Abstract
Bisphenol A (BPA), a chemical widely used to manufacture plastics, is estrogenic and capable of disrupting sex differentiation. However, recent in vitro studies have shown that BPA can also antagonize T3 activation of the T3 receptor. The difficulty in studying uterus-enclosed mammalian embryos has hampered the analysis on the direct effects of BPA during vertebrate development. This study proposed to identify critical T3 pathways that may be disrupted by BPA based on molecular analysis in vivo. Because amphibian metamorphosis requires T3 and encompasses the postembryonic period in mammals when T3 action is most critical, we used this unique model for studying the effect of BPA on T3-dependent vertebrate development at both the morphological and molecular levels. After 4 d of exposure, BPA inhibited T3-induced intestinal remodeling in premetamorphic Xenopus laevis tadpoles. Importantly, microarray analysis revealed that BPA antagonized the regulation of most T3-response genes, thereby explaining the inhibitory effect of BPA on metamorphosis. Surprisingly, most of the genes affected by BPA in the presence of T3 were T3-response genes, suggesting that BPA predominantly affected T3-signaling pathways during metamorphosis. Our finding that this endocrine disruptor, well known for its estrogenic activity in vitro, functions to inhibit T3 pathways to affect vertebrate development in vivo and thus not only provides a mechanism for the likely deleterious effects of BPA on human development but also demonstrates the importance of studying endocrine disruption in a developmental context in vivo.
Genome-wide gene expression analysis shows that the endocrine disruptor bisphenol A, an estrogenic compound, interferes with postembryonic development by blocking mainly thyroid hormone signaling.
Endocrine disruption by environmental contaminants poses a great concern for global ecology and human health. Endocrine disrupting compounds (EDCs) have been defined as exogenous substances that alter function(s) of the endocrine system and consequently cause adverse health effects in an intact organism, or its progeny, or (sub)populations (1,2,3). Some EDCs act as antiestrogenic and antiandrogenic agents to affect reproductive function and sexual development (4), suggesting that EDCs are responsible for the increased appearance of reproductive health problems in both human and wildlife. In humans, the trend for increased breast and testicular cancers, reduced sperm counts, and early puberty has been attributed to increased exposure to EDCs (5,6,7). In wildlife, decreased species populations and increased animal malformations, including feminization and hermaphroditism, have been reported worldwide (8,9,10,11). There is also increasing concern that EDCs may affect other endocrine systems, such as the T3 system.
T3 plays a central role in vertebrate development, growth, and metabolism (12,13,14,15,16,17,18). The effects of EDCs on T3 signaling will undoubtedly pose a threat to human and wildlife health (19,20,21,22). Keyed by the discovery of nuclear T3 receptors (TRs) that function as transcription factors, recent advances have been made in examining the mechanisms of T3 action at the molecular level (12,13,15,23,24,25,26,27,28,29,30,31). Concurrently, studies have also revealed a broad array of EDCs that can bind to TR and affect T3-regulated gene expression in vitro (32). However, the lack of a suitable in vivo model to study EDCs’ effects on TR function in vertebrate development impedes our understanding on whether and how persistent exposure to these bioaccumulative compounds affects human health.
One such compound is bisphenol A (BPA), an established EDC of the reproductive system. BPA is used in the production of plastics and has widespread applicability, making its manufacturing and processing an important economical factor as well as a source of BPA release into the environment (33,34,35,36,37,38,39,40). BPA studies have primarily focused on its estrogenic activity (4,41). Recently based on extensive review of the existing data, the National Toxicology Program of the National Institutes of Health raised concerns for neural and behavioral effects of BPA in fetuses, infants, and children at the currently allowed human exposures (www.niehs.nih.gov/news/media/questions/sya-bpa.cfm#2). The concerns from this reviewing panel were primarily focused on the estrogenic effects of BPA, even though the role of estrogens on mammalian neural development is unclear. On the other hand, neural and behavioral development is dependent on T3, raising the possibility that the developmental effects of BPA in humans may be manifested through the T3 pathway. Given the possible cross talks between the T3 and estrogenic pathways (42,43), BPA may indirectly affect T3 signaling by influencing estrogenic pathways. On the other hand, in vitro studies have shown that BPA can bind to and antagonize T3 activation of TR (44), and a study using cultured mouse oligodendrocyte precursor cells found that BPA inhibited T3-induced differentiation (45). In addition, a study with rats showed that BPA exposure during development produced an endocrine profile similar to that observed in patients with T3 resistance syndrome (46).
The ability of BPA to bind to both estrogen and thyroid receptors to elicit disruption makes it very difficult to study the actions of BPA during mammalian development. Suitable alternative in vivo models are urgently needed to evaluate the effects of BPA on T3 function during development. Amphibian metamorphosis represents an attractive model due to its absolute dependence on T3 but not estrogens (14,15), although sex steroids can alter larval development in amphibians (47,48,49). Recent studies have shown that BPA blocks metamorphosis and affects T3-signaling in amphibians (50,51,52,53). In addition, it has been shown that BPA suppresses TRH-induced release of thyroid-stimulating hormone and prolactin in adult bullfrog pituitary cells, suggesting that BPA can disrupt the hypothalamic-pituitary-thyroid axis (54). On the other hand, BPA has also been shown to induce feminization in Xenopus laevis tadpoles (55,56), although a different study failed to produce this effect (57).
Here we propose the use of X. laevis metamorphosis as a model to investigate whether and how BPA affects T3-dependent vertebrate development. To date, little molecular analyses have been carried out to determine how BPA affects either metamorphosis or other postembryonic developmental processes in vertebrates. Because changes in gene expression often precede morphological changes, we aimed to use microarray technology to determine the signaling transduction pathways underlying any metamorphic effects of BPA. We chose the intestine as the model system because it represents an organ that persists throughout metamorphosis but undergoes extensive but well-characterized remodeling (58,59). It is important to note that gene regulation by T3 through TR is not only necessary but also sufficient for intestinal remodeling and other metamorphic processes (60). Furthermore, because the metamorphic process can easily be manipulated by controlling the availability of T3 via the tadpole rearing water, the influence of maternal hormones and the difficulty to manipulate the uterus-enclosed mammalian embryo are avoided.
Our molecular analysis indicates that BPA, even though mainly known as an estrogenic compound, predominantly disrupts T3-signaling pathways during metamorphosis, resulting in delayed metamorphosis. Our results suggest that similar adverse effects of BPA on human development by disrupting T3 pathways is likely and argue for the importance of studying endocrine disruption in the developmental context in vivo. They also highlight the power of combining morphological and molecular analyses of amphibian metamorphosis for studying endocrine disruption in development.
Materials and Methods
Animals
Tadpoles of X. laevis used in this study were purchased from NASCO (Fort Atkinson, WI). The animals were exposed to a 12-h light, 12-h dark photoperiod (lights on at 0700 h) and were fed spirulina, a fresh water alga, at 1000 h. Animal studies were approved by National Institute of Child Health and Human Development Animal Use and Care Committee.
Chemicals
T3, BPA, and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis. MO). All exposure treatments were conducted in 0.1% DMSO solution.
Oocyte injections and luciferase assays
Microinjection experiments were performed as described (61,62). Briefly, a reporter construct, TRE-Luc, harboring the T3-dependent X. laevis TRβA promoter driving the firefly luciferase reporter was microinjected (0.33 ng/oocyte) into the nuclei of X. laevis oocytes together with a plasmid harboring the control Renilla luciferase reporter. In vitro-transcribed mRNAs encoding TRβ and retinoid X receptor (RXR)-α were coinjected (1.15 ng/oocyte for TRβ and RXRα) into the cytoplasm. After overnight incubation in the presence or absence of BPA and/or T3, oocytes were assayed for luciferase activity.
Animal exposures to BPA
Experiment 1
BPA exposures were performed in a static-renewal system based on previous studies (51,52,56). Before exposure, test animals were acclimatized to laboratory conditions at 23–24 C for 24 h. During the acclimatization and exposure periods, the animals were not fed to eliminate dietary influence on metamorphosis progression (note that tadpoles undergoing metamorphosis or T3 treatment do not feed) (15). Ten premetamorphic X. laevis tadpoles (stage 54) were randomly transferred into 1-liter tanks containing dechlorinated water. Animals were subsequently exposed to conditions with 2 nm T3, 0.1 or 10 μm BPA, or the combination of 2 nm T3 and 0.1 or 10 μm BPA; the corresponding control group contained DMSO vehicle. The two concentrations of BPA used in this study are known to interfere with T3 action in vitro (44) and physiologically relevant for human infants (0–12 months) (within 24 h, estimated infant intake is 13 μg/kg body weight or 60 nm, calculated based on the assumption that: 1) BPA uptake is equivalent to BPA metabolized and excreted by the body within the 24 h and 2) BPA is equally distributed throughout the body with a density of 1; the Environmental Protection Agency has set safe level of exposure to 50 μg/kg per day in the United States) (www.niehs.nih.gov/news/media/questions/sya-bpa.cfm#2). Exposure treatment was conducted for 21 d at 23–24 C under 12-h light, 12-h dark cycle conditions. Water changes and chemical replacement were performed every other day. The developmental stages of the animals from each group were examined every 3 d. During the experiments, tadpoles were anesthetized in 0.02% 3-aminobenzoic acid ethyl ester (Sigma) and photographed under a stereomicroscope (Olympus, Tokyo, Japan) for gross morphology analysis. Each treatment was repeated at least three times (10 tadpoles/replicate) using tadpoles derived from different sets of adults.
Experiment 2
To study the effect of BPA on T3-induced gene expression during development, a short-term exposure experiment was performed. Acclimatization and exposure conditions were performed as described above. Groups of 10 premetamorphic X. laevis tadpoles (stage 54) were randomly placed into four tanks with 1 liter of dechlorinated water. Animals were subsequently exposed to conditions with the DMSO vehicle, 2 nm T3, 10 μm BPA, or the combination of 2 nm T3 and 10 μm BPA. Exposure treatment was conducted for 4 d at 23–24 C under 12-h light, 12-h dark cycle conditions. Water changes and chemical replacement were performed after 2 d of exposure. Each treatment was replicated three times (10 tadpoles/replicate).
RNA isolation and microarray analysis
At the end of the 4-d treatment period, the intestine from 10 tadpoles were isolated and pooled for each of the three biological replicates per treatment. RNA was isolated and subjected to cDNA array (slides AMADID 013665; Agilent, Santa Clara, CA) analysis by using a two-color reference design system as described (63,64) (also see supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). To identify significantly regulated genes, we performed ANOVA across all treatments and used a false discovery rate of 10% or less for multivariate correction (65,66,67).
Real-time PCR quantification
This was performed as described (63) using the three RNA samples as in the microarray as well as another from other tadpoles treated under the same conditions to confirm the microarray data. Real-time quantitative RT-PCR (qRT-PCR) was carried out using FAM-labeled Taqman probes for some genes (supplemental Table S1) with cDNA standards made from whole-body total RNA from tadpoles at stages 50–66. The expression level of each gene was normalized to that of the control gene, ribosomal protein L8 (rpl8). Additional genes were analyzed with SYBR Green I dye (supplemental Table S2), and the expression level of each gene was normalized to that of the control gene, elongation factor-1α (EF-1α). In a preliminary experiment, we observed that the levels of rpl8 and EF1α were not different in intestine samples from control and chemically treated tadpoles. For data analysis, intergroup comparisons were performed with ANOVA followed by Fisher’s protected least significant difference test; P ≤ 0.05 was considered to be statistically significant.
Histology
The intestines were dissected, flushed and fixed in Bouin’s fluid for 24 h, rinsed in 0.6× PBS and stored in 70% ethanol. Paraffin embedded 5-μm-thick sections were serially collected on glass slides and stained with hematoxylin-eosin.
Results
BPA suppresses T3-induced transcription
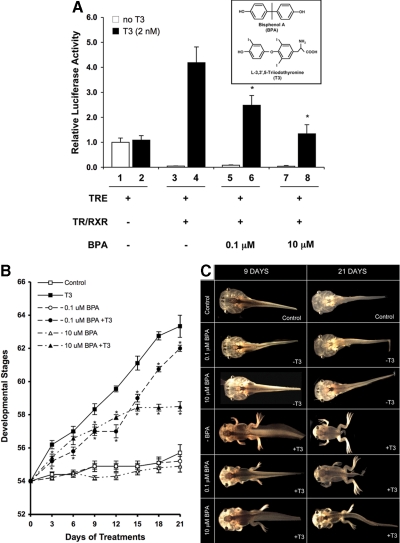
BPA is known to bind to and antagonize T3-dependent activation of mammalian TR (44). To investigate whether BPA influences X. laevis TR in vivo, we analyzed the effect of BPA on TR-dependent luciferase reporter (TRE-Luc) expression in the reconstituted frog oocyte system, a model to study T3-mediated transcription in the context of chromatin (61). In the absence of T3, overexpressed TRβ and RXRα repressed the promoter, as reported (62,68), whereas in the presence of T3, the promoter was activated (Fig. 1A). BPA inhibited the transcriptional activation of the promoter by T3 but had little effect on the promoter in the absence of T3 (Fig. 1A). In the absence of TRβ and RXRα, BPA had no significant effect on the promoter activity (data not shown). Thus, BPA may function as an inhibitor of gene activation by T3 to affect X. laevis development.
Figure 1.
BPA inhibits TR-mediated transcription and T3-induced metamorphosis. A, The mRNAs for TR/RXR (1.15 ng/oocyte) were injected into the cytoplasm of 20 oocytes as indicated. After 4 h of incubation, the TRE-Luc reporter vector (TRE) together with the control Renilla luciferase plasmid were coinjected into the nucleus of the oocytes. The oocytes were incubated overnight with or without T3 (2 nm) and indicated amounts of BPA. After incubation, the oocytes were lysed and subjected to dual-luciferase assays. The relative activity of the reporter vs. that of the control was plotted with the basal luciferase activity set to 1 (lane 1, in the absence of T3). The bars represent the means ± se of at least two independent experiments performed in quadruplicates. *, P ≤ 0.05 vs. lane 4 in the presence of T3. Insert, Structural comparison of BPA (upper panel) and T3 (lower panel). B, Dose- and time-dependent inhibition of T3-induced X. laevis metamorphosis by BPA. The average developmental stages of the animals were plotted every third day, and each point represents the mean ± se. An asterisk indicates significant differences in the development stages between T3 and T3+BPA treatment groups (P ≤ 0.05). Note only one time point for one treatment group did not show a statistically significant difference in development progression compared with T3-treated animals (d 6; 10 μm BPA+T3). There was no significant difference in development between control (DMSO vehicle control) and BPA-only-treated animals. C, Representative profile of tadpoles housed in water (DMSO vehicle control), T3 (2 nm), BPA (0.1 or 10 μm), or combinations of BPA (0.1 or 10 μm) and T3 (2 nm). Groups of 10 tadpoles at stage 54 (d 0) were used for each treatment. Tadpoles representing the typical stage in each treatment were photographed and the gross morphology observed for 9- and 21-d-treated tadpoles are presented here.
BPA inhibits T3-induced metamorphosis
To study the effect of BPA on development, premetamorphic tadpoles were treated with BPA, T3, or a combination of both (Fig. 1, B and C). Gross morphology was monitored to determine the developmental stages every 3 d for a 21-d study period. Treatments of premetamorphic tadpoles with T3 resulted in well-established morphological changes (15), and the inhibition of these changes by BPA could be observed as early as 3 d (Fig. 1B and supplemental Fig. S2). At the end of the 21-d study, T3-treated animals had metamorphosed to stage 64, whereas the control animals reached only stage 56 (Fig. 1C). No significant stage difference was observed between control (DMSO) and BPA-treated animals. The tadpoles that were exposed to combined T3 and BPA (T3+BPA) were significantly delayed in metamorphosis compared with the T3-treated animals, and this effect of BPA was dose dependent (Fig. 1B).
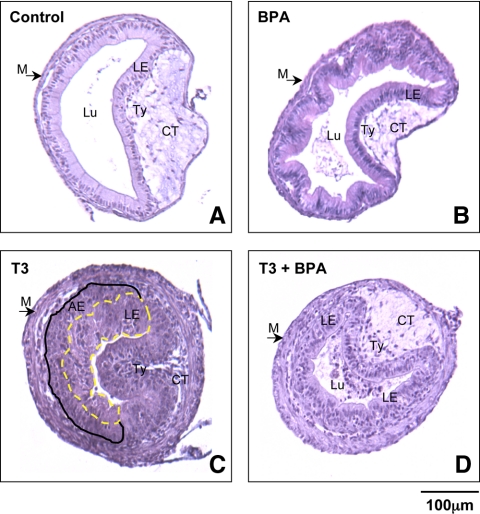
To study the effect of BPA on the remodeling of visceral organs during development, we analyzed the intestine, a model organ that has been well characterized morphologically and molecularly (58,59,63,69). After 4 d of treatment, control intestinal cross-sections had thin muscle layers around the exterior, a thin layer of connective tissue, and a simple inner epithelium with a single in-folding, the typhlosole, which contains the majority of connective tissue in the larval intestine (Fig. 2A). Little morphological change occurred after 4 d of BPA treatment, and these intestinal samples were comparable with those of the untreated samples (Fig. 2B). In the presence of T3, the overall length of the intestine shortened (data not shown). Histological examination of the T3-treated intestine revealed the well-documented tissue remodeling responses to T3, such as the increased thickness in muscle and connective tissue layers (Fig. 2C) (59). In the presence of T3+BPA, very little morphological change occurred (Fig. 2D).
Figure 2.
In the presence of BPA, intestinal remodeling is delayed during T3-induced metamorphosis as early as 4 d after treatment. Tadpoles of the same size and at the same stage (stage 54) were treated with T3 to initiate the metamorphic process. Four days later, the intestines were isolated, fixed, and the sections stained with hematoxylin-eosin. Representative control (A), BPA- (B), T3- (C), and T3+BPA (D)-treated tadpoles are shown. Note that the control, BPA, and T3+BPA intestine remained largely typical of tadpole intestine, as seen by the presence of a thin muscle layer, little connective tissue, and little or no adult intestinal precursor cells. Histology of T3-treated intestines revealed increased muscle layer thickness, proliferation of connective tissue, and the appearance of adult epithelial cells (the larval epithelial cells are surround by a yellow dashed ring, whereas the appearance of adult epithelial cells are represented between a black solid and the yellow dashed line). This experiment was repeated four times with similar results. Scale bar, 100 μm. AE, Adult epithelium; CT, connective tissue; LE, larval epithelium; Lu, lumen; M, muscle; Ty, typhlosole.
BPA inhibits T3-induced gene expression
To investigate whether BPA inhibits the expression of T3-response genes, we first determined whether known T3 response genes were affected by BPA. Total RNA was isolated from the intestine and qRT-PCR was performed. The expression of three early, direct T3 response genes, TRβ, stromelysin-3 (ST3), and T3-responsive basic leucine zipper transcription factor (TH/bZIP) was significantly higher in the T3-treated tadpoles than the control or BPA-treated counterparts after 4 d (Fig. 3A). The expression level of ST3 and TH/bZIP were significantly reduced in the combined T3+BPA group compared with the T3-only group, although BPA had little effect on the T3 induction of TRβ. The expression of two late, likely indirect T3 response genes, matrix metalloproteinase (MMP)-2 and the tissue inhibitor of metalloproteinase (TIMP)-2, were also significantly reduced in the T3+BPA-treated animals compared with the T3-treated animals. The expression of the third late response gene, bone morphogenetic protein (BMP)-4, in the T3+BPA-treated group was not significantly different from either the T3-treated or control group, although there was significant difference between the control and T3-treated groups (Fig. 3B). (Note that because different T3 response genes have different T3 regulation kinetics, it is possible that TRβ and BMP4 are affected by BPA at different time points). These results suggest that BPA inhibits the expression of known T3-response genes.
Figure 3.
The relative expression of known T3-inducible genes was reduced in the intestine of animals exposed to BPA. The cDNA was generated from total RNA of tadpoles treated as in Fig. 2 and subjected to qRT-PCR. A, Direct T3-response genes ST3, TH/bZIP, and TRβ were examined. B, Late T3-response genes MMP-2, TIMP2, and BMP4 were examined. The results are expressed as fold induction of the transcript with respect to the control gene, rpl8. The expected increase in relative levels of transcript with respect to rpl8 was observed in the presence of T3. For graphical presentation, results were expressed as fold induction as compared with the DMSO vehicle control. Data are shown as means ± se (n = 3; pooled samples of 10 intestines for each treatment). In the animals treated with T3+BPA, the expression levels of ST3, TH/bZIP, MMP-2, and TIMP2 genes were significantly reduced in the intestine. An asterisk indicates significant differences in mRNA expression levels between T3 and T3+BPA treatment groups (P ≤ 0.05).
BPA predominantly affects T3-signaling pathways in the intestine
To investigate whether BPA indeed inhibits T3-induced metamorphosis by blocking the T3-signaling pathways, we performed a genome-wide analysis by profiling gene expression in the intestine with a 60-mer oligonucleotide microarray (cDNA array). Because the phenotypes of BPA exposed tadpoles were similar with BPA at either concentration and reproducible, we performed the subsequent molecular analysis at the higher dose to detect the relatively small changes in gene expression caused by BPA. Total RNA was isolated from the intestine of tadpoles treated for 4 d with control solution (DMSO), BPA (10 μm), T3 (2 nm), and combined T3 (2 nm) + BPA (10 μm). For cDNA array analysis, we used a two-color labeling system, with Cy3-labeled experimental sample and Cy5-labeled universal control made of RNA isolated from whole animals of different metamorphic stages as the internal reference (supplemental Fig. S1). For each treatment group, three biological replicates, each consisting of 10 pooled intestine samples, were used. Quality control of the data were performed as previously described (63).
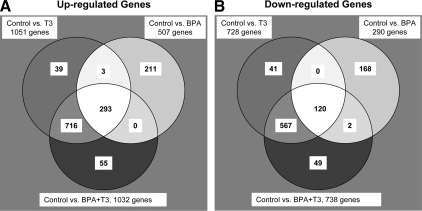
To identify significantly regulated genes, we performed ANOVA across all treatment groups with statistical significance of 10% false discovery rate with the fold change cutoff value set at 1.1 or greater for the regulated genes. Note that a relatively low cutoff was chosen because the effect of BPA on gene regulation was expectedly small. Because of the reproducibility of the cDNA array and the use of three biological replicates/treatment, it was possible to obtain statistically significant changes at this fold change cutoff. Of the 21,654 genes represented on the microarray, we found 1874 significantly regulated genes. There were 1051 and 728 genes significantly up- and down-regulated, respectively, in the T3-treated intestines compared with the controls (Fig. 4, A and B, respectively; supplemental Tables 3 and S4, respectively). Many of the genes that were differentially regulated by T3 after 4 d were similar to those reported previously (63) (data not shown). The gene regulation profiles of the T3 and combined T3+BPA samples were remarkably similar. The highest number of shared regulated genes was recorded between these two groups, in which 716 and 567 genes were exclusively shared up- and down-regulated, respectively (Fig. 4, A and B, respectively). There were 293 up-regulated genes common to all three treatments (BPA, T3, and combined T3+BPA) and 120 common down-regulated genes. Of the total number of regulated genes on the array, 211 of these genes were exclusively up-regulated and 168 of these genes were exclusively down-regulated in the BPA-only-treated group (supplemental Tables S5 and S6, respectively), suggesting that BPA can affect genes independent of the T3 pathway during development.
Figure 4.
Venn diagrams showing the number of up- and down-regulated genes for the treatment groups in comparison with that of the control group. A, A total of 1317 genes were up-regulated in response to treatment with the exogenous compounds, T3 and/or BPA. There were 293 genes commonly up-regulated in all three treatment groups, and 39, 211, and 55 genes were up-regulated only in treatment groups for T3, BPA, or T3+BPA, respectively. There were 716 genes commonly up-regulated between T3 and T3+BPA groups, three genes between T3- and BPA-only groups, and none between BPA and T3+BPA groups. B, A total number of 947 genes were down-regulated in response to treatment with the exogenous compounds, T3 and/or BPA. There were 120 genes commonly down-regulated in all three treatment groups, and 41, 168, and 49 genes were down-regulated only in treatment groups for T3, BPA, or T3+BPA, respectively. There were 567 genes commonly down-regulated between T3 and T3+BPA groups, no genes between T3- and BPA-only groups, and two genes between BPA and T3+BPA groups.
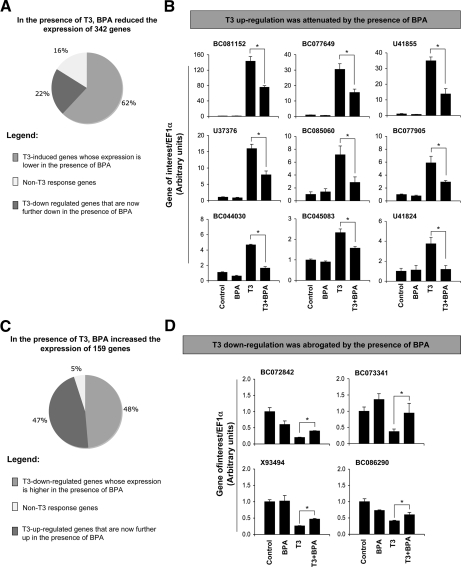
Given the inhibitory effects of BPA on all aspects of T3-induced metamorphosis, it seemed surprising that most of the up- or down-regulated genes in the T3 group were also similarly affected in the T3+BPA-treated group in comparison with the controls (Fig. 4). However, as shown above, BPA only partially blocked the regulation of established T3-response genes (Fig. 3). Thus, it is likely that BPA may globally attenuate the magnitude of T3-regulation to inhibit metamorphosis. To test this, we analyzed the microarray data and compared the expression levels of individual genes between T3- and T3+BPA-treated groups to identify T3-dependent genes whose expression was affected by the presence of BPA. Of the 21,654 genes on the microarray, 342 genes had decreased expression in the presence of BPA in the T3-treated intestines compared with T3-only-treated animals (supplemental Table S7). Among these BPA down-regulated transcripts, most (62%) of these genes were identified as T3-induced genes (compared with vehicle treated control), revealing an attenuation of T3-dependent gene activation by BPA (Fig. 5A). The remaining down-regulated genes in the T3+BPA treatment group relative to T3 alone could be subdivided into genes known to be down-regulated by T3 (when compared with vehicle treated control) whose expression was now, in the presence of BPA, further repressed (22%) and genes whose expression did not have any known T3 dependency (16%). To validate the repression of the T3-induced genes by BPA, 10 of the genes were analyzed by qRT-PCR across all treatment groups with total RNA isolated independently from that used in the microarray. The BPA regulation of all selected genes was confirmed by qRT-PCR, of which nine are represented here (Fig. 5B).
Figure 5.
Analysis and verification of genes newly identified by the microarray, whose expression was disrupted by BPA in the presence of T3. A, When the gene expression levels in the T3-treated group were compared with those in the T3+BPA-treated group, expression levels of 342 genes were reduced in the presence of BPA, most of which (60%) were T3-induced genes (i.e. their expression was up-regulated by T3 when compared with the control, untreated group). B, Verification of BPA regulation of T3-induced genes whose T3 induction was reduced by BPA. All up-regulated T3 genes tested by qRT-PCR showed that in the presence of BPA, the expression levels were reduced, confirming the findings from the microarray. C, When the gene expression levels in the T3-treated group were compared with those in the T3+BPA-treated group, the expression of 159 genes was increased by BPA, of which 48% were genes that were down-regulated by T3 (when compared with the control, untreated group) and had their down-regulation abrogated by BPA. D, Verification of BPA regulation of T3-regulated genes whose down-regulation was abrogated by the presence of BPA. All four T3-down-regulated genes tested by qRT-PCR showed that in the presence of BPA, their expression levels were partially reversed as observed by the microarray. For graphical presentation, the qRT-PCR results were expressed as fold induction as compared with the DMSO vehicle controls (control = 1), after normalization with the housekeeping gene, EF1α. Data are shown as means ± se (n = 3; pooled samples of 10 intestines for each treatment). An asterisk indicates significant differences in mRNA expression levels between T3 and T3+BPA treatment groups (P ≤ 0.05). GenBank accession numbers are shown above each chart.
In the presence of BPA+T3, 159 genes had enhanced expression when compared with T3 treatment alone (supplemental Table S8). Of these genes, 48% were down-regulated in the presence of T3 (when compared with vehicle treated control), revealing an abrogation of T3-dependent gene repression by BPA (Fig. 5C). The remaining genes showing enhanced expression in the T3+BPA treatment group relative to T3 alone, included genes known to be up-regulated by T3 (when compared with vehicle treated control), which now in the presence of BPA were further enhanced (47%), and genes that did not have any known T3 dependency (5%). Again, qRT-PCR was used to confirm the regulation of T3-response genes by BPA as found by the cDNA array. Here four genes were analyzed by qRT-PCR, and their regulation by BPA was confirmed (Fig. 5D).
The above qRT-PCR results thus confirmed the findings from microarray analysis. More importantly, the microarray results demonstrate that BPA functions mainly by inhibiting T3-pathways because most of the BPA-affected genes were T3-response genes whose regulation by T3 was attenuated by BPA.
The antimetamorphic effects of BPA are associated with inhibition of T3-dependent gene regulation programs
Whereas the major effects of BPA is the inhibition of T3 signaling pathways, it is possible that the antimetamorphic effects of BPA may be due to effects of BPA on genes independent of T3. Thus, we analyzed the genes that were regulated by T3 after 4 d of treatment. Of the total number of T3 up-regulated genes (1051), 33% of these genes were down-regulated by BPA (data not shown). Conversely, of the total number of T3 down-regulated genes (728), 36% of these genes were up-regulated by BPA (data not shown). Interestingly, when we ranked the T3-induced genes from most dramatically regulated to the least regulated, we found that the majority of the 50 most dramatically T3 up-regulated genes (≥2.5-fold induction by T3) were inhibited by BPA (Fig. 6A). Similarly, the T3 repression of most of the 50 dramatically T3 down-regulated genes (≥1.9-fold repression by T3) was reduced/abrogated by BPA (Fig. 6B). Moreover, by incorporating microarray data of T3-responsive genes in the tail, hindlimb, and brain (64), we observed that the vast majority of these dramatically regulated genes that are also induced by T3 in other organs were inhibited by BPA in the presence of T3 (Fig. 6A). Conversely, of the 12 genes that are known to be down-regulated by T3 in multiple organs, T3 repression of seven genes was abrogated by BPA (Fig. 6B). These results suggest that BPA inhibits most of the genes highly up-regulated by T3. The reason for our failure to detect BPA inhibition of genes less significantly up-regulated by T3 was most likely because their regulation by T3 was approaching the lower limit of the cDNA array analysis, thus making the regulation by BPA fall below the detection limit. Because gene regulation by T3-bound TR is both necessary and sufficient for amphibian metamorphosis, these results suggest that BPA inhibits metamorphosis because it blocks most of the T3-signaling pathways.
Figure 6.
BPA inhibits the genes most significantly regulated by T3. A, The expression of most of the top 50 significantly T3-up-regulated genes in the intestine is reduced by BPA. The genes in shade are attenuated by BPA. B, Most of the top 50 significantly T3-down-regulated genes in the intestine have their T3-dependent repression reduced by the presence of BPA. The genes in shade are abrogated by BPA. *, The gene is also significantly regulated by T3 in the tail (t), limb (l), and brain (b), respectively. NP, Gene not present in the earlier cDNA array used for the analysis of the organs t, l, and b (64). Blanks under the other organs indicate genes are not significantly regulated by T3 in t, l, and b.
Discussion
In the present study, we characterized for the first time global gene expression changes associated with BPA exposure by using amphibian metamorphosis as our experimental model. This model was favorable over mammalian models because the in vivo screening process was quicker and the influence of maternal hormones and the difficulty in manipulating the uterus-enclosed embryo were eliminated. Whereas BPA was able to regulate many genes in premetamorphic tadpoles in the absence of T3, there was no detectible morphologic phenotype, making it difficult to determine the significance. We thus focused our analysis on the effect of BPA during metamorphosis, i.e. when T3 is also present. Our microarray analysis revealed novel findings. First, BPA inhibited the regulation of most T3-dependent responsive genes, which presumably underlie the inhibition of metamorphosis by BPA, which was not evident from limited analyses in earlier studies. Second and more importantly, BPA predominantly affected T3-signaling pathways during metamorphosis, although the influence of BPA on estrogen-signaling pathways in metamorphosing tadpoles cannot be dismissed. Our findings thus point to the critical need, even for EDCs of known effects, to have suitable developmental models to analyze the potential effects of EDCs on human embryonic and postembryonic development.
Of the two BPA concentrations used in this study, the lower concentration (0.1 μm) closely resembled the estimated BPA exposure level in human infants (see Materials and Methods). Both doses inhibited TRβ-induced transcription in the frog oocyte system. Furthermore, whereas the two doses ranged 100-fold, both inhibited T3-induced metamorphosis reproducibly with the higher dose resulting in a more dramatic inhibition. These findings are in strong support that BPA acts as a T3 antagonist in vivo. Given that high levels of T3 are critical for human development, especially during the late-embryonic and neonatal period that share many similarities with frog metamorphosis (12,13,14,17,19,70,71), our results argue that BPA represents a serious risk to human development through disruption of T3 signaling pathways.
Using microarray, we found that after 4 d of treatment, the regulation of about 33% of the T3-induced genes and 36% of the T3-repressed genes were inhibited by BPA. Interestingly, the majority of the most dramatically T3-regulated genes were affected in the presence of BPA. Many of these genes are early and/or direct target genes of T3 and are likely important for metamorphosis. For example, ST3 is a direct T3 target gene and has been shown to be necessary for apoptosis and tissue morphogenesis during intestinal metamorphosis (72,73). Thus, its inhibition by BPA may contribute to the blockage of intestinal remodeling during metamorphosis by BPA. In addition, most of these BPA-affected T3-response genes are ubiquitously regulated by T3 in different organs as suggested by our metatissue analysis. All these and the fact that gene regulation by T3-bound TR is necessary and sufficient for amphibian development (60,74) strongly argue that the BPA inhibition of these most dramatically T3-regulated genes, including ST3, is the underlying cause for the inhibition of metamorphosis by BPA. The failure to observe significant effects by BPA on many less dramatically regulated T3-response genes is presumably due to the difficulty to detect the relatively small changes in their expression caused by BPA with microarray analysis.
Whereas one may expect that BPA inhibit metamorphosis by disrupting T3 signaling, it is surprising that the vast majority of the genes affected by BPA are T3-response genes. Of the BPA down-regulated genes in the presence of T3, 60% were T3-induced genes whose activation by T3 was now reduced/eliminated by BPA. Conversely, about 50% of the BPA up-regulated genes in the presence of T3 were T3-down-regulated genes whose down-regulation by T3 was reduced/eliminated by BPA. Only about 20% of the BPA-regulated genes in the presence of T3 were completely independent of T3-signaling process. Our studies thus indicate that developmental context has a major role in determining the pathways by which BPA interacts in vivo. In this regard, it is worth noting that T3, but not other hormones, is the causative agent of amphibian metamorphosis and hence intestinal remodeling (15). Whereas it is possible that potential cross talks between TR and estrogen receptor (ER)-signaling pathways (42,43,75) may allow BPA to affect T3-pathway through ER, the fact that most of the BPA regulated genes are T3-response genes argue against this. In addition, as discussed above, most of the dramatically T3-regulated genes are affected by BPA, suggesting that BPA is likely targeting TRs directly during metamorphosis. Currently there are no data on the expression profiles of estrogens and ERα in the intestine during development, although ERα mRNA could be detected in whole-body premetamorphic tadpoles and were up-regulated after prolonged T3 treatment in the liver (76,77,78). Our microarray analysis showed no regulation by BPA in the expression of two known estrogen-response genes, ERα (AY310905, L20736) and steroid-5-α-reductase (BQ732157) (76,79), from BPA or combined T3+BPA treatments. Furthermore, treatment with T3 alone did not change their gene expression, suggesting that there does not appear to be any cross-regulation between estrogens and T3 in the metamorphic intestine. It is possible that the lack of significant ER in the tadpole intestine may be the underlying cause for the observed dominant effects of BPA on T3-signaling process during metamorphosis in this study.
In summary, our findings demonstrate that BPA, which is one of the most prevalent chemicals for daily use, suppresses transcriptional activity of ligand-bound TR during vertebrate development. Moreover, genome-wide analysis leads to two major conclusions. First, the inhibitory effect of BPA on metamorphosis is due to the inhibition of the T3 pathway. Endocrine disruptor studies normally focus on the regulation of one or a few genes; the pathways involving these genes may or may not have any significant contribution to the biological effects of the disruptor. This argues for genome-wide molecular analysis of the effect of endocrine disruptors. Second, the major effect of BPA in developing tadpoles is on the T3, but not estrogenic pathways, which would be expected based on previous BPA studies in vitro and in adult animals, although estrogenic pathways are also likely to be affected by BPA. This argues that the effects of an endocrine disruptor are tissue and developmental stage dependent and that in vivo studies coupled with genome-wide molecular gene regulation analysis are needed to assess the biological effects of an endocrine disruptor and the underlying molecular mechanism. Our findings further demonstrate the unique advantages of combining morphological analysis with genome-wide gene expression studies in amphibians to determine the molecular pathways that underlie a developmental consequence of an EDC, especially for those affecting T3 pathways. The diverse array of EDCs that may disrupt T3 levels and the potential for concurrent exposure to many of these compounds make it imperative to use in vivo developmental models to appreciate the effects of EDCs on vertebrate development. This will help to ensure that important environmental health and developmental consequences of EDC exposure are not overlooked.
Supplementary Material
Footnotes
This work was supported in part by the Intramural Research Program, National Institute of Child Health and Human Development, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 19, 2009
Abbreviations: BMP, Bone morphogenetic protein; BPA, bisphenol A; DMSO, dimethyl sulfoxide; EDC, endocrine disrupting compound; EF-1α, elongation factor-1α; ER, estrogen receptor; MMP, matrix metalloproteinase; rpl8, ribosomal protein L8; qRT-PCR, quantitative RT-PCR; RXR, retinoid X receptor; ST3, stromelysin-3; TH/bZIP, T3-responsive basic leucine zipper transcription factor; TIMP, tissue inhibitor of metalloproteinase; TR, T3 receptor.
References
- Guillete Jr LJ 2006 Endocrine disrupting contaminants—beyond the dogma. Environ Health Perspect 114:9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, Skakkebaek NE, Main KM 2006 Environmental chemicals and thyroid function. Eur J Endocrinol 154:599–611 [DOI] [PubMed] [Google Scholar]
- Crews D, McLachlan JA 2006 Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology 147:S4–S10 [DOI] [PubMed] [Google Scholar]
- Maffini MV, Rubin BS, Sonnenschein C, Soto AM 2006 Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol 254–255:178–186 [DOI] [PubMed] [Google Scholar]
- Wolff MS, Weston A 1997 Breast cancer risk and environmental exposures. Environ Health Perspect 105:891–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Jørgensen N, Carlsen E, Peterson PM, Giwercman A, Andersen A-G, Jensen TK, Andersson A-M, Müller J 1998 Germ cell cancer and disorders of spermatogenesis: an environmental connection? APMIS 106:3–12 [DOI] [PubMed] [Google Scholar]
- Jensen TK, Toppari J, Keiding N, Skakkebaek NE 1995 Do environmental estrogens contribute to the decline in male reproductive health? Clin Chem 41:1896–1901 [PubMed] [Google Scholar]
- Ouellet M, Bonin J, Rodrigue J, DesGranges JL, Lair S 1997 Hindlimb deformities (ectromelia, ectrodactyly) in free-living anurans from agricultural habitats. J Wildl Dis 33:95–104 [DOI] [PubMed] [Google Scholar]
- Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL 2000 Quantitative evidence for global amphibian population declines. Nature 404:752–755 [DOI] [PubMed] [Google Scholar]
- Hayes TB, Case P, Chui S, Chung D, Haeffele C, Haston K, Lee M, Mai VP, Marjuoa Y, Parker J, Tsui M 2006 Pesticide mixtures, endocrine disruption, and amphibian declines: are we understanding the impact? Environ Health Perspect 114:40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A 2003 Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environ Health Perspect 111:568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar MA 1993 Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14:184–193 [DOI] [PubMed] [Google Scholar]
- Yen PM 2001 Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- Tata JR 1993 Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays 15:239–248 [DOI] [PubMed] [Google Scholar]
- Shi Y-B 1999 Amphibian metamorphosis: from morphology to molecular biology. New York: John Wiley, Sons, Inc. [Google Scholar]
- Atkinson BG 1994 Metamorphosis: model systems for studying gene expression in postembryonic development. Dev Genet 15:313–319 [Google Scholar]
- Hetzel BS 1989 The story of iodine deficiency: an international challenge in nutrition. Oxford, UK: Oxford University Press [Google Scholar]
- Denver RJ 1996 Neuroendocrine control of amphibian metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG, eds. Metamorphosis postembryonic reprogramming of gene expression in amphibian and insect cells. San Diego: Academic Press; 433–464 [Google Scholar]
- Howdeshell KL 2002 A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect 110:337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump D, Werry K, Veldhoen N, Van Aggelen G, Helbing CC 2002 Exposure to the herbicide acetochlor alters thyroid hormone-dependent gene expression and metamorphosis in Xenopus laevis. Environ Health Perspect 110:1199–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T 2002 Clues from wildlife to create and assay for thyroid system disruption. Environ Health Perspect 110:363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT 2007 Environmental chemicals impacting the thyroid: targets and consequences. Thyroid 17:811–817 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM 1995 The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- Ito M, Roeder RG 2001 The TRAP/SMCC/Mediator complex and thyroid hormone receptor function. Trends Endocrinol Metab 12:127–134 [DOI] [PubMed] [Google Scholar]
- Rachez C, Freedman LP 2000 Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene 246:9–21 [DOI] [PubMed] [Google Scholar]
- Zhang J, Lazar MA 2000 The mechanism of action of thyroid hormones. Annu Rev Physiol 62:439–466 [DOI] [PubMed] [Google Scholar]
- Burke LJ, Baniahmad A 2000 Co-repressors 2000. FASEB J 14:1876–1888 [DOI] [PubMed] [Google Scholar]
- Jones PL, Shi Y-B 2003 N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors. In: Workman JL, ed. Current topics in microbiology and immunology: protein complexes that modify chromatin. Berlin: Springer-Verlag; 237–268 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW 2001 Nuclear receptors, coregulators, ligands, and selective receptor modulators: making sense of the patchwork quilt. Ann NY Acad Sci 949:3–5 [DOI] [PubMed] [Google Scholar]
- Rachez C, Freedman LP 2001 Mediator complexes and transcription. Curr Opin Cell Biol 13:274–280 [DOI] [PubMed] [Google Scholar]
- McKinney JD, Waller CL 1998 Molecular determinants of hormone mimicry: halogenated aromatic hydrocarbon environmental agents. J Toxicol Environ Health B Crti Rev 1:27–58 [DOI] [PubMed] [Google Scholar]
- Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, Guillette Jr LJ 2007 An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol 24:225–239 [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL 2005 Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 113:391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I 2002 Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect 110:A703–A707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Yokouchi Y, Otsuki A, Itoh H 2001 Depth profiles of volatile halogenated hydrocarbons in seawater in the Bay of Bengal. Chemosphere 45:371–377 [DOI] [PubMed] [Google Scholar]
- Reporter CM 1999 ChemExpo chemical profile: bisphenol A. New York: Schnell Publishing Co. [Google Scholar]
- Lewis JB, Rueggeberg FA, Lapp CA, Ergle JW, Schuster GS 1999 Identification and characterization of estrogen-like components in commercial resin-based dental restorative materials. Clin Oral Invest 3:107–113 [DOI] [PubMed] [Google Scholar]
- Staples CA, Dorn PB, Klecka GM, O'Block ST, Harris LR 1998 A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 36:2149–2173 [DOI] [PubMed] [Google Scholar]
- Howe SR, Borodinsky L, Lyon RS 1998 Potential exposure to bisphenol A from food-contact use of epoxy coated cans. J Coat Technol 70:69–74 [Google Scholar]
- vom Saal FS, Hughes C 2005 An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect 113:926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan NS, Duarte P, Wade MG, Lean DR, Trudeau VL 2008 Estrogenic exposure affects metamorphosis and alters sex rations in the northern leopard frog (Rana pipiens): identifying critically vulnerable periods of development. Gen Comp Endocrinol 156:515–523 [DOI] [PubMed] [Google Scholar]
- Hogan NS, Crump KL, Duarte P, Lean DR, Trudeau VL 2007 Hormone cross-regulation in the tadpole brain: developmental expression profiles and effect of T3 exposure on thyroid hormone- and estrogen-responsive gene in Rana pipiens. Gen Comp Endocrinol 154:5–15 [DOI] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K 2002 Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab 87:5185–5190 [DOI] [PubMed] [Google Scholar]
- Seiwa C, Nakahara J, Komiyama T, Katsu Y, Iguchi T, Asou H 2004 Bisphenol A exerts thyroid-hormone-like effects on mouse oligodendrocyte precursor cells. Neuroendocrinology 80:21–30 [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C 2005 Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 146:607–612 [DOI] [PubMed] [Google Scholar]
- Gray KM, Janssens PA 1990 Gonadal hormones inhibit the induction of metamorphosis by thyroid hormones in Xenopus laevis tadpoles in vivo, but not in vitro. Gen Comp Endocrinol 77:202–211 [DOI] [PubMed] [Google Scholar]
- Richards CM, Nace GW 1978 Gynogenetic and hormonal sex reversal used in tests of the XX-XY hypothesis of sex determination in Rana pipiens. Growth 42:319–331 [Google Scholar]
- Hayes TB 1997 Steroids as potential modulators of thyroid hormone activity in anuran metamorphosis. Am Zool 37:185–194 [Google Scholar]
- Iwamuro S, Sakakibara M, Terao M, Ozawa A, Kurobe C, Shigeura T, Kato M, Kikuyama S 2003 Teratogenic and anti-metamorphic effects of bisphenol A on embryonic and larval Xenopus laevis. Gen Comp Endocrinol 133:189–198 [DOI] [PubMed] [Google Scholar]
- Iwamuro S, Yamada M, Kato M, Kikuyama S 2006 Effects of bisphenol A on thyroid hormone-dependent up-regulation of thyroid hormone receptor α and β and down-regulation of retinoid X receptor γ in Xenopus tail culture. Life Sci 279:2165–2171 [DOI] [PubMed] [Google Scholar]
- Goto Y, Kitanura S, Kashiwagi K, Oofusa K, Tooi O, Yoshizato K, Sato J, Ohta S, Kashiwagi A 2006 Suppression of amphibian metamorphosis by bisphenol A and related chemical substances. J Health Sci 52:160–168 [Google Scholar]
- Fini JB, Le Mevel S, Turque N, Palmier K, Zalko D, Cravedi JP, Demeneix BA 2007 An in vivo multiwell-based fluorescent screen for monitoring vertebrate thyroid hormone disruption. Environ Sci Technol 41:5908–5914 [DOI] [PubMed] [Google Scholar]
- Kaneko M, Okada R, Yamamoto K, Nakamura M, Mosconi G, Polzonetti-Magni AM, Kikuyama S 2008 Bisphenol A acts differently from and independently of thyroid hormone in suppressing thyrotropin release from the bullfrog pituitary. Gen Comp Endocrinol 155:574–580 [DOI] [PubMed] [Google Scholar]
- Kloas W, Lutz I, Einspanier R 1999 Amphibians as a model to study endocrine disruptors: II. Estrogenic activity of environmental chemicals in vitro and in vivo. Sci Total Environ 225:59–68 [DOI] [PubMed] [Google Scholar]
- Levy G, Lutz I, Krüger A, Kloas W 2004 Bisphenol A induces feminization in Xenopus laevis tadpoles. Environ Res 94:102–111 [DOI] [PubMed] [Google Scholar]
- Pickford DB, Hetheridge MJ, Caunter JE, Hall AT, Hutchinson TH 2003 Assessing chronic toxicity of bisphenol A to larvae of the African clawed frog (Xenopus laevis) in a flow-through exposure system. Chemosphere 53:223–235 [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shi YB 2007 Regulation of adult intestinal epithelial stem cell development by thyroid hormone during Xenopus laevis metamorphosis. Dev Dyn 236:3358–3368 [DOI] [PubMed] [Google Scholar]
- Shi YB, Ishizuya-Oka A 1996 Biphasic intestinal development in amphibians: embryogenesis and remodeling during metamorphosis. Curr Top Dev Biol 32:205–235 [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Fu L, Shi YB 2006 Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol 145:1–19 [DOI] [PubMed] [Google Scholar]
- Wong J, Shi YB 1995 Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem 270:18479–18483 [DOI] [PubMed] [Google Scholar]
- Amano T, Leu K, Yoshizato K, Shi Y-B 2002 Thyroid hormone regulation of a transcriptional coactivator in Xenopus laevis: implication for a role in postembryonic tissue remodeling. Dev Dyn 223:526–535 [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Heimeier RA, Das B, Washington T, Shi Y-B 2007 Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol 303:576–590 [DOI] [PubMed] [Google Scholar]
- Das B, Cai L, Carter MG, Piao YL, Sharov AA, Ko MS, Brown DD 2006 Gene expression changes at metamorphosis induce by thyroid hormone in Xenopus laevis tadpoles. Dev Biol 291:342–355 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y 1995 Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B 57:289–300 [Google Scholar]
- Hamatani T, Carter MG, Sharov AA, Ko MS 2004 Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell 6:117–131 [DOI] [PubMed] [Google Scholar]
- Sharov AA, Dudekula DB, Ko MS 2005 A web-based tool for principal component and significance analysis of microarray data. Bioinformatics 21:2548–2549 [DOI] [PubMed] [Google Scholar]
- Tomita A, Buchholz DR, Shi YB 2004 Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol Cell Biol 24:3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Cai L, Brown DD 2005 Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc Natl Acad Sci USA 102:3720–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreale de Escobar G 2001 The role of thyroid hormone in fetal neurodevelopment. J Pediatr Endocrinol Metab 14:1453–1462 [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F 2004 Role of thyroid hormone during early brain development. Eur J Endocrinol 151:U25–U37 [DOI] [PubMed] [Google Scholar]
- Fu L, Ishizuya-Oka A, Buchholz DR, Amano T, Matsuda H, Shi YB 2005 A causative role of stromelysin-3 in extracellular matrix remodeling and epithelial apoptosis during intestinal metamorphosis in Xenopus laevis. J Biol Chem 280:27856–27865 [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Li Q, Amano T, Damjanovski S, Ueda S, Shi YB 2000 Requirement for matrix metalloproteinase stromelysin-3 in cell migration and apoptosis during tissue remodeling in Xenopus laevis. J Cell Biol 150:1177–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havis E, Le Mevel S, Dubois GM, Shi DL, Scanlan TS, Demeneix BA, Sachs LM 2006 Unliganded thyroid hormone receptor is essential for Xenopus laevis eye development. EMBO J 25:4943–4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau VL, Turque N, Le Mével S, Alliot C, Gallant N, Coen L, Pakdel F, Demeneix B 2005 Assessment of estrogenic endocrine-disrupting chemical actions in the brain using in vivo somatic gene transfer. Environ Health Perspect 113:329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögi C, Levy G, Lutz I, Kloas W 2002 Functional genomics and sexual differentiation in amphibians. Comp Biochem Physiol B Biochem Mol Biol 133:559–570 [DOI] [PubMed] [Google Scholar]
- Tata JR, Baker BS, Machuca I, Rabelo EM, Yamauchi K 1993 Autoinduction of nuclear receptor genes and its significance. J Steroid Biochem Mol Biol 46:105–119 [DOI] [PubMed] [Google Scholar]
- Rabelo EM, Tata JR 1993 Thyroid hormone potentiates estrogen activation of vitellogenin genes and autoinduction of estrogen receptor in adult Xenopus hepatocytes. Mol Cell Endocrinol 96:37–44 [DOI] [PubMed] [Google Scholar]
- Urbatzka R, Lutz I, Kloas W 2007 Aromatase, steroid-5α-reductase type 1 and type 2 mRNA expression in gonads and in brain of Xenopus laevis during ontogeny. Gen Comp Endocrinol 53:280–288 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.