Abstract
Renin is the rate-limiting enzyme in renin-angiotensin system (RAS) activation. We sought to determine the impact of renin inhibition on whole-body insulin sensitivity and skeletal muscle RAS, oxidative stress, insulin signaling, and glucose transport in the transgenic TG(mRen2)27 rat (Ren2), which manifests increased tissue RAS activity, elevated serum aldosterone, hypertension, and insulin resistance. Young (aged 6–9 wk) Ren2 and age-matched Sprague Dawley control rats were treated with aliskiren [50 mg/kg · d, ip] or placebo for 21 d and administered an ip glucose tolerance test. Insulin metabolic signaling and 2-deoxyglucose uptake in soleus muscle were examined in relation to tissue renin-angiotensin-aldosterone system [angiotensin (Ang) II, mineralocorticoid receptor (MR), and Ang type I receptor (AT1R)] and measures of oxidative stress as well as structural changes evaluated by light and transmission electron microscopy. Ren2 rats demonstrated systemic insulin resistance with decreased skeletal muscle insulin metabolic signaling and glucose uptake. This was associated with increased Ang II, MR, AT1R, oxidative stress, and reduced tyrosine insulin receptor substrate-1 phosphorylation, protein kinase B/(Akt) phosphorylation and glucose transporter-4 immunostaining. The Ren2 also demonstrated perivascular fibrosis and mitochondrial remodeling. Renin inhibition improved systemic insulin sensitivity, insulin metabolic signaling, and glucose transport along with normalization of Ang II, AT1R, and MR levels, oxidative stress markers, fibrosis, and mitochondrial structural abnormalities. Our data suggest that renin inhibition improves systemic insulin sensitivity, skeletal muscle insulin metabolic signaling, and glucose transport in Ren2 rats. This is associated with reductions in skeletal muscle tissue Ang II, AT1R, and MR expression; oxidative stress; fibrosis; and mitochondrial abnormalities.
Renin inhibition improves insulin resistance, skeletal muscle insulin metabolic signaling, and glucose transport in a rodent model of renin-angiotensin-aldosterone system activation.
Activation of the renin-angiotensin-aldosterone system (RAAS) has been linked to increased production of reactive oxygen species (ROS) in numerous tissues, including skeletal muscle and cardiovascular tissue (1,2). Excessive oxidative stress may result in impairment of intracellular insulin signaling, constituting a potential pathway by which RAAS activation induces insulin resistance (2,3,4). Existent data suggest that angiotensin II (Ang II) and aldosterone signaling through an Ang type 1 receptor (AT1R) or mineralocorticoid receptor (MR), respectively, mediate these detrimental effects on skeletal muscle insulin metabolic signaling and glucose transport (1,2,5). One important mechanism by which activation of AT1R and the MR inhibits insulin metabolic signaling is through activation of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymatic complex (1,2,6). Resulting increases in ROS can activate redox sensitive serine (Ser) kinases which, in turn, can decrease insulin metabolic signaling (4,5).
RAAS activation involves production of preprorenin, followed by glycosylation and removal of a signal peptide, to form prorenin and finally renin (7). Ang I results from cleavage of angiotensinogen by renin, and Ang II is produced by the action of the angiotensin converting enzyme on Ang I. Increase in Ang II , in turn, stimulates aldosterone production (7). Renin is the rate-limiting enzyme in the generation of Ang II (8), making it a particularly attractive therapeutic target for specific RAAS blockade at an early stage.
Aliskiren is a potent direct renin inhibitor, with high specificity for human and mouse renin (9). Because of this species specificity, aliskiren cannot be studied effectively in conventional rat models. To circumvent this issue, we used the transgenic TG(mRen2)27 rat (Ren2), which harbors the mouse renin gene and is a model of excessive tissue renin-angiotensin system (RAS) activity and high plasma levels of aldosterone (5,6). This transgenic rodent model also develops hypertension and systemic insulin resistance (2,10,11,12). Use of the Ren2 rat allows for interrogation of the specific role of the RAAS because it contributes to hypertension and insulin resistance. We previously reported that AT1R, MR blockade and ROS scavenging treatment strategies improve whole-body glucose tolerance and skeletal muscle insulin-stimulated glucose transport in the Ren2 rodent model (1,2,5).
Accordingly, in this investigation, we hypothesized that in vivo direct renin inhibition would correct the skeletal muscle RAAS abnormalities and attenuate tissue oxidative stress, thereby improving insulin metabolic signaling, glucose transport, and systemic insulin sensitivity in young insulin-resistant Ren2 rats.
Materials and Methods
Animals and treatments
All animal procedures were approved by the University of Missouri animal care and use committees and housed in accordance with National Institutes of Health guidelines. Transgenic Ren2 rats (6–9 wk of age) and age-matched Sprague Dawley (SD) littermates were randomly assigned to untreated (Ren2-C and SD-C, respectively) (n = 6 each) or aliskiren-treated (Ren2-A and SD-A) (n = 6 each) paradigms. Ren2-A and SD-A animals were treated with aliskiren 50 mg/kg · d (ip injection) for 21 d.
Systolic blood pressure (SBP)
Restraint conditioning was initiated before blood pressure measurements. SBP was measured in triplicate on separate occasions throughout the day, using the tail-cuff method (Student oscillometric recorder; Harvard Systems, Holliston, MA) before initiation of treatment and on d 19 or 20 before the animals were killed at 21 d.
Intraperitoneal glucose tolerance test (IPGTT)
Animals were fasted overnight, weighed, and anesthetized with Nembutal (35 mg/kg ip). IPGTT was performed as previously described (5). The insulin resistance index was calculated as the product of areas under the glucose and insulin curves (AUCGlucose × AUCInsulin) (13): n = 4 for SD-C; n = 4 for SD-A; n = 3 for Ren2-C; n = 5 for Ren2-A.
Glucose transport
2-Deoxyglucose (2-DOG) transport was measured in isolated soleus strips incubated in the presence or absence of a maximally effective dose of insulin (100 nm) as previously described (2,12). Briefly, soleus muscles were dissected into longitudinal strips and incubated for 45 min in preincubation buffer. After additional incubation for 15 min with or without insulin, strips were transferred to a rinse buffer for 10 min. Thereafter individual strips were transferred to flasks containing oxygenated incubation buffer with or without insulin for 20 min. Samples were analyzed with a scintillation counter (Beckman, Palo Alto, CA) set for dual channel detection (3H and 14C): n = 6 for SD-C; n = 6 for SD-A; n = 4 for Ren2-C; n = 5 for Ren2-A.
Quantification of insulin receptor substrate (IRS)-1, Akt, and glucose transporter 4 (GLUT-4)
Four-micrometer soleus cross-sections (n = 6 for all groups) were incubated overnight with rabbit anti-IRS-1 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), anti-pIRS1 [Tyr941; 1:50 (phosphatidylinositol 3-kinase binding site); Upstate Biotechnology, Inc., Lake Placid, NY]; rabbit anti-Akt (1:50; Cell Signaling Technology, Beverly, MA); anti-Akt (Thr308 1:50; Cell Signaling Technology); and mouse anti-GLUT-4 (1:100; Santa Cruz). After washing several times, the sections were incubated with Alexa-fluor donkey antirabbit 647 for IRS-1 and Akt and donkey antimouse for GLUT-4, washed again, stained with 1:2000 4′,6′-diamino-2-phenylindole (DAPI) for 15 min, and mounted with mowiol. Under a biphoton confocal microscope, images were captured with laser confocal microscopy (LSM) imaging system and the signal intensities were analyzed with MetaVue (5).
Markers of oxidative stress
NADPH oxidase activity
NADPH oxidase activity was determined in plasma membrane fractions as previously described (1,6,14,15). Aliquots of soleus muscle membrane and cytosolic fractions (12.5–100 mg proteins) were incubated with NADPH (100 mm) at 37 C. NADPH oxidase activity was determined by measuring the conversion of radical detector (Cayman Chemical, Ann Arbor, MI) in the absence and presence of NADPH inhibitor diphenylene iodonium sulfate (500 μm) using spectrophotometric (450 nm) techniques (n = 4 for SD-C; n = 4 for SD-A; n = 3 for Ren2-C; n = 3 for Ren2-A).
NADPH oxidase subunit immunostaining
Harvested soleus muscle tissue was prepared as previously described (5). Sections were incubated with 1:100 dilution of anti-p22phox, p47phox, Nox2 (Santa Cruz), and 1:200 of anti-Rac1 (Upstate) antibodies in 10-fold diluted blocking agent (primary antibodies) overnight. After washing, sections were incubated with 1:300 Alexa-fluor rabbit antigoat 647 (Molecular Probes, Eugene OR) for p22, p47 Nox2, and Rac1 for 4 h. The slides were washed and incubated with 1:2000 DAPI for 10 min and mounted with mowiol. The slides were examined under a biphoton confocal microscope (LSM, 510 MLO; Zeiss, Thornwood, NY), and the images were captured with a LSM imaging system, and the signal intensities were analyzed with MetaVue (n = 4 for SD-C; n = 4 for SD-A; n = 3 for Ren2-C; n = 3 for Ren2-A).
3-Nitrotyrosine immunostaining
3-Nitrotyrosine was quantified as previously described (14,16). Samples were incubated with 1:150 primary rabbit polyclonal antinitrotyrosine antibody overnight (Chemicon, Temecula, CA). Sections were then washed and incubated with secondary antibodies, linked, and labeled with Strepavidin for 30 min each. After several rinses with distilled water, diaminobenzidine was applied for 10 min, sections again rinsed and stained with hematoxylin for 30 sec, rehydrated, and mounted with a permanent media. The slides were inspected under a bright-field (50i; Nikon, Tokyo, Japan) microscope and the ×40 images captured with a cool snapcf camera and intensities measured with MetaView (Boyce Scientific Inc., Gary Summit, MO; n = 4 for SD-C; n = 4 for SD-A; n = 3 for Ren2-C; n = 3 for Ren2-A).
Rho kinase activity assay
A commercially available, ELISA-based RhoA activity assay (G-LISA; Cytoskeleton, Denver, CO) was used to measure relative RhoA activity in soleus muscle after experimental treatments. Tissue lysates were processed as per the manufacturer protocol and then incubated in microwells to which the rhotekin binding domain peptide was bound. Active RhoA was detected using indirect immunodetection followed by a colorimetric reaction measured by absorbance at 490 nm (n = 4 for SD-C; n = 4 for SD-A; n = 3 for Ren2-C; n = 3 for Ren2-A).
Immunohistochemical quantification of Ang II, AT1R, and MR
Four-micrometer sections of soleus muscle (n = 6 for all groups) were dewaxed in CitriSolv (Fisher Scientific, Pittsburgh, PA) and rehydrated in ethanol series and HEPES wash buffer. The epitopes were retrieved in citrate buffer (pH 6) at 95 C for 25 min. Nonspecific binding sites were blocked by goat blocker for 4 h. Sections were incubated overnight with goat polyclonal Ang II (1:100) (Santa Cruz Biotechnology), rabbit polyclonal AT1R (1:100) (Santa Cruz Biotechnology), and mouse monoclonal rMR-365 (1:20). Later, sections were washed (3 × 15 min) and incubated with Alexa-fluor 647 donkey antigoat, antirabbit, and antimouse, respectively (Invitrogen, Eugene, OR) for 4 h. After washing thoroughly, the sections were incubated with 1:2000 DAPI for 15 min. Finally, sections were washed and mounted with mowiol. The slides were checked under a biphoton confocal microscope and the images were captured with an LSM imaging system, and the signal intensities were analyzed with MetaVue.
Western blot analysis
Western blots were performed as previously described (1,2) under denaturing condition (SDS-PAGE) using the Movex mini-cell (Invitrogen, Carlsbad, CA) (n = 3 for all groups). Briefly, 4 and 9% of acrylamide were used for stacking and resolving gels, respectively. Eighteen micrograms of total protein of cytosolic and plasma membrane fractions of gastrocnemius homogenates of different groups of animals were loaded in each well of 15-well gels and run at 120 MA for 1.5 h. Proteins were then transferred from the gels to a nitrocellulose membrane by using wet transfer system. Nonspecific sites were blocked with 1% nonfat dry milk for 1 h, and then the blocks were incubated with 1:500 anti AT1R (Santa Cruz) and 1:200 anti rMR-365 overnight (courtesy Dr. Celso Gomez-Sanchez, University of Mississippi at Jackson, Jackson, MS). After washing, the membranes were incubated with 1:10,000 of goat antirabbit horseradish peroxidase for AT1R and goat antimouse horseradish peroxidase for MR for 1 h (secondary antibodies). After washing, visualization was accomplished using Super Signal West Pico (Pierce and Warriner, Chester, UK) chemiluminescent as a substrate. Signal intensities were measured using the Fluor-S multi-Imager system with Quantity One Software (Bio-Rad Laboratories, Hercules, CA).
Light microscopic quantification of perivascular fibrosis
To evaluate perivascular fibrosis, fixed paraffin sections of soleus (n = 6 for all groups) from different treatments were evaluated with Verhoeff-van Gieson staining. Slides were analyzed with a Nikon 50i microscope and ×4, ×10, and ×40 magnification; images were captured with a cool snapcf. camera. Morphometric analysis was performed using MetaVue software (5,6).
Transmission electron microscopy (TEM)
Sections of soleus muscle were thinly sliced and placed in primary fixative [2% glutaraldehyde, 2% paraformaldehyde in 0.1 m Na cadylate buffer (pH 7.35)] and then in osmium tetroxide as previously described (1) (n = 4 for all groups). The ultrathin sections on grids were then stained with 5% uranyl acetate and Sato’s triple lead stain. A TEM (1200-EX; Jeol Ltd., Tokyo, Japan) was used to view and capture images for ultrastructural studies.
Mitochondrial quantification
The harvested soleus tissue (n = 6 for all groups) was prepared as previously described (16). Soleus tissue of four animals from each treatment group was used for analysis. Sections were incubated with mouse antihuman complex IV subunit 1 antibody (Mitosciences, Eugene, OR) 3 μg/ ml in 10-fold diluted blocker. After washing with HEPES wash buffer, the sections were incubated with 1:300 goat-antimouse Alexa-fluor 647 (Invitrogen). After 4 h, the sections were washed and incubated with 1:2000 DAPI. After 10 min, slides were washed and mounted with mowiol. The slides were checked with a multiphoton confocal system (Carl Zeiss), images were captured by LSM imaging system, enhanced with Photoshop (Adobe Systems, San Jose, CA), and mitochondria quantified by MetaMorph (Molecular Devices, Downington, PA).
Statistics
All results are presented as means ± se. ANOVA with Fisher’s least significant differences and unpaired t test was performed as appropriate.
Results
SBP
At initiation and end of treatment, SBPs were higher in Ren2 compared with SD controls. At the end of the treatment period there was a significant increase in SBP in Ren2 compared with the beginning. Treatment with aliskiren resulted in SBP reduction in the Ren2 compared with the controls as previously reported (16).
Systemic insulin sensitivity
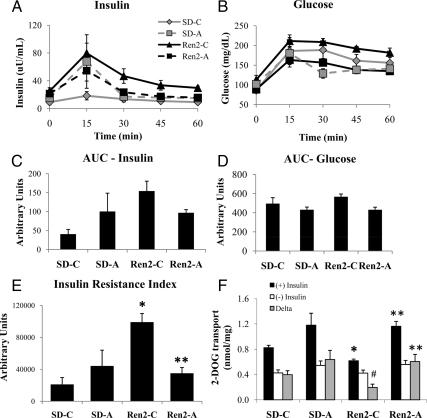
As a measure of insulin resistance (2), the insulin resistance index was increased in Ren2 compared with SD controls (99.1 × 103 ± 10790.2 vs. 21.2 × 103 ± 8591.5 arbitrary units, P < 0.05). In vivo treatment with aliskiren improved whole-body insulin resistance in Ren2 (35.6 × 103 ± 72.6 × 103 arbitrary units, P < 0.05) compared with untreated Ren2 rats. Furthermore, the insulin resistance index in aliskiren-treated Ren2 was similar to treated SD rats (35.1 × 103 ± 72.6 × 103 vs. 44.2 × 103 ± 19.8 × 103 arbitrary units, P > 0.05) (Fig. 1, A–C).
Figure 1.
Direct renin inhibition improves systemic insulin resistance and improves insulin-stimulated glucose uptake in the Ren2 rat. Insulin sensitivity was measured during an IPGTT performed after overnight fast on d 21. Samples for serum insulin (A) and glucose (B) were obtained and AUCs were calculated (C and D, respectively). The insulin resistance index (E) was calculated as the product of the AUC for glucose and insulin. Values presented as means ± se. *, P < 0.05 compared with SD-C; **, P < 0.05 when aliskiren-treated Ren2 (Ren2-A) are compared with Ren2 controls (Ren2-C). F, Insulin-stimulated 2-DOG uptake analyzed in ex vivo soleus muscle strips in the absence and presence of a maximally effective dose of insulin (100 nm). Values presented as means ± se. *, P < 0.05 compared with SD-C; #, P = 0.06 when compared with SD-C; **, P < 0.05 when Ren2-A are compared with Ren2-C.
Skeletal muscle insulin-stimulated glucose uptake
Skeletal muscle 2-DOG uptake was measured in the absence and presence of maximally effective doses of insulin (Fig. 1D). In response to insulin, 2-DOG uptake was decreased in untreated Ren2 rats relative to skeletal muscle from SD controls (0.62 ± 0.03 vs. 0.82 ± 0.04 mmol/mg per 20 min, P < 0.05). In vivo renin inhibition improved insulin-stimulated glucose uptake when compared with placebo-treated Ren2 animals (0.95 ± 0.08 mmol/mg per 20 min, P < 0.05). Insulin-stimulated incremental (δ) glucose uptake in untreated Ren2 was lower compared with untreated SD animals (δ: 0.20 ± 0.05 vs. 0.40 ± 0.06 mmol/mg per 20 min muscle, P = 0.06). Treatment with aliskiren in Ren2 rats increased insulin-stimulated glucose uptake (δ: 0.51 ± 0.10 mmol/mg muscle per 20 min, P < 0.05) relative to untreated Ren2 controls (Fig. 1D).
Skeletal muscle IRS-1, Akt, and GLUT-4 immunostaining
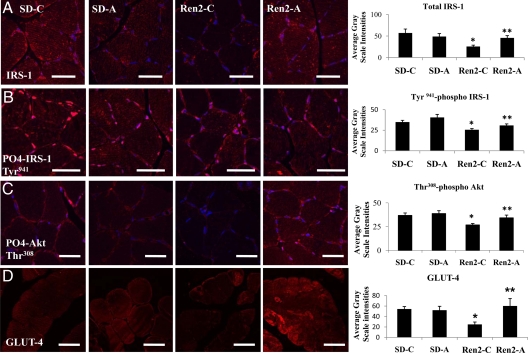
Total and Tyr941 phosphorylated IRS-1 was reduced in Ren2 (25.5 ± 2.0 and 25.6 ± 1.5 average gray-scale intensities, respectively) compared with SD controls (57.0 ± 5.3 and 34.9 ± 2.2 average gray-scale intensities, respectively; P < 0.05). There were substantial increases in total and Tyr941-phosphorylated IRS-1 in the treated Ren2 (45.6 ± 3.0 and 30.8 ± 1.9 average gray-scale intensities, respectively; P < 0.05) (Fig. 2, A and B). Similarly, there were reductions in total and Thr308 phosphorylated Akt in Ren2 (16.4 ± 0.8 and 26.7 ± 1.5 average gray-scale intensities, respectively) compared with SD controls (27.9 ± 0.9 and 3.8 ± 2.6 average gray-scale intensities respectively, P < 0.05). After treatment, Thr308 phosphorylated Akt increased relative to Ren2 controls (34.2 ± 2.9 average gray-scale intensity, P < 0.05) (Fig. 2C). GLUT-4 immunostaining was reduced in the Ren2 compared with SD controls (24.9 ± 4.6 vs. 54.3 ± 4.7 average gray-scale intensities, P < 0.05) and was increased in the aliskiren-treated Ren2 (60.2 ± 14.2 average gray-scale intensity, P < 0.05) (Fig. 2D).
Figure 2.
Direct renin inhibition improves IRS-1, Akt, and GLUT-4 in soleus muscle of the Ren2 rat. A, Representative fluorescent images of total IRS-1 and quantification of converted signal intensities to the right. B, Representative fluorescent images of Tyr-phosphorylated (Tyr941) IRS-1 and quantification of converted signal intensities. C, Representative fluorescent images of threonine (Thr30)-phosphorylated Akt and quantification of converted signal intensities to the right. D, Representative fluorescent images of GLUT-4 and quantification of converted signal intensities to the right. Values presented as means ± se. *, P < 0.05 compared with SD-C; **, P < 0.05 when aliskiren-treated Ren2 (Ren2-A) are compared with Ren2 controls (Ren2-C). Scale bar, 50 μm.
Skeletal muscle oxidative stress
NADPH oxidase
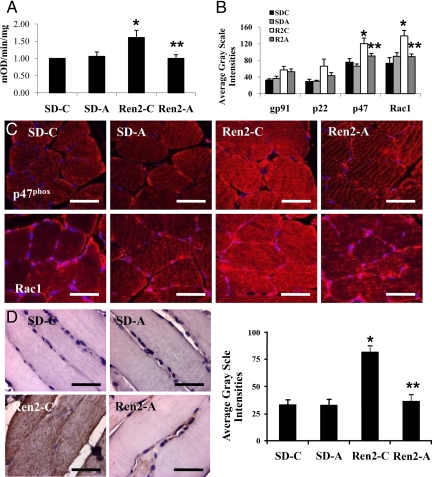
NADPH oxidase activity was elevated in the Ren2 compared with SD controls (6.3 ± 0.2 vs. 4.1 ± 0.3 mOD/mg · min, P < 0.05) and decreased in the aliskiren-treated Ren2 animals (4.8 ± 0.7 mOD/mg · min, P < 0.05) (Fig. 3A). Analysis of NADPH oxidase subunits demonstrated increased p47phox and Rac1 immunostaining in Ren2 animals, which were significantly reduced by aliskiren treatment (120.0 ± 13.9 vs. 90.5 ± 6.0, and 138.8 ± 13.3 vs. 89.0 ± 6.5 average gray-scale intensities, respectively, P < 0.05) (Fig. 3, B and C).
Figure 3.
Direct renin inhibition reduces NADPH oxidase activity and subunits in soleus muscle of the Ren2 rat. A, Total NADPH oxidase activity. B, Measures of intensities of immunohistochemical analysis of NADPH oxidase subunits as depicted by representative images in C. D, 3-Nitrotyrosine immunostaining and corresponding measures of intensity. Values presented as means ± se. *, P < 0.05 compared with SD-C; **, P < 0.05 when aliskiren-treated Ren2 (Ren2-A) are compared with Ren2 controls (Ren2-C). Scale bar, 50 μm.
Reactive nitrogen species as a marker of peroxynitrite formation
3-Nitrotyrosine immunostaining was increased in Ren2 compared with SD controls (81.7 ± 5.9 vs. 32.2 ± 5.2 average gray-scale intensities, P < 0.05). Treatment with aliskiren reduced 3-nitrotyrosine immunostaining in Ren2 rats (36.2 ± 6.1 average gray-scale intensity, P < 0.05) (Fig. 3D).
Redox-sensitive ROK activity
Redox-sensitive ROK activity was evaluated via RhoA activation. There were increases in ROK in Ren2 compared with SD controls (0.871 ± 0.07 vs. 0.690 ± 0.104 μg/ml). Treatment with aliskiren did not significantly reduce RhoA activity (0.91 ± 0.05 μg/ml).
Skeletal muscle Ang II, AT1R, and MR immunostaining
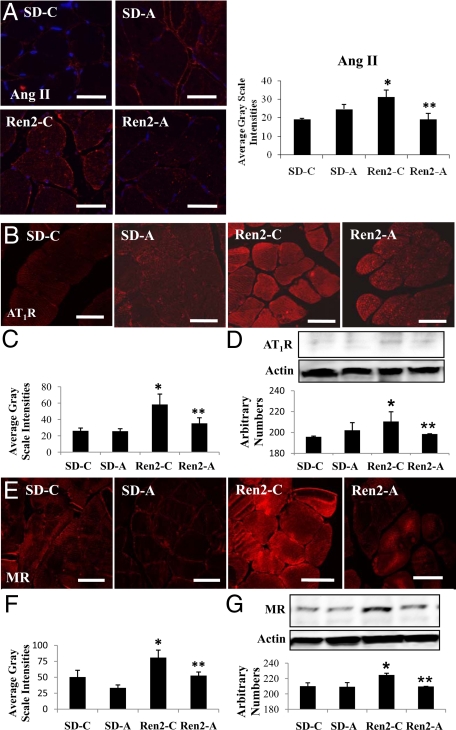
Ang II immunostaining was increased in Ren2 controls (31.9 ± 2.2 average gray-scale intensities, P < 0.05) compared with SD controls and aliskiren-treated SD (19.3 ± 0.4 vs. 24.7 ± 1.6 average gray-scale intensities, respectively; P > 0.05). Aliskiren treatment reduced Ang II immunostaining (19.2 ± 1.8 average gray-scale intensities, P < 0.05) (Fig. 4A). In corollary, there were increases in AT1R by immunostaining and Western blot in untreated Ren2 compared with SD controls, reduced with aliskiren treatment (Fig. 4, B–D). A parallel trend was observed for MR immunostaining and Western blot analysis (Fig. 4, E–G).
Figure 4.
Direct renin inhibition attenuates RAS activation in soleus muscle of the Ren2 rat. A, Representative images of immunohistochemistry analysis of Ang II in soleus and corresponding measures of intensities to the right. B, Representative images of immunohistochemistry analysis for AT1R. C, Corresponding measures of intensities below. D, Western blot of AT1R with corresponding densitometry analysis below. E, Representative images of immunohistochemistry analysis for MR and corresponding measures of intensities below (F). G, Western blot of MR with corresponding densitometry analysis below. Values presented as means ± se. *, P < 0.05 compared with Sprague Dawley controls (SD-C); **, P < 0.05 when aliskiren-treated Ren2 (Ren2-A) are compared with Ren2 controls (Ren2-C). Scale bar, 50 μm.
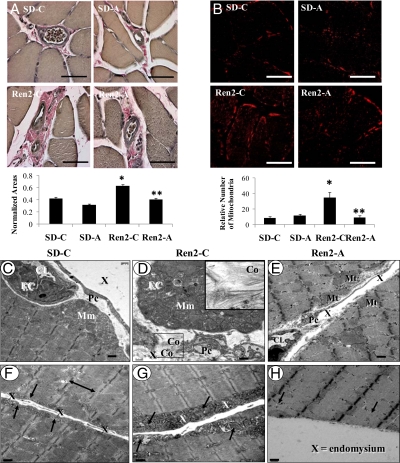
Skeletal muscle remodeling: fibrosis and mitochondrial changes
On light microscopy, perivascular fibrosis was apparent in untreated Ren2 animals compared with SD controls (0.63 ± 0.02 vs. 0.43 ± 0.02 normalized areas, P < 0.05) and improved after treatment with aliskiren (0.41 ± 0.02 normalized area, P < 0.05) (Fig. 5A). TEM analysis corroborated these findings and demonstrated pericapillary fibrosis with significant increases in mitochondria. The Ren2 tissue displayed early organized collagen deposition adjacent to capillary endothelial cells and pericyte foot processes within the interstitial endomysium (Fig. 5, D and G). These changes were not found in the aliskiren-treated Ren2 rats (Fig. 5, E and H). On immunohistochemical analysis, the number of mitochondria was higher in Ren2 animals relative to SD controls (34.93 ± 7.01 vs. 3.75 ± 1.68 relative number of mitochondria, P < 0.05) and decreased with aliskiren treatment (4.46 ± 1.99 relative number of mitochondria, P < 0.05) (Fig. 5B). TEM corroborated increased mitochondrial mounding interdigitating subsarcolemmal mitochondria in the Ren2 (Fig. 5, D and G), not observed in the aliskiren-treated Ren2 (Fig. 5, E and H).
Figure 5.
Direct renin inhibition attenuates fibrosis and ultrastructural mitochondrial abnormalities in the Ren2 rat. A, Representative images of Verhoeff-von Gieson (VVG) staining of soleus to demonstrate interstitial perivascular fibrosis and corresponding measures of area. B, Immunohistochemistry for number of mitochondria and measures of intensities below. Values presented as means ± se. *, P < 0.05 compared with SD-C; **, P < 0.05 when aliskiren-treated Ren2 (Ren2-A) are compared with Ren2 controls (Ren2-C). Scale bar, 50 μm. C, Representative TEM images of typical mitochondrial mounding (Mm) that is present in the vicinity of interstitial endomysial capillaries found in the soleus in the SD-C. Note the endothelial cell (EC) surrounding the capillary lumen (CL) and the pericyte foot process (Pc) traversing the endomysium (X). D, Marked increased in interdigitating mitochondria found within the Mm in the Ren2-C. Note the organized early collagen deposition (Co) adjacent to a capillary EC within the X and Pc. Insert (i), Exploded image of the early organized collagen fibrils indicative of early pericapillary fibrosis found in the Ren2. E, Abrogation of the pericapillary Mm found in the Ren2-A. Note the absence of the Mm in the vicinity of the interstitial endomysial capillary (CL) and the marked decrease in subsarcolemmal mitochondria (Mt). Also note the long connecting Pc traversing the interstitial X. F, Subsarcolemmal mitochondria (arrows) in the SD-C on either side of the interstitial X between two soleus skeletal muscle myocytes. Sarcomeres are bounded by Z line (double arrows). G, Marked increased in subsarcolemmal Mt (arrows) in the Ren2 untreated rat model. Note the marked increase in subsarcolemmal Mt compared with A and B. H, Representative image of the Ren2-A. Note the abrogation of the subsarcolemmal Mt (arrows). Magnification, ×2500. Scale bar, 0.5 μm.
Discussion
This investigation explored the effect of direct renin inhibition on insulin-stimulated glucose transport in skeletal muscle in a rodent model of increased tissue RAS activation, elevated plasma levels of aldosterone, and enhanced oxidative stress. We observed that young transgenic rats overexpressing the mouse renin gene are insulin resistant compared with age-matched SD littermates, an observation consistent with previous studies (2,5,13,16). In concert with systemic insulin resistance, there were increases in skeletal muscle tissue Ang II, AT1R, and MR receptor levels as well as NADPH oxidase-mediated increases in ROS. These alterations in tissue RAAS were accompanied by reductions in tyrosine (Tyr)-phosphorylation of IRS-1, Akt phosphorylation, GLUT-4 expression, and insulin-stimulated glucose transport. Insulin metabolic signaling and glucose transport in Ren2 skeletal muscle were improved by in vivo treatment with a direct renin inhibitor, as previously observed with treatment with an AT1R and MR blockade (2,5). This investigation further demonstrated that this improvement in insulin mediated glucose transport in skeletal muscle was accompanied by increases in tissue IRS-1, Tyr phosphorylation of IRS-1, and enhanced Akt phosphorylation and GLUT 4 expression.
Our observations are consistent with the notion that accentuated muscle RAAS in the Ren2 model leads to increased NADPH oxidase activity/ROS production and activation of redox-sensitive Ser kinases. These kinases, which can induce Ser and reduce Tyr phosphorylation of IRS-1 (4,17,18), have been reported to be activated by Ang II and aldosterone and to be activated by increases in NADPH generation of ROS (19,20,21,22,23). Tyr phosphorylation of IRS-1 is critical for insulin metabolic signaling. In contrast, Ser IRS-1 phosphorylation results in either proteosomal degradation or conformational changes that lead to reduced engagement with phosphoinositol kinase and downstream phosphorylation/activation of Akt (3). In the current study, total IRS-1, Tyr941 IRS phosphorylation, Thr308 Akt phosphorylation/activation, and expression of GLUT-4 were diminished in Ren 2 skeletal muscle, and this abnormality was corrected after in vivo treatment with the direct renin inhibitor, aliskerin.
Our observations suggest that the RAAS in skeletal muscle tissue is dependent on renin activation in this tissue. Renin is the rate-limiting enzyme in tissue RAAS activation, and the fact that angiotensinogen is its only naturally occurring substrate allows for specific inhibition (16). A recently described active tissue renin receptor could conceptually increase the efficiency of angiotensinogen conversion to angiotensin I (24). Activation of this tissue receptor could further contribute to the observed abnormalities in the Ren2 skeletal muscle and contributes to insulin resistance as well as cardiovascular and kidney diseases (25). Aliskiren blocks the activation of RAS at the initial rate-limiting step of renin activation (26). In this context, a potential benefit of blocking renin activation would be to reduce renin binding to renin/prorenin receptors, which has been shown to have pathological consequences in other tissues (9,27,28).
To the best of our knowledge, this is the first investigation demonstrating a beneficial effect of renin inhibition on skeletal muscle insulin metabolic signaling and glucose uptake. These data complement our recent report that renin inhibition improves pancreas β-cell NADPH oxidase activity, IRS-1, IRS-2, and Akt as well as abnormal β-cell insulin content and whole-body insulin resistance (16), Collectively, both bodies of work suggest an impact of overactive local RAAS on not only glucose metabolism through effects on insulin production/secretion and insulin metabolic signaling but also a potential role for renin inhibition in improving systemic insulin sensitivity and glucose metabolism via decreases in tissue oxidative stress.
Increased oxidative stress links RAAS activation and impaired insulin signaling (22). Analysis of NADPH oxidase subunits demonstrated increased p47phox and Rac1 immunostaining in Ren2 animals, which were significantly reduced by aliskiren treatment. The resulting increase in ROS can contribute to reduced muscle insulin metabolic signaling by several mechanisms including the activation of redox-sensitive Ser kinases. Results of the current investigation complement prior work demonstrating that in vivo treatment of young Ren2 rats with a ROS scavenger, AT1R blockade, and MR antagonist not only decreased skeletal muscle NADPH oxidase activity and ROS generation but also improved systemic and skeletal muscle insulin sensitivity (5).
The existing results confirm previous observations of increased RAS activity in skeletal muscle of untreated Ren2 animals, including increased Ang II and AT1R levels (2,5), which in our experiments were improved by renin inhibition. However, the demonstration of MR expression in skeletal muscle of the Ren2 is another novel finding of our study. In this regard, increased tissue Ang II signaling through AT1R has previously been shown to increase MR expression in the heart (14,22,29). These observations support an important role for MR in the pathogenesis of insulin resistance in the setting of increased skeletal muscle RAS activity and oxidative stress. This adds to previous observations from our laboratory that in vivo blockade of MR resulted in improved insulin-stimulated glucose transport and signaling as well as systemic insulin resistance in the Ren2 rat (5). Collectively these data suggest the existence of a positive feedback mechanism of RAS activity and MR expression (30).
Similar to previous studies, several structural abnormalities were observed by light and TEM in Ren2 skeletal muscle, including early perivascular fibrosis and increased number and structural abnormalities of subsarcolemmal mitochondria (5). Similar observations have been reported in insulin-resistant humans, in whom collagen deposition is increased in skeletal muscle (31). Comparable fibrotic changes have been described recently in pancreas (16), skeletal muscle (32), and cardiovascular tissue of insulin-resistant rodents (33) and were improved after RAAS blockade, supporting a role for mitochondrial dysfunction in the pathogenesis of RAAS-mediated insulin resistance.
Our immunohistochemistry and TEM data suggest Ren2 skeletal muscle exhibits increased mitochondrial biogenesis and subsarcolemmal mitochondria structural abnormalities, which were largely corrected by direct renin inhibition. Increased mitochondrial density might result in increased ROS production of ROS via citrate synthase and the electron transport chain (34,35). As opposed to studies in sedentary obese and diabetic patients, our analysis derives from highly physically active and nonobese rodents, which might explain the finding of increased mitochondria biogenesis in the Ren2 animals compared with reports in humans.
In summary, the results of this investigation provide novel findings regarding Ang II, AT1R, and MR expression in skeletal muscle tissue and provides new evidence for a beneficial effect of direct renin inhibition on skeletal muscle insulin metabolic signaling.
Acknowledgments
We thank Dr. Carlos M. Ferrario (Wake Forest University School of Medicine, Winston-Salem, NC) for the male transgenic Ren2 rats and male Sprague Dawley controls kindly provided through the Transgenic Core Facility supported in part by National Institutes of Health Grant P-01 HL-51952. The MR antibody was courteously provided by Drs. Celso and Elise Gomez-Sanchez (University of Mississippi, Oxford, MS). We also acknowledge Lama Appesh for her valuable work in glucose uptake studies.
Footnotes
This work was supported by National Institutes of Health Grants R01 HL73101-01A1 (to J.R.S.) and P01 HL-51952 (to C.F.); Veterans Affairs Merit System 0018 (to J.R.S.) awards and VISN 15 (to A.T.W.-C.); Missouri Kidney Program (to A.T.W.-C.); and Novartis Pharmaceuticals (to J.R.S. and A.T.W.-C.).
Disclosure Summary: J.R.S. and A.T.W.-C. report having received grant funding from Novartis, and J.R.S. reports being on advisory board for Novartis and Forest Laboratories. G.L., J.H., C.M., M.R.H., J.R., K.P., and C.F. have nothing to declare.
First Published Online February 26, 2009
Abbreviations: Ang, Angiotensin; AT1R, Ang type 1 receptor; AUC, area under the curve; DAPI, 4′,6′-diamino-2-phenylindole; 2-DOG, 2-deoxyglucose; GLUT-4, glucose transporter 4; IPGTT, ip glucose tolerance test; IRS, insulin receptor substrate; LSM, laser confocal microscopy; MR, mineralocorticoid receptor; NADPH, nicotinamide adenine dinucleotide phosphate; RAAS, renin-angiotensin-aldosterone system; RAS, renin-angiotensin system; Ren2, TG(mRen2)27 rat; Ren2-A, aliskiren-treated Ren2; Ren2-C, untreated Ren; ROS, reactive oxygen species; SBP, systolic blood pressure; SD, Sprague Dawley; SD-A, aliskiren-treated SD; SD-C, SD control; Ser, serine; TEM, transmission electron microscopy; Tyr, tyrosine.
References
- Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS 2006 Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 281:35137–35146 [DOI] [PubMed] [Google Scholar]
- Blendea MC, Jacobs D, Stump CS, McFarlane SI, Ogrin C, Bahtyiar G, Stas S, Kumar P, Sha Q, Ferrario CM, Sowers JR 2005 Abrogation of oxidative stress improves insulin sensitivity in the Ren-2 rat model of tissue angiotensin II overexpression. Am J Physiol Endocrinol Metab 288:E353–E359 [DOI] [PubMed] [Google Scholar]
- Hitomi H, Kiyomoto H, Nishiyama A, Hara T, Moriwaki K, Kaifu K, Ihara G, Fujita Y, Ugawa T, Kohno M 2007 Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension 50:750–755 [DOI] [PubMed] [Google Scholar]
- Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, Kim JK, Lee SW, Kim YB 2005 Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab 2:119–129 [DOI] [PubMed] [Google Scholar]
- Lastra G, Whaley-Connell A, Manrique C, Habibi J, Gutweiler AA, Appesh L, Hayden MR, Wei Y, Ferrario C, Sowers JR 2008 Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am J Physiol Endocrinol Metab 295:E110–E116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptergrove GM, Clark SE, Stump CS, Ferrario CM, Sowers JR 2007 NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension 50:384–391 [DOI] [PubMed] [Google Scholar]
- Griendling KK, Murphy TJ, Alexander RW 1993 Molecular biology of the renin-angiotensin system. Circulation 87:1816–1828 [DOI] [PubMed] [Google Scholar]
- Blundell T, Sibanda BL, Pearl L 1983 Three-dimensional structure, specificity and catalytic mechanism of renin. Nature 304:273–275 [DOI] [PubMed] [Google Scholar]
- Rahuel J, Rasetti V, Maibaum J, Rueger H, Göschke R, Cohen NC, Stutz S, Cumin F, Fuhrer W, Wood JM, Grütter MG 2000 Structure-based drug design: the discovery of novel nonpeptide orally active inhibitors of human renin. Chem Biol 7:493–504 [DOI] [PubMed] [Google Scholar]
- Rebuffat P, Rocco S, Andreis PG, Neri G, Nowak KW, Peters J, Opocher G, Mazzocchi G, Mantero F, Nussdorfer GG 1995 Morphology and function of the adrenal zona glomerulosa of transgenic rats TGR (mREN2) 27: effects of prolonged sodium restriction. J Steroid Biochem Mol Biol 54:155–162 [DOI] [PubMed] [Google Scholar]
- Sander M, Bader M, Djavidani B, Maser-Gluth C, Vecsei P, Mullins J, Ganten D, Peters J 1992 The role of the adrenal gland in hypertensive transgenic rat TGR(mREN2)27. Endocrinology 131:807–814 [DOI] [PubMed] [Google Scholar]
- Sloniger JA, Saengsirisuwan V, Diehl CJ, Kim JS, Henriksen EJ 2005 Selective angiotensin II receptor antagonism enhances whole-body insulin sensitivity and muscle glucose transport in hypertensive TG(mREN2)27 rats. Metabolism 54:1659–1668 [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M 2001 Selective angiotensin II receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension 38:884–890 [DOI] [PubMed] [Google Scholar]
- Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR 2007 Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology 148:3773–3780 [DOI] [PubMed] [Google Scholar]
- Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump C, Ferrario C, Sowers JR 2007 Angiotensin II-mediated oxidative stress promotes myocardial tissue remodeling in the transgenic (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab 293:E355–E363 [DOI] [PubMed] [Google Scholar]
- Habibi J, Whaley-Connell A, Hayden MR, DeMarco VG, Schneider R, Sowers SD, Karuparthi P, Ferrario CM, Sowers JR 2008 Renin inhibition attenuates insulin resistance, oxidative stress, and pancreatic remodeling in the transgenic Ren2 rat. Endocrinology 149:5643–5653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T, Wakino S, Homma K, Yoshioka K, Tatematsu S, Hasegawa K, Takamatsu I, Sugano N, Hayashi K, Saruta T 2006 Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J 20:169–171 (First Published 2 November 2005; 10.1096/fj.05-4497fje) [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Yamada M, Imakiire T, Kushiyama T, Higashi K, Hyodo N, Yamamoto K, Oda T, Suzuki S, Miura S 2007 A Rho-kinase inhibitor, fasudil, prevents development of diabetes and nephropathy in insulin-resistant diabetic rats. J Endocrinol 192:595–603 [DOI] [PubMed] [Google Scholar]
- Gardner CD, Eguchi S, Reynolds CM, Eguchi K, Frank GD, Motley ED 2003 Hydrogen peroxide inhibits insulin signaling in vascular smooth muscle cells. Exp Biol Med 228:836–842 [DOI] [PubMed] [Google Scholar]
- Hirono Y, Yoshimoto T, Suzuki N, Sugiyama T, Sakurada M, Takai S, Kobayashi N, Shichiri M, Hirata Y 2007 Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system. Endocrinology 148:1688–1696 [DOI] [PubMed] [Google Scholar]
- Jin L, Ying Z, Webb RC 2004 Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol 287:H1495–H1500 [DOI] [PubMed] [Google Scholar]
- Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR 2007 Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 293:H2009–H2023 [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Yoshimoto T, Tsuchiya K, Gochou N, Hirono Y, Tateno T, Fukai N, Shichiri M, Hirata Y 2005 Aldosterone induces angiotensin converting enzyme gene expression via a JAK2-dependent pathway in rat endothelial cells. Endocrinology 146:3900–3906 [DOI] [PubMed] [Google Scholar]
- Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD 2002 Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109:1417–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Zhang Y, Moe G, Naik G, Gilbert RE 2007 Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia 50:2398–2404 [DOI] [PubMed] [Google Scholar]
- Fisher ND, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK 2008 Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation 117:3199–3205 [DOI] [PubMed] [Google Scholar]
- Nguyen G, Danser AHJ 2008 Prorenin and (pro)renin receptor: a review of available data from in vitro studies and experimental models in rodents. Exp Physiol 93:557–563 [DOI] [PubMed] [Google Scholar]
- Jan Danser AH 2006 Prorenin: back into the arena. Hypertension 47:824–826 [DOI] [PubMed] [Google Scholar]
- Carey RM, Siragy HM 2003 Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 24:261–271 [DOI] [PubMed] [Google Scholar]
- de Resende MM, Kauser K, Mill JG 2006 Regulation of cardiac and renal mineralocorticoid receptor expression by captopril following myocardial infarction in rats. Life Sci 78:3066–3073 [DOI] [PubMed] [Google Scholar]
- Berria R, Wang L, Richardson DK, Finlayson J, Belfort R, Pratipanawatr T, De Filippis EA, Kashyap S, Mandarino LJ 2006 Increased collagen content in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab 290:E560–E565 [DOI] [PubMed] [Google Scholar]
- Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J 2008 Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med 36:1548–1554 [DOI] [PubMed] [Google Scholar]
- Zaman AK, Fujii S, Goto D, Furumoto T, Mishima T, Nakai Y, Dong J, Imagawa S, Sobel BE, Kitabatake A 2004 Salutary effects of attenuation of angiotensin II on coronary perivascular fibrosis associated with insulin resistance and obesity. J Mol Cell Cardiol 37:525–535 [DOI] [PubMed] [Google Scholar]
- Trask AJ, Jessup JA, Chappell MC, Ferrario CM 2008 Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol 294:H2242–H2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H 2003 Glucose toxicity in (2)-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 52:581–587 [DOI] [PubMed] [Google Scholar]