vannier B, Milgrom E 1992 Two-subunit structure of the human thyrotropin receptor. Proc Natl Acad Sci USA 89:3765–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM 2003 The thyrotropin receptor autoantigen in Graves’ disease is the culprit as well as the victim. J Clin Invest 111:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JA, Gianoukakis AG, Salehi S, Moorhead J, Rao PV, Khan MZ, McGregor AM, Smith T, Banga JP 2006 Monoclonal pathogenic antibodies to the TSH receptor in Graves’ disease with potent thyroid stimulating activity but differential blocking activity activate multiple signaling pathways. J Immunol 176:5084–5092 [DOI] [PubMed] [Google Scholar]
- Mizutori Y, Saitoh O, Eguchi K, Nagayama Y 2006 Adenovirus encoding the thyrotropin receptor A-subunit improves the efficacy of dendritic cell-induced Graves’ hyperthyroidism in mice. J Autoimmun 26:32–36 [DOI] [PubMed] [Google Scholar]
- Kaneda T, Honda A, Hakozaki A, Fuse T, Muto A, Yoshida T 2007 An improved Graves’ disease model established by using in vivo electroporation exhibited long-term immunity to hyperthyroidism in BALB/c mice. Endocrinology 148:2235–2244 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Jaume JC, McLachlan SM, Rapoport B 1997 Engineering the human thyrotropin receptor ectodomain from a non-secreted form to a secreted, highly immunoreactive glycoprotein that neutralizes autoantibodies in Graves’ patients’ sera. J Biol Chem 272:18959–18965 [DOI] [PubMed] [Google Scholar]
- Chazenbalk G, McLachlan S, Pichurin P, Yan XM, Rapoport B 2001 A “prion-like” shift between two conformational forms of a recombinant thyrotropin receptor A subunit module: purification and stabilization using chemical chaperones of the form reactive with Graves’ autoantibodies. J Clin Endocrinol Metab 86:1287–1293 [DOI] [PubMed] [Google Scholar]
- Pichurin PN, Chen CR, Chazenbalk GD, Aliesky H, Pham N, Rapoport B, McLachlan SM 2006 Targeted expression of the human thyrotropin receptor A-subunit to the mouse thyroid: Insight into overcoming the lack of response to A-subunit adenovirus immunization. J Immunol 176:668–676 [DOI] [PubMed] [Google Scholar]
- McLachlan SM, Nagayama Y, Pichurin PN, Mizutori Y, Chen CR, Misharin A, Aliesky HA, Rapoport B 2007 The link between Graves’ disease and Hashimoto’s thyroiditis: a role for regulatory T cells. Endocrinology 148:5724–5733 [DOI] [PubMed] [Google Scholar]
- Kappler JW, Roehm N, Marrack P 1987 T cell tolerance by clonal elimination in the thymus. Cell 49:273–280 [DOI] [PubMed] [Google Scholar]
- Derbinski J, Schulte A, Kyewski B, Klein L 2001 Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2:1032–1039 [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Cooke A, Peterson P, Rich T 2008 Death in the AIRE. Trends Immunol 29:306–312 [DOI] [PubMed] [Google Scholar]
- Finnish-German APECED Consortium 1997 An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 17:399–403 [DOI] [PubMed] [Google Scholar]
- Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N 1997 Positional cloning of the APECED gene. Nat Genet 17:393–398 [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D 2002 Projection of an immunological self shadow within the thymus by the aire protein. Science 298:1395–1401 [DOI] [PubMed] [Google Scholar]
- Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kämpe O, Eskelin P, Pelto-Huikko M, Peltonen L 2002 Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet 11:397–409 [DOI] [PubMed] [Google Scholar]
- Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C 2005 Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med 202:805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki S, Oshikawa K, Mouri Y, Hirota F, Matsushima A, Yano M, Han H, Bando Y, Izumi K, Matsumoto M, Nakayama KI, Kuroda N, Matsumoto M 2006 Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. J Clin Invest 116:1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Ferrer-Francesch X, Domínguez O, Juan M, Foz-Sala M, Pujol-Borrell R 1998 Transcription of a broad range of self-antigens in human thymus suggests a role for central mechanisms in tolerance toward peripheral antigens. J Immunol 161:5918–5929 [PubMed] [Google Scholar]
- Li HS, Carayanniotis G 2005 Detection of thyroglobulin mRNA as truncated isoform(s) in mouse thymus. Immunology 115:85–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Hosoi Y, Negishi T, Kamiya Y, Miyashita K, Yamada M, Iriuchijima T, Yokoo H, Yoshida I, Tsushima Y, Mori M 1996 Thymic hyperplasia in patients with Graves’ disease. Identification of thyrotropin receptors in human thymus. J Clin Invest 98:2228–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton CM, Joba W, Spitzweg C, Heufelder AE, Bahn RS 1997 Thyrotropin receptor expression in adrenal, kidney, and thymus. Thyroid 7:879–884 [DOI] [PubMed] [Google Scholar]
- Murakami M, Hosoi Y, Araki O, Morimura T, Imamura M, Ogiwara T, Mizuma H, Mori M 2001 Expression of thyrotropin receptors in rat thymus. Life Sci 68:2781–2787 [DOI] [PubMed] [Google Scholar]
- Chen CR, Aliesky H, Pichurin PN, Nagayama Y, McLachlan SM, Rapoport B 2004 Susceptibility rather than resistance to hyperthyroidism is dominant in a thyrotropin receptor adenovirus-induced animal model of Graves’ disease as revealed by BALB/c-C57BL/6 hybrid mice. Endocrinology 145:4927–4933 [DOI] [PubMed] [Google Scholar]
- Kekäläinen E, Tuovinen H, Joensuu J, Gylling M, Franssila R, Pöntynen N, Talvensaari K, Perheentupa J, Miettinen A, Arstila TP 2007 A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol 178:1208–1215 [DOI] [PubMed] [Google Scholar]
- Chen CR, Aliesky HA, Guo J, Rapoport B, McLachlan SM 2006 Blockade of costimulation between T cells and antigen-presenting cells: an approach to suppress murine Graves’ disease induced using thyrotropin receptor-expressing adenovirus. Thyroid 16:427–434 [DOI] [PubMed] [Google Scholar]
- Saitoh O, Nagayama Y 2006 Regulation of Graves’ hyperthyroidism with naturally occurring CD4+CD25+ regulatory T cells in a mouse model. Endocrinology 147:2417–2422 [DOI] [PubMed] [Google Scholar]
- Saitoh O, Abiru N, Nakahara M, Nagayama Y 2007 CD8+CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves’ hyperthyroidism in a murine model. Endocrinology 148:6040–6046 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Wang Y, Guo J, Hutchison JS, Segal D, Jaume JC, McLachlan SM, Rapoport B 1999 A mouse monoclonal antibody to a thyrotropin receptor ectodomain variant provides insight into the exquisite antigenic conformational requirement, epitopes and in vivo concentration of human autoantibodies. J Clin Endocrinol Metab 84:702–710 [DOI] [PubMed] [Google Scholar]
- Morris JC, Bergert ER, McCormick DJ 1993 Structure-function studies of the human thyrotropin receptor. Inhibition of binding of labeled thyrotropin (TSH) by synthetic human TSH receptor peptides. J Biol Chem 268:10900–10905 [PubMed] [Google Scholar]
- Pichurin P, Schwarz-Lauer L, Braley-Mullen H, Paras C, Pichurina O, Morris JC, Rapoport B, McLachlan SM 2002 Peptide scanning for thyrotropin receptor T-cell epitopes in mice vaccinated with naked DNA. Thyroid 12:755–764 [DOI] [PubMed] [Google Scholar]
- McLachlan SM, Aliesky HA, Pichurin PN, Chen CR, Williams RW, Rapoport B 2008 Shared and unique susceptibility genes in a mouse model of Graves’ disease determined in BXH and CXB recombinant inbred mice. Endocrinology 149:2001–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misharin A, Hewison M, Chen CR, Lagishetty V, Aliesky HA, Mizutori Y, Rapoport B, McLachlan SM 2009 Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology 150:1051–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kont V, Laan M, Kisand K, Merits A, Scott HS, Peterson P 2008 Modulation of Aire regulates the expression of tissue-restricted antigens. Mol Immunol 45:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D, Benoist C 2007 A decade of AIRE. Nat Rev Immunol 7:645–650 [DOI] [PubMed] [Google Scholar]
- Patibandla SA, Fan JL, Prabhakar BS, Seetharamaiah GS 1999 Comparison of immune responses to extracellular domains of mouse and human thyrotropin receptor. J Autoimmun 13:205–213 [DOI] [PubMed] [Google Scholar]
- Chen Z, Benoist C, Mathis D 2005 How defects in central tolerance impinge on a deficiency in regulatory T cells. Proc Natl Acad Sci USA 102:14735–14740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su MA, Giang K, Zumer K, Jiang H, Oven I, Rinn JL, Devoss JJ, Johannes KP, Lu W, Gardner J, Chang A, Bubulya P, Chang HY, Peterlin BM, Anderson MS 2008 Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest 118:1712–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ST, Lucassen AM, Gough SC, Powell EE, Undlien DE, Pritchard LE, Merriman ME, Kawaguchi Y, Dronsfield MJ, Pociot F 1995 Susceptibility to human type 1 diabetes at IDDM2 is determined by tandem repeat variation at the insulin gene minisatellite locus. Nat Genet 9:284–292 [DOI] [PubMed] [Google Scholar]
- Giraud M, Taubert R, Vandiedonck C, Ke X, Lévi-Strauss M, Pagani F, Baralle FE, Eymard B, Tranchant C, Gajdos P, Vincent A, Willcox N, Beeson D, Kyewski B, Garchon HJ 2007 An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature 448:934–937 [DOI] [PubMed] [Google Scholar]
- Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L, Goodnow CC 2004 Gene dosage—limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med 200:1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan QG, Tung K, Eisenman D, Setiady Y, Eckenrode S, Yi B, Purohit S, Zheng WP, Zhang Y, Peltonen L, She JX 2007 The autoimmune regulator directly controls the expression of genes critical for thymic epithelial function. J Immunol 178:7173–7180 [DOI] [PubMed] [Google Scholar]
- Nithiyananthan R, Heward JM, Allahabadia A, Barnett AH, Franklyn JA, Gough SC 2000 A heterozygous deletion of the autoimmune regulator (AIRE1) gene, autoimmune thyroid disease, and type 1 diabetes: no evidence for association. J Clin Endocrinol Metab 85:1320–1322 [DOI] [PubMed] [Google Scholar]
- Meyer G, Donner H, Herwig J, Bohles H, Usadel KH, Badenhoop K 2001 Screening for an AIRE-1 mutation in patients with Addison’s disease, type 1 diabetes, Graves’ disease and Hashimoto’s thyroiditis as well as in APECED syndrome. Clin Endocrinol (Oxf) 54:335–338 [DOI] [PubMed] [Google Scholar]
- Cetani F, Barbesino G, Borsari S, Pardi E, Cianferotti L, Pinchera A, Marcocci C 2001 A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J Clin Endocrinol Metab 86:4747–4752 [DOI] [PubMed] [Google Scholar]
- Perniola R, Filograna O, Greco G, Pellegrino V 2008 High prevalence of thyroid autoimmunity in Apulian patients with autoimmune polyglandular syndrome type 1. Thyroid 18:1027–1029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.