Abstract
Exogenous glucocorticoids act within the hindbrain to enhance the arterial pressure response to acute novel stress. Here we tested the hypothesis that endogenous glucocorticoids act at hindbrain glucocorticoid receptors (GR) to augment cardiovascular responses to restraint stress in a model of stress hyperreactivity, the borderline hypertensive rat (BHR). A 3- to 4-mg pellet of the GR antagonist mifepristone (Mif) was implanted over the dorsal hindbrain (DHB) in Wistar-Kyoto (WKY) and BHRs. Control pellets consisted of either sham DHB or subcutaneous Mif pellets. Rats were either subjected to repeated restraint stress (chronic stress) or only handled (acute stress) for 3–4 wk, then all rats were stressed on the final day of the experiment. BHR showed limited adaptation of the arterial pressure response to restraint, and DHB Mif significantly (P ≤ 0.05) attenuated the arterial pressure response to restraint in both acutely and chronically stressed BHR. In contrast, WKY exhibited a substantial adaptation of the pressure response to repeated restraint that was significantly reversed by DHB Mif. DHB Mif and chronic stress each significantly increased baseline plasma corticosterone concentration and adrenal weight and reduced the corticosterone response to stress in all rats. We conclude that endogenous corticosterone acts via hindbrain GR to enhance the arterial pressure response to stress in BHR, but to promote the adaptation of the arterial pressure response to stress in normotensive rats. Endogenous corticosterone also acts in the hindbrain to restrain corticosterone at rest but to maintain the corticosterone response to stress in both BHR and WKY rats.
Keywords: corticosterone, brain, nucleus of the solitary tract, hypothalamic-pituitary-adrenal axis, chronic stress
exaggerated cardiovascular responses to acute stress and chronic stress increase the risk for hypertension and cardiovascular disease (1, 3, 13, 15, 31, 38, 42, 58, 68). Acute stress rapidly increases blood pressure, heart rate, plasma glucocorticoid concentration, and blood glucose levels (8), while chronic or repeated stress is associated with increases in baseline blood pressure and glucocorticoids (17). Chronically elevated glucocorticoids increase morbidity and mortality from cardiovascular disease and alterations in the glucocorticoid receptor (GR), and in glucocorticoid metabolizing enzymes are associated with hypertension and cardiovascular disease in humans (28, 30, 35, 36, 39, 48, 49, 61, 65, 67, 70, 71). Thus, chronic stress-induced elevations in glucocorticoids likely contribute to the adverse effects of stress on cardiovascular health.
The mechanisms by which glucocorticoids modulate cardiovascular stress responses are not fully understood. A review by Sapolsky et al. (52) concluded that glucocorticoids act permissively to enhance the arterial pressure response to many physical stressors, in part by supporting the peripheral effects of catecholamines. Other studies indicate that chronic, systemic elevations in glucocorticoids enhance cardiovascular and catecholamine responses to acute novel psychological stress (33, 64). We demonstrated that chronic administration of the glucocorticoid corticosterone to the dorsal hindbrain (DHB) enhances the blood pressure and glucose responses to acute novel stress, without altering the systemic corticosterone or heart rate responses (56). Thus, exogenously administered glucocorticoids can act within the brain to modulate the cardiovascular response to psychological stress. The goal of the present study was to test the hypothesis that endogenous corticosterone also acts within the DHB to enhance the cardiovascular and glucose responses to both acute and repeated (chronic) stress. Borderline hypertensive rats (BHR) and normotensive outbred Wistar-Kyoto (WKY) rats were subjected to either a single or repeated episodes of restraint stress. BHR are the offspring of female spontaneously hypertensive rats (SHR) and normotensive male WKY rats (51). SHR exhibit exaggerated cardiovascular responses to acute stress and develop glucocorticoid-dependent hypertension (20, 41, 50). BHR also have enhanced stress reactivity and are susceptible to stress-induced hypertension (21, 51). Thus, we hypothesized that endogenous glucocorticoids would exert a greater influence on stress responsiveness in BHR relative to WKY rats.
Both rats and humans secrete glucocorticoids that bind to two receptor subtypes, the mineralocorticoid receptor (MR) and the GR (46). MR are sometimes colocalized with an enzyme, 11β-hydroxysteroid dehydrogenase type II, that degrades corticosterone; in the absence of this enzyme, MR are occupied by glucocorticoids even at baseline hormone concentrations (7, 46). Glucocorticoids have a lower affinity for GR compared with MR, and GR are only fully occupied at very high concentrations of glucocorticoids (7). This study focused on the role of GR in mediating the effects of elevated endogenous glucocorticoids on stress reactivity, and tested the hypothesis that endogenous corticosterone acts in the hindbrain via GR to enhance the blood pressure response to stress selectively in BHR. Actions of corticosterone within the DHB were chronically blocked by implantation of a 3- to 4-mg pellet of the GR antagonist mifepristone (Mif), as previously described (55). Control animals received either DHB sham pellets or subcutaneous Mif.
METHODS
General
Physiological data were obtained from 57 male BHR (385 ± 4 g body wt) and 46 outbred male WKY rats (414 ± 6 g body wt) purchased from Taconic Farms. Twenty additional male WKY rats (440 ± 9 g body wt; Taconic Farms) and 6 male Sprague-Dawley rats (487 ± 25 g body wt; Charles River) were used to estimate brain Mif levels. All animals were allowed at least 1 wk to recover from transport prior to being subjected to any procedures and were then randomly assigned to treatment groups. All animals were housed in American Association for Accreditation of Laboratory Animal Care International (AAALAC) accredited animal care facilities on a 12:12-h light-dark cycle and maintained on a normal sodium diet. Food and water were provided ad libitum. Experimental protocols were approved by the Institutional Animal Care and Use Committees at the University of Missouri Kansas City, Kansas City, MO and the University of Florida, and were performed with strict adherence to all AAALAC and National Institutes of Health and National Research Council guidelines.
Surgical Procedures
Animals used for the physiological experiments underwent two surgical procedures: implantation of pellets either on the DHB or subcutaneously, and implantation of arterial catheters. The additional animals underwent pellet implantation only (n = 20), pellet implantation followed by adrenalectomy (n = 4), or no surgery (n = 2). All surgical procedures were performed using aseptic technique with the depth of anesthesia maintained such that there was no reflex response to pinching the hind paw. Rats were placed in warm, padded cages following surgery and monitored until they could move about and groom normally. Buprenorphine (0.05 to 0.1 mg/kg sc), nalbuphine (4 mg/kg sc), or carprofen (5 mg/kg sc) was given as needed to alleviate postsurgical pain. Rats were singly housed following implantation of arterial catheters.
Pellet implantation.
Selective chronic blockade of DHB GR was achieved by implantation of small pellets of the GR receptor antagonist Mif (Sigma-Aldrich) on the surface of the DHB as previously described and validated (55). Briefly, powdered Mif was melted and pipetted into a mold to form 3- to 4-mg pellets with the approximate dimensions of 1.5 mm (l) × 1.75 mm (w) × 1.0 mm (h). Sham pellets were made of hardened Silastic (Kwik-Sil; World Precision Instruments) and carved to the same dimensions as the Mif pellets. Previous results indicate that Silastic pellets serve as an appropriate control in this model (55). To control for systemic effects of the DHB Mif, 3- to 4-mg pellets were implanted subcutaneously. Pellets were implanted using Domitor (metatomidine hydrochloride, 0.5 mg/kg ip; Pfizer Animal Health, Exton, PA) and ketamine (75 mg/kg ip; Fort Dodge Animal Health, Fort Dodge, IA) or inhaled isoflurane (2–3% in 100% oxygen at a flow rate of 1 l/min) anesthesia. Prophylactic penicillin (600,000 U/kg sc Pen-Pro-G; Henry Schein) was given to each rat. Animals were placed in a stereotaxic headframe with the head slightly ventroflexed, and a midline incision was made between the caudal aspect of the occipital bone and the first vertebra. Subcutaneous Mif pellets were implanted at this location. To implant Mif or sham DHB pellets, a small hole was made in the dura, and the pia was removed from the dorsal surface of the hindbrain. The bottom surface of the DHB pellet was coated with mineral oil to assist diffusion of the Mif into the brain. The pellet was placed on the surface of the hindbrain with approximately one-third of the pellet caudal to calamus scriptorius. The pellet was secured in place with a drop of Vetbond surgical glue and covered with a thin layer of Silastic gel (Kwik-Sil; World Precision Instruments). Rats anesthetized with Dormitor-ketamine received 6 to 8 ml of saline subcutaneously to replace lost fluid and Antisedan (1 mg/kg ip atipamezole hydrochloride) was administered to reverse the anesthesia.
Catheter implantation.
Rats were anesthetized with inhaled isoflurane (2–4% isoflurane in oxygen at a 1-L/min flow rate), and a small skin incision was made to expose the femoral artery as previously described (54). A Teflon-tipped catheter was introduced into the artery and advanced to the descending aorta until the tip was estimated to be 1–2 cm below the left renal artery. The catheter was tunneled subcutaneously to exit between the scapulae, filled with sterile heparin (1,000 U/ml), and closed with a sterile plug.
Adrenalectomy.
The adrenals were removed via a retroperitoneal approach under isoflurane anesthesia through small bilateral dorsal flank incisions. Adrenalectomized rats were offered both 0.45% saline and 7% sucrose to drink ad libitum.
Experimental Protocol
Physiological experiments were performed in six experimental groups in each rat strain: rats with DHB sham, DHB Mif, or systemic Mif pellets were subjected to either chronic (i.e., repeated) or acute (i.e., once only) restraint stress. Restraint stress was achieved by placing rats in clear Plexiglas restrainers. Repeatedly, stressed rats were weighed and subjected to restraint stress 5 to 7 days per week for 1–2 h per restraint session for 21 to 25 days, beginning 4–7 days following pellet implantation. The time of day of restraint was also varied so that the stress was not entirely predictable. Acutely stressed rats were weighed each day that the corresponding chronically stressed rats were restrained. Arterial catheters were then implanted in all rats, and the rats were brought to the laboratory daily in their home cages for the next 4 days for the measurement of arterial pressure. Chronically stressed rats continued to be stressed daily while acutely stressed rats were stressed only on the final day. Arterial pressure was measured by connecting each catheter via extension tubing to a pressure transducer (Maxxim Medical) so the rat was free to move normally within the cage until it was placed in the restrainer. The arterial pressure signal was processed using a MacLab system (ADInstruments) connected to a Macintosh computer. Mean arterial pressure and heart rate were calculated online. Arterial pressure was recorded continuously for 3 h each day with the last hour of the final day used to determine baseline arterial pressure and heart rate. Following the baseline arterial pressure measurement on the final day, all rats were subjected to 1 h of restraint stress. In addition, blood samples (300 μl) were obtained from the arterial catheter following the baseline period prior to restraint and at 10 and 60 min during restraint for the measurement of blood glucose (One Touch Ultra glucose meter; LifeScan, Johnson & Johnson) and plasma corticosterone. The blood used for measurement of corticosterone was placed in heparinized tubes and kept in ice. The blood samples were then centrifuged, and the plasma removed and stored at −20°C or less until assayed for plasma corticosterone using the MP Biomedicals rat 125I-RIA kit. Rats were euthanized with an overdose of inhaled isoflurane and both adrenals, and the abdominal fat pad were removed and weighed.
Assessment of Brain Mif levels
Immunohistochemical assay.
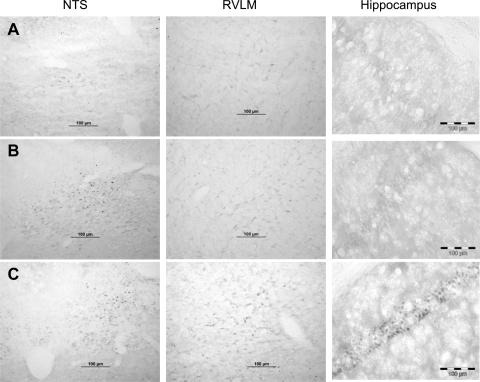
Mif and corticosterone both bind to the GR and cause the subsequent translocation of the ligand-bound receptor from the cytoplasm to the nucleus (22, 45). We previously used immunohistochemical identification of GR to demonstrate that 4 days of treatment with DHB Mif or corticosterone caused nuclear translocation of the GR within the DHB, but not in the ventral hindbrain or the forebrain (55). The present experiment utilized the same technique to determine the effect of prolonged DHB Mif treatment on nuclear translocation of the GR. Rats were treated with either DHB sham or DHB Mif pellets as described above. Six or 8 wk later they were deeply anesthetized and perfused transcardially with phosphate-buffered saline followed by 4% paraformaldehyde, and the brains were harvested. Three to 5 days prior to perfusion they were adrenalectomized to eliminate endogenous corticosterone. Untreated adrenal-intact rats were restraint stressed for 60 min to stimulate endogenous corticosterone release and were then anesthetized and perfused as described above. These rats were used as a positive control for GR nuclear translocation. Immunohistochemistry was performed as previously described using a rabbit anti-GR antibody (1:1,000 PA1–511, Affinity Bioreagents) that has a higher affinity for the occupied GR compared with the unoccupied receptor (55). Representative photomicrographs from adrenalectomized rats treated for 6 wk with either a DHB sham or a DHB Mif pellet are provided in Fig. 1 (rows A and B, respectively). Figure 1, row C shows photomicrographs from an adrenal-intact rat stressed just prior to perfusion. The DHB sham-treated rats exhibited light cytoplasmic staining for the GR in the DHB, including the nucleus of the solitary tract (NTS; a prominent nucleus in the DHB), the ventral hindbrain including the rostral ventrolateral medulla, and the hippocampus. DHB Mif-treated rats showed dark nuclear GR staining in the NTS, but only lighter cytoplasmic staining in the rostral ventrolateral medulla, and the hippocampus. Adrenal-intact stressed rats showed dark nuclear staining in all three regions of the brain. These results demonstrate that the DHB pellet can produce prolonged delivery of Mif to the DHB, but that Mif was not present in the ventral medulla or hippocampus in sufficient quantities to cause GR translocation to the nucleus that was detectable using immunohistochemistry. Therefore, any effects of Mif from the DHB Mif pellets were likely due to blockade of DHB GR.
Fig. 1.
Immunohistochemical localization of occupied glucocorticoid receptors (GR). Rats were treated with dorsal hindbrain (DHB) sham (row A) or DHB mifepristone (Mif) (row B) pellets for a total of 6 wk and were adrenalectomized at least 72 h prior to collection of the brains. Each row contains data from a single animal. The rat in row C was adrenal-intact and subjected to restraint stress for 60 min just prior to collection of the brain. Immunohistochemistry was performed using an antibody that has higher affinity for the occupied, compared with the unoccupied, GR. Scale bars are all equal to 100 μm. NTS, nucleus of the solitary tract; RVLM, rostral ventrolateral medulla.
HPLC.
We also attempted to measure tissue concentrations of Mif by HPLC following extraction of the Mif from brain tissue. Rats were treated with either DHB sham (n = 7), DHB Mif (n = 6) or subcutaneous Mif (n = 7) pellets as described above, and 4 wk later they were then deeply anesthetized with isoflurane and the brains rapidly removed and frozen. Samples of brain tissue were obtained from the DHB, the ventral hindbrain, and the forebrain with average weights of 28 ± 2 mg, 27 ± 2 mg, and 36 ± 2 mg, respectively. The tissue samples were homogenized in ethanol/saline (1:1), extracted twice with methylene chloride, and the samples were then dried under vacuum. The samples were reconstituted in acetonitrile/water (60:40) and analyzed by reversed-phase HPLC (Phenomenex Ultracarb 5 ODS; 150 × 4.60 mm, 1.2 ml/min, 100 μl injection volume; UV detection at 254 nm) using acteonitrile-water (60% vol/vol) and calibration samples ranging from 0.5 to 10 μg/ml (r2 = 0.997). Initial tests ensured that Mif and corticosterone did not coelute (retention times of 10.3 and 2.5 min, respectively). The limit of quantification (LOQ) was defined as the lowest concentration included in the calibration curve (0.5 μg/ml; equivalent to 50 ng per injection), and peak height was approximately fourfold higher than background noise. Based on the average tissue weight and lower limit of the assay, the minimum detectable concentration of the DHB samples was ∼1,800 ng Mif per gram of brain tissue. Each DHB tissue sample from the DHB Mif-treated rats was run separately. Some samples had apparent peaks at 10.3 min, but only one was above LOQ. When all ventral hindbrain samples from DHB Mif-treated rats were combined, a small Mif peak was detected; however, this was below the LOQ. Forebrain samples from all of the DHB Mif-treated rats were likewise combined, and there was no detection of a Mif peak. Samples from the subcutaneous Mif-treated rats were run together as described above, and no indication of a Mif peak was observed. No further attempts were made to obtain precise measurements of brain Mif concentrations due to the relative insensitivity of the assay.
Statistical Analysis
Group data are expressed as means ± SE. The data from the DHB sham and subcutaneous Mif animals were not significantly different, so the results from these animals were collapsed into a single control group. Data were initially analyzed by three- or four-way ANOVA. The between-subject factors were strain (BHR compared with WKY), stress (acute compared with chronic) and the effect of Mif (control compared with DHB Mif). When applicable, the time was also included as a repeated measure. When the overall ANOVA detected significant interactions, additional ANOVA was performed on the appropriate subgroups of data. Mean arterial pressure and heart rate values during stress were averaged into 5-min bins, and the changes from baseline were calculated. The data were divided into 2 × 30-min time segments for analysis. Adrenal weights were normalized to body weight on the day of euthanasia for quantification and analysis. Body weights for the first 3 wk of stress or corresponding period for the acutely stressed rats (i.e., the period prior to surgical implantation of the vascular catheters) were pooled into 2-day periods, and changes from day 1 were calculated for analysis. Significance was accepted at P ≤ 0.05.
RESULTS
Blood Pressure and Heart Rate
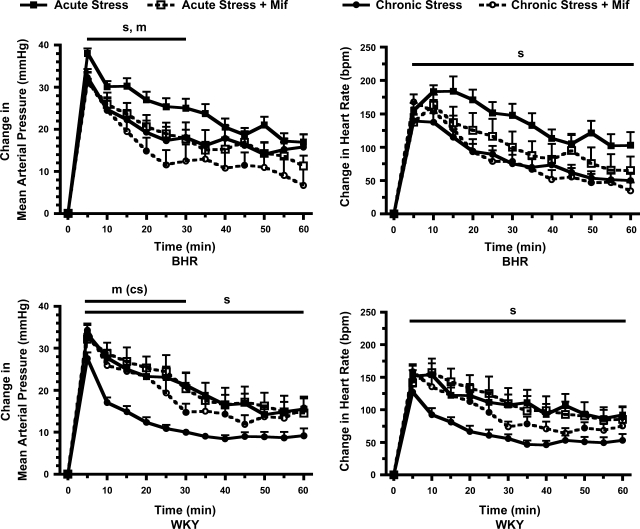
Baseline mean arterial pressure was higher, and baseline heart rate was lower in BHR relative to WKY rats (Table 1). Chronic stress did not significantly alter baseline heart rate or mean arterial pressure. DHB Mif treatment had no significant effect on baseline arterial pressure, but reduced baseline heart rate selectively in chronically stressed WKY rats. Restraint stress produced a rapid, significant increase in mean arterial pressure and heart rate in all groups of rats (Fig. 2). The chronically stressed WKY rats had a significantly smaller increase in arterial pressure compared with the acutely stressed WKY rats during the entire 60 min of restraint, whereas chronically stressed BHR had a significantly smaller arterial pressure response to restraint only during the first 30 min of stress. Thus, chronically stressed BHR rats exhibited less adaptation of the arterial response to restraint stress compared with WKY rats. The effects of DHB Mif treatment on the arterial pressure response to stress also differed between rat strains. DHB Mif attenuated the arterial pressure increase during acute restraint in BHR, but had no effect on this response in WKY rats. DHB Mif also attenuated the arterial pressure increase during restraint in chronically stressed BHR, but enhanced this response in chronically stressed WKY rats. These data indicate that endogenous corticosterone acted in BHR to enhance the arterial pressure response to either novel or repeated restraint stress, but acted in the opposite way in WKY rats to promote the adaptation of the blood pressure response to repeated stress. The heart rate response to stress was significantly attenuated in chronically compared with acutely stressed rats throughout the 60 min of restraint in both strains, and DHB Mif did not significantly alter the heart rate response.
Table 1.
Average baseline mean arterial pressure and heart rate
| Control | Mifepristone | |||
|---|---|---|---|---|
| Mean Arterial Pressure, mmHg | ||||
| WKY acute stress | 110±2 (n= 11) | 115±4 (n=7) | ||
| WKY chronic stress | 109±1 (n=18) | 104±2 (n=7) | ||
| BHR acute stress | 122±2*(n=16) | 125±3*(n=9) | ||
| BHR chronic stress | 121±3*(n=17) | 123±4*(n=9) | ||
| Heart Rate, beats/min | ||||
| WKY acute stress | 312±6 (n=11) | 322±12 (n=7) | ||
| WKY chronic stress | 320±5 (n=18) | 295±9†(n=7) | ||
| BHR acute stress | 295±7*(n=16) | 305±5*(n=9) | ||
| BHR chronic stress | 293±7*(n=17) | 292±5*(n=9) | ||
Values are mean ± SE.
P < 0.05 for an overall effect of strain; mean arterial pressure was higher and heart rate lower in borderline hypertensive rats (BHR) compared with Wistar-Kyoto (WKY) rats.
P < 0.05 for effect of mifepristone to reduce heart rate selectively in chronically stressed WKY rats.
Fig. 2.
Changes in mean arterial pressure (left) and heart rate (right) in response to 60 min of restraint stress in borderline hypertensive rats (BHR) (top) and Wistar-Kyoto (WKY) rats (bottom). Control rats, treated with either a DHB sham pellet or subcutaneous Mif pellet, are shown in solid lines and symbols. DHB Mif-treated rats are shown in dashed lines and open symbols. Chronically stressed rats (circles) were exposed to repeated restraint, while acutely stressed rats (squares) were restrained only on the final day of the experiment. Restraint stress produced a rapid, significant increase in mean arterial pressure and heart rate in all groups of rats (for clarity this statistical significance is not noted in the figure). sP ≤ 0.05 for main effect of chronic compared with acute stress; mP ≤ 0.05 for main effect for control compared with DHB Mif treatment; m(cs)P ≤ 0.05 for significant effect of DHB Mif only in the chronically stressed rats. Baseline values and number of rats per group are in Table 1.
Corticosterone and Glucose
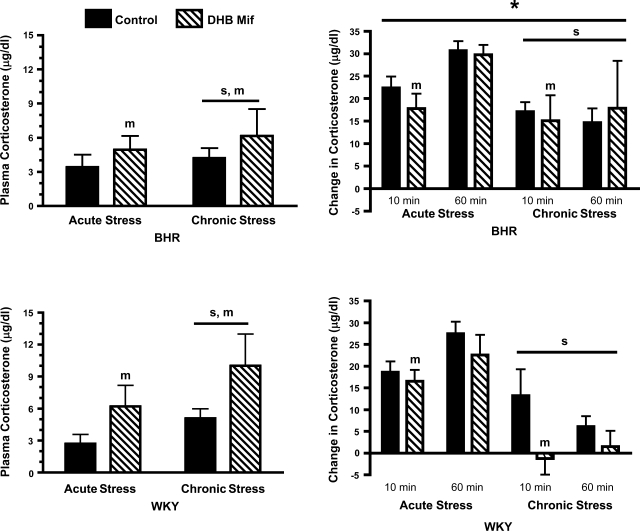
Baseline plasma corticosterone was significantly increased by both chronic stress and DHB Mif treatment, with no difference between rat strains (Fig. 3). The increase in plasma corticosterone at both 10 and 60 min of restraint stress was significantly greater in BHR compared with WKY rats, although chronic stress blunted the restraint stress-induced increase in corticosterone in both strains. DHB Mif treatment had an overall effect to reduce the corticosterone response to stress at 10 min of restraint in both acutely and chronically stressed rats, an effect that was independent of rat strain.
Fig. 3.
Baseline plasma corticosterone (left) and change in plasma corticosterone (right) at 10 and 60 min of restraint stress in BHR (top) and WKY rats (bottom). Control rats were treated with either a DHB sham pellet or subcutaneous Mif pellet. Chronically stressed rats were exposed to repeated restraint, while acutely stressed rats were restrained only on the final day of the experiment. sP ≤ 0.05 for main effect of chronic compared with acute stress; mP ≤ 0.05 for main effect for control compared with DHB Mif treatment; *P ≤ 0.05 for a difference between BHR and WKY rats (i.e., effect of strain). The number of animals per group is as follows: acute stress control WKY = 11, BHR = 15; acute stress Mif WKY = 7, BHR = 9; chronic stress control WKY = 16, BHR = 17; chronic stress Mif WKY = 7, BHR = 7.
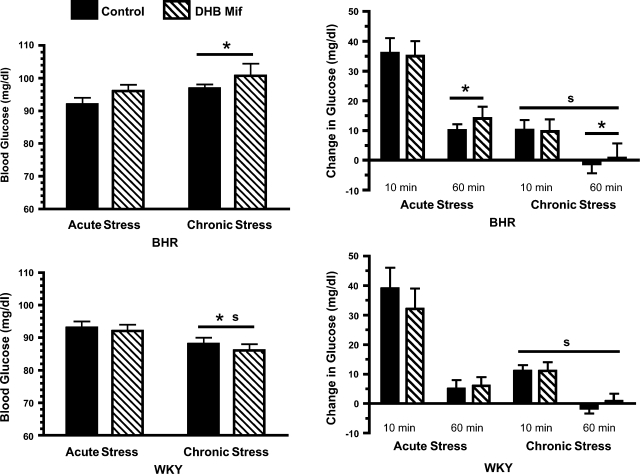
Chronic stress significantly (P = 0.04) lowered baseline plasma glucose in WKY rats, while it tended to increase plasma glucose in BHR (P = 0.09; Fig. 4). As a result, baseline glucose was higher in chronically stressed BHR compared with chronically stressed WKY rats. The restraint stress-induced increase in plasma glucose was significantly attenuated in all chronically stressed rats compared with acutely stressed rats. DHB Mif treatment had no effects on plasma glucose in these experiments.
Fig. 4.
Baseline blood glucose (left) and change in blood glucose (right) at 10 and 60 min of restraint stress in BHR (top) and WKY rats (bottom). Control rats were treated with either a DHB sham pellet or subcutaneous Mif pellet. Chronically stressed rats were exposed to repeated restraint, while acutely stressed rats were restrained only on the final day of the experiment. sP ≤ 0.05 for main effect of chronic compared with acute stress; *P ≤ 0.05 for a difference between BHR and WKY rats (i.e., effect of strain). The number of rats per group are the same as for arterial pressure (Table 1).
Adrenal and Body Weights
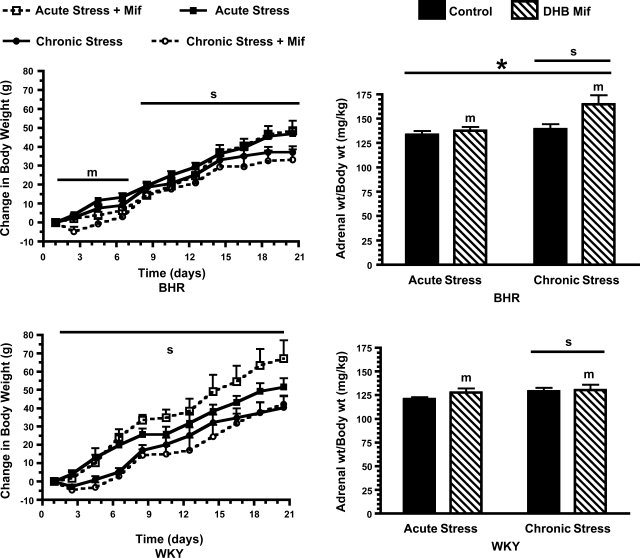
Adrenal gland weight was greater in BHR compared with WKY rats. Chronic stress and DHB Mif treatment each significantly increased adrenal weight in both BHR and WKY rats (Fig. 5). Chronic stress significantly attenuated body weight gain during the entire duration of repeated stress in WKY rats, but only after the first 7 days of stress in BHR. DHB Mif treatment reduced body weight gain in BHR rats during the initial phase of treatment, but had no significant effect on body weight gain in WKY rats.
Fig. 5.
Change in body weight from the first day of stress (left) and adrenal weight (right) for BHR (top) and WKY rats (bottom). Control rats, treated with either a DHB sham pellet or subcutaneous Mif pellet, are shown in solid lines and symbols or solid black bars. DHB Mif-treated rats are shown in dashed lines and open symbols or striped bars. Chronically stressed rats were exposed to repeated restraint, while acutely stressed rats were restrained only on the final day of the experiment. sP ≤ 0.05 for main effect of chronic compared with acute stress; mP ≤ 0.05 for main effect for control compared with DHB Mif treatment; *P ≤ 0.05 for a difference between BHR and WKY rats (i.e., effect of strain). The number of animals per group is as follows: acute stress control: WKY = 12, BHR = 16; acute stress Mif: WKY = 7, BHR = 10; chronic stress control: WKY = 18, BHR = 20; chronic stress Mif: WKY = 9/8 (body wt/adrenal wt), BHR = 10/9 (body wt/adrenal wt).
DISCUSSION
Effects of Rat Strain and Endogenous Corticosterone on Responses to Acute Stress
These experiments reveal that endogenous corticosterone can act within the hindbrain in rats to modulate cardiovascular and hypothalamic-pituitary-adrenal (HPA) axis function, but the effects are influenced by the stress state and genetic background (strain) of the animal. We previously demonstrated that chronic treatment of the DHB with exogenous corticosterone increased the arterial pressure and glucose responses to acute restraint stress in normotensive rats (56). The present study demonstrates that blockade of DHB GR with Mif attenuates the arterial pressure response to acute restraint stress in BHR, but has no affect on this response in WKY rats. BHR had a larger glucose response to stress compared with WKY rats, but there were no effects of DHB Mif on this response. The data suggest that in BHR, but not WKY rats, endogenous corticosterone acts via GR within the DHB to enhance the arterial pressure response to acute stress even in the absence of a background level of chronic stress. This cannot be due to increased baseline corticosterone in BHR relative to WKY rats, since the baseline values for corticosterone were similar between the two strains of rats. DHB Mif increased baseline corticosterone similarly in both BHR and WKY rats, so this increase in corticosterone also cannot explain the differential effect of Mif on the arterial pressure response to acute stress in the two rat strains. However, the BHR had a larger corticosterone response to stress compared with the WKY rats, indicating some enhanced activity of the HPA axis in these animals. The BHR also had larger adrenals compared with the WKY rats, but we did not determine whether this corresponded to a greater glucocorticoid synthesizing capacity of the BHR adrenals. Qualitative comparison of the data in Fig. 2 reveals that blockade of DHB GR in the BHR reduced the arterial pressure response to acute stress to the level observed in control-treated WKY rats, suggesting that endogenous corticosterone may mediate the enhanced stress reactivity observed in the BHR.
Effects of Rat Strain and Endogenous Corticosterone on Responses to Chronic Stress
Repeated restraint increased adrenal weight and baseline plasma corticosterone in both BHR and WKY rats, indicating that this protocol produced chronic stress in both rat strains (7, 43). However, the two strains of rats exhibited differential physiological responses to the chronic stress. WKY rats showed greater adaptation to the repeated stress compared with BHR, as evidenced by a significantly greater reduction in the arterial pressure response and an apparently larger reduction in the corticosterone response to repeated restraint (Figs. 2 and 3). Baseline glucose was significantly reduced by chronic stress in WKY rats, but not in BHR (Fig. 4), and chronic stress had a greater effect to attenuate body weight gain in WKY rats compared with BHR. Furthermore, the role of endogenous corticosterone in chronically stressed rats was different in the two rat strains. Blockade of DHB GR inhibited the adaptation of the arterial pressure response repeated stress in the WKY rats, effectively increasing the arterial pressure response to repeated restraint. These data extend previous findings that systemic corticosterone can promote the adaptation of the heart rate, immediate early gene expression, and the initial amygdalar corticotropin releasing hormone responses to repeated psychological stress (6, 57). Corticosterone is possibly facilitating the acquisition of the memory of the stress, since activation of GR within the NTS enhances memory consolidation (47). In contrast, the blood pressure response to stress in the BHR exhibited little adaptation with repeated exposure even in the absence of GR blockade, and Mif inhibited the arterial pressure response to restraint even in the chronically stressed rats. Others have reported that BHR fail to show adaptation of immediate early gene responses to repeated stress (44). The action of DHB Mif to inhibit body weight gain in the BHR is consistent with recent reports that corticosterone can act within the brain to stimulate food intake, especially in response to stress (9). The reasons for between-strain differences in stress responsiveness and adaptation are currently unknown.
Effects of DHB Mif on Plasma Corticosterone
We have previously shown that chronic treatment with DHB Mif elevated baseline plasma corticosterone concentration in the evening, but not in the morning (55). Similar effects of Mif on circadian glucocorticoid secretion have been reported with intracerebroventricular administration of Mif in rats and systemic administration in humans (23, 66). Data from the present study demonstrate that endogenous glucocorticoids act at DHB GR to inhibit baseline corticosterone secretion, but to enhance the corticosterone response to stress. These results are consistent with other studies demonstrating that the NTS both tonically inhibits baseline HPA axis function (24) and can mediate stimulation of the HPA axis (4, 40). The finding that corticosterone can act at DHB GR to maintain the corticosterone response to repeated psychological stress is also in agreement with a study by Laugero et al. (34) reporting positive feedback of centrally administered corticosterone on the HPA axis activity with repeated restraint stress. The present results identify the DHB as one site of action for this positive feedback.
Chronic Delivery of Mif to the DHB
Other investigators have administered glucocorticoid agonists and antagonists into the brain to investigate the central nervous system actions of glucocorticoids on blood pressure regulation, but the results have been varied due in part to differences in dose, stress level of the animals, and route of administration (26, 27, 59, 60, 62, 69). Most of the previous studies have utilized intracerebroventricular administration of these drugs, confounding interpretation of the results since glucocorticoids might have opposing effects on blood pressure at different locations within the central nervous system. The approach used in this study circumvents some of the problems with intracerbroventricular administration by providing chronic local increases in Mif. We previously demonstrated that DHB Mif reduced arterial pressure in rats with systemic corticosterone treatment but not in control rats, while systemic Mif at the same dose had no effect (55). The immuohistochemical localization of the GR to the nucleus only in the DHB (Fig. 1) strongly suggests that the Mif from the DHB pellets did not diffuse to other brain regions in quantities sufficient to bind GR. The HPLC data suggest that some Mif may have diffused into the ventral hindbrain, and we cannot completely rule out a role for ventral hindbrain GR in mediating the effects reported in this paper. The HPLC results and the immunohistochemistry analysis both indicate that Mif did not reach the forebrain GR. A short diffusion distance within brain tissue has been reported for structurally similar compounds (18). Mif also acts as an antagonist at progesterone receptors. However, we previously demonstrated that the cardiovascular effects of systemic Mif in male rats were abolished after elimination of endogenous corticosterone by adrenalectomy (53). The present results do not rule out a role for MR in mediating the effects of DHB corticosterone on the stress response. In fact an important role for MR is likely since it is known that activation of central MR increases baseline blood pressure and sympathetic activity, and enhances stress reactivity (2, 19, 25, 63).
The region of the DHB includes two areas that are important for cardiovascular regulation: the area postrema and the NTS (37). There are no reports that GRs are expressed in the area postrema, so the NTS is the most likely site of action for Mif in the present study. The catecholaminergic neurons in the NTS express a high density of GR (29). NTS catecholaminergic neurons are activated with psychological stress and project to forebrain areas that are important for cardiovascular regulation including the paraventricular nucleus of the hypothalamus (10, 11). Further studies are needed to determine the role of these and other NTS neurons in the cardiovascular responses to acute and chronic stress.
Perspectives
There is growing evidence that both stress and glucocorticoids contribute to the etiology of cardiovascular disease; however, the role of glucocorticoids in the stress response has long been a matter of debate (1, 3, 5, 13, 15, 28, 30, 31, 35, 36, 38, 39, 42, 48, 49, 52, 58, 61, 65, 67, 68, 70, 71). Recent research has led to the general hypothesis that stress-induced increases in glucocorticoids act in the brain to prepare the organism for future events (12, 52). With chronic stress, that could include attenuation of the cardiovascular response to a familiar, nonthreatening stress, while enhancing the response to a novel stress that might prove to be a threat to survival. If the chronic stress is prolonged, this could also lead to a state of constant vigilance that includes an increase in baseline blood pressure. However, little is known regarding central actions of chronic increases in glucocorticoids on cardiovascular regulation during stress, and the present study provides novel insight into this area of investigation. There is also considerable variability among people in their sensitivity to stress and to glucocorticoids, making some humans susceptible and some resistant to the adverse effects of stress (12, 14, 16, 32). It is intriguing that the BHR showed increased stress reactivity and poor adaptation of the cardiovascular response to repeated stress relative to the normotensive rats, and blockade of DHB GR attenuated the blood pressure response to stress selectively in BHR. Understanding the intertwined roles of glucocorticoids, stress, and genetic susceptibility in the development of cardiovascular disease will lead to improved methods for treatment and prevention.
GRANT
This study was supported by National Heart, Lung, and Blood Institute Grant R01-HL-076807.
Acknowledgments
The authors thank Kathy Vernon, Stephanie McCloud, and Mayur Narasimhan for help conducting the experiments described in the manuscript and Dr. Yun He for performing the assays for plasma corticosterone.
REFERENCES
- 1.Agyemang C, van Hooijdonk C, Wendel-Vos W, Ujcic-Voortman JK, Lindeman E, Stronks K, Droomers M. Ethnic differences of environmental stressors on blood pressure and hypertension in the Netherlands. BMC Public Health doi: 10.1186/1471-2458-1118. [DOI] [PMC free article] [PubMed]
- 2.Bing SH, Wang HL, Leenen FHH. Chronic central infusion of aldoseterone leads to sympathetic hyperactivity and hypertension in Dahl S but not Dahl R rats. Am J Physiol Heart Circ Physiol 288: H517–H524, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Branth S, Ronquist G, Stridsberg M, Hambraeus L, Kindgren E, Olsson R, Carlander D, Arnetz B. Development of abdominal fat and incipient metabolic syndrome in young healthy men exposed to long-term stress. Nutr Metab Cardiovasc Dis 17: 427–435, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Buller K, Xu Y, Dayas C, Day T. Dorsal and ventral medullary catecholamine cell groups contribute differentially to systemic interleukin-1β-induced hypothalamic pituitary adrenal axis responses. Neuroendocrinology 73: 129–138, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress 10: 213–219, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Cook CJ Glucocorticoid feedback increases the sensitivity of the limbic system to stress. Physiol Behav 75: 455–464, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Dallman MF Stress update: adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends Endocrinol Metab 4: 62–69, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol 4: 517–526, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gome F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA 100: 11696–11701, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 14: 1143–1152, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Dayas CV, Buller KM, Day TA. Medullary neurons regulate hypothalamic corticotropin-releasing factor cell responses to an emotional stressor. Neuroscience 105: 707–719, 2001. [DOI] [PubMed] [Google Scholar]
- 12.de Kloet RE, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev 6: 463–475, 2005. [DOI] [PubMed] [Google Scholar]
- 13.De Vogli R, Ferrie JE, Chandola T, Kivmaki M, Marmot MG. Unfairness and health: evidence from the Whitehall II study. J Epidemiol Community Health 61: 513–518, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeRijk R, de Kloet R. Corticosteroid receptor genetic polymorphisms and stress responsivity. Endocrine 28: 263–269, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Deter HC, Bleecher A, Weber CS. Cardiovascular reactivity of patients with essential and renal hypertension in an emotion-triggering interview. Behav Med 32: 117–125, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Duma D, Jewell CM, Cidlowski JA. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J Steroid Biochem Mol Biol 102: 11–21, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Kaye D, El-Osta A, Guo L, Barton D, Pier C, Brenchley C, Dawood T, Jennings G, Lambert E. Human sympathetic nerve biology. Parallel influences of stress and epignentics in essential hypertension and panic disorder. Ann NY Acad Sci 1148: 338–348, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Francis AB, Pace TWW, Ginsberg AB, Rubin BA, Spencer RL. Limited brain diffusion of the glucocorticoid receptor agonist RU28362 following ICV administration: implications for ICV drug delivery and glucocorticoid negative feedback in the hypothalamic-pituitary-adrenal axis. Neuroscience 141: 1503–1515, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol 281: H2241–H2251, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Friese RS Common genetic mechanisms of blood pressure elevation in two independent rodent models of human essential hypertension. Am J Hypertens 18: 633–652, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs LC, Hoque AM, Clarke NL. Vascular and hemodynamic effects of behavioral stress in borderline hypertensive and Wistar-Kyoto rats. Am J Physiol Regul Integr Comp Physiol 274: R375–R382, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Funder JW Mineralocorticoids, glucocorticoids, receptors and response elements. Science 259: 1132–1133, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Gaillard RC, Riondel A, Muller AF, Herrmann W, Baulieu EE. RU 486: A steroid with antiglucocorticosteroid activity that only disinhibits the human pituitary-adrenal system at a specific time of day. Proc Natl Acad Sci USA 81: 3879–3882, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gieroba ZJ, Fullerton MJ, Funder JW, Blessing WW. Medullary pathways for adrenocorticotropic hormone and vasopressin secretion in rabbits. Am J Physiol Regul Integr Comp Physiol 262: R1047–R1056, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Sanchez EP Intracerebroventricular infusion of aldosterone induces hypertension in rats. Endocrinology 118: 819–823, 1986. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Sanchez EP, Venkataraman MT, Thwaites D, Fort C. ICV infusion of corticosterone antagonizes ICV-aldosterone hypertension. Am J Physiol Endocrinol Metab 258: E649–E653, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Grunfeld JP, Eloy L. Glucocorticoids modulate vascular reactivity in the rat. Hypertension 10: 608–618, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Hammer F, Stewart PM. Cortisol metabolism in hypertension. Best Practice Res Clin Endocrinol Metabol 20: 337–353, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, Verhofstad A, Gustafsson JA. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci USA 83: 9779–9783, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huerta C, Lanes SF, Rodriguez LAG. Respiratory medications and the risk of cardiac arrhythmias. Epidemiology 16: 360–366, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Izzo JI, Black HR. Hypertension Primer (3rd ed.). Philadelphia, PA: Lippincott, Williams & Wilkins, 2003.
- 32.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci 8: 1450–1457, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Kvetnansky R, Pacak K, Fukahara K, Viskupic E, Hiremagalur B, Nankova B, Goldstein DS, Sabban EL, Kopin IJ. Sympathoadrenal system in stress, interaction with the hypothalamic-pituitary-adrenocortical system. Ann NY Acad Sci 771: 131–158, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Laugero KD, Gomez F, Manalo S, MFD. Corticosterone infused intracerebroventricularly inhibits energy storage and stimulates the hypothalamo-pituitary axis in adrenalectomized rats drinking sucrose. Endocrinology 143, 2002. [DOI] [PubMed]
- 35.Lin RCY, Wang XL, Morris BJ. Association of coronary artery disease with glucocorticoid receptor N363S variant. Hypertension 41: 404–407, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Lin RCY, Wang YSW, Morris BJ. Association and linkage analyses of glucocorticoid receptor gene markers in essential hypertension. Hypertension 34: 1186–1192, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Loewy AD, Spyer KM. Central regulation of autonomic functions. New York: Oxford University Press, 1990, p. 390.
- 38.Lupien SJ, King S, Meany MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol 13: 653–676, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Magiakou MA, Smyrnaki P, Chrousos GP. Hypertension in Cushing's syndrome. Best Practice Res Clin Endocrinol and Metab 20: 467–482, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Matta SG, Foster CA, Sharp BM. Selective administration of nicotine into catecholaminergic regions of rat brainstem stimulates adrenocorticotropin secretion. Endocrinology 133: 2935–2942, 1993. [DOI] [PubMed] [Google Scholar]
- 41.McDougall SJ, Paull JRA, Widdop RE, Lawrence AJ. Restraint stress: differential cardiovascular responses in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 35: 126–129, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Ohlin B, Goran B, Rosvall M, Nilsson PM. Job strain in men, but not women, predicts a significant rise in blood pressure after 65 years of follow-up. J Hypertens 25: 525–531, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Ottenweller JE, Servatius RJ, Natelson BH. Repeated stress persistently elevates morning, but not evening, plasma corticosterone levels in male rats. Physiol Behav 55: 337–340, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Palmer AA, Printz MP. Strain differences in fos expression following airpuff startle in spontaneously hypertensive and Wistar Kyoto rats. Neuroscience 89: 965–978, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Rajpert EJ, Lemaigre FP, Eliard PH, Place M, Lafontaine DA, Economidis IV, Belayew A, Martial JA, Rousseau GG. Glucocorticoid receptors bound to the antagonist RU486 are not downregulated despite their capacity to interact in vitro with defined gene regions. J Steroid Biochem 26: 513–520, 1987. [DOI] [PubMed] [Google Scholar]
- 46.Rashid S, Lewis G. The mechanisms of differential glucocorticoid and mineralocorticoid action in the brain and peripheral tissue. Clin Biochem 38: 401–409, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Roozendaal B, Williams CL, McGaugh JL. Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: involvement of the basolateral amygdala. Eur J Neurosci 11: 1317–1323, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Rosmond R, Chagnon YC, Holm G, Chagnon M, Perusse L, Lindell K, Carlsson B, Bouchard C, Bjorntorp P. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic-pituitary-adrenal axis. Obes Res 8: 211–218, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, Nuzzo V, Lombardi G. Subclinical Cushing's syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab 85: 1440–1448, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Ruch W, Baumann JB, Hausler A, Otten UH, Siegl H, Girard J. Importance of the adrenal cortex for development and maintenance of hypertension in spontaneously hypertensive rats. Acta Endocrinol 105: 417–424, 1984. [DOI] [PubMed] [Google Scholar]
- 51.Sanders BJ, Lawler JE. The borderline hypertensive rat (BHR) as a model for environmentally-induced hypertension: a review and update. Neurosci Biobehav Rev 16: 207–217, 1992. [DOI] [PubMed] [Google Scholar]
- 52.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory and preparative actions. Endocrine Rev 21: 55–89, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Scheuer DA, Bechtold AG. Glucocorticoids modulate baroreflex control of heart rate in conscious normotensive rats. Am J Physiol Regul Integr Comp Physiol 282: R475–R483, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Scheuer DA, Bechtold AG. Glucocorticoids potentiate central actions of angiotensin to increase arterial pressure. Am J Physiol Regul Integr Comp Physiol 280: R1719–R1726, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am J Physiol Heart Circ Physiol 286: H458–H467, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Scheuer DA, Bechtold AG, Vernon KA. Chronic activation of dorsal hindbrain corticosteroid receptors augments the arterial pressure response to acute stress. Hypertension 49: 127–133, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stamp J, Herbert J. Corticosterone modulates autonomic responses and adaptation to immediate-early gene expression to repeated restraint. Neuroscience 107: 465–479, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Willemsen G, Kirschbaum C, Marmot MG. Socioeconomic status and stress-related biological responses over the working day. Psychosom Med 65: 461–470, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi H, Takeda K, Ashizawa H, Inoue A, Yoneda S, Yoshimura M, Ijichi H. Centrally induced cardiovascular and sympathetic responses to hydrocortisone in rats. Am J Physiol Heart Circ Physiol 245: H1013–H1018, 1983. [DOI] [PubMed] [Google Scholar]
- 60.Tonolo G, Soro A, Madeddu P, Troffa C, Melis MG, Patteri G, Pinna Parpaglia P, Sabino G, Maioli M, Glorioso N. Effect of chronic intracerebroventricular dexamethasone on blood pressure in normotensive rats. Am J Physiol Endocrinol Metab 264: E843–E847, 1993. [DOI] [PubMed] [Google Scholar]
- 61.Ukkola O, Rosmond R, Tremblay A, Bouchard C. Glucocorticoid receptor Bcl I variant is associated with an increased atherogenic profile in response to long-term overfeeding. Atherosclerosis 157: 221–224, 2001. [DOI] [PubMed] [Google Scholar]
- 62.van Acker SA, Fluttert MF, Sibug RM, de Kloet ER. Intracerebroventricular administration of a glucocorticoid receptor antagonist enhances the cardiovascular responses to brief restraint. Eur J Pharmacol 430: 87–91, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Van den Berg DTWM, De Jong W, de Kloet ER. Mineralocorticoid antagonist inhibits stress-induced blood pressure response after repeated daily warming. Am J Physiol Endocrinol Metab 267: E921–E926, 1994. [DOI] [PubMed] [Google Scholar]
- 64.van den Buuse M, van Acker SA, Fluttert MF, de Kloet ER. Involvement of corticosterone in cardiovascular responses to an open-field novelty stressor in freely moving rats. Physiol Behav 75: 207–215, 2002. [DOI] [PubMed] [Google Scholar]
- 65.van der Hooft CS, Heeringa J, Brusselle GG, Hofman A, Witteman JCM, Kingma JH, Sturkenboom MCJM, Stricker BHC. Corticosteroids and the risk of atrial fibrillation. Arch Intern Med 165: 1016–1020, 2006. [DOI] [PubMed] [Google Scholar]
- 66.van Haarst AD, Oitzl MS, Workel JO, de Kloet ER. Chronic brain glucocorticoid receptor blockade enhances the rise in circadian and stress-induced pituitary-adrenal activity. Endocrinology 137: 4935–4943, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Walker BR Glucocorticoids and cardiovascular disease. Eur J Endocrinol 157: 545–559, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Wang HX, Leineweber C, Kirkeeide R, Svane B, Schenck-Gustafsson K, Theorell T, Orth-Gomer K. Psychosocial stress and atherosclerosis: family work and stress accelerate progression of coronary disease in women. The Stockholm female coronary angiography study. J Intern Med 261: 245–254, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Wang LL, Ou CC, Chan JYH. Receptor-independent activation of GABAergic neurotransmission and receptor-dependent nontranscriptional activation of phosphatidylinositol 3-kinase/protein kinase Akt pathway in short-term cardiovascular actions of dexamethasone at the nucleus tractus solitarii of the rat. Mol Pharmacol 67: 489–498, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Watt GCM, Harrap SB, Foy CJW, Holton DW, Edwards HV, Davidson HR, Conner JM, Lever AF, Fraser R. Abnormalities of glucocorticoid metabolism and the renin-angiotensin system: a four-corners approach to the identification of genetic determinants of blood pressure. J Hypertens 10: 473–482, 1992. [DOI] [PubMed] [Google Scholar]
- 71.Whitworth JA, Mangos GJ, Kelley JJ. Cushing, cortisol and cardiovascular disease. Hypertension 36: 912–916, 2000. [DOI] [PubMed] [Google Scholar]