Abstract
Although ovarian nerve growth factor (NGF) facilitates follicular development and ovulation, an excess of the neurotrophin in the rodent ovary reduces ovulatory capacity and causes development of precystic follicles. Here we show that ovarian NGF production is enhanced in patients with polycystic ovarian syndrome (PCOS) and that transgenically driven overproduction of NGF targeted to the ovary results in cystic morphology, when accompanied by elevated LH levels. NGF levels are increased in the follicular fluid from PCOS ovaries and in the culture medium of granulosa cells from PCOS patients, as compared with non-PCOS patients. Ovaries from transgenic mice carrying the NGF gene targeted to thecal-interstitial cells by the 17α-hydroxylase gene promoter produce more NGF than wild-type (WT) ovaries and are hyperinnervated by sympathetic nerves. Antral follicle growth is arrested resulting in accumulation of intermediate size follicles, many of which are apoptotic. Peripubertal transgenic mice respond to a gonadotropin challenge with a greater increase in plasma 17-hydroxyprogesterone, estradiol, and testosterone levels than WT controls. Transgenic mice also exhibit a reduced ovulatory response, delayed puberty, and reduced fertility, as assessed by a prolonged interval between litters, and a reduced number of pups per litter. Sustained, but mild, elevation of plasma LH levels results in a heightened incidence of ovarian follicular cysts in transgenic mice as compared with WT controls. These results suggest that overproduction of ovarian NGF is a component of polycystic ovarian morphology in both humans and rodents and that a persistent elevation in plasma LH levels is required for the morphological abnormalities to appear.
Mice with genetically induced excess of nerve growth factor suffer from reproductive abnormalities similar to those of PCOS and show increased susceptibility to developing ovarian follicular cysts.
Polycystic ovarian syndrome (PCOS) is a complex endocrine disorder characterized by hyperandrogenism, chronic anovulation, and polycystic ovaries (1,2). More than 90% of women affected by the syndrome have abnormal levels of circulating gonadotropins, characterized by a selective elevation of LH in the presence of normal FSH values, resulting in an increased LH to FSH ratio (3,4). Many PCOS patients are also obese and develop metabolic and cardiovascular abnormalities similar to those observed in metabolic syndrome (1,2).
Although the presence of polycystic ovaries is considered as a key diagnostic trait of PCOS (1,2,5), a sizable fraction (16–25%) of apparently normal-cycling women with regular menstrual cycles (6) and a high prevalence of healthy adolescents (7) exhibit features of polycystic ovarian morphology (PCOM), in the absence of any of the other manifestations of PCOS. Importantly, near half of asymptomatic adolescents with PCOM show signs of ovarian dysfunction that, without being associated with hyperandrogenemia, is nonetheless consistent with the presence of a subclinical PCOS condition (7). A hallmark of this dysfunction is an enhanced 17-hydroxyprogesterone response to a GnRH challenge (7,8). A major implication of these findings is that adolescents affected by PCOM are at risk for PCOS (8).
The mechanisms underlying the development of PCOM have not been identified. Because these morphological changes become first evident at the time of puberty (7,8), in the presence of normal but rising LH levels, they may be set in motion by intraovarian factors working in concert with a changing gonadotropin input to the gland. The finding that adult women with PCOM have elevated androgen levels in the face of normal gonadotropin levels (6) suggest that once the morphological changes have been established at puberty, they are maintained in the absence of abnormal gonadotropin secretion. Inferentially, these findings also emphasize the importance of local, intraovarian factors as key contributors to the etiology of PCOM. Although, among the plethora of peptides and steroids produced by the ovary, androgens appear to play a key role in the etiology of PCOS (4,9), they may not be a causative factor in PCOM because this morphology is not associated with changes in androgen production (7,8).
Studies in rats raised the possibility that a deranged production of nerve growth factor (NGF), a neurotrophin produced by ovarian nonneuronal cells (10), may contribute to the development of PCOM. Under normal conditions, NGF acting via high-affinity tyrosine receptor kinase A (trkA) receptors facilitates the ovulatory process (11,12,13). However, an excess of NGF, instead of being beneficial, has been shown to initiate pathological changes in both endocrine and nonendocrine tissues (14,15,16). The ovary is no exception because the development of follicular cysts in rats treated with estradiol valerate (EV) is associated with sustained overproduction of NGF in the gland (17,18). These studies also demonstrated that immunoneutralizing NGF actions in conjunction with blocking the synthesis of p75NTR, the common neurotrophin receptor, partially restored the normal dynamics of antral follicular growth, estrous cyclicity, and ovulatory capacity in EV-treated rats (17). Additional studies showed that a selective increase in intraovarian NGF content via grafting of cells genetically engineered to produce NGF initiated several of the structural and functional alterations associated with the development of follicular cysts in the rat ovary, including appearance of precystic structures, an increase in the number of apoptotic follicles, and hyperandrogenemia (19). Thus, ovarian NGF may not only contribute to regulating normal follicle growth, but if produced at persistently elevated levels, it may also initiate ovarian pathology. A potential contribution of an excess of intraovarian NGF to human PCOM can be inferred from the findings of sympathetic hyperinnervation of the ovary from PCOS patients (20,21). Sympathetic hyperactivity is a hallmark of NGF overexpression in peripheral tissues (14,15,16), including the ovary (17,18). Furthermore, sympathetic hyperactivity in PCOS has been suggested by indirect methods (22) and direct measurement of sympathetic nerve activity (23).
In the present study, we show that the follicular fluid from ovaries of PCOS patients contains more NGF than the fluid from non-PCOS subjects and isolated granulosa cells from PCOS patients produce more NGF than granulosa cells from ovaries from non-PCOS individuals. In humans, NGF is produced by both granulosa and thecal cells (24). To determine whether a chronic excess of endogenous NGF production is capable of causing ovarian pathology, we generated transgenic mice carrying the NGF gene under the control of the 17-α hydroxylase/C17–20 lyase (17α-OH) promoter. Because this gene is specifically expressed in androgen-producing cells (25), these animals show selective overexpression of NGF in thecal/interstitial cells of the ovary, the predominant site of NGF production in rodents (11). The results show that the ovaries of NGF-overexpressing transgenic mice have an accumulation of antral follicles, which appear to be arrested at a medium-intermediate stage. This developmental arrest is accompanied by enhanced granulosa cell apoptosis, increased androgen production in response to increased FSH-like activity [pregnant mare serum gonadotropin (PMSG)] and a heightened prevalence of follicular cysts in response to a sustained elevation of serum LH-like levels [human chorionic gonadotropin (hCG)]. In keeping with these findings, NGF overproducers have reduced fertility and shortened reproductive life span. Preliminary reports of these findings have been published (26,27).
Materials and Methods
Patients
Human granulosa cells and follicular fluid were obtained from follicular aspirates of women undergoing routine in vitro fertilization at the praxis of Assisted Reproductive Technologies GmbH (Bogenhausen, München, Germany) as described (28,29,30,31). The use of human samples for research purposes was approved by the ethics committee of the University of Munich, and written consent from the patients was obtained. Samples were obtained from two groups of patients undergoing this procedure, i.e. women with clinical PCOS and women without PCOS. Aspirates from different follicles of each individual patient were pooled and treated as one sample (n = 11 patients per group). After centrifugation at 560 × g for 3 min, follicular fluid samples were frozen at −80 C until NGF assay. Granulosa cells from both groups (n = 5 patients per group) were cultured as previously described (28,29,30,31). Additional details are presented as supplemental Materials and Methods, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
Animals and tissue collection
Transgenic mice were generated at the Oregon Health and Science University Transgenic/Gene Targeting Core by pronuclear microinjection of the transgene construct into fertilized eggs from B6D2F1/J mice (The Jackson Laboratory, Bar Harbor, ME), followed by transfer of the injected eggs into the uterus of a surrogate mother, using standard procedures (32). Animal usage was duly approved by the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center. For description of method of genotyping and propagation, see supplemental Materials and Methods. Two lines, 2380 and 2456, showed an enhanced level of expression and exhibited similar phenotypes. The 2456 line (henceforth referred to as 17NF) was selected for further study and was bred to homozygosity.
Assessment of puberty and fertility
Wild-type (WT) and transgenic mice were weaned and setup in groups of four or five females per cage at 21 d of age. The females were then observed daily for vaginal opening. After vaginal opening, vaginal lavages were collected and observed daily to record the age at first estrus. A true estrus was considered to have occurred only when the cornified cells in the vaginal lavages were replaced by at least 2 d of lavages containing predominately leukocytes (33,34). Ovulation was also confirmed by observation of corpora lutea in ovaries after collection and histological preparation. Other mice were set up in breeding cages containing one adult WT male and two 30-d-old females (WT or transgenic) to determine the interval to the first fertile cycle and the interval between fertile cycles. Fertility of the mice was measured by the number of litters produced and the number of pups/litter born over 1 yr. Cyclicity in adult mice was assessed by daily vaginal lavages for 3 wk.
Assessment of ovulatory capacity
Ovulatory capacity was determined by injecting WT and transgenic mice (age, 27.4 ± 0.4 d) with 5 IU of PMSG, ip (Sigma-Aldrich, St. Louis, MO) followed 48 h later by hCG, ip (Sigma-Aldrich; 1, 2.5, and 5.0 IU). The number of ovulated oocytes present in the oviduct was determined 24 h later by puncturing the wall of the oviduct with a fine pair of scissors under a stereomicroscope and extruding the oocytes onto a glass slide for counting.
Cyst formation in response to hCG
Immature mice injected with a single dose of PMSG (5 IU/mouse, ip) followed by 7 d of hCG infusion (250 mIU/h) have been shown to develop large cystic follicles (35). Because all mice treated with this dose of hCG exhibit follicular cysts (35), we tested three doses of the gonadotropin in WT mice (250, 125, and 50 mIU/h) to identify a dose that would be minimally effective. Because approximately 25% of mice exposed to the 50 mIU/h dose had cysts, we selected the 50 mIU hCG/h dose to compare the effect it had on cyst formation in 17NF and WT mice. Immature mice (24–27 d of age) were injected with 5 IU PMSG (Sigma-Aldrich) ip and at the same time received between the scapulae a sc implanted microosmotic pump (model 1007D; Alzet Corp., Palo Alto, CA) delivering 50 mIU/h hCG (Sigma-Aldrich). The ovaries were collected 1 wk later, fixed in Kahle’s fixative, and processed for morphological analysis. The whole ovary was sectioned at 6 μm, and one every tenth section was stained with Weigert’s iron hematoxylin and counterstained with picric acid methyl blue. The numbers of antral, precystic, and cystic follicles were counted in each of these sections. Cystic follicles and type III follicles were defined as described in supplemental Materials and Methods.
Supplemental materials and methods
Details concerning NGF assays, transgene construction, in vitro testing of the construct, ribonuclease protection assay, in situ hybridization, Western blots, immunohistochemistry, ovarian morphometric analysis, assessment of apoptosis, hormone measurement, and statistical analysis are presented in supplemental Materials and Methods.
Results
The content of NGF is increased in the ovarian follicular fluid from PCOS patients, and PCOS granulosa cells produce more NGF than cells from non-PCOS ovaries
Follicular fluid, collected from PCOS patients undergoing in vitro fertilization, showed almost a 2-fold increase in mean NGF levels with respect to follicular fluid obtained from non-PCOS ovaries (Fig. 1A). Granulosa cells collected from follicular aspirations and cultured in serum-free medium for 24 h (after the culture was established in serum containing medium for 72–96 h) also produced more NGF than control granulosa cells (Fig 1B).
Figure 1.
NGF content is increased in ovarian follicular fluid from PCOS patients, and granulosa cells from PCOS ovaries produce more NGF than granulosa cells from non-PCOS ovaries A, NGF levels are greater in the ovarian follicular fluid of PCOS patients than the fluid of non-PCOS subjects. *, P < 0.05 (Mann-Whitney rank sum test) vs. non-PCOS group. B, Granulosa cells from PCOS ovaries produce more NGF than granulosa cells from non-PCOS ovaries. The cells, collected during follicle aspiration, were placed in culture for 3–4 d in serum-containing medium. At this time, the medium was replaced with serum-free medium and the cells were incubated for an additional 24 h period before NGF measurement. NGF values are expressed as picograms per arbitrary unit (AU) of ATP (an indirect assessment of viability). *, P < 0.05 (t test) vs. normal group. Numbers on top of bars are numbers of subjects per group.
In vitro testing of the 17α-OH-human NGF (hNGF) transgene construct
Because of the above finding, we sought to establish a rodent model in which production of NGF in the ovary is endogenously enhanced. Although in humans NGF is produced by both thecal-interstitial cells and granulosa cells (24), in rodents NGF appears to be mostly produced by thecal-interstitial cells (36). Consequently, we generated transgenic mice in which expression of the hNGF gene is targeted to thecal-interstitial cells by the mouse 17α-OH promoter (supplemental Fig. 1A).
The transgene construct was transiently transfected into two cell lines (MA-10 and MLTC) derived from Leydig cells. Both cell lines released substantial amounts of NGF into the medium after transfection, but the increase was more pronounced in the case of the MLTC cells (supplemental Fig. 2B).
Selection of founders
After pronuclear injection, the offspring of the surrogate mothers were tested for genomic incorporation of the transgene and for ovarian expression of hNGF mRNA, as outlined in supplemental Materials and Methods. As hemizygotes, two lines showed selective ovarian expression of the transgene mRNA. Both lines had seemingly normal reproductive characteristics. Age at vaginal opening, age at first estrus, interval between litters, and fertility based on the number of pups produced by each dam were all similar to WT controls. One of the lines (17NF) was then bred to homozygosity and used for all subsequent studies.
Transgenic expression of hNGF mRNA and hNGF protein
Ribonuclease protection assay showed the presence of hNGF mRNA only in 17NF mice and no detectable expression in WT tissues (supplemental Fig. 2A). The primary site of transgene expression was the ovary and, to a much lesser extent, the adrenal gland. Traces of hNGF mRNA were also detected in the kidney and liver (supplemental Fig. 2A). Because ribonuclease digestion of the cRNA probe was incomplete, a smaller undigested fragment is present in the digested probe lane (lane D) as well as all other sample lanes. No hNGF mRNA was detected in the hypothalamus, hippocampus, and cerebral cortex. There was no enhancement of transgene mRNA expression after PMSG treatment (supplemental Fig. 2A).
Ovarian NGF content, as assessed by ELISA, was markedly increased in 17NF mice (supplemental Fig. 2B). As in the case of hNGF mRNA, there was no change in ovarian NGF content after PMSG treatment. There was no change in overall ovarian weight in the transgenic mice, and as expected, ovarian weight increased equally in 17NF and WT mice in response to PMSG (supplemental Fig. 2C). Consistent with the fact that transgene expression in the construct is driven by the 17α-OH promoter, hNGF mRNA transcripts were selectively localized to the thecal-interstitial compartment of the ovary, as assessed by in situ hybridization (supplemental Fig. 2, D and E).
NGF overproduction increases the extrinsic sympathetic input to the ovary
Tyrosine hydroxylase (TH) in the ovary is mostly confined to sympathetic nerves (10,37). Thus, the level of TH in the ovary can be regarded as a measure of the sympathetic tone to the gland. In keeping with earlier observations showing that NGF produced in the ovary is required for the integrity of the extrinsic ovarian sympathetic innervation (38,39), the ovaries from 17NF mice exhibited a marked increase in TH content in comparison with WT mice, as determined by Western blot analysis (supplemental Fig. 3, A and B). An immunohistochemical evaluation of these changes revealed that this increase is, for the most part, due to a greater density of TH-positive nerve fibers coursing through the interstitial tissue and surrounding ovarian follicles (supplemental Fig. 3, C–F).
Ovarian overproduction of NGF delays the timing of puberty and compromises adult reproductive capacity
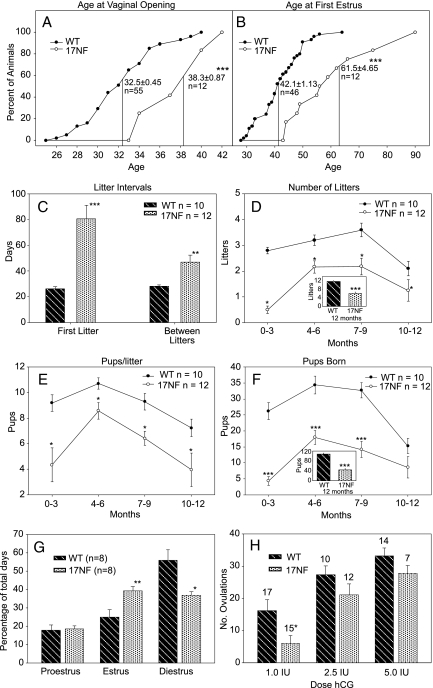
17NF mice showed a delay in puberty as assessed by the age at vaginal opening (Fig. 2A) and the age at first estrus (Fig. 2B). The delay in pubertal development resulted in an increase in the interval between exposure to a male and delivery of the first litter in 17NF mice compared with WT mice (Fig. 2C). There was a continued reduction in fecundity throughout the year in the 17NF mice as shown by an increase in the interval between litters (Fig. 2C), a reduction in the number of litters born (Fig. 2D), a reduced number of pups per litter (Fig. 2E), and an overall reduction of the total number of pups born (Fig. 2F). Adult mice exhibited an increase number of days they spend in estrus and a reduction of days in diestrus (Fig. 2G), whereas the days in proestrus were equivalent between the two groups. At diestrus, progesterone (P4) levels were 3-fold lower in 17NF mice (data not shown), suggesting either a failure to ovulate or reduction in the number of functional corpora lutea.
Figure 2.
Transgenic mice overexpressing NGF in the ovary exhibit delayed puberty and reduced fertility; estrous cyclicity is disrupted and the ovulatory response to gonadotropins is reduced in 17NF mice. A, Vaginal opening (mean ± sem shown on graph) was delayed in mice with enhanced ovarian NGF expression. ***, P < 0.001 (t test) vs. WT mice. B, After vaginal opening, daily vaginal lavages were used to determine the age at first estrus, which was also delayed in transgenic mice. ***, P < 0.001 (Mann-Whitney rank sum test) vs. WT mice. The discrepancy in the number of WT animals in A and B was because some the animals were mistakenly identified as being in estrus and thus were not included in B. C, Transgenic and WT (B6D2) females were set up in breeding cages with WT (B6D2) males at 30 d of age. The interval from cage setup to first litter was delayed (***, P < 0.001; t test) as was the intervals between subsequent litters. **, P < 0.01 (t test) vs. WT. D, The number of litters born per female during 3-month intervals in transgenic mice was reduced compared with WT mice (*, P < 0.05; one-way repeated measures ANOVA) (inset, summary of data for 12 months; ***, P < 0.001; t test). E, The number of pups/litter per female was reduced in transgenic mice compared with WT animals. *, P < 0.05 (Friedman repeated measures ANOVA). F, The total number of pups born per female was reduced in the transgenic mice both quarterly (***, P < 0.001; one way repeated measures ANOVA) and for the entire year (inset; ***, P < 0.001; t test). G, Young adult (60 d of age) 17NF mice spend more days in estrus (**, P < 0.01; t test) and fewer days in diestrus. *, P < 0.05 (Mann-Whitney rank sum test) vs. age-matched WT mice. H, The ovulatory response of 17NF mice to a priming PMSG injection followed by a low dose of hCG (1 IU) was lower than that of WT animals. *, P < 0.05 (Mann-Whitney rank sum test). This reduced response was not apparent at higher doses of hCG (2.5 and 5 IU hCG). Numbers on top of bars are number of subjects per group.
Ovarian overproduction of NGF decreases ovulatory capacity
Using a single ip dose of PMSG (5 IU), followed 2 d later by a single ip injection of different doses of hCG, we found that the ovulatory response to the lowest dose of hCG (1 IU) was reduced in immature 27-d-old 17NF mice in comparison with WT controls (Fig. 2H). This deficiency was overcome by higher doses (2.5 and 5 IU) of hCG (Fig. 2H).
Ovarian overproduction of NGF results in arrested follicular development and apoptosis of antral follicles
To determine whether an excess of NGF in the ovary results in alterations of follicular growth, we performed a morphometric analysis of ovaries from immature 28-d-old 17NF and WT mice. The most salient morphological feature of the transgenic ovaries was an abundance of medium size antral follicles, accompanied by a paucity of large preovulatory follicles (Fig. 3, A and B). Quantitative analysis of these changes showed that the total number of follicles/ovary was increased in 17NF mice (Fig. 3C). Morphological evaluation of granulosa cell integrity (see Materials and Methods for details) demonstrated that this increase is due to a higher incidence of atretic follicles in the transgenic ovaries and not to changes in the number of healthy follicles (Fig. 3D). Similarly, when the size distribution of follicles was evaluated, no statistical differences were observed in the number of healthy follicles (Fig. 3E). However, the number of atretic follicles measuring 100–200 and 202–300 μm was increased in 17NF ovaries (Fig. 3F), a change that is consistent with the morphological aspect of the transgenic ovaries (Fig. 3B).
Figure 3.
The ovaries of 17NF mice contain more antral follicles of an intermediate size than the ovaries of WT mice. A, Microphotograph of a WT ovary. B, Microphotograph of a 17NF ovary. The ovaries were collected from 32-d-old mice, fixed in Kahle’s solution, embedded in paraffin, sectioned at 6 μm, stained with Weigert’s iron hematoxylin, and counterstained with picric acid-methyl blue. Bars, 300 μm. C, The total number of follicles per ovary was greater in 17NF mice than WT controls. *, P < 0.05 (t test). D, The number of antral atretic follicles but not that of healthy follicles was also increased in 17NF mice. *, P < 0.05 (ANOVA, Student-Newman-Keuls multiple comparison of means). E, The ovaries of 17NF mice tended to have more healthy follicles measuring 101–200 μm in diameter than WT ovaries. F, The transgenic ovaries had significantly more atretic follicles in the range of 101–200 and 201–300 μm than WT controls. *, P < 0.05 (ANOVA, Student-Newman-Keuls multiple comparison of means).
Granulosa cell apoptosis is increased in 17NF transgenic ovaries
To confirm these morphological findings, we subjected ovarian sections from 17NF and WT mice to a terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay. As shown in Fig. 4, there were more apoptotic follicles in the ovaries from transgenic mice (Fig. 4B) than in WT ovaries (Fig. 4A). The degree of apoptosis (i.e. the number of apoptotic granulosa cells per follicle) appeared to be also increased (Fig. 4, C–E). No positive cells were seen in sections incubated without the terminal deoxynucleotidyl transferase enzyme solution (not shown). Quantitation of these changes demonstrated that the increased number of TUNEL-positive follicles in 17NF ovaries was significantly greater than in WT ovaries (Fig. 4F). In addition, the degree of apoptosis per follicle was significantly greater in 17NF ovaries than WT glands, as measured by comparing the variance of the fluorescent intensity between the two groups (48,899 ± 20,707 vs. 8,830 ± 2,701 for the 17NF and WT group, respectively).
Figure 4.
The ovaries of 17NF transgenic mice exhibit more apoptotic follicles than WT controls, as assessed by TUNEL assay. A, WT ovary. B, 17NF ovary. Bars, 300 μm. C, Example of follicle from a WT ovary showing some TUNEL-positive granulosa cells. D, Typical atretic follicle from a 17NF ovary exhibiting numerous TUNEL-positive granulosa cells. E, Another example of granulosa cell apoptosis in a 17NF ovary. Bars, 100 μm. F, Ovaries from 17NF mice have a similar number of healthy antral follicles but more antral apoptotic follicles than WT ovaries. **, P < 0.01 (t test).
17NF mice show an increased ovarian steroid response to PMSG stimulation
To determine whether 17NF mice display changes in circulating steroid and/or gonadotropin levels resembling those detected in PCOM/PCOS patients, we measured basal serum levels of P4, 17-hydroxyprogesterone (17-OHP4), androstenedione (Δ4), testosterone (T4), and estradiol (E2), in addition to LH and FSH, in 28- to 32-d-old late juvenile mice. In addition, the same steroids were measured in late juvenile mice stimulated with PMSG. With the exception of a modest increase in basal P4 levels (Fig. 5A), basal serum levels of all other steroids measured were similar in WT and 17NF mice (Fig. 5, A–D). In contrast to this relative lack of changes in basal steroid levels, clear differences emerged after PMSG stimulation. The 17NF mice responded to PMSG with a blunted P4 elevation compared with WT mice (Fig. 5A) but had a greater 17-OHP4, T4, and E2 response to the gonadotropin (Fig. 5, B–D). Resembling subjects with PCOM (6,7), basal serum LH and FSH levels were similar in WT and 17NF mice (Fig. 5, E and F), suggesting that an overproduction of ovarian NGF more closely mimic the pathology of PCOM (which does not include a change in circulating gonadotropin levels) than that of PCOS (that includes central activation of LH secretion).
Figure 5.
Basal serum levels of sex steroids and gonadotropins are similar in juvenile (J; 28–30 d old) 17NF and WT mice but differ substantially after PMSG stimulation. A, Basal serum P4 levels are modestly increased in 17NF mice (*, P < 0.05; t test), but the P4 response to PMSG is decreased (**, P < 0.01; t test) in these animals in comparison with WT controls. B, The 17-OHP4 response to PMSG is greater in 17NF mice than in WT controls. *, P < 0.05 (t test). C, Serum Δ4 levels are similar in both groups stimulated with PMSG, but the T4 response is increased in 17NF mice. ***, P < 0.001 (Mann-Whitney rank sum test). D, The E2 response to PMSG is also greater in 17NF mice than WT controls. ***, P < 0.001 (Mann-Whitney rank sum test). E, Serum LH levels are similar in the two groups. F, FSH levels are also similar. Numbers on top of bars are number of subjects per group.
17NF mice showed increased formation of follicular cysts in response to hCG stimulation
Because 17NF transgenic animals showed normal serum LH levels, we considered the possibility that isolated ovarian overexpression of NGF can arrest antral follicular development to a medium size but cannot induce conversion of these intermediate size follicles into cysts, unless circulating levels of LH are also increased because it occurs in humans developing PCOM at the time of puberty (7). To examine this possibility, we treated 24- to 27-d-old 17NF and WT mice with PMSG (5 IU; single ip injection) followed by sustained delivery of hCG using osmotic minipumps. The pump was set to deliver 50 mIU/h of hCG, a dose that in initial experiments was found to induce formation of cystic follicles at a low frequency (25%) in WT mice. Consistent with the aforementioned premise, 17NF mice responded to this treatment with the formation of more antral follicles (Fig. 6, A–C), precystic follicles (Fig, 6, D–F), and cysts (Fig. 6, G–I) than WT mice.
Figure 6.
The ovaries from 17NF transgenic mice respond more pronouncedly than WT ovaries to a persistent, but mild elevation, in circulating levels of LH-like activity. A sustained elevation in circulating LH-like activity was achieved by treating juvenile (24–27 d old) 17NF and WT mice with a low dose of hCG (50 mIU/h) delivered for 7 d via osmotic minipumps. To simulate a peripubertal condition preceding the exposure to hCG, all animals received a single dose of PMSG (5 IU/mouse) before initiating the hCG treatment. A–C, Total number of antral follicles (examples denoted by arrows in A and B) is increased in 17NF ovaries. ***, P < 0.001 (t test). D–F, The number of precystic type III follicles (examples denoted by arrows in D and E) is also increased. **, P < 0.01 (t test). G–I, Likewise, ovaries from 17NF mice have more follicular cysts (arrows in G and H) than WT controls. *, P < 0.05 (t test). Bars in microphotographs, 250 μm. Numbers on top of bars are number of subjects per group.
Discussion
The present results demonstrate that the ovaries from PCOS patients produced more NGF than non-PCOS control ovaries. This difference was demonstrated both in vivo by a greater accumulation of the neurotrophin in the follicular fluid of PCOS ovaries compared with non-PCOS glands and in vitro by the higher levels of NGF detected in the culture medium of granulosa cells from PCOS patients than the medium of granulosa cells from non-PCOS controls. Our results also show that when an intraovarian excess of NGF was simulated in mice via targeted transgenic overexpression of the neurotrophin to the ovary, antral follicle growth was arrested at an intermediate stage and granulosa cell atresia was accelerated, but no follicular cysts were formed. Such cysts developed in young animals only if the targeted overexpression of NGF was accompanied by a sustained, but mild, elevation in circulating LH-like levels.
An excessive production of NGF may directly affect ovarian function via trkA receptors and p75NTR located on ovarian cells and indirectly via its well-established trophic actions on the ovarian innervation (reviewed in Ref. 10). As in other peripheral tissues exposed to an excessive production of NGF, such as lung (14), skin (15), and pancreas (16), the ovaries of 17NF mice are hyperinnervated by catecholaminergic fibers, suggesting that the sympathetic tone to the gland (10,37) is enhanced by NGF. The findings that ovarian denervation (40) and neutralization of NGF actions (17) reduce the incidence of follicular cysts in animals in which cyst formation was induced by estradiol valerate support this notion.
The reproductive deficits observed in 17NF animals are consistent with the morphological changes resulting from the overproduction of NGF. Antral follicular growth is arrested at an intermediate stage, and this deficiency correlates well with delayed puberty, lower ovulatory response to gonadotropins, and lower fecundity observed in these mice. The failure of antral follicles to progress toward a preovulatory stage in 17NF mice appears be related to an increased rate of granulosa cell death. Because cystic follicles contain very few (or even just one) layer of granulosa cells, granulosa cell death is likely an early event in the natural history of a cystic follicle. In recent studies, we observed that an apoptotic signal-regulating kinase-1/stathmin-mediated pathway is activated in granulosa cells of 17NF mice (27). Because this pathway is used by extracellular agents such as TNFα to promote cell death (41,42) and TNFα expression is increased in 17NF ovaries (27), excessive production of NGF by thecal-interstitial cells may promote follicular atresia by setting in motion a cell death signaling pathway unrelated to that used by the low-affinity p75NTR to promote cell death in the nervous system (43). A direct role for p75NTR in mediating granulosa cell apoptosis appears less likely because expression of this gene is very low or undetectable in granulosa cells of the rodent ovary (44).
During adolescence, PCOM develops in the presence of increasing plasma gonadotropin levels, an endocrine scenario broadly mimicked by two of our experimental paradigms aimed at increased circulating gonadotropin levels on a background of intraovarian NGF excess: a brief elevation in circulating gonadotropin levels resulting from a single administration of PMSG and a sustained elevation of LH levels to nonpreovulatory values resulting from the long-term administration of hCG via minipumps. Whereas a PMSG challenge uncovered a steroidal hyperresponsiveness to the gonadotropin similar to that seen in humans (see below), the second paradigm revealed that an overproduction of ovarian NGF increases the susceptibility of the gonads to the cyst-inducing activity of an excess of circulating LH.
As in adolescents with PCOM (7), basal circulating androgen levels are normal in peripubertal 17NF mice. Also, as seen in adult women with PCOM (6), basal plasma levels of LH, FSH, and E2 are unchanged in 17NF mice with respect to WT controls. A distinct difference between adolescents with PCOM and healthy subjects is an enhanced 17-OHP4 response of the PCOM patients to a GnRH agonist (7). Resembling this feature, 17NF mice release more 17-OHP4 than WT mice after stimulation with PMSG. However, 17NF mice also respond to PMSG with a greater increase in T4 and E2 levels, a response more characteristically seen in adult PCOM subjects challenged with hCG in the case of T4 (6) and adolescents with PCOS (7) in the case of E2.
The lack of changes in basal and PMSG-stimulated serum Δ4 levels observed in 17NF mice was unexpected because of a previous study in rats showing an increase in basal serum Δ4 levels in animals receiving intraovarian grafts of NGF-producing cells (19). Although this difference may be species related, it could also be caused by the different ages of the animals used. In the present study, Δ4 was measured on d 28–30; in the earlier study, it was measured 60 d after grafting the cells on postnatal d 28. This age-related difference is remarkably similar to that of PCOM adolescents, who, in contrast to adult women (6,45), show no elevation in basal plasma T4 or Δ4 levels (7).
Our data suggest that establishment of an ovarian cystic morphology requires both a persistent increase in circulating LH levels and a changed intraovarian environment resulting from gonadotropin-independent alterations in the production of paracrine factors required for normal follicle growth. Although a plethora of intraovarian factors may be involved (46,47), our results suggest that NGF is one such factor. Earlier studies performed in rats showed increased NGF production in the ovaries of rats in which follicular cysts were induced by EV treatment (17,18) and in rats in which intraovarian NGF production was induced by grafting cells genetically engineered to produce the neurotrophin (19). Resembling the present results, overproduction of NGF by the grafted cells did not result in cyst formation (19), giving further credence to the idea that deregulation of intraovarian NGF production plays either a permissive or priming role in the development of follicular cysts.
Could this interaction between gonadotropins and a target tissue factor be involved in the manifestation of human disorders such as PCOM and/or the ovarian component of PCOS? A basic observation and several clinical findings suggest that an excess of ovarian NGF might indeed be an abnormality contributing to PCOM and PCOS. The basic observation is the well-established trophic effect that NGF exerts on the sympathetic innervations of various peripheral organs (48), including the ovary (10). The clinical findings made in PCOS patients include an augmented density of catecholaminergic nerves in the ovary (20,21), an increased sympathetic nerve activity (23), and a diminished peripheral catecholamine metabolism (22). These conditions and the ability of wedge resection (a procedure that disrupts the extrinsic innervation to the gland) to induce ovulatory cycles in PCOS (49,50,51) suggest that the sympathetic outflow to the human polycystic ovary is increased.
Our results showing that ovarian production of NGF is elevated in adult human PCOS ovaries raise the possibility that an intraovarian excess of NGF not only facilitates the development of PCOM but also persists in cases when PCOM evolves to become a distinguishing feature of PCOS. This interpretation is consistent with the notions that PCOM without hyperandrogenemia in asymptomatic adolescents represents subclinical PCOS (7) and that adolescents affected by PCOM are at risk for PCOS (8). Because NGF is elevated in the follicular fluid of subjects treated with ovulatory doses of gonadotropins to induce ovulation, this elevation may merely reflect an augmented response to surge-like levels of gonadotropins instead of an increase in basal NGF production. However, the increased capacity of isolated granulosa cells to produce NGF after several days in culture suggests that basal, gonadotropin-independent NGF production is increased in PCOS. The mechanism by which granulosa cells in culture retain a memory of their in vivo status remains to be identified. Earlier studies using sheep ovaries showed that the follicular fluid of medium-large follicles contains more NGF than the fluid of small follicles and that gonadotropins stimulate the in vitro production of NGF by isolated follicles (52). These results and those of the present study suggest that follicular production of NGF is likely to increase in response to either a preovulatory surge of LH or a sustained, nonsurge elevation in circulating LH levels, like that seen during puberty or PCOS.
In summary, the present report demonstrates that ovarian production of NGF is increased in PCOS patients and that transgenic overexpression of the neurotrophin targeted to the mouse ovary results in morphological and functional abnormalities leading to ovulatory deficiency, gonadotropin stimulated hyperandrogenemia and hypersensitivity of the gland to develop follicular cysts in response to LH. Altogether, these observations suggest a contribution of ovarian NGF to the pathology of PCOM and PCOS in humans.
Supplementary Material
Acknowledgments
We thank Dieter Berg and Ulrike Berg (Assisted Reproductive Technologies Bogenhausen, München, Germany) for providing human follicular aspirates and Sandra Raffael for expert technical assistance. We also thank Maria E. Costa for her excellence in performing the in situ hybridization and immunohistochemical studies. We also thank Dr. Anda Cornea (director of the Oregon National Primate Research Center imaging core) for her help with the analysis of the TUNEL results.
Footnotes
This work was supported by National Institutes of Health Grant HD24870 (to S.R.O.), Grant HD18185 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to S.R.O.), and Grant RR00163-49 for the operation of the Oregon National Primate Research Center (to G.A.D. and S.R.O.). C.G.-R. was a visiting scientist supported by a fellowship from National Institute of Child Health and Human Development Grant TW/HD00668 from the Fogarty International Training and Research in Population and Health. Morphological studies were supported by Fondo Nacional de Desarrollo Científico y Tecnológico Grant 1061143 (to A.P.). Studies involving human PCOS samples were supported by Grant MA 1080/17-1 from the Deutsche Forschungsgemeinschaft (to A.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 5, 2009
Abbreviations: Δ4, Androstenedione; E2, estradiol; EV, estradiol valerate; hCG, human chorionic gonadotropin; hNGF, human NGF; NGF, nerve growth factor; 17α-OH, 17-α hydroxylase/C17–20 lyase; 17-OHP4, 17-hydroxyprogesterone; P4, progesterone; PCOM, polycystic ovarian morphology; PCOS, polycystic ovarian syndrome; PMSG, pregnant mare serum gonadotropin; T4, testosterone; TH, tyrosine hydroxylase; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling; WT, wild type.
References
- Ehrmann DA 2005 Polycystic ovary syndrome. N Engl J Med 352:1223–1236 [DOI] [PubMed] [Google Scholar]
- Norman RJ, Dewailly D, Legro RS, Hickey TE 2007 Polycystic ovary syndrome. Lancet 370:685–697 [DOI] [PubMed] [Google Scholar]
- Hall JE, Taylor AE, Hayes FJ, Crowley Jr WF 1998 Insights into hypothalamic-pituitary dysfunction in polycystic ovary syndrome. J Endocrinol Invest 21:602–611 [DOI] [PubMed] [Google Scholar]
- McCartney CR, Eagleson CA, Marshall JC 2002 Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med 20:317–326 [DOI] [PubMed] [Google Scholar]
- Dunaif A, Thomas A 2001 Current concepts in the polycystic ovary syndrome. Annu Rev Med 52:401–419 [DOI] [PubMed] [Google Scholar]
- Adams JM, Taylor AE, Crowley Jr WF, Hall JE 2004 Polycystic ovarian morphology with regular ovulatory cycles: insights into the pathophysiology of polycystic ovarian syndrome. J Clin Endocrinol Metab 89:4343–4350 [DOI] [PubMed] [Google Scholar]
- Mortensen M, Rosenfield RL, Littlejohn E 2006 Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab 91:3786–3790 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL 2007 Clinical review: identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab 92:787–796 [DOI] [PubMed] [Google Scholar]
- Azziz R 2003 Androgen excess is the key element in polycystic ovary syndrome. Fertil Steril 80:252–254 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Paredes A, Romero C, Dees WL, Ojeda SR 2004 Neural and neurotrophic control of ovarian development. In: Leung P, Adashi E, eds. The ovary. 2nd ed. San Diego: Academic Press; 3–23 [Google Scholar]
- Dissen GA, Hill DF, Costa ME, Les Dees CW, Lara HE, Ojeda SR 1996 A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology 137:198–209 [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Dissen GA, Parrott JA, Hill DF, Mayerhofer D, Garfield RE, Costa ME, Skinner MK, Ojeda SR 1996 Involvement of nerve growth factor in the ovulatory cascade: TrkA receptor activation inhibits gap-junctional communication between thecal cells. Endocrinology 137:5662–5670 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Parrott JA, Skinner MK, Hill DF, Costa ME, Ojeda SR 2000 Direct effects of nerve growth factor on thecal cells from antral ovarian follicles. Endocrinology 141:4736–4750 [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Graham RM, Finkelstein JB, Nguyen K-PT, Gozal D, Friedman M 1998 Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol 18:149–157 [DOI] [PubMed] [Google Scholar]
- Davis BM, Fundin BT, Albers KM, Goodness TP, Cronk KM, Rice FL 1997 Overexpression of nerve growth factor in skin causes preferential increases among innervation to specific sensory targets. J Comp Neurol 387:489–506 [DOI] [PubMed] [Google Scholar]
- Edwards RH, Rutter WJ, Hanahan D 1989 Directed expression of NGF to pancreatic β cells in transgenic mice leads to selective hyperinnervation of the islets. Cell 58:161–170 [DOI] [PubMed] [Google Scholar]
- Lara HE, Dissen GA, Leyton V, Paredes A, Fuenzalida H, Fiedler JL, Ojeda SR 2000 An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the rat. Endocrinology 141:1059–1072 [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Lundeberg T, Waldenström U, Manni L, Aloe L, Gunnarsson S, Janson PO 2000 Effects of electro-acupuncture on nerve growth factor and ovarian morphology in rats with experimentally induced polycystic ovaries. Biol Reprod 63:1497–1503 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Lara HE, Leyton V, Paredes A, Hill DF, Costa ME, Martinez-Serrano A, Ojeda SR 2000 Intraovarian excess of nerve growth factor increases androgen secretion and disrupts estrous cyclicity in the rat. Endocrinology 141:1073–1082 [DOI] [PubMed] [Google Scholar]
- Heider U, Pedal I, Spanel-Borowski K 2001 Increase in nerve fibers and loss of mast cells in polycystic and postmenopausal ovaries. Reprod Endocrinol 75:1141–1147 [DOI] [PubMed] [Google Scholar]
- Semenova II 1969 Adrenergic innervation of ovaries in Stein-Leventhal syndrome. Vestn Akad Med Nauk 24:58–62 [PubMed] [Google Scholar]
- Garcia-Rudaz C, Armando I, Levin G, Escobar ME, Barontini M 1998 Peripheral catecholamine alterations in adolescents with polycystic ovary syndrome. Clin Endocrinol (Oxf) 49:221–228 [DOI] [PubMed] [Google Scholar]
- Sverrisdóttir YB, Mogren T, Kataoka J, Janson PO, Stener-Victorin E 2008 Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am J Physiol Endocrinol Metab 294:E576–E581 [DOI] [PubMed] [Google Scholar]
- Salas C, Julio-Pieper M, Valladares M, Pommer R, Vega M, Mastronardi C, Kerr B, Ojeda SR, Lara HE, Romero C 2006 Nerve growth factor-dependent activation of trkA receptors in the human ovary results in synthesis of FSH receptors and estrogen secretion. J Clin Endocrinol Metab 91:2396–2403 [DOI] [PubMed] [Google Scholar]
- Gore-Langton RE, Armstrong DT 1994 Follicular steroidogenesis and its control. In: Knobil E, Neill JD, eds. The physiology of reproduction. 2nd ed. New York: Raven Press, Ltd.; 571–627 [Google Scholar]
- Dissen GA, Ojeda SR, Transgenic expression of nerve growth factor (NGF) targeted to ovarian androgen-producing cells delays pubertal development and compromises fertility. Program of the 86th Annual Meeting of The Endocrine Society, New Orleans, LA, 2004, p 212 (Abstract P242) [Google Scholar]
- Garcia-Rudaz C, Mayerhofer A, Ojeda SR, Dissen GA, An excessive ovarian production of nerve growth factor (NGF) facilitates the development of polycystic ovarian morphology in mice and is a discernible feature of polycystic ovarian syndrome (PCOS) in humans. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008 (Abstract P483) [Google Scholar]
- Mayerhofer A, Fritz S, Grünert R, Sanders SL, Duffy DM, Ojeda SR, Stouffer RL 2000 D1-Receptor, DARPP-32, and PP-1 in the primate corpus luteum and luteinized granulosa cells: evidence for phosphorylation of DARPP-32 by dopamine and human chorionic gonadotropin. J Clin Endocrinol Metab 85:4750–4757 [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Hemmings Jr HC, Snyder GL, Greengard P, Boddien S, Berg U, Brucker C 1999 Functional dopamine-1 receptors and DARPP-32 are expressed in human ovary and granulosa luteal cells in vitro. J Clin Endocrinol Metab 84:257–264 [DOI] [PubMed] [Google Scholar]
- Fritz S, Kunz L, Dimitrijevic N, Grünert R, Heiss C, Mayerhofer A 2002 Muscarinic receptors in human luteinized granulosa cells: activation blocks gap junctions and induces the transcription factor early growth response factor-1. J Clin Endocrinol Metab 87:1362–1367 [DOI] [PubMed] [Google Scholar]
- Kunz L, Rämsch R, Krieger A, Young KA, Dissen GA, Stouffer RL, Ojeda SR, Mayerhofer A 2006 Voltage-dependent K(+) channel acts as sex steroid sensor in endocrine cells of the human ovary. J Cell Physiol 206:167–174 [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R 2003 Manipulating the mouse embryo: a laboratory manual. 3rd ed. New York: Cold Spring Harbor Laboratory Press [Google Scholar]
- Prevot V, Rio C, Cho GJ, Lomniczi A, Heger S, Neville CM, Rosenthal NA, Ojeda SR, Corfas G 2003 Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci 23:230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronardi C, Smiley GG, Raber J, Kusakabe T, Kawaguchi A, Matagne V, Dietzel A, Heger S, Mungenast AE, Cabrera R, Kimura S, Ojeda SR 2006 Deletion of the Ttf1 gene in differentiated neurons disrupts female reproduction without impairing basal ganglia function. J Neurosci 26:13167–13179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemade RV, Carrette O, Larsen WJ, Markoff E 2002 Involvement of nitric oxide and the ovarian blood follicle barrier in murine follicular cyst development. Fertil Steril 78:1301–1308 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Mayerhofer A, Ojeda SR 2000 Neurotrophins and the ovulatory process: a role for NGF and trkA? In: Adashi EY, ed. Ovulation: evolving scientific and clinical concepts. Norwell, MA: Springer, New York; 167–174 [Google Scholar]
- Burden HW 1985 The adrenergic innervation of mammalian ovaries. In: Ben-Jonathan N, Bahr JM, Weiner RI, eds. Catecholamines as hormone regulators. New York: Raven Press; 261–278 [Google Scholar]
- Lara HE, Hill DF, Katz KH, Ojeda SR 1990 The gene encoding nerve growth factor is expressed in the immature rat ovary: effect of denervation and hormonal treatment. Endocrinology 126:357–363 [DOI] [PubMed] [Google Scholar]
- Lara HE, McDonald JK, Ojeda SR 1990 Involvement of nerve growth factor in female sexual development. Endocrinology 126:364–375 [DOI] [PubMed] [Google Scholar]
- Rosa-E-Silva, Guimaraes MA, Padmanabhan V, Lara HE 2003 Prepubertal administration of estradiol valerate disrupts cyclicity and leads to cystic ovarian morphology during adult life in the rat: role of sympathetic innervation. Endocrinology 144:4289–4297 [DOI] [PubMed] [Google Scholar]
- Mizumura K, Takeda J, Hashimoto S, Horie T, Ichijo H 2006 Identification of Op18/stathmin as a potential target of ASK1–p38 MAP kinase cascade. J Cell Physiol 206:363–370 [DOI] [PubMed] [Google Scholar]
- Vancompernolle K, Boonefaes T, Mann M, Fiers W, Grooten J 2000 Tumor necrosis factor-induced microtubule stabilization mediated by hyperphosphorylated oncoprotein 18 promotes cell death. J Biol Chem 275:33876–33882 [DOI] [PubMed] [Google Scholar]
- Dechant G, Barde YA 2002 The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci 5:1131–1136 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Hill DF, Costa ME, Ma YJ, Ojeda SR 1991 Nerve growth factor receptors in the peripubertal rat ovary. Mol Endocrinol 5:1642–1650 [DOI] [PubMed] [Google Scholar]
- Wachs DS, Coffler MS, Malcom PJ, Shimasaki S, Chang RJ 2008 Increased androgen response to follicle-stimulating hormone administration in women with polycystic ovary syndrome. J Clin Endocrinol Metab 93:1827–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen E, Laven JS, Dommerholt HB, Polman J, van Rijt C, van den HC, Westland J, Mosselman S, Fauser BC 2004 Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol Endocrinol 18:3050–3063 [DOI] [PubMed] [Google Scholar]
- Giudice LC 1999 Growth factor action on ovarian function in polycystic ovary syndrome. Endocrinol Metab Clin North Am 28:325–339, vi [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R 1987 The nerve growth factor 35 years later. Science 237:1154–1162 [DOI] [PubMed] [Google Scholar]
- Yen SSC 1991 Chronic anovulation caused by peripheral endocrine disorders. In: Yen SSC, Jaffe RB, eds. Reproductive endocrinology: physiology, pathophysiology and clinical management. Philadelphia: W. B. Saunders, Co.; 576–630 [Google Scholar]
- Vaitukaitis JL 1983 Polycystic-ovary syndrome—what is it? N Engl J Med 309:1245–1246 [DOI] [PubMed] [Google Scholar]
- Nakamura Y 1990 Treatment of polycystic ovary syndrome: an overview. Horm Res 33(Suppl 2):31 [DOI] [PubMed] [Google Scholar]
- Mattioli M, Barboni B, Gioia L, Lucidi P 1999 Nerve growth factor production in sheep antral follicles. Domest Anim Endocrinol 17:361–371 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.