Abstract
GABAA receptor agonists act in the suprachiasmatic nucleus (SCN) to reset circadian rhythms during the day but inhibit the ability of light to reset rhythms during the night. In the present study, we examined whether these paradoxical differences in the effect of GABAA receptor stimulation on the circadian system are mediated by separate GABAA receptor subtypes. 4,5,6,7-Tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP), a GABAA receptor agonist, preferentially activates GABAA receptors in extrasynaptic locations. THIP, muscimol (a GABAA agonist), or vehicle were microinjected into the SCN region of Syrian hamsters free-running in constant darkness during the mid-subjective day, early subjective night, or late subjective night. The subjective night injections were followed by a light pulse or sham control. Behavioral phase shifts of wheel running rhythms and both Period1 (Per1) and Per2 mRNA levels in the SCN were assessed. Animals that received THIP during the subjective day did not exhibit significant phase alterations. During the early and late subjective night, however, THIP abolished the phase-shifting effects of light and the ability of light to increase Per1 and Per2 mRNA levels. The ability of N-methyl-d-aspartic acid to phase-shift wheel running rhythms was also attenuated by THIP. Together these data demonstrate that THIP does not produce phase shifts during the subjective day, but does inhibit the ability of light to produce phase shifts. Thus, extrasynaptic GABAA receptors appear to play a role in regulating light input to the SCN, while a different population of GABAA receptors appears to be responsible for daytime effects of GABA.
Keywords: suprachiasmatic nucleus, GABA, THIP, phase shift, circadian rhythm
in mammals, the synchronization of physiology and behavior with the earth's 24-h period (i.e., entrainment) is maintained by a master circadian clock, the suprachiasmatic nucleus (SCN). Cells within the SCN generate endogenous rhythms through an inhibitory feedback loop composed of the transcription and translation of core circadian clock genes (12, 14, 27). The SCN is reset daily to match the earth's period by light and other environmental cues (zeitgebers) acting to alter the expression of these clock genes and their products (2, 12, 27, 43). Environmental time cues are only capable of resetting the circadian system at discrete phases of the circadian cycle. Photic cues reset the timing of the clock during the night phase of the endogenous rhythm, while a number of nonphotic stimuli reset the clock during the day (19, 34).
The classical inhibitory neurotransmitter γ-aminobutyric acid (GABA) (8, 11, 29, 32), its receptors (16, 35, 41), synthesis enzymes (21, 22, 53), and transporters (4) are in nearly every cell in the SCN. There is substantial evidence both in vivo and in vitro that GABA plays a major role in the regulation of SCN neurons. Electrophysiological evidence indicates that tonic input, mediated by the GABAA receptor, is received by nearly every SCN cell (10, 26) and that the source of this GABA seems to be both within and outside the SCN (24, 50). These local GABA connections have been implicated in synchronizing electrical activity in SCN cells and regional cell groups (3, 28, 45, 50, 51). Other investigations have implicated GABA in the entrainment pathway of the SCN. Activation of GABAA receptors in the SCN phase shifts the circadian clock during the subjective day (20, 46), while activation of either GABAA or GABAB receptors inhibits light input during the night (17, 18, 36, 38).
GABAA receptors in the brain are heterogeneous and exist as pentomeric constructs of at least 18 possible subunits. Functional receptors are formed from specific arrangements of subunits with the particular combination of subunits determining the pharmacological profile and/or cellular location of the receptor (9, 15, 54). We therefore hypothesized that different subpopulations of GABAA receptors in the SCN are responsible for the multiple actions of GABA. To date, there have been no investigations to examine whether multiple GABAA receptor subunit constructs are responsible for the multiple actions of GABA on circadian processes. In this study we focus on a subtype of GABAA receptors possessing the δ-subunit. This subunit is responsible for directing the receptors to extrasynaptic locations where they are believed to respond to tonic levels of GABA in the extracellular space (40). 4,5,6,7-Tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) is a GABAA receptor agonist that preferentially activates these extrasynaptic receptors which are found primarily in areas of the hippocampus, cortex, and thalamus, but its potential ability to modulate circadian processes remains ambiguous (5–7, 41, 42, 48). The present study took advantage of site-directed injections of THIP in the SCN to investigate the role of these extrasynaptic receptors in GABA's action on the circadian system.
MATERIALS AND METHODS
Animals and surgery.
Adult male Syrian hamsters (Mesocricetus auratus; Charles River Laboratories, Wilmington, MA; 130–150 g) were group housed (6 per cage) in polycarbonate cages (20 × 40 × 20 cm) in a 14:10-h light-dark cycle until the time of surgery (at least 7 days after arrival). Food and water were available ad libitum. All procedures and protocols were approved by Morehouse School of Medicine Institutional Animal Care and Use Committee.
Animals were deeply anesthetized with a cocktail of ketamine (120 mg/kg) and xylazine (25 mg/kg) before a 26-gauge guide cannula (11 mm total length) aimed at the SCN was stereotaxically implanted. The skull was leveled before implantation using bregma and lambda as reference points. Coordinates were 0.9 mm anterior and 1.7 mm lateral to bregma. With a 32-gauge injection needle inserted into the cannula, the final depth of injection was 7.2 mm below dura. A 10-degree angle toward the midline was used for implantations. The cannula was affixed to the skull using stainless steel screws and cranioplastic cement.
Experimental design.
Animals were maintained in a 14:10-h light-dark cycle for 7 days following surgery. They were then transferred to individual cages (20 × 40 × 20 cm) equipped with running wheels (16 cm diameter). Each wheel revolution activated a microswitch on the outside of the cage that was monitored continuously by a computer using VitalView software and hardware (Minimitter, Bend, OR). Animals were then transferred to constant darkness and allowed to establish stable free-running rhythms. Hamsters were in continuous darkness for at least 2 wk before receiving a 200-nl microinjection of THIP (22 nmol), muscimol (4.4 nmol), or vehicle. Both THIP and muscimol are GABAA receptor agonists. The rank order potency (EC50) for these agonists has been estimated from two types of δ-subunit-containing receptor and is muscimol > GABA > THIP, with the magnitude of the differences dependent on the receptor construct (1, 6, 49). THIP acts as a superagonist at receptors with the δ-subunit and elicits a 50% greater peak response than either GABA or muscimol (1, 6, 49). Drugs were administered at circadian time (CT) 6, 13.5 or 20 (CT 12 is defined by convention as activity onset). A phase response curve was constructed for THIP by treatments at additional time points (CT 0, 2, 10, and 16). Microinjections of drugs dissolved in 0.9% saline were given using a 16-mm, 32-gauge needle attached by polyethylene tubing to a 1-μl Hamilton syringe. Injections were given to hamsters gently restrained by hand in dim red illumination (<5 lux). The needle was left in place for at least 20 s after injection. Microinjections during the night were followed by a light pulse (15 min, 730 μW/cm2; fluorescent tube), sham light pulse, or microinjection of N-methyl-d-aspartic acid (NMDA; CT 13.5 only; 5 mM/200 nl). Light was administered by transferring the animal's cage to a light-tight ventilated box in the same room. Sham light pulses were delivered by moving the animal's cage to a shelf below the light-treatment box for 15 min. Each animal received two treatments separated by 10 days. Separate cohorts of animals were used in a counterbalanced within-subjects design at each time point and for NMDA. Animals in the phase response curve cohort received two treatments of THIP at randomly assigned phases. At the end of each study, brains were sectioned (50 μm), mounted on slides, stained with cresyl violet, and examined using a light microscope to verify the site of injection.
Cohorts of animals treated with THIP (22 nmol) at CT 13.5 and 20 were assigned for in situ hybridization processing. These animals were killed by rapid decapitation 3 h following injections at CT 6 or 1.5 h following injections at CT 13.5 and 20. Brains were quickly removed under dim red light and frozen on dry ice. Coronal cryostat sections (20 μm) collected in four sets on Superfrost Plus slides (Fisher) were used for in situ hybridization. Whole and sectioned brains were stored at −80°C.
In situ hybridization.
These methods have been described previously (13, 39). A fragment of hamster Per1 cDNA (donated by Dr. Joseph Takahashi, Northwestern University, Evanston, IL) or mouse Per2 cDNA (donated by Dr. Steven M. Reppert, University of Massachusetts Medical School, Worcester, MA), was linearized and used as a template to synthesize [35S]UTP-labeled riboprobes. Sections mounted on slides were fixed in 4% paraformaldehyde, acetylated by 0.25% acetic anhydride in 0.1 M triethanolamine, and dehydrated in ethanol. Each slide was then hybridized in a solution consisting of 10 mM DTT, [35S]hPer1 or mPer2 riboprobes, and buffer (50% formamide, 100 mg/ml dextran sulfate, 2× SSC, 1× Denhardt's solution, 0.5 mg/ml tRNA, 0.5 mg/ml Heparin sodium salt, and 0.4 mg/ml ssDNA), and then incubated overnight at 55°C. For posthybridization, sections were washed (50% formamide in 2 × SSC at 52°C) and treated with RNase A (1 μg/ml) in a bath at 37°C. The slides were then air-dried and exposed to Kodak BioMax MR films with 14C microscales (Amersham Life Science, Little Chalfont, Buckinghamshire, UK) for 7–14 days at room temperature.
Phase shift.
Phase shifts were calculated using the linear regression method. Regression lines were calculated from activity onsets for both the 7 days preceding treatment and days 3–7 following treatment (day 0 = day of treatment). These regressions were used to predict the onset on day 0, and the difference between these two predicted onsets was the phase shift. To determine statistical significance (ascribed at P < 0.05), Student's t-test for paired samples or ANOVA was used. Post hoc pairwise comparisons were made using the Tukey's multiple comparisons test.
RESULTS
Early subjective night.
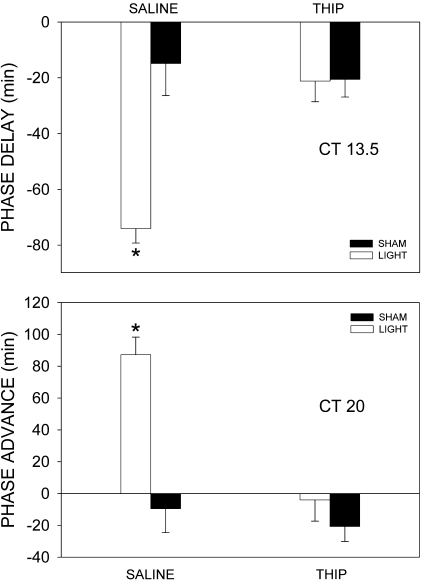
THIP prevented the phase-altering effects of light when microinjected into the SCN region during the subjective night. A light pulse delivered in early night (CT 13.5; i.e., 1.5 h after activity onset) to animals pretreated with saline, resulted in significant phase delays of 74 ± 10 min (mean ± SE; n = 7; Figs. 1 and 2) compared with sham-treated animals receiving saline (15 ± 8 min; n = 6). When hamsters were treated with THIP just prior to light, this effect was abolished and reduced to levels not significantly different from sham light-pulse treated controls [2-way ANOVA with repeated measures on the drug factor; interaction drug × light F(1,11) = 18.75; P = 0.001; drug main effect, F(1,11) = 12.96; P = 0.004; light main effect, F(1,11) = 6.6; P = 0.026; light group n = 6, sham group n = 7].
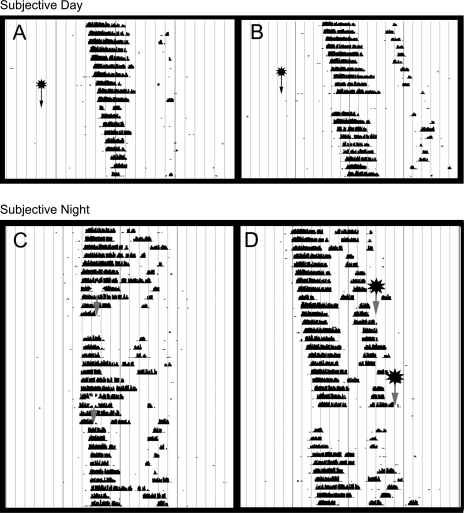
Fig. 1.
Representative actograms depicting the actions of 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) and muscimol. During the mid-subjective day circadian time 6 (CT 6) THIP (22 nmol) did not result in any significant phase alterations (A), while muscimol (4.4 nmol) resulted in large-phase advances of wheel-running activity (B; arrows denote time of treatment). THIP (22 nmol) did abolish the phase-delaying (C) and phase-advancing (D) effects of light (each animal received a microinjection of THIP or saline followed by a light pulse in counterbalanced order; stars with arrows denote time of treatment; THIP top arrows; saline, bottom arrows). Each horizontal line represents 1 day; black bars are the number of wheel revolutions recorded in 5-min bins.
Fig. 2.
THIP inhibits light-induced behavioral phase shifts in the early and late subjective night. THIP (22 nmol) delivered to the suprachiasmatic nucleus (SCN) region just prior to a light pulse during the early subjective night (CT 13.5) inhibited phase delays so that they were not different from saline-treated control animals. THIP administered just prior to a light pulse in the late subjective night (CT 20) significantly inhibited the resulting phase advances. In both the early and late night, the effect of light in saline-treated animals was significantly greater than all other groups. Bars represent means ± SE; *P < 0.05.
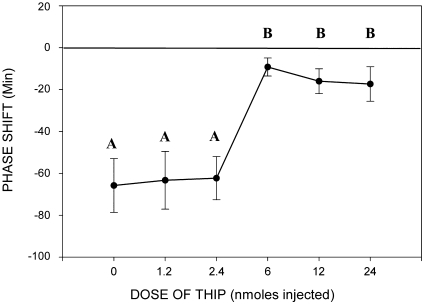
In a separate study, we found this inhibitory action of THIP to be dose dependent (Fig. 3). Cohorts of hamsters received five different concentrations of THIP (n = 9, saline; n = 7, 1.2 nmoles; n = 9, 2.4 nmoles; n = 9, 6.0 nmoles; n = 6, 12.0 nmoles; n = 9, 24.0 nmoles) prior to a light pulse at CT 13.5. A block of light-induced phase delays was produced by the three highest doses of THIP [6 nmoles; ANOVA; F(5,46) = 7.7; P < 0.001; Tukey's honestly significant difference post hoc comparison saline vs. 6 nmoles, P = 0.018]. Saline treatment and the two low doses of THIP had no effect on light-induced phase shifts.
Fig. 3.
Dose response for the inhibition of light phase shifts by THIP. THIP delivered to the SCN region in the early night (CT 13.5) inhibited light-induced phase delays in wheel-running activity. A complete block of light's effect was produced by the three highest doses of THIP investigated. The y-axis shows phase shifts in the circadian rhythm of wheel-running activity following a light pulse and THIP A,BDifferent letters indicate statistically significant differences, P < 0.05.
NMDA release in the SCN induced by light is part of the photic phase-shifting pathway (33). To elucidate where THIP acts in this pathway, we investigated the ability of THIP to block the effects of light downstream of this NMDA release. During the early night a microinjection of NMDA (5 mM/200 nl) and saline resulted in phase-delays of wheel-running activity of 87 ± 9 min. This was reduced by approximately one-third when THIP immediately preceded NMDA [61 ± 9 min, paired samples t-test; t(6) = 2.15; P = 0.04].
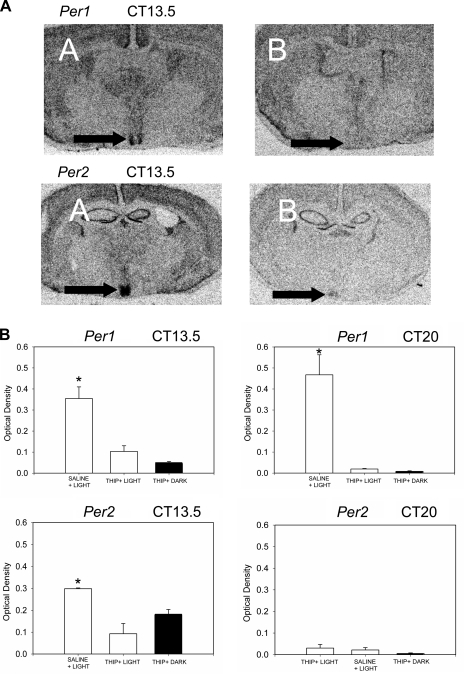
To determine whether THIP acts by attenuating light-induced Per mRNA expression, we examined mRNA levels of Per1 and Per2 in animals treated at CT 13.5. THIP treatment prior to a light pulse prevented Per1 and Per2 mRNA increases in the SCN [Per1 ANOVA treatment effect F(2.11) = 14.01, P = 0.002, light+THIP n = 3, sham+THIP n = 4, light+saline n = 5; Per2 ANOVA treatment effect F(2.9) = 21.02, P = 0.001, light+THIP n = 3, sham+THIP n = 3, light+saline n = 4; Fig. 4]. Per1 and Per2 mRNA levels in animals that received THIP and light were not significantly different from animals that received THIP with sham light pulses (P = 0.458, Per1; P = 0.10, Per2, Tukey's multiple comparison test).
Fig. 4.
THIP inhibits light-induced period mRNA expression during the subjective night. A: representative autoradiograms. Per1 and Per2 mRNA increases induced by light in the early night (CT 13.5; A) were abolished in animals pretreated with THIP (B). Arrows indicate the SCN. B: THIP (22 nmol) delivered to the SCN region just prior to a light pulse during the early night (CT 13.5) and late night (CT 20) inhibited light-induced increases in Per1 mRNA. THIP delivered to the SCN region just prior to a light pulse during the early subjective night (CT 13.5) also inhibited light-induced increases in Per2 mRNA. The effect of light in saline-treated animals for Per1 at both time points and Per2 in the early night was significantly greater than all other groups. There were no significant differences between any of the Per2 mRNA groups in the late night. Bars represent means ± SE; *P < 0.05.
Late subjective night.
Phase advances in THIP pretreated animals pulsed with light during the late night (CT 20) were also blocked compared with animals that received saline and light [two-way ANOVA with repeated measures on the drug factor; interaction drug × light F(1,6) = 7.425, P = 0.034; drug main effect, F(1,6) = 12.189, P = 0.013; light main effect, F(1,6) = 34.963, P = 0.001; light group n = 4, sham group n = 4; Figs. 1 and 2]. A sham light pulse combined with THIP had little phase-altering effect. Per1 but not Per2 mRNA levels were significantly increased by light treatment at CT 20 [Per1 ANOVA treatment effect, F(2,11) = 24.8, P < 0.001, light+THIP n = 3; sham+THIP n = 4; light+saline n = 5; Per2 ANOVA treatment effect, F(2,9) = 0.274, P = 0.768, light+THIP n = 5, sham+THIP n = 2, light+saline, n = 3]. This increase in Per1 was completely blocked by THIP (Fig. 4). THIP alone had no effect on either Per1 or Per2 mRNA expression.
Subjective day.
Microinjections of THIP (22 nmol) during the midday (CT 6) produced small phase shifts that were not significantly different from vehicle [Tukey's post hoc comparison THIP (22 nmol) vs. saline, P = 0.963]. Three doses were examined for THIP (22 nmol, 18 ± 11 min, n = 2; 720 pmol −10 ± 32 min, n = 2; 22 pmol, −17 ± 11 min, n = 2; 200 nl injection); however, none elicited significant alterations in phase. The dose of 22 nmol was near the limit of solubility for THIP, thus higher doses were not attempted. We also confirmed the well-characterized ability of the GABAA agonist muscimol to induce phase advances at this CT. Muscimol treatment at CT 6 resulted in phase advances of 57 ± 8 min (n = 4), which were significantly greater than those produced by THIP [1 ± 6 min, 22 nmol; ANOVA, F(2,16) = 5.202, P = 0.02; Tukey's test P = 0.02] and saline (5 ± 14 min; Tukey's test P = 0.03).
Phase shifts induced by THIP alone were investigated across the circadian day to generate a phase-response curve (n = 3–8 /time point). THIP had little effect on its own, and induced only small alterations in phase across the circadian day. We did not determine the significance of these shifts due to the lack of vehicle control injections for comparison. (CT 0, n = 3, 30 ± 15; CT 2, n = 3, 4 ± 8; CT 6, n = 7, 1 ± 6; CT 10, n = 8, −22 ± 9; CT 13, n = 7, −19 ± 8; CT 16, n = 6, −39 ± 7; CT 20, n = 4, −21 ± 10).
DISCUSSION
THIP abolished the ability of light to phase-shift behavioral rhythms and alter Per gene expression in the SCN during the early and late subjective night. Microinjection of THIP in the SCN region immediately prior to a light pulse prevented both behavioral phase delays during the early night, and behavioral phase advances during the late night. In the SCN, THIP blocked the light-induced increases in Per1 and Per2 mRNA in the early night and Per1 in the late night. During the midday, however, THIP microinjection had no phase-altering effect. These data suggest that THIP-sensitive GABAA receptors are responsible for the inhibitory effects of GABA on light, but not the direct actions of GABA agonists during the day.
We next investigated the role of THIP in the neuronal pathway responsible for light inhibition. The retinohypothalamic tract, a direct retinal projection, is responsible for conveying light information to the SCN. Glutamate is a primary neurotransmitter in the retinohypothalamic tract and NMDA-type glutamate receptor activation is part of the pathway by which light phase shifts the SCN (30, 31, 33). Initially, we hypothesized that THIP acted presynaptically on optic nerve terminals in the SCN to inhibit glutamate release induced by light. However, the ability of THIP to attenuate the phase-shifting effects of NMDA indicates that THIP also acted downstream of glutamate's action. In addition, THIP blocked light-induced Per1 and Per2 mRNA increases indicating that it acts upstream of changes in Period mRNA. Therefore, our evidence suggests that THIP attenuated phase shifts during the subjective night by acting directly on cells in the SCN to prevent Per1 and Per2 expression and phase shifts. This photic inhibitory effect of THIP, however, was incomplete. Thus, we cannot eliminate the possibility that THIP is also acting through presynaptic inhibition or other mechanisms reducing photic input to the SCN.
The actions of THIP differed from the actions of muscimol on the circadian system when administered during the subjective day. THIP microinjected during the day did not have any phase-altering effect. Muscimol induces large-phase advances at this time (23, 46), a finding that was replicated in the present study. We cannot rule out the possibility that our concentration of THIP was too low to cause phase advances during the day. However, if higher concentrations of drug were needed to elicit phase alterations, one possible explanation would be that several populations of receptors are present and are activated at different agonist concentrations.
During the night, both THIP and muscimol prevented the ability of light to phase-shift behavioral activity and block light-induced Per1 and Per2 mRNA increases in the SCN. These findings suggest that a subpopulation of GABAA receptors selectively sensitive to THIP inhibited the ability of light to reset circadian timing, while a separate population of GABAA receptors is responsible for the ability of GABA to cause phase shifts. Agonists and positive allosteric modulators of the GABAA receptor possess the well-characterized ability to induce phase advances during the day and inhibit the effects of light during the night (17, 18, 20, 36, 37, 39, 46, 47, 52). The present study demonstrates that THIP is unique among GABAA receptor agonists in its ability to inhibit the effects of light at night without direct phase-altering effects during the subjective day. These data support the hypothesis that there are two distinct subpopulations of GABAA receptors in the SCN, one selectively sensitive to THIP but both sensitive to muscimol, and that the THIP sensitive subpopulation is involved in the ability of GABA to block light-induced phase shifts.
Several labs have investigated the regional distribution of GABAA receptor subunits in the rodent brain. Of these, only one mouse study has looked for the δ-subunit in the SCN. The δ-subunit was found to be undetectable by Western blot (41). However, we have found no studies which looked for the δ-subunit in the hamster SCN. A comparison of studies that have looked for the presence of other GABA receptor subunits in the SCN indicates a large degree of both species and technique differences (16, 25, 35, 41). It will therefore be important to confirm the existence of the δ-subunit protein in the SCN. Despite this, the current data provide evidence for the δ-subunit's presence in the SCN. The potency of THIP is estimated to be 10-fold greater for the δ-subunit (1, 5, 49). Thus, the ability of the SCN to respond to THIP provides strong evidence that the δ-subunit is expressed in the SCN.
Our present understanding of the process by which the SCN integrates environmental stimuli is incomplete. These investigations suggest a novel mechanism by which the SCN may filter these entraining stimuli. GABAA receptor activation in the SCN is necessary and sufficient for entraining stimuli to reset circadian timing during the day (20, 46, 47). The data in the current study, however, provides evidence supporting the hypothesis that not all GABAA receptors are involved in these advances. These data suggest that receptors possessing the δ-subunit inhibit the effects of light during the dark phase of the circadian cycle, and other uncharacterized receptors are responsible for phase shifts generated during the subjective day. The finding that both photic and NMDA-induced phase shifts are inhibited by THIP indicates that the receptors activated by THIP are on cells downstream of the NMDA synapse. It is therefore possible that the δ-subunit containing receptors, preferential targets of THIP, are expressed on retinorecipient cells of the SCN. The δ-subunit is believed to direct GABAA receptors to an extrasynaptic location where these receptors respond to tonic levels of GABA (40, 44). This tonic GABA in the SCN may modulate the response of SCN cells to light.
Perspectives and Significance
Rhythmic sensitivity to light is a fundamental characteristic of the mammalian circadian system. These data provide evidence that GABA may drive this rhythmic filtering of light input. The data demonstrate that THIP prevents photic entrainment during the night, consistent with the effects of other GABAA agonists. However, THIP, unlike other GABAA agonists, does not produce phase shifts during the day. A specific subset of GABAA receptors possessing the δ-subunit are activated by THIP. These receptors are located in extrasynapic locations and respond to ambient GABA in the extracellular space. Therefore, GABA in SCN is likely acting specifically on an extrasynaptic (δ-subunit containing) subpopulation of receptors to block light's influence on the circadian system. These results suggest that rhythmicity in GABAergic neuronal signaling is a plausible method for the circadian system to manage light information. Furthermore, rhythmicity in the activation of extrasynaptic GABAA receptors seems a likely mechanism contributing to this rhythmic filtering of light input.
GRANTS
This study was supported, in part, by National Institutes of Health Grant NS-34194 and a research grant from the Investigator-Initiated Studies Program of Merck.
DISCLOSURES
Opinions in this manuscript are those of the authors and do not necessarily represent those of Merck.
Acknowledgments
The authors thank Laura Mathews, Lennisha Pinkney, and Shanon Belle for their expert technical assistance.
REFERENCES
- 1.Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. α4β3Δ GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem 276: 38934–38939, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht U Invited review: regulation of mammalian circadian clock genes. J Appl Physiol 92: 1348–1355, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol 15: 886–893, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of γ-aminobutyric acid and γ-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. J Comp Neurol 506: 708–732, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Boehm SL, Homanics GE, Blednov YA, Harris RA. δ-Subunit containing GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridin-3-ol. Eur J Pharmacol 541: 158–162, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3Δ GABAA receptors. Br J Pharmacol 136: 965–974, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caruncho HJ, Puia G, Mohler H, Costa E. The density and distribution of six GABAA receptor subunits in primary cultures of rat cerebellar granule cells. Neuroscience 67: 583–593, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Castel M, Morris JF. Morphological heterogeneity of the GABAergic network in the suprachiasmatic nucleus, the brain's circadian pacemaker. J Anat 196: 1–13, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chebib M, Johnston GA. The “ABC” of GABA receptors: a brief review. Clin Exp Pharmacol Physiol 26: 937–940, 1999. [DOI] [PubMed] [Google Scholar]
- 10.De Jeu M, Pennartz C. Circadian modulation of GABA function in the rat suprachiasmatic nucleus: excitatory effects during the night phase. J Neurophysiol 87: 834–844, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Decavel C, Van den pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol 302: 1019–1037, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Dunlap JC Molecular bases for circadian clocks. Cell 96: 271–290, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Ehlen JC, Novak CM, Karom MC, Gamble KL, Paul KN, Albers HE. GABAA receptor activation suppresses Period 1 mRNA and Period 2 mRNA in the suprachiasmatic nucleus during the mid-subjective day. Eur J Neurosci 23: 3328–3336, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Field MD, Maywood ES, O'Brien JA, Weaver DR, Reppert SM, Hastings MH. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron 25: 437–447, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Frolund B, Ebert B, Kristiansen U, Liljefors T, Krogsgaard-Larsen P. GABA(A) receptor ligands and their therapeutic potentials. Curr Top Med Chem 2: 817–832, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Gao B, Fritschy JM, Moore RY. GABAA-receptor subunit composition in the circadian timing system. Brain Res 700: 142–156, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie CF, Huhman KL, Babagbemi TO, Albers HE. Bicuculline increases and muscimol reduces the phase-delaying effects of light and VIP/PHI/GRP in the suprachiasmatic region. J Biol Rhythms 11: 137–144, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Gillespie CF, Mintz EM, Marvel CL, Huhman KL, Albers HE. GABAA and GABAB agonists and antagonists alter the phase-shifting effects of light when microinjected into the suprachiasmatic region. Brain Res 759: 181–189, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Hastings MH, Duffield GE, Ebling FJ, Kidd A, Maywood ES, Schurov I. Non-photic signalling in the suprachiasmatic nucleus. Biol Cell 89: 495–503, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Huhman KL, Babagbemi TO, Albers HE. Bicuculline blocks neuropeptide Y-induced phase advances when microinjected in the suprachiasmatic nucleus of Syrian hamsters. Brain Res 675, 333–336. 1995. [DOI] [PubMed] [Google Scholar]
- 21.Huhman KL, Hennessey AC, Albers HE. Rhythms of glutamic acid decarboxylase mRNA in the suprachiasmatic nucleus. J Biol Rhythms 11: 311–316, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Huhman KL, Jasnow AM, Sisitsky AK, Albers HE. Glutamic acid decarboxylase mRNA in the suprachiasmatic nucleus of rats housed in constant darkness. Brain Res 851: 266–269, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Huhman KL, Marvel CL, Gillespie CF, Mintz EM, Albers HE. Tetrodotoxin blocks NPY-induced but not muscimol-induced phase advances of wheel-running activity in Syrian hamsters. Brain Res 772: 176–180, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Jiang ZG, Yang Y, Liu ZP, Allen CN. Membrane properties and synaptic inputs of suprachiasmatic nucleus neurons in rat brain slices. J Physiol 499: 141–159, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jobst EE, Robinson DW, Allen CN. Potential pathways for intercellular communication within the calbindin subnucleus of the hamster suprachiasmatic nucleus. Neuroscience 123: 87–99, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kim YI, Dudek FE. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: inhibitory synaptic mechanisms. J Physiol 458: 247–260, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King DP, Takahashi JS. Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci 23: 713–742, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron 25: 123–128, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Makara GB, Rappay G, Stark E. Autoradiographic localization of 3H-γ-aminobutyric acid in the medial hypothalamus. Exp Brain Res 22: 449–455, 1975. [DOI] [PubMed] [Google Scholar]
- 30.Mintz EM, Albers HE. Microinjection of NMDA into the SCN region mimics the phase shifting effect of light in hamsters. Brain Res 758: 245–249, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci 19: 5124–5130, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett 150: 112–116, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Rev 51: 1–60, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Mrosovsky N, Reebs SG, Honrado GI, Salmon PA. Behavioral entrainment of circadian rhythms. Experientia 45, 696–702. 1989. [DOI] [PubMed] [Google Scholar]
- 35.Naum OG, Fernanda RM, Golombek DA. Rhythmic variation in γ-aminobutyric acidA-receptor subunit composition in the circadian system and median eminence of Syrian hamsters. Neurosci Lett 310: 178–182, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Novak CM, Albers HE. Circadian phase alteration by GABA and light differs in diurnal and nocturnal rodents during the day. Behav Neurosci 118: 498–504, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Novak CM, Albers HE. Novel phase-shifting effects of GABAA receptor activation in the suprachiasmatic nucleus of a diurnal rodent. Am J Physiol Regul Integr Comp Physiol 286: R820–R825, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Novak CM, Ehlen JC, Huhman KL, Albers HE. GABAB receptor activation in the suprachiasmatic nucleus of diurnal and nocturnal rodents. Brain Res Bull 63: 531–535, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Novak CM, Ehlen JC, Paul KN, Fukuhara C, Albers EL. Light and GABAA receptor activation alter Period mRNA levels in the SCN of diurnal Nile grass rats. Eur J Neurosci 284: 2843–2852, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18: 1693–1703, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Hara BF, Andretic R, Heller HC, Carter DB, Kilduff TS. GABAA, GABAC, and NMDA receptor subunit expression in the suprachiasmatic nucleus and other brain regions. Brain Res Mol Brain Res 28: 239–250, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63: 647–676, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Saxena NC, MacDonald RL. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci 14: 7077–7086, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirakawa T, Honma S, Katsuno Y, Oguchi H, Honma KI. Synchronization of circadian firing rhythms in cultured rat suprachiasmatic neurons. Eur J Neurosci 12: 2833–2838, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Smith RD, Inouye S, Turek FW. Central administration of muscimol phase-shifts the mammalian circadian clock. J Comp Physiol [A] 164: 805–814, 1989. [DOI] [PubMed] [Google Scholar]
- 47.Smith RD, Turek FW. β-Methyl carboline, a benzodiazepine inverse agonist, attenuates the effect of triazolam on the circadian rhythm of locomotor activity. Experientia 45: 334–337, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience 80: 987–1000, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Storustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther 316: 1351–1359, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Strecker GJ, Wuarin JP, Dudek FE. GABAA-mediated local synaptic pathways connect neurons in the rat suprachiasmatic nucleus. J Neurophysiol 78: 2217–2220, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Tominaga K, Shibata S, Hamada T, Watanabe S. GABAA receptor agonist muscimol can reset the phase of neural activity rhythm in the rat suprachiasmatic nucleus in vitro. Neurosci Lett 166: 81–84, 1994. [DOI] [PubMed] [Google Scholar]
- 52.Turek FW, Losee-Olson S. A benzodiazepine used in the treatment of insomnia phase-shifts the mammalian circadian clock. Nature 321: 167–168, 1986. [DOI] [PubMed] [Google Scholar]
- 53.Van den pol AN γ-Aminobutyrate, gastrin releasing peptide, serotonin, somatostatin, and vasopressin: ultrastructural immunocytochemical localization in presynaptic axons in the suprachiasmatic nucleus. Neuroscience 17: 643–659, 1986. [DOI] [PubMed] [Google Scholar]
- 54.Whiting PJ, Bonnert TP, McKernan RM, Farrar S, le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann NY Acad Sci 868: 645–653, 1999. [DOI] [PubMed] [Google Scholar]