Abstract
We tested the hypothesis that maternal consumption of dietary fat, independent from obesity, increases serum leptin in neonatal pups and predisposes them to adult obesity. Female rats either were fed a high-fat (HF) diet or a low-fat (LF) diet or were fed the HF diet but pair fed (PF) to the caloric intake of the LF group for 4 wk before breeding and throughout gestation and lactation. Dams consuming the HF diet had increased adiposity and were hyperphagic. At weaning, pups born to obese dams had significantly higher body fat and serum leptin levels and reduced insulin tolerance compared with offspring of LF-fed dams. Pups were weaned onto a chow diet until 8 wk of age, when they were then fed either HF or LF diet. At 18 wk of age, offspring from obese HF dams weighed more than offspring from nonobese LF or PF dams, and offspring eating HF diet weighed significantly more than those eating LF diet. Consequently, HF-fed offspring of obese HF dams weighed the most and LF-fed offspring from obese HF dams were similar in weight to HF-fed offspring from nonobese LF dams. These data suggest that maternal obesity exerts an independent effect on offspring body weight that is of similar magnitude as the effect of the offspring's adult diet. Furthermore, there was no difference in body weight between the nonobese LF and PF offspring on either diet. Together, these data suggest that maternal adiposity, and not dietary fat per se, induces hyperleptinemia and insulin resistance in offspring, as well as an increased body weight that persists into adulthood.
Keywords: perinatal environment, programming, leptin
obesity is increasing in the general population but is increasing at a higher rate in women than men (20, 25, 49), and obesity is also increasing in children (1). It is predicted that if current trends continue 86.3% of adults will be overweight by 2030 (49). Maternal obesity can have many adverse outcomes, including labor and delivery complications, fetal and neonatal death, maternal hypertension and preeclampsia, and gestational diabetes (7, 14, 31, 45). In addition to these acute risks to the obese mother, it is increasingly evident that maternal obesity also has adverse outcomes in offspring, and that these negative outcomes extend into adulthood and include obesity and cardiovascular disease. A growing body of evidence indicates that epigenetic effects induced during the perinatal period produce persistent developmental adaptations in structure, physiology, and metabolism (4, 32). This process of “fetal programming” has led to many unfavorable outcomes. For example, the maternal and perinatal environment influences neural development and cognitive function, because neonatal handling increases cognitive function while prenatal exposures to cigarette smoke, cocaine, and alcohol impair cognitive function (10, 41, 46, 52). Likewise, maternal stress, smoking during pregnancy, and perinatal ambient temperature all predispose the offspring to obesity (15, 27, 34, 50, 51). Work in animal models indicates that maternal overnutrition, maternal undernutrition, and neonatal overfeeding predispose offspring to obesity (24, 29, 37, 40, 47, 48).
While both maternal obesity and maternal high fat intake have been described as contributing to obesity of adult offspring (5, 26, 40, 43), these two factors have never been examined separately. In the present work we assessed the contribution of these factors by allowing mothers to consume a high-fat diet but preventing obesity by using a pair-feeding model. Therefore, these experiments tested whether maternal high fat intake, independent of maternal obesity, would increase the predisposition toward obesity in adult offspring.
MATERIALS AND METHODS
Animal maintenance and breeding.
Twenty 8-wk-old virgin female Long-Evans rats (Harlan Laboratories, Indianapolis, IN) were singly housed in shoebox cages in a room with a 12:12-h light-dark cycle. Water was provided ad libitum through an automated watering system. Rats were fed either a high-fat (HF) diet or a low-fat (LF) diet or were fed the HF diet but were pair fed (PF) to the caloric intake of the LF group. The HF diet (Research Diets D12492) consisted of 60% fat energy, 20% carbohydrate energy, and 20% protein energy and had an energy density of 5.24 kcal/g. The LF diet (Research Diets D12450B) consisted of 10% fat energy, 70% carbohydrate energy, and 20% protein energy, with an energy density of 3.85 kcal/g. After 4 wk of eating these diets, rats were bred with 10-wk-old virgin male chow-fed Long-Evans rats. Dams continued on their respective diets throughout both gestation and lactation. Food intake was measured daily in the dams.
Within 24 h of birth, litter size was adjusted to 10 pups per litter and sexes were distributed as equally as possible (HF 5.2 ± 0.8, LF 5.5 ± 0.3, and PF 5.2 ± 0.7 males/litter). At 3 wk of age, one male pup/litter was euthanized by decapitation and trunk blood and brain and adipose tissue were collected. The remaining male pups were weaned onto chow and multihoused in shoebox cages until 8 wk of age. Because of the possibility of sexual dimorphism, only male pups were utilized in the studies reported in this article.
At 8 wk of age, pups from each litter were divided into two groups and fed either a HF or a LF diet until 20 wk of age. At that time, rats were euthanized and trunk blood and brain and adipose tissue were collected. The Institutional Animal Care and Use Committee approved all of the experimental protocols, which were consistent with National Institutes of Health guidelines on the use of experimental animals.
Body weight and body composition analysis.
Body weight was measured weekly in the dams before conception and during gestation. During lactation, both the dams and the pups were weighed on days 2, 7, 14, and 20 postpartum. Pups were then weighed weekly throughout the remainder of the study. Body composition was measured in the pups at weaning with a Bruker minispec LF90 time-domain NMR analyzer and then weekly through 18 wk of age.
Milk and blood collection from dams.
Milk was collected from the dams at 7 days postpartum. Pups were separated from the dams for 30 min. Dams were then anesthetized with isoflurane and injected intraperitoneally with 4 IU of oxytocin (Parkedale Pharmaceuticals, Rochester, MI) to stimulate milk flow. Five minutes after oxytocin injection, milk was expressed and collected into a Pasteur pipette. Milk samples were then stored at −20°C until analysis. Blood was also collected at this time, via the lateral tail vein.
Insulin tolerance test.
An insulin tolerance test (ITT) was performed on the offspring at weaning, at 8 wk of age, and at 18 wk of age. Two rats from each litter received a dose of 0.5 U/kg body mass of insulin intraperitoneally after a 4-h fast. When tested at 18 wk of age, there was both a rat eating high fat and a rat eating low fat from each litter. Blood samples were collected from the tail of lightly restrained animals and analyzed with a commercially available glucometer (AccuCheck Advantage, Roche, Mannheim, Germany). Samples were drawn immediately before injection of insulin and at 30-min intervals for 2 h after injection.
Intraperitoneal glucose tolerance test.
An intraperitoneal glucose tolerance test (IPGTT) was performed on the offspring at 20 wk of age. Two rats from each litter, one on each diet, received a dose of 2.5 mg/kg body mass of a 50% glucose solution intraperitoneally after an overnight fast. Blood samples were collected from the tail of lightly restrained animals and analyzed with a commercially available glucometer (AccuCheck Advantage, Roche). Samples were drawn immediately before injection of insulin and at 30-min intervals for 2 h after injection.
Assays.
Trunk blood was allowed to clot at 4°C overnight and then centrifuged at 3,000 g for 30 min, and serum was collected and stored at −80°C. Serum leptin was analyzed with a radioimmunoassay kit (Linco Research, St. Louis, MO). Glucose was measured on an AccuCheck Advantage glucometer (Roche).
RNA analysis.
mRNA was isolated from hypothalamus and brown adipose tissue (BAT) by the modified guanidinium-isothiocyanate method (17) with Tri Reagent (GIBCO BRL, Gaithersburg, MD) Samples were quantified by spectrophotometry, checked for quality on an agarose gel, and then DNase treated. The mRNA levels were determined by quantitative real-time RT-PCR in the ABI-Prism 7900 HT Sequence Detection system (Applied Biosystems, Foster City, CA), using cyclophilin as an internal standard. Primers were as follows: cyclophilin, forward: ggagatggcacaggaggaaa, reverse: cccatagtgcttcagcttgaagtt; β3-adrenergic receptor (β3AR), forward: gaggcaacctgctggtaatca, reverse: aacacgttggttatggtctgtagtct; uncoupling protein 1 (UCP1), forward: ggacctacaatgcttacagagttatagc, reverse: tcgtccctttccacagtgttg; neuropeptide Y (NPY), forward: ccgctctgcgacactacatc, reverse: aatcagtgtctcagggctggat; agouti-related protein (AGRP), forward: agctttggcagaggtgcta, reverse: aggactcgtgcagccttacac; proopiomelanocortin (POMC), forward: agaggccactgaacatcttcgt, reverse: tgtagcagaatctcggcatcttc.
Adipocyte cell size and number.
A subset of five rats/group was used to measure adipocyte cell size and number as described by Smith and colleagues (3). Fifty milligrams of the retroperitoneal fat pad was washed in saline and then fixed in osmium tetraoxide solution (31 mg/ml of 0.2 M collidine HCl buffer). At the time of analysis, samples were diluted with 154 mM NaCl and dissociated over 1 wk by the addition of 10 ml of 8 M urea in 154 mM NaCl. The sample was then filtered through a 250-μm nylon filter into a weighed beaker. The volume was increased to 300 ml with 154 mM NaCl containing 0.1% Triton X-100. The cells were counted on a Multisizer-3 (Beckman Coulter, Fullerton, CA) using a 400-μm aperture (dynamic linear range 12–320 μm) and presented as the geometric mean.
Data analysis.
Data were analyzed by two-way ANOVA via the general linear model (GLM) procedure in the SAS software package (SAS V8, SAS Institute, Cary, NC). When experiment-wide tests for the main effects of maternal diet, offspring diet, or their interaction were significant, post hoc comparisons were made with the LSMEANS statement with the PDIFF option and thus represent least significant difference tests for preplanned comparisons. All data are expressed as means ± SE, with a probability value of <0.05 considered statistically significant.
RESULTS
Dam body weight, food intake, and body composition analysis.
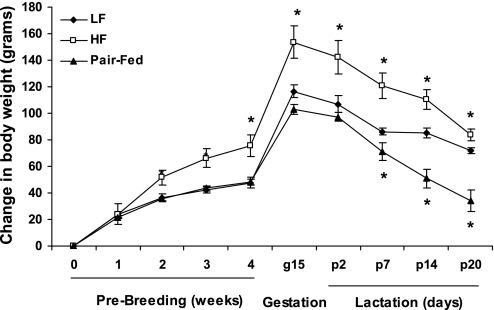
Female rats were fed a HF or LF diet for 4 wk before breeding and continued on this diet throughout gestation and lactation. By 2 wk on the HF diet the HF group weighed significantly more than the LF and PF groups, and this difference continued through the end of lactation (Fig. 1). HF rats also had a higher average daily food intake (71.24 ± 3.59 kcal/day, n = 7) during the 4 wk of diet before breeding compared with LF (56.61 ± 1.86 kcal/day, n = 7; P < 0.0005) and PF (56.00 ± 0.54 kcal/day, n = 6; P < 0.0005) rats.
Fig. 1.
Body weight gain in dams before breeding, during gestation, and during lactation. Female Long-Evans rats were fed a high-fat (HF) diet or a low-fat (LF) diet or were pair fed to the caloric intake of the LF group for 4 wk and then bred, continuing on their respective diets throughout lactation. Data are expressed as mean ± SE body weights with 6 or 7 rats per group. *P < 0.05 compared with LF group. g, Gestation day; p, postpartum day.
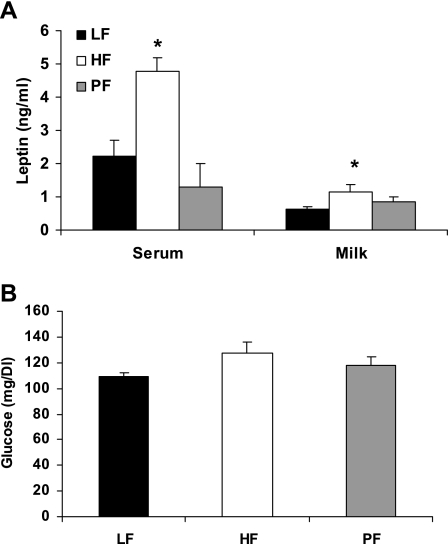
Maternal obesity had significant effects on both serum and milk leptin during lactation. There were significant differences between the obese HF group and both the nonobese LF and PF groups in serum leptin at day 7 and a significant difference between the LF and HF groups in milk leptin (Fig. 2). By day 21, there was no difference in serum leptin between LF (2.59 ± 0.33 ng/ml, n = 4) and HF (2.79 ± 0.46 ng/ml, n = 4; P = 0.5) groups, but there was still a significant difference between the HF and PF (0.99 ± 0.28 ng/ml, n = 3; P = 0.01) groups. At day 21 there was also a difference between the LF and PF groups (P = 0.05). Serum glucose levels are also shown in Fig. 2; there were no significant differences between the groups at day 21.
Fig. 2.
Effect of HF diet on dams during lactation. Female Long-Evans rats were fed a HF diet or a LF diet or were pair fed (PF) to the caloric intake of the LF group for 4 wk and then bred, continuing on their respective diets throughout lactation. A: serum and milk were collected at postpartum day 7, and leptin levels were measured. Values are expressed as mean ± SE leptin values, with 3–6 animals per group. B: serum glucose was measured at weaning. Values are expressed as mean ± SE glucose values, with 3 or 4 animals per group. *Difference from LF group, P < 0.05.
Offspring adiposity and insulin responsiveness from birth to weaning.
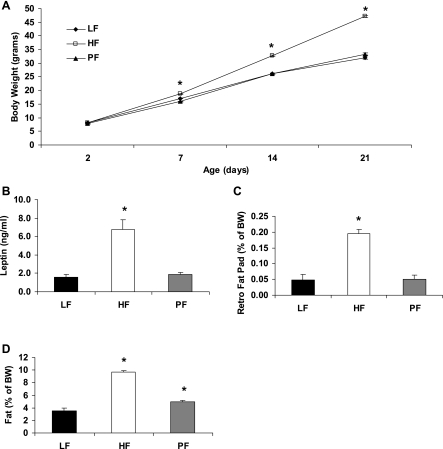
Differences in adiposity in the pups from birth through weaning are shown in Fig. 3. There were no differences in birth weight or litter size (data not shown). By 7 days of age pups born to maternally obese HF dams weighed significantly more than those born to nonobese LF or PF dams, and this difference continued to diverge through weaning. Serum leptin levels were significantly higher in pups from obese HF-fed dams than in pups from nonobese LF- or PF-fed dams. As with body weight and serum leptin, retroperitoneal fat pads and whole body adiposity (NMR) were significantly higher in pups from an obese HF-fed dam compared with both nonobese LF- and PF-fed dams (Fig. 3). Additionally, the offspring of the PF group had a significantly higher percentage of body fat than the offspring of the LF group, as measured by NMR.
Fig. 3.
Measures of adiposity from male offspring of dams fed HF or LF at weaning. Female Long-Evans rats were fed a HF diet or a LF diet or were pair fed (PF) to the caloric intake of the LF group for 4 wk and then bred, continuing on their respective diets throughout lactation. A: offspring body weight from birth until weaning of 26–34 animals per group. B: serum leptin levels measured at weaning of male offspring; 5 or 6 animals per group. C: weight of retroperitoneal fat pad expressed as mean ± SE % of body weight; 5 or 6 animals per group. D: mean ± SE % of body fat per gram of body weight measured via NMR at weaning; 22–25 animals per group. *Difference from LF group, P < 0.05.
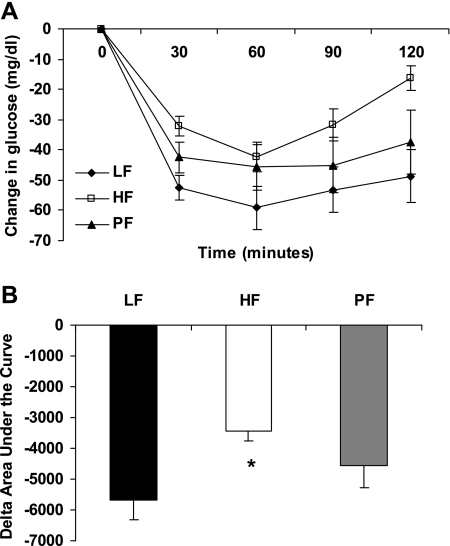
Insulin sensitivity was measured in the pups on the day of weaning via ITT (Fig. 4). Pups from obese HF-fed dams exhibited a reduced insulin tolerance compared with pups from nonobese LF or PF dams, with pups from PF dams exhibiting a response that was intermediate between the LF and HF groups. However, baseline serum glucose levels were not different between offspring of nonobese LF dams (117.6 ± 3.4 mg/dl, n = 12) and either the HF (113.5 ± 3.2 mg/dl, n = 10) or PF offspring (125.3 ± 3.7 mg/dl, n = 9).
Fig. 4.
Effect of maternal obesity on insulin sensitivity at weaning. An insulin tolerance test (ITT) was performed on offspring of obese dams fed HF or nonobese LF and PF dams after a 4-h fast. A: mean ± SE change in glucose levels of weanling rats after receiving 0.5 U/kg body mass of insulin; 9–12 animals per group. B: mean ± SE change (Δ) in area under ITT curve; 9–12 animals per group. *Difference from LF group, P < 0.05.
Offspring adiposity, food intake, and insulin responsiveness while eating chow.
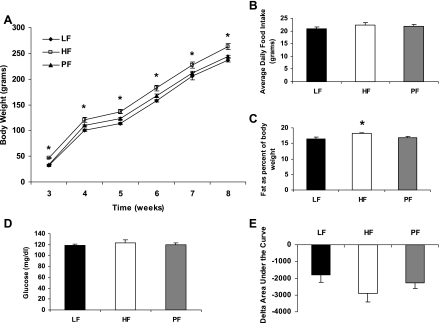
After weaning, the offspring were fed a chow diet for 5 wk. Offspring of obese HF-fed dams weighed significantly more at weaning, and this difference persisted for the 5-wk period (Fig. 5). However, there was no difference in the rate of body weight change between the groups during this time (LF 205.1 ± 4.2 g, n = 20; HF 216.3 ± 5.8 g, n = 20; PF 209.7 ± 3.5 g, n = 20; P = 0.235). There was also no difference in average daily food intake between the groups (Fig. 5). NMR performed after 5 wk of chow indicated that offspring from obese HF dams had a significantly higher percentage of body fat (Fig. 5). However, an ITT performed at this time suggests no significant difference in the baseline glucose values or area under the curve (Fig. 5).
Fig. 5.
Effect of maternal obesity on offspring while eating a chow diet. A: mean ± SE body weight of offspring from obese HF-fed dams or nonobese LF or PF dams from 3 to 8 wk of age while eating chow. B: average ± SE daily food intake of these offspring during the 5 wk on chow. C: mean ± SE % of body fat per gram of body weight measured via NMR at 8 wk of age. There were 20 animals per group in A–C. D: mean ± SE 4-h fasted glucose at 8 wk of age; 10–12 animals per group. E: an ITT was performed after a 4-h fast at 8 wk of age. Mean ± SE change in area under the curve for the ITT is shown; 10–12 animals per group. *Difference from LF group, P < 0.05.
Phenotype of adult offspring.
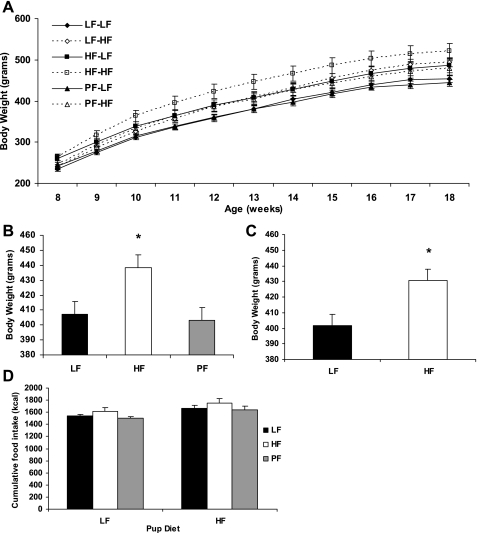
After 5 wk of chow intake (8 wk of age), offspring were assigned to either a HF or LF diet. During this period, body weights progressively diverged such that pups born to obese HF dams and consuming a HF diet weighed significantly more than any other group, whereas pups born to nonobese LF or PF dams and consuming LF weighed the least (Fig. 6). Intermediate between these groups were those pups that either were born to obese HF mothers or consumed HF in adulthood. Statistically, body weight was significantly affected by maternal obesity (P < 0.01) and offspring diet (P = 0.005), but the interaction between these effects was not significant, indicating that the effects were independent and additive. Similarly, there was a significant main effect of maternal obesity (P < 0.05) and offspring diet (P < 0.005) on cumulative food intake, but no interaction (P = 0.97). Feed efficiency (gain/feed) was also analyzed, and the results are in Table 1. All of the offspring eating a HF diet had a significantly higher feeding efficiency than their maternal diet counterpart eating a LF diet.
Fig. 6.
Effect of maternal obesity on body weight and food intake of adult offspring. Offspring of obese HF-fed dams or nonobese LF or PF dams were fed a HF or LF diet starting at 8 wk of age. A: body weight ± SE of the offspring from 8 to 18 wk of age, with 10 animals per group. LF-LF, PF-LF, pups born to nonobese LF or PF dams and consuming LF; HF-HF, pups born to obese HF dams and consuming HF; LF-HF, PF-HF, HF-LF, pups born to nonobese LF mothers and consuming HF in adulthood. B: main effects of maternal obesity. C: main effects of offspring diet. D: cumulative food intake of the offspring from 8 to 18 wk of age; 10 animals per group. *Significant main effect, P < 0.05.
Table 1.
Effect of maternal obesity on offspring at 20 wk of age
|
LF Offspring |
HF Offspring
|
Effects
|
||||||
|---|---|---|---|---|---|---|---|---|
| LF | HF | PF | LF | HF | PF | Dam | Pup | |
| Gene expression level | ||||||||
| Hypothalamus (n) | 10 | 9 or 10 | 10 | 9 | 9 or 10 | 10 | ||
| NPY | 0.90±0.03 | 1.18±0.09* | 0.86±0.06 | 0.98±0.05 | 0.93±0.05† | 0.95±0.06 | P < 0.05 | NS |
| AGRP | 1.06±0.07 | 1.14±0.11 | 0.89±0.10 | 1.01±0.07 | 1.01±0.10 | 0.96±0.10 | NS | NS |
| POMC | 1.01±0.09 | 1.10±0.09 | 0.81±0.08 | 1.07±0.09 | 1.09±0.09 | 1.12±0.08 | NS | P < 0.1 |
| BAT (n) | 10 | 10 | 10 | 9 | 10 | 9 or 10 | ||
| β3AR | 0.88±0.07 | 1.18±0.14* | 0.99±0.09 | 0.68±0.05 | 1.01±0.10* | 0.93±0.04 | P < 0.005 | NS |
| UCP1 | 0.74±0.1 | 0.66±0.1 | 0.60±0.1 | 1.5±0.1† | 1.5±0.1† | 1.4±0.1† | NS | P < 0.0001 |
| Adult offspring phenotype (n) | 10 | 10 | 10 | 9 | 10 | 10 | ||
| Feeding efficiency, gain/feed | 0.143±0.003 | 0.139±0.003 | 0.135±0.004 | 0.154±0.00† | 0.147±0.003† | 0.142±0.002† | P < 0.05 | P < 0.0005 |
| Retroperitoneal fat pad, % of BW | 1.0±0.04 | 1.2±0.1 | 1.0±0.1 | 1.5±0.1† | 1.7±0.1*† | 1.4±0.1† | P < 0.005 | P < 0.0001 |
| Leptin, ng/ml | 9.0±0.52 | 10.1±0.4 | 10.2±0.5 | 9.4±0.6 | 10.9±0.7 | 10.9±0.9 | P < 0.1 | NS |
| Retroperitoneal fat pad cells (n) | 5 | 4 | 5 | 5 | 5 | 5 | ||
| Mean cell size, μl | 1.2±0.1 | 1.4±0.1 | 1.4±0.04 | 1.5±0.4† | 1.6±0.1 | 1.5±0.1 | NS | P < 0.01 |
| Cell number, cells/mg tissue | 1,887±184 | 1,581±108 | 1,856±106 | 1,566±86 | 1,936±189 | 1,751±220 | NS | NS |
Data are from high-fat (HF)-fed and low fat (LF)-fed offspring of HF-fed, LF-fed, and HF-fed nonobese (PF) dams. Gene expression data are expressed as mean ± SE ratio of the gene to cyclophilin. BAT, brown adipose tissue; NPY, neuropeptide Y; AGRP, agouti-related protein; POMC, proopiomelanocortin; β3AR, β3-adrenergic receptor; UCP1, uncoupling protein 1. Feeding efficiency is expressed as mean ± SE ratio of gain in body weight (BW) over 10 wk of HF or LF feeding to cumulative food intake over the same period. Weight of the retroperitoneal fat pad is expressed as mean ± SE % of BW. Serum data are expressed as mean ± SE serum levels (ng/ml). Cell size is expressed as mean ± SE cell size in microliters, while cell number is expressed as mean ± SE number of cells per milligram of tissue.
Maternal difference in same offspring diet group,
offspring difference in same maternal treatment group (P < 0.05); NS, not significant.
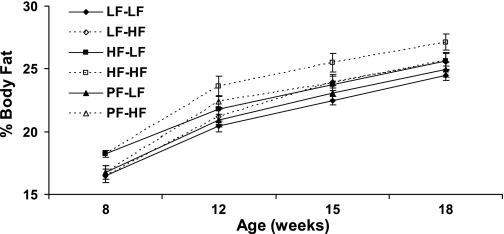
In addition to body weight, there were significant differences in body adiposity in the offspring after eating a HF or LF diet (Fig. 7), with a significant main effect of maternal obesity (P < 0.01) and of offspring diet (P < 0.005) but no interaction between the two. In the offspring of the obese HF-fed dams, there a significant difference in percent body fat between the offspring eating a HF and LF diet. Offspring of obese HF-fed dams eating a HF diet had significantly higher percent body fat than offspring of either nonobese LF or PF dams eating a HF diet. Significant main effects of maternal obesity (P < 0.005) and offspring diet (P < 0.0001) were also detected for body adiposity, as measured by retroperitoneal fat pad weights (Table 1). Fat pad weights were significantly higher in offspring eating a HF diet compared with offspring eating a LF diet within the same maternal diet group, while offspring of obese HF dams eating a HF diet also had higher fat pad weights than offspring of nonobese LF dams eating a HF diet. There was no interaction between maternal obesity and offspring diet on retroperitoneal fat pad weights, again indicating that the two main effects interacted additively. Adipocyte cell size and number were also measured in the retroperitoneal fat pad (Table 1). There was no effect of either maternal obesity or offspring diet on adipocyte number, nor was there an effect of maternal obesity on adipocyte cell size. However, there was a significant effect of offspring diet on mean cell size, with offspring of nonobese LF fed dams eating a HF diet having a larger mean cell size then those eating a LF diet. Serum leptin levels were not significantly different between groups (Table 1).
Fig. 7.
Effect of maternal obesity on offspring adiposity. Offspring of obese HF-fed dams or nonobese LF or PF dams were fed a HF or LF diet starting at 8 wk of age. Body fat as % of body mass was measured at 8, 12, 15, and 18 wk of age. There were 10 animals per group, except for 20 animals in the 8-wk group.
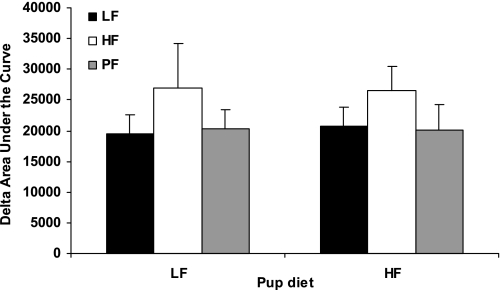
After the offspring had been on their respective LF or HF diet for 10 wk, an ITT was performed. There were no differences in baseline glucose, nor was there a significant effect of maternal obesity or offspring diet on insulin sensitivity (data not shown). However, because there was a trend toward an effect of maternal obesity (P = 0.11), an IPGTT was performed. Again, there was no difference in baseline glucose (data not shown) and no effect of either maternal obesity nor offspring diet but a trend toward an effect of maternal obesity was detected. (Fig. 8).
Fig. 8.
Effect of maternal obesity on an intraperitoneal glucose tolerance test (IPGTT) performed at 20 wk of age on offspring from obese HF-fed dams and nonobese LF or PF dams. Rats were fasted overnight. Mean ± SE change in area under the curve for the IPGTT is shown; 4–6 animals per group.
After the offspring were killed at 20 wk of age, hypothalamic RNA was isolated and expression of NPY, AGRP, and POMC was analyzed (Table 1). Levels of hypothalamic NPY were higher in LF-fed offspring of obese HF-fed dams compared with both offspring of LF and PF dams. They were also higher compared with HF-fed offspring of obese HF-fed dams. There were no differences in hypothalamic AGRP expression. There was a significant main effect of offspring diet for hypothalamic POMC, but there were no significant individual differences of interest.
RNA was also isolated from BAT and levels of UCP1 and β3AR were analyzed (Table 1). Offspring of obese HF-fed dams that were eating a LF diet had higher levels of β3AR expression than offspring of nonobese LF-fed dams that were eating a LF diet. Likewise, in offspring eating a HF diet, those born to obese HF-fed dams had higher β3AR expression than those born to nonobese LF-fed dams. UCP1 expression was not affected by maternal diet but was affected by offspring diet. All of the pups eating a HF diet had significantly higher levels of UCP1 than their maternal diet counterparts eating a LF diet.
DISCUSSION
The present experiments confirm previous studies indicating that consumption of a high-fat diet before and during pregnancy leads to obesity in the adult offspring of that pregnancy. In addition, the present work extends these observations by demonstrating that it is maternal obesity and not the consumption of dietary fat per se that is the primary mechanism driving offspring obesity. When mothers were fed a HF diet but restricted to the caloric intake of the LF group (PF), they failed to become obese and this prevention of maternal obesity resulted in normal body weight homeostasis in their adult offspring. Contrastingly, ad libitum maternal consumption of the HF diet resulted in obese dams whose offspring weighed more by 7 days of age. This trend continued throughout the remainder of the study, such that these offspring were heavier in adulthood than offspring of nonobese dams whether they were fed a LF or a HF diet. Maternally obese dams had higher levels of leptin both in serum and in milk during lactation than nonobese dams. Offspring of obese dams were also less sensitive to insulin at weaning than offspring of nonobese dams, although this effect was lost by 8 wk of age. Perhaps most remarkable, our data indicate that exposure to maternal obesity produces an increase in body weight that is similar in magnitude to the effect of the offspring actually eating a HF diet in adulthood. These effects of maternal obesity and offspring HF diet acted additively, such that getting a single exposure (either in utero or in adulthood) produced an increase in body weight, while being exposed to both resulted in a further increase in body weight and adiposity.
Increased body weight in the offspring of obese dams at weaning appears to be due to an increase in body fat, because results from both the NMR and retroperitoneal fat pad weights indicate increased adiposity but no difference in lean mass. The increase in body weight and body fat is still seen at 8 wk of age, despite the offspring being maintained on chow for 5 wk after weaning. Thus maternal obesity produces an increase in body weight and adiposity at weaning that persists well into adulthood. When the offspring are then placed on LF or HF diets, the groups diverge and a clear pattern emerges. In this pattern, the lightest offspring are those that are exposed to neither maternal obesity nor the HF diet in adulthood (offspring from nonobese LF and PF dams and eating a LF diet). Contrastingly, the heaviest group is those offspring that were both born to obese mothers and actively eating the HF diet. These offspring in essence receive a double dose, once from their mothers and once in adulthood. Finally, the remaining groups stratify to a weight that is intermediate between these extremes. These offspring were either born to an obese mother and ate LF in adulthood or were born to a nonobese mother but were placed on the HF diet in adulthood. Thus these intermediate groups reflect offspring that, in effect, only received a single dose of the HF diet. Most interestingly, these data indicate that maternal obesity produces an increase in body weight that is similar in magnitude to the increase that is induced by HF diet consumption in adulthood. Thus the offspring that were born to an obese mother were as heavy as those eating a HF diet, even though the former group was never exposed to a HF diet in adulthood.
While other studies have demonstrated that maternal obesity or maternal fat intake programs obesity in the offspring (5, 26, 40, 43), not all work leads to the same conclusions. In one case a high-fat diet was fed to dams without observing any effect of body weight in the offspring (11), while other studies either have not seen a difference at weaning or have observed a lower body weight at weaning in offspring of high-fat-fed mothers (6, 39, 40). However, our data replicated other studies showing an increase in body weight at weaning of high-fat-fed offspring (16, 21). The varied results from different studies can be explained by the use of different fat sources (lard, vegetable oil, cafeteria diet, margarine, etc) and the different times and length of time that the dams are exposed to the diets, thus illustrating the possibility of several developmental windows.
The increase in body weight in offspring of obese dams could reflect increased food intake or decreased energy expenditure. Food intake was significantly increased in the offspring of obese dams and, as such, likely contributed to their increased weight gain. However, the overall magnitude of this hyperphagia was relatively modest, leading us to also examine energy expenditure by assessing feed efficiency and BAT thermogenesis. The higher feed efficiency observed in offspring eating a HF diet indicates a reduced energy expenditure, which would explain their increased weight gain. Unfortunately, the data from BAT do not provide such a clear picture. High-fat diets have been demonstrated to suppress the expression of β3ARs in adipose tissue (18, 38). While values of β3ARs in BAT are trending lower in the offspring eating a HF diet compared with those eating a LF diet, maternal obesity does not follow the same pattern. Offspring of the obese dams eating a HF diet have significantly higher levels of BAT β3AR than the offspring of dams eating a LF diet. Even levels of β3AR in offspring of the PF dams who were eating a HF diet but were nonobese are higher, though not significantly, than those of offspring of the LF rats. These results would suggest a higher level of sympathetic activity in the offspring of dams eating a HF diet. However, results of UCP1 message in BAT do not support a higher level of sympathetic activity in offspring of HF-fed dams, because maternal obesity had no effect on UCP1 levels. Additionally, offspring eating a HF diet had significantly higher levels of BAT UCP1, which was also unexpected and does not match the feeding efficiency data. Ultimately, these differences will be best studied with a metabolic chamber, which we hope to do in the future.
Increases in body adiposity can be due to an increase in adipocyte size and adipocyte cell number. Increased cell size is generally believed to be the most important factor in determining obesity (8, 23). However, there is evidence that increased cell number is associated with an increased predisposition to obesity (22). Furthermore, recent work by Spalding et al. (42) shows not only that fat cell number is an important determinant in adult obesity but also that this number is fixed during childhood. We therefore chose to assess both cell size and number in retroperitoneal fat pads, to specifically test the hypothesis that maternal obesity might have a programming effect on adipose tissue, inducing hyperplasia that could predispose offspring to obesity. Interestingly, we observed no effect of maternal obesity on either adipocyte size or number. Work by Caluwaerts et al. (12) did show an effect of maternal obesity on cell size at weaning but not at 8 wk of age. Thus these data are not consistent with the hypothesis that maternal obesity programs an increase in adipocyte number. Consistent with previous reports (2, 36, 40), we did detect an effect of offspring HF diet consumption on adipocyte size. There was no effect on cell number, but results differ in the literature about the effect of a HF diet on hyperplasia (2, 19, 28, 33, 40). Because rat strain and fat cell depot examined also have an effect on cell size (35), these strain and depot variations could account for the variable results.
The present work provides evidence that supports a model in which the obese maternal environment produces permanent programming effects on the offspring. Yet the specific components of the obese maternal environment that produce these changes are not fully clear. In rodents, the central nervous system continues to develop during the first few weeks of postnatal life. Included in this process are leptin-sensitive arcuate nucleus neurons, whose projections to downstream brain areas mature within the first 2 wk of birth (9). Leptin replacement during the first 2 wk of life is sufficient to permanently rescue underdeveloped hypothalamic architecture in ob/ob mice, indicating that leptin action during this critical period permanently organizes the structure of neuronal pathways regulating energy homeostasis (9). Offspring from HF dams in the present work exhibited significantly increased circulating leptin levels, and these altered leptin levels are thus positioned to influence the structure of these neuronal pathways. The most likely mechanism driving these increases in circulating leptin is the increased body adiposity of the pups themselves. However, obese HF dams also exhibited increased levels of leptin within milk. Considering that leptin given orally to neonates is absorbed into the bloodstream (13), and that leptin given to dams during lactation or gestation influences weight gain in the offspring (30, 44), it remains possible that variations in milk leptin levels could contribute to alterations in circulating leptin in the pups. Regardless of the mechanism, these data clearly indicate that pups born to HF-fed, obese mothers exhibit increased levels of circulating leptin, consistent with the possibility that this hyperleptinemia induces permanent changes in the development of leptin-sensitive circuits within the brain.
Perspectives and Significance
The work described here supports the concept of nutritional programming and indicates that offspring of maternally obese dams weigh more and exhibit increased body adiposity compared with offspring of nonobese dams. In addition, the present work extends this observation by effectively distinguishing between the direct effects of a high-fat diet and effects that are secondary to maternal obesity. Because the offspring of HF-fed nonobese (PF) dams were similar to the offspring of LF dams, these data clearly indicate that consumption of a high-fat diet itself (i.e., in the absence of obesity) is insufficient to predispose offspring to obesity. It is instead maternal obesity that is required for this programming effect, indicating that some feature of the obese maternal environment induces a permanent structural or functional change in the offspring, with this change predisposing the offspring to obesity.
GRANTS
This work was partially supported by National Institutes of Health Clinical Nutrition Research Unit (1P30 DK-07246) and COBRE (RR-021945) center grants and Grant NS-051570 to C. D. Morrison.
Acknowledgments
We thank Dr. Steve Smith and David Hymel for measuring adipocyte cell size and number.
REFERENCES
- 1.Adair LS Child and adolescent obesity: epidemiology and developmental perspectives. Physiol Behav 94: 8–16, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Aslan H, Altunkaynak BZ, Altunkaynak ME, Vuraler O, Kaplan S, Unal B. Effect of a high fat diet on quantitative features of adipocytes in the omentum: an experimental, stereological and ultrastructural study. Obes Surg 16: 1526–1534, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Azuma K, Heilbronn LK, Albu JB, Smith SR, Ravussin E, Kelley DE. Adipose tissue distribution in relation to insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 293: E435–E442, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ Fetal origins of coronary heart disease. BMJ 311: 171–174, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayol SA, Farrington SJ, Stickland NC. A maternal “junk food” diet in pregnancy and lactation promotes an exacerbated taste for “junk food” and a greater propensity for obesity in rat offspring. Br J Nutr 98: 843–851, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Beck B, Kozak R, Moar KM, Mercer JG. Hypothalamic orexigenic peptides are overexpressed in young Long-Evans rats after early life exposure to fat-rich diets. Biochem Biophys Res Commun 342: 452–458, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya S, Campbell DM, Liston WA. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health 7: 168, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjorntorp P Effects of age, sex, and clinical conditions on adipose tissue cellularity in man. Metabolism 23: 1091–1102, 1974. [DOI] [PubMed] [Google Scholar]
- 9.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304: 108–110, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus). Horm Behav 46: 30–38, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Buckley AJ, Keseru B, Briody J, Thompson M, Ozanne SE, Thompson CH. Altered body composition and metabolism in the male offspring of high fat-fed rats. Metabolism 54: 500–507, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Caluwaerts S, Lambin S, van Bree R, Peeters H, Vergote I, Verhaeghe J. Diet-induced obesity in gravid rats engenders early hyperadiposity in the offspring. Metabolism 56: 1431–1438, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Casabiell X, Pineiro V, Tome MA, Peino R, Dieguez C, Casanueva FF. Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab 82: 4270–4273, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Chen A, Feresu SA, Fernandez C, Rogan WJ. Maternal obesity and the risk of infant death in the United States. Epidemiology 20: 82–83, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. Int J Epidemiol 35: 121–130, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology 149: 5348–5356, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Collins S, Daniel KW, Petro AE, Surwit RS. Strain-specific response to beta3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology 138: 405–413, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Faust IM, Mrosovsky N. Resistance to adipocyte hyperplasia in ground squirrels given high-fat diets. Am J Physiol Regul Integr Comp Physiol 253: R576–R579, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES, Zhao G, Li C, Pearson WS, Mokdad AH. Trends in obesity and abdominal obesity among hypertensive and nonhypertensive adults in the United States. Am J Hypertens 21: 1124–1128, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Guo F, Jen KL. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav 57: 681–686, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab 5: 299–311, 1976. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PR, Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. J Lipid Res 13: 2–11, 1972. [PubMed] [Google Scholar]
- 24.Jones AP, Assimon SA, Friedman MI. The effect of diet on food intake and adiposity in rats made obese by gestational undernutrition. Physiol Behav 37: 381–386, 1986. [DOI] [PubMed] [Google Scholar]
- 25.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 32: 1431–1437, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Khan I, Dekou V, Hanson M, Poston L, Taylor P. Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation 110: 1097–1102, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Koupil I, Toivanen P. Social and early-life determinants of overweight and obesity in 18-year-old Swedish men. Int J Obes (Lond) 32: 73–81, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Lemonnier D Sex difference in the number of adipose cells from genetically obese rats. Nature 231: 50, 1971. [DOI] [PubMed] [Google Scholar]
- 29.Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol Regul Integr Comp Physiol 275: R1374–R1379, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Lins MC, de Moura EG, Lisboa PC, Bonomo IT, Passos MC. Effects of maternal leptin treatment during lactation on the body weight and leptin resistance of adult offspring. Regul Pept 127: 197–202, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Lu GC, Rouse DJ, DuBard M, Cliver S, Kimberlin D, Hauth JC. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. Am J Obstet Gynecol 185: 845–849, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Lucas A Programming by early nutrition: an experimental approach. J Nutr 128: 401S–406S, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Morange PE, Lijnen HR, Alessi MC, Kopp F, Collen D, Juhan-Vague I. Influence of PAI-1 on adipose tissue growth and metabolic parameters in a murine model of diet-induced obesity. Arterioscler Thromb Vasc Biol 20: 1150–1154, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Mueller BR, Bale TL. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol Behav 88: 605–614, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Obst BE, Schemmel RA, Czajka-Narins D, Merkel R. Adipocyte size and number in dietary obesity resistant and susceptible rats. Am J Physiol Endocrinol Metab 240: E47–E53, 1981. [DOI] [PubMed] [Google Scholar]
- 36.Petrescu O, Cheema AF, Fan X, Bradbury MW, Berk PD. Differences in adipocyte long chain fatty acid uptake in Osborne-Mendel and S5B/Pl rats in response to high-fat diets. Int J Obes (Lond) 32: 853–862, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, Dorner G. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res 836: 146–155, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Prpic V, Watson PM, Frampton IC, Sabol MA, Jezek GE, Gettys TW. Adaptive changes in adipocyte gene expression differ in AKR/J and SWR/J mice during diet-induced obesity. J Nutr 132: 3325–3332, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Rolls BJ, Rowe EA. Pregnancy and lactation in the obese rat: effects on maternal and pup weights. Physiol Behav 28: 393–400, 1982. [DOI] [PubMed] [Google Scholar]
- 40.Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 294: R528–R538, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Singer LT, Minnes S, Short E, Arendt R, Farkas K, Lewis B, Klein N, Russ S, Min MO, Kirchner HL. Cognitive outcomes of preschool children with prenatal cocaine exposure. JAMA 291: 2448–2456, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature 453: 783–787, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab 291: E792–E799, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Stocker CJ, Wargent E, O'Dowd J, Cornick C, Speakman JR, Arch JR, Cawthorne MA. Prevention of diet-induced obesity and impaired glucose tolerance in rats following administration of leptin to their mothers. Am J Physiol Regul Integr Comp Physiol 292: R1810–R1818, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Thompson DR, Clark CL, Wood B, Zeni MB. Maternal obesity and risk of infant death based on Florida birth records for 2004. Public Health Rep 123: 487–493, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toro R, Leonard G, Lerner JV, Lerner RM, Perron M, Pike GB, Richer L, Veillette S, Pausova Z, Paus T. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacology 33: 1019–1027, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279: E83–E87, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology 149: 1906–1913, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 16: 2323–2330, 2008. [DOI] [PubMed] [Google Scholar]
- 50.White CL, Braymer HD, York DA, Bray GA. Effect of a high or low ambient perinatal temperature on adult obesity in Osborne-Mendel and S5B/Pl rats. Am J Physiol Regul Integr Comp Physiol 288: R1376–R1384, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Wideroe M, Vik T, Jacobsen G, Bakketeig LS. Does maternal smoking during pregnancy cause childhood overweight? Paediatr Perinat Epidemiol 17: 171–179, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Willford J, Leech S, Day N. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcohol Clin Exp Res 30: 1051–1059, 2006. [DOI] [PubMed] [Google Scholar]