Abstract
Brief anesthesia, such as after exposure to high levels of carbon dioxide, prior to decapitation is considered a more humane alternative for the euthanasia of rodents, compared with use of decapitation alone. Studies of the levels of certain stress hormones in plasma such as corticosterone and ACTH have supported the use of this method of euthanasia in endocrinological and molecular studies. In the current study, rats were briefly exposed to a chamber filled with carbon dioxide until recumbent (20–25 sec), immediately killed via decapitation, and trunk blood collected; findings were compared with rats killed via decapitation with no exposure to carbon dioxide. RIAs were used to measure arginine vasopressin (AVP) and ACTH immunoreactivity (ir) in plasma. Whereas ACTH-ir levels remained steady after brief exposure to carbon dioxide (in accordance with results of other investigators), AVP-ir levels were increased by more than an order of magnitude. These results were confirmed by quantitative capillary-liquid chromatography-mass spectrometry, indicating this observation of rapid increase in plasma AVP-ir levels is not due to nonspecific recognition by the antibody used in the RIA. Likewise, using capillary-liquid chromatography-mass spectrometry, we observed a rapid increase in plasma oxytocin levels after carbon dioxide exposure. These surprising findings have important implications for the design and interpretation of studies involving brief carbon dioxide exposure prior to decapitation as well as those with euthanasia resulting from carbon dioxide-induced asphyxiation.
Brief carbon dioxide exposure prior to decapitation leads to dramatic release of vasopressin, and this method of euthanasia is therefore unsuitable for certain endocrinological investigations.
In the euthanization of animals, various factors must be considered, not the least of which is the humane treatment of the animals, but also the safety and comfort of the laboratory workers and the possible effects of the euthanasia method on any posthumous measurements that may be germane to the study (1). A common method of killing rodents is asphyxiation using carbon dioxide, which has been recommended for some time by the Universities Federation for Animal Welfare and the American Veterinary Medical Association (AVMA, Schaumburg, IL, http://www.avma.org/issues/animal_welfare/euthanasia.pdf; and see Ref. 2). In addition to use in asphyxiation-induced euthanasia, exposure to CO2 for shorter time periods results in anesthesia. Exposure to CO2 until the animal has become recumbent, followed by rapid decapitation is also a common method for euthanasia and is more suitable for endocrinological measurements (4). This method has been shown to have minimal effect on the levels of certain hormones related to stress response, with only a slight decrease in the plasma levels of corticosterone and prolactin, in rats (4). Similarly, exposure to CO2 for 30 sec did not lead to changes in plasma levels of ACTH, although longer exposure did lead to elevations (5). The use of anesthetics, including CO2, before decapitation, has been suggested as a more ethical method for euthanasia than decapitation alone (1,4).
Since the initial enumeration of antidiuretic hormone [also known as arginine vasopressin (AVP)] and its ability to induce antidiuresis and alter arterial pressure, AVP has been intensely studied and characterized, and shown to have a role in various systems (6). A number of early pioneering studies focused on the release of AVP into plasma induced by pharmacological agents, including anesthetics (7). There have been studies focusing on the effects of CO2 as an agent in euthanasia, either as an anesthetic before decapitation or as the primary inducer of death via asphyxiation, on several behavioral and endocrine parameters (4,5,8,9,10,11), but to our knowledge the effect on AVP at the time of death after such brief exposure has not been previously addressed. More prolonged exposure to CO2 as well as hypoxic conditions (low O2) in hypercapnia/hypoxia studies has been addressed, and large increases in plasma vasopressin were observed (12,13,14). We report here a dramatic and very rapid increase in the plasma levels of AVP, as measured by RIA as well as capillary-liquid chromatography mass spectrometry, after exposure to CO2, which serves as a cautionary note for the design and interpretation of studies incorporating CO2 exposure for the purposes of euthanasia.
Materials and Methods
Animals
Male Fischer and Lewis rats (13–18 wk, 200–250 g) were used in all studies herein and purchased from Charles River Laboratories (Wilmington, MA). Rats were singly housed in stress-minimized rooms, with a 12-h light, 12-h dark cycle, and food and water were provided ad libitum. All animals were housed for at least 1 wk, with daily handling, before studies. Animals were housed and euthanized in a manner approved by The Rockefeller University Institutional Animal Care and Use Committee. Two groups of animals were studied. One was rapidly decapitated immediately upon removal from the home cage and trunk blood collected in ice-cold EDTA-coated tubes. The other group was placed in a chamber that was precharged with CO2. The characteristics of the chamber were 18 in. (length) × 12 in. (width) × 18 in. (height) plexiglas, with a metal grid approximately 8 in. from the floor. Approximately 1 lb of dry ice pellets were placed on the floor of the chamber and allowed to sublime for at least 5 min with the chamber lid (equipped with small air holes for emission of ambient air) closed. The level of CO2 in the chamber after 5 min preequilibration with dry ice was observed to be 100% (±10%), using Gastec calibrated ultrahigh-range carbon dioxide calorimetric detection tubes (Nextteq, Tampa, FL); a similar chamber setup for CO2-induced recumbency has previously been reported to have a chamber level of 97% CO2 (8). Animals were monitored until recumbent (defined as animal being prostrate and unresponsive), which for all rats occurred within a time range of 20–25 sec. The animals were then rapidly decapitated and trunk blood collected. Plasma was obtained by centrifuging the tubes at 3100 × g for 15 min at 4 C.
RIA
After collection, plasma was divided into fractions for mass spectrometry (see below), AVP RIA, and ACTH RIA. For ACTH, RIA was performed using untreated plasma, with a kit from Diasorin (Stillwater, MN), in a manner similar to our previous studies (15,16). AVP RIA was performed using a kit from Phoenix Pharmaceuticals (Belmont, CA). Plasma (500 μl) was dried down using a vacuum centrifuge and reconstituted in binding buffer, and the RIA was then performed according to the manufacturer’s instructions.
Capillary-liquid chromatography-mass spectrometry
Plasma (100 μl) was diluted 1:1 with 2% trifluoroacetic acid and filtered using centricon filters (molecular weight cutoff 10 kDa; Millipore, Billerica, MA) to remove proteins. Approximately 150 μl were recovered, with 50 μl remaining as the retentate. Capillary liquid chromatography was performed using in-house packed reversed-phase capillary columns. The analytical column (75 μm inner diameter, 5 cm length) was packed using PicoTip Emitter fused silica capillaries (New Objective, Woburn, MA) and 5 μm C18 beads, and the concentrating precolumn (75 μm inner diameter, 5 cm length) was packed using Integrafrit fused silica capillaries (New Objective) and 15 μm C18 beads. Then 150 μl of diluted, filtered plasma was loaded onto the precolumn, manually using a pressure bomb. A Pharmacia (Uppsala, Sweden) SMART system delivered a gradient of mobile phase A (1% acetic acid in water) and B (1% acetic acid in 80% acetonitrile/20% water): 0 min, 0% B, ramping to 100% B at 17 min, holding at 100% B until 23 min, returning to 0% B at 28 min, followed by a 15-min equilibration phase. The flow rate of the system was set to 30 μl/min, with a flow split resulting in column flow of approximately 100 nl/min, measured using graduated capillaries.
The capillary liquid chromatography system was interfaced with a LTQ ion trap mass spectrometer (Thermo Scientific, Waltham, MA). The mass spectrometric method was set up to record, serially, a mass spectrum (ms) scan, with a m/z range of 300-2000, followed by data-independent ms2 and ms3 scans for AVP (ms2 precursor ion 1 m/z 543.0; ms3 precursor ion 1 m/z 543.0, precursor ion 2 m/z 328.3), oxytocin (ms2 precursor ion 1 m/z 504.5; ms3 precursor ion 1 m/z 504.5, precursor ion 2 m/z 285.2), and ACTH (ms2 precursor ion 1 m/z 764.7; ms3 precursor ion 1 m/z 764.7, precursor ion 2 m/z 884.4). In all cases, the collision-induced activation level was set to 35.0% according to the manufacturer’s nomenclature, with an activation time of 30.0 msec, and the window for precursor ion 1 was set to a width of m/z = 2.0, and the window for precursor ion 2 was set to a width of m/z = 1.0. The mass spectral data were analyzed using the Thermo Scientific software package Xcaliber. For standard runs, AVP was obtained from Phoenix Pharmaceuticals; oxytocin was synthesized by the Rockefeller University Proteomics Resource Center.
Results
RIA
RIAs were conducted for the measurement of ACTH and AVP in rat plasma, from both Fischer and Lewis rats (Fig. 1). Our laboratory has extensive experience with ACTH RIA (15,16). The AVP RIA has not been used prior by our laboratory, although it has been used by many other groups. Whereas ACTH levels did not change on this brief exposure of the rats to CO2, the levels of AVP increased drastically by greater than an order of magnitude (P < 0.005 for both strains of rats).
Figure 1.
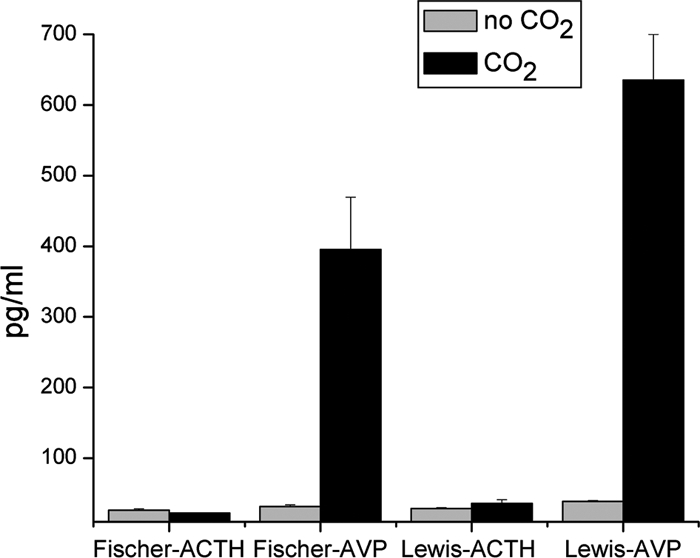
Plasma from Lewis and Fischer rats, either decapitated without CO2 (n = 3 for all groups) or exposed for 20–25 sec before decapitation, was used for RIAs of ACTH and AVP. Whereas AVP showed no change, plasma levels of AVP increased by more than 10-fold in each strain of rat.
Mass spectrometry
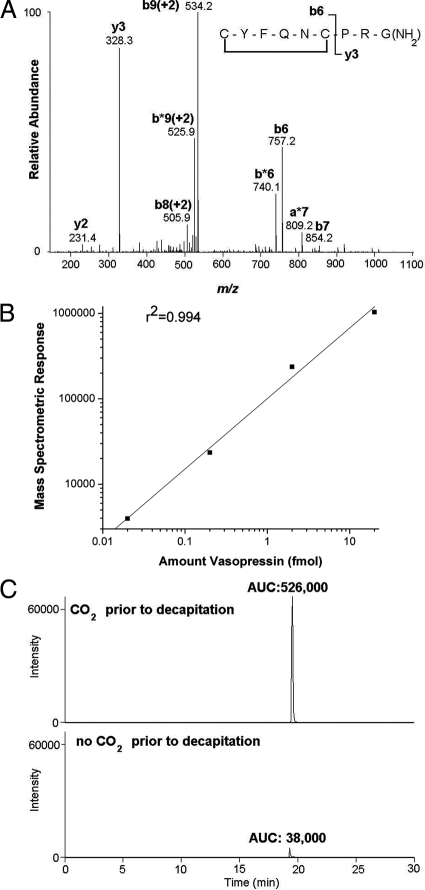
The fragmentation pattern of AVP analysis was determined using AVP standards prepared in PBS immediately before loading onto the column for mass spectrometric analysis (Fig. 2A). For purposes of quantification of AVP, we used the integrated peak area of a mass chromatogram using ms2 tandem mass spectra (precursor ion − vasopressin [M+2H]+2 = 543.0 m/z, observing y3 product ion = 328.3 m/z). A standard curve was generated over the range of 20 amol to 20 fmol of AVP in PBS by monitoring the integrated peak intensity from the ms2 mass chromatogram; we observed a linear relationship of the integrated peak area over 3 orders of magnitude (Fig. 2B). Rats exposed to CO2 gave a consistently higher integrated peak intensity corresponding to AVP compared with rats killed without CO2, with representative chromatograms shown in Fig. 2C. Identification of the indicated peak as endogenous AVP was further verified by the ms3 spectrum (data not shown).
Figure 2.
Mass spectrometric detection of AVP. A, Fragmentation pattern of AVP: the most prominent fragments resulting from internal cleavage sites correspond to cleavage of the N-terminal cyclic moiety from the C-terminal tripeptide PRGamide, resulting in the y3 and b6 ions. B, AVP standards (0.02, 0.2, 2, and 20 fmol) were loaded in succession onto the capillary-liquid chromatography column and the eluent monitored via tandem mass spectrometry. Ms2, mass chromatogram: doubly charged ion of AVP served as the precursor ion: m/z, 543.0 [ms2 precursor ion 543.0 m/z; product ion monitoring of 328.2 m/z]. The base peak was set to correspond to the y3 product ion (328.3), and the resulting peaks were integrated using Xcaliber software (Thermo Scientific). The integrated peak areas are plotted vs. the amount of AVP loaded onto the column; a linear relationship for the amounts tested was found (r2 = 0.994). C, Capillary-liquid chromatography-mass spectrometric detection of endogenous AVP in plasma. Ms2 mass chromatograms [ms2 precursor ion 543.0 m/z; product ion monitoring of 328.2 m/z] of plasma from representative rats exposed to CO2 (upper plot) or decapitated without CO2 exposure (lower plot), with the base peak set to the y3 ion, 328.3. Integration of the peaks is indicated by the resulting area under the curve (AUC). The ms2 spectra were compared with the spectra from standard AVP (A) to confirm that the peaks shown correspond to authentic endogenous AVP.
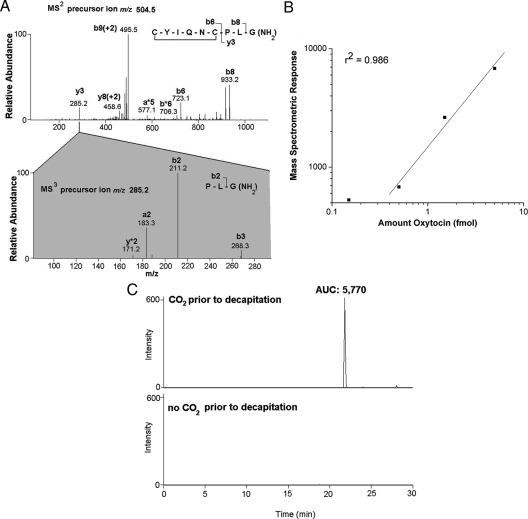
In rats that were subject to CO2 anesthesia before decapitation, oxytocin was unambiguously identified in plasma using mass spectrometry, based on the ms2 and ms3 fragmentation patterns (Fig. 3A). The background of the mass chromatogram using ms2 tandem mass spectra (precursor ion − oxytocin [M+2H]+2 = 504.5 m/z, observing y3 product ion = 285.2 m/z) was too high for use in the quantification of oxytocin in plasma; hence, we used ms3 tandem mass spectra (precursor ion 1 − oxytocin [M+2H]+2 = 504.5 m/z, precursor ion 2 − y3 fragment ion = 285.2 m/z, observing b2 product ion = 211.2 m/z). A standard curve of oxytocin in PBS indicated the linearity of integrated peak area of the ms3 product ion 211.2 above 0.5 fmol (Fig. 3B). We observed oxytocin in the plasma of rats exposed to CO2, but not in rats that were not exposed, indicating that the levels of oxytocin present basally in plasma are below the detection limits of the method (Fig. 3C). In comparison with the standard curve of oxytocin (Fig. 3B), the integrated peak areas observed from the plasma of rats exposed to CO2 correspond to at least 10-fold greater than the detection limits, indicating the extent of increase in plasma oxytocin induced by CO2 exposure is likely greater than 10-fold, in concordance with the increase in AVP.
Figure 3.
Capillary-liquid chromatography-mass spectrometric detection of endogenous oxytocin in plasma. A, Ms2 and ms3 spectra of oxytocin in a representative rat exposed to CO2, with m/z for precursor ions: 504.5 in the ms2 spectrum (doubly charged oxytocin), 285.2 in the ms3 spectrum (the y3 product ion of the oxytocin precursor ion), showing clear correspondence to endogenous oxytocin, with observed ions marked on the inset peptide backbone fragmentation diagram. B, Using oxytocin standard peptide in PBS, we determined the linearity and estimated the detection limits of oxytocin. With 5 fmol, the area under the curve (AUC) of the integrated peak of oxytocin ms3 [precursor ion 1 = 504.5 m/z, doubly charged oxytocin; precursor ion 2 = 285.2 m/z, y3 product ion, product ion monitoring of 211.2 m/z], was 6820. The AUC was linear as the concentration was decreased an order of magnitude, to 0.5 (AUC of 0.5 fmol, 680) (r2 = 0.986). Measurements of AUC less than 600 were not reliable, without correspondence to concentration and spectra typically lacking other fragment ions corresponding to the 285.2 product ion (A). Although the AUC for 0.15 fmol of oxytocin is plotted, it was not included in the regression line. C, Ms3 chromatograms [precursor ion 1 = 504.5 m/z, doubly charged oxytocin; precursor ion 2 = 285.2 m/z, y3 product ion, product ion monitoring of 211.2 m/z], for representative rats exposed to CO2 (upper chromatogram) or not exposed to CO2 (lower chromatogram). There was no oxytocin detected, using ms3 fragmentation criteria, in the absence of CO2, whereas we observed oxytocin with AUC of 5770 after CO2 exposure.
Although we monitored ACTH using ms2 and ms3, we did not detect ACTH in either group of animals, indicating that the plasma levels of ACTH are below the detection limits of the capillary-liquid chromatography-mass spectrometric method (data not shown).
Discussion
In our laboratory, investigating stress responsivity in drug abuse, we have been using CO2 before decapitation for a number of years in our studies of rodent models of drug abuse, with measurements of plasma levels of hormones such as corticosterone and ACTH as common end points (15,16). Recently we observed that after exposure to CO2, the levels of vasopressin in plasma were considerably higher than otherwise unprovoked levels in plasma reported in the literature, which are typically in the range of 0.5 to 5 pm (17,18,19). We therefore compared the effects of CO2 exposure vs. the absence of CO2 exposure to investigate this discrepancy. The rapidity and extent of AVP plasma increase are very dramatic and were unanticipated.
The use of RIA to measure AVP in plasma has a long history and has been used to show perturbations of AVP levels in plasma due to a variety of stimuli: pharmacological, cardiovascular, osmotic, etc. (20). The surprising results presented here, however, raise the possibility of artifactual cross-reactivity. The use of mass spectrometry for the detection of endogenous AVP confirmed that there was indeed a large, dramatic increase in the plasma levels of AVP after brief CO2 exposure (Fig. 2C). The fragmentation of vasopressin and oxytocin (Figs. 2A and 3A) are similar to that previously observed by other investigators (21,22). The standard curve (Fig. 2B) obtained for AVP present in PBS indicates that the integrated signal of the y3 ion (m/z = 328.3) in tandem mass spectra of AVP is a reliable measure of AVP levels. The curve demonstrates linearity of the integrated peak intensity of AVP in the ms2 mass chromatogram greater than 3 orders of magnitude (also found to be linear in another recent mass spectrometric study of AVP) (21). Although relative levels of AVP can be determined, we note that the absolute levels of AVP in plasma cannot be discerned by comparison with the standard curve shown in Fig. 2B; the presence of other peptides in plasma are expected to alter the extent of vasopressin ionization and hence the peak intensities.
The use of mass spectrometry in this instance provided the additional advantage of allowing us to also determine the presence of oxytocin in the blood after CO2 exposure. We did not have oxytocin antibodies or radioiodinated standards immediately available to perform RIA of oxytocin; the mass spectral parameters corresponding to oxytocin were included in the mass spectrometric method essentially as a hunch, given the relationship of oxytocin to vasopressin. Because the baseline levels of oxytocin were not detectable, the extent of increase cannot be accurately determined, although the results of a comparison of standards (Fig. 3B) indicate the oxytocin increase to be at least 10-fold. Although the basal plasma levels of oxytocin and AVP are generally comparable (19), the relative sensitivity of our method is sufficient for detection of basal AVP but not basal oxytocin.
The demonstration of a large increase in plasma oxytocin levels strengthens the notion that CO2 induces a significant change in the hormone levels of plasma and adds a further note of caution in the use of CO2 in the euthanasia of animals. Note that in the case of oxytocin, ms2 was not sufficient for detection of oxytocin above background, but ms3 unambiguously shows the presence of this peptide after CO2 exposure. The use of ms3 for the detection of peptides has similarly been recently used for the detection of endogenous enkephalins (23). Further improvements in sample preparation, including the use of higher levels of plasma, will be investigated with an eye toward the detection of basal levels of oxytocin. No ACTH was observed in any sample using mass spectrometry, indicating the levels of ACTH were below the detection limits of mass spectrometry in both the absence and presence of CO2 exposure, results not inconsistent with the results of the RIA for AVP.
Previous studies have also investigated hypothalamic-pituitary-adrenal (HPA) axis activation through the measurement of ACTH levels after CO2 exposure. After 2 min of exposure to CO2, ACTH levels are increased severalfold (5,24). Hackbarth et al. (5), in a comparative study, with different times of CO2 exposure, found that ACTH levels had not yet begun to rise after 30 sec of CO2 exposure, in concordance with the results found here (Fig. 1). Several previous studies in our laboratory using similarly brief (<30 sec) CO2-induced anesthesia before decapitation of rodents demonstrated plasma ACTH and corticosterone levels, which are likewise not consistent with pronounced HPA axis activation (15,16,25,26), although it should be noted these studies did not directly compare animals with and without CO2 exposure. It is possible that the delay in CO2-induced ACTH increase is due to a requirement for prior release of an ACTH secretogogue, such as corticotrophin releasing factor; because AVP has been shown to be an ACTH secretogogue (27), it may be that the substantial increase in AVP levels observed here is the primary factor for the rise in ACTH levels observed after 2 min CO2 exposure (5,24).
Whereas the results of the current study were entirely unanticipated, there are reports of investigations that are in concordance with the AVP release observed herein. In addition to the hypoxia/hypercapnia studies in canines (13,14), which were done using longer exposures, brief CO2 exposure in rats, although generally found to have modest effects on hormonal levels (4), results in significant analgesia, for up to an hour after CO2 exposure (10). The mediator of this analgesia was not determined, but it was shown to be both nonopioidergic, because it was not blocked by naloxone, and dependent on the presence of an intact pituitary gland, because it was blocked by neurohypophysectomy. Because intravenous AVP at high doses in rats induces nonopioidergic analgesia, also for up to an hour (28), the present results suggest that the release of high levels of endogenous vasopressin possibly serves as the mediator of the CO2-induced analgesia.
The investigation of CO2 effects on plasma hormone levels in man (29,30,31,32,33,34) correspond to some degree with the findings in animals, although the extent of exposure and the time course of plasma sampling are necessarily different. In a study by Kaye et al. (31), exposure to a single breath of 35% CO2 resulted in a significant increase in ACTH in as little as 2 min, similarly to that described in rodents after longer and higher levels of CO2 (5,24), although the increase is much more modest, as should be expected. These investigators did not find an increase in AVP at the 2-min time point. Certainly there are a number of differences between their study and the current one, including CO2 concentration, exposure duration, sampling time, and species studied, which likely accounts for this difference.
Our finding of a dramatic CO2-induced increase in AVP and oxytocin has important implications for the use of CO2 in methods of euthanasia in studies involving endocrinological measurements. Currently we can but speculate on the potential mechanisms underlying this rapid CO2-induced increase in AVP and CO2. The regulatory mechanism may involve mediation by glutamate (35), catecholamines (36,37), serotonin (30,38), or acetylcholine (39), possibly after preliminary activation of carotid chemoreceptors (40). It is possible that hypotension plays a role. Future investigations into the mechanism of CO2-induced increases in AVP may yield insights into other effects of CO2, such as panic-associated behaviors and later increases in HPA activity (29,30,31,32,33,34,36).
Acknowledgments
The authors thank Dr. Yan Zhou and Dr. Eduardo Butelman for helpful conversations regarding the findings of this study as well as Dr. Beatrix Ueberheid for valuable guidance regarding the capillary-liquid chromatography mass spectrometric instrumentation.
Footnotes
This work was supported by National Institutes of Health-National Institute on Drug Abuse Grant P60-DA05130 (to M.J.K.) and National Institutes of Health-National Center for Research Resources Grant RR00862 (to B.T.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 12, 2009
For editorial see page 2505
Abbreviations: AVP, Arginine vasopressin; HPA, hypothalamic-pituitary-adrenal; ms, mass spectrum.
References
- Danneman PJ 2000 Euthanasia. In: Silverman J, Suckow MA, Murthy S, eds. The IACUC handbook. New York: CRC Press; 251–276 [Google Scholar]
- Ewbank R 1983 Is CO2 euthanasia humane? Nature 305:268 [Google Scholar]
- Stricker EM, Sved AF 2002 Controls of vasopressin secretion and thirst: similarities and dissimilarities in signals. Physiol Behav 77:731–736 [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Kelley ST 1991 Sedation by exposure to a gaseous carbon dioxide-oxygen mixture: application to studies involving small animal species. Lab Anim Sci 41:80–82 [PubMed] [Google Scholar]
- Hackbarth H, Küuppers N, Bohnet W 2000 Euthanasia of rats with carbon dioxide—animal welfare aspects. Lab Anim 34:91–96 [DOI] [PubMed] [Google Scholar]
- Urban IJA, Burbach JPH, De Wied D, eds. 1998 Advances in brain vasopressin. Progress in brain research. Vol. 119. New York: Elsevier [Google Scholar]
- Heller H, Ginsburg M 1966 Secretion, metabolism and fate of the posterior pituitary hormones. In: Harris GW, Donovan BT, eds. The pituitary gland. Vol. 3. Berkeley, CA: University of California Press; 330–373 [Google Scholar]
- Blackshaw JK, Fenwick DC, Beattie AW, Allan DJ 1988 The behavior of chickens, mice and rats during euthanasia, carbon dioxide and ether. Lab Anim 22:67–75 [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Berger UV, Sharma M, Paul CA 1994 Effects of carbon dioxide-induced anesthesia on cholinergic parameters in rat-brain. Lab Anim Sci 44:369–371 [PubMed] [Google Scholar]
- Mischler SA, Alexander M, Battles AH, Raucci Jr JA, Nalwalk JW, Hough LB 1994 Prolonged antinociception following carbon dioxide anesthesia in the laboratory rat. Brain Res 640:322–327 [DOI] [PubMed] [Google Scholar]
- Danneman PJ, Stein S, Walshaw SO 1997 Human and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci 47:376–385 [PubMed] [Google Scholar]
- Heyes MP, Farber MO, Manfredi F, Robertshaw D, Weinberger M, Fineberg M, Robertson G 1982 Acute effects of hypoxia on renal and endocrine function in normal humans. Am J Physiol 243:R265–R270 [DOI] [PubMed] [Google Scholar]
- Rose Jr CE, Anderson RJ, Carey RM 1984 Antidiuresis and vasopressin release with hypoxemia and hypercapnia in conscious dogs. Am J Physiol 247:R127–R134 [DOI] [PubMed] [Google Scholar]
- Wang BC, Sundet WC, Goetz KL 1984 Vasopressin in plasma and cerebrospinal fluid of dogs during hypoxia or acidosis. Am J Physiol 247:E449–E455 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yuferov VP, Spangler R, Maggos CE, Ho A, Kreek MJ 1998 Effects of memantine alone and with acute ‘binge’ cocaine on hypothalamo-pituitary-adrenal activity in the rat. Eur J Pharmacol 352:65–71 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ 2008 Involvement of arginine vasopressin and VIb receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology 33:226–236 [DOI] [PubMed] [Google Scholar]
- Dogterom J, van Wimersma Greidanus TB, De Wied D 1978 Vasopressin in cerebrospinal fluid and plasma of man, dog, and rat. Am J Physiol 234:E463–E467 [DOI] [PubMed] [Google Scholar]
- Patchev VK, Kalogeras KT, Zelazowski P, Wilder RL, Chrousos GP 1992 Increased plasma concentrations, hypothalamic content, and in vitro release of arginine vasopressin in inflammatory disease-prone, hypothalamic corticotropin-releasing factor hormone-deficient Lewis rats. Endocrinology 131:1453–1457 [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Vecsemyés M, Laczi F, Bíró E, Szabó G, Kovács GL 1992 Effects of cocaine on the contents of neurohypophyseal hormones in the plasma and in different brain structures in rats. Neuropeptides 23:27–31 [DOI] [PubMed] [Google Scholar]
- Forsling ML 1985 Measurement of vasopressin in body fluids. Basic Clin Endocrinol 6:161–192 [Google Scholar]
- Shipkova P, Drexler DM, Langish R, Smalley J, Salyan ME, Sanders M 2008 Application of ion trap technology to liquid/chromatography/mass spectrometry quantitation of large peptides. Rapid Commun Mass Spectrom 22:1359–1366 [DOI] [PubMed] [Google Scholar]
- Janaky T, Szabo P, Kele Z, Balaspiri L, Varga C, Galfi M, Vecsernyes M, Gaspar L, Juhasz A, Laszlo FA 1998 Identification of oxytocin and vasopressin from neurohypophyseal cell culture. Rapid Comm Mass Spectrom 12:1765–1768 [DOI] [PubMed] [Google Scholar]
- Baseski HM, Watson CJ, Cellar NA, Shackman JG, Kennedy RT 2005 Capillary liquid chromatography with MS3 for the determination of enkephalins in microdialysis samples from the striatum of anesthetized and freely-moving rats. J Mass Spectrom 40:146–153 [DOI] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio, Herman JP 2005 Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol 289:E823–E828 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Franck J, Spangler R, Maggos CE, Ho A, Kreek MJ 2000 Reduced hypothalamic POMC and anterior pituitary CRF1 receptor mRNA levels after acute, but not chronic, daily “binge” intragastric alcohol administration. Alcohol Clin Exp Res 24:1575–1582 [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ 2003 Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge’ cocaine and withdrawal. Brain Res 964:187–199 [DOI] [PubMed] [Google Scholar]
- Nichols Jr B, Guillemin R 1959 Endogenous and exogenous vasopressin on ACTH release. Endocrinology 64:914–920 [DOI] [PubMed] [Google Scholar]
- Bernston GG, Berson BS 1980 Antinociceptive effects of vasopressin or systemic administration of vasopressin in the rat. Life Sci 26:455–459 [DOI] [PubMed] [Google Scholar]
- Argyropoulos SV, Bailey JE, Hood SD, Kendrick AH, Rich AS, Laszlo G, Nash JR, Lightman SL, Nutt DJ 2002 Inhalation of 35% CO2 results in activation of the HPA axis in healthy volunteers. Psychoneuroendocrinology 27:715–729 [DOI] [PubMed] [Google Scholar]
- Hood SD, Hince DA, Robinson H, Cirillo M, Christmas D, Kaye JM 2006 Serotonin regulation of the human stress response. Psychoneuroendocrinology 31:1087–1097 [DOI] [PubMed] [Google Scholar]
- Kaye J, Buchanan F, Kendrick A, Johnson P, Lowry C, Bailey J, Nutt D, Lightman S 2004 Acute carbon dioxide exposure in healthy adults: evaluation of a novel means of investigating the stress response. J Neuroendocrinol 16:256–264 [DOI] [PubMed] [Google Scholar]
- van Duinen MA, Schruers KR, Maes M, Griez EJ 2005 CO2 challenge results in hypothalamic-pituitary-adrenal activation in healthy volunteers. J Psychopharmol 19:243–247 [DOI] [PubMed] [Google Scholar]
- van Duinen MA, Schruers KR, Maes M, Griez EJ 2007 CO2 challenge induced HPA axis activation in panic. Int J Neuropsychopharmacol 10:797–804 [DOI] [PubMed] [Google Scholar]
- Wetherell MS, Crown AL, Lightman SL, Miles JN, Kaye J, Vedhara K 2006 The four-dimensional stress test: psychological, sympathetic-adrenal-medullary, parasympathetic and hypothalamic-pituitary-adrenal responses following inhalation of CO2. Psychoneuroendocrinology 31:736–747 [DOI] [PubMed] [Google Scholar]
- Amano M, Asari T, Kubo T 1994 Excitatory amino acid receptors in the rostral ventrolateral medulla mediate hypertension induced by carotid body chemoreceptor stimulation. Naun Schmied Arch Pharmacol 349:549–554 [DOI] [PubMed] [Google Scholar]
- Bailey JE, Argyropoulos SV, Lightman SL, Nutt DJ 2003 Does the brain noradrenaline network mediate the effects of the CO2 challenge? J Psychopharmacol 17:252–259 [DOI] [PubMed] [Google Scholar]
- Stocker SD, Wilson ME, Madden CJ, Lone U, Sved AF 2006 Intravenous 6-hydroxydopamine attenuates vasopressin and oxytocin secretion stimulated by hemorrhage and hypotension but not hyperosmolality in rats. Am J Physiol 291:R59–R67 [DOI] [PubMed] [Google Scholar]
- Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA 2005 Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol 19:327–341 [DOI] [PubMed] [Google Scholar]
- Bisset GW, Chowdrey HS 1984 A cholinergic link in the reflex release of vasopressin by hypotension in the rat. J Physiol 354:523–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsling ML, Ullmann E 1974 Release of vasopressin during hypoxia. J Physiol 241:35P–36P [PubMed] [Google Scholar]