Abstract
Sighs, a well-known phenomenon in mammals, are substantially augmented by hypoxia and hypercapnia. Because (d-Ala2,N-Me-Phe4,Gly-ol)-enkephalin (DAMGO), a μ-receptor agonist, injected intravenously and locally in the caudal medullary raphe region (cMRR) decreased the ventilatory response to hypoxia and hypercapnia, we hypothesized that these treatments could inhibit sigh responses to these chemical stimuli. The number and amplitude of sighs were recorded during three levels of isocapnic hypoxia (15%, 10%, and 5% O2 for 1.5 min) or hypercapnia (3%, 7%, and 10% CO2 for 4 min) to test the dependence of sigh responses on the intensity of chemical drive in anesthetized and spontaneously breathing rats. The role of μ-receptors in modulating sigh responses to 10% O2 or 7% CO2 was subsequently evaluated by comparing the sighs before and after 1) intravenous administration of DAMGO (100 μg/kg), 2) microinjection of DAMGO (35 ng/100 nl) into the cMRR, and 3) intravenous administration of DAMGO after microinjection of d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP, 100 ng/100 nl), a μ-receptor antagonist, into the cMRR. Hypoxia and hypercapnia increased the number, but not amplitude, of sighs in a concentration-dependent manner, and the responses to hypoxia were significantly greater than those to hypercapnia. Systemic and local injection of DAMGO into the cMRR predominantly decreased the number of sighs, while microinjection into the rostral and middle MRR had no or limited effects. Microinjecting CTAP into the cMRR significantly diminished the systemic DAMGO-induced reduction of the number of sighs in response to hypoxia, but not to hypercapnia. Thus we conclude that hypoxia and hypercapnia elevate the number of sighs in a concentration-dependent manner in anesthetized rats, and this response is significantly depressed by activating systemic μ-receptors, especially those within the cMRR.
Keywords: hypercapnia, hypoxia
sighs were first noted in humans by Haldane in 1919 (22) and have been a subject of interest for nearly a century. Sighs occur in awake, sleeping, and anesthetized states of humans and animals (18, 23, 24, 34, 39, 43, 57) and are characterized by a spontaneously augmented inspiration on top of the preceding normal tidal volume (7). Several investigators have indicated that carotid chemoreceptors and vagal airway mechanoreceptors are critical for the genesis of sighs (3, 4, 20, 46, 55) because bilateral vagotomy or carotid sinus denervation diminished or eliminated sighs in cats (7), rabbits (31), and rats (3, 30). Although the functional significance and possible implications of sighs are not fully understood, they are thought to play a role in maintaining a healthy lung condition by reopening collapsed alveoli (3, 20, 46), causing increased lung compliance and preventing atelectasis (36). Recent studies also suggest that sudden infant death syndrome (SIDS) may be associated with the failure to sigh (25). The number of sighs is greatly augmented by hypoxia and hypercapnia in anesthetized cats (7), awake dogs (7) and unanesthetized dogs (3, 20, 55). Interestingly, systemic administration of morphine, a μ-receptor agonist, substantially attenuated the ventilatory responses to these chemical challenges (48, 58). It also reduced the number of sighs in patients in postoperative analgesia (14), indicating a modulatory effect of μ-receptors on sighs. However, it has not been explored whether systemic opioids are able to inhibit sigh responses to hypoxia and hypercapnia and, if so, which central sites' μ-receptors are involved.
There are several lines of evidence implying a possible involvement of medullary raphe region (MRR) μ-receptors in modulating sigh responses to hypercapnia and hypoxia. First, the MRR expresses abundant μ-receptors (12), and our recent studies show that MRR μ-receptors are capable of profoundly depressing ventilation and the ventilatory responses to hypoxia (60) and hypercapnia (61). Second, neurons within the MRR project extensively to the respiration-related nuclei, including the pre-Bötzinger complex (pre-BötC) (15, 16) and nucleus tractus solitarii (6, 54, 56), which have substantial synaptic inputs from vagal airway mechanoreceptors (32, 33) and carotid chemoreceptors (8, 13, 17). Third, sighs are significantly reduced in SIDS victims (25), in whom μ-receptors seem to play an important role (10, 50, 51) and an abnormality in the MRR has been observed (26, 41). To date, direct evidence of MRR μ-receptor participation in opioid-induced depression of sighs is lacking.
To address these issues, we tested in anesthetized and spontaneously breathing rats whether 1) hypercapnia and hypoxia augment the number and amplitude of sighs in a concentration-dependent manner; 2) systemic or local injection of (d-Ala2,N-Me-Phe4,Gly-ol)-enkephalin (DAMGO), a selective μ-receptor agonist, into the MRR alters sighs during hypoxia and hypercapnia; and 3) systemic DAMGO-induced attenuation of sigh responses to hypoxia and hypercapnia is diminished by a previous microinjection of d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP), a μ-receptor antagonist, into the MRR.
MATERIALS AND METHODS
The experimental protocols were approved by the Institutional Animal Care and Use Committee of Lovelace Respiratory Research Institute, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, USA. The experiments were performed in tracheotomized and spontaneously breathing adult male Sprague-Dawley rats (350–450 g).
General animal preparation.
Rats (n = 46) were initially anesthetized with urethane (1,200 mg/kg ip), and supplemental urethane was administered, if needed, to reach an adequate level of anesthesia at which rats exhibited neither eyeblink nor limb-withdrawal reflex throughout the experiment. The animal preparation was similar to that we previously reported (61). Briefly, the right femoral vein and artery were cannulated, the former for drug administration and the latter for monitoring mean arterial blood pressure (BP) and heart rate (HR). The trachea below the larynx was exposed through a midline incision, tracheotomized by blunt dissection, and cannulated. A pneumotachograph was connected to the tracheal cannula to record airflow and for a setup that allowed the rat to be exposed to different chemical challenges. The pneumotachograph had a linear flow-pressure relationship in the range of 2–20 ml/s, a flow resistance of 0.046 cmH2O·ml−1·s, and a dead space of 0.2 ml. During isocapnic hypoxia, CO2 was added to maintain the end-tidal CO2 pressure (PetCO2) within 2-mmHg deviation from the baseline value (53). PetCO2 was measured via a CO2 analyzer (MicroCapStar end-tidal CO2 analyzer, model 15-10000; CWE, Ardmore, PA) connected to a side port of the tracheal cannula. Animals were placed into a rigid metal frame with their heads fixed and centered in a stereotaxic apparatus (model 1404, Kopf, Tujunga, CA). A hole (∼10-mm diameter) was drilled at the midline of the skull in some rats for microinjection of DAMGO or CTAP into the MRR. The animals' core temperature was monitored with a rectal probe and maintained at 36.5–37.5°C with a heat pad and a radiant heat lamp.
Hypercapnic and hypoxic exposure.
Hyperoxia (30% O2 balanced with nitrogen) was applied to serve as the control level at which a sigh was rarely observed. To test the hypoxic concentration dependence of sighs, the animal was exposed to 15%, 10%, and 5% O2 (balanced with varied percentages of nitrogen) for 1.5 min, because brief hypoxia is believed to act mainly on the carotid body (5). With respect to hypercapnia, 3%, 7%, and 10% CO2 (balanced with 30% O2 and varied percentages of nitrogen) for 4 min, respectively, were applied. This exposure was chosen because it would primarily stimulate central chemoreceptors, and <4 min of hypercapnic exposure failed to sufficiently evoke sighs, especially under lower CO2 concentration, in our pilot study. A 3-min interval was allowed for recovery between two chemical challenges. Subsequently, to clarify the effect of intravenously or locally injected DAMGO on sighs during hypoxia or hypercapnia, the middle, rather than higher, levels of chemical stimulations (10% O2 followed by 7% CO2) were chosen to avoid severe impact of these stimulations.
Systemic administration or microinjection of DAMGO.
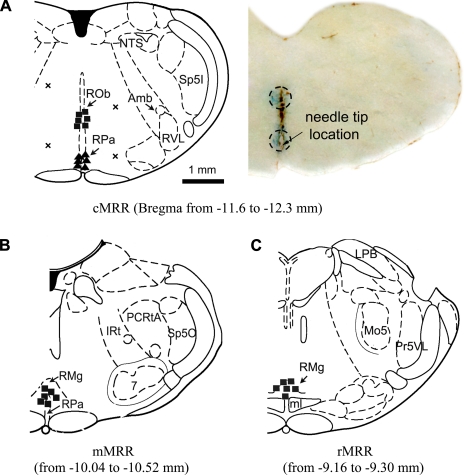
To evaluate the role of systemic μ-receptors in modulating sigh responses to hypoxia and hypercapnia, rats were exposed to these stimulations before and after intravenous injection of DAMGO (100 μg/kg). This dose was demonstrated in our previous studies to substantially depress the ventilatory response to hypoxia (60) and hypercapnia (61). For microinjection, a 0.5-μl microneedle with the tip (OD 0.25 mm) (Hamilton, Reno, NV) prefilled with DAMGO (Sigma-Aldrich, St. Louis, MO) was inserted into the selected MRR region. DAMGO (0.35 μg/μl) was made in a solution of 0.9% saline containing 1% Chicago Sky Blue (Sigma, St. Louis, MO). According to the rat stereotaxic atlas of Paxinos and Watson (1998) and earlier studies (61), the MRR, extending from 9 to 12 mm caudal to the bregma, was divided into three subregions: rostral, middle, and caudal MRR (rMRR, mMRR, and cMRR), located at 9.0, 10.5, and 12.0 mm caudal to the bregma, respectively. The rMRR contained the magnus nucleus (RMg), the mMRR contained the RMg and its neighboring pallidus nucleus (RPa), and the cMRR contained the obscurus nucleus (ROb) and RPa. The central sites for the mMRR and rMRR were localized 9 mm ventral to the cerebellar surface, and each site received a 100-nl microinjection. Because the two nuclei in the cMRR were located separately and distantly, two injections (100 nl each) were given. The first injection was given when the needle was placed into the site 8.3 mm ventral to the cerebellar surface, corresponding to the center of the ROb. After this injection, the needle was advanced 1 mm deeper, corresponding to the RPa, and was followed by the second injection. This relative large volume of microinjection (100 nl) was chosen for two reasons. First, according to the rat stereotaxic atlas of Paxinos and Watson (1998), each of the three subnuclei (RMg, RPa, ROb) covers a relatively large area. For example, the RMg includes a region ∼0.5–1 mm wide, centered at the middle, extending from the base of the brain to 1 mm dorsal, and extending ∼2 mm from caudal to rostral. Previous studies have calculated that microinjection of a volume of 100 nl into the brain stem could spread as far as 1 mm (29, 37). Second, microinjection of this volume (100 nl) into the raphe (38) and hypothalamus (11) in rats has been used by other investigators recently. Microinjection of DAMGO was made purposely outside the cMRR in two rats to test the unique role of the cMRR. The microinjections were located at 1 mm left of midline and 7.5 mm and 9 mm ventral to the cerebellar surface, respectively, in one rat and at 1 mm right of midline and 8.3 mm and 9.3 mm ventral to the cerebellar surface, respectively, in another rat. To block μ-receptors in the cMRR, microinjections of CTAP (100 ng/100 nl containing 1% Chicago Sky Blue), a μ-receptor antagonist, were made in the cMRR (100 nl).
Experimental protocol.
Our experiments were performed in seven groups of anesthetized and spontaneously breathing rats. Systemic administration of DAMGO alone was conduced in group I (n = 6), in which animals were initially exposed to three degrees of hypoxia and hypercapnia to test the concentration dependence of sigh responses on these challenges. Subsequently, the exposures to 10% O2 and 7% CO2 were repeated before and 5 and 30 min after intravenous injection of DAMGO. The effects of microinjection of DAMGO/CTAP into the MRR were evaluated in five groups of rats (n = 6 each group in groups II-V and n = 5 in group VI); 10% O2 and 7% CO2 were performed before and 5, 30, and 60 or up to 120 min after microinjection of DAMGO into the cMRR (group II), mMRR (group III), and rMRR (group IV), respectively. Because only activation of cMRR μ-receptors inhibited sigh responses in our pilot studies, the role of blocking local μ-receptors in the systemic DAMGO-induced inhibition of sighs was studied in group V. In these cases, sigh responses to hypoxia and hypercapnia were repeated four times. In other words, the exposures were applied before and after systemic DAMGO, and the same protocols were repeated 2 h later, with the exception that CTAP (100 ng/100 nl) was microinjected into the cMRR before systemic DAMGO. The 2-h interval was chosen because systemic DAMGO has been reported to have an ∼15-min half-life in mammals (52, 61). To verify the effect of local CTAP alone on sigh responses to hypoxia and hypercapnia, the same hypoxic and hypercapnic exposures were performed before and after microinjection of CTAP (100 ng/100 nl) into the cMRR in group VI. Animals in group VII (n = 11) served as sham-operated controls, in which intravenous and local injection of vehicle instead of agents was conducted in nine animals and microinjection of DAMGO was made outside of the cMRR in two rats.
Identification of microinjection sites.
After completing the experiments, all animals were euthanized by an overdose of anesthetic. The brain stem was removed and fixed by soaking in 4% paraformaldehyde (pH 7.4) for at least 36 h at 4°C and subsequently sectioned at a 40-μm thickness with a slicing machine (Leica, CM 1850, Microsystems, Nussioch, Germany). The area marked by Chicago Sky Blue was identified under a microscope, and the center of the stained area was utilized as the microinjection location.
Data acquisition and statistical analysis.
Raw data of the airflow signal, BP, HR, PetCO2, and rectal temperature were digitized, monitored, and recorded with a PowerLab/8sp (model ML 785; ADInstruments, Colorado Springs, CO) connected to a computer using PowerLab Chart 5 software. The airflow signals were integrated to generate tidal volume (Vt), respiratory frequency (f), and minute ventilatory volume (V̇e). Sighs were defined as an augmented spontaneous inspiration with Vt being at least twofold greater than that of the preceding normal breath (21). Sigh amplitudes were calculated by the augmented inspiration (phase II) above the preceding eupneic Vt (phase I), because phase II, unlike phase I, was relatively constant and largely independent of chemical stimuli (7). All data are presented as means ± SE. Student's t-test was used to compare the difference between the hypoxia-induced and hypercapnia-induced respiratory variables and sigh responses and the sigh responses before and after microinjection of CTAP alone into the cMRR. One-way analysis of variance (ANOVA) for repeated measures was employed to compare 1) the number and amplitude of sighs during different degrees of hypoxic or hypercapnic exposures; 2) the number and amplitude of sighs during 10% O2 and 7% CO2 before and several time points after administration of DAMGO; and 3) the hypoxia- or hypercapnia-evoked sigh responses (Δ% change from control, baseline value) before and after DAMGO alone and coupled with CTAP. Fisher's least significant difference post test was used if the overall ANOVA (an omnibus test) had a P value <0.05. STATISTICA 6.0 software (StatSoft, Tulsa, OK) was employed for statistical analysis. Difference was considered significant at a P value <0.05.
RESULTS
Hypoxia and hypercapnia increased the number of sighs in a concentration-dependent manner.
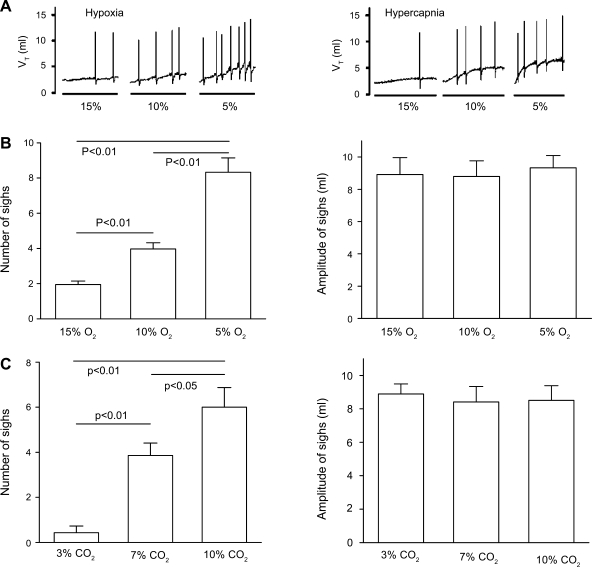
Sighs were rarely observed during eupneic breathing, while various concentrations of hypoxia and hypercapnia increased the number, but not the amplitude, of sighs in a concentration-dependent manner (Fig. 1). Previous studies have indicated a stronger sigh response to hypoxia than hypercapnia in cats by comparing the number of sighs at similar Vt levels (7) . To estimate the differences in sigh responses to hypoxia and hypercapnia in rats, we compared the responses of sighs and respiratory variables between the two chemical challenges. Table 1 shows that the averaged V̇e and Vt responses to the 1.5-min hypoxia were strikingly lower than those of the 4-min hypercapnia (P < 0.01), but the number of sighs induced by hypoxia was significantly greater than that of hypercapnia. Additionally, the amplitude of sighs in response to hypoxia and hypercapnia was not markedly different.
Fig. 1.
Effects of different hypoxic and hypercapnic degrees on sighs. A: representative recordings of sighs induced by 3 different hypoxic (1.5 min) and hypercapnic (4 min) exposures. To emphasize the sigh responses, the moving average of the tidal volume (Vt) is presented and the spikes reflect sighs. B and C: grouped responses of sigh number (left) and amplitude (right) to hypoxia (B) or hypercapnia (C). Data are presented as means ± SE; n = 6.
Table 1.
Ventilation and sigh responses to hypoxia or hypercapnia
| V̇e, ml/min | f, breaths/min | Vt, ml | No. of sighs | Amplitude of sighs, ml | |
|---|---|---|---|---|---|
| Hypoxia | 485±24 | 127±3 | 3.84±0.45 | 4.75±0.57 | 9.45±0.48 |
| Hypercapnia | 674±47† | 130±6 | 5.16±0.38† | 3.43±0.32* | 8.66±0.85 |
Values are means ± SE; n = 6 rats. V̇e, minute ventilatory volume; f, respiratory frequency; Vt, tidal volume. All data were averaged from 3 degrees of 1.5-min hypoxia (15%, 10%, and 5% O2) and 4-min hypercapnia (3%, 7%, and 10% CO2).
P < 0.05,
P < 0.01 compared between hypoxia and hypercapnia.
Systemic DAMGO decreased the sigh responses to hypoxia and hypercapnia.
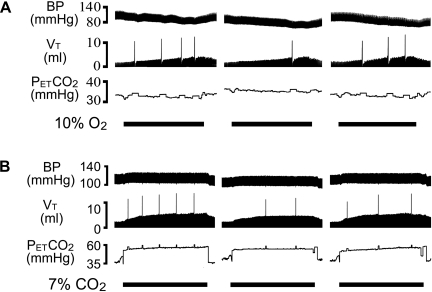
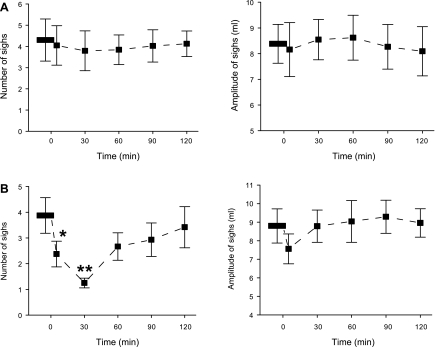
Intravenous administration of DAMGO decreased responses of both number and amplitude of sighs to hypoxia and hypercapnia, but the effects were much greater on the number than on the amplitude. Typical recordings and the corresponding group data are exhibited in Figs. 2 and 3, respectively. As shown, systemic DAMGO significantly depressed the number and amplitude of sighs in response to hypoxia by 65% and 14%, respectively, and the responses to hypercapnia by 55% and 17%, respectively. These DAMGO-induced depressions lasted no longer than 30 min. In sharp contrast, intravenous injection of vehicle did not change the responses of the number and amplitude of sighs to hypoxia and hypercapnia.
Fig. 2.
Representative recordings exhibiting the impact of systemic (d-Ala2,N-Me-Phe4,Gly-ol)-enkephalin (DAMGO; 100 μg/kg) on hypoxia (A)- or hypercapnia (B)-induced changes in sigh number and amplitude. In each panel, the chemical challenges (10% O2 for 1.5 min and 7% CO2 for 4 min) were applied before (left) and 5 min (center) and 30 min (right) after systemic DAMGO. Traces from top to bottom are arterial blood pressure (BP), Vt, and end-tidal pressure of carbon dioxide (PetCO2), with stimulating durations of chemical challenges indicated.
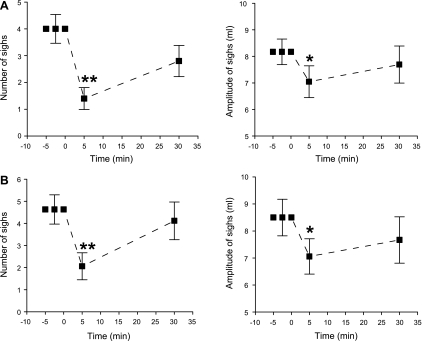
Fig. 3.
Effect of systemic DAMGO (100 μg/kg) on sigh number (left) and amplitude (right) induced by 10% O2 (A) or 7% CO2 (B). Data are presented as means ± SE; n = 6. *P < 0.05, **P < 0.01 compared with before DAMGO. “0” on x-axis indicates onset of intravenous administration of DAMGO.
Microinjection of DAMGO into the cMRR attenuated sighs during hypoxia and hypercapnia.
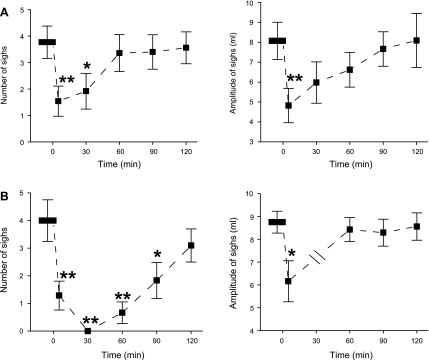
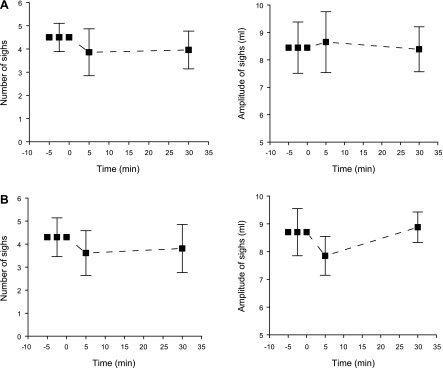
Similar to systemic DAMGO, microinjection of DAMGO into the cMRR attenuated responses of both sigh number and amplitude to hypoxia and hypercapnia, with a greater effect on number (Fig. 4). Five minutes after microinjection, the number and amplitude of sighs in response to hypoxia were strikingly attenuated by 59% and 40%, respectively, and in response to hypercapnia by 68% and 30%, respectively. Microinjection of the same volume of vehicle into the cMRR had no effect on these responses (Table 2). Different from systemic DAMGO, DAMGO microinjection-induced depression of sigh responses usually lasted longer (>60 min). In particular, hypercapnia-induced responses of sigh number were abolished 30 min after microinjection of DAMGO, and the inhibitory effect lasted for 90 min. To test the unique role of the cMRR, DAMGO was administered into regions outside the cMRR in two rats (Fig. 5). These microinjections did not make a remarkable difference in the number of sighs (4.02 ± 0.98 vs. 3.89 ± 0.92 during hypoxia; 4.16 ± 0.82 vs. 4.08 ± 0.94 during hypercapnia) or the amplitude of sighs (9.04 ± 0.79 vs. 8.83 ± 0.86 ml during hypoxia; 8.88 ± 0.82 vs. 8.62 ± 0.76 ml during hypercapnia).
Fig. 4.
Effect of microinjection of DAMGO into the caudal medullary raphe region (cMRR) on the number (left) and amplitude (right) of sighs induced by 10% O2 (A) or 7% CO2 (B). Data are presented as means ± SE; n = 6. *P < 0.05, **P < 0.01 compared with before DAMGO. “0” on x-axis indicates onset of intravenous DAMGO administration. Note that there was no amplitude response to 7% CO2 at the 30-min point in B because of the absence of a sigh at that time point.
Table 2.
Sigh responses to hypoxia and hypercapnia before and after microinjection of vehicle into caudal or middle medullary raphe region
|
10% O2 |
7% CO2
|
|||
|---|---|---|---|---|
| No. of sighs per 1.5 min | Amplitude of sighs, ml | No. of sighs per 4 min | Amplitude of sighs, ml | |
| cMRR (n=5) | ||||
| Before | 3.83±0.53 | 9.15±0.52 | 4.05±0.46 | 8.78±0.81 |
| After | 4.08±0.91 | 8.86±0.85 | 4.13±0.52 | 9.05±0.97 |
| mMRR (n=4) | ||||
| Before | 4.01±0.58 | 9.01±0.82 | 3.94±0.41 | 8.83±0.89 |
| After | 3.78±0.73 | 9.26±0.94 | 4.16±0.63 | 8.67±0.87 |
Values are means ± SE for n rats. cMRR, caudal medullary raphe region; mMRR, middle medullary raphe region.
Fig. 5.
Diagram showing the sites where the microinjections occurred. A, left: cartoon of a coronal slice. ▪ and ▴, Locations of the microinjections in the cMRR of 6 rats;×, 4 microinjections outside the cMRR in 2 rats. A, right: representative slice containing the cMRR. Areas stained by Chicago Sky Blue are circled. B and C: ▪, locations of the microinjections in the middle MRR (mMRR; B) and rostral MRR (rMRR; C), respectively. Amb, nucleus ambiguus; IRt, intermediate reticular nucleus; LPB, lateral parabrachial nucleus; ml, medial lemniscus; Mo5, motor 5 nucleus; NTS, nucleus of solitary tract; PCRtA, parvicellular reticular nucleus alpha; Pr5VL, ventrolateral part of principal sensory 5 nucleus; ROb, raphe obscurus nucleus; RMg, raphe magnus nucleus; RPa, raphe pallidus nucleus; RVL, rostroventrolateral reticular nucleus; 7, facial nucleus; Sp5I, interpolar part of spinal 5 nucleus; Sp5O, oral part of spinal 5 nucleus.
Changes in sigh responses after microinjection of DAMGO into the mMRR or rMRR were limited.
The data presented in Fig. 6 indicated that microinjecting DAMGO into the mMRR only significantly attenuated the responses of sigh number to hypercapnia by 38% and 67% 5 and 30 min after microinjection, respectively. This microinjection failed to alter the responses of sigh number and amplitude to hypoxia and the amplitude responses to hypercapnia. Again, microinjecting the same volume of vehicle into this region did not lead to remarkable alterations in sigh responses (Table 2). When the microinjection was made in the rMRR (Fig. 7), no significant changes in the responses of sigh number or amplitude to hypoxia or hypercapnia were observed. The locations of the microinjections made in the mMRR and rMRR are also illustrated in Fig. 5.
Fig. 6.
Influence of DAMGO microinjection into the mMRR on the responses of sigh number (left) and amplitude (right) to 10% O2 (A) and 7% CO2 (B). Data are presented as means ± SE; n = 6. *P < 0.05, **P < 0.01 compared with before DAMGO. “0” on x-axis indicates onset of intravenous DAMGO administration.
Fig. 7.
Effect of DAMGO microinjection into the rMRR on the responses of sigh number (left) and amplitude (right) to 10% O2 (A) and 7% CO2 (B). Data are presented as means ± SE; n = 6. “0” on x-axis indicates onset of intravenous DAMGO administration.
Blocking cMRR μ-receptors diminished the systemic DAMGO-induced attenuation of sigh responses.
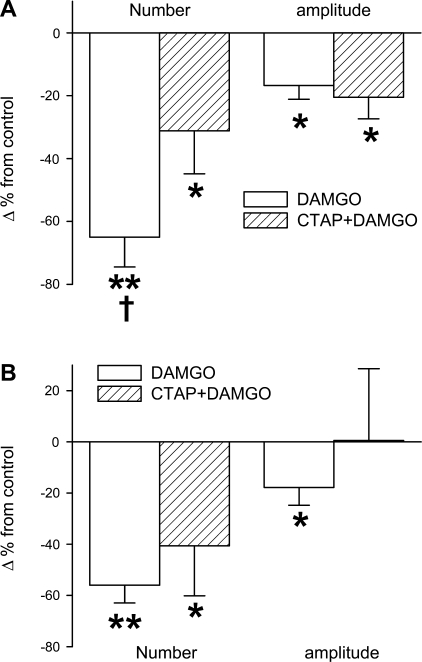
This study was performed in two groups of rats. In group V, we first tested the effects of systemic DAMGO on hypoxia- and hypercapnia-induced sighs and found that DAMGO significantly inhibited the responses of sigh number and amplitude to hypoxia and hypercapnia, similar to those mentioned above. Second, we examined whether locally blocking cMRR μ-receptors by microinjecting CTAP into the cMRR, with the same protocol described above, would affect this systemic DAMGO-induced change. As illustrated in Fig. 8A, the systemic DAMGO-induced attenuation of sigh number rather than amplitude in response to hypoxia was significantly diminished from 65% to 31% after CTAP microinjection. In other words, the systemic DAMGO-induced depression of the sigh number during hypoxia was diminished by 52% after blockade of cMRR μ-receptors. Although local blockade of cMRR μ-receptors tended to decrease the systemic DAMGO-induced depression of the sigh number during hypercapnia, this change did not reach significance (Fig. 8B). The effects of microinjection of CTAP alone into the cMRR on sigh responses were evaluated in group VI. We found that the microinjection did not significantly affect the responses of sighs to hypoxia (3.93 ± 0.46 vs. 4.13 ± 0.64 for number; 8.85 ± 0.91 vs. 9.16 ± 0.89 ml for amplitude) and hypercapnia (4.01 ± 0.31 vs. 3.82 ± 0.53 for number; 8.79 ± 0.81 vs. 9.13 ± 0.86 ml for amplitude).
Fig. 8.
Comparison of the systemic DAMGO-induced attenuation of the responses of sigh number (left) and amplitude (right) to 10% O2 (A) or 7% CO2 (B) before and after microinjection of d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) into the cMRR. All variables are presented as % change (Δ%) from control (indicated as “0” on y-axis). Data are means ± SE; n = 6. *P < 0.05, **P < 0.01 compared with control; †P < 0.05 between DAMGO-induced changes before and after CTAP microinjection.
DISCUSSION
Similar to previous studies reported in anesthetized cats (7) and awake dogs (45), we found that hypoxia and hypercapnia increased the number, but not amplitude, of sighs in a concentration-dependent manner in anesthetized rats. Although hypoxia induces sighs associated with hypotension, this transient hypotension is unlikely a major trigger of sighs. First, it was reported that hypotension remained while sighs disappeared in bilateral vagotomized rats exposed to hypoxia (3, 30). Second, hypercapnia is also able to increase sighs without decreasing BP (61), which is the same in the present study (see Fig. 2B). Third, systemic injection of DAMGO did not markedly alter hypoxia-induced hypotension but dramatically reduced hypoxia-induced sighs (see Fig. 2A). To estimate whether sigh responses to hypoxia and hypercapnia differed, we compared sighs and respiratory variable responses to hypoxia and hypercapnia owing to the dependence of the number of sighs on Vt (7). We found that compared with hypercapnia, hypoxia with a shorter exposure period (1.5 min vs. 4 min) produced lower V̇e and Vt responses but generated a greater number of sighs, suggesting that hypoxia is much more powerful than hypercapnia in producing sighs, which is in agreement with previous studies in cats (7, 20). A stronger hypoxic stimulating effect than hypercapnia on sigh genesis may be due to the fact that carotid chemoreceptor inputs play an important role in facilitating sighs (3, 20, 55).
Our major finding is that intravenous and local injection of DAMGO into the cMRR decreased the number and amplitude of sighs induced by hypoxia or hypercapnia. Systemic DAMGO significantly depressed the responses of sigh number and amplitude to hypoxia by 65% and 14%, respectively, and the responses to hypercapnia by 55% and 17%, respectively. These results agree with an early observation (14) in which morphine used systemically in patients for postoperative analgesia reduced the number of sighs. Because hypoxia and hypercapnia stimulate sighs primarily via elevating their number, it is understandable that DAMGO mainly affects the number of sighs. Given that our study was performed in anesthetized rats, we cannot rule out the possible interaction between the anesthetic and DAMGO on sighs. However, it was reported that the number of sighs was not strikingly influenced by anesthesia in rats (3). Similar to systemic DAMGO, microinjection of DAMGO into the cMRR attenuated responses of both sigh number and amplitude to hypoxia and hypercapnia, with a greater effect on the number. In sharp contrast, the same microinjections made into the mMRR or rMRR had no effect on sighs, with the exception that the former attenuated the response of sigh number to hypercapnia. Clearly, our results demonstrate that activating MRR μ-receptors, especially those within the cMRR, depresses chemical stimulation-induced sighs.
An interesting finding is that microinjection of CTAP, a μ-receptor antagonist, into the cMRR diminishes the systemic DAMGO-depressed responses of sigh number to hypoxia, with no significant effect on the amplitude responses. Moreover, unexpectedly, the CTAP microinjection failed to significantly change the systemic DAMGO-depressed hypercapnia-induced sighs. The absence of a CTAP effect may be due to the fact that at the same dose of the systemic DAMGO, the role of cMRR μ-receptors in inhibiting the hypercapnia-induced sighs is less important than in depressing the hypoxia-induced sighs, although a local high dose of DAMGO could diminish both chemical stimuli-induced sighs.
In the present study, systemic DAMGO-induced attenuation of sighs is not fully eliminated by blocking cMRR μ-receptors, which leads to a postulation that μ-receptors in other regions, in addition to the cMRR, also participate in controlling sighs. Previous studies have pointed out that both vagal mechanoreceptor and carotid chemoreceptor inputs are critical for evoking sighs (3, 7, 30, 31). These, along with the presence of heavy expression of μ-receptors on vagal afferents (nodose ganglion) (1, 27, 40) and the carotid body (35), raise a possible peripheral μ-receptor contribution to systemic DAMGO-induced depression of sighs. Another possibility is that other central μ-receptors may be involved owing to a wide distribution of μ-receptors in the brain stem (12, 44), including the pre-BötC, which is thought to be critical for the genesis of sighs (28, 49). Because of the presence of the mutual projections between the MRR and the pre-BötC (15, 16), further studies are needed to define whether the MRR functions as a sigh generator and/or a relay station on the ascending or descending pathway of the sigh generator. Hypoxia (42) and hypercapnia (19) could promote central release of opioids; however, microinjection of CTAP alone into the cMRR did not significantly alter the sighs during hypoxia and hypercapnia in this study. These results allow us to believe that the endogenously released opioids in cMRR by hypoxia and hypercapnia contribute little to modulate the sigh responses. Nevertheless, our results suggest that cMRR μ-receptors play a crucial role in modulating systemic DAMGO-induced attenuation of the response of sigh number to hypoxia.
Sighs are thought to maintain a healthy lung condition by reopening collapsed alveoli (3, 9, 20, 46), increasing lung compliance and preventing atelectasis (36). Opioids used in postoperative analgesia increased pulmonary morbidity, including atelectasis, pulmonary infection, and overall pulmonary complications (2, 47). More importantly, the majority of sighs during sleep are associated with electroencephalographic signs of arousal (43, 59) that is critical for breaking an apnea in obstructive sleep apnea syndrome or SIDS (25). Our finding that cMRR μ-receptors play an important role in depressing sighs during hypoxia and hypercapnia is consistent with the abnormality of the MRR and elevated endogenous cerebral opioid in SIDS (10, 26, 41, 50, 51). Determining the role of cMRR μ-receptors in hypoxic and hypercapnic modulation of sighs helps us understand participation of these receptors in atelectasis and respiratory disorders, including sleep apnea and breathing mechanisms in SIDS.
In conclusion, we found that hypoxia and hypercapnia selectively increased the number of sighs in a concentration-dependent manner. Systemic or local administration of DAMGO into the cMRR predominantly depressed the responses of sigh number to hypoxia and hypercapnia. These results suggest that cMRR μ-receptors play an important role in depressing sighs elicited by hypoxia and hypercapnia in anesthetized rats.
Perspectives
Hypoxia and hypercapnia elevate the number of sighs in a concentration-dependent manner, and this response is significantly depressed by activation of systemic μ-receptors, especially those within the cMRR.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-074183 and by American Lung Association Fellowship RT-83131-N.
Acknowledgments
The authors thank Dr. Jianguo Zhuang (Associate Research Scientist, Pathophysiology Program, Lovelace Respiratory Research Institute) for his assistance with the experiment setup and statistics.
REFERENCES
- 1.Aicher SA, Goldberg A, Sharma S, Pickel VM. Mu-opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. J Comp Neurol 422: 181–190, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne JC, Carr DB, deFerranti S, Suarez T, Lau J, Chalmers TC, Angelillo IF, Mosteller F. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg 86: 598–612, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett D Jr. Origin and regulation of spontaneous deep breaths. Respir Physiol 12: 230–238, 1971. [DOI] [PubMed] [Google Scholar]
- 4.Bendixen HH, Smith GM, Mead J. Pattern of ventilation in young adults. J Appl Physiol 19: 195–198, 1964. [DOI] [PubMed] [Google Scholar]
- 5.Bisgar GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Regulation of Breathing, edited by Dempsey JA, Pack AL. New York: Dekker, 1995, p. 617.
- 6.Bonham AC Neurotransmitters in the CNS control of breathing. Respir Physiol 101: 219–230, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Cherniack NS, von Euler C, Glogowska M, Homma I. Characteristics and rate of occurrence of spontaneous and provoked augmented breaths. Acta Physiol Scand 111: 349–360, 1981. [DOI] [PubMed] [Google Scholar]
- 8.Ciriello J, Hochstenbach SL, Roder S. Central projections of baroreceptor and chemoreceptor afferent fibers in the rat. In: Nucleus of the Solitary Tract, edited by Barraco IRA. Boca Raton, FL: CRC, 1994, p. 35–50.
- 9.Collier CR, Mead J. Pulmonary exchange as related to altered pulmonary mechanics in anesthetized dogs. J Appl Physiol 19: 659–664, 1964. [DOI] [PubMed] [Google Scholar]
- 10.Coquerel A, Buser M, Tayot J, Pfaff F, Matray F, Proust B. Beta-endorphin and neurotensin in brainstem and cerebrospinal fluid in the sudden infant death syndrome. Neurochem Int 20: 97–102, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Deolindo M, Pelosi GG, Tavares RF, Aguiar Correa FM. The ventrolateral periaqueductal gray is involved in the cardiovascular response evoked by l-glutamate microinjection into the lateral hypothalamus of anesthetized rats. Neurosci Lett 430: 124–129, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol 367: 375–402, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Donoghue S, Felder RB, Jordan D, Spyer KM. The central projections of carotid baroreceptors and chemoreceptors in the cat: a neurophysiological study. J Physiol 347: 397–409, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egbert LD, Bendixen HH. Effect of morphine on breathing pattern. A possible factor in atelectasis. JAMA 188: 485–488, 1964. [DOI] [PubMed] [Google Scholar]
- 15.Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res 513: 35–42, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Ellenberger HH, Feldman JL. Origins of excitatory drive within the respiratory network: anatomical localization. Neuroreport 5: 1933–1936, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res 572: 108–116, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Fleming PJ, Goncalves AL, Levine MR, Woollard S. The development of stability of respiration in human infants: changes in ventilatory responses to spontaneous sighs. J Physiol 347: 1–16, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda T, Hisano S, Toyooka H. Moderate hypercapnia-induced anesthetic effects and endogenous opioids. Neurosci Lett 403: 20–23, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Glogowska M, Richardson PS, Widdicombe JG, Winning AJ. The role of the vagus nerves, peripheral chemoreceptors and other afferent pathways in the genesis of augmented breaths in cats and rabbits. Respir Physiol 16: 179–196, 1972. [DOI] [PubMed] [Google Scholar]
- 21.Golder FJ, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Augmented breath phase volume and timing relationships in the anesthetized rat. Neurosci Lett 373: 89–93, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haldane JS, Meakins JC, Priestley JG. The effects of shallow breathing. J Physiol 52: 433–453, 1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoch B, Bernhard M, Hinsch A. Different patterns of sighs in neonates and young infants. Biol Neonate 74: 16–21, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Hoppenbrouwers T, Hodgman JE, Arakawa K, McGinty DJ, Mason J, Harper RM, Sterman MB. Sleep apnea as part of a sequence of events: a comparison of three months old infants at low and increased risk for sudden infant death syndrome (SIDS). Neuropadiatrie 9: 320–337, 1978. [DOI] [PubMed] [Google Scholar]
- 25.Kahn A, Blum D, Rebuffat E, Sottiaux M, Levitt J, Bochner A, Alexander M, Grosswasser J, Muller MF. Polysomnographic studies of infants who subsequently died of sudden infant death syndrome. Pediatrics 82: 721–727, 1988. [PubMed] [Google Scholar]
- 26.Kinney HC, Filiano JJ, White WF. Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. J Neuropathol Exp Neurol 60: 228–247, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Li JL, Kaneko T, Mizuno N. Effects of peripheral nerve ligation on expression of mu-opioid receptor in sensory ganglion neurons: an immunohistochemical study in dorsal root and nodose ganglion neurons of the rat. Neurosci Lett 214: 91–94, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci 3: 600–607, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Lipski J, Bellingham MC, West MJ, Pilowsky P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods 26: 169–179, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Marshall JM, Metcalfe JD. Cardiovascular changes associated with augmented breaths in normoxia and hypoxia in the rat. J Physiol 400: 15–27, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto S, Takeda M, Saiki C, Takahashi T, Ojima K. Effects of vagal and carotid chemoreceptor afferents on the frequency and pattern of spontaneous augmented breaths in rabbits. Lung 175: 175–186, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Mazzone SB, Canning BJ. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol 283: R86–R98, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 569: 559–573, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGinty DJ, London MS, Baker TL, Stevenson M, Hoppenbrouwers T, Harper RM, Sterman MB, Hodgman J. Sleep apnea in normal kittens. Sleep 1: 393–412, 1979. [PubMed] [Google Scholar]
- 35.McQueen DS, Ribeiro JA. Inhibitory actions of methionine-enkephalin and morphine on the cat carotid chemoreceptors. Br J Pharmacol 71: 297–305, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mead J, Collier C. Relation of volume history of lungs to respiratory mechanics in anesthetized dogs. J Appl Physiol 669–678, 1959.
- 37.Mitra J, Dev NB, Trivedi R, Amini S, Ernsberger P, Cherniack NS. Intramedullary sodium cyanide injection on respiratory and vasomotor responses in cats. Respir Physiol 93: 71–82, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Nucci TB, Branco LG, Gargaglioni LH. 5-HT1A, but not 5-HT2 and 5-HT7, receptors in the nucleus raphe magnus modulate hypoxia-induced hyperpnoea. Acta Physiol (Oxf) 193: 403–414, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Orem J, Trotter RH. Medullary respiratory neuronal activity during augmented breaths in intact unanesthetized cats. J Appl Physiol 74: 761–769, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Ozaki N, Sengupta JN, Gebhart GF. Differential effects of mu-, delta-, and kappa-opioid receptor agonists on mechanosensitive gastric vagal afferent fibers in the rat. J Neurophysiol 83: 2209–2216, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol 59: 377–384, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Pearce W Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol 100: 731–738, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Padilla R, West P, Kryger MH. Sighs during sleep in adult humans. Sleep 6: 234–243, 1983. [DOI] [PubMed] [Google Scholar]
- 44.Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci 21: 5281–5288, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reininger EJ, Segall P. Effect of inhalation of O2 and CO2 gas mixtures on spontaneous gasps and apnea in unanesthetized dogs. Physiologist 13: 290, 1970. [Google Scholar]
- 46.Reynolds LB Jr. Characteristics of an inspiration-augmenting reflex in anesthetized cats. J Appl Physiol 17: 683–688, 1962. [DOI] [PubMed] [Google Scholar]
- 47.Rigg JR Pulmonary atelectasis after anaesthesia: pathophysiology and management. Can Anaesth Soc J 28: 305–313, 1981. [DOI] [PubMed] [Google Scholar]
- 48.Romberg R, Olofsen E, Sarton E, Teppema L, Dahan A. Pharmacodynamic effect of morphine-6-glucuronide versus morphine on hypoxic and hypercapnic breathing in healthy volunteers. Anesthesiology 99: 788–798, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Solomon IC, Edelman NH, Neubauer JA. Pre-Bötzinger complex functions as a central hypoxia chemosensor for respiration in vivo. J Neurophysiol 83: 2854–2868, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Storm H, Rognum TO, Reichelt KL. Inverse relationship between beta-endorphin immunoreactivity in cerebrospinal fluid and nucleus tractus solitarius in sudden infant death. Eur J Pediatr 153: 381–386, 1994. [DOI] [PubMed] [Google Scholar]
- 51.Storm H, Rognum TO, Saugstad OD, Reichelt KL. Elevated beta-endorphin immunoreactivity in the cerebrospinal fluid in victims of sudden infant death correlates with hypoxanthine in vitreous humour. Eur J Pediatr 152: 935–938, 1993. [DOI] [PubMed] [Google Scholar]
- 52.Szeto HH, Lovelace JL, Fridland G, Soong Y, Fasolo J, Wu D, Desiderio DM, Schiller PW. In vivo pharmacokinetics of selective mu-opioid peptide agonists. J Pharmacol Exp Ther 298: 57–61, 2001. [PubMed] [Google Scholar]
- 53.Tatsumi K, Hannhart B, Pickett CK, Weil JV, Moore LG. Influences of gender and sex hormones on hypoxic ventilatory response in cats. J Appl Physiol 71: 1746–1751, 1991. [DOI] [PubMed] [Google Scholar]
- 54.Thor KB, Helke CJ. Serotonin- and substance P-containing projections to the nucleus tractus solitarii of the rat. J Comp Neurol 265: 275–293, 1987. [DOI] [PubMed] [Google Scholar]
- 55.Tschirgi RD, Gerard RW. The carotid-mandibular reflex in acute respiratory failure. Am J Physiol 150: 358–364, 1947. [DOI] [PubMed] [Google Scholar]
- 56.Voss MD, De Castro D, Lipski J, Pilowsky PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol 295: 208–218, 1990. [DOI] [PubMed] [Google Scholar]
- 57.Waggener TB, Frantz ID 3rd, Stark AR, Kronauer RE. Oscillatory breathing patterns leading to apneic spells in infants. J Appl Physiol 52: 1288–1295, 1982. [DOI] [PubMed] [Google Scholar]
- 58.Weil JV, McCullough RE, Kline JS, Sodal IE. Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal man. N Engl J Med 292: 1103–1106, 1975. [DOI] [PubMed] [Google Scholar]
- 59.Wulbrand H, McNamara F, Thach BT. Suppression of sigma spindle electroencephalographic activity as a measure of transient arousal after spontaneous and occlusion-evoked sighs and startles. Pediatr Res 44: 767–773, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid mu-receptors in the medullary raphe region (MRR) attenuates the ventilatory response to hypoxia in anesthetized rats (Abstract). FASEB J 22: 1172.3, 2008. [Google Scholar]
- 61.Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid mu receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology 107: 288–297, 2007. [DOI] [PubMed] [Google Scholar]