Abstract
The androgen receptor (AR) is an important signaling molecule in multiple tissues, yet its mode of action and cell-specific activities remain enigmatic. AR function has been best studied in the prostate, in which it is essential for growth and homeostasis of the normal organ as well as each stage of cancer development. Investigation of mechanisms responsible for continued AR action that evolve during prostate cancer progression or after hormonal management of the disease have been instructive in defining AR signaling pathways. In the current paper, we use sequence similarity and the collocation of somatic mutations in prostate cancer to define residues 501–535 of the AR amino-terminal domain as an important mediator of receptor function. Specifically, the 501–535 region is required for optimal interaction of the amino-terminal domain with both the p160 coactivator, nuclear receptor coactivator-2, and the AR-ligand binding domain in the amino/carboxyl (N/C) interaction. The N/C interaction is decreased by deletion of the 501–535 region but is distinct from deletion of the 23FQNLF27 peptide in that it does not affect the capacity of the AR to activate transcription from a chromatin integrated reporter or recruitment of the receptor to androgen-responsive loci in vivo. Collectively, we have been able to outline two classes of N/C-deficient AR variant that are divergent in their capacity to act in a chromatin context, thereby further defining the interplay between N/C interaction and coregulator recruitment via multiple receptor domains. These mechanisms are likely to be key determinants of the cell and promoter specific activities of the AR.
Studies of the androgen receptor reveal a novel amino terminal domain important in receptor function and inter-domain communication, and distinguish variants capable of productive engagement with chromatin.
The androgen receptor (AR) is a class III nuclear receptor, which in humans comprises all of the steroid receptors including the estrogen receptor-α and -β, progesterone, mineralocorticoid, and the glucocorticoid receptors as well as the estrogen-related receptor-1 and -2 (1). The cellular genomic actions of AR arise from a signaling cascade involving association with chaperone proteins, ligand binding, nuclear translocation, and DNA binding as well as association with coregulatory molecules resulting in subsequent regulation of the transcription of target genes. The AR protein contains four modular domains each performing distinct but interrelated roles in the cascade (2). The AR DNA binding domain (DBD) is highly conserved among the nuclear receptors and consists of two cysteine-rich zinc fingers that directly bind to specific DNA sequences in the genome (3). A short, nonconserved, flexible hinge region separates the DBD from the AR ligand binding domain (LBD). The LBD upon agonist binding adopts a conformation that results in the formation of a hydrophobic pocket known as activation function (AF)-2. The formation of the AF2 pocket is a common characteristic to all of the nuclear receptors and has been shown to have a high affinity for binding LxxLL-type peptides in many nuclear receptor coregulators (4). The long, structurally flexible amino-terminal domain (NTD) is the most evolutionarily divergent region of the nuclear receptors (5,6) and also interacts with members of the p160 coactivator family as well as the corepressor silencing mediator of retinoid and thyroid receptors and the general transcriptional machinery (7,8,9,10). These interactions induce conformational changes within this NTD that lead to a more closed, active conformation (5). Indeed, interaction with one of the p160 coactivators is thought to be an essential step in AR transcriptional activation (11).
The 23FQNLF27 peptide in the AR NTD is essential for the amino-carboxyl (N/C) interaction, which is a rapid agonist-induced contact between the NTD and the AF2 surface in the LBD (12,13,14). The AF2 surface of the AR is unique among the class III nuclear receptors in that it preferentially binds FxxLF peptides over the LxxLL-type, predominantly due to a charge clamp formed by the positively charged residues K715, K718 and R724 and the negatively charged residues E707, E891, and E895 (15). This finding has led to the hypothesis that the N/C interaction is adopted in preference to coregulator binding at the AR AF2 (15). Nevertheless, the AR AF2 retains the potential to bind p160 and other coactivators via LxxLL-type peptides, and the AR remains transcriptionally responsive to them (15). This has resulted in uncertainty regarding the mechanism and manner of p160 binding in the context of the strong N/C interaction and whether they contribute to or compete with the N/C interaction (16,17). More recently, live cell imaging with the full-length AR suggests the N/C interaction may occur before nuclear import to prevent untimely or inappropriate association of AR with LxxLL- and FxxLF-containing coregulators (18,19). Whereas these findings can be interpreted as the AR N/C interaction and p160 binding being mutually exclusive, interaction between the NTD and LBD can be rescued for an N/C-deficient AR variant (i.e. 23FQNLF27 to 23AQNAA27) by overexpression of nuclear receptor coactivator (NCOA)-2 (16). This finding suggests that these two events may not be mutually exclusive. In any case, these results require the AR NTD to be the critical determinant of the AR transcriptional response due to the occupancy of the AF2 pocket by N/C or coregulator interactions.
Prostate cancer is a leading cause of cancer-related death in Western males. In advanced stages of the disease, many men receive systemic hormonal treatment termed androgen ablation therapy (AAT), which involves blockade of testicular androgen synthesis and reducing circulating androgens (20). Whereas AAT leads to a reduction in AR signaling, it has also been combined with the administration of an AR-specific antagonist to more specifically block the actions of the AR at a cellular level (combined androgen blockade). Whereas patients initially respond well to these hormonal therapies, disease progression is inevitable, after which there are limited treatments (20).
It is now clear that the AR is a key determinant of prostate cancer cell growth despite hormonal therapy. Several mechanisms have been demonstrated to be responsible for continued AR signaling during AAT, including altered expression of key coregulators or chaperones and somatic mutation of the AR gene (21). Numerous studies have identified that AR gene mutations can result in promiscuous, more sensitive, or superactive receptors (22,23,24). The majority of mutations identified to date collocate to functionally important subdomain structures within the AR LBD (22). Although several studies have now identified missense mutations in the AR NTD in prostate tumors after hormonal therapy (25,26,27), regions of functional importance in this flexible domain remain poorly defined.
In this study, we report that somatic AR mutations identified in advanced prostate cancer cluster to a previously identified evolutionary conserved region in the AR NTD between amino acids 497 and 555 (6) and formally identify AR amino acids 513–535 as a site of collocation for somatic variation in prostate cancer. AR variants in this region exhibit divergent responses in different cell types, with a subset exhibiting enhanced coactivation by p160 coactivators specifically in prostate cancer cells. Deletion of amino acids 501–535 results in an AR with increased transactivation activity on a transiently transfected reporter element and decreased ability to form an N/C interaction and interact with NCOA2, but retains the capacity to bind endogenous androgen-responsive regions and activate transcription in a chromatin setting in mammalian cells.
Materials and Methods
Plasmids
Expression vectors pCMV-AR3.1, pVP16-ARNTD (1–538), GAL4DBD-ARLBD (644–917), pBS-SK, the androgen-responsive ARR3-tk-Luc, pCMV-ARΔDBD, and GAL4-responsive pGK-1 have been described previously (28,29). pM-GRIP1, pSG5-SRC1a (NCOA1), pSG5-GRIP1 (NCOA2), and pSG5-AIB1 (NCOA3) plasmids were a gift from Professor M. Stallcup (University of Southern California, Los Angeles, CA), and pcDNA3.1-AR.d-LBD was a gift from Professor Gerhard Coetzee (University of Southern California). pVP16-SRC1a (NCOA1) and pVP16-AIB1 (NCOA3) were created via amplification of the 5′ coding region using primers listed in supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org, followed by subcloning the 3′ regions of the coding sequences using the restriction endonucleases PmlI/PsiI and TthIII1/PsiI, respectively. pcDNA3.1-AR.1–555, pcDNA3.1-AR.1–535, and pcDNA3.1-AR.1–500 were created via amplification of the NTD using primers listed in supplemental Table 1, followed by replacement of the majority of the region with a sequence-verified AR NTD by subcloning with AflII/Bsu36I. Substitution variants were created using the PCR megaprimer method in pCMV-AR3.1. Deletion of amino acids 501–535 and AR NTD substitution variants were created by primer overlap extension in pCMV-AR3.1 and pVP16-ARNTD (1–538), respectively (supplemental Table 1). Base changes, ligation sites, and amplified sequences were confirmed by automated DNA sequencing; AR numbering is according to that published elsewhere (30). The ARR3 promoter element was PCR amplified from pGL3-ARR3-tk-luc using PB3LucS 5′-TATTGCTAGCGCTCAGATCCAAGCTGGAGCT-3′ and PB3LucAS 5′-TATTGATATCTACCAACAGTACCGGAATGCCA-3′ and subcloned into pGL4.14 (Promega Corp., Madison, WI) using the restriction endonucleases NheI/EcoRV.
Cell culture, transactivation and interaction assays, and immunoblot analysis
COS-1 cells (American Type Culture Collection, Manassas, VA) and the PC3 cell subline PC3AR+, described elsewhere (28), were maintained in RPMI 1640 medium supplemented with 5% fetal bovine serum. Transactivation and coactivator assays were performed in PC3AR+ cells (10,000 cells/well in 96-well plates) transfected with 100 ng ARR3-tk-Luc and 0.5 ng pCMV-AR3.1 or equivalent molar amount of AR variant. Coactivator assays included 50 molar excess of pSG5-SRC-1 (NCOA1), pSG5-GRIP1 (NCOA2), or pSG5-AIB1 (NCOA3). Mammalian 2-hybrid N/C interaction assays were performed in COS-1 cells (10,000 cells/well in 96 well plates) transfected with 25ng pGK-1, 5 ng pVP16-ARNTD (1–538) or equivalent molar amount of variant, and GAL4DBD:ARLBD (644–917). Modified N/C interaction assays were performed in COS-1 cells (10,000 cells/well in 96-well plates) using 5 ng pcDNA3.1-AR.D-LBD and 5 ng pcDNA3.1-AR.1–555 or molar equivalent of pcDNA3.1-AR.1–535 or pcDNA3.1-AR.1–500. Mammalian two-hybrid p160 interaction assays were performed using 5 ng VP16-GRIP1, VP16-AIB1, or VP16-SRC1a and 5 ng GAL4DBD:AR (1–555) or molar equivalent of GAL4DBD-AR (1–555)Δ35αα with 25 ng pGK-1 reporter per well. In all experiments, total DNA and molar expression vector concentration was controlled using prokaryotic pBS-SK and empty vector, respectively. Sextuplicate wells were independently transfected in all experiments and luciferase assays followed 24 h of treatment with 10 nm 5α-dihydrotestosterone (DHT) or vehicle (EtOH) as previously described (28). Lysates from replicates were pooled and equal volumes immunoblotted using AR-N20 antisera (Santa Cruz Biotechnology, Santa Cruz, CA) as previously described (28) to act as a verification of relative expression to wild-type AR (wtAR) of the AR variants within the transactivation assays.
Establishment of stable cell lines
PC3AR+ cells (5 × 106) were electroporated with 20 μg pGL4.14-ARR3LUC plasmid or empty pGL4.14 with a 2-mm gap cuvette (BTX International, Middle Cove, New South Wales, Australia) on a square wave electroporator ECM 630 (BTX International) at 280 V for 2 msec. Each electroporation reaction was plated on a 10-cm tissue culture plate. After 48 h, growth medium containing 120 μg/ml hygromycin B in charcoal stripped growth medium was placed on each of the plates. Medium was changed every 48 h and plates were left until visible colonies had formed. Single colonies were transferred to each well on 24-well plates and were subsequently propagated into flasks. Empty vector clones were diagnosed via PCR amplification of genomic DNA, whereas ARR3-Luc containing clones were diagnosed via transient transfection with 100 ng AR expression plasmid and transactivation assay as described above.
Transient chromatin immunoprecipitation (ChIP)
PC3AR+ cells (3 × 106) were plated in 150-mm2 plates for 48 h and then transfected with 10 μg pCMV-AR or AR variant expression plasmid using Lipofectamine2000 (Invitrogen, Melbourne, Victoria, Australia) for 4 h. Medium was replaced with phenol red-free RPMI 1640 containing 5% dextran charcoal-stripped fetal calf serum for 16–24 h and stimulated for 1 h with 10 nm DHT or equivalent vehicle (ethanol). Cells were fixed for 10 min with 1% formaldehyde, washed twice in ice-cold PBS, scraped into 500 μl PBS containing 1× protease inhibitor cocktail (Sigma-Aldrich, Sydney, New South Wales, Australia), collected by centrifugation, and lysed in 700 μl sodium dodecyl sulfate (SDS) lysis buffer [1% SDS (wt/vol), 10 mm EDTA (pH 8.1), 50 mm Tris-HCl (pH 8.1), 2× protease inhibitor cocktail]. Cells were sonicated to produce DNA fragment lengths of 300-1500 bp and insoluble cell material removed by centrifugation (4 C, 10 min, 14,500 × g). Two hundred microliters of soluble chromatin were diluted in 1.8 ml ice-cold dilution buffer [0.01% SDS (wt/vol), 1.1% Triton X-100, 1.2 mm EDTA (pH 8.1), 16.7 mm Tris-HCl (pH 8.1), 167 mm NaCl, and 1× protease inhibitor cocktail] and precleared for 1 h at 4 C with 45 μl of 50:50 protein G sepharose slurry in 1× Tris/EDTA buffer (pH 8.1) (GE Healthcare, Rydalmere, New South Wales, Australia) and 200 μg of yeast tRNA (Sigma-Aldrich).
Samples were centrifuged 4 C for 30 sec at 14,500 × g and the supernatant transferred to fresh, ice-cold tubes. Immunoprecipitations were performed at 4 C overnight by adding to each sample 4 μg AR N-20 (Santa Cruz Biotechnology) or normal rabbit IgG (Santa Cruz Biotechnology) antisera. Immunoprecipitated chromatin was bound for 1 h at 4 C to 45 μl 50:50 protein G sepharose slurry beads in the presence of 200 μg of tRNA, which were then collected at 4 C by centrifugation at 5000 × g. Beads were washed for 5 min with each of the following ice-cold solutions: once with low-salt immune complex wash buffer [0.1% SDS (wt/vol), 1% Triton X-100, 2 mm EDTA (pH 8.1), 20 mm Tris-HCl (pH 8.1), and 150 mm NaCl]; once with high-salt immune complex wash buffer [0.1% SDS (wt/vol), 1% Triton X-100, 2 mm EDTA (pH 8.1), 20 mm Tris-HCl (pH 8.1), and 500 mm NaCl]; once with LiCl immune complex wash buffer [0.25 m LiCl, 1% Igepal CA-630, 1% sodium deoxycholate, 1 mm EDTA (pH 8.1), and 10 mm Tris-HCl (pH 8.1)]; and twice with 1× Tris/EDTA buffer (pH 8.1). Ten percent of the collected immunoprecipitated complexes were placed into denaturing SDS-PAGE loading buffer for immunoblot analysis. Protein-DNA complexes were eluted from beads with two 15-min room temperature washes in a total of 500 μl elution buffer (1% SDS, 0.1 m NaHCO3). DNA-protein cross-links were reversed by addition of NaCl to a final concentration of 200 mm and incubation overnight at 65 C. EDTA (pH 8.1) and Tris-HCl (pH 6.5) were added to final concentrations of 10 and 40 mm, respectively, after which samples were treated first for 1 h with ribonuclease I (New England Biolabs, Ipswich, MA) at 37 C and then for 1.5 h with proteinase K at 45 C. DNA was purified by phenol-chloroform extraction and ethanol precipitation, and resuspended in 100 μl RT-PCR grade H2O.
Samples were assayed in triplicate by real-time PCR using 5 μl DNA, iQ SYBR Green supermix (Bio-Rad Laboratories, Gladesville, New South Wales, Australia) and a final concentration of 4 μm of primers: the previously described androgen-responsive region of the FKBP51 enhancer (31), 5′-GCTCTGACTTATTGTTCTCTTACTGCCC-3′, 5′-TTGCTGTCAGCACATCGAGTTCA-3′; nonspecific, 5′-AGACACACTGTCACTTGGGCAA-3′, 5′-GTCTAGGTCTTGGCAGCGTGTTA-3′. PCR parameters were 95 C for 3 min and then 55 cycles at 95 C for 15 sec, 60 C for 15 sec, and 72 C for 30 sec. All quantitative RT-PCRs were followed by melt curve analysis to determine specificity of the amplification products.
Results
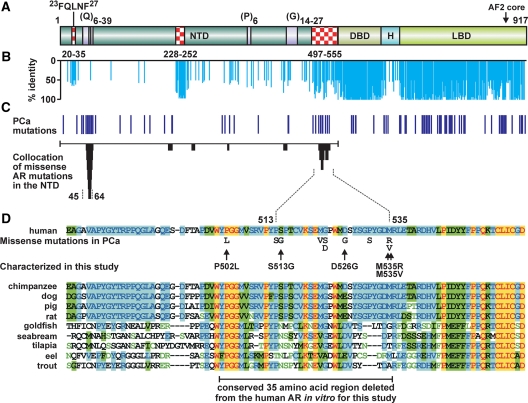
Collocation of somatic AR mutations in prostate cancer to small areas of the LBD has been instructive in defining subdomain structures, coregulator binding sites, and AR functionality important in this disease (22,32). Whereas somatic mutations have also been proposed to cluster to regions of the AR NTD (22), there has until recently been too few individual mutations to formally assign regions of collocation on which we might infer biological function. With recent reports bringing the number of AR mutations identified in the AR NTD in prostate cancer specimens to about 40 (24,25,26,27), we are now able to assign formal collocation for two AR NTD regions using stringent criteria (Fig. 1C). The first centers over the polymorphic glutamine repeat between amino acids 45 and 64, the length of which has previously been inversely associated with risk of prostate cancer and also previously been reported as a site for somatic repeat number contraction and expansion in prostate cancer (33). The second lies between amino acids 513 and 535 and is the site of 10 individual mutations identified in advanced prostate cancers after relapse on combined androgen blockade (26) (Fig. 1D). Amino acids 497–555 comprise one of only three small regions of the AR NTD with high amino acid sequence identity between species (Fig. 1, A and B) and has previously been identified as one of the most evolutionarily conserved regions of the AR NTD (6). This region has been implicated in vitro in directly interacting with and suppressing DNA binding of the isolated AR DBD to androgen-response elements (34) and in the interaction of the receptor with a p160 coregulator protein, NCOA2 (35). These findings suggest a role for this conserved region in aspects of AR function that may include signaling events before DNA binding or on in vivo androgen-responsive regions.
Figure 1.
Somatic AR NTD mutations in advanced prostate cancer formally collocate to the polyglutamine repeat region and a region of unknown function with high identity between species. A, Schematic of the AR protein depicting the amino terminal (NTD), DBD, hinge (H), LBD domains, polymorphic Q, G, and P repeats, and the 23FQNLF27 peptide involved in the AR N/C interaction. Regions of high identity between species in the AR NTD are indicated by checked boxes and delineated by amino acid numbers. B, Percent amino acid identity for all 24 full-length ARs from different species (http://www.ncbi.nlm.nih.gov) performed using the ClustalW algorithm. Species, sequences used, and accession numbers are listed in supplemental Table 2. C, Position AR mutations identified in prostate cancer and the two regions of the NTD formally ascribed here as regions to which mutations collocate. Collocation analysis was performed as we previously described for the LBD (25). D, Collocation between amino acids 500 and 535 in the AR NTD. Amino acid sequence of this region, the position and nature of somatic missense mutations detected in previous studies of prostate cancer (24,25,26,27), and alignment diverse species is shown (yellow highlight, identical; green highlight, similarity; blue highlight, conserved; green on white, weakly similar).
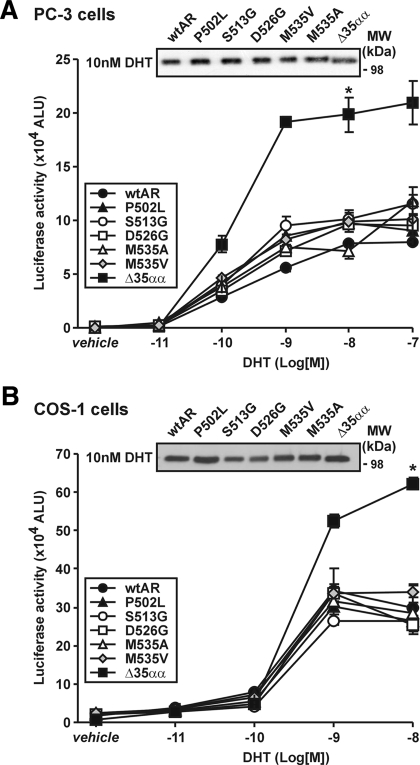
The AR NTD is responsible for the majority of the transcriptional competence of the receptor (36). Consequently, we first assessed the effect of candidate somatic AR prostate cancer mutations between amino acids 502 and 535 on AR transactivation activity. In prostate cancer PC-3 cells, transactivation activity of most of the AR variants was found to be modestly higher than wtAR in response to 0.1 and 1 nm DHT in each of several independent assays (Fig. 2A). AR immunoblot analysis performed directly on transactivation assay lysates demonstrated that transfection efficiency or protein levels were not responsible for these differences (Fig. 2A). Activity of these same AR variants in nonprostate cancer COS-1 cells was comparable with wtAR at all concentrations of DHT investigated (Fig. 2B). In contrast to the above results, however, deletion of the entire 501–535 region (i.e. ARΔ35αα) resulted in an increase in AR transactivation activity at DHT concentrations 1 nm and above in both cell lines (Fig. 2, A and B).
Figure 2.
Somatic missense AR mutations in advanced prostate cancer demonstrate cell-specific differences in activity in comparison with wtAR. A, Transactivation activity of wild-type and variant ARs on the ARR3-tk-luc reporter in transfected PC3 cells with vehicle or 0.01–100 nm DHT. Data represent the mean ± sem arbitrary light units (ALU) of six independently transfected wells and are representative of at least three independently performed experiments. *, P < 0.01 on Mann Whitney U analysis for ARΔ35αα compared with wtAR. Lysates not assayed for luciferase activity were pooled for each variant at 10 nm DHT and assessed by immunoblot analysis for equal relative AR protein levels. B, COS-1 cells were transfected, treated, assayed, and immunoblotted as described in A. MW, Molecular weight.
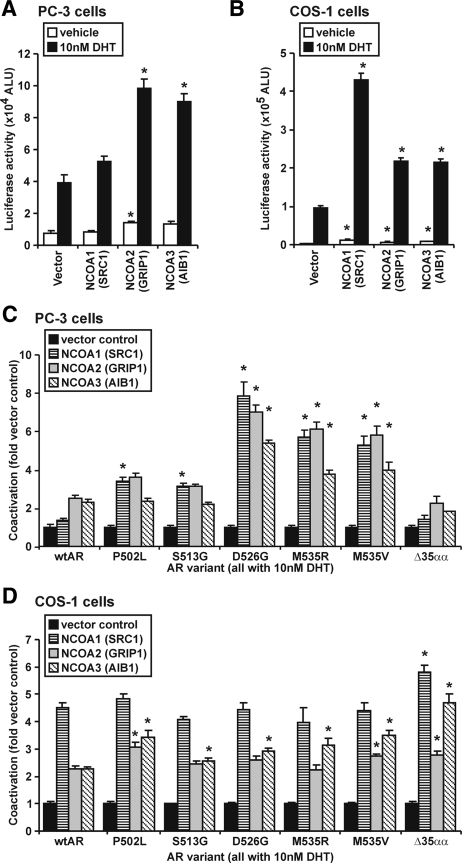
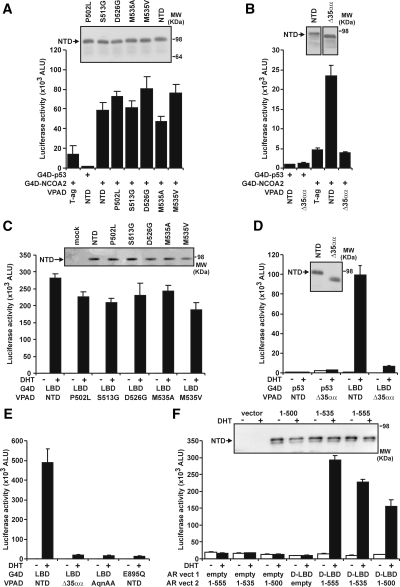
Divergent activity in the different cell types might be due to differential expression between the cell lines or functional output of coregulators such as the p160 family, NCOA1 [steroid receptor coactivator (SRC)-1], NCOA2 [SRC2/glucocorticoid-interacting protein (GRIP)-1/transcription intermediary factor-2], or NCOA3 [SRC3/amplified in breast cancer-1 receptor-associated coactivator (RAC3)] (37). Overexpression of each p160 protein robustly enhanced wtAR activity in COS-1 cells but at best only modestly in prostate cancer PC-3 cells (Fig. 3, A and B). Moreover, the response of a subset of 502–535 AR variants (D526G, M535A, and M535V) was enhanced compared with wtAR in prostate cancer PC-3 cells, whereas the variants exhibited a more modest enhancement of transactivation activity compared with wtAR in COS-1 (Fig. 3, C and D). Deletion of amino acids 501–535 (ARΔ35αα) did not confer increased response of the AR to p160s in PC-3 cells and was modestly enhanced in response to NCOA1 and NCOA3 in COS-1 cells (Fig. 3, C and D). Whereas these results may be indicative of potential differences in the overexpression levels of the p160 proteins between the cell lines, these results nevertheless indicate that the 502–535 region of the AR may be a mediator of the interaction between the AR NTD and the p160 coactivators. To assess this, we used a number of AR variants depicted in Fig. 4. Whereas the apparent interaction of NCOA2 with the AR NTD alone was not significantly affected by the individual 502–535 AR variants (Fig. 5A), it was dramatically reduced in comparison with wtAR when the 501–535 region was removed (i.e. for AR NTDΔ35αα) (Fig. 5B).
Figure 3.
A subset of the AR variants exhibit increased enhancement by the p160 coactivators in comparison with wtAR in prostate cancer cells. A, AR activity on ARR3-tk-luc reporter in PC3 cells cotransfected with a 50-fold excess of p160 coactivators as indicated or an equivalent molar concentration of the empty vector control. Treatment was with 10 nm DHT. Data are presented as in Fig. 2A. *, P < 0.05 on Mann Whitney U analysis in comparison with similarly treated wtAR cotransfected with empty vector. B, COS-1 cells were plated, transfected, and treated as described in A. C, Effect of coregulators on wtAR or AR variants in PC3 cells performed as in A. Data represent fold effect of coregulator empty vector control for each variant and is the mean ± sem of four independently transfected wells. *, P < 0.05 on Mann Whitney U analysis compared with wtAR with the same coactivator. D, COS-1 cells were plated, transfected, treated, assayed, and presented as described in C. All data are representative of at least three independently performed experiments.
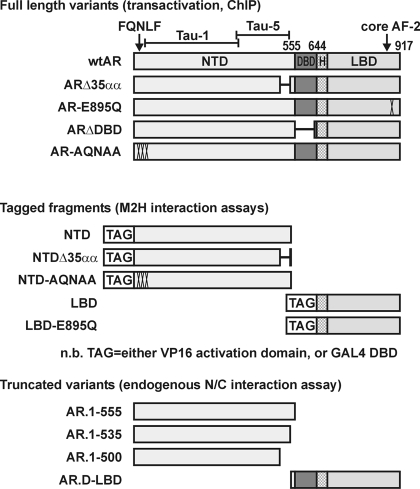
Figure 4.
Schematic of AR variants used in Figs. 5 and 6. AR.D-LBD, AR variant containing the DBD and LBD; TAG, either the GAL4 DNA binding or VP16 activation domains (G4D or VPAD).
Figure 5.
Deletion of amino acids 501–535 of the AR disrupts the N/C interaction and the AR NTD NCOA2 interaction. A, Interaction assay for NCOA2 performed in COS-1 cells using VP16-tagged AR NTD variants, GAL4 DBD tagged NCOA2 and pGK-1 reporter. All wells were controlled for molar equivalent of expression plasmid and total DNA transfected per well. Data were assayed and immunoblot performed as described in Fig. 2A. T-ag, VP16 large T antigen. B, Interaction of ARΔ500–535 with NCOA2 performed as in A. C, N/C interaction assay in COS-1 cells using VP16-tagged AR-NTD variants, GAL4-tagged AR LBD, and pGK-1 reporter. Treatment was with vehicle or 10 nm DHT, and cells were lysed, assayed, and immunoblotted as in A. D and E, N/C assays for ARΔ500–535 and other AR variants performed as in C. F, Native N/C interaction assay performed in COS-1 cells transfected with untagged AR DBD LBD and variant AR NTD fragments as indicated. Data were assayed and immunoblot performed as described in Fig. 2A. All data are representative of at least three independently performed experiments. ALU, Arbitrary light units; G4D, GAL4 DNA-binding domain; MW, molecular weight; vect, vector; VPAD, VP16 activation domain.
Because previous reports suggest a link between the AR N/C and coregulator interactions (16,19), we interrogated N/C for each AR variant. Whereas individual AR NTD variants exhibit only a marginally reduced capacity to form an N/C interaction compared with wtAR (Fig. 5C), N/C was substantially decreased for AR NTDΔ35αα (Fig. 5D). This dramatic effect was equivalent to previously published N/C-deficient AR variants mutated in either the NTD interaction peptide (Fig. 5F) (i.e. 23FQNLF27 to 23AQNAA27) or AF2 (i.e. AR-E895Q) (Fig. 5E) (14,32). In contrast, experiments using an androgen response element and nontagged AR variant fragments consisting of portions of the AR NTD demonstrated that whereas amino acids 500–535 contribute to the N/C interaction, the removal of these amino acids did not result in complete abrogation of the interaction (Fig. 5F).
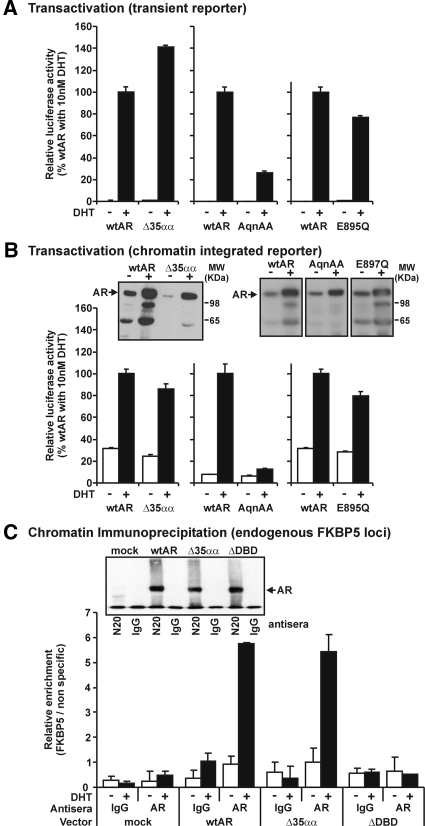
To assess the relationship between the N/C interaction and transactivation activity of full-length receptors, we first analyzed the ability of AR variants to activate a transiently transfected reporter gene in human prostate cancer cells. As demonstrated in Fig. 6A, deletion of amino acids 501–535 and the AR-E895Q mutation of AF2 resulted in an increase and decrease, respectively, in AR transactivation capacity on the transiently transfected probasin promoter. Whereas the AR-E895Q variant has previously been shown to abolish the N/C interaction, it is thought to still exhibit the ability to bind coregulators such as NCOA2 to the AF2 surface (32,38). In contrast to these variants, the AR-23AQNAA27 variant exhibited a more dramatic decrease in transactivation activity to 30% of that observed in response to wtAR (Fig. 6A). Next, we tested whether the ability to form an N/C interaction is required for AR activity on a chromatin integrated promoter. To assess the activity of these N/C-deficient variants in a chromatin context, we created a PC-3 cell line containing a stably integrated androgen-responsive luciferase reporter construct. Whereas the AR-23AQNAA27 N/C-deficient variant almost abolished DHT-induced activity in a chromatin context, both N/C-impaired ARΔ35αα and AR-E895Q variants were capable of activating transcription in chromatin at a similar level to wtAR (Fig. 6B). All variants in this assay exhibited similar increases to wtAR in steady-state levels in response to DHT as measured by immunoblot analysis. An increase in steady-state levels is not therefore a hallmark of an N/C interaction in this assay.
Figure 6.
The ability to form an N/C interaction does not relate to the ability to induce exogenous or endogenous transactivation activity A, Activity of AR variants in transiently transfected PC3 cells with vehicle or 10 nm DHT treatment performed as described in Fig. 2A. B, Activity of AR variants on chromatin integrated ARR3 reporter. PC3-ARR3-C13 cells were transfected with 100 ng AR expression vector and treated, assayed, and immunoblotted as described in A. Data are representative of results also obtained in an independent derived stable transfected clone. C, Interaction of AR variants with DNA in quantitative transient ChIP analysis. ChIP was performed in PC3 cells transfected in 150 mm2 plates with AR variants and treated for 1 h vehicle or 10 nm DHT. Data represent the relative enrichment of the FKBP5 locus for vehicle (open bars) and DHT (closed bars) over a nonspecific genomic DNA region from the KLK3 locus by quantitative real-time PCR. Data are representative of three independently performed transient ChIP experiments. Inset shows AR immunoblot of immunoprecipitated material for AR variants. MW, Molecular weight.
Finally, to assess the capacity of ARΔ35αα to bind to DNA, we undertook transient ChIP assays in PC-3 cells. As expected, we were not able to detect binding of an AR variant deleted of the DBD (ARΔDBD) at the canonical androgen-responsive element (ARE) in the enhancer region of FKBP5. ARΔ35αα, in contrast, exhibited a similar DHT-induced enrichment to wtAR at this locus (Fig. 6C). Consequently, it appears as if the ARΔ35αα variant is not defective in its capacity to interact with canonical AREs in a chromatin context.
Discussion
The AR NTD is regarded as the major determinant of AR transcriptional activity, but its component regions and their specific functions are only loosely defined. Here we used evolutionary conservation of the AR and the collocation of prostate cancer-derived somatic mutations to define the 501–535 region of the AR NTD as potentially biologically significant.
The hormonal management of prostate cancer provides an environment in which mechanisms of continued AR action are selected (25). For example, AR LBD variants such as T877A or W743L exhibit agonist responses to the canonical AR antagonists hydroxyflutamide and bicalutamide, respectively (39,40), and have been detected in tumors from patients treated with these same agents (25). Whereas arising in a similar hormonally selective environment, the activity of the 501–535 AR variants is distinct from those above in that their response to bicalutamide and hydroxyflutamide is similar to wtAR (data not shown). Our results suggest that the region between amino acids 501 and 535 is an important contributor to interaction of the AR NTD with the p160 coactivator, NCOA2, and involved in formation or maintenance of an optimal N/C interaction. Consistent with disruption or variation in these contacts, several of the somatic variants from prostate cancer that lie within the 501–535 region exhibit a cell context-specific transactivation phenotype and increased response to the p160 coactivators. Other prostate cancer-derived somatic NTD variants behave similarly, including AR-E235G and ARpolyQ2L (which contains two nonconsecutive leucine residues interrupting the polyglutamine tract) (32,41). The polyQ2L mutation resides in a second region of the AR NTD to which somatic prostate cancer mutations collocate, and E235G occurs in another of the short evolutionary conserved regions of NTD termed the signature sequence (Fig. 1) (6,41). Altered response to coregulators is by no means unique to AR NTD variants: recent studies suggest that even LBD T877A and W743L variants promote differential coregulator activities in comparison with wtAR (42). Consequently, altered interaction with or response to coregulators may be common mechanism of treatment escape by many AR variants. Indeed, expression of p160 family members and a host of other AR coregulators is commonly altered in advanced and/or metastatic prostate cancers compared with primary disease (43,44).
There are two possible explanations why interaction of NCOA2 with the 501–535 AR variants was similar to wtAR, yet a subset of these variants exhibited enhanced response to all three of the p160 coactivators. In the first instance, two interaction surfaces on the AR have been described for NCOA2; the AF2 region in the LBD and NTD amino acids 351–537 (35), both of which also engage in the formation of the N/C interaction. The use of partial proteins in the context of a mammalian two-hybrid interaction assay, although a valuable screening tool, may not be sufficient to capture the complex interactions occurring on the full-length AR molecule with and without ligand binding. On the other hand, mutations may alter the interaction of normally bound p160s with secondary coactivators such as coiled-coil activator or cAMP response element-binding protein/p300, thereby affecting the synergistic response (45,46).
A previous report using partial proteins in an in vitro assay reported that coexpression of an AR fragment consisting of residues 479–590 decreased binding of an isolated AR DBD to an ARE, and decreased transactivation activity of the full-length AR using a transfected reporter (34). In contrast, our results using the full-length receptor and responsive elements in a native chromatin context indicate that deletion of residues 501–535 does not affect the capacity of AR to bind to a canonical hormone response element or mediate transactivation in vivo. It is possible that mechanisms such as altered N/C interaction or coregulator binding could compensate for the effect on DNA binding seen in the in vitro setting or that this region may be more important in the binding of the receptor to specific androgen-responsive or selective DNA elements.
Several studies have reported the potential involvement of coactivators in the N/C interaction (8,16,47). Recent results indicated that the N/C forms rapidly after ligand binding in the cytoplasm and persists until the AR molecule becomes immobilized on DNA in nucleus. In this later state, the strength of the N/C is relaxed thereby allowing receptor interaction with FxxLF-containing coactivators such as the p160s at AF2 (18,19). By demonstrating that coregulator binding and N/C interaction occur at different points in the AR signaling cascade, that finding resolved the apparent paradox of how both interactions were apparently essential for AR function but strongly antagonistic. In the current study, we demonstrate that amino acids 501–535 of the AR NTD, although not essential for the N/C interaction, make an important contribution to the overall interaction as well as being involved in the interaction with the p160 coactivator, NCOA2. Consequently, our results support the modified theory in which AR N/C and coregulator interactions are competitive but occur in a temporal and spatial manner using multiple, and perhaps redundant, interaction domains.
Our finding that the outcome from the mammalian two-hybrid N/C interaction assay is more dramatic than observed with a native assay involving untagged partial AR constructs reveals that although useful, the widely used mammalian two-hybrid assay may not reflect the magnitude of the interaction in the native situation. Nevertheless, our finding that deletion of amino acids 500–535 results in compromised activity in both the native assay and the mammalian two-hybrid interaction assay strongly supports a role for this region in the optimal N/C interaction.
The suggestion that N/C interaction is required for AR activity in vivo was based on inactivating germline AR mutations that abolish the N/C interaction. Genetically male carriers of these mutations present phenotypically as females in the complete androgen insensitivity syndrome (48). These observations are supported by a study in Xenopus ova demonstrating that the N/C-deficient AR-23AQNAA27 variant is inactive on a chromatin integrated reporter element (49) and that competing FQNLF-containing peptides block the AR N/C interaction and ultimately lead to the inhibition of prostate cancer cell growth (50). In this study, we demonstrate in mammalian cells that activity of AR-23AQNAA27 is severely compromised on both a chromatinized and transiently transfected androgen response element, whereas the activity of the N/C-deficient AR-E895Q variant on both templates is similar to that of ARΔ35αα and wtAR. Therefore, decreased transactivation activity is not a hallmark of an N/C-impaired AR variant. Rather, it appears that there are two classes of N/C-deficient AR variants, those directly affecting surfaces required for formation of the N/C (e.g. 23FQNAA27) and that severely compromise AR function, and those in other surfaces that contribute to the overall N/C interaction (e.g. E895Q and mutations in residues 501–535) that retain transactivation capacity regardless of the chromatin context. Future experiments will importantly determine how these two classes of N/C-deficient variants differentially affect overall AR biological action.
In summary, the use of evolutionary sequence conservation and the collocation of naturally occurring prostate cancer mutations has allowed us to define the 501–535 region of the flexible AR-NTD as an important region for NCOA2 contact and for the N/C interaction. Moreover, we show that only a subset of N/C-deficient AR variants compromise receptor activity on a chromatinized template. These findings support the notion that N/C and NCOA2 interactions compete for similar binding surfaces across the AR and occur in a synergistic but temporal manner during the AR signaling process.
Supplementary Material
Acknowledgments
We thank Ms. Michelle Newman for her technical contribution and Dr. Margaret Centenera for helpful comments on the manuscript.
Footnotes
This work was supported by Grant YI02 from the Prostate Cancer Foundation of Australia (to G.B.), Grant 453662 from The National Health and Medical Research Council of Australia (to W.D.T. and V.R.M.), Grant PC060443 from the U.S. Department of Defense (to W.D.T. and V.R.M.), Grant P50 CA92629 from the National Institutes of Health (to H.I.S.), Memorial Sloan Kettering Cancer Center Special Program in Research Excellence in prostate cancer (to H.I.S.), PepsiCo Foundation (to H.I.S.), and Prostate Cancer Foundation (to H.I.S.). G.B. holds a National Health Medical Research Council C. J. Martin Biomedical Fellowship. E.F.N. holds a Freemasons Foundation postdoctoral fellowship.
Current address for N.L.M.: Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, Texas.
Current address for A.C.: Cell Culture Process Development, Lonza Biologics Plc, Berkshire, United Kingdom.
Disclosure Summary: The authors have nothing to declare.
First Published Online March 12, 2009
Abbreviations: AAT, Androgen ablation therapy; AF, activation function; AR, androgen receptor; ARE, androgen-responsive element; ChIP, chromatin immunoprecipitation; DBD, DNA binding domain; DHT, 5α-dihydrotestosterone; GRIP, glucocorticoid-interacting protein; LBD, ligand binding domain; N/C, amino/carboxyl; NCOA, nuclear receptor coactivator; NTD, amino-terminal domain; SDS, sodium dodecyl sulfate; SRC, steroid receptor coactivator; wtAR, wild-type AR.
References
- Laudet V 1997 Evolution of the nuclear receptor superfamily: early diversification from an ancestral orphan receptor. J Mol Endocrinol 19:207–226 [DOI] [PubMed] [Google Scholar]
- Jenster G, van der Korput JA, Trapman J, Brinkmann AO 1992 Functional domains of the human androgen receptor. J Steroid Biochem Mol Biol 41:671–675 [DOI] [PubMed] [Google Scholar]
- Shaffer PL, Jivan A, Dollins DE, Claessens F, Gewirth DT 2004 Structural basis of androgen receptor binding to selective androgen response elements. Proc Natl Acad Sci USA 101:4758–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heery DM, Kalkhoven E, Hoare S, Parker MG 1997 A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733–736 [DOI] [PubMed] [Google Scholar]
- McEwan IJ, Lavery D, Fischer K, Watt K 2007 Natural disordered sequences in the amino terminal domain of nuclear receptors: lessons from the androgen and glucocorticoid receptors. Nucl Recept Signal 5:e001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JW, Kelley DB 1998 Evolution of the androgen receptor: structure-function implications. Bioessays 20:860–869 [DOI] [PubMed] [Google Scholar]
- Alen P, Claessens F, Verhoeven G, Rombauts W, Peeters B 1999 The androgen receptor amino-terminal domain plays a key role in p160 coactivator-stimulated gene transcription. Mol Cell Biol 19:6085–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen T, Palvimo JJ, Jänne OA 1997 Interaction between the amino- and carboxyl-terminal regions of the rat androgen receptor modulates transcriptional activity and is influenced by nuclear receptor coactivators. J Biol Chem 272:29821–29828 [DOI] [PubMed] [Google Scholar]
- Callewaert L, Van Tilborgh N, Claessens F 2006 Interplay between two hormone-independent activation domains in the androgen receptor. Cancer Res 66:543–553 [DOI] [PubMed] [Google Scholar]
- Dotzlaw H, Papaioannou M, Moehren U, Claessens F, Baniahmad A 2003 Agonist-antagonist induced coactivator and corepressor interplay on the human androgen receptor. Mol Cell Endocrinol 213:79–85 [DOI] [PubMed] [Google Scholar]
- Shang Y, Myers M, Brown M 2002 Formation of the androgen receptor transcription complex. Mol Cell 9:601–610 [DOI] [PubMed] [Google Scholar]
- Burd CJ, Petre CE, Moghadam H, Wilson EM, Knudsen KE 2005 Cyclin D1 binding to the androgen receptor (AR) NH2-terminal domain inhibits activation function 2 association and reveals dual roles for AR corepression. Mol Endocrinol 19:607–620 [DOI] [PubMed] [Google Scholar]
- Bai S, He B, Wilson EM 2005 Melanoma antigen gene protein MAGE-11 regulates androgen receptor function by modulating the interdomain interaction. Mol Cell Biol 25:1238–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Kemppainen JA, Wilson EM 2000 FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. J Biol Chem 275:22986–22994 [DOI] [PubMed] [Google Scholar]
- He B, Wilson EM 2003 Electrostatic modulation in steroid receptor recruitment of LXXLL and FXXLF motifs. Mol Cell Biol 23:2135–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC, Buchanan G, Butler LM, Prescott J, Henderson M, Tilley WD, Coetzee GA 2005 GRIP1 mediates the interaction between the amino- and carboxyl-termini of the androgen receptor. Biol Chem 386:69–74 [DOI] [PubMed] [Google Scholar]
- He B, Wilson EM 2002 The NH2-terminal and carboxyl-terminal interaction in the human androgen receptor. Mol Genet Metab 75:293–298 [DOI] [PubMed] [Google Scholar]
- Schaufele F, Carbonell X, Guerbadot M, Borngraeber S, Chapman MS, Ma AAK, Miner JN, Diamond MI 2005 The structural basis of androgen receptor activation: Intramolecular and intermolecular amino-carboxy interactions. Proc Natl Acad Sci USA 102:9802–9807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Royen ME, Cunha SM, Brink MC, Mattern KA, Nigg AL, Dubbink HJ, Verschure PJ, Trapman J, Houtsmuller AB 2007 Compartmentalization of androgen receptor protein-protein interactions in living cells. J Cell Biol 177:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloway M, Roach 3rd M 2005 Prostate cancer progression after therapy of primary curative intent. Cancer 104:2310–2322 [DOI] [PubMed] [Google Scholar]
- Scher HI, Sawyers CL 2005 Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 23:8253–8261 [DOI] [PubMed] [Google Scholar]
- Buchanan G, Greenberg NM, Scher HI, Harris JM, Marshall VR, Tilley WD 2001 Collocation of androgen receptor gene mutations in prostate cancer. Clin Cancer Res 7:1273–1281 [PubMed] [Google Scholar]
- Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F 2007 The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res 67:4514–4523 [DOI] [PubMed] [Google Scholar]
- Gottlieb B, Beitel LK, Wu JH, Trifiro M 2004 The androgen receptor gene mutations database (ARDB): 2004 update. Hum Mutat 24:102 [DOI] [PubMed] [Google Scholar]
- Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD 2004 Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer 11:459–476 [DOI] [PubMed] [Google Scholar]
- Hyytinen ER, Haapala K, Thompson J, Lappalainen I, Roiha M, Rantala I, Helin HJ, Jänne OA, Vihinen M, Palvimo JJ, Koivisto PA 2002 Pattern of somatic androgen receptor gene mutations in patients with hormone-refractory prostate cancer. Lab Invest 82:1591–1598 [DOI] [PubMed] [Google Scholar]
- Haapala K, Hyytinen ER, Roiha M, Laurila M, Rantala I, Helin HJ, Koivisto PA 2001 Androgen receptor alterations in prostate cancer relapsed during a combined androgen blockade by orchiectomy and bicalutamide. Lab Invest 81:1647–1651 [DOI] [PubMed] [Google Scholar]
- Buchanan G, Craft PS, Yang M, Cheong A, Prescott J, Jia L, Coetzee GA, Tilley WD 2004 PC-3 cells with enhanced androgen receptor signaling: a model for clonal selection in prostate cancer. Prostate 60:352–366 [DOI] [PubMed] [Google Scholar]
- Butler LM, Centenera MM, Neufing PJ, Buchanan G, Choong CS, Ricciardelli C, Saint K, Lee M, Ochnik A, Yang M, Brown MP, Tilley WD 2006 Suppression of androgen receptor signaling in prostate cancer cells by an inhibitory receptor variant. Mol Endocrinol 20:1009–1024 [DOI] [PubMed] [Google Scholar]
- Tilley WD, Buchanan G, Hickey TE, Bentel JM 1996 Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin Cancer Res 2:277–285 [PubMed] [Google Scholar]
- Magee JA, Chang LW, Stormo GD, Milbrandt J 2006 Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology 147:590–598 [DOI] [PubMed] [Google Scholar]
- Buchanan G, Yang M, Cheong A, Harris JM, Irvine RA, Lambert PF, Moore NL, Raynor M, Neufing PJ, Coetzee GA, Tilley WD 2004 Structural and functional consequences of glutamine tract variation in the androgen receptor. Hum Mol Genet 13:1677–1692 [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Stampfer MJ, Krithivas K, Brown M, Dahl D, Brufsky A, Talcott J, Hennekens CH, Kantoff PW 1997 The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci USA 94:3320–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GZ, Wang H, Wang Z 2003 Identification of a highly conserved domain in the androgen receptor that suppresses the DNA-binding domain-DNA interactions. J Biol Chem 278:14956–14960 [DOI] [PubMed] [Google Scholar]
- Irvine RA, Ma H, Yu MC, Ross RK, Stallcup MR, Coetzee GA 2000 Inhibition of p160-mediated coactivation with increasing androgen receptor polyglutamine length. Hum Mol Genet 9:267–274 [DOI] [PubMed] [Google Scholar]
- McEwan I 2004 Molecular mechanisms of androgen receptor-mediated gene regulation: structure-function analysis of the AF-1 domain. Endocr Relat Cancer 11:281–293 [DOI] [PubMed] [Google Scholar]
- Gregory CW, Fei X, Ponguta LA, He B, Bill HM, French FS, Wilson EM 2004 Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem 279:7119–7130 [DOI] [PubMed] [Google Scholar]
- Esteéanez-Perpiñá E, Moore JM, Mar E, Delgado-Rodrigues E, Nguyen P, Baxter JD, Buehrer BM, Webb P, Fletterick RJ, Guy RK 2005 The molecular mechanisms of coactivator utilization in ligand-dependent transactivation by the androgen receptor. J Biol Chem 280:8060–8068 [DOI] [PubMed] [Google Scholar]
- Duff J, McEwan IJ 2005 Mutation of histidine 874 in the androgen receptor ligand-binding domain leads to promiscuous ligand activation and altered p160 coactivator interactions. Mol Endocrinol 19:2943–2954 [DOI] [PubMed] [Google Scholar]
- Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, Miyamoto M 2003 Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res 63:149–153 [PubMed] [Google Scholar]
- Han G, Buchanan G, Ittmann M, Harris JM, Yu X, Demayo FJ, Tilley W, Greenberg NM 2005 Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proc Natl Acad Sci USA 102:1151–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke GN, Parker MG, Bevan CL 2007 Mechanisms of androgen receptor activation in advanced prostate cancer: differential co-activator recruitment and gene expression. Oncogene 27:2941–2950 [DOI] [PubMed] [Google Scholar]
- Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM 2007 Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int J Cancer 120:719–733 [DOI] [PubMed] [Google Scholar]
- Buchanan G, Ricciardelli C, Harris JM, Prescott J, Yu ZC, Jia L, Butler LM, Marshall VR, Scher HI, Gerald WL, Coetzee GA, Tilley WD 2007 Control of androgen receptor signaling in prostate cancer by the cochaperone small glutamine rich tetratricopeptide repeat containing protein α. Cancer Res 67:10087–10096 [DOI] [PubMed] [Google Scholar]
- Yang CK, Kim JH, Li H, Stallcup MR 2006 Differential use of functional domains by coiled-coil coactivator in its synergistic coactivator function with β-catenin or GRIP1. J Biol Chem 281:3389–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Hong H, Huang SM, Irvine RA, Webb P, Kushner PJ, Coetzee GA, Stallcup MR 1999 Multiple signal input and output domains of the 160-kilodalton nuclear receptor coactivator proteins. Mol Cell Biol 19:6164–6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CL, Chen YL, Ting HJ, Lin WJ, Yang Z, Zhang Y, Wang L, Wu CT, Chang HC, Yeh S, Pimplikar SW, Chang C 2005 Androgen receptor (AR) NH2- and COOH-terminal interactions result in the differential influences on the AR-mediated transactivation and cell growth. Mol Endocrinol 19:350–361 [DOI] [PubMed] [Google Scholar]
- Thompson J, Saatcioglu F, Jänne OA, Palvimo JJ 2001 Disrupted amino- and carboxyl-terminal interactions of the androgen receptor are linked to androgen insensitivity. Mol Endocrinol 15:923–935 [DOI] [PubMed] [Google Scholar]
- Li J, Fu J, Toumazou C, Yoon HG, Wong J 2006 A role of the amino-terminal (N) and carboxyl-terminal (C) interaction in binding of androgen receptor to chromatin. Mol Endocrinol 20:776–785 [DOI] [PubMed] [Google Scholar]
- Minamiguchi K, Kawada M, Ohba S, Takamoto K, Ishizuka M 2004 Ectopic expression of the amino-terminal peptide of androgen receptor leads to androgen receptor dysfunction and inhibition of androgen receptor-mediated prostate cancer growth. Mol Cell Endocrinol 214:175–187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.