Abstract
The proinflammatory consequences of obesity are thought to be due, in part, to macrophage infiltration into adipose tissue. There are, however, potential antiinflammatory consequences of obesity that include obesity-associated up-regulation of IL-1 receptor antagonist (IL-1RA). Here we show that obesity-associated up-regulation of IL-1RA speeds recovery from hypoxia. We found that high-fat diet-fed (HFD) mice recovered from acute hypoxia 5 times faster than normal-diet-fed (ND) mice. HFD mice had a 10-fold increase in serum IL-1RA when compared with ND mice. White adipose tissue (WAT) was a significant source of IL-RA, generating 330 ± 77 pg/mg protein in HFD mice as compared with 15 ± 5 pg/mg protein in ND mice. Peritoneal macrophages isolated from HFD mice showed little difference in IL-1RA production when compared with ND mice, but WAT macrophages from HFD mice generated 11-fold more IL-1RA than those from ND mice. When ND mice were given an ip transfer of the stromal vascular fraction portion of WAT from HFD mice, serum IL-1RA increased 836% and recovery from acute hypoxia was faster than in mice that did not receive a stromal vascular fraction transfer. To determine whether IL-1RA was important to this accelerated recovery, ND mice were administered exogenous IL-1RA prior to hypoxia, and their recovery matched that of HFD mice. Inversely, when IL-1RA was immunoabsorbed in HFD mice with IL-1RA antiserum, recovery from acute hypoxia was attenuated. Taken together these data demonstrate that HFD-induced obesity speeds recovery from hypoxia due to obesity-associated up-regulation of IL-1RA.
Recovery from acute hypoxia is accelerated in obese mice due to obesity-associated up-regulation of the anti-inflammatory molecule IL-1 receptor antagonist.
Obesity is a state of increased lipid accumulation in white adipose tissue (WAT) that is linked to not only weight gain but also chronic, low-grade inflammation (1). Long seen as just a passive storage tissue, fat is now considered an active endocrine organ that regulates satiety, metabolism, reproduction, and immune function (2). In addition to its elaboration of lipid molecules, fat is a pleiotropic source of proteins, hormones, chemokines, and cytokines (3,4). WAT, similar to many solid organs, is comprised of an eponymous cell, i.e. the adipocyte, supported by a fibrovascular stroma (4) containing capillaries (5) and tissue based macrophages (6). These resident adipose tissue macrophages (AtMφs) of the stromal vascular fraction (SVF) appear integral to obesity and its associated complications (7). Conversely, weight loss leads to a reduction in AtMφ numbers and obesity-associated inflammation (7).
With tissue-based macrophages, the local microenvironment contributes substantially to phenotypic heterogeneity, and this heterogeneity extends beyond intertissue differences to intratissue disparities (8). In general, macrophage activation is functionally defined as classical or alternative (9). However, between these defined activation states there exists a cornucopia of variations (8,10). The phenotype of AtMφs is not clearly defined, but there appears to be an activation state difference between AtMφs resident to WAT and those recruited to WAT during/after the onset of obesity (11). In addition, it has been suggested that the phenotype of recruited AtMφs in obesity skews to alternative (12) due to: 1) decreased expression/elaboration of IL-8, cyclooxygenase-2 (13), IL-6, inducible nitric oxide synthase, and C-C chemokine receptor 2 (11); 2) increased endocytic activity; and 3) augmented expression/elaboration of IL-10 and IL-1 receptor antagonist (IL-1RA) (14).
Although conventional wisdom associates obesity with a proinflammatory state (15), up-regulation of antiinflammatory mediators, such as IL-10 and IL-1RA, appear linked to adiposity as well. In obese humans, serum IL-1RA is increased (16) as it is in high-fat diet-fed (HFD) rodent models, Obesity also increases WAT-based IL-1RA (17,18). Whereas IL-1RA antagonizes the effect of IL-1 (19), its role in obesity, like that of IL-1, is not clear (20). In humans and mice, the IL-1 receptor appears to have an antiobesity effect with IL-1 receptor-1 knockout mice developing mature-onset obesity (21). Interestingly, IL-1 knockout mice have a wild-type phenotype and do not exhibit signs of obesity (weight gain, increased epididymal white adipose tissue, and leptin) unless it is coupled to a knockout of IL-6 (22). In contrast, IL-1RA knockout mice are resistant to the development of obesity and have a lean phenotype (23,24). Taken together, these data indicate that in mice, IL-1RA through the IL-1 receptor 1 promotes fatness.
In humans, obesity is associated with improved survival for certain diseases and conditions including heart failure (25,26), coronary artery disease (27,28), chronic kidney disease (29), and rheumatoid arthritis (30,31). In terms of mechanism, the enhanced survival in rheumatoid arthritis is the easiest to understand because anti-IL-1 therapy in the form of a derivitized IL-1RA (anakinra) is used clinically in the treatment of rheumatoid arthritis (32). Given that obesity is associated with increased serum levels of endogenous IL-1RA, it would seem reasonable to expect that obese patients would be protected from or have blunted complications to rheumatoid arthritis. We have recently shown that acute hypoxia up-regulates IL-1 in the brain and that this brain-based increase in IL-1β is responsible for behavioral deficits found during recovery from acute hypoxia (33). We also found that blocking IL-1 signaling through MyD88 knockout or inhibiting IL-1β production in the brain via intracerebroventricular administration of a caspase-1 inhibitor hastens mouse recovery from acute hypoxia (33). Here we tested whether obesity-associated IL-1RA up-regulation is beneficial to acute hypoxia recovery in a mouse model of obesity.
Materials and Methods
Materials
All reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except as follows and where indicated: fetal calf serum (0.05 ng/ml, 0.48 U/ml endotoxin; Atlanta Biologicals, Atlanta, GA); Bio-Rad protein assay (500-0006) and Bio-Plex (X60000ZM08; Bio-Rad Laboratories, Hercules, CA); IL-1RA ELISA (MRA00) and leptin ELISA (MOB00; R&D Systems, Minneapolis, MN); collagenase type I (no. 4196; Worthington Biochemical Corp., Freehold, NJ); 60% fat diet (D12492; Research Diets, Inc., New Brunswick, NJ); sheep antirat IL-1RA serum (National Institute for Biological Standards and Control, Hertfordshire, UK); normal preimmune sheep serum (GTX73209, GeneTex, Inc. (San Antonio, TX); and anakinra (Amgen, Thousand Oaks, CA).
Animals
Animal use was conducted in accordance with the Guide for the Care and Use of Laboratory Animals as we have described (33). C56BL/6J male mice were bred in-house. Mice were housed in standard shoe box cages and allowed NIH 5K52 LabDiet pelleted food (Purina Mills, St. Louis, MO) and water ad libitum. Housing temperature (72 F) and humidity (45–55%) were controlled as was a 12-h reversed dark, 12-h light cycle (2000-0800 h). Obesity was induced by feeding a high-fat diet (60% fat) immediately after weaning for 12 wk. Normal-diet mice were fed a diet containing 10% fat for the same period of time.
Hypoxia
Hypoxia was induced as we have described (33,34). In brief, mice were placed in a 12- × 6- × 4-in. plastic container connected to a S. J. Smith & Co. (Davenport, IA)-certified gas cylinder containing 8% oxygen and 92% nitrogen for 2 h. Normoxic controls were tested in a similar 12- × 6- ×-4 in. plastic container with atmospheric air was blown into it at the same rate as mice receiving the 8% oxygen/92% nitrogen mix. Hypoxia was terminated by returning mice to their home cages in atmospheric air conditions.
Recovery from hypoxia
Hypoxia-induced social withdrawal was used to measure recovery from hypoxia, as we have described (33,34). In brief, a 3- to 4-wk-old novel, conspecific juvenile (challenge) mouse was enclosed in a 3- × 3-in. wire mesh cage that was placed in the home cage of the adult (test) mouse for 5 min immediately before hypoxia (−2 h) and for 5 min at 0, 2, 6, and 10 h after return of the test mouse to normal oxygen. Duration of test mouse-initiated exploratory behavior of the challenge mouse was determined from the video records. To control for mouse-to-mouse variability in baseline social exploration and allow comparison of relative changes in social exploration levels, the prehypoxia exposure (−2 h) measurement was used as an internal control for each mouse. Results are expressed as percentages of the baseline measurement.
Peritoneal lavage
As we previously described (35), mice were killed by CO2 asphyxiation and the peritoneum lavaged using 1 ml of ice-cold PBS (pH 7.4). Lavage fluid was clarified via centrifugation at 16,000 × g for 10 min. ELISAs were performed on clarified lavage fluid in 96-well plates at room temperature.
Peritoneal macrophage (PerMφ) isolation
As we previously described (35), mice were killed by CO2 asphyxiation. Peritoneal cells were collected by peritoneal lavage using 10 ml of ice-cold growth medium [RPMI 1640 supplemented with 10% fetal calf serum, 2 g/liter sodium bicarbonate, 110 mg/liter sodium pyruvate, 62.1 mg/liter penicillin, 100 mg/liter streptomycin, and 10 mm HEPES (pH 7.4)]. Lavage cells were pelleted and resuspended in 10 ml of hypertonic red blood cell lysis buffer [142 mm NaCl, 1 mm KHCO3, and 118 mm NaEDTA (pH 7.4)] at room temperature for 5 min and then mixed 1:1 with growth medium and repelleted and resuspended at 37 C. Cells were plated on plastic at 0.5 × 06 cells/ml, and after 1 h plates were washed twice to remove nonadherent cells, resulting in greater than 80% pure macrophages as confirmed by CD11b staining and morphology. Cells were incubated in fresh growth media cultured at 37 C in a 5% CO2 environment. For in vitro hypoxia experiments, macrophages were cultured in a modular incubator chamber (Billups-Rothenberg, Inc., Del Mar, CA) in an 8% oxygen/92% nitrogen environment at 37 C.
SVF isolation
Perigonadal WAT was collected and minced in 20 ml of Hanks’ balanced salt solution containing collagenase type I (1 mg/ml) and incubated with shaking for 1.5 h at 37 C. Suspensions were centrifuged at 500 × g for 5 min to separate buoyant layer from pellet. The pellet was resuspended in 10 ml of hypertonic RBC lysis buffer for 5 min at room temperature and then repelleted and resuspended in 500 μl of sterile saline for ip injection (pooled SVF from three HFD mice) or mixed 1:1 with growth media and plated on plastic for macrophage isolation. For macrophage isolation, cells were allowed to adhere for 1 h and then washed twice to remove nonadherent cells. Adherent cells were cultured in growth media at 37 C.
Blood glucose, total cholesterol, and triglycerides
Blood was collected from either the tail vein (blood glucose) or inferior vena cava (cholesterol and triglycerides). Briefly, mice were fasted for 8 h and blood glucose levels measured using a One Touch Ultra glucometer (Johnson & Johnson, New Brunswick, NJ) per the manufacturer’s instructions. Cholesterol and triglycerides were measured after a 14-h fast on a UniCel DxC 800 Synchron clinical system (Beckman Coulter, Fullerton, CA).
Cytokine measurements
For blood cytokine levels, inferior vena cava serum was analyzed by BioPlex for IL-1β, IL-4, IL-6, IL-10, IL-12 (p40), IL-12 (p70), IL-13, interferon-γ, and TNF-α and by ELISA for IL-1RA and leptin, all per the manufacturer’s instructions. For tissue cytokine levels, 75 mg of the indicated tissue were collected into 500 μl of ice-cold homogenization buffer [1% Triton X-100, 100 mm NaCl, 50 mm NaF, 1 mm dithiothreitol, 25 mm benzamidine, 1 mm phenylmethylsulfonyl fluoride, 1:1000 Protease Inhibitor Cocktail Set III, no. 539134 (Calbiochem, La Jolla, CA), 2 mm sodium orthovanadate, 250 nm okadaic acid, and 50 mm Tris (pH 7.4)]. Tissues were ground with a tissue tearor (BioSpec Products, Bartlesville, OK) and the homogenates centrifuged at 10,000 × g (4 C) for 10 min. Cytokine levels were measured in the clarified lysates and normalized to total tissue protein as measured by Bio-Rad protein assay.
RNA isolation and reverse transcription
Total RNA was extracted into TRIzol (Invitrogen, Carlsbad, CA). Reverse transcription was preformed with high-capacity cDNA reverse transcription kits (Applied Biosystems, Foster City, CA) per the manufacturer’s instructions. To minimize interassay variation, all RNA samples were reverse transcribed simultaneously.
Real-time PCR
Real-time PCR was performed as we have described (33,35). In brief, TaqMan gene expression primer for IL-1α (Mm00439620_m1), IL-1β (Mm0043228_m1), IL-1RA (Mm00446185_m1), and IL-1R2 (Mm00439622_m1) were used in real-time RT-PCR performed on a 7900 HT Fast real-time PCR system (Applied Biosystems) using TaqMan universal PCR master mix. To normalize gene expression, a parallel amplification of endogenous glyceraldehyde-3-phosphate dehydrogenase (Mm999999615_g1) was performed with TaqMan gene expression primer. Reactions with no reverse transcriptase and no template were included as negative controls. Relative quantitative evaluation of target gene levels was performed by comparing ΔCts, where Ct is the threshold concentration.
Statistical analysis
Data are presented as mean ± sem. The experimental design for hypoxia recovery experiments was a completely randomized design, with a 2 × 2 factorial arrangement of treatments (two levels of pretreatment and two levels of treatment). All data were analyzed using SAS Inst PROC MIXED procedures (SAS, Cary, NC). The statistical model for hypoxia recovery included the effects of diet/anakinra/SVF transfer/IL-1RA antiserum and hypoxia and time, with time as a repeated measure, and the interactions of diet/anakinra/SVF transfer/IL-1RA antiserum × hypoxia × time. Post hoc comparisons of individual group means were performed with the Tukey’s test. Where indicated, experimental data were analyzed by ANOVA using SAS. Statistical significance was denoted at P < 0.05.
Results
Obesity speeds recovery from acute hypoxia
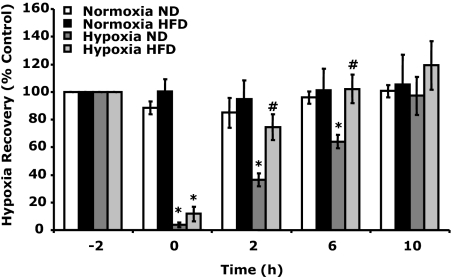
Mice fed a HFD for 12 wk after weaning had a 37, 57, and 46% increase in body weight, fasting blood glucose, and total cholesterol, respectively (supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) when compared with mice fed a normal diet (ND). Figure 1 demonstrates that HFD mice recovered faster from acute hypoxia than ND mice. HFD mice had significantly improved recovery from hypoxia at 2 h (75 ± 9 vs. 36 ± 5%) and 6 h (101 ± 9 vs. 64 ± 9%) after hypoxia compared with ND mice. Three-way ANOVA (phenotype × hypoxia × time) revealed a significant diet × hypoxia interaction [F (1,16) = 2.98, P < 0.05] and a hypoxia × time interaction [F (3,48) = 33.73, P < 0.0001] but no diet × hypoxia × time interaction.
Figure 1.
HFD-induced obesity speeds recovery from acute hypoxia. Baseline behavior was measured in ND and HFD mice immediately before hypoxia exposure (−2 h). Mice were then placed in either a normoxic or hypoxic environment for 2 h. Behavioral recovery from hypoxia was measured 0, 2, 6, and 10 h after return of animals to normoxia. Results are expressed as percentages of the baseline measurement, means ± sem: n = 6. *, P < 0.05, hypoxia vs. normoxia; #, P < 0.05, ND vs. HFD.
IL-1 and its counterregulatory proteins are altered by obesity
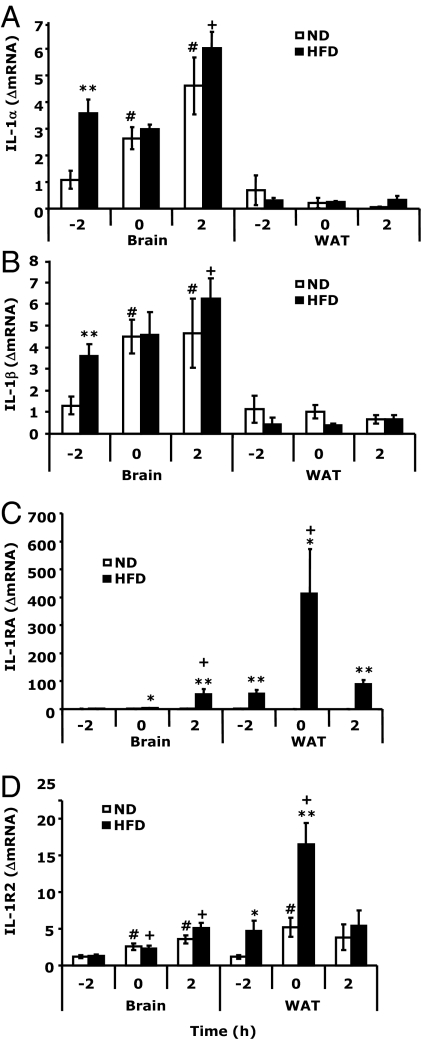
In the brain (Fig. 2, A and B), HFD mice had increased mRNA expression of IL-1α and -β basally when compared with ND mice [3.57 ± 0.53 vs. 1.09 ± 0.34 ΔmRNA, F (1,5) = 17.32, P < 0.005 and 3.59 ± 0.56 vs. 1.30 ± 0.42 ΔmRNA, F (1,11) = 11.26, P < 0.005, respectively]. Hypoxia increased IL-1α and -β mRNA expression in the brain but not differentially in HFD and ND mice. WAT mRNA expression of IL-1α and IL-1β was not impacted by obesity or hypoxia. Figure 2C demonstrates that WAT from HFD mice had increased basal expression of IL-1RA mRNA [55.38 ± 12.72 vs. 1.21 ± 0.32 ΔmRNA HFD vs. ND, F (1,10) = 21.73, P < 0.0005] and that IL-1RA mRNA was up-regulated by hypoxia significantly more in HFD than ND mice [412.30 ± 159.53 vs. 0.48 ± 0.16 HFD vs. ND at 0 h, F (1,14) = 7.33, P < 0.01; and 89.05 ± 15.74 vs. 0.37 ± 0.13 HFD vs. ND at 2 h, F (1,8) = 67.73, P < 0.0005]. In the brain, IL-1RA mRNA increased 42-fold 2 h after hypoxia in HFD mice but only 2-fold in ND mice. IL-1R2 mRNA expression (Fig. 2D) showed a similar pattern as IL-1RA in WAT [4.73 ± 1.38 vs. 1.17 ± 0.27 HFD vs. ND, basally, F (1,10) = 6.46, P < 0.05, and 16.48 ± 2.94 vs. 5.21 ± 1.34 HFD vs. ND, at 0 h, F (1,14) = 13.91, P < 0.005]. In the brain, IL-1R2 mRNA increased after hypoxia but showed no diet differential. Finally, supplemental Table 2 shows that IL-1RA was increased 11-fold in the serum of HFD mice basally when compared with ND animals. This was coupled to a 1.4-fold increase in leptin.
Figure 2.
IL-1 and its counterregulatory proteins are altered by HFD-induced obesity. IL-1α (A), IL-1β (B), IL-1RA (C), and IL-1R2 (D) mRNA were measured in brain and perigonadal WAT by real-time RT-PCR. Results are expressed as relative change in target mRNA to glyceraldehyde-3-phosphate dehydrogenase (ΔmRNA) and as means ± sem: n = 6–8. *, P < 0.05, **, P < 0.001 ND vs. HFD; #, P < 0.05 −2 h vs. 0 h, 2 h ND; +, P < 0.05 −2 h vs. 0 h, 2 h HFD.
WAT in HFD mice is a key source of IL-1RA
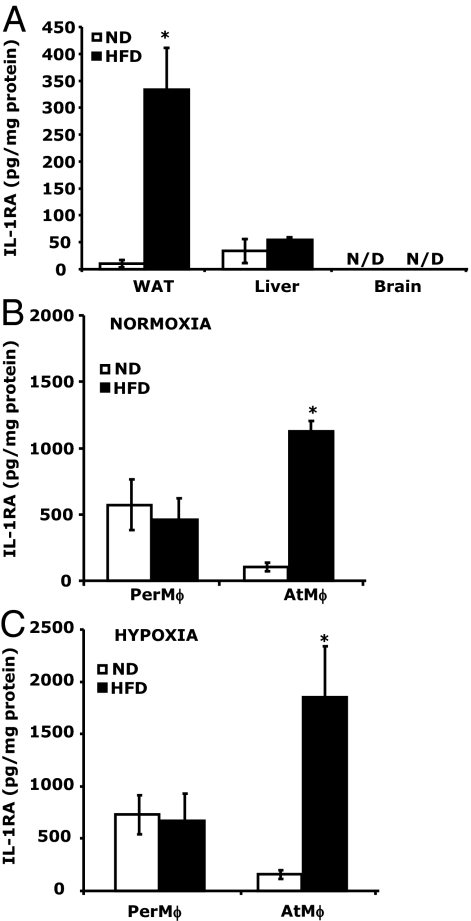
Figure 3A demonstrates that IL-1RA protein expression (per milligram of tissue) was 22-fold greater [330.61 ± 77.01 vs. 14.82 ± 4.93 pg/mg protein, F (1,4) = 25.81, P < 0.005] in perigonadal WAT from HFD mice than ND mice. Liver from HFD and ND mice showed similar amounts of IL-1RA (54.33 ± 4.74 vs. 33.79 ± 22.03 pg/mg protein, HFD vs. ND). In brain, IL-1RA protein was not detectable. Supplemental Table 3 demonstrates the expression of IL-1RA, IL-1R2, IL-1α, and IL-1β mRNA expression in perigonadal, sc, perirenal, and mesenteric fat from ND and HFD mice. Because macrophages are a critical source of IL-1RA, we isolated AtMφs to examine their IL-1RA production. Figure 3C demonstrates that AtMφs from HFD mice produced 11-fold more IL-1RA than AtMφs from ND mice (103 ± 42 vs. 1129 ± 107 pg/mg protein per 3 h). PerMφ production of IL-1RA from HFD mice did not show a difference compared with ND mice (456.49 ± 168.01 vs. 572.38 ± 192.86 pg/mg protein per 3 h, HFD vs. ND). Finally, a hypoxic environment did not appear to significantly impact AtMφ or PerMφ IL-1RA production in either HFD or ND mice.
Figure 3.
WAT in HFD mice is a key source of IL-1RA. A, IL-1RA protein was measured in WAT, liver, and brain tissue homogenates by ELISA. Results are expressed as means ± sem: n = 4. *, P < 0.05; **, P < 0.005, ND vs. HFD. B, IL-1RA protein produced by PerMφs and AtMφs cultured in vitro for 2 h in normoxic conditions was measured by ELISA. Results are expressed as means ± sem: n = 3. *, P < 0.05, ND vs. HFD. C, IL-1RA protein produced by PerMφs and AtMφs cultured in vitro for 2 h in hypoxic conditions was measured by ELISA. Results are expressed as means ± sem: n = 3. *, P < 0.05, ND vs. HFD.
Accelerated hypoxia recovery can be transferred from HFD mice to ND mice
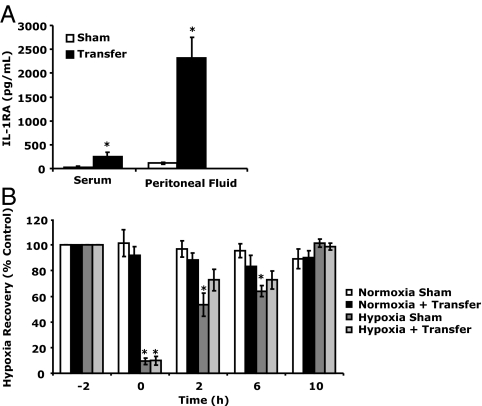
SVF isolated from HFD mice and then transferred to the peritoneum of ND mice increased peritoneal and blood levels of IL-1RA in ND mice 20-fold (115 ± 19 vs. 2322 ± 432 pg/ml per 3 h) and 9-fold (29.58 ± 24.31 vs. 251.43 ± 86.41 pg/ml per 3 h), respectively (Fig. 4A). Figure 4B demonstrates that ND mice administered SVF from HFD mouse recovered faster from acute hypoxia than sham-transferred ND mice. Immediately after hypoxia, SVF and sham-transferred mice showed similar recovery (9.84 ± 3.49 vs. 9.39 ± 2.47%, SVF vs. sham). Two hours after hypoxia, SVF-transferred mice had recovered from hypoxia (72.71 ± 8.25 vs. 88.20 ± 5.49%, hypoxia vs. normoxia), whereas sham-transferred animals had not (53.44 ± 9.27 vs. 96.91 ± 6.43%, hypoxia vs. normoxia). This delay in recovery was still evident in sham-treated animals 6 h after hypoxia (64.01 ± 4.32 vs. 95.51 ± 5.41%, hypoxia vs. normoxia). Three-way ANOVA (phenotype × hypoxia × time) revealed a significant hypoxia × time interaction [F (2,36) = 45.29, P < 0.0001] but no treatment × hypoxia × time interaction.
Figure 4.
Accelerated hypoxia recovery can be transferred from HFD mice to ND mice. A, ND mice were ip injected with (transfer) or without (sham) HFD mouse SVF. Serum and peritoneal fluid IL-1RA protein was measured by ELISA 3 h after transfer. Results are expressed as means ± sem: n = 3. *, P < 0.05 sham vs. transfer. B, ND mice were ip injected with (transfer) or without (sham) SVF mouse stromal vascular fraction 3 h before hypoxia exposure. Baseline behavior was measured in sham and transferred mice immediately before hypoxia exposure (−2 h). Mice were then placed in either a normoxic or hypoxic environment for 2 h. Behavioral recovery from hypoxia was measured 0, 2, 6, and 10 h after return of animals to normoxia. Results are expressed as means ± sem: n = 3–5. *, P < 0.05 normoxia vs. hypoxia.
Exogenous administration of IL-1RA improves recovery from hypoxia
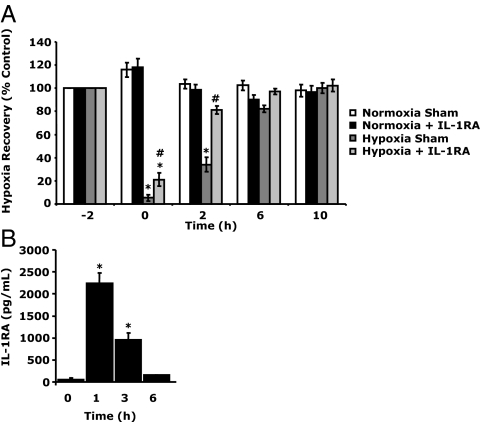
To ensure that IL-1RA was important to hypoxia recovery, ND mice were administered anakinra (a derivatized recombinant IL-1RA) (32) 1 h before hypoxia. Figure 5A shows that IL-1RA-treated ND mice recovered faster from acute hypoxia than sham-treated ND mice. Immediately after hypoxia, IL-1RA-treated ND mice were less recovered (21.17 ± 5.77 vs. 5.24 ± 2.65%, IL-1RA vs. sham). At 2 h after hypoxia, IL-1RA-treated ND mice had recovered from hypoxia (81.29 ± 3.46 vs. 98.72 ± 4.32%, hypoxia vs. normoxia), whereas sham-treated mice were had not (34.07 ± 6.39 vs. 103.54 ± 3.91%, hypoxia vs. normoxia). At 6 h after hypoxia, both IL-1RA and sham-treated ND mice had recovered. Three-way ANOVA (phenotype × hypoxia × time) revealed a significant IL-1RA × hypoxia × time [F (2,32) = 4.21, P < 0.05], IL-1RA × hypoxia interaction [F (1,16) = 50.35, P < 0.0001], and a hypoxia × time interaction [F (2,32) = 116.51, P < 0.0001]. Figure 5B demonstrates the half-life of serum IL-1RA in ND mice administered IL-1RA. Before IL-1RA administration, ND mice had serum IL-1RA levels of 50.28 ± 38.11 pg/ml. At 1 and 3 h after IL-1RA, ND mouse IL-1RA was increased to 2239.67 ± 238.94and 953.77 ± 154.08 pg/ml, respectively. At 6 h after IL-1RA, serum IL-1RA had returned to normal (190.57 ± 55.67 pg/ml).
Figure 5.
Exogenous administration of IL-1RA improves recovery from hypoxia. A, ND mice were injected sc with (IL-1RA) or without (sham) anakinra (1.4 mg/kg) 1 h before hypoxia exposure. Baseline behavior was measured in sham and anakinra (IL-1RA) administered to mice immediately before hypoxia exposure (−2 h). Mice were then placed in either a normoxic or hypoxic environment for 2 h. Behavioral recovery from hypoxia was measured 0, 2, 6, and 10 h after return of animals to normoxia. Results are expressed as means ± sem: n = 6. *, P < 0.05, hypoxia vs. normoxia; #, P < 0.05 IL-1RA vs. sham. B, ND mice were injected with IL-1RA, as in A, and serum IL-1RA protein was measured by ELISA, at the times indicated. Results are expressed as means ± sem: n = 3–4. *, P < 0.005 vs. 0 h.
Accelerated hypoxia recovery in HFD mice is reliant on IL-1RA
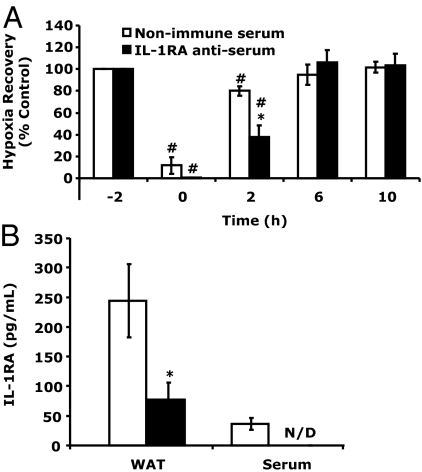
To determine the importance of IL-1RA to hypoxia recovery in HFD mice, HFD mice were administered IL-1RA antiserum to immune absorb IL-1RA. Figure 6A shows that HFD mice treated with IL-1RA antiserum 2 h before hypoxia exposure were delayed in their recovery compared with HFD mice treated with normal sheep serum. Immediately after hypoxia (0 h), IL-1RA antiserum and normal sheep serum-treated HFD mice were similarly impacted (0.34 ± 0.41 vs. 11.67 ± 7.82%, IL-1RA antiserum vs. normal sheep serum). At 2 h after hypoxia, HFD mice treated with IL-1RA antiserum were markedly delayed in their recovery compared with normal sheep serum-treated HFD mice (37.92 ± 10.54 vs. 80.16 ± 4.35%, IL-1RA antiserum vs. normal sheep serum). At 6 h after hypoxia, both IL-1RA antiserum and normal sheep serum-treated HFD mice had recovered. Two-way ANOVA (treatment × time) revealed a significant treatment × time interaction [F (3,12) = 16.91, P < 0.0001]. Figure 6B demonstrates that IL-1RA anti-serum was able to significantly reduce both serum and perigonadal WAT IL-1RA in HFD mice [serum 36.59 ± 9.84 pg/ml vs. nondetectable, normal sheep serum vs. IL-1RA antiserum and WAT 244.48 ± 61.80 vs. 77.65 ± 27.98 pg/mg protein, normal sheep serum vs. IL-1RA antiserum, F (1,4) = 9.07, P < 0.05].
Figure 6.
Accelerated hypoxia recovery in HFD mice is reliant on IL-1RA. A, HFD mice were injected ip with IL-1RA antiserum or without nonimmune serum 1 h before hypoxia exposure. Baseline behavior was measured in nonimmune serum and IL-1RA antiserum administered to mice immediately before hypoxia exposure (−2 h). Mice were then placed in either a normoxic or hypoxic environment for 2 h. Behavioral recovery from hypoxia was measured 0, 2, 6, and 10 h after return of animals to normoxia. Results are expressed as means ± sem: n = 3. *, P < 0.05 nonimmune serum vs. IL-1RA antiserum: #, P < 0.05 vs. −2 h. B, IL-1RA protein was measured in serum and WAT from the mice in A immediately after the 10-h time point. Results are expressed as means ± sem: n = 3. *, P < 0.05.
Discussion
An obesity paradox was first described more than 7 yr ago by Horwich et al. (25), who noted that higher body mass index was not a risk factor for increased mortality in heart failure but was in fact associated with improved survival. Given today’s antifat cultural environment (36), this assertion by Horwich et al. is especially controversial, but recent work has supported an obesity paradox in heart failure (25,26). Whereas Habbu et al. (37) questioned the validity of an obesity survival advantage in heart failure, their counterargument is weakened by the poor prognosis seen in those who suffer from cardiac cachexia (38). Therefore, it is not entirely surprising that increased fatness, and hence energy stores, might mitigate demise in heart failure. Very few studies have looked at this paradox in severely obese populations, but even Habbu et al. (37) support the notion of a U-shaped outcomes curve in which overweight and mildly obese populations have the best outcomes.
In general, improved survival tied to body mass index is often explained by vague notions of heightened nutritional status, in which increased calorie consumption is linked to a greater intake of protein and other essential nutrients. An antagonistic finding to the obesity paradox is that, in mice, calorie restriction is associated with increased life span (39,40). Whether calorie restriction is beneficial to humans is unclear (41,42), especially in that weight loss occurring at 65 yr of age or older precipitates decreased health-related quality of life (43) and survival (44,45). Obesity ties in to this quandary because it is regarded as a proinflammatory state (15), and many of the leading causes of death in those 65 yr of age and older are associated with inflammation including heart disease, diabetes, Alzheimer’s disease, and nephritis (46). Fat from overweight and obese individuals tends to be infiltrated by macrophages (6) and serum proinflammatory cytokines like TNF-α, IL-1, and IL-6 can be elevated in obesity (3,47). We demonstrate that HFD mice were 38.5% heavier then our ND mice and had fasting blood glucoses and total cholesterols 57 and 46% than our ND mice, respectively. Interestingly, we did not see increases in serum TNF-α, IL-1, or IL-6 (in fact, IL-6 was down 51.5%). This is not suppressing because elevations in some or all of these cytokines in serum are variable (48,49). In addition, it appears that a proinflammatory serum cytokine profile is better correlated with degree of insulin resistance in diabetes than with the weight (47). We did, however, find brain-based and fat depot-based basal increases in both IL-1α and IL-1β mRNA in HFD mice. The brain findings are very interesting because obesity is associated with a variety of conditions that may be caused by brain-based proinflammation or brain-based proinflammatory cytokines, including depression (50) and Alzheimer’s disease (51).
As expected, HFD mice demonstrated an increase in serum leptin (52,53). Leptin, importantly, drives expression of IL-1RA in both monocytes (54) and macrophages (34). This ability of leptin to enhance IL-1RA production underscores the antiinflammatory potential of a HFD and the resulting fatness. Leptin-dependent up-regulation of IL-1RA appears to be a direct result of leptin in that leptin through its receptor long form uses the ERK/MAPK pathway and the nuclear factor-κB/PU.1 binding site of the IL-1RA promoter (55). In addition, Janus kinase-2 recruitment to Tyr985 may be pivotal to leptin-induced IL-1RA because Tyr985 plays an important role in leptin-induced full activation of ERK (56). In our study, feeding a HFD increased serum leptin by 38% but increased serum IL-1RA by nearly 1100%. IL-1RA was also increased in perigondal and perirenal WAT of HFD mice, likely due to a direct effect of WAT leptin on adipose tissue-based macrophages, as we recently reported (34). Interestingly, IL-1R2 mRNA was increased in perigonadal and perirenal WAT of HFD mice. Like IL-1RA, the IL-1 decoy receptor (IL-1R2) counteracts IL-1 signaling (57); however, it is not yet clear whether leptin directly drives expression of IL-1R2.
Acute hypoxia is potent activator of the IL-1 arm of the neuroimmune system (58), and loss of IL-1 production or signaling speeds recovery from acute hypoxia (33). Importantly, interruption of IL-1 signaling also protects the brain from ischemic necrosis induced by carotid artery ligation in mice (59). Also, disruption of IL-1 signaling via administration of IL-1RA to stroke victims may have a beneficial impact on stroke recovery (60). We found that HFD mice recovered faster from acute hypoxia than ND mice. This finding was somewhat surprising for two reasons. First, HFD mice had a basal up-regulation of IL-1α and IL-1β mRNA in the brain without a corresponding increase in basal IL-1R2 or IL-1RA. Second, we have previously shown that obese db/db mice, which have an increased basal up-regulation of IL-1β in the brain, have a marked recovery prolongation when exposed to acute hypoxia (33,34). A critical difference between db/db mice and HFD mice is functional leptin signaling and the failure of db/db mice to up-regulate either IL-1R2 or IL-1RA in response to hypoxia (33). Significantly, when ob/ob mice are administered leptin, systemic IL-1RA in response to hypoxia is restored, as is a normal recovery from acute hypoxia (34).
Finally, how important is IL-1RA to acute hypoxia recovery in HFD mice? We found that peripheral administration of anakinra to ND mice speeds ND mouse recovery from acute hypoxia. Interestingly, transfer of the WAT SVF from HFD mice to ND mice also speeds hypoxia recovery. The impact of the SVF transfer on hypoxia recovery was not as great as that seen with anakinra because of an inability to achieve a serum level of IL-1RA that matched that of anakinra administration. This limitation in achievable IL-1RA titer was due to the number of mice required. To generate a serum IL-1RA level of 2000 pg/ml (which was seen with anakinra), the SVF of about 24 HFD mice would have been needed because we found that the SVF of three HFD mice generated a serum IL-1RA level of 250 pg/ml. Therefore, to ensure that IL-1RA was important to accelerated hypoxia recovery in HFD mice, IL-1RA was immunoabsorbed in vivo with anti-IL-1RA antiserum. When HFD mice were administered anti-IL-1RA, they were 50% less recovered from acute hypoxia at 2 h after hypoxia than HFD mice administered nonimmune serum. In addition, IL-1RA antiserum reduced HFD mouse serum IL-1RA to undetectable. Taken together, our results indicate that, in acute hypoxia, HFD-associated up-regulation of IL-1RA is critical to accelerated recovery. Furthermore, because hypoxia is a key contributor to cardiac dysfunction and death in heart failure (61), it is likely that a significant component of the obesity paradox of Horwich et al. (25) is tied to obesity-dependent up-regulation of IL-1RA and the ability of IL-1RA to counterregulate IL-1 driven peripheral and neuroinflammation.
Supplementary Material
Footnotes
This work was supported by grants from the American Heart Association (Predoctoral Fellowship to C.L.S), National Institutes of Health (DK64862 and NS58525 to G.G.F.) and University of Illinois Agricultural Experiment Station (to G.G.F.). This work was also supported in part by a grant from the United States Department of Homeland Security, Assistance to Firefighters Grants Office, Research and Development Grant EMW-2006-FP-02459.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 12, 2009
Abbreviations: AtMφ, Adipose tissue macrophage; HFD, high-fat diet-fed; IL-1RA, IL-1 receptor antagonist; ND, normal-diet-fed; PerMφ, peritoneal macrophage; SVF, stromal vascular fraction; WAT, white adipose tissue.
References
- Lago F, Dieguez C, Gómez-Reino J, Gualillo O 2007 Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol 3:716–724 [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS 2004 Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89:2548–2556 [DOI] [PubMed] [Google Scholar]
- Hauner H 2005 Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc 64:163–169 [DOI] [PubMed] [Google Scholar]
- Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW 2004 Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 145:2273–2282 [DOI] [PubMed] [Google Scholar]
- Meliga E, Strem BM, Duckers HJ, Serruys PW 2007 Adipose-derived cells. Cell Transplant 16:963–970 [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW 2003 Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K 2005 Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54:2277–2286 [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR 2005 Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964 [DOI] [PubMed] [Google Scholar]
- Gordon S 2003 Alternative activation of macrophages. Nat Rev Immunol 3:23–35 [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M 2008 Macrophage activation and polarization. Front Biosci 13:453–461 [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR 2007 Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56:16–23 [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A 2008 Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab 4:619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C, Galitzky J, Lafontan M, Karpe F, Frayn KN, Bouloumié A 2008 Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 117:806–815 [DOI] [PubMed] [Google Scholar]
- Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Györi G, Zlabinger GJ, Stulnig TM 2007 Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 31:1420–1428 [DOI] [PubMed] [Google Scholar]
- Guest CB, Gao Y, O'Conner JC, Freund GG 2007 Obesity and Immunity. In: Ader R, ed. Pyschoneuroimmunology. 4th ed. San Diego: Elsevier; 993–1012 [Google Scholar]
- Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM 2002 IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab 87:1184–1188 [DOI] [PubMed] [Google Scholar]
- Juge-Aubry CE, Somm E, Giusti V, Pernin A, Chicheportiche R, Verdumo C, Rohner-Jeanrenaud F, Burger D, Dayer JM, Meier CA 2003 Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes 52:1104–1110 [DOI] [PubMed] [Google Scholar]
- Somm E, Cettour-Rose P, Asensio C, Charollais A, Klein M, Theander-Carrillo C, Juge-Aubry CE, Dayer JM, Nicklin MJ, Meda P, Rohner-Jeanrenaud F, Meier CA 2006 Interleukin-1 receptor antagonist is upregulated during diet-induced obesity and regulates insulin sensitivity in rodents. Diabetologia 49:387–393 [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL 1992 Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci USA 89:9117–9120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier S, Darakhshan F, Hajduch E 2006 IL-1 receptor antagonist in metabolic diseases: Dr. Jekyll or Mr. Hyde? FEBS Lett 580:6289–6294 [DOI] [PubMed] [Google Scholar]
- García MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, Horn M, Ahrén B, Enerback S, Ohlsson C, Wallenius V, Jansson JO 2006 Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes 55:1205–1213 [DOI] [PubMed] [Google Scholar]
- Chida D, Osaka T, Hashimoto O, Iwakura Y 2006 Combined interleukin-6 and interleukin-1 deficiency causes obesity in young mice. Diabetes 55:971–977 [DOI] [PubMed] [Google Scholar]
- Matsuki T, Horai R, Sudo K, Iwakura Y 2003 IL-1 plays an important role in lipid metabolism by regulating insulin levels under physiological conditions. J Exp Med 198:877–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somm E, Henrichot E, Pernin A, Juge-Aubry CE, Muzzin P, Dayer JM, Nicklin MJ, Meier CA 2005 Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes 54:3503–3509 [DOI] [PubMed] [Google Scholar]
- Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH 2001 The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol 38:789–795 [DOI] [PubMed] [Google Scholar]
- Hall JA, French TK, Rasmusson KD, Vesty JC, Roberts CA, Rimmasch HL, Kfoury AG, Renlund DG 2005 The paradox of obesity in patients with heart failure. J Am Acad Nurse Pract 17:542–546 [DOI] [PubMed] [Google Scholar]
- Galal W, van Domburg RT, Feringa HH, Schouten O, Elhendy A, Bax JJ, Awara AM, Klein J, Poldermans D 2007 Relation of body mass index to outcome in patients with known or suspected coronary artery disease. Am J Cardiol 99:1485–1490 [DOI] [PubMed] [Google Scholar]
- Uretsky S, Messerli FH, Bangalore S, Champion A, Cooper-Dehoff RM, Zhou Q, Pepine CJ 2007 Obesity paradox in patients with hypertension and coronary artery disease. Am J Med 120:863–870 [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB 2005 Survival advantages of obesity in dialysis patients. Am J Clin Nutr 81:543–554 [DOI] [PubMed] [Google Scholar]
- Escalante A, Haas RW, del Rincón I 2005 Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med 165:1624–1629 [DOI] [PubMed] [Google Scholar]
- Kremers HM, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE 2004 Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum 50:3450–3457 [DOI] [PubMed] [Google Scholar]
- Waugh J, Perry CM 2005 Anakinra: a review of its use in the management of rheumatoid arthritis. Biodrugs 19:189–202 [DOI] [PubMed] [Google Scholar]
- Johnson DR, O'Connor JC, Hartman ME, Tapping RI, Freund GG 2007 Acute hypoxia activates the neuroimmune system which diabetes exacerbates. J Neurosci 27:1161–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry CL, Kramer JM, York JM, Freund GG 2009 Behavioral recovery from acute hypoxia is reliant on leptin. Brain Behav Immun 23:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry CL, O'Connor JC, Kramer JM, Freund GG 2007 Augmented lipopolysaccharide-induced TNF-α production by peritoneal macrophages in type 2 diabetic mice is dependent on elevated glucose and requires p38 Map kinase. J Immunol 178:663–670 [DOI] [PubMed] [Google Scholar]
- Carr D, Friedman MA 2005 Is obesity stigmatizing? Body weight, perceived discrimination, and psychological well-being in the United States. J Health Soc Behav 46:244–259 [DOI] [PubMed] [Google Scholar]
- Habbu A, Lakkis NM, Dokainish H 2006 The obesity paradox: fact or fiction? Am J Cardiol 98:944–948 [DOI] [PubMed] [Google Scholar]
- Anker SD, Steinborn W, Strassburg S 2004 Cardiac cachexia. Ann Med 36:518–529 [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D 1986 The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr 116:641–654 [DOI] [PubMed] [Google Scholar]
- Gajjar A, Kubo C, Johnson BC, Good RA 1987 Influence of extremes of protein and energy intake on survival of B/W mice. J Nutr 117:1136–1140 [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E, Pennington CALERIE Team 2006 Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295:1539–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley DP, Kirkwood TB 2006 Caloric restriction does not enhance longevity in all species and is unlikely to do so in humans. Biogerontology 7:165–168 [DOI] [PubMed] [Google Scholar]
- Keller HH 2004 Nutrition and health-related quality of life in frail older adults. J Nutr Health Aging 8:245–252 [PubMed] [Google Scholar]
- Bales CW, Buhr G 2008 Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc 9:302–312 [DOI] [PubMed] [Google Scholar]
- Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP, Cardiovascular Study Research Group 2001 Weight change in old age and its association with mortality. J Am Geriatr Soc 49:1309–1318 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2008 WISQARS leading causes of death. Atlanta: Centers for Disease Control and Prevention [Google Scholar]
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G 2001 Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280:E745–E751 [DOI] [PubMed] [Google Scholar]
- O'Rourke RW, Kay T, Lyle EA, Traxler SA, Deveney CW, Jobe BA, Roberts Jr CT, Marks D, Rosenbaum JT 2006 Alterations in peripheral blood lymphocyte cytokine expression in obesity. Clin Exp Immunol 146:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF 2003 Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52:812–817 [DOI] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB 2004 Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry 65:634–651, quiz 730 [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ 2008 Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol 63:652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes RC, Blondet A, Birkenkamp-Demtroeder K, Tirard J, Orntoft TF, Gertler A, Durand P, Naville D, Bégeot M 2003 Study of the alteration of gene expression in adipose tissue of diet-induced obese mice by microarray and reverse transcription-polymerase chain reaction analyses. Endocrinology 144:4773–4782 [DOI] [PubMed] [Google Scholar]
- Lin S, Thomas TC, Storlien LH, Huang XF 2000 Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord 24:639–646 [DOI] [PubMed] [Google Scholar]
- Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier CA 2001 Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab 86:783–791 [DOI] [PubMed] [Google Scholar]
- Dreyer MG, Juge-Aubry CE, Gabay C, Lang U, Rohner-Jeanrenaud F, Dayer JM, Meier CA 2003 Leptin activates the promoter of the interleukin-1 receptor antagonist through p42/44 mitogen-activated protein kinase and a composite nuclear factor κB/PU. 1 binding site. Biochem J 370:591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks AS, Davis SM, Bates SH, Myers Jr MG 2000 Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275:14563–14572 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Muzio M, Ghezzi P, Colotta F, Introna M 1996 Negative regulators of the interleukin-1 system: receptor antagonists and a decoy receptor. Int J Clin Lab Res 26:7–14 [DOI] [PubMed] [Google Scholar]
- Johnson DR, Sherry CL, York JM, Freund GG 2008 Acute hypoxia, diabetes, and neuroimmune dysregulation: converging mechanisms in the brain. Neuroscientist 14:235–239 [DOI] [PubMed] [Google Scholar]
- Rothwell N 2003 Interleukin-1 and neuronal injury: mechanisms, modification, and therapeutic potential. Brain Behav Immunol 17:152–157 [DOI] [PubMed] [Google Scholar]
- Mulcahy NJ, Ross J, Rothwell NJ, Loddick SA 2003 Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol 140:471–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano FJ 2005 Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.