Abstract
Adipocyte insulin resistance can be caused by proximal insulin signaling defects but also from postreceptor mechanisms, which in large are poorly characterized. Adipocytes exposed for 18 h to the HIV protease inhibitor nelfinavir manifest insulin resistance characterized by normal insulin-stimulated tyrosine phosphorylation of the insulin receptor and insulin receptor substrate proteins, preserved in vitro phosphatidylinositol 3-kinase (PI 3-kinase) assay activity but impaired activation of PKB/Akt and stimulation of glucose uptake. Here we aimed to assess whether impaired PKB/Akt activation is indeed rate limiting for insulin signaling propagation in response to nelfinavir and the mechanism for defective PKB/Akt activation. Nelfinavir treatment of 3T3-L1 adipocytes impaired the insulin-stimulated translocation and membrane fusion of myc-glucose transporter (GLUT)-4-green fluorescent protein (GFP) reporter. Phosphorylation of PKB/Akt substrates including glycogen synthase kinase-3 and AS160 decreased in response to nelfinavir, and this remained true, even in cells with forced generation of phosphatidylinositol-3,4,5-trisphohphate (PIP3) by a membrane-targeted active PI 3-kinase, confirming that impaired PKB/Akt activation was rate limiting for insulin signal propagation. Cells expressing a GFP-tagged pleckstrin homology domain of general receptors for phosphoinositides 1, which binds PIP3, revealed intact PIP3-mediated plasma membrane translocation of this reporter in nelfinavir-treated cells. However, expression of a membrane-targeted catalytic subunit of PI 3-kinase failed to induce myc-GLUT4-GFP translocation in the absence of insulin, as it did in control cells. Conversely, a membrane-targeted and constitutively active PKB/Akt mutant was normally phosphorylated on S473 and T308, confirming intact PKB/Akt kinases activity, and induced myc-GLUT4-GFP translocation. Collectively, nelfinavir uncovers a postreceptor mechanism for insulin resistance, caused by interference with the sensing of PIP3 by PKB/Akt, leading to impaired GLUT4 translocation and membrane fusion.
Insensitivity of Akt/PKB to PI-3,4,5-P3 constitutes a mechanism for adipocyte insulin resistance, demonstrating Akt/PKB activation as a rate-limiting step in signal propagation towards GLUT4 translocation.
Despite substantial progress in our understanding of insulin signaling, the molecular basis for insulin resistance in adipocytes, a condition present in various common conditions like obesity and diabetes, still remains enigmatic. Impairment in early insulin signaling events, like the tyrosine phosphorylation of the insulin receptor and of insulin receptor substrates (IRS), has been documented both in vivo and in various experimental models (1,2,3,4). Yet it remains unclear whether defective early insulin signaling constitutes the rate-limiting step for the downstream propagation of the insulin-initiated signal. Furthermore, various inducers of insulin resistance, including ceramide (5,6), oxidative stress (7), and GH (8), do so without affecting insulin receptor activation, suggesting that signaling step(s) more distal to the insulin receptor and its immediate substrates are the functional break points in insulin action. One such candidate is the propagation of the signal from phosphatidylinositol 3-kinase (PI 3-kinase) to PKB/Akt, a process consisting of several events, including the generation of the major PI 3-kinase product phosphatidylinositol-3,4,5-trisphosphate (PIP3), the recruitment of PKB/Akt and its upstream kinase phosphoinosotide-dependent kinase (PDK)-1 to the plasma membrane through their pleckstrin homology (PH) domains, and a dual phosphorylation of PKB/Akt on Thr308 by PDK1 and Ser473 by the mammalian target of rapamycin (mTOR)-signaling complex 2 (9,10,11,12). Indeed, impaired insulin-stimulated PKB/Akt phosphorylation has been noted in insulin-resistant adipocytes, whether or not upstream signaling defects are present (1,13). Given that activation of PKB/Akt is likely a required step for most of insulin’s key actions (9), it is possible that insulin-induced PKB/Akt activation constitutes a critical integration nexus in the insulin signaling cascade. Several molecular mechanisms to explain impaired PKB/Akt activation despite normal activation of PI 3-kinase (as assessed in an in vitro kinase assay) have been proposed. They include up-regulated activity of protein phosphatase 2A (PP2A; a major PKB/Akt phosphatase) (14), defective subcellular localization of PI 3-kinase and/or PKB/Akt (13,15), impaired in vivo PIP3 generation despite normal in vitro kinase activity (16), phosphorylation of PKB/Akt on its PH domain by protein kinase C (PKC)-ξ (17,18), and complex formation between PKB/Akt and Drosophila Tribbles homologue protein 3 (TRB3) (19).
Despite all of the above, several studies questioned the possibility that PKB/Akt is indeed the reason for impaired insulin action in insulin resistance conditions (20). Even when its activity is diminished, a large fractional inhibition of PKB/Akt activity (>80%) was required to attenuate glucose transporter (GLUT)-4 translocation in muscle cells (21). Because most inducers of insulin resistance impair PKB/Akt activation to a lesser degree, it remains possible that under some circumstances other signaling steps constitute the rate-limiting step(s) in insulin action (22).
Nelfinavir is an HIV protease inhibitor used as part of the antiretroviral chemotherapeutic cocktail. Insulin resistance and accompanying diabetes and accelerated atherosclerosis have been reported as serious side effects of this drug and other members of this class of protease inhibitors (23). Nelfinavir-induced insulin resistance has been successfully recapitulated in cellular adipocyte models (24,25,26,27). These studies demonstrated that although HIV protease inhibitors acutely inhibit GLUT4 function without affecting insulin signaling (26,28,29,30), when adipocytes are exposed to nelfinavir for 18 h, an insulin signaling defect is induced characterized by normal proximal signaling events, intact insulin-stimulated activation of PI 3-kinase (as measured by an in vitro kinase assay), but with impaired phosphorylation on both Ser and Thr residues of PKB/Akt and a resulting defective activation of the enzyme. A previous study ruled out increased PP2A activity or elevated expression of the PIP3 phosphatases [phosphatase and tensin homolog deleted from chromosome 10 (PTEN) and SH2-containing inositol 5′-phosphate 2 (SHIP2) as underling mechanism (25), leaving the mechanism for defective PI 3-kinase to PKB/Akt signal transmission unresolved. Here we undertook to investigate the molecular mechanisms for nelfinavir-induced impairment in PKB/Akt activation, and to establish that defective PKB/Akt sensing of insulin-stimulated generation of PIP3 underlies the impaired ability of insulin to recruit GLUT4 to the plasma membrane.
Materials and Methods
Materials and reagents
Tissue culture medium, serum, and antibiotic solutions were obtained from Biological Industries (Beit-Haeemek, Israel). Recombinant human insulin was from Novo Nordisk (Bagsvaerd, Denmark). Anti-PKB/Akt, anti-pSer473 Akt/PKB, anti-pThr308 Akt/PKB, anti-pAkt substrate (PAS), anti-AS160, anti-pSer21/9 glycogen synthase kinase (GSK)-3α/β were purchased from Cell Signaling (Beverly, MA.). Anti-c-Myc was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin was from Sigma (St. Louis, MO). Alexa Fluor 555 antibody was from Molecular Probes (Eugene, OR). Nelfinavir was supplied by Roche Pharmaceuticals (Tel Aviv, Israel). The myc-GLUT4-enhanced green fluorescent protein and green fluorescent protein (GFP)-general receptor for phosphoinositides (GRP)-PH constructs have been described previously (16,31). The myrAKT plasmid was a kind gift from Dr. Michael Quon (National Institutes of Health, Bethesda, MD). The p110-CAAX construct was described previously (32).
Cell culture and treatment
3T3-L1 preadipocytes (American Type Culture Collection, Manassas, VA) were cultured and induced to differentiate as we previously reported (7,13). Cells were used 6–10 d after differentiation induction (for electroporation cells were maintained in medium supplemented with insulin until d 6 after differentiation induction). Nelfinavir treatment was performed by adding the drug to serum-free DMEM supplemented with 0.5% RIA-grade BSA, to a final concentration of 30 μmol/liter, for 18 h. The final nelfinavir medium concentration was achieved by the appropriate dilution of a 100 mmol/liter stock solution prepared in 100% ethanol.
Electroporation
Cells from 100-mm plates of 3T3-L1 adipocytes 6 d after differentiation were collected after gentle trypsinization and three washes (by centrifugation) in PBS into two to three electroporation cuvettes, into which 50 μg of DNA [GRP1-PH-GFP and GFP GLUT4myc)] or 150 μg p110-CAAX and myr-Akt plasmids (for cotransfection experiments) were added. Cells were electroporated using a Gene Pulser II (Bio-Rad Laboratories, Hercules, CA) at 0.16 kV and 950 μF. After electroporation, cells were plated on collagen IV-coated glass coverslips in 6-well plates and allowed to recover in DMEM supplemented with 10% serum for 4 h before treatment with nelfinavir.
GRP1(PH)-GFP redistribution
Transfected 3T3-L1 adipocytes were starved for 18 h and treated for 10 min with or without 100 nmol/liter insulin prepared in serum-free medium. Cells were washed in PBS and fixed with 4% paraformaldehyde (PFA) in PBS for 5 min at 4 C and an additional 25 min at room temperature. Excess PFA was then quenched for 10 min with 100 mmol/liter glycine in PBS, and coverslips were mounted onto glass slides. The cells were imaged using a LSM 510 confocal microscope (Zeiss, Jena, Germany).
GLUT4 redistribution assay
Transfected 3T3-L1 adipocytes were starved for 18 h and treated for 20 min with or without 100 nmol/liter insulin in serum-free medium. Cells were washed in ice-cold PBS and incubated for 10 min in blocking buffer containing 5% goat serum at 4 C. Cells were incubated with primary anti-myc antibody at 1:100 dilution in blocking buffer, and the antibody was detected using antimouse IgG conjugated with Alexa 555 in 1:750 dilution. The cells were then fixed with 4% PFA in PBS for 5 min at 4 C and an additional 25 min at room temperature. Paraformaldehyde was quenched for 10 min in 100 mmol/liter glycine in PBS. The cells were imaged using a Zeiss LSM 510 confocal microscope.
Quantitation of Myc-GLUT4-GFP translocation
Images of 20–30 individual adipocyte cells (rounded morphology with visible lipid droplets) per experiment were analyzed for their GFP and myc staining morphology. Images were taken using the same acquisition and gain parameters throughout the experiment. The rim score of GFP (total Myc-GLUT4-GFP) or myc (GLUT4 molecules fused with the plasma membrane) was rated using the following scoring system: 0, no rim; 1, light-intensity and/or discontinuous rim staining; and 2, strong and continuous rim staining, similar to the approach used in (33). The myc to GFP fluorescence ratio was done as reported elsewhere (34). Rating was performed being blinded to the specific cell treatment.
Cell lysates and Western blots
Cells were rinsed three times with PBS and incubated in the absence or presence of 100 nmol/liter insulin for 7 min and then scraped into 0.25 ml/well of ice-cold lysis buffer [50 mmol/liter Tris-HCl (pH 7.5), 0.1% (vol/vol) Triton X-100, 1 mmol/liter EDTA, 1 mmol/liter EGTA, 50 mmol/liter NaF, 10 mmol/liter sodium-glycerophosphate, 5 mmol/liter sodium pyrophosphate, 1 mmol/liter sodium vanadate, 0.1% (vol/vol) 2-mercaptoethanol, and inhibitors (1:1000 dilution of protease inhibitor cocktail; Sigma)]. Lysate protein content was determined using the Bio-Rad Bradford procedure. Proteins were resolved by SDS-PAGE and subjected to Western blot analyses, followed by quantification using image analysis software (ImageGauge version 4.0; Fuji, Tokyo, Japan). In each experiment the intensity of the band derived from control cells was assigned a value of 1 arbitrary unit, and the intensity of all treatment groups was expressed as fold of control.
Statistical analysis
Data are expressed as means ± se. Each treatment was compared with the control, and statistical significance between two groups was evaluated using the Student’s t test. P < 0.05 was considered significant.
Results
Nelfinavir impairs insulin-stimulated translocation and fusion of GLUT4 with the plasma membrane and inhibits PKB/Akt activation
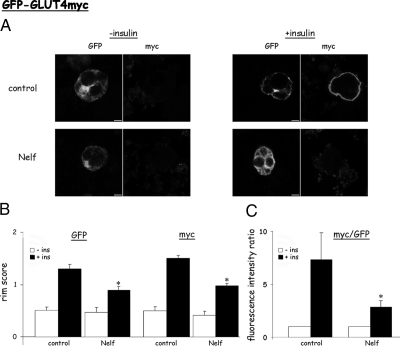
To dissect out the signaling defect responsible for impaired insulin-stimulated translocation of GLUT4 to the plasma membrane, we used a single-cell assay to assess GLUT4 translocation. 3T3-L1 adipocytes were electroporated with a plasmid encoding a myc-GLUT4-GFP dual-tagged transporter that serves as a single cell quantitative reporter of GLUT4 fusion with the plasma membrane. Because the myc epitope is inserted in the first exofacial loop of GLUT4, it is externalized upon fusion of vesicles containing myc-GLUT4-GFP with the plasma membrane (PM), making it available for labeling with a myc antibody in nonpermeabilized cells. In control cells in the absence of insulin, cells expressing myc-GLUT4-GFP were identified by their green fluorescence, which is mostly distributed in perinuclear regions (Fig. 1A, upper left panels). Myc staining is low or undetectable, consistent with a low level of fused GLUT4 in the PM under basal conditions. In response to insulin GLUT4 vesicles translocated to the PM forming a GFP rim, and in addition, a rim of externalized myc became observable, consistent with insulin-induced GLUT4 vesicle fusion with the PM (Fig. 1A, upper right panels). Cells exposed for 18 h to nelfinavir showed decreased insulin-induced GLUT4 translocation (as assessed by GFP rims) and GLUT4 vesicle fusion (as assessed by myc rims). These observations using a single-cell assay were quantified as described in Materials and Methods, and the results are depicted in Fig. 1, B and C. The single-cell assay is consistent with previously published observations, which demonstrated nelfinavir-induced impairment in insulin-stimulated GLUT4 translocation using both subcellular fractionation approach and the PM lawn assay (25,27).
Figure 1.
Nelfinavir impairs insulin-induced translocation and fusion of GLUT4 in 3T3-L1 adipocytes observed in a single-cell assay. Differentiated 3T3-L1 adipocytes were electroporated in the presence of a plasmid encoding a GFP-tagged GLUT4myc (Myc-GLUT4-GFP) and then treated without or with 30 μmol/liter nelfinavir (Nelf) for 18 h. The cells were then washed and incubated for 20 min without or with 100 nmol/liter insulin (ins), followed by fixing the cells without permeabilization, and immunostaining with anti-myc antibodies to detect surface-exposed GLUT4 molecules. A, Shown are representative images obtained with a laser confocal microscope at the middle height of the cells. Bar, 5 μm. Twenty to 30 individual cells per experiment and eight individual experiments were analyzed for their GFP and myc rim staining (B) or the ratio between the red (myc) and green (GFP) fluorescence (C), as described in Materials and Methods. *, P < 0.05 compared with insulin-stimulated control cells.
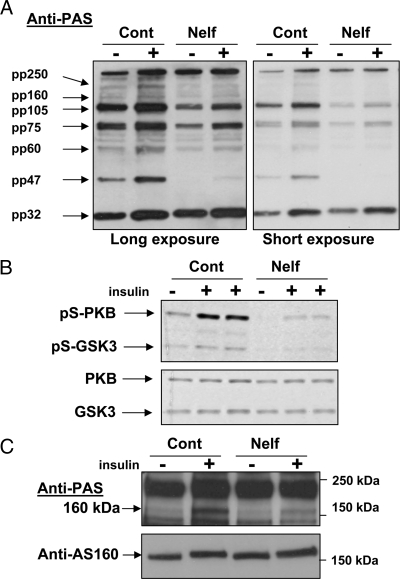
We have previously shown, that nelfinavir-induced insulin resistance was associated with normal insulin receptor and IRS tyrosine phosphorylation as well as normal (or even exaggerated) PI 3-kinase activity in response to insulin stimulation (27). Yet PKB/Akt phosphorylation on both Ser473 and Thr308 and its activity were impaired (25). Because a marked inhibition of PKB/Akt activation was reported to be required to impair signal transmission downstream of PKB/Akt, we assessed whether signaling steps downstream of PKB/Akt were affected. For this we used the anti-PAS antibody. This antibody recognizes proteins phosphorylated on Ser/Thr residues within PKB/Akt’s consensus sequence motif R/KxR/KxxR/KxS/T (35). Two exposures of these blots are presented to enable the visualization of both highly and less abundant PKB/Akt substrates, without signal saturation (Fig. 2A). As can be seen, acute insulin stimulation increased the intensity of bands observable with PAS antibody, consistent with previous reports (35). Nelfinavir treatment blunted insulin-stimulated phosphorylation of most of these bands, although to varying degrees (densitometry analysis revealed that nelfinavir treatment inhibited the mean net insulin stimulated increase in the intensity of the various bands by 33 to more than 90%). Consistent with the decrease in PKB/Akt Ser473 phosphorylation, nelfinavir impaired insulin-induced phosphorylation of GSK3 on Ser9 without significantly altering the total amount of either PKB/Akt or GSK3 (Fig. 2B). Furthermore, nelfinavir attenuated insulin-stimulated phosphorylation of a 160-kDa Akt substrate, identified as AS160 [Fig. 2C (35)]. This PKB/Akt substrate has been implicated in downstream signal propagation from PKB/Akt to GLUT4 translocation and fusion (36,37,38). Collectively, these data demonstrate that nelfinavir-induced impairment in insulin-stimulated GLUT4 translocation and fusion with the PM is readily observable by a single-cell assay and provides evidence that it can be attributed to impaired insulin signaling downstream from PKB/Akt.
Figure 2.
Insulin-stimulated phosphorylation of PKB/Akt and its downstream substrates is impaired by nelfinavir (Nelf). 3T3-L1 adipocytes were treated without or with 30 μm nelfinavir for 18 h and then washed and stimulated for 10 min with insulin. Cell lysates and Western blot analysis were performed as described in Materials and Methods, and membranes were blotted with antibody against PAS (A); anti-pSer473-Akt/PKB antibody and anti-pSer9-GSK3 antibody or antibodies against total Akt/PKB and GSK3 (B); or anti-PAS and anti-AS160 antibodies (C). Shown are blots representative of three to five independent experiments. Anti-PAS blots in A are shown after both long and short exposure to depict various phosphorylated PAS below signal saturation conditions. Cont, Control; pS, pSer 9.
Nelfinavir does not impair insulin-induced PIP3-mediated translocation to the plasma membrane, and a membrane-targeted active PI 3-kinase cannot bypass the defect in GLUT4 translocation induced by nelfinavir
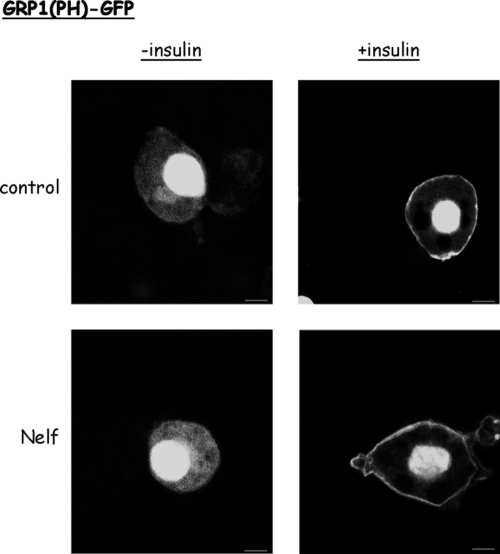
Because impaired in situ PIP3 generation could be defective even when PI 3-kinase activity is intact as assessed by an in vitro kinase assay (16), we sought to functionally estimate insulin-stimulated PIP3 production in intact cells using a single-cell assay approach. For this, cells were electroporated in the presence of a plasmid encoding a fusion protein composed of GFP and the PH domain of GRP1. Because this PH domain has a rather selective affinity to PIP3 binding (compared with other PIPs) (39), the translocation of GRP1(PH)-GFP from the cytosol to the cell periphery upon insulin stimulation is a reliable functional measure of insulin-induced PIP3 generation in intact cells. Consistent with previous reports, this fusion protein strongly localizes with the cell nucleus, but this localization does not reflect PIP3 binding (40). Therefore, the extranuclear localization of GRP1(PH)-GFP is used for assessing PIP3 production. In the absence of insulin stimulation, GRP1(PH)-GFP exhibited a diffuse cytosolic localization in both control and nelfinavir-treated cells (Fig. 3, left panels). In response to insulin, GRP1(PH)-GFP localized to the cell periphery. Remarkably, nelfinavir treatment did not appreciably affect insulin-induced GRP1(PH)-GFP relocalization (Fig. 3, right column). Similar observations were obtained when GRP1(PH)-GFP was introduced to 3T3-L1 adipocytes by microinjection followed by live-cell microscopy (data not shown).
Figure 3.
Insulin-stimulated PI3,4,5P3 generation is comparable between nelfinavir-treated and control cells. 3T3-L1 adipocytes were electroporated in the presence of plasmids encoding the GFP-tagged PH domain of GRP1 to detect PI3,4,5P3 in intact cells. Nelfinavir (Nelf) treatment (when indicated) was for 18 h with 30 μmol/liter. Cells were rinsed and then left unstimulated or stimulated for 10 min with insulin and then fixed and visualized by a laser confocal microscope. Shown are representative cells from three different experiments.
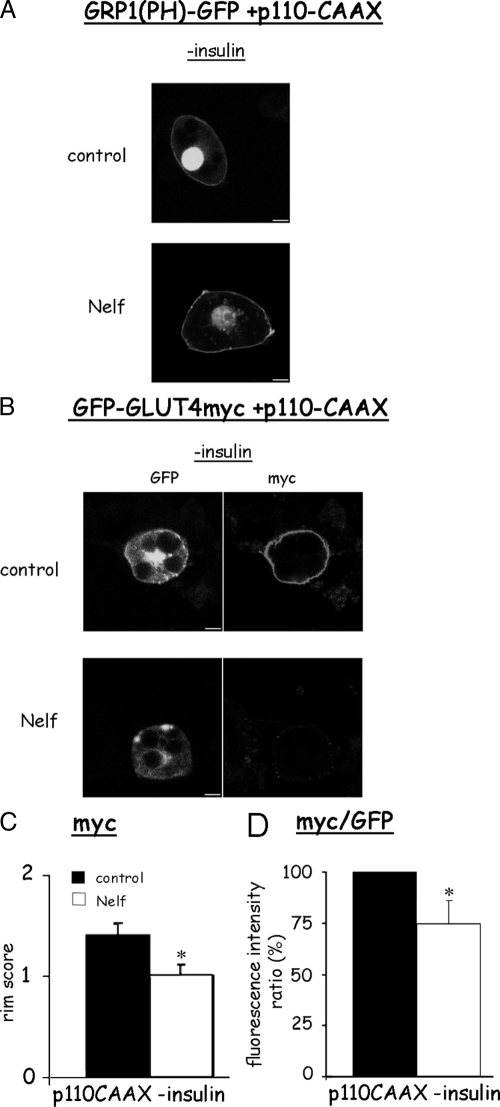
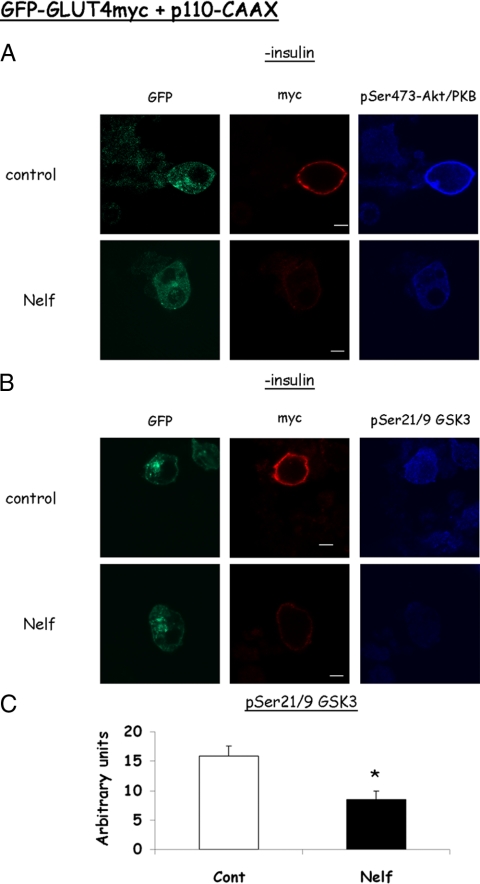
To determine whether forced PM generation of PIP3 could overcome nelfinavir-induced defect in insulin signaling, we used a p110 catalytic subunit of PI 3-kinase targeted to the PM by virtue of a CAAX motif (32). When coexpressed with GRP1(PH)-GFP, this kinase caused the translocation of GRP1(PH)-GFP to the cell periphery in the absence of insulin, confirming p110-CAAX-mediated PIP3 generation (Fig. 4A). Nelfinavir-treated cells showed a similar GRP1(PH)-GFP rim, suggesting that nelfinavir did not decrease PIP3 production through increasing PIP3 phosphatases like phosphatase and tensin homolog deleted from chromosome 10 (PTEN) or SHIP2, as previously proposed (25). In control cells expressing p110-CAAX along with myc-GLUT4-GFP, this forced PIP3 generation resulted in GLUT4 translocation in the absence of insulin, which was equivalent in magnitude to that observed in Fig. 1 (Fig. 4, B and C). Yet in cells treated with nelfinavir, this forced PIP3 generation did not overcome the inhibitory effect on GLUT4 PM fusion. Furthermore, Akt and GSK3 phosphorylation in p110-CAAX-expressing cells were still impaired in nelfinavir-treated cells (Fig. 5), confirming that Akt activation remained the limiting step in signal propagation to GLUT4, even under forced PIP3 generation. Collectively, these data strongly suggest that nelfinavir does not induce impairment in insulin-stimulated PIP3 generation and that the signaling defect is attributed to a signaling step(s) downstream of PIP3 generation, such as the activation of Akt/PKB.
Figure 4.
GLUT4 externalization by a membrane targeted active PI 3-kinase is impaired by nelfinavir (Nelf). 3T3-L1 adipocytes were electroporated in the presence of plasmids encoding a membrane-targeted catalytic subunit of PI 3-kinase (p110-CAAX) along with GRP1(PH)-GFP (A) or Myc-GLUT4-GFP (B–D). The cells were treated (when indicated) with 30 μm nelfinavir for 18 h but were not further stimulated with insulin. Shown are representative confocal cell images from three independent experiments for A and eight for B–D. C, Rim score. D, myc/GFP (red/green) fluorescence ratio. *, P < 0.05.
Figure 5.
Insulin-stimulated phosphorylation of Akt and of GSK3 are decreased in nelfinavir-treated-, p110-CAAX-, and myc-GLUT4-GFP-expressing adipocytes. 3T3-L1 adipocytes expressing a membrane-targeted catalytic subunit of PI 3-kinase (p110-CAAX) and myc-GLUT4-GFP were generated and treated or not with nelfinavir (Nelf) as in the legend for Fig. 4. After immunostaining surface myc in nonpermeabilized cells, cells were permeabilized and incubated with anti-pSer473-PKB/Akt (A) or pSer9-GSK3 (B), followed by an alexa 633-conjugated secondary antibody. Confocal microscopy was performed, with acquisition in three channels. These experiments were repeated three times, and quantification of the intensity of the pGSK signal in nelfinavir treated and control cells (n = 30 in each experiment) is shown in C in arbitrary units. *, P < 0.05.
Active PKB/Akt rescues the nelfinavir-induced inhibition of GLUT4 translocation
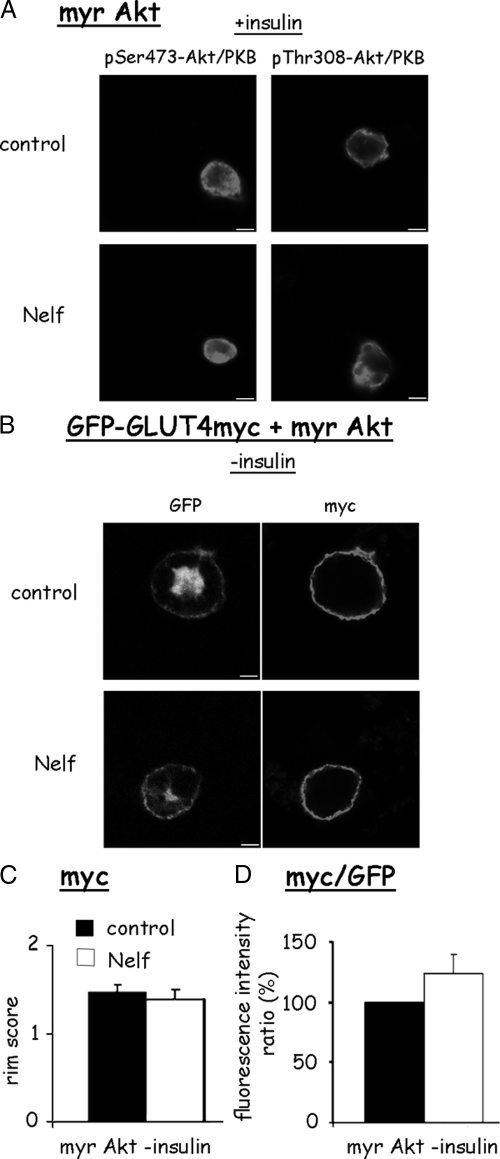
Previously, subcellular fractionation revealed that impaired insulin-stimulated translocation of PKB/Akt to the PM accompanied the nelfinavir-induced defect in GLUT4 translocation (25). To examine the hypothesis that this failure of PKB/Akt to arrive and become phosphorylated at the PM in response to insulin was responsible for the impairment in GLUT4 translocation, we used a membrane targeted active PKB/Akt by virtue of a myristoylation sequence (myr-Akt). We first assessed whether this PM-targeted Akt would be phosphorylated in nelfinavir-treated cells, reflecting on the activity of PKB/Akt kinases (PDK1 and mammalian target of rapamycin (mTOR)-signaling complex 2 (TORC2)). As shown in Fig. 6A, nelfinavir did not attenuate PKB/Akt phosphorylation of myr-Akt on either Ser473 or Thr308. This suggests that the PI 3-kinase to PKB/Akt signal transmission defect induced by nelfinavir is not due to impaired activity of upstream PKB/Akt kinases. When coexpressed with myc-GLUT4-GFP, myr-Akt expression resulted in GLUT4 PM fusion in the absence of insulin to an extent equivalent in non-myr-Akt expressing cells stimulated by insulin (Fig. 6, B and C, compared with Fig. 1). In contrast to the effect observed with p110-CAAX, the ability of myr-Akt to induce GLUT4 translocation was preserved in nelfinavir-treated cells. This finding suggests that a PKB/Akt that is active irrespective of PIP3 generation can circumvent the insulin signaling defect induced by nelfinavir.
Figure 6.
Membrane-targeted PKB/Akt induces GLUT4 externalization in both control and nelfinavir-treated 3T3-L1 adipocytes. 3T3-L1 adipocytes were electroporated in the presence of plasmids encoding a membrane-targeted PKB/Akt (myr Akt) and Myc-GLUT4-GFP. Cells were then treated or not with 30 μm nelfinavir (Nelf) for 18 h. A, After rinsing the cells, they were stimulated for 10 min with 100 nm insulin, after which cells were fixed, permeabilized, and stained with anti-pS473-PKB/Akt or anti-pT308-PKB/Akt. B, Cells were left unstimulated with insulin, fixed, and stained for surface myc. C and D, Rims of myc staining or the myc to GFP fluorescence intensity ratio, respectively, were rated as described in Materials and Methods, and results of 20–30 cells in each of eight independent experiments are shown.
Discussion
Impaired activation of PKB/Akt as a functional break point in insulin signal propagation
Defective proximal insulin signaling steps, such as decreased insulin receptor activation and reduced tyrosine phosphorylation of IRS molecules, are frequently proposed as cellular mechanisms for insulin resistance, both in vivo and in cell models. However, in some cases decreases in early signaling events are not rate limiting for signal propagation, suggesting that the functional signaling defect(s) is further downstream (20,41). In other situations, including in response to nelfinavir, early signaling steps including the activation of PI 3-kinase are intact (or even exaggerated), but signaling steps from PKB/Akt and downstream are impaired (5,6,13,27). Applying the same logic as for early signaling events, defective insulin-induced PKB/Akt activation still does not prove that this step is indeed the cause of insulin resistance. This is particularly true in muscle cells, in which only a marked decrease in PKB/Akt activation achieved by expression of mutated PKB/Akt translated into impaired downstream events (GLUT4 translocation) (21). In these cells in response to a hydrogen peroxide-generating system, decrease in the activation (GTP loading) of Rac1 displayed a closer dose-response relationship to the attenuation of GLUT4 translocation than the decrease in PKB/Akt phosphorylation (22). This suggests the possibility that parallel signaling steps to PKB/Akt activation, which are also downstream of PI 3-kinase, may constitute the actual rate-limiting step for signal propagation. On the other hand, the present study suggests that in 3T3-L1 adipocytes treated with nelfinavir, PKB/Akt is indeed a functional break point in insulin signal transduction toward GLUT4 translocation: signaling steps downstream of the activation of this key kinase, including phosphorylation of GSK3, AS160, and other PKB/Akt substrates were all decreased (Fig. 2), even in cells with forced generation of PIP3 by a constitutively active PI 3-kinase (Fig. 5). Moreover, a membrane-targeted active PKB/Akt was able to promote GLUT4 translocation in nelfinavir-treated adipocytes as in control cells (Fig. 6). These findings strongly support the notion that PKB/Akt activity in situ is rate limiting for insulin signal propagation in response to nelfinavir.
Impaired PIP3 sensing by PKB/Akt as a putative mechanism for insulin resistance
Even when a causative role is assigned for impaired activation of PKB/Akt in insulin resistance, multiple mechanisms may underlie this phenomenon. For example, PKB/Akt may lack the signals that are required for its activation, such as PIP3 generation or the localization and activation of its upstream kinases (PDK1 and PDK2). In response to nelfinavir, both steps seem functionally unaffected because PIP3-mediated translocation of GRP1(PH)-GFP to the plasma membrane seems intact (Fig. 3), and when PKB/Akt was targeted to the plasma membrane, it was phosphorylated normally on both Ser473 and Thr308 sites, suggesting intact PKB/Akt kinases activity (Fig. 6). Downstream to its activation, steady-state levels of PKB/Akt phosphorylation may still be decreased if a PKB/Akt phosphatase (like PP2A) would be activated. Yet previous studies ruled out this mechanism in response to nelfinavir (25). Hence, one is left with the option that after nelfinavir treatment, despite apparently normal PIP3 generation in response to insulin, PKB/Akt remains unresponsive to this event, thereby failing to translocate to the plasma membrane and to become phosphorylated and activated by PKB/Akt kinases. Thus, with the ever-increasing appreciation of the complexity of the insulin signaling cascades, it appears that multiple functional defects may contribute to the overall phenomenon of insulin resistance. This may underscore one reason for the difficulty in finding effective therapies to overcome insulin resistance. In the case of insulin resistance of adipocytes in response to nelfinavir, the mechanism appears to be the failure of PKB/Akt to sense insulin-induced PIP3 generation.
Why does PKB/Akt become insensitive to PIP3 generated in the cell? One potential mechanism was suggested in muscle cells exposed to ceramide, implicating hyperactivated PKCξ in the phosphorylation of PKB directly on its Thr34 within its PH domain (the protein domain responsible for PIP3 sensing) (17,18). We were unable to demonstrate that this mechanism is operational to any appreciable degree in 3T3-L1 adipocytes: Although PKCξ could be coimmunoprecipitated with PKB/Akt, this association was not regulated by insulin, and nelfinavir did not seem to increase the phosphorylation of PKCξ (data not shown). An alternative molecular mechanism is complex formation between PKB/Akt and TRB3. This protein has been recently shown as a physiologically relevant off switch for insulin action through the inhibition of PKB/Akt in liver (19) [although this view was challenged by other observations (42,43)]. The probability that this mechanism underlies the effect of nelfinavir was supported by several lines of reasoning. First, nelfinavir-induced insulin resistance in adipocytes is accompanied by accelerated basal lipolysis rate (27), and increased TRB3 expression was recently proposed also as a mechanism for increase in lipolysis (44). Second, in cancer cells nelfinavir was suggested to induce endoplasmic reticulum stress (45), which is an inducer of TRB3 (46). Third, nelfinavir treatment during adipogenesis was shown to inhibit adipocyte differentiation (47), as does overexpression of TRB3 (48). However, although nelfinavir increased endogenous TRB3 protein content in 3T3-L1 adipocytes (supplementary Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), inhibiting this effect of nelfinavir by cycloheximide (data not shown) or using small interfering RNA (supplementary Fig. 1) did not prevent the decrease in PKB/Akt phosphorylation. Finally, oxidative/nitrative posttranslational modifications of PKB/Akt may impair its insulin-stimulated activation (49,50). Intriguingly, nelfinavir was proposed to induce increased radical generation and oxidative stress (24,51), and we found that an superoxide dismutase, superoxide dismutase/catalase-mimetic agent [Mn (III) tetrakis (4-benzoic acid) poryphrin chlorid (MnTBAP)] can protect cells from nelfinavir-induced impairment in PKB/Akt activation (24) and defective GLUT4 translocation (data not shown). Future studies should reveal whether and which oxidative/nitrative modifications of PKB/Akt are caused by nelfinavir. Furthermore, whether oxidative/nitrative stress(es) can induce insulin resistance despite normal PIP3 generation remains to be demonstrated.
In summary, we describe a postreceptor mechanism for adipocyte insulin resistance in response to nelfinavir, a drug associated with lipodystrophy and systemic insulin resistance. The underling mechanism involves oxidation-induced insensitivity of PKB/Akt to PIP3 generated in response to insulin.
Supplementary Material
Footnotes
This work was supported by Israel Science Foundation Grants 912-05 and 1254-06 (to A.R. and N.B.) and National Institutes of Health Grant DK33823 (to J.E.P.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 29, 2009
Abbreviations: GLUT, Glucose transporter; IRS, insulin receptor substrate; GFP, green fluorescent protein; GRP, general receptors for phosphoinositides; GSK, glycogen synthase kinase; PAS, pAkt substrate; PDK, phosphoinosotide-dependent kinase; PFA, paraformaldehyde; PH, pleckstrin homology; PI 3-kinase, phosphatidylinositol 3-kinase; PIP3, phosphatidylinositol-3,4,5-trisphosphate; PKC, protein kinase C; PM, plasma membrane; PP2A, protein phosphatase 2A; TRB3, Drosophila Tribbles homologue proteins 3.
References
- Danielsson A, Ost A, Lystedt E, Kjolhede P, Gustavsson J, Nystrom FH, Strålfors P 2005 Insulin resistance in human adipocytes occurs downstream of IRS1 after surgical cell isolation but at the level of phosphorylation of IRS1 in type 2 diabetes. FEBS J 272:141–151 [DOI] [PubMed] [Google Scholar]
- Engelman JA, Berg AH, Lewis RY, Lisanti MP, Scherer PE 2000 Tumor necrosis factor α-mediated insulin resistance, but not dedifferentiation, is abrogated by MEK1/2 inhibitors in 3T3-L1 adipocytes. Mol Endocrinol 14:1557–1569 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM 1996 IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 271:665–668 [DOI] [PubMed] [Google Scholar]
- Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF 2001 Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 107:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers SA, Garza LA, Zhou H, Birnbaum MJ 1998 Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol 18:5457–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CN, O'Brien L, Brindley DN 1998 Effects of cell-permeable ceramides and tumor necrosis factor-α on insulin signaling and glucose uptake in 3T3-L1 adipocytes. Diabetes 47:24–31 [DOI] [PubMed] [Google Scholar]
- Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N 1998 Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 47:1562–1569 [DOI] [PubMed] [Google Scholar]
- Takano A, Haruta T, Iwata M, Usui I, Uno T, Kawahara J, Ueno E, Sasaoka T, Kobayashi M 2001 Growth hormone induces cellular insulin resistance by uncoupling phosphatidylinositol 3-kinase and its downstream signals in 3T3-L1 adipocytes. Diabetes 50:1891–1900 [DOI] [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR 2001 PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114:2903–2910 [DOI] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA 2004 Structure, regulation and function of PKB/AKT–a major therapeutic target. Biochim Biophys Acta 1697:3–16 [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM 2006 Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev Cell 11:859–871 [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM 2006 Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22:159–168 [DOI] [PubMed] [Google Scholar]
- Tirosh A, Potashnik R, Bashan N, Rudich A 1999 Oxidative stress disrupts insulin-induced cellular redistribution of insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. J Biol Chem 274:10595–10602 [DOI] [PubMed] [Google Scholar]
- Cazzolli R, Carpenter L, Biden TJ, Schmitz-Peiffer C 2001 A role for protein phosphatase 2A-like activity, but not atypical protein kinase Cζ, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes 50:2210–2218 [DOI] [PubMed] [Google Scholar]
- Ogihara T, Asano T, Katagiri H, Sakoda H, Anai M, Shojima N, Ono H, Fujishiro M, Kushiyama A, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Gotoh Y, Komuro I, Fujita T 2004 Oxidative stress induces insulin resistance by activating the nuclear factor-κB pathway and disrupting normal subcellular distribution of phosphatidylinositol 3-kinase. Diabetologia 47:794–805 [DOI] [PubMed] [Google Scholar]
- Yang C, Watson RT, Elmendorf JS, Sacks DB, Pessin JE 2000 Calmodulin antagonists inhibit insulin-stimulated GLUT4 (glucose transporter 4) translocation by preventing the formation of phosphatidylinositol 3,4,5-trisphosphate in 3T3L1 adipocytes. Mol Endocrinol 14:317–326 [DOI] [PubMed] [Google Scholar]
- Powell DJ, Hajduch E, Kular G, Hundal HS 2003 Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ-dependent mechanism. Mol Cell Biol 23:7794–7808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrich P, Neuscheler D, Melzer M, Hennige AM, Häring HU, Lammers R 2007 The Par6α/aPKC complex regulates Akt1 activity by phosphorylating Thr34 in the PH-domain. Mol Cell Endocrinol 268:30–36 [DOI] [PubMed] [Google Scholar]
- Du K, Herzig S, Kulkarni RN, Montminy M 2003 TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300:1574–1577 [DOI] [PubMed] [Google Scholar]
- Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB 1999 Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest 104:733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, Klip A 1999 Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol 19:4008–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JeBailey L, Wanono O, Niu W, Roessler J, Rudich A, Klip A 2007 Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes 56:394–403 [DOI] [PubMed] [Google Scholar]
- Rudich A, Ben-Romano R, Etzion S, Bashan N 2005 Cellular mechanisms of insulin resistance, lipodystrophy and atherosclerosis induced by HIV protease inhibitors. Acta Physiol Scand 183:75–88 [DOI] [PubMed] [Google Scholar]
- Ben-Romano R, Rudich A, Etzion S, Potashnik R, Kagan E, Greenbaum U, Bashan N 2006 Nelfinavir induces adipocyte insulin resistance through the induction of oxidative stress: differential protective effect of antioxidant agents. Antivir Ther 8:1051–1060 [PubMed] [Google Scholar]
- Ben-Romano R, Rudich A, Tirosh A, Potashnik R, Sasaoka T, Riesenberg K, Schlaeffer F, Bahan N 2004 Nelfinavir-induced insulin resistance is associated with impaired plasma membrane recruitment of the PI 3-kinase effectors Akt/PKB and PKCζ. Diabetologia 47:1107–1117 [DOI] [PubMed] [Google Scholar]
- Ben-Romano R, Rudich A, Török D, Vanounou S, Riesenberg K, Schlaeffer F, Klip A, Bashan N 2003 Agent and cell-type specificity in the induction of insulin resistance by HIV protease inhibitors. AIDS 17:23–32 [DOI] [PubMed] [Google Scholar]
- Rudich A, Vanounou S, Riesenberg K, Porat M, Tirosh A, Harman-Boehm I, Greenberg AS, Schlaeffer F, Bashan N 2001 The HIV protease inhibitor nelfinavir induces insulin resistance and increases basal lipolysis in 3T3-L1 adipocytes. Diabetes 50:1425–1431 [DOI] [PubMed] [Google Scholar]
- Hruz PW, Murata H, Mueckler M 2001 Adverse metabolic consequences of HIV protease inhibitor therapy: the search for a central mechanism. Am J Physiol Endocrinol Metab 280:E549–E553 [DOI] [PubMed] [Google Scholar]
- Murata H, Hruz PW, Mueckler M 2000 The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem 275:20251–20254 [DOI] [PubMed] [Google Scholar]
- Rudich A, Konrad D, Török D, Ben-Romano R, Huang C, Niu W, Garg RR, Wijesekara N, Germinario RJ, Bilan PJ, Klip A 2003 Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia 46:649–658 [DOI] [PubMed] [Google Scholar]
- Watson RT, Khan AH, Furukawa M, Hou JC, Li L, Kanzaki M, Okada S, Kandror KV, Pessin JE 2004 Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is GGA dependent. EMBO J 23:2059–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa K, Sharma PM, Nakashima N, Huang Y, Huver E, Boss GR, Olefsky JM 1999 Membrane-targeted phosphatidylinositol 3-kinase mimics insulin actions and induces a state of cellular insulin resistance. J Biol Chem 274:14306–14314 [DOI] [PubMed] [Google Scholar]
- Randhawa VK, Ishikura S, Talior-Volodarsky I, Cheng AW, Patel N, Hartwig JH, Klip A 2008 GLUT4 vesicle recruitment and fusion are differentially regulated by Rac, AS160, and Rab8A in muscle cells. J Biol Chem 283:27208–27219 [DOI] [PubMed] [Google Scholar]
- Williams D, Pessin JE 2008 Mapping of R-SNARE function at distinct intracellular GLUT4 trafficking steps in adipocytes. J Cell Biol 180:375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE 2002 A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277:22115–22118 [DOI] [PubMed] [Google Scholar]
- Sano H, Kane S, Sano E, Mîiinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE 2003 Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278:14599–14602 [DOI] [PubMed] [Google Scholar]
- Gonzalez E, McGraw TE 2006 Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell 17:4484–4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong FS, Bilan PJ, Klip A 2007 The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes 56:414–423 [DOI] [PubMed] [Google Scholar]
- Klarlund JK, Tsiaras W, Holik JJ, Chawla A, Czech MP 2000 Distinct polyphosphoinositide binding selectivities for pleckstrin homology domains of GRP1-like proteins based on diglycine versus triglycine motifs. J Biol Chem 275:32816–32821 [DOI] [PubMed] [Google Scholar]
- Patel N, Rudich A, Khayat ZA, Garg R, Klip A 2003 Intracellular segregation of phosphatidylinositol-3,4,5-trisphosphate by insulin-dependent actin remodeling in L6 skeletal muscle cells. Mol Cell Biol 23:4611–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Karlsson HK, Zierath JR 2004 Insulin signaling defects in type 2 diabetes. Rev Endocr Metab Disord 5:111–117 [DOI] [PubMed] [Google Scholar]
- Iynedjian PB 2005 Lack of evidence for a role of TRB3/NIPK as an inhibitor of PKB-mediated insulin signalling in primary hepatocytes. Biochem J 386:113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Latres E, Liu R, Thabet K, Murphy A, Valenzeula D, Yancopoulos GD, Stitt TN, Glass DJ, Sleeman MW 2007 Genetic deletion of Trb3, the mammalian Drosophila tribbles homolog, displays normal hepatic insulin signaling and glucose homeostasis. Diabetes 56:1350–1356 [DOI] [PubMed] [Google Scholar]
- Qi L, Heredia JE, Altarejos JY, Screaton R, Goebel N, Niessen S, Macleod IX, Liew CW, Kulkarni RN, Bain J, Newgard C, Nelson M, Evans RM, Yates J, Montminy M 2006 TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science 312:1763–1766 [DOI] [PubMed] [Google Scholar]
- Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, Abu-Asab MS, Borojerdi J, Warfel NA, Gardner ER, Danish M, Hollander MC, Kawabata S, Tsokos M, Figg WD, Steeg PS, Dennis PA 2007 Nelfinavir, a lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res 13:5183–5194 [DOI] [PubMed] [Google Scholar]
- Corcoran CA, Luo X, He Q, Jiang C, Huang Y, Sheikh MS 2005 Genotoxic and endoplasmic reticulum stresses differentially regulate TRB3 expression. Cancer Biol Ther 4:1063–1067 [DOI] [PubMed] [Google Scholar]
- Dowell P, Flexner C, Kwiterovich PO, Lane MD 2000 Suppression of preadipocyte differentiation and promotion of adipocyte death by HIV protease inhibitors. J Biol Chem 275:41325–41332 [DOI] [PubMed] [Google Scholar]
- Bezy O, Vernochet C, Gesta S, Farmer SR, Kahn CR 2007 TRB3 blocks adipocyte differentiation through the inhibition of C/EBPβ transcriptional activity. Mol Cell Biol 27:6818–6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, de Oliveira MG, Velloso LA, Curi R, Saad MJ 2005 S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes 54:959–967 [DOI] [PubMed] [Google Scholar]
- Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M 2005 S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem 280:7511–7518 [DOI] [PubMed] [Google Scholar]
- Vincent S, Tourniaire F, El Yazidi CM, Compe E, Manches O, Plannels R, Roche R 2004 Nelfinavir induces necrosis of 3T3F44-2A adipocytes by oxidative stress. J Acquir Immune Defic Syndr 37:1556–1562 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.