Abstract
Familial tumoral calcinosis is characterized by ectopic calcifications and hyperphosphatemia. The disease is caused by inactivating mutations in fibroblast growth factor 23 (FGF23), Klotho (KL), and uridine diphosphate-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 3 (GALNT3). In vitro studies indicate that GALNT3 O-glycosylates a phosphaturic hormone, FGF23, and prevents its proteolytic processing, thereby allowing secretion of intact FGF23. In this study we generated mice lacking the Galnt3 gene, which developed hyperphosphatemia without apparent calcifications. In response to hyperphosphatemia, Galnt3-deficient mice had markedly increased Fgf23 expression in bone. However, compared with wild-type and heterozygous littermates, homozygous mice had only about half of circulating intact Fgf23 levels and higher levels of C-terminal Fgf23 fragments in bone. Galnt3-deficient mice also exhibited an inappropriately normal 1,25-dihydroxyvitamin D level and decreased alkaline phosphatase activity. Furthermore, renal expression of sodium-phosphate cotransporters and Kl were elevated in Galnt3-deficient mice. Interestingly, there were sex-specific phenotypes; only Galnt3-deficient males showed growth retardation, infertility, and significantly increased bone mineral density. In summary, ablation of Galnt3 impaired secretion of intact Fgf23, leading to decreased circulating Fgf23 and hyperphosphatemia, despite increased Fgf23 expression. Our findings indicate that Galnt3-deficient mice have a biochemical phenotype of tumoral calcinosis and provide in vivo evidence that Galnt3 plays an essential role in proper secretion of Fgf23 in mice.
Galnt3-deficient mice provide in vivo evidence that Galnt3 plays an essential role in proper secretion of Fgf23.
Tumoral calcinosis (also referred to as hyperphosphatemic familial tumoral calcinosis, Online Mendelian Inheritance in Man 211900) is an autosomal recessive disorder, characterized by the development of often severe ectopic calcifications in soft tissues. Calcific masses are typically found around major joints such as the hip, shoulder, and knee. Biochemical abnormalities associated with tumoral calcinosis include hyperphosphatemia secondary to increased renal tubular phosphate reabsorption and elevated or inappropriately normal 1,25-dihydroxyvitamin D [1,25(OH)2D] levels. Furthermore, this disorder is occasionally associated with diaphysitis, hyperostosis, dental abnormalities, and angioid streaks of the retina.
Previous molecular genetic analyses demonstrated that tumoral calcinosis can result from biallelic inactivating mutations in genes encoding fibroblast growth factor 23 (FGF23) (1,2,3,4), uridine diphosphate-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 3 (GALNT3) (5,6,7,8), or Klotho (KL) (also referred to as α-Klotho) (9). FGF23 is a hormone that promotes renal phosphate excretion by decreasing phosphate reabsorption in the proximal tubule, and also reduces circulating 1,25(OH)2D by both decreasing biosynthesis and increasing metabolism of 1,25(OH)2D (10). GALNT3 is a Golgi-associated enzyme that initiates mucin-type O-glycosylation of mature polypeptides. GALNT3 O-glycosylates FGF23 in a furin-like convertase recognition sequence and prevents proteolytic processing of FGF23, thereby allowing secretion of intact FGF23 (11,12). Loss-of-function mutations in FGF23 also destabilize FGF23 and make it susceptible to proteolysis (13). Therefore, dysfunction of either FGF23 or GALNT3 decreases circulating intact, bioactive FGF23, which leads to hyperphosphatemia and ultimately to ectopic calcifications. FGF23 requires an additional cofactor, KL, to bind and signal through its cognate fibroblast growth factor receptors (FGFRs) (14,15). Thus, a mutation in KL impairs FGF23 signaling through FGFRs and causes severe tumoral calcinosis with multiple abnormalities in mineral homeostasis (9).
Diminished Kl expression in mice results in a phenotype characterized by osteopenia, skin and muscle atrophy, pulmonary emphysema, infertility, hypoactivity, ectopic vascular and soft-tissue calcifications, and death by 12 wk of age (16). Biochemical abnormalities of Kl-deficient mice include severe hyperphosphatemia, hypercalcemia, hypoglycemia, and increased serum levels of 1,25(OH)2D (15,16,17,18). Of significance, the Kl-deficient phenotype largely overlaps with the phenotype of Fgf23-null mice (15,19,20), underscoring the observed direct interactions between KL, FGF23, and its cognate FGFRs (9,14,15).
Premature death of Fgf23-null and Kl-deficient mice indicates that these mice have a more severe phenotype than that seen in humans with tumoral calcinosis. Therefore, the goal of this study was to create a more appropriate animal model of tumoral calcinosis by deleting the Galnt3 gene in mice.
Materials and Methods
Generation of Galnt3-deficient mice
A genomic clone (230G18) harboring the Galnt3 gene was isolated from the 129 female mouse genomic library (RPCI-22 129S6/SvEvTac mouse bacterial artificial chromosome library) at the Roswell Park Cancer Institute (Buffalo, NY). The 5′ and 3′ fragments flanking Galnt3 exons 2 and 3 were amplified from this clone, using PfuUltra HF DNA polymerase (Stratagene, La Jolla, CA): 5′-ACT CCC AAA GGC AGG TTT CT-3′ and 5′-CCA GGG AAA ACA AAA GCA AA-3′ (5′ arm); and 5′-CCA CCT GTG GAG ATT TGC TT-3′ and 5′-ATG TCA GGG GCA TTG AAG AC-3′ (3′ arm). The PCR products were digested with BamHI and NheI (5′ arm) and KpnI and NsiI (3′ arm), and cloned into the pKO-Neor/LoxP vector (a generous gift from Dr. Weinian Shou at Indiana University, Indianapolis, IN). Embryonic stem (ES) cells (CCE916 ES cell line) derived from the 129SvEv strain were electroporated with linearized targeting vector and selected with G418 and gancyclovir. Genomic DNA from the selected clones was analyzed by Southern blot using the 3′ probe that contained exon 4 (supplemental Fig. 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), and targeted ES cell lines were expanded and injected into C57BL/6J blastocysts. Two male chimeras that transmitted the disrupted Galnt3 through germ line were mated to C57BL/6J females to generate F1 offspring. F2 offspring (C57BL/6J-129SvEv hybrid) generated from the F1 heterozygous mice were used for all studies.
All animals were maintained on NIH-31 Mouse/Rat Sterilizable Diet (Harlan Teklad, Madison, WI), which contains 1.19% calcium, 0.93% phosphorus, and 4.21 IU/g vitamin D3. The study was approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
PCR genotyping
Genomic DNA was extracted from mouse tails, using the Gentra Puregene Tissue Kit (QIAGEN, Inc., Valencia, CA). Galnt3 genotypes of the mice were determined by quadruplex PCR, using the Multiplex PCR Kit (QIAGEN). PCR primers were: Galnt3 F1, 5′-CCA TGA GCA CCT CTG TGA GA-3′ (Galnt3/neomycin); Galnt3 R1, 5′-GTT TGC CGG TAA AAG GGT TT-3′ (Galnt3); Neo R1, 5′-TCG CCT TCT TGA CGA GTT CT-3′ (neomycin); Galnt3 F2, 5′-TAG CAG TGG TGC AGA AAT GG-3′ (Galnt3); Galnt3 R2, 5′-TGT GGC GTC TTG AAA GTC AG-3′ (Galnt3/neomycin); and Neo F1, 5′-TGT CTG TTG TGC CCA GTC AT-3′ (neomycin).
Immunoblotting analysis
Harvested kidneys and femurs were homogenized in radioimmunoprecipitation assay buffer (Sigma-Aldrich Corp., St. Louis, MO) containing protease inhibitors (Complete Mini Tablet; Roche Applied Science, Indianapolis, IN). The homogenized tissues were then centrifuged at 10,000 × g for 20 min at 4 C. Supernatants (protein extracts) from femurs were used to immunoprecipitate Fgf23 with human FGF23 antibody [FC1, a generous gift from Drs. Hisashi Hasegawa and Yuji Yamazaki at Kirin Pharma Co., Ltd., Gunma, Japan (21)] and Protein G Sepharose 4 Fast Flow (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Supernatants (kidneys) or immunoprecipitates (femurs) were electrophoresed on Tris-HCl Ready Gel precast gels (Bio-Rad Laboratories, Inc., Hercules, CA) and blotted onto nitrocellulose or polyvinylidene fluoride membranes. Membranes were probed with an antibody raised against human GALNT3 (a generous gift from Drs. Ulla Mandel and Henrik Clausen at University of Copenhagen, Copenhagen, Denmark) or human FGF23 (FC1) and then with horseradish peroxidase-conjugated antimouse IgG antibody (eBioscience, San Diego, CA, or GE Healthcare Bio-Sciences). Immunoblots were developed with ECL Western Blotting Detection Reagents (GE Healthcare Bio-Sciences).
Transferase assay
Kidney samples (43–49 mg) were homogenized in 0.5 ml buffer as described (22) except that the Tris-maleate concentration was 10 mm, the dextran molecular weight was 200,000, and the buffer contained 1× Protease Inhibitor Cocktail III (Calbiochem, San Diego, CA). The homogenate was diluted 5-fold with water and centrifuged for 1 h at 257,551 × g. The pellet was resuspended in 300 μl extraction buffer [2.5% Triton X-100, 50 mm sodium cacodylate (pH 6.6), 50 mm β-mercaptoethanol, and 1× protease inhibitor cocktail] and stirred for 1 h. The sample was centrifuged at 16,100 × g for 15 min, and the supernatant was collected for the assay.
Assays (10 μl) containing 2 μl of the kidney extract were done in duplicate as described (23) but contained 10 mm CDP-choline to inhibit pyrophosphatase activity. The acceptor peptide used for the assay (RGPGRAFVTIGKIGNMR, 1 mm) is based on the HIV gp120 protein and is selective for GALNT3 (24). EA2 is a nonselective peptide, which is derived from a rat submandibular gland mucin (PTTDSTTPAPTTK) (25).
Serum biochemistry measurement
Blood samples were collected from the mice under anesthesia by cardiac puncture. Routine serum biochemistries were determined by standard methods at the General Clinical Research Center of Indiana University School of Medicine. Plasma 1,25(OH)2D concentrations were determined using a RIA kit (DiaSorin Inc., Stillwater, MN). Plasma Pth concentrations were determined using the Mouse Intact PTH ELISA kit (Immutopics International, San Clemente, CA). Serum Fgf23 concentrations were determined using the FGF23 ELISA kit (Kainos Laboratories Inc., Tokyo, Japan) that recognizes only intact Fgf23.
Histological analysis
Kidney, skin, testis, and bone tissues were harvested from Galnt3-deficient mice and age-matched littermate controls. Harvested tissue samples were fixed in 10% neutral-buffered formalin and embedded in paraffin. Paraffin sections (3–4 μm) were stained with hematoxylin-eosin or von Kossa.
Femurs were fixed in 10% neutral-buffered formalin for 5 d and embedded in methyl methacrylate. Four-micrometer sections were cut from diaphyses (midshaft) and distal femurs, and stained with hematoxylin-eosin, von Kossa/MacNeal, and tartate-resistant acid phosphatase (TRAP).
Radiological analysis
Radiographs were obtained using a piXarray 100 Digital Specimen Radiography System (Bioptics, Inc., Tucson, AZ). Image acquisition was performed at 24 kV voltage and 0.4 sec exposure time.
Bone mineral densities (BMDs) of whole body (including tail), left leg, and extracted femur were measured by dual-energy x-ray absorptiometry (DXA), using a PIXImus2 densitometer (Lunar Corp, Madison, WI). Coefficient of variation on 11 measurements of a frozen mouse specimen was 0.57% for BMD.
Bone parameters of the femur were also assessed using micro-computed tomography (micro-CT) (SkyScan 1172; SkyScan, Kontich, Belgium) using a 60-kV source voltage and 6-μm pixel size. Trabecular bone properties such as bone volume/trabecular volume (BV/TV), trabecular number (Tb.N), and trabecular thickness (Tb.Th), were assessed on 1 mm (∼166 slices) in the distal metaphysis. For cortical bone parameters, bone area (BA) and cross-sectional moment of inertia were assessed on a single slice of the diaphysis.
Gene expression analysis
Right femurs and kidneys were harvested from wild-type, heterozygous, and homozygous mice, and stored in RNAlater RNA Stabilization Reagent (QIAGEN). Total RNA was extracted from the whole tissues, using TRIzol reagent (Invitrogen Corp., Carlsbad, CA). First-strand cDNA was synthesized from the RNA, using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Intron-spanning PCR primers for Actb, Fgf23, Gapdh, Kl, Slc34a1, and Slc34a3 were designed using Primer3 v.0.4.0 (Whitehead Institute for Biomedical Research, Cambridge, MA; http://fokker.w.mit.edu/cgi-bin/primer3/primer3_www.cgi; Ref. 26). Primer sequences or TaqMan gene expression assays used in quantitative real-time PCR are shown in supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. Target gene transcripts were amplified from the synthesized cDNA using the iTaq SYBR Green Supermix with ROX (Bio-Rad Laboratories) or TaqMan Gene Expression Master Mix (Applied Biosystems) in the ABI-PRISM 7000 Sequence Detection System (Applied Biosystems). Relative gene expression was determined by analyzing the data using the relative standard curve method. Either Actb or Gapdh was used as an internal control in the experiments.
Statistical analysis
Means and sem values were calculated for all outcome measures by genotype. Where the measurements were highly skewed, analyses were performed on both the original and log units. Because the conclusions did not change with log transformations, all results are presented in original units. General linear models were used to test for overall differences among genotypes; where appropriate, covariates were included, or subgroup analyses (e.g. within sex or same age) were conducted. Where there were multiple measurements on each mouse, mice were treated as random effects in the models. Within each model, specific comparisons of the homozygous and heterozygous mice to the wild-type control mice were made, and P values less than 0.05 were reported without adjustment for multiple testing. For comparison of body weight between genotypes, age was treated as a categorical variable so no parametric function of age was assumed. Both body weight and DXA measurements were analyzed by sex; all other measurements were analyzed with roughly equal numbers of males and females combined because there was no sex-specific difference. When there were three age groups of mice, the between-genotype comparisons were made at each age.
Results
Generation of Galnt3-deficient mice
We recently reported two GALNT3 mutations in exons 2 and 3 (exons 1 and 2 in the original publication) that resulted in hyperphosphatemic tumoral calcinosis (6). Exon 2 contains the initiation codon, and exon 3 encodes a part of the glycosyl transferase family 2 domain. Thus, to generate a null mutation in the mouse Galnt3 gene, we constructed a targeting vector that would replace the mouse counterparts of these two exons with a cassette encoding neomycin resistance by homologous recombination (supplemental Fig. 1). Homologous recombination with the targeting vector occurred at a low frequency in ES cells (two out of 240 colonies screened). The two independently derived ES cell clones produced male chimeras that transmitted the altered Galnt3 allele through the germ line. Because F1 heterozygous carriers were viable and fertile, F2 offspring from F1 crosses were used for subsequent studies.
Southern blotting analysis and PCR genotyping confirmed the absence of the Galnt3 gene in mice homozygous for the deletion allele (supplemental Fig. 2A). Galnt3 mRNA was detected in the kidneys of wild-type mice by quantitative RT-PCR analysis; Galnt3 expression was reduced to almost half in heterozygous mice and to an undetectable level in homozygous mice (supplemental Fig. 2B). In immunoblotting analysis the amount of kidney Galnt3 protein was also reduced by half in heterozygous mice and was undetectable in animals homozygous for the mutant allele (supplemental Fig. 2C). In addition, the extract of homozygous mouse kidneys had significantly reduced glycosyltransferase activity on the HIV gp120 protein, which is a selective substrate for Galnt3 (24), whereas there was no effect on the nonselective control peptide (supplemental Fig. 2D).
Phenotype of Galnt3-deficient mice
Gross phenotype
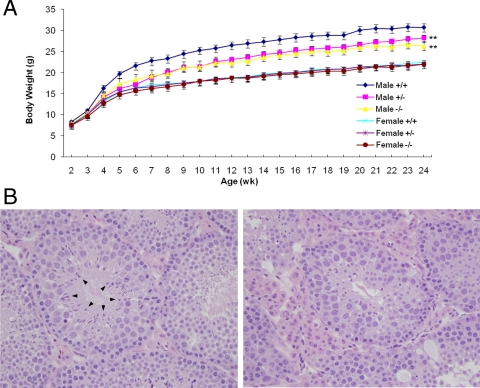
Mice lacking Galnt3 were outwardly normal at birth and developed into adulthood. These mice appear to have a normal life expectancy. Wild-type, heterozygous, and homozygous females grow at the same rate (Fig. 1A). However, heterozygous and homozygous males had slower growth compared with wild-type males.
Figure 1.
Mouse growth and fertility. A, Growth curves. The data are presented as mean body weight ± sem at each age for six sex-by-genotype groups (n = 13–24 per group). **, P < 0.01 (overall comparison with same-sex wild-type control mice). B, Histological analysis of the testis. Wild-type (left) and Galnt3-deficient (right) mice at 23 wk of age. Arrowheads indicate spermatozoa in the seminiferous tubules of wild-type mice. Hematoxylin-eosin staining. Magnifications, ×400.
Fertility
Mating between F1 heterozygotes produced a roughly equal number of male [272 (48.2%)] and female [292 (51.8%)] pups. The mutant allele was inherited in a mendelian fashion, and expected numbers of wild-type [156 (27.7%)], heterozygous [277 (49.1%)], and homozygous [131 (23.2%)] F2 offspring were born. Thus, fecundity and fertility were normal in mice heterozygous for the introduced mutation. However, four breeding pairs between homozygous F2 mice failed to produce any F3 offspring after 12 wk. When the four homozygous females, originally caged with homozygous males, were introduced to wild-type males, all four had an expected number of pups (averaging six pups per litter). In contrast, inverse mating between the same homozygous males and wild-type females did not produce any offspring after 15–30 wk.
Because various crosses suggested that homozygous males have reproductive problems, histological sections of testes were analyzed. Compared with wild-type mice, there was a marked decrease in spermatozoa in the seminiferous tubules of Galnt3-deficient mice (Fig. 1B). In addition, the tubules in Galnt3-deficient mice were narrower, and their visible spermatozoa had abnormal morphology.
Histological and radiographic findings
Homozygous Galnt3 mice showed no sign of ectopic calcifications by radiographic imaging, even after 1 yr (data not shown). Histological analyses of the skin and kidney at 24 wk of age were normal.
Although alkaline phosphatase was significantly decreased in Galnt3-deficient mice (see below), no histological abnormalities were apparent in diaphyses and distal femurs in various stains (Fig. 2A). However, radiographs of ex vivo femurs showed that many Galnt3-deficient mice appeared to have femurs that were denser than wild-type mice (Fig. 2B). To corroborate this finding, weight-adjusted whole body BMD was significantly higher in homozygous males, compared with wild-type control mice (Table 1). The BMD of the left leg and extracted femurs were also higher in homozygous males (data not shown). However, there was no statistically significant difference among the three genotypic groups in females.
Figure 2.
Skeletal phenotype of Galnt3-deficient mice at 24 wk. A, Bone histology. von Kossa (top) and TRAP (bottom) staining of femoral sections. Magnifications, ×25 and ×200 respectively. B, Plain radiographs of left femurs. C, Micro-CT analysis of male femurs.
Table 1.
Weight-adjusted whole body BMD
| +/+ | +/− | −/− | |
|---|---|---|---|
| Males | 0.056 ± 0.001 | 0.057 ± 0.001 | 0.062 ± 0.001a |
| Females | 0.056 ± 0.001 | 0.052 ± 0.001 | 0.057 ± 0.002 |
The data are presented as mean ± sem (number of animals: six to nine per group).
P < 0.01 (vs. wild-type control mice).
Micro-CT analysis showed that Galnt3-deficient male mice had significantly higher distal femur BV/TV (+41.5%) compared with wild-type littermates (Table 2 and Fig. 2C). This higher BV/TV was manifested through higher Tb.Th (+20%) and Tb.N (+16.5%). Diaphyseal cortical BA (+23.4%) and cross-sectional moment of inertia (+50.5%) were both significantly higher in Galnt3-deficient mice compared with wild-type littermates.
Table 2.
Micro-CT measurements of male femurs
| +/+ | −/− | |
|---|---|---|
| Three-dimensional trabecular analyses | ||
| Tissue volume (mm3) | 1.77 ± 0.05 | 2.14 ± 0.06a |
| Bone volume (mm3) | 0.22 ± 0.02 | 0.37 ± 0.04b |
| Percent bone volume (%) | 12.38 ± 1.25 | 17.51 ± 1.65 |
| Tissue surface (mm2) | 9.62 ± 0.15 | 10.84 ± 0.27b |
| Bone surface (mm2) | 16.31 ± 1.65 | 22.86 ± 0.83b |
| Trabecular pattern factor (1/mm) | 21.22 ± 1.06 | 14.89 ± 2.19 |
| Tb.Th (mm) | 0.051 ± 0.002 | 0.061 ± 0.006 |
| Tb.N (1/mm) | 2.48 ± 0.34 | 2.89 ± 0.07 |
| Apparent density (bone plus marrow) (pixel intensity) | 38.40 ± 1.77 | 46.09 ± 2.30 |
| Bone density (mineralization) (pixel intensity) | 119.14 ± 1.04 | 126.01 ± 3.17 |
| Two-dimensional cortical analyses | ||
| BA (mm2) | 0.86 ± 0.02 | 1.06 ± 0.05b |
| Bone perimeter (mm) | 9.87 ± 0.28 | 11.39 ± 0.82 |
| Polar moment of inertia (mm4) | 0.46 ± 0.03 | 0.69 ± 0.07b |
| Bone density (mineralization) (pixel intensity) | 175.95 ± 1.24 | 176.29 ± 0.99 |
The data are presented as mean ± sem (number of animals: three males per group).
P < 0.01 (vs. wild-type control mice).
P < 0.05 (vs. wild-type control mice).
Serum biochemistry
Serum profiles were comparable between wild-type and heterozygous mice at 3, 6, and 12 wk. However, homozygous deletion of Galnt3 affected multiple serum parameters (Fig. 3). Compared with wild-type and heterozygous mice, serum phosphorus levels in homozygous mice were higher (∼2.5 mg/dl) at all three ages (Fig. 3A). Serum calcium levels in homozygous mice were also elevated, but the difference reached statistical significance only at 6 wk (Fig. 3B). Serum alkaline phosphatase activity in Galnt3-deficient mice was significantly lower starting at 3 wk and continued through 12 wk (Fig. 3C). Serum glucose was not different among the three genotypic groups (Fig. 3D). Because Galnt3-deficient mice had blood urine nitrogen (BUN) levels equivalent to wild-type and heterozygous mice (Fig. 3E), biochemical abnormalities in homozygous mice are not likely due to differences in renal function. Plasma Pth levels at 12 wk were also significantly lower in homozygous mice (Fig. 3F). At 24 wk, 1,25(OH)2D concentrations in Galnt3-deficient mice were inappropriately normal in the face of predicted hyperphosphatemia (Fig. 3G). Importantly, Galnt3-deficient mice derived from independent chimeras (i.e. two separate ES cell clones) had similar serum biochemistries (Fig. 3, A–E). There were no differences between males and females (data not shown).
Figure 3.
Comparison of serum biochemical values. A–E, Serum biochemistries measured on two independent lines, line 1 (L1) at 3, 6, and 12 wk, and line 2 (L2) at 12 wk (within-group n ≥10). ALP, alkaline phosphatase. F, PTH concentration in line 1 at 12 wk [wild-type n = 9 (white bar), heterozygote n = 12 (gray bar), homozygote n = 10 (black bar)]. G, 1,25(OH)2D concentration in line 1 at 24 wk (wild-type n = 13, heterozygote n = 15, homozygote n = 8). The data are represented as mean ± sem. *, P < 0.05 and **, P < 0.01 vs. wild-type control mice of the same age and line.
Fgf23 gene expression and serum Fgf23 concentration
As an appropriate response to hyperphosphatemia, Fgf23 gene expression in the femur was approximately 14-fold higher in homozygous mice than wild-type and heterozygous counterparts (Fig. 4A). However, circulating intact Fgf23 levels in Galnt3-deficient mice were 50–60% lower than those of wild-type and heterozygous mice at all three ages (Fig. 4B). Immunoblotting analysis of Fgf23 in the femur revealed a marked increase in C-terminal Fgf23 fragments and an undetectable level of intact Fgf23 in Galnt3-deficient mice, whereas unaffected littermates had only detectable levels of intact Fgf23 (Fig. 4C). The apparent disparity between low-intact Fgf23 in serum and undetectable-intact Fgf23 in bone suggests that the amount of C-terminal Fgf23 fragments in Galnt3-deficient mice is so much greater than that of intact Fgf23 that it even masked 40–50% of intact Fgf23 produced in these mice.
Figure 4.
Discordance between Fgf23 gene expression and circulating Fgf23 concentration. A, Relative Fgf23 gene expression in the femur. B, Serum intact Fgf23 concentration (n = 10–15 animals per genotype). The same 6-wk-old animals used in A were also used in B. The data are presented as mean ± sem. White bar, wild-type. Gray bar, heterozygote. Black bar, homozygote. **, P < 0.01 vs. wild-type control mice of the same age. C, Detection of Fgf23 by immunoblotting. Recombinant (Rec) FGF23 was used as a positive control; no homogenate was used as a negative (Neg) control. C-term, C-Terminal.
Expression of genes involved in phosphate homeostasis
Galnt3 deficiency also affected expression of several genes involved in phosphate homeostasis. Consistent with increased phosphate reabsorption, sodium-phosphate cotransporters IIa and IIc (encoded by Slc34a1 and Slc34a3, respectively) were up-regulated in the kidneys isolated from homozygous mice, but only Slc34a1 expression at 12 wk reached statistical significance (Fig. 5, A and B). However, expression of genes encoding enzymes involved in vitamin D metabolism (Cyp27b1 and Cyp24a1) was not substantially different among the three genotypes (Fig. 5, C and D). Importantly, expression of Fgfr cofactor, Kl, was significantly increased in the kidney of homozygous mice (Fig. 5E).
Figure 5.
Relative renal expression of genes involved in phosphate homeostasis. A, Slc34a1, sodium-phosphate cotransporter IIa. B, Slc34a3, sodium-phosphate cotransporter IIc. C, Cyp24a1, 24-hyroxylase. D, Cyp27b1, 1-α-hydroxylase. E, Kl. The data are presented as mean fold change ± sem (n = 10–12 animals per group). White bar, wild-type; gray bar, heterozygote; black bar, homozygote. *, P < 0.05 and **, P < 0.01 vs. wild-type control mice of the same age.
Discussion
In this study we generated an animal model of hyperphosphatemic tumoral calcinosis by replacing the mouse Galnt3 gene with the neomycin-resistance gene. Galnt3-deficient mice did not develop any ectopic calcifications at major joints, even after 1 yr of observation. However, these mice had marked hyperphosphatemia and low-serum Fgf23 levels, despite increased Fgf23 gene expression in bone. Consistent with decreased Fgf23 concentrations, expression of renal sodium-phosphate cotransporters was up-regulated in these animals [although only NaPi-IIa (Slc34a1) expression in 12 wk old mice reached statistical significance], resulting in the observed hyperphosphatemia. Plasma 1,25(OH)2D concentration was inappropriately normal at 24 wk. However, because we did not measure 1,25(OH)2D levels at the earlier time points, we cannot completely exclude the possibility that elevated 1,25(OH)2D levels had also contributed to hyperphosphatemia. These data demonstrated that the lack of Galnt3 activity in mice leads to a biochemical phenotype reflecting impaired biological activity of Fgf23, as observed in tumoral calcinosis patients.
Under normal physiological circumstances, hyperphosphatemia increases serum Fgf23 concentrations (27), which then inhibit renal phosphate reabsorption and 1,25(OH)2D synthesis. In accord with this negative feedback mechanism, Galnt3-deficient mice had a 14-fold increase in Fgf23 gene expression in bone but had only 40–50% of serum Fgf23 concentrations compared with wild-type and heterozygous mice. Galnt3-deficient mice also had a significant amount of C-terminal Fgf23 fragments but an undetectable level of intact Fgf23 in bone. Similarly, tumoral calcinosis patients with inactivating mutations in GALNT3 (and also FGF23) have low or undetectable levels of intact FGF23 but highly elevated C-terminal fragments (1,4,28,29,30), indicating increased FGF23 expression. Furthermore, recent in vitro studies (11,12) demonstrated that Galnt3 O-glycosylates Fgf23 to prevent cleavage by intracellular furin-like convertases, allowing secretion of intact Fgf23. Together, the lack of Galnt3 in both Galnt3-deficient mice and tumoral calcinosis patients drastically destabilizes Fgf23 and decreases secretion of full-length Fgf23 despite appropriate up-regulation of the Fgf23 gene. This leads to reduced Fgf23-Kl-Fgfr complex formation and ultimately to impaired Fgf23 signaling. Therefore, O-glycosylation of Fgf23 by Galnt3 is a crucial posttranslational step in proper secretion of Fgf23 and, thus, regulation of phosphate homeostasis.
Although the present study demonstrates that Galnt3 is essential for proper Fgf23 secretion, two major questions remain. First, it is unclear how a fraction of intact Fgf23 is secreted in Galnt3-deficient mice (and tumoral calcinosis patients). In the absence of Galnt3, Fgf23 may be glycosylated by other members of the GalNAc transferase family, such as Galnt6, which has similar substrate specificity to Galnt3 (24). It is also possible that due to the increased Fgf23 expression, a small fraction of unglycosylated or underglycosylated Fgf23 may “escape” from degradation by furin-like convertases. Second, it is unclear whether Fgf23 produced in Galnt3-deficient mice functions as well as that from wild-type mice. Underglycosylated Fgf23 from mutant mice may have reduced potency, compared with the fully glycosylated form of Fgf23. The mechanism of partial Fgf23 secretion and effect of glycosylation on Fgf23 bioactivity are currently active areas of investigation.
Observed phenotypes of Galnt3-deficient mice suggest that reduced Fgf23 levels increased phosphate reabsorption but had no effect on 1,25(OH)2D synthesis. However, this is in agreement with a phenotype of tumoral calcinosis patients, who have hyperphosphatemia with elevated or normal levels of 1,25(OH)2D. The most likely explanation for an apparently normal 1,25(OH)2D level in Galnt3-deficient mice is that although low Fgf23 would increase 1,25(OH)2D synthesis, low Pth counteracted to reduce 1,25(OH)2D synthesis. It should be noted that in the setting of hyperphosphatemia, which suppresses 1,25(OH)2D concentrations, a normal 1,25(OH)2D concentration is inappropriate.
Galnt3-deficient mice had consistently low-serum alkaline phosphatase, which is not a phenotype associated with tumoral calcinosis in humans. However, this decreased alkaline phosphatase may reflect the increased bone mineralization seen in these mice. Of note, GALNT3 mutations also cause a variant of tumoral calcinosis, named hyperostosis-hyperphosphatemia syndrome, which is characterized by cortical hyperostosis, usually without ectopic calcifications (11,31,32).
Interestingly, Galnt3-deficient mice had increased Kl expression in the kidney. In contrast, renal Kl expression was significantly decreased in Fgf23 transgenic mice (33) and in a murine model of X-linked hyperphosphatemia (Hyp mice) (34), both of which have hypophosphatemia due to increased circulating Fgf23. These observations suggest that Kl expression may be regulated by serum phosphate, Fgf23, or a yet unidentified factor.
Mice deficient in Kl, Fgf23, and Galnt3 all develop hyperphosphatemia and increased or inappropriately normal 1,25(OH)2D levels (supplemental Table 2). In contrast, there are several differences between these mice. First, Fgf23-null mice and Kl-deficient mice exhibited shortened lifespan and multiple additional phenotypes, such as pulmonary emphysema, skin atrophy, and neural degeneration (16,19,35). However, Galnt3-deficient mice, in concert with tumoral calcinosis patients, have not exhibited any of these features, even after 1 yr. Second, Galnt3-deficient mice did not develop abnormal calcifications seen in Kl-deficient mice and Fgf23-null mice. Since these calcifications are thought to be secondary to increased serum phosphate and 1,25(OH)2D concentrations, biochemical abnormalities in Galnt3-deficient mice may not be severe enough to cause calcifications. Third, there were multiple differences in serum biochemistries (supplemental Table 2). Kl-deficient mice and Fgf23-null mice developed severe hypercalcemia, whereas Galnt3-null mice had mostly normal serum calcium. In addition, both Kl-deficient mice and Fgf23-null mice are hypoglycemic (16,19). In contrast, Galnt3-deficient mice had normal glucose concentrations. These phenotypic differences could be explained by differing functional roles of the mutated genes or mouse strain specificity. However, the most likely explanation lies in the amount of biological Fgf23 signaling. Fgf23-null mice have no Fgf23 production, and Fgf23 signaling in Kl-null mice is severely impaired in the absence of Kl. In contrast, Galnt3 deficiency did not lead to the complete loss of Fgf23 signaling because some intact Fgf23 was secreted. This partial Fgf23 secretion, and, therefore, milder hyperphosphatemia, was most likely enough to prevent the development of features only seen in the other two knockout mice.
Both Kl- and Fgf23-deficient mice die within 12 wk of life; however, milder hyperphosphatemia due to some Fgf23 signaling allows Galnt3-deficient mice to survive beyond 1 yr. Likewise, patients with tumoral calcinosis (regardless of mutations in FGF23, GALNT3, or KL) survive into adulthood, likely because of some FGF23 signaling. Furthermore, consistent with biochemical profiles in these patients, Galnt3-deficient mice have hyperphosphatemia and inappropriately normal 1,25(OH)2D but lack other biochemical features (low glucose and hypercalcemia) seen in mice lacking Fgf23 or Kl (supplemental Table 2). These observations suggest that Galnt3-deficient mice most closely resemble familial tumoral calcinosis in humans, particularly in terms of serum biochemistries. The apparently normal lifespan of Galnt3-deficient mice is also a major advantage, rendering it possible to study a long-term effect of low Fgf23 concentration as well as hyperphosphatemia on the body. However, it should be noted that Galnt3-deficient mice may have a phenotype independent of Fgf23 signaling because Galnt3 is likely involved in glycosylation of other proteins.
In conclusion, we demonstrated that a homozygous loss of Galnt3 in mice resulted in impaired Fgf23 secretion, leading to hyperphosphatemia and inappropriately normal 1,25(OH)2D concentrations, as observed in patients with tumoral calcinosis. Despite the increased Fgf23 production, the ablation of Galnt3 diminished circulating intact Fgf23 concentrations necessary for normal phosphate homeostasis. Therefore, glycosylation of Fgf23 by Galnt3 is a crucial step in proper secretion of full-length Fgf23 and regulation of phosphate homeostasis.
Supplementary Material
Acknowledgments
We thank Drs. Erik A. Imel and Kenneth E. White for critical review of this manuscript, Drs. Keith W. Condon and Carrie L. Phillips for analyzing histological slides, Dr. Alexander G. Robling and Shana Ellis for assistance with the PIXImus2 densitometer, Ronald M. McClintock for analyzing serum biochemistries, Dr. Matthew R. Allen for micro-CT analysis, and Leah R. Curry and Amie K. Gray for technical assistance.
Footnotes
This work was supported by National Institutes of Health grants R01 AR42228 and S10 RR023710, and in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 12, 2009
Abbreviations: BA, Bone area; BMD, bone mineral density; BUN, blood urine nitrogen; BV/TV, bone volume/trabecular volume; 1,25(OH)2D, 1,25-dihydroxyvitamin D; DXA, dual-energy x-ray absorptiometry; ES, embryonic stem; FGF23, fibroblast growth factor 23; FGFR, fibroblast growth factor receptor; GALNT3, uridine diphosphate-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 3; KL, Klotho; micro-CT, micro-computed tomography; Tb.N, trabecular number; Tb.Th, trabecular thickness; TRAP, tartate-resistant acid phosphatase.
References
- Araya K, Fukumoto S, Backenroth R, Takeuchi Y, Nakayama K, Ito N, Yoshii N, Yamazaki Y, Yamashita T, Silver J, Igarashi T, Fujita T 2005 A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J Clin Endocrinol Metab 90:5523–5527 [DOI] [PubMed] [Google Scholar]
- Benet-Pagès A, Orlik P, Strom TM, Lorenz-Depiereux B 2005 An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet 14:385–390 [DOI] [PubMed] [Google Scholar]
- Chefetz I, Heller R, Galli-Tsinopoulou A, Richard G, Wollnik B, Indelman M, Koerber F, Topaz O, Bergman R, Sprecher E, Schoenau E 2005 A novel homozygous missense mutation in FGF23 causes familial tumoral calcinosis associated with disseminated visceral calcification. Hum Genet 118:261–266 [DOI] [PubMed] [Google Scholar]
- Larsson T, Yu X, Davis SI, Draman MS, Mooney SD, Cullen MJ, White KE 2005 A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab 90:2424–2427 [DOI] [PubMed] [Google Scholar]
- Campagnoli MF, Pucci A, Garelli E, Carando A, Defilippi C, Lala R, Ingrosso G, Dianzani I, Forni M, Ramenghi U 2006 Familial tumoral calcinosis and testicular microlithiasis associated with a new mutation of GALNT3 in a white family. J Clin Pathol 59:440–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Lyles KW, Econs MJ 2005 A novel GALNT3 mutation in a pseudoautosomal dominant form of tumoral calcinosis: evidence that the disorder is autosomal recessive. J Clin Endocrinol Metab 90:2420–2423 [DOI] [PubMed] [Google Scholar]
- Specktor P, Cooper JG, Indelman M, Sprecher E 2006 Hyperphosphatemic familial tumoral calcinosis caused by a mutation in GALNT3 in a European kindred. J Hum Genet 51:487–490 [DOI] [PubMed] [Google Scholar]
- Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E 2004 Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 36:579–581 [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ 2007 A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest 117:2684–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imel EA, Econs MJ 2005 Fibroblast growth factor 23: roles in health and disease. J Am Soc Nephrol 16:2565–2575 [DOI] [PubMed] [Google Scholar]
- Frishberg Y, Ito N, Rinat C, Yamazaki Y, Feinstein S, Urakawa I, Navon-Elkan P, Becker-Cohen R, Yamashita T, Araya K, Igarashi T, Fujita T, Fukumoto S 2007 Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res 22:235–242 [DOI] [PubMed] [Google Scholar]
- Kato K, Jeanneau C, Tarp MA, Benet-Pagès A, Lorenz-Depiereux B, Bennett EP, Mandel U, Strom TM, Clausen H 2006 Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem 281:18370–18377 [DOI] [PubMed] [Google Scholar]
- Larsson T, Davis SI, Garringer HJ, Mooney SD, Draman MS, Cullen MJ, White KE 2005 Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology 146:3883–3891 [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M 2006 Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T 2006 Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774 [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI 1997 Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51 [DOI] [PubMed] [Google Scholar]
- Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y 2003 Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol 17:2393–2403 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fujimori T, Nabeshima Y 2002 Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1α-hydroxylase gene. Endocrinology 143:683–689 [DOI] [PubMed] [Google Scholar]
- Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T 2004 Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Jüppner H, Lanske B 2004 Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 23:421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S 2002 Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87:4957–4960 [DOI] [PubMed] [Google Scholar]
- Hagen FK, Gregoire CA, Tabak LA 1995 Cloning and sequence homology of a rat UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase. Glycoconj J 12:901–909 [DOI] [PubMed] [Google Scholar]
- Hagen FK, Ten Hagen KG, Beres TM, Balys MM, VanWuyckhuyse BC, Tabak LA 1997 cDNA cloning and expression of a novel UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase. J Biol Chem 272:13843–13848 [DOI] [PubMed] [Google Scholar]
- Bennett EP, Hassan H, Mandel U, Hollingsworth MA, Akisawa N, Ikematsu Y, Merkx G, van Kessel AG, Olofsson S, Clausen H 1999 Cloning and characterization of a close homologue of human UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J Biol Chem 274:25362–25370 [DOI] [PubMed] [Google Scholar]
- Albone EF, Hagen FK, VanWuyckhuyse BC, Tabak LA 1994 Molecular cloning of a rat submandibular gland apomucin. J Biol Chem 269:16845–16852 [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ 2000 Primer 3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, eds. Bioinformatics methods and protocols: methods in molecular biology. Totowa, NJ: Humana Press; 365–386 [DOI] [PubMed] [Google Scholar]
- Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA 2005 Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146:5358–5364 [DOI] [PubMed] [Google Scholar]
- Garringer HJ, Fisher C, Larsson TE, Davis SI, Koller DL, Cullen MJ, Draman MS, Conlon N, Jain A, Fedarko NS, Dasgupta B, White KE 2006 The role of mutant UDP-N-acetyl-α-D-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J Clin Endocrinol Metab 91:4037–4042 [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Imel EA, Sorenson AH, Severe R, Knudson P, Harris GJ, Shaker JL, Econs MJ 2006 Tumoral calcinosis presenting with eyelid calcifications due to novel missense mutations in the glycosyl transferase domain of the GALNT3 gene. J Clin Endocrinol Metab 91:4472–4475 [DOI] [PubMed] [Google Scholar]
- Garringer HJ, Mortazavi SM, Esteghamat F, Malekpour M, Boztepe H, Tanakol R, Davis SI, White KE 2007 Two novel GALNT3 mutations in familial tumoral calcinosis. Am J Med Genet A 143:2390–2396 [DOI] [PubMed] [Google Scholar]
- Frishberg Y, Topaz O, Bergman R, Behar D, Fisher D, Gordon D, Richard G, Sprecher E 2005 Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J Mol Med 83:33–38 [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Guigonis V, Imel EA, Courouble M, Heissat S, Henley JD, Sorenson AH, Petit B, Lienhardt A, Econs MJ 2007 Novel GALNT3 mutations causing hyperostosis-hyperphosphatemia syndrome result in low intact fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab 92:1943–1947 [DOI] [PubMed] [Google Scholar]
- Marsell R, Krajisnik T, Göransson H, Ohlsson C, Ljunggren O, Larsson TE, Jonsson KB 2008 Gene expression analysis of kidneys from transgenic mice expressing fibroblast growth factor-23. Nephrol Dial Transplant 23:827–833 [DOI] [PubMed] [Google Scholar]
- Meyer MH, Dulde E, Meyer Jr RA 2004 The genomic response of the mouse kidney to low-phosphate diet is altered in X-linked hypophosphatemia. Physiol Genomics 18:4–11 [DOI] [PubMed] [Google Scholar]
- Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B 2006 Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J 20:720–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.