Abstract
Partial exchange transfusion with a cell-free hemoglobin (Hb) polymer during transient middle cerebral artery occlusion (MCAO) reduces infarct volume but fails to increase blood flow, as might be expected with the induced decrease in hematocrit. In ischemic brain, endothelin antagonists are known to produce vasodilation. In nonischemic brain, pial arterioles constrict after Hb exchange transfusion, and the constriction is blocked by an inhibitor of 20-HETE synthesis. We tested the hypothesis that a 20-HETE synthesis inhibitor and an endothelin A receptor antagonist increase pial arteriolar dilation after Hb exchange transfusion during MCAO. Pial arteriolar diameter was measured in the ischemic border region of the distal MCA border region through closed cranial windows in anesthetized rats subjected to the filament model of MCAO. During 2 h of MCAO, pial arteriolar dilation gradually subsided from 37 ± 3 to 7 ± 5% (±SE). Compared with residual dilation at 2 h of MCAO with vehicle superfusion (14 ± 3%), loss of dilation was not prevented by superfusion of a 20-HETE synthesis inhibitor (21 ± 5%), partial Hb exchange transfusion (7 ± 5%) that decreased hematocrit to 23%, or a combination of the two (5 ± 5%). However, loss of dilation was prevented by superfusion of an endothelin A receptor antagonist with (35 ± 4%) or without (32 ± 5%) Hb transfusion. Pial artery constriction during reperfusion was attenuated by HET0016 alone and by BQ610 with or without Hb transfusion. Systemic administration of the endothelin antagonist during prolonged MCAO increased blood flow in the border region. Thus loss of pial arteriolar dilation in the ischemic border region during prolonged MCAO depends on endothelin A receptor activation, and this effect was independent of the presence of cell-free Hb polymers in the plasma. In contrast to previous work in nonischemic brain, inhibition of oxygen-dependent 20-HETE synthesis does not significantly influence the pial arteriolar response to polymeric Hb exchange transfusion during focal ischemia.
Keywords: blood substitutes, BQ610, hemoglobin-based oxygen carrier, HET0016, rat, stroke
partial exchange transfusion with cell-free hemoglobin (Hb) provides the opportunity to decrease hematocrit and blood viscosity without a proportional decrease O2 carrying capacity. Such a strategy with αα-cross-linked tetrameric Hb was shown to increase cerebral blood flow (CBF) during middle cerebral artery (MCA) occlusion (MCAO) in the rat when the exchange transfusion was performed before ischemia (3). However, exchange transfusion with another cross-linked Hb after the onset of MCAO in the cat failed to produce an immediate increase in CBF (24). One limitation of the use of cross-linked tetramers is that they can extravasate in peripheral vascular beds (13), where they can then readily scavenge nitric oxide (NO), generate the release of endothelin, and produce hypertension and peripheral vasoconstriction that can be attenuated with an endothelin receptor A (ETA) antagonist (7–9, 28, 33). The increase in plasma endothelin concentration seen in patients transfused with αα-cross-linked tetrameric Hb after the onset of stroke (30) may have contributed to the lack of efficacy of this compound in clinical stroke (29).
To minimize peripheral extravasation, a compound containing a large polymer composed of many Hb tetramers was designed in which the polymerization agent was not retained. Unlike cross-linked tetramers (28, 33), this polymer, designated zero-link bovine Hb (ZL-HbBv), did not appear in renal lymph and did not produce peripheral vasoconstriction or arterial hypertension (13, 14). Exchange transfusion with ZL-HbBv after the onset of MCAO in mice reduced infarct volume (14). However, the distribution of intraischemic CBF was not improved despite the decrease in hematocrit. Collectively, these observations raise the possibility of arteriolar constriction that counteracts the decrease in blood viscosity and prevents a decrease cerebrovascular resistance.
In the absence of ischemia, partial exchange transfusion with ZL-HbBv resulted in constriction of pial arterioles, and this constriction appeared to offset the decrease in blood viscosity associated with a decrease in hematocrit because cerebrovascular resistance and CBF were unchanged (25). The constriction was reversed to dilation when plasma viscosity was increased, thereby suggesting that the constriction is a homeostatic response that prevents overoxygenation of the brain when blood viscosity is low. Interestingly, the constrictor response to ZL-HbBv exchange transfusion was not blocked by an inhibitor of NO synthase (22), and NO-dependent vasodilator responses to acetylcholine and ADP were preserved (21, 23). Thus, cell-free Hb in the plasma may not scavenge a physiologically greater amount of endothelially generated NO than does Hb in the red blood cell. However, the constrictor response to ZL-HbBv exchange transfusion was blocked by 20-hydroxyeicosatetraenoic acid (20-HETE) synthesis inhibitors, including N-hydroxy-N′-(4-n-butyl-2-methylphenyl)formamidine (HET0016) (22). Whereas 20-HETE promotes vasoconstriction during increases in arterial pressure (5), the role of 20-HETE synthesis in limiting vasodilation during ischemia without Hb transfusion is unclear. Administration of HET0016 or other 20-HETE inhibitors did not increase laser-Doppler flux (LDF) recorded over the densely ischemic lateral cortex during MCAO, although LDF was improved by 3 h of reperfusion (4, 20, 26). However, if transfusion of cell-free Hb improves intraischemic oxygenation in arterioles, then O2-dependent synthesis of 20-HETE from arachidonic acid (6) may limit the degree of vasodilation during ischemia as it does without ischemia (22).
Several pieces of evidence suggest that vasodilation during MCAO is not maximal and that additional dilation is possible. For example, infusion of l-arginine can produce vasodilation (16), and topical administration of ETA antagonists during MCAO can transiently dilate cat pial arterioles (19). However, little information exists on the natural time course of pial arteriolar dilation during prolonged MCAO in the rat. In the present study, we focused on pial arterioles in the distal MCA region of parietal cortex near the anterior cerebral artery (ACA) watershed region. In this region, the direction of flow is typically reversed during MCAO because the region becomes supplied through anastomoses with the ACA. We determined the time course of changes in pial arteriolar diameter during 2 h of MCAO in the rat and whether exchange transfusion with ZL-HbBv during MCAO affected the time course. On the basis of the evidence that 20-HETE synthesis is involved in pial artery constriction after ZL-HbBv exchange transfusion and that ETA antagonists can dilate pial arteries during focal ischemia, we postulated that 20-HETE and ETA receptors may influence pial arterial dilation after ZL-HbBv transfusion during MCAO. We tested the hypothesis that topical superfusion of the 20-HETE synthesis inhibitor HET0016 or the peptidergic ETA receptor antagonist BQ610 [homopiperidinyl-carbonyl-Leu-d-Trp (CHO)-d-Trp-ONH4] increases pial artery diameter during prolonged MCAO when the continuous superfusion was started before the ZL-HbBv transfusion. BQ610 has been used by others to reduce postischemic leukocyte adhesion (12).
METHODS
Surgical preparation.
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee. In male Wistar rats (∼300 g; Harlan Laboratories, Indianapolis, IN), anesthesia was induced with 5% isoflurane in O2-enriched air and maintained with 1.5–2% isoflurane during surgery and the experimental protocol. The right femoral artery was cannulated to monitor mean arterial blood pressure (MABP) and arterial blood gases; the left femoral artery was cannulated to withdraw blood during the exchange transfusion; and the femoral vein was cannulated for transfusion. The rat was placed on a Plexiglas cradle that permitted the body to be rotated onto its side while remaining in a head holder. The lungs were mechanically ventilated through a tracheostomy, and end-tidal CO2 was monitored. MCAO was induced by the intraluminal filament technique (36). Using a lateral approach in the neck, we ligated the right common carotid, external carotid, and pterygopalantine arteries. A 4-0 monofilament with a blunted tip was inserted into the stump of the external carotid artery for later advancement through the internal carotid artery.
With the rat in the prone position in a head holder, the skull was exposed to construct a cranial window. The periosteum was removed, and a 3- to 4-mm-wide craniotomy was made over the right parietal cortex. A plastic ring was secured with dental acrylic to the skull surrounding the craniotomy. The exposed dura mater was kept moist with artificial cerebrospinal fluid (aCSF) that was bubbled with 6% O2 and 6% CO2 and contained (in mM) 151 Na+, 3 K+, 1.3 Ca2+, 0.6 Mg2+, 134 Cl−, 24.6 HCO3−, 6 urea, and 3.7 glucose. The dura was incised, gently retracted, and cut to expose the pial surface. The window was filled with warmed aCSF, and a glass coverslip was cemented to the plastic ring. The plastic ring had an inflow and outflow port, a port for monitoring pressure, and a thermistor for monitoring fluid temperature. Rectal and window temperatures were maintained with a heating lamp throughout the surgical procedure and experiment. Pial arteriolar diameter was measured through the closed cranial window by intravital microscopy (Zeiss Axiohead System II microscope, Oberkochen, Germany) and a digital camera (Fast1394, QImaging, Surrey, BC, Canada) connected to a computer for image analysis (Metamorph software, Universal Imaging, Downingtown, PA). A LDF probe was placed on the right temporal bone that had been previously thinned by drilling a small hole lateral to the cranial window.
Experimental protocol.
Forty-five minutes after the completion of surgery, the window was flushed over a 5-min period with aCSF, and baseline measurements of MABP, arteriolar diameter, arterial blood gases, and Hb concentration were obtained. MCAO was produced by advancing the filament to achieve a stable reduction in LDF by >60% over lateral cortex. The early change in arteriolar diameter was measured at 10 min of MCAO. Except for a time control group with no superfusion, the window was superfused with aCSF, vehicle, or drug at a rate of 200 μl/min for 10 min starting at 15 min of MCAO. The superfusion rate was decreased to 17 μl/min for the remainder of the experiment. At 25 min of MCAO, measurements of arteriolar diameter were repeated. In some groups, an exchange transfusion was performed starting at 35 min of MCAO at a rate of 0.5 ml/min for 15 min followed by a maintenance infusion of 1 ml/h for the remainder of the experiment. The solution contained ∼6% ZL-HbBv and 5% human serum albumin to maintain oncotic pressure. Details of the production of ZL-HbBv have been described previously (13). Reperfusion was initiated at 120 min of MCAO by withdrawal of the filament. Arteriolar diameter measurements were repeated at 60, 75, 90, 105, and 120 min of MCAO and at 10 and 20 min of reperfusion. Arterial blood gases and Hb concentration measurements were repeated at 30, 90, and 120 min of MCAO. After 20 min of reperfusion, the heart was arrested by intravenous injection of KCl.
Drugs were applied by superfusion of the cranial window to ensure adequate delivery to the artery that was examined. Because the superfusion could possibly clear vasoactive agents produced during ischemia, comparisons first were made between groups of rats with no cranial window superfusion (n = 9) and superfusion with aCSF (n = 9). In the second experiment, the window was superfused with either 0.1% ethanol vehicle (n = 9) or 1 μmol/l of HET0016 (n = 8). In the third experiment, comparisons were made between groups transfused with ZL-HbBv after superfusion with either 0.1% ethanol (n = 9) or 1 μmol/l HET0016 (n = 8). In a fourth experiment, comparisons were made among groups superfused with 0.02% DMSO vehicle and no transfusion (n = 9), 3 μmol/l BQ610 and no transfusion (n = 8), or 3 μmol/l BQ610 and ZL-HbBv transfusion (n = 7).
To determine whether BQ610 was capable of increasing CBF in the ischemic border region, a separate group of five rats was studied without cranial window measurements of arteriolar diameter. LDF was measured in the border region (4 mm caudal and 3 mm lateral to bregma) and in the ischemic core (0 mm rostral and 10 mm lateral to bregma). The skull was thinned at both sites without exposing the dura mater, and LDF probes were held in a fixed position for the duration of MCAO. At 90 min of MCAO, 0.5 μmol/kg of BQ610 was injected intravenously and the percent change in LDF was measured. A time control group of five rats was also studied.
Statistical analysis.
The percent change in diameter from the preischemic baseline was calculated for each arteriole (baseline diameter = 48 ± 17 μm). Statistical analysis was performed by using the average percent change of four to seven pial arterioles per rat, with the sample size as the number of rats. On the basis of the 7% standard deviation of the initial change in diameter and an average sample size of eight, differences equivalent to 11% of baseline diameter could be detected between two groups with an estimated power of 80%. For each experiment, two-way ANOVA was performed. If a significant interaction occurred between treatment groups and time, then comparisons among groups were performed at individual time points with the Newman-Keuls multiple range test. Measurements of LDF before and after BQ610 administration were compared by paired t-test. A significance level of 0.05 was used in all tests.
RESULTS
MABP was relatively stable throughout the observation period in all groups (Table 1), and exchange transfusion with ZL-HbBv did not produce significant hypertension. In all groups, arterial Po2 was kept above 100 Torr, arterial Pco2 was relatively constant at ∼40 Torr, and arterial pH remained at ∼7.40 throughout MCAO (Table 2).
Table 1.
Arterial blood pressure before and during MCAO in rats whose cortical surface was superfused with vehicle or drug via a cranial window starting at 15 min of MCAO
| Treatment |
MCAO Duration, min |
|||
|---|---|---|---|---|
| Baseline | 30 | 90 | 120 | |
| No superfusion | 99±2 | 100±1 | 101±1 | 99±1 |
| CSF superfusion | 101±1 | 101±1 | 99±1 | 99±1 |
| 0.1% ethanol superfusion | 107±3 | 108±2 | 109±3 | 108±3 |
| HET0016 superfusion | 100±1 | 101±1 | 100±1 | 100±1 |
| 0.1% ethanol superfusion/Hb transfusion | 100±1 | 101±2 | 103±2 | 101±2 |
| HET0016 superfusion/Hb transfusion | 100±1 | 100±1 | 110±7 | 101±3 |
| 0.02% DMSO superfusion | 103±2 | 101±1 | 101±1 | 101±1 |
| BQ610 superfusion | 101±1 | 101±1 | 100±1 | 101±1 |
| BQ610 superfusion/Hb transfusion | 101±1 | 101±1 | 101±1 | 103±1 |
Values are means ± SE. MCAO, middle cerebral artery occlusion; CSF, cerebrospinal fluid. Some groups of rats also underwent hemoglobin (Hb) exchange transfusion at 35–50 min.
Table 2.
Arterial Po2, Pco2, and pH at baseline and at 120 min of MCAO in rats whose brains were superfused with vehicle or drug via a cranial window starting at 15 min of MCAO
| Treatment |
Po2, Torr |
Pco2, Torr
|
pH
|
|||
|---|---|---|---|---|---|---|
| Baseline | 120 min | Baseline | 120 min | Baseline | 120 min | |
| No superfusion | 137±9 | 156±8 | 39±1 | 37±1 | 7.39±0.01 | 7.40±0.01 |
| CSF superfusion | 142±10 | 156±7 | 37±1 | 38±1 | 7.42±0.01 | 7.41±0.01 |
| 0.1% ethanol superfusion | 141±10 | 158±5 | 39±1 | 39±1 | 7.42±0.01 | 7.43±0.01 |
| HET0016 superfusion | 135±6 | 161±11 | 39±1 | 38±1 | 7.39±0.01 | 7.41±0.01 |
| 0.1% ethanol superfusion/Hb transfusion | 132±7 | 144±12 | 40±1 | 39±2 | 7.38±0.01 | 7.35±0.03 |
| HET0016 superfusion/Hb transfusion | 138±12 | 128±11 | 40±2 | 38±1 | 7.38±0.01 | 7.37±0.01 |
| 0.02% DMSO superfusion | 144±13 | 123±15 | 41±1 | 41±1 | 7.39±0.01 | 7.39±0.01 |
| BQ610 superfusion | 162±10 | 146±8 | 38±1 | 41±1 | 7.40±0.01 | 7.38±0.01 |
| BQ610 superfusion/Hb transfusion | 149±7 | 155±12 | 41±1 | 41±1 | 7.40±0.01 | 7.39±0.01 |
Values are means ± SE. Some groups of rats also underwent Hb exchange transfusion at 35–50 min.
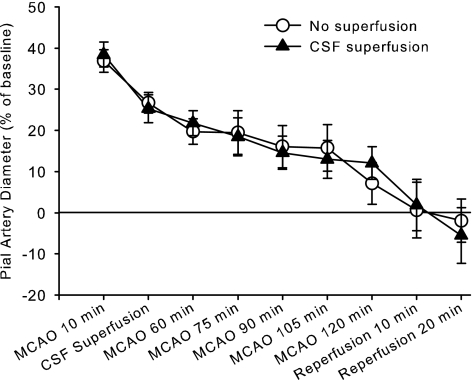
Pial arterioles had increased in diameter by 30–40% at 10 min after MCAO (Fig. 1). However, this dilation subsided over the 2-h period of MCAO. Reperfusion resulted in a relative constriction. The gradual loss of dilation during sustained MCAO occurred whether or not the cranial window was superfused with aCSF starting at 15 min of MCAO.
Fig. 1.
Percent change in pial arteriolar diameter (±SE) during 2 h of middle cerebral artery occlusion (MCAO) and 20 min of reperfusion with either no superfusion of the cranial window (n = 9) or continuous superfusion of cerebrospinal fluid (CSF; n = 9). Two-way ANOVA indicated no significant interaction between group treatment and time (P = 0.99).
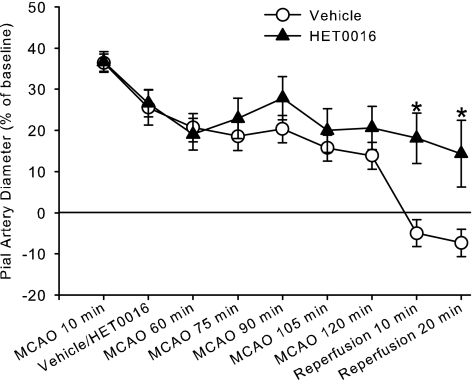
Loss of dilation also occurred during prolonged MCAO in groups superfused with 0.1% ethanol (vehicle for HET0016) or HET0016 (Fig. 2). Although two-way ANOVA indicated a significant interaction of time and HET0016 superfusion, comparisons at individual time points revealed significant differences only during reperfusion. The constrictor response to reperfusion was attenuated by HET0016 superfusion.
Fig. 2.
Percent change in pial arteriolar diameter (±SE) during 2 h of MCAO and 20 min of reperfusion with continuous superfusion of vehicle (0.1% ethanol, n = 9) or 1 μM HET0016 (n = 8) starting at 15 min of MCAO. Two-way ANOVA indicated a significant interaction between treatment groups and time (P = 0.033); *P < 0.05 from vehicle superfusion by Newman-Keuls multiple range test.
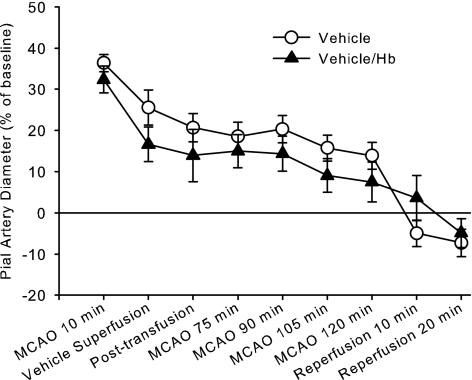
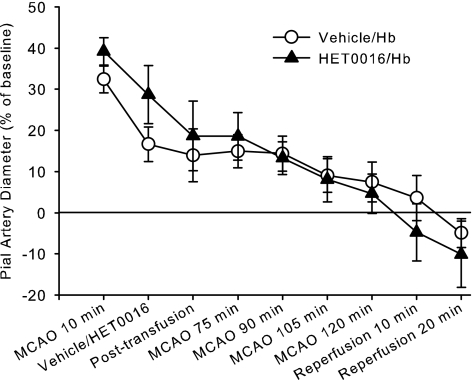
Exchange transfusion with ZL-HbBv starting at 35 min of MCAO resulted in a decrease in hematocrit from 38 ± 1 to 23 ± 1% and in arterial Hb concentration from 12.3 ± 0.2 to 8.1 ± 0.3 g/dl. Transfusion did not significantly alter the time course of pial arteriolar diameter changes during MCAO or reperfusion (Fig. 3). Moreover, initiating HET0016 superfusion before exchange transfusion with ZL-HbBv did not prevent the decrease in diameter, and ANOVA indicated no significant interaction of HET0016 treatment with time in these two Hb-transfused groups (Fig. 4).
Fig. 3.
Percent change in pial arteriolar diameter (±SE) during 2 h of MCAO and 20 min of reperfusion with continuous superfusion of vehicle (0.1% ethanol) starting at 15 min of MCAO. One group underwent zero-link bovine Hb (ZL-HbBv) exchange transfusion at 35 min of MCAO (n = 9) and another group was not transfused (n = 9). Two-way ANOVA indicated no significant interaction between treatment groups and time (P = 0.47).
Fig. 4.
Percent change in pial arteriolar diameter (±SE) during 2 h of MCAO and 20 min of reperfusion with continuous superfusion of vehicle (0.1% ethanol, n = 9) or 1 μM HET0016 (n = 8) starting at 15 min of MCAO followed by ZL-HbBv exchange transfusion at 35 min. Two-way ANOVA indicated no significant interaction between treatment groups and time (P = 0.54).
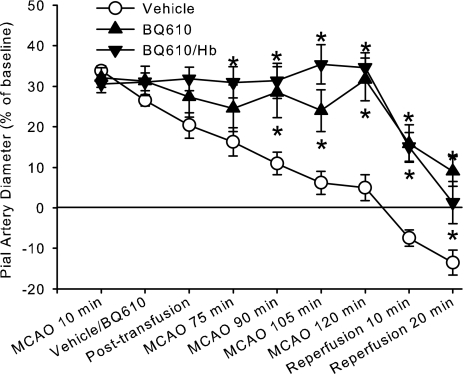
As in other groups, loss of dilation occurred when the window was superfused with 0.02% DMSO, the vehicle for BQ610 (Fig. 5). In comparing this effect of time with that of the group superfused with CSF alone, two-way ANOVA indicated no significant interaction between CSF and 0.02% DMSO superfusion treatments over time (P < 0.90). However, the group superfused with BQ610 maintained significantly greater arteriolar diameter than did the vehicle group by 90 min of MCAO and thereafter. When ZL-HbBv was exchange transfused after BQ610 superfusion, diameter was sustained at the initial 10-min value throughout MCAO. The change in diameter from the preischemic baseline became significantly greater than that in the vehicle group by 75 min of MCAO and thereafter. In both groups superfused with BQ610, the extent of dilation at 2 h of MCAO was not different from the initial dilation before BQ610 superfusion had commenced.
Fig. 5.
Percent change in pial arteriolar diameter (±SE) during 2 h of MCAO and 20 min of reperfusion with continuous superfusion of vehicle (0.02% DMSO, n = 9) or 3 μM BQ610 starting at 15 min of MCAO followed by no transfusion (n = 8) or ZL-HbBv exchange transfusion (n = 7) at 35 min. Two-way ANOVA indicated a significant interaction between treatment groups and time (P < 0.001); *P < 0.05 from vehicle superfusion by Newman-Keuls multiple range test.
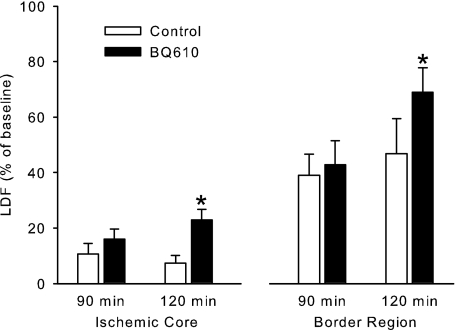
To determine whether BQ610 administration can increase CBF during MCAO, the drug was injected intravenously at 90 min of MCAO. Delayed injection during MCAO permitted a paired statistical analysis of LDF within the same animal at a time when pial arterioles in the border region had exhibited decreased dilation and should be capable of additional dilation. The drug was administered systemically to provide delivery to both intraparenchymal and extraparenchymal blood vessels during LDF monitoring. Intravenous injection of BQ610 did not significantly change MABP (104 ± 2 to 107 ± 2 mmHg) but increased LDF in the cortical ischemic core and border region (Fig. 6). In a time control group, LDF was not significantly increased over the same time period.
Fig. 6.
Laser-Doppler flux (LDF), expressed as a percent of preischemic baseline (±SE), in the cortical ischemic core and border region at 90 and 120 min of MCAO in a time control group (n = 5) and a group injected intravenously with BQ610 (n = 5) after the 90-min measurement. *P < 0.05 from the corresponding 90-min value.
DISCUSSION
This study revealed several major findings. First, pial arterioles in the distal MCA distribution area near the ACA border region exhibited a gradual loss of dilation during the course of the 2-h MCAO. Second, decreasing hematocrit by exchange transfusion with cell-free polymeric Hb after MCAO did not alter the loss of pial arteriolar dilation. Third, superfusion of a 20-HETE synthesis inhibitor did not increase pial diameter after Hb exchange transfusion during MCAO as it did in nonischemic pial arterioles (22). Fourth, the ETA receptor antagonist was equally effective at preventing the loss of dilation whether or not the rats were transfused with cell-free Hb during MCAO.
In a study by Patel et al. (19) that used chloralose-anesthetized cats with an open cranial window superfused with mineral oil, pial arteries in the penumbral region initially dilated after MCAO. However, by 30 min of MCAO, the responses became heterogeneous, with some animals displaying sustained arterial dilation and others showing constriction to diameters as much as 50% smaller than those of preischemic baseline. In our model with closed cranial windows in isoflurane-anesthetized rats, we observed initial pial arteriolar dilation that subsided after 10 min of MCAO. The decrease in dilation was more gradual than that seen in the study on cats and usually did not diminish to values significantly below preischemic baseline. Although differences in species, anesthetic, and methodology may account for quantitative differences between ours and this previous study, both studies are qualitatively consistent with the concept that vasodilation is submaximal during prolonged MCAO. Because the loss of vasodilation was observed in the group without continuous superfusion of the cranial window and in the three groups superfused with aCSF or each vehicle, the loss of vasodilation was a robust finding in our model, and the superfusion protocol did not appear to substantially dilute locally released vasoactive agents.
Exchange transfusion of ZL-HbBv in nonischemic animals produced pial arteriolar constriction that offset the decrease in blood viscosity and resulted in no change in CBF (25). This constriction was blocked by acute superfusion with 1 μmol/l of HET0016 (22), the same concentration that we used here. Because 20-HETE synthesis is oxygen dependent (6, 10), the vasoconstrictor response to decreased blood viscosity and a plasma-based O2 carrier was attributed to increased O2-dependent 20-HETE synthesis (22). Previous work indicated that exchange transfusion with ZL-HbBv did not produce the increase in intraischemic blood flow that would be expected with a decrease in hematocrit (14). Thus we postulated that the Hb-based O2 carrier might improve oxygenation sufficiently to increase 20-HETE production and restrict pial arteriolar dilation during MCAO. However, we found that loss of pial arteriolar dilation during MCAO still occurred after ZL-HbBv exchange transfusion and that HET0016 superfusion started before the transfusion failed to block the loss of dilation. Thus 20-HETE synthesis did not appear to be a major factor that limited dilation when oxygenation was expected to be improved by ZL-HbBv.
Inhibition of 20-HETE synthesis has been found to decrease infarct size in a variety of MCAO models (4, 15, 17, 20). Because 20-HETE is produced by CYP 4A in cerebral vascular smooth muscle (4) and because 20-HETE is a cerebral vasoconstrictor, tissue rescue by 20-HETE synthesis inhibitors might result from vasodilation. However, previous studies did not find a significant increase in LDF in lateral cortex where ischemia is dense (4, 20). An increase in intraischemic LDF was not detected over penumbral cortex after administration of a 20-HETE synthesis inhibitor, which retained neuroprotective properties when administered at reperfusion (26). The present finding that superfusion of HET0016 over the pial surface did not significantly attenuate the loss of pial arteriolar dilation during MCAO also supports the concept that protection by 20-HETE synthesis inhibition is not mediated by intraischemic vasodilation. Protection could be mediated by a vascular mechanism during reperfusion (20), consistent with greater dilation seen during early reperfusion in our experiment, or possibly by mitigating effects of 20-HETE on neurons and glia. Reoxygenation may increase O2-dependent 20-HETE synthesis and constrain vasodilation during reperfusion. Maintaining pial arteries in a vasodilated state during reperfusion with HET0016 should help overcome the poor reflow phenomenon among capillaries during early reperfusion.
Loss of vasodilation during prolonged MCAO could be attributed to decreased release of vasodilator mediators, increased release of vasoconstrictors, decreased vasoactivity to released mediators, and decreased intraluminal distending pressure, which is known to fall to levels below 20 mmHg during MCAO (34). The observations in cats that perivascular microinjections of nonpeptidergic endothelin antagonists produce immediate, transient dilation of pial arterioles that either had remained dilated or had constricted below preischemic baseline implies that locally released endothelin contributed to submaximal dilation (19). On the basis of these observations, we continuously superfused an endothelin antagonist to demonstrate that pial arteriolar diameter could be maintained in a vasodilated state during prolonged MCAO. Superfusion with the peptidergic ETA antagonist BQ610 beginning at 15 min of MCAO prevented subsequent loss of dilation. Moreover, intravenous injection of BQ610 at 90 min of MCAO, a time at which pial arteriolar dilation had subsided, produced an increase in CBF in the ischemic border region. The increase in CBF implies that loss of pial arteriolar dilation is associated with constrained CBF. Moreover, the potential effect on CBF could be greater than that observed because the low intraischemic CBF may have limited the delivery of BQ610. Collectively, these results are consistent with local release of endothelin as a major component of submaximal dilation in the ischemic border region and lend further support to other studies that have shown improved perfusion with endothelin antagonists (11, 18, 35). The improved pial artery diameter during reperfusion with BQ610 superfusion also suggests that endothelin antagonists could improve reflow. Because other studies have demonstrated that endothelin antagonist administration results in decreased infarct volume (11, 18, 32), the effect of BQ610 on infarct volume was not pursued in the present study.
Transfusion of cross-linked tetrameric Hb produces peripheral vasoconstriction that can be attenuated by NO synthase inhibition or an endothelin antagonist. Because the tetramers can extravasate, they are likely to scavenge NO and decrease NO-dependent inhibition of peripheral endothelin release. However, the large Hb polymers that we used here do not readily extravasate or produce peripheral vasoconstriction and thus appear less likely to stimulate endothelin release. We did not determine whether the ZL-HbBv augments endothelin release from the peripheral vasculature or ischemia-induced endothelin release from the cerebral vasculature. However, the observation that loss of pial arteriolar dilation was not significantly augmented by ZL-HbBv transfusion suggests that any augmentation of endothelin release by ZL-HbBv was not physiologically significant. Furthermore, BQ610 was equally potent in blocking the loss of dilation with and without ZL-HbBv transfusion. Without Hb transfusion, cerebral ischemia has been reported to increase plasma and cerebral tissue endothelin (2, 37), although the time course has not been well studied. We did not measure the time course of plasma endothelin during MCAO to determine whether the levels correlated with the time course of pial arterial diameter. However, the diameter difference between vehicle and BQ610 superfused groups increased progressively, which implies that the effect of endothelin also increased over time. Mean arterial pressure and blood gases were unchanged during MCAO and did not confound interpretation of the data.
We conclude that rescue of ischemic tissue by ZL-HbBv exchange transfusion is constrained by submaximal dilation mediated by endothelin rather than by 20-HETE synthesis. However, this constraint is not substantially greater than that without ZL-HbBv transfusion. Tissue rescue previously reported with ZL-HbBv transfusion is likely related to improved oxygenation within parenchyma rather than to extraparenchymal vasodilation.
PERSPECTIVES AND SIGNIFICANCE
Much effort has been directed at therapeutics that directly protect neurons from ischemia. Strategies to improve O2 delivery during the acute phase of stroke also offer great potential for limiting brain damage. Transfusion of cell-free Hb is one such strategy, but efficacy may be limited if collateral vessels are not maximally dilated. The present study provides one of the first systematic analysis of the time course of pial arterial diameter in the ischemic border region during prolonged focal cerebral ischemia. We found a time-dependent loss of the initial pial arterial dilation and that exchange transfusion of the cell-free Hb polymer ZL-HbBv did not affect this loss of dilation. Whereas O2-dependent synthesis of the vasoconstrictor 20-HETE in vascular smooth muscle limits vasodilation induced by ZL-HbBv exchange transfusion in nonischemic brain (22), 20-HETE synthesis does not appear to be responsible for the loss of dilation during prolonged ischemia, although it may play a greater role during reoxygenation. In contrast, endothelin appears to play a more prominent role during the ischemic period in limiting vasodilation with or without ZL-HbBv transfusion. Because endothelial NO synthase becomes downregulated during MCAO (1, 16, 27, 31), it is interesting to speculate that increased endothelin release is related to endothelial NO synthase dysfunction. Thus strategies directed at improving endothelial NO synthase function or blocking ETA receptors may be useful adjunct therapies with Hb-based O2 carriers for augmenting O2 delivery in acute ischemic stroke.
GRANTS
This study was supported by grants from the National Institutes of Health (NS38684 and HL59996).
DISCLOSURES
R. C. Koehler and H. Kwansa were paid consultants to Oxyvita, Inc., holder of the licensing rights to the zero-link bovine hemoglobin polymer. The terms of this arrangement were managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Acknowledgments
The authors thank Claire F. Levine for editorial assistance.
REFERENCES
- 1.Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest 117: 1961–1967, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barone FC, Globus MYT, Price WJ, White RF, Storer BL, Feuerstein GZ, Busto R, Ohlstein EH. Endothelin levels increase in rat focal and global ischemia. J Cereb Blood Flow Metab 14: 337–342, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Cole DJ, Schell RM, Przybelski RJ, Drummond JC, Bradley K. Focal cerebral ischemia in rats: effect of hemodilution with α-α cross-linked hemoglobin on CBF. J Cereb Blood Flow Metab 12: 971–976, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Dunn KM, Renic M, Flasch AK, Harder DR, Falck JR, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295: H2455–H2465, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res 87: 60–65, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Gebremedhin D, Yamaura K, Harder DR. Role of 20-HETE in the hypoxia-induced activation of Ca2+-activated K+ channel currents in rat cerebral arterial muscle cells. Am J Physiol Heart Circ Physiol 294: H107–H120, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Gulati A, Sen AP, Sharma AC, Singh G. Role of ET and NO in resuscitative effect of diaspirin cross-linked hemoglobin after hemorrhage in rat. Am J Physiol Heart Circ Physiol 273: H827–H836, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Gulati A, Sharma AC, Singh G. Role of endothelin in the cardiovascular effects of diaspirin cross-linked hemoglobin. Crit Care Med 24: 137–147, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Gulati A, Singh G, Rebello S, Sharma AC. Effect of diaspirin crosslinked and stroma-reduced hemoglobin on mean arterial pressure and endothelin-1 concentration in rats. Life Sci 56: 1433–1442, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Harder DR, Narayanan J, Birks EK, Liard JF, Imig JD, Lombard JH, Lange AR, Roman RJ. Identification of a putative microvascular oxygen sensor. Circ Res 79: 54–61, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Legos JJ, Lenhard SC, Haimbach RE, Schaeffer TR, Bentley RG, McVey MJ, Chandra S, Irving EA, Andrew AP, Barone FC. SB 234551 selective ET(A) receptor antagonism: perfusion/diffusion MRI used to define treatable stroke model, time to treatment and mechanism of protection. Exp Neurol 212: 53–62, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Lehmberg J, Putz C, Furst M, Beck J, Baethmann A, Uhl E. Impact of the endothelin-A receptor antagonist BQ 610 on microcirculation in global cerebral ischemia and reperfusion. Brain Res 961: 277–286, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Matheson B, Kwansa HE, Bucci E, Rebel A, Koehler RC. Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol 93: 1479–1486, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Mito T, Nemoto M, Kwansa H, Sampei K, Habeeb M, Murphy SJ, Bucci E, Koehler RC. Decreased damage from transient focal cerebral ischemia by transfusion of zero-link hemoglobin polymers in mouse. Stroke 40: 278–284, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyata N, Seki T, Tanaka Y, Omura T, Taniguchi K, Doi M, Bandou K, Kametani S, Sato M, Okuyama S, Cambj-Sapunar L, Harder DR, Roman RJ. Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N′-hydroxyimido formamide], on hemorrhagic and ischemic stroke. J Pharmacol Exp Ther 314: 77–85, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Morikawa E, Rosenblatt S, Moskowitz MA. l-Arginine dilates rat pial arterioles by nitric oxide-dependent mechanisms and increases blood flow during focal cerebral ischemia. Br J Pharmacol 107: 905–907, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omura T, Tanaka Y, Miyata N, Koizumi C, Sakurai T, Fukasawa M, Hachiuma K, Minagawa T, Susumu T, Yoshida S, Nakaike S, Okuyama S, Harder DR, Roman RJ. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke 37: 1307–1313, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Patel TR, Galbraith S, Graham DI, Hallak H, Doherty AM, McCulloch J. Endothelin receptor antagonist increases cerebral perfusion and reduces ischaemic damage in feline focal cerebral ischaemia. J Cereb Blood Flow Metab 16: 950–958, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Patel TR, Galbraith S, McAuley MA, McCulloch J. Endothelin-mediated vascular tone following focal cerebral ischaemia in the cat. J Cereb Blood Flow Metab 16: 679–687, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Poloyac SM, Zhang Y, Bies RR, Kochanek PM, Graham SH. Protective effect of the 20-HETE inhibitor HET0016 on brain damage after temporary focal ischemia. J Cereb Blood Flow Metab 26: 1551–1561, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Qin X, Kwansa H, Bucci E, Dore S, Boehning D, Shugar D, Koehler RC. Role of heme oxygenase-2 in pial arteriolar response to acetylcholine in mice with and without transfusion of cell-free hemoglobin polymers. Am J Physiol Regul Integr Comp Physiol 295: R498–R504, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin X, Kwansa H, Bucci E, Roman RJ, Koehler RC. Role of 20-HETE in the pial arteriolar constrictor response to decreased hematocrit after exchange transfusion of cell-free polymeric hemoglobin. J Appl Physiol 100: 336–342, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebel A, Cao S, Kwansa H, Dore S, Bucci E, Koehler RC. Dependence of acetylcholine and ADP dilation of pial arterioles on heme oxygenase after transfusion of cell-free polymeric hemoglobin. Am J Physiol Heart Circ Physiol 290: H1027–H1037, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebel A, Ulatowski JA, Joung K, Bucci E, Traystman RJ, Koehler RC. Regional cerebral blood flow in cats with cross-linked hemoglobin transfusion during focal cerebral ischemia. Am J Physiol Heart Circ Physiol 282: H832–H841, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Rebel A, Ulatowski JA, Kwansa H, Bucci E, Koehler RC. Cerebrovascular response to decreased hematocrit: effect of cell-free hemoglobin, plasma viscosity, and CO2. Am J Physiol Heart Circ Physiol 285: H1600–H1608, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Renic M, Klaus JA, Omura T, Kawashima N, Onishi M, Miyata N, Koehler RC, Harder DR, Roman RJ. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab 29: 629–639, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke 36: 2251–2257, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampei K, Ulatowski JA, Asano Y, Kwansa H, Bucci E, Koehler RC. Role of nitric oxide scavenging in vascular response to cell-free hemoglobin transfusion. Am J Physiol Heart Circ Physiol 289: H1191–H1201, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena R, Wijnhoud AD, Carton H, Hacke W, Kaste M, Przybelski RJ, Stern KN, Koudstaal PJ. Controlled safety study of a hemoglobin-based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke 30: 993–996, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Saxena R, Wijnhoud AD, Man in 't Veld AJ, van den Meiracker AH, Boomsma F, Przybelski RJ, Koudstaal PJ. Effect of diaspirin cross-linked hemoglobin on endothelin-1 and blood pressure in acute ischemic stroke in man. J Hypertens 16: 1459–1465, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Shin HK, Salomone S, Potts EM, Lee SW, Millican E, Noma K, Huang PL, Boas DA, Liao JK, Moskowitz MA, Ayata C. Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow Metab 27: 998–1009, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatlisumak T, Carano RAD, Takano K, Opgenorth TJ, Sotak CH, Fisher M. A novel endothelin antagonist, A-12772, attenuates ischemic lesion size in rats with temporary middle cerebral artery occlusion. Stroke 29: 850–858, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Ulatowski JA, Nishikawa T, Matheson-Urbaitis B, Bucci E, Traystman RJ, Koehler RC. Regional blood flow alterations after bovine fumaryl ββ-crosslinked hemoglobin transfusion and nitric oxide synthase inhibition. Crit Care Med 24: 558–565, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi S, Kobayashi S, Yamashita K, Kitani M. Pial arterial pressure contribution to early ischemic brain edema. J Cereb Blood Flow Metab 9: 597–602, 1989. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Belayev L, Zhao W, Irving EA, Busto R, Ginsberg MD. A selective endothelin ET(A) receptor antagonist, SB 234551, improves cerebral perfusion following permanent focal cerebral ischemia in rats. Brain Res 1045: 150–156, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Chen TY, Kirsch JR, Toung TJ, Traystman RJ, Koehler RC, Hurn PD, Bhardwaj A. Kappa-opioid receptor selectivity for ischemic neuroprotection with BRL 52537 in rats. Anesth Analg 97: 1776–1783, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Ziv I, Fleminger G, Djaldetti R, Achiron A, Melamed E, Sokolovsky M. Increased plasma endothelin-1 in acute ischemic stroke. Stroke 23: 1014–1016, 1992. [DOI] [PubMed] [Google Scholar]