Abstract
CCL19 and CCL21 are endogenous agonists for the seven-transmembrane receptor CCR7. They are equally active in promoting G protein stimulation and chemotaxis. Yet, we find that they result in striking differences in activation of the G protein-coupled receptor kinase (GRK)/ß-arrestin system. CCL19 leads to robust CCR7 phosphorylation and β-arrestin2 recruitment catalyzed by both GRK3 and GRK6 whereas CCL21 activates GRK6 alone. This differential GRK activation leads to distinct functional consequences. Although each ligand leads to β-arrestin2 recruitment, only CCL19 leads to redistribution of β-arrestin2-GFP into endocytic vesicles and classical receptor desensitization. In contrast, these agonists are both capable of signaling through GRK6 and β-arrestin2 to ERK kinases. Thus, this mechanism for “ligand bias” whereby endogenous agonists activate different GRK isoforms leads to functionally distinct pools of β-arrestin.
Keywords: CCR7, CCL19, CCL21, chemokine, arrestin
Traditional agonists of seven-transmembrane receptors (7TMRs) lead to the simultaneous activation of G protein- and β-arrestin-dependent signaling pathways through a series of broadly conserved biochemical steps (1). Receptor activation promotes the dissociation of heterotrimeric G proteins into free Gα and Gβγ subunits and subsequent second messenger signaling. Agonist-activated 7TMRs undergo phosphorylation by G protein-coupled receptor kinases (GRKs), and this promotes the binding of β-arrestins (2). β-Arrestins desensitize 7TMR-mediated G protein signaling at several mechanistic levels including direct steric hindrance of the receptor by β-arrestin attachment (3), recruitment of 2nd messenger-degrading enzymes (4, 5), and by acting as a scaffold for proteins that facilitate receptor internalization (6).
β-Arrestin binding also initiates additional signaling programs through coordinated recruitment of signaling intermediates to activated receptors. For instance, β-arrestin-dependent signaling to MAP kinase has been extensively characterized and is demonstrable for many 7TMRs (7, 8). Proteomic approaches predict an “interactome” of hundreds of additional binding partners (9).
Recently, “β-arrestin-biased” synthetic ligands have been described for the angiotensin 1A receptor (10), β2-adrenergic receptor (β2AR) (11, 12), and parathyroid hormone receptor (13). These ligands each cause receptor phosphorylation and β-arrestin-mediated effects in the absence of G protein activation. The mechanism of ligand bias is poorly understood although biophysical studies suggest that agonists and receptors oscillate among multiple conformations, some of which may be capable of differential signaling (14, 15).
Given the complexity of β-arrestin function, the traditional concept of GRKs as redundant kinases is likely overly simplistic. GRKs 2, 3, 5, and 6 are ubiquitously expressed. The extent to which they provide redundancy versus functional specialization is largely unknown, but evidence for the latter has recently emerged in some receptor systems. For instance, GRK5/6 appear to be uniquely required for β-arrestin-mediated MAP kinase signaling (16, 17).
The chemokine receptor CCR7 is particularly important for immune responses requiring the coordinated interaction of various cell types within lymphoid tissues (18, 19). It is unique for its high expression on mature, naïve T lymphocytes and is vital for homeostatic recirculation of these cells through secondary lymphoid tissues. CCR7 has two endogenous ligands, CCL19 and CCL21, which have evolved from a series of complex gene duplications and retain 32% amino acid sequence homology (20–22). They are predicted to be structurally similar except that CCL21 has a conserved motif consisting of an additional 37 amino acids at its C terminus (23). CCL19 and CCL21 have been shown to have similar binding affinities (Kd ≈100 pM), equal efficacy for G protein activation, calcium flux, and chemotactic responses (20–22, 24–26). Yet, some signaling characteristics appear to be unique to CCL19-activated CCR7. For instance, CCL19 but not CCL21 is reported to lead to receptor desensitization (20), internalization (27, 28), receptor degradation (29), and rapid dendrite extension (30). Recently, Kohout et al. (31) have reported that CCL19 but not CCL21 also leads to receptor phosphorylation and β-arrestin recruitment. Here, we examine the role of individual GRKs in orchestrating the differential signaling of CCR7 with particular emphasis on β-arrestin-mediated functions.
Results
Gi/o Protein Signaling.
CCL19 and CCL21 have been shown to have equal binding affinities for CCR7 and equivalent efficacy and potency for the activation of the Gi/o subfamily of G proteins (21, 24, 25). To confirm this in live cells, we designed an assay to study Gi/o-mediated inhibition of adenylate cyclase by using the cAMP biosensor ICUE2 (32, 33). Human embryonic kidney (HEK) cells stably coexpressing CCR7 and ICUE2 were treated with forskolin (50 μM) in the absence or presence of saturating concentrations of CCL19 (100 nM) or CCL21 (100 nM). Forskolin stimulation results in a large decrease in ICUE2 FRET, indicating an increase in intracellular cAMP concentration (Fig. 1A). Coincubation of forskolin with either CCR7 ligand led to a blunted response indicative of simultaneous Gi/o signaling. There was no difference between CCL19 and CCL21 for the inhibition of forskolin responsiveness, indicating that these ligands have comparable efficacy for Gi/o signaling.
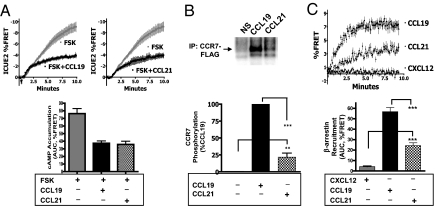
Fig. 1.
CCL19 and CCL21 lead to equivalent G protein responses but differential CCR7 phosphorylation and ß-arrestin2 recruitment. (A) HEK-293 cells stably expressing CCR7 and the cAMP biosensor ICUE2 were stimulated with 50 μM forskolin in the presence or absence of saturating concentrations of CCL19 (100 nM) or CCL21 (100 nM). cAMP accumulation was measured as the change in ICUE2 FRET ratio. (Upper) Change in FRET (mean ± SE, n = 4). (Lower) Integrated response after 10 min. (B) HEK-293 cells stably expressing CCR7-FLAG were labeled with 32Pi and stimulated at 37 °C for 10 min with or without 100 nM CCL19 or 100 nM CCL21 as indicated. (Upper) Representative image showing 32Pi incorporation. (Lower) Bar graph that represents the mean ± SE from six independent experiments. (C) Live HEK-293 cells stably expressing CCR7-CFP and ß-arrestin2-YFP were stimulated with 100 nM CXCL12, 100 nM CCL21, or 100 nM CCL19, and the recruitment of ß-arrestin2 was measured by FRET. Data represent the mean ± SE from six experiments. Statistical significance was determined by paired two-tailed t tests. **, P < 0.01; ***, P < 0.001.
CCR7 Phosphorylation and β-Arrestin2 Recruitment.
The ability to undergo agonist-induced phosphorylation is a hallmark of 7TMRs and an important, proximate indicator of GRK function. To compare CCL19 and CCL21 for bulk receptor phosphorylation, we analyzed the incorporation of 32Pi into CCR7-FLAG after 10 min of stimulation. CCL19 (100 nM) led to robust CCR7 phosphorylation (Fig. 1B), whereas CCL21 (100 nM) mediated a much smaller but consistent level of CCR7 phosphorylation (21.6 ± 6.1% of that induced by CCL19).
Next, we sought to compare β-arrestin2 recruitment with CCR7 in response to CCL19 and CCL21. Live HEK cells stably expressing CCR7-CFP and β-arrestin2-YFP were stimulated with 100 nM CCL19 or 100 nM CCL21, and the time course of β-arrestin recruitment to the receptor was analyzed by FRET (Fig. 1C). CCL19 promoted a robust interaction between the receptor and β-arrestin2 whereas the effect of CCL21 was weak by comparison.
Taken together, these findings demonstrate that CCL21 is, in fact, a biased ligand with equivalent efficacy for G protein-related activity but differential activation of the GRK/β-arrestin system. Although differences in receptor phosphorylation can be the result of either kinase or phosphatase activity, in this case the proximate step in the differential activation of the GRK/β-arrestin system appears to be unequal kinase activity. Importantly, however, we found that the bias between CCR7 ligands is not absolute, and CCL21 did lead to detectable bulk phosphorylation and β-arrestin recruitment.
β-Arrestin2 Trafficking and Desensitization.
β-Arrestin recruitment to 7TMRs generally occurs in one of two patterns (34). “Class A” recruitment leads to β-arrestin2-GFP-forming puncta at the cell membrane. In contrast, “class B” receptors traffic with β-arrestin2-GFP into early endosomes. To compare the two ligands for their pattern of β-arrestin2 recruitment, HEK cells were transfected with CCR7 and β-arrestin2-GFP. Cells were stimulated with CCL19 (100 nM) or CCL21 (100 nM) for 60 min, and live cells were visualized by confocal microscopy. Approximately 60% of GFP-expressing cells showed β-arrestin2-GFP redistribution after treatment with CCL19. This β-arrestin trafficking in response to CCL19 was observed exclusively as a class B pattern (Fig. 2A). In contrast, stimulation with CCL21 resulted in no evident redistribution of β-arrestin2-GFP, neither class A nor class B, suggesting that this interaction is even more transient than that typically seen for class A receptors.
Fig. 2.
CCL19, but not CCL21, leads to ß-arrestin2 trafficking to endocytic vesicles and desensitization. (A) HEK-293 cells were transiently transfected with CCR7 and ß-arrestin2-GFP. Cells were left unstimulated or stimulated with 100 nM CCL19 or 100 nM CCL21 for 1 h. Live cells were imaged by confocal microscopy, and representative images from three independent experiments are shown. (B) HEK-293 cells stably expressing CCR7 and the cAMP biosensor ICUE2 were pretreated with 100 nM CCL19 or 100 nM CCL21 for 1 h as indicated. Cells were washed and stimulated with forskolin alone (50 μM) or 100 nM CCL21 + forskolin. The bar graft shows cAMP responses as measured by the integrated ICUE2 FRET after 10 min. Statistical significance was determined by paired two-tailed t tests. **, P < 0.01.
β-Arrestin recruitment classically leads to agonist-induced receptor desensitization. To compare the CCR7 ligands for their ability to induce desensitization, HEK cells stably expressing CCR7 and ICUE2 were preincubated with CCL19 (100 nM) or CCL21 (100 nM) for 60 min. Cells were then washed, and Gi/o activity was assessed by restimulation with CCL21 (100 nM) and forskolin (50 μM). We found that preincubation with CCL19 leads to complete desensitization of CCR7 toward subsequent G protein activation (Fig. 2B). In contrast, desensitization was not observed after preincubation with CCL21.
β-Arrestin2-Mediated MAPK Signaling.
In addition to desensitization, β-arrestins also function as scaffolds to facilitate signaling pathways such as ERK activation. Next, we sought to compare CCL19 and CCL21 for their ability to activate MAP kinase in a β-arrestin-dependent fashion.
Similar to other Gi-coupled receptors, we find that CCR7-mediated ERK activation is ablated by preincubation with pertussis toxin (Fig. S1). Thus, G protein activation appears to be a prerequisite for MAP kinase signaling. CCR7 mediated ERK is also highly dependent on β-arrestins (Fig. 3). Activation of ERK was dependent on β-arrestin2 and was opposed by β-arrestin1 expression. This is a pattern of β-arrestin dependence consistent with other similarly studied 7TMRs such as the angiotensin 1A receptor (7, 35). Surprisingly, we did not observe differential β-arrestin-mediated phospho-ERK between CCL19 and CCL21. This was unexpected in light of the preferential GRK/β-arrestin activation seen with CCL19. This led us to investigate further the proximate steps of CCR7 signaling to determine the contribution of individual GRK isoforms for CCR7 phosphorylation, β-arrestin recruitment, and β-arrestin-mediated signaling.
Fig. 3.
CCL19 and CCL21 lead to ß-arrestin2-dependent MAP kinase. HEK-293 cells stably expressing CCR7 were transfected with either control siRNA or siRNA specific to ß-arrestin1, ß-arrestin2, or both ß-arrestins. ß-Arrestin expression was determined by immunoblotting. Cells were serum starved for 2 h and then stimulated with 100 nM CCL19 or 100 nM CCL21 at 37 °C for 5 min. Lysates were prepared, separated by SDS/PAGE, and immunoblotted for phospho-ERK and total ERK. Phospho-ERK levels were normalized to total ERK levels and expressed as a percentage of CCL19-mediated ERK. Statistical significance was determined by paired two-tailed t tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
GRK Specificity for CCR7 Phosphorylation.
Next, we examined the effect of individual GRKs on CCR7 phosphorylation. HEK cells stably expressing CCR7-FLAG were transfected with control siRNA or siRNA specific for GRK2, GRK3, or GRK6. (GRK5 expression could not be assessed reliably by using available antibodies.) The effect of siRNA on the expression of individual GRKs was specific and typically resulted in >90% reduction in protein expression (Fig. 4A).
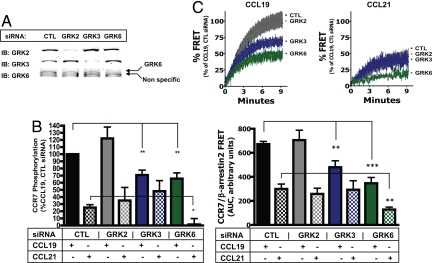
Fig. 4.
Differential phosphorylation and β-arrestin recruitment by CCL19 and CCL21 are caused by strict specificity and bias among GRK isoforms for activated CCR7. (A) HEK-293 cells stably expressing CCR7-FLAG or CCR7-CFP/ß-arrestin2-YFP were transfected with control siRNA or siRNA specific for GRK2, GRK3, or GRK6. A representative immunoblot showing the effect of siRNA on GRK expression is shown. (B) Agonist-induced phosphorylation for each condition is expressed as a percentage of CCL19-mediated phosphorylation in control siRNA-treated cells. The mean ± SE from four to nine independent experiments is represented by the bar graph and demonstrates the effect of GRK expression on CCR7 bulk phosphorylation. (C) Cells were stimulated with 100 nM CCL19 or 100 nM CCL21, and the recruitment of ß-arrestin2 was measured by FRET and normalized to CCL19 responses in control transfected cells. The bar graft shows the effect of GRK expression on the integrated FRET response after 10 min in response to each ligand. Data represent the mean ± SE from three to eight independent experiments, each done in duplicate. Statistical significance was determined by paired two-tailed t tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001).
Agonist-induced receptor phosphorylation in response to CCL19 (100 nM) and CCL21 (100 nM) was measured by analyzing the incorporation of 32Pi into CCR7-FLAG after 10 min of stimulation (Fig. 4B). We found that loss of GRK2 did not have an appreciable effect on either CCL19- or CCL21-mediated phosphorylation. In contrast, GRK3 siRNA led to a significant reduction in CCL19-mediated phosphorylation, but there was no loss of CCL21-mediated phosphorylation. Surprisingly, loss of GRK6 led to a significant reduction of phosphorylation in response to both CCL19 and CCL21 and in fact ablated phosphorylation induced by CCL21.
GRK Specificity for ß-Arrestin Recruitment.
Next, we determined the effect of individual GRKs on agonist-induced β-arrestin recruitment by FRET. HEK cells stably expressing CCR7-CFP and β-arrestin2-YFP were transfected with control siRNA or siRNA specific for GRK2, GRK3, or GRK6. Cells were then stimulated with CCL19 (100 nM) or CCL21 (100 nM), and the recruitment of β-arrestin2 to CCR7 was measured by FRET (Fig. 4C). Loss of GRK2 had no appreciable effect on either CCL19- or CCL21-mediated β-arrestin recruitment. β-Arrestin recruitment in the absence of GRK3 after CCL19 stimulation was reduced, whereas that after CCL21 stimulation was not. The absence of GRK6 resulted in reduced β-arrestin recruitment to both CCL19 and CCL21.
These data are striking in that they demonstrate that CCL19 and CCL21 activation leads to differential GRK specificity for the activated CCR7. Therefore, the differential bulk phosphorylation and β-arrestin recruitment between CCR7 ligands is not caused by a general preference of GRKs for the CCL19-activated receptor. Rather, GRK3 is differentially active in response to CCL19 whereas GRK6 activity is observed in response to either ligand.
GRK6 but Not GRK3 Is Necessary for MAPK Signaling.
We next sought to test directly whether GRK isoforms can specifically promote β-arrestin2-dependent MAP kinase in a nonredundant fashion. HEK cells expressing CCR7 were treated with GRK-specific siRNAs, stimulated with CCL19 (100 nM) and CCL21 (100 nM), and ERK activation was determined by immunoblotting.
We found that the activation of ERK in response to CCR7 stimulation is highly dependent on GRK expression (Fig. 5). GRK6 inhibition led to nearly complete loss of ERK activation in response to either CCL19 or CCL21. Yet, EGF-stimulated ERK was preserved in these cells treated with GRK6 siRNA (Fig. S2).
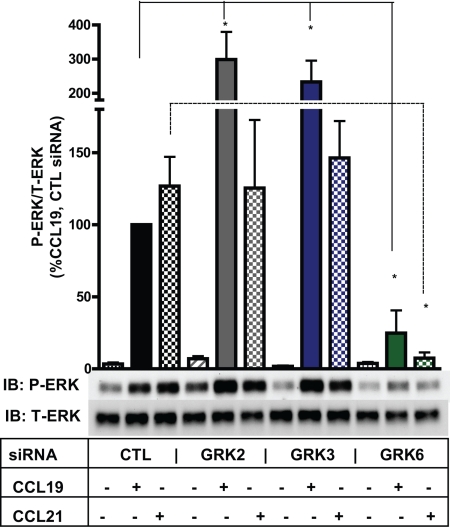
Fig. 5.
MAP kinase signaling by both CCL19 and CCL21 is highly dependent on GRK expression. HEK cells stably expressing CCR7 were transfected with control siRNA or siRNA specific for GRK2, GRK3, or GRK6. Cells were serum starved and then stimulated with 100 nM CCL19 or 100 nM CCL21 at 37 °C for 5 min. The bar graph shows the effect of GRK expression on CCL19- and CCL21-mediated ERK activation. Data represent the mean ± SE from three independent experiments. Statistical significance was determined by paired two-tailed t tests. *, P < 0.05.
Overall, this data is consistent with our earlier findings that the ligands of CCR7 both lead to similar GRK6-dependent CCR7 phosphorylation, GRK6-dependent β-arrestin recruitment, and β-arrestin-dependent MAP kinase signaling. In addition, we observed that loss of GRK2 or GRK3 led to increased P-ERK in response to CCL19. Whether this is because of unopposed GRK6 activity or implies a negative regulatory function by these enzymes is unknown. These data provide further evidence that GRK3 and GRK6 are not functionally redundant enzymes and only the latter leads to MAP kinase signaling.
Discussion
The purpose of this study was to investigate the differential activation of the GRK/β-arrestin system by the endogenous ligands of CCR7. We found that CCL19 behaves as a traditional agonist, leading to robust agonist-induced G protein activation, CCR7 phosphorylation, β-arrestin recruitment, desensitization of CCR7, and β-arrestin2-dependent activation of MAP kinase. In contrast, activation of CCR7 by CCL21 leads to equivalent G protein activity but markedly less bulk phosphorylation and β-arrestin2 recruitment. Thus, these results agree in principle with those of Kohout et al. (31) and suggest that CCR7 ligands are indeed natural biased agonists.
CCL21 contains an extra 3-kDa motif at its C terminus that is conserved in both mouse and humans (20–24). This motif could account for the differential signaling described here by a variety of mechanisms, including tethering CCL21 to extracellular glycosaminoglycans or by differentially inducing ligand oligomerization. Alternatively, because the amino acids of the N terminus of CCL19 have been shown to regulate receptor activation (36–38), subtle differences in the primary structure in this region may account for differential signaling. Thus, additional studies using chimeric ligands could test the relative importance of these motifs for CCR7 ligand bias.
In contrast to Kohout et al. (31), however, we find that CCR7 ligand bias is not absolute. Although weak relative to CCL19, CCL21 does lead to CCR7 phosphorylation and β-arrestin recruitment.
Next, we tested the functional consequence of this modest GRK/β-arrestin activation by CCL21 compared with CCL19. Although CCL21 was incapable of causing desensitization, it was fully capable of activating β-arrestin-dependent ERK. This apparent paradox led us to investigate the effects of individual GRKs on CCR7 signaling. Specifically, we sought to use this system to address two interrelated issues pertaining to GRK redundancy. The first question is whether substrate specificity exists among the ubiquitously expressed GRKs for CCR7 phosphorylation. The second is whether GRK isoforms that are specific for a receptor differentially affect the eventual function of β-arrestin.
Discovery of GRK Bias.
We inhibited the expression of individual GRK isoforms with siRNA to determine the relative contributions of each kinase to CCL19- and CCL21-mediated CCR7 phosphorylation. GRK2 did not phosphorylate CCR7 in response to either ligand despite robust expression in HEK-293 cells. However, loss of GRK3 leads to a substantial reduction in bulk receptor phosphorylation and β-arrestin recruitment but only in response to CCL19, and not CCL21. However, GRK6 was active in response to either ligand and contributed equally to bulk phosphorylation in response to CCL21 and CCL19. Therefore, despite similar Gi/o protein activation with these ligands, GRK3 activity is unique to CCL19 and leads to additional bulk phosphorylation and β-arrestin recruitment. Conversely, GRK6 is catalytically active in a relatively unbiased manner for each of these ligands. Thus, we found marked GRK specificity for activated CCR7, but also biased GRK utilization by its endogenous ligands. Differential GRK phosphorylation despite equal G protein activation, as observed here by CCR7 ligands, provides a mechanism of ligand bias. We speculate that other examples of GRK and β-arrestin bias by endogenously expressed ligands will emerge, particularly among promiscuous receptors.
GRK Isoforms Determine β-Arrestin Function.
The GRK specificity and bias of CCR7 is an attractive system to compare the functional consequences of individual GRKs. Thus, we sought to determine whether GRKs differentially affected β-arrestin activity. We found that total β-arrestin recruitment, as measured by FRET, approximately correlated with the extent of bulk phosphorylation. However, assays of β-arrestin function did not correlate directly with these bulk phosphorylation responses. For instance, although both GRK3 and GRK6 lead to β-arrestin recruitment in response to CCL19 activation of the receptor, only the loss of GRK6, but not GRK3, abrogated MAP kinase signaling. Yet, although CCL21 mediated receptor phosphorylation, β-arrestin recruitment, and was fully sufficient for β-arrestin-dependent MAP kinase signaling, it did not induce desensitization. Thus, desensitization and β-arrestin-mediated signaling are not activated proportionately to each other, but instead are promoted independently by GRK3 and GRK6, respectively.
The effects of GRK bias observed in this study add important insight to our understanding of β-arrestins as multifunctional proteins. An expanding number of β-arrestin binding partners and cellular functions have been identified. Yet, less is known about how these processes are coordinated at the molecular level. The data presented here reveal that 7TMRs are capable of a remarkable degree of GRK specificity, even in response to ligands with equivalent G protein responses. These data also highlight the emerging concept that the functions of β-arrestins may be prespecified by the particulars of the GRK–receptor interaction. Thus, individual GRK isoforms may lead to functionally distinct pools of β-arrestin, presumably by virtue of phosphorylating distinct sites on the receptor. These GRK-specific phosphorylation patterns may establish a “bar code” of sorts that instructs the β-arrestin partners as to which functions to perform, i.e., which conformation to adopt.
Although chemokines were discovered for their shared structural and functional features, unique properties of individual chemokines have emerged as well. This study expands our appreciation of the complex specialization of these ligands. The ligand bias of CCR7 appears to give tissues the ability to control the balance of distinct β-arrestin-dependent functions within infiltrating immune cells based on the relative concentration of CCL19 and CCL21 in the extracellular milieu. Leukocytes may in turn have varied responses according to their cellular GRK profile. Thus, this study reveals a mechanism of biased agonism and shows that ligands are able to engage the GRK/β-arrestin system selectively with more precision and functional distinction than previously appreciated.
Experimental Procedures
Materials.
Human CCL19 and CCL21 were purchased from R&D Systems. 125I-labeled CCL19 was obtained from PerkinElmer. Cell culture supplies, anti-FLAG beads, and forskolin were from Sigma. Anti-phospho-MAPK p42/44 antibody was purchased from Cell Signaling Technology. Anti-MAPK p44 antibody was from Upstate. GeneSilencer was from Gene Therapy Systems. Chemiluminescent detection was performed with SuperSignal West Pico reagent (Pierce).
Plasmids and Cell Culture.
CCR7-FLAG has been described in ref. 31. β-Arrestin2-mYFP has been described in ref. 39. CCR7-mCFP was generated by PCR amplification of human CCR7 containing a diglycine linker in place of the stop codon, flanked by HindIII and XhoI restriction sites. This product was then subcloned into the pcDNA3.1-mCFP vector. CCR7-pTRE2-hyg was generated by PCR amplification of human CCR7, flanked by BamHI and SalI sites. This product was then subcloned into pTRE2-hyg (Clontech). All plasmids were amplified in bacteria, kit purified (Qiagen), and validated by capillary electrophoresis sequencing.
Cell Culture.
HEK-293 cells were purchased from American Type Culture Collection and maintained in minimum essential Eagle's medium (Sigma) plus 10% (vol/vol) FBS (Sigma) and 1% (vol/vol) penicillin/streptomycin (Sigma). Cells were transfected with FuGENE 6 (Roche Applied Science) according to the manufacturer's instructions. Where applicable, G418 (500 μg/mL; Sigma), hygromycin (250 μg/mL), or zeocin (300 μg/mL; Invitrogen) was added for selection and maintenance of stable clones.
siRNA Transfection.
Chemically synthesized double-stranded siRNA duplexes (with 3′ dTdT overhangs) were purchased from Xeragon and transfected as described (16, 17, 40). The siRNA sequences used in this study have been validated elsewhere (13, 16, 35, 40).
ICUE2 cAMP Assay.
The cAMP biosensor ICUE2 assay was performed as described (32, 33). HEK-293 cells stably expressing CCR7-pTRE2-hyg and ICUE2 were stimulated with 50 μM forskolin in the presence or absence of 100 nM CCL19 or 100 nM CCL21. cAMP accumulation was measured as the percentage change in ICUE2-corrected FRET.
CCR7 Phosphorylation.
HEK-293 cells stably expressing CCR7-FLAG were labeled with 32Pi and stimulated at 37 °C for 10 min with 100 nM CCL19 or 100 nM CCL21. CCR7-FLAG was immunoprecipitated, separated by SDS/PAGE, and analyzed by phosphorimaging as described (39, 41). Basal phosphorylation was subtracted from agonist-induced phosphorylation, and each condition was then expressed as a percentage of the CCL19-mediated response. When done in conjunction with siRNA transfection, CCR7 surface expression was measured by radioligand binding by using 125I-labeled CCL19 to ensure that equal amounts of receptor were added for immunoprecipitations.
CCR7/β-Arrestin2 FRET.
HEK cells stably expressing β-arrestin2-YFP and CCR7-CFP were stimulated at 37 °C, and β-arrestin2-YFP recruitment by FRET was determined as described in ref. 39.
β-Arrestin2-GFP Redistribution.
HEK cells were transiently cotransfected with CCR7-FLAG and β-arrestin2-GFP at a ratio of 5:1 by using FuGENE 6 according to the manufacturer's instructions. Cells were split and grown on dishes containing glass coverslips. Cells were stimulated with 100 nM CCL19 or 100 nM CCL21 for 60 min and imaged by confocal microscopy as described in ref. 41.
ERK Activation.
HEK cells stably expressing CCR7-pTRE2-hyg were grown at low confluence and split to coated 24-well plates. Cells were serum-starved for 2 h and stimulated with 100 nM CCL19 and 100 nM CCL21 for 5 min at 37 °C, washed, harvested directly into 4× SDS sample buffer, and analyzed by immunoblotting after SDS/PAGE.
Supplementary Material
Acknowledgments.
We thank Donna Addison and Elizabeth Hall for secretarial assistance, Diane Sawyer and Lucie Langevin for technical assistance, and Seungkirl Ahn and Jennifer Daum for critical review of the manuscript. This work was supported in part by National Institutes of Health Grants HL16037 and HL70631 (to R.J.L.). R.J.L. is an Investigator with the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904361106/DCSupplemental.
References
- 1.Lefkowitz RJ, Whalen EJ. β-Arrestins: Traffic cops of cell signaling. Curr Opin Cell Biol. 2004;16:162–168. doi: 10.1016/j.ceb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. β-Arrestin: A protein that regulates β-adrenergic receptor function. Science. 1990;v248 doi: 10.1126/science.2163110. p1547(1544) [DOI] [PubMed] [Google Scholar]
- 3.Lohse MJ, et al. Receptor-specific desensitization with purified proteins: Kinase dependence and receptor specificity of β-arrestin and arrestin in the β2-adrenergic receptor and rhodopsin systems. J Biol Chem. 1992;267:8558–8564. [PubMed] [Google Scholar]
- 4.Perry SJ, et al. Targeting of cyclic AMP degradation to β2-adrenergic receptors by β-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 5.Nelson CD, et al. Targeting of diacylglycerol degradation to M1 muscarinic receptors by β-arrestins. Science. 2007;315:663–666. doi: 10.1126/science.1134562. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson SSG. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 7.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-Arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 8.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of β-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 9.Xiao K, et al. Functional specialization of β-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei H, et al. Independent β-arrestin2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wisler JW, et al. A unique mechanism of β-blocker action: Carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drake MT, et al. β-Arrestin-biased agonism at the β2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 13.Gesty-Palmer D, et al. Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 14.Kenakin T. Pharmacological proteus? Trends Pharmacol Sci. 1995;16:256–258. doi: 10.1016/s0165-6147(00)89037-9. [DOI] [PubMed] [Google Scholar]
- 15.Kobilka BK, Deupi X. Conformational complexity of G protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, et al. Functional antagonism of different G protein-coupled receptor kinases for β2-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren X-R, et al. Different G protein-coupled receptor kinases govern G protein and β2-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Förster R, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 19.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: Balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida R, et al. Secondary lymphoid tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J Biol Chem. 1998;273:7118–7122. doi: 10.1074/jbc.273.12.7118. [DOI] [PubMed] [Google Scholar]
- 21.Willimann K, et al. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur J Immunol. 1998;28:2025–2034. doi: 10.1002/(SICI)1521-4141(199806)28:06<2025::AID-IMMU2025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 22.Nagira M, et al. Molecular cloning of a novel human CC chemokine secondary lymphoid tissue chemokine that is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J Biol Chem. 1997;272:19518–19524. doi: 10.1074/jbc.272.31.19518. [DOI] [PubMed] [Google Scholar]
- 23.Hromas R, et al. Isolation and characterization of Exodus-2, a novel C-C chemokine with a unique 37-amino acid carboxyl-terminal extension. J Immunol. 1997;159:2554–2558. [PubMed] [Google Scholar]
- 24.Campbell JJ, et al. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3β receptor CCR7. J Cell Biol. 1998;141:1053–1059. doi: 10.1083/jcb.141.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan SK, McGrath DA, Grigoriadis D, Bacon KB. Pharmacological and signaling analysis of human chemokine receptor CCR-7 stably expressed in HEK-293 cells: High-affinity binding of recombinant ligands MIP-3β and SLC stimulates multiple signaling cascades. Biochem Biophys Res Commun. 1999;263:685–690. doi: 10.1006/bbrc.1999.1442. [DOI] [PubMed] [Google Scholar]
- 26.Ngo VN, Lucy Tang H, Cyster JG. Epstein–Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naïve T cells and activated B cells. J Exp Med. 1998;188:181–191. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byers MA, et al. Arrestin 3 Mediates endocytosis of CCR7 following ligation of CCL19 but not CCL21. J Immunol. 2008;181:4723–4732. doi: 10.4049/jimmunol.181.7.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bardi G, Lipp M, Baggiolini M, Loetscher P. The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur J Immunol. 2001;31:3291–3297. doi: 10.1002/1521-4141(200111)31:11<3291::aid-immu3291>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 29.Otero C, Groettrup M, Legler DF. Opposite fate of endocytosed CCR7 and its ligands: Recycling versus degradation. J Immunol. 2006;177:2314–2323. doi: 10.4049/jimmunol.177.4.2314. [DOI] [PubMed] [Google Scholar]
- 30.Yanagawa Y, Onoe K. CCL19 induces rapid dendritic extension of murine dendritic cells. Blood. 2002;100:1948–1956. doi: 10.1182/blood-2002-01-0260. [DOI] [PubMed] [Google Scholar]
- 31.Kohout TA, et al. Differential desensitization, receptor phosphorylation, β-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J Biol Chem. 2004;279:23214–23222. doi: 10.1074/jbc.M402125200. [DOI] [PubMed] [Google Scholar]
- 32.Violin JD, et al. β2-Adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem. 2008;283:2949–2961. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 33.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci USA. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, β-arrestin1, and β-arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 35.Ahn S, Wei H, Garrison TR, Lefkowitz RJ. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by β-arrestins 1 and 2. J Biol Chem. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- 36.Ott TR, Pahuja A, Nickolls SA, Alleva DG, Struthers RS. Identification of CC chemokine receptor 7 residues important for receptor activation. J Biol Chem. 2004;279:42383–42392. doi: 10.1074/jbc.M401097200. [DOI] [PubMed] [Google Scholar]
- 37.Ott TR, et al. The N-terminal domain of CCL21 reconstitutes high affinity binding, G protein activation, and chemotactic activity to the C-terminal domain of CCL19. Biochem Biophys Res Commun. 2006;348:1089–1093. doi: 10.1016/j.bbrc.2006.07.165. [DOI] [PubMed] [Google Scholar]
- 38.Ott TR, et al. Determinants of high-affinity binding and receptor activation in the N terminus of CCL-19 (MIP3β) Biochemistry. 2004;43:3670–3678. doi: 10.1021/bi035895h. [DOI] [PubMed] [Google Scholar]
- 39.Violin JD, Ren X-R, Lefkowitz RJ. G protein-coupled receptor kinase specificity for β-arrestin recruitment to the β2-adrenergic receptor revealed by fluorescence resonance energy transfer. J Biol Chem. 2006;281:20577–20588. doi: 10.1074/jbc.M513605200. [DOI] [PubMed] [Google Scholar]
- 40.Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of β2-arrestins demonstrated by RNA interference. Proc Natl Acad Sci USA. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shenoy SK, et al. β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.