Abstract
Hyperexcitability of C-fiber bladder afferent pathways has been proposed to contribute to urinary frequency and bladder pain in chronic bladder inflammation including interstitial cystitis. However, the detailed mechanisms inducing afferent hyperexcitability after bladder inflammation are not fully understood. Thus, we investigated changes in the properties of bladder afferent neurons in rats with bladder inflammation induced by intravesical application of hydrochloric acid. Eight days after the treatment, bladder function and bladder sensation were analyzed using cystometry and an electrodiagnostic device of sensory function (Neurometer), respectively. Whole cell patch-clamp recordings and immunohistochemical staining were also performed in dissociated bladder afferent neurons identified by a retrograde tracing dye, Fast Blue, injected into the bladder wall. Cystitis rats showed urinary frequency that was inhibited by pretreatment with capsaicin and bladder hyperalgesia mediated by C-fibers. Capsaicin-sensitive bladder afferent neurons from sham rats exhibited high thresholds for spike activation and a phasic firing pattern, whereas those from cystitis rats showed lower thresholds for spike activation and a tonic firing pattern. Transient A-type K+ current density in capsaicin-sensitive bladder afferent neurons was significantly smaller in cystitis rats than in sham rats, although sustained delayed-rectifier K+ current density was not altered after cystitis. The expression of voltage-gated K+ Kv1.4 α-subunits, which can form A-type K+ channels, was reduced in bladder afferent neurons from cystitis rats. These data suggest that bladder inflammation increases bladder afferent neuron excitability by decreasing expression of Kv1.4 α-subunits. Similar changes in capsaicin-sensitive C-fiber afferent terminals may contribute to bladder hyperactivity and hyperalgesia due to acid-induced bladder inflammation.
Keywords: urinary bladder, hyperalgesia, inflammation, C-fiber afferent, potassium channel
it has been demonstrated that afferent pathways innervating the urinary bladder consist of myelinated Aδ-fibers and unmyelinated C-fibers and that hyperexcitability of C-fibers in bladder afferent pathways contributes to bladder overactivity and/or bladder pain under pathological conditions such as painful bladder syndrome/interstitial cystitis (PBS/IC) (60, 61).
Voltage-gated K+ (Kv) currents are major determinants of neuronal excitability. Kv currents in sensory neurons are divided into two major categories; i.e., sustained delayed rectifier-type K+ (KDR) and transient A-type K+ (KA) currents (19, 21, 30, 57). KA currents in sensory neurons including dorsal root ganglion (DRG) cells can be further subdivided into at least two different subtypes based on their inactivation kinetics (i.e., fast- and slow-decaying KA currents) (3, 17, 37). A reduction in KDR and/or KA currents is reportedly involved in hypersensitivity of afferent pathways under various pathological conditions (1, 18). We have also reported that bladder inflammation for 2 wk increased excitability of capsaicin-sensitive DRG neurons innervating the urinary bladder due to a reduction in slow-decaying KA currents without affecting KDR currents (59).
Kv channels are formed by subfamily-specific tetramerization of channel subunits composed of ion-conducting α-subunits and auxiliary β-subunits (32). For instance, individual expression of Kv1.4 and other Kv1-family α-subunits generates homotetramers with rapidly-inactivating and sustained current kinetics, respectively (52). Coexpression of Kv1.4 and other Kv1-family α-subunits results in formation of heteromeric complexes with intermediate current kinetics (42). Transcripts for various α-subunits in Kv1 subfamily have been identified in DRG (24, 25, 56). Previous studies have indicated that Kv1.1 and Kv1.2 α-subunits are expressed in both small- and medium/large-sized DRG neurons; whereas Kv1.4 α-subunits are preferentially expressed in small-sized DRG neurons, the majority of which also express TRPV1, indicating that Kv1.4 constitutes a major fraction of α-subunits in small-sized, nociceptive DRG neurons (43). Furthermore, slow-decaying KA currents, but not fast-decaying KA currents, in DRG neurons are partially reduced by α-dendrotoxin, a blocker for Kv1.1 and Kv1.2 channels (17, 18, 21, 40, 56). These findings suggest that slow-decaying KA currents in C-fiber sensory neurons are generated by heteromeric channels formed with Kv1.4 and Kv1.1/Kv1.2 α-subunits.
Previous studies have shown reductions in Kv α-subunits in rats with neuropathic pain induced by spinal nerve ligation (43) and sciatic nerve injury (25, 56). However, a molecular mechanism responsible for a reduction of KA currents and hyperexcitability of bladder afferent pathways after bladder inflammation remains to be elucidated. Hence, the present study was performed to clarify the mechanisms inducing bladder dysfunction and hyperexcitability of bladder afferent neurons in cystitis rats, especially focusing on Kv channel function.
MATERIALS AND METHODS
All animal experiments were in accordance with institutional guidelines and approved by the Institutional Animal Care and Use Committees (IACUC) at the University of Pittsburgh and Taiho Pharmaceutical, Japan, and with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Female Sprague-Dawley rats (170–220 g) (Hilltop, Pittsburgh, PA; Charles River Laboratories, Japan) were used for this study and were housed under a 12:12-h light-dark cycle with free access to water and food.
Production of cystitis model.
Bladder inflammation was induced according to a previously used method (26). Briefly, a polyethylene catheter (PE-50) was inserted transurethrally into the bladder under pentobarbital (50 mg/kg ip) anesthesia. Following withdrawal of urine from the bladder, 0.4 N hydrochloric acid (HCl) was infused into the bladder in a volume of 0.2 ml and kept at least for 90 s. Sham rats were treated with saline instead of HCl. An antibiotic (Cefazolin; 7 mg/kg) and an analgesic (buprenorphine, 0.2 mg/kg) were injected intramuscularly after the intravesical treatment to suppress posttreatment infection and pain, respectively. Experiments were performed 8 days after intravesical treatment.
For whole cell patch-clamp recordings and immunohistochemical studies, Fast Blue (1% wt/vol) (EMS Chemie, Zürich, Switzerland) was injected into the bladder wall 4–5 days before HCl instillation into the bladder to identify afferent neurons innervating the bladder by retrograde axonal transport of the fluorescent dye as previously described (59). Briefly, the bladder was exposed by a midline lower abdominal incision, and the dye was injected with a 30-gauge needle at four to six sites (total volume, 20 μl) on the surface of the bladder under isoflurane anesthesia. At each injection site, the needle was kept in place for 20–30 s and any leakage of dye was removed by application of cotton swab. The injection site was then rinsed with saline, and the incision was closed.
Histological analysis.
Under pentobarbital (80 mg/kg ip) anesthesia, the bladder was removed and fixed with 4% paraformaldehyde in 0.1 M PBS. Transverse sections were then prepared from the middle part of the bladders, embedded in paraffin wax, cut into 4-μm sections, and then stained with hematoxylin-eosin, Alcian blue-safranin (for mast cells), or Luna (for eosinophils) staining. The numbers of mast cells and eosinophils were counted in the entire field of a randomly selected section of the bladder with ×100 magnification in a blinded fashion.
Continuous cystometry.
A PE-50 catheter was implanted through the dome of the bladder under urethane anesthesia (1.0 g/kg sc). The catheter was connected to a pressure transducer for recording intravesical pressure and a syringe pump for infusing saline (3.0 ml/h) into the bladder. Micturition volume was continuously measured using an electrical scale positioned under the animal to collect fluid released from the urethral orifice. Intravesical pressure and micturition volume were recorded using a PowerLab System (AD Instruments, Bella Vista, Australia). In some animals, capsaicin was subcutaneously administered in three divided doses (50 and 25 mg/kg on the first day and 50 mg/kg on the second day) over a 2-day period to desensitize the majority of C-fiber afferents that are sensitive to capsaicin 5 days before cystometry (47). C-fiber desensitization was confirmed by negative response to an eye wipe test before the experiments (53).
Measurement of bladder sensation.
Two silver wire electrodes attached to a specially designed balloon catheter was inserted into the empty bladder through the urethral orifice under isoflurane anesthesia. The tip of one silver wire electrode was positioned on the surface of the balloon to have contact with the intraluminal surface of the bladder when the balloon catheter was inflated by filling with saline (0.2 ml). The other electrode tip was positioned on the catheter distal to the balloon to have contact with the bladder neck. Rats were then placed in a Bollman-type cage and recovered from anesthesia. Electrical stimuli of two different sine-wave pulses (250 or 5 Hz) with increasing intensities (range: 0–300 μA) were applied through the electrodes using a Neurometer CPT/C (Neurotron, Baltimore, MD) (4), and the animal responses were recorded (2, 27, 39). When vocalization and/or sudden body movements were observed as current intensities were increased, the stimulation was immediately stopped and the intensity was recorded as current perception thresholds (CPT) of bladder sensation at each frequency of stimulation (27, 39).
Whole cell patch-clamp recordings.
Freshly dissociated neurons from L6-S1 DRG were prepared from isoflurane-anesthetized rats as described previously (59). Briefly, L6-S1 DRG were removed, minced, and incubated for 25 min at 35°C in 5 ml DMEM (Sigma, St. Louis, MO) containing 0.3 mg/ml trypsin (Type III, Sigma), 1 mg/ml collagenase (Type I, Sigma), and 0.1 mg/ml deoxyribonuclease (Type IV, Sigma). Trypsin inhibitor (Type II-S, Sigma) was then added to neutralize the enzyme activity. Individual DRG cell bodies were isolated by trituration and plated on poly-l-lysine-coated 35-mm culture dishes. Whole cell patch-clamp recordings were performed at room temperature (20–22°C) on each Fast Blue-positive neuron within 10 h after dissociation. The internal solution contained (in mM): 140 KCl, 1 CaCl2, 2 MgCl2, 11 EGTA, 10 HEPES, 2 Mg-ATP, and 0.4 Tris-GTP adjusted to pH 7.4 with KOH. Patch electrodes had resistances of 3–5 MΩ when filled with the internal solution. Neurons were superfused at a flow rate of 2.0 ml/min with an external solution containing (in mM): 150 NaCl, 5 KCl, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 d-glucose, adjusted to pH 7.4 with NaOH. For the isolation of K+ currents following the evaluation of action potential characteristics, the external solution was changed to one containing (in mM): 150 choline-Cl, 5 KOH, 0.03 CaCl2, 10 HEPES, 3 MgCl2, and 10 d-glucose, adjusted to pH 7.4 with Tris-base. All recordings were made with an Axopatch 700B patch-clamp amplifier and controlled by Clampex software (Axon Instruments, Sunnyvale, CA). Data were then analyzed by pCLAMP software (Axon Instruments). Cell membrane capacitances were obtained by reading the value for whole cell input capacitance neutralization directly from the amplifier. In current-clamp recordings, data are presented from neurons that exhibited resting membrane potentials greater than −40 mV and action potentials that overshot 0 mV. In voltage-clamp recordings, the filter was set to −3 dB at 2,000 Hz. Leak currents were subtracted by P/4 pulse protocol, and the series resistance was compensated by 50–60%. The voltage error did not exceed 5 mV after compensation of the series resistance, and a charging time constant of the voltage clamp was < 300 μs, which was faster than gating properties of outward K+ currents in this study. In a protocol examining firing characteristics, action potentials were elicited by depolarizing current pulses (duration 800 ms) at intensities that were kept at just suprathreshold for inducing a single action potential during a 50-ms depolarizing stimulus pulse. Capsaicin-sensitive neurons were identified by capsaicin (500 nM)-induced inward currents in voltage-clamp recordings at the end of patch-clamp recordings in each cell. Capsaicin was dissolved in the normal external solution containing 10% ethanol and 10% Tween 80 at a concentration of 5 mM and then diluted in the external solution before experiments. No effects were detected by application of ethanol and Tween 80 in concentrations as high as 0.2%.
Immunohistochemistry.
Eight days after HCl or vehicle treatment, rats were deeply anesthetized with pentobarbital (80 mg/kg ip) and perfused through the left ventricle with 300 ml cold oxygenated PBS, followed by a fixative solution consisting of 4% paraformaldehyde in 0.1 M PBS. L6 DRGs were then removed and postfixed for 8 h in the same fixative solution. The tissues were placed in PBS containing increasing concentrations of sucrose (10, 20, and 30%) at 4°C for cryoprotection, frozen in mounting medium, and sectioned at 30-μm thickness. After mounting on gelatin-coated slides, the sections were washed and incubated with antibodies for Kv1.2 or Kv1.4 α-subunits (Alomone Lab, Israel) for 24 h at 4°C, followed by visualization with anti-rabbit IgG antibody conjugated to Cy3 for 2 h at room temperature. Images were obtained with a fluorescent microscope and the IPLab Spectram.
In randomly selected DRG sections (6 sections per rat, n = 3 rats), labeling intensity measurements were made on all cell profiles that exhibited a nucleus using Scion Image software (Scion, Frederick, MD). Kv α-subunit staining intensity of each neuron was estimated by subtracting nonspecific background staining. For the measurement of labeling intensity, the nuclear region was excluded. Mean labeling intensity of Kv α-subunits was calculated in dye-labeled bladder afferent neurons as well as unlabeled afferent neurons, and the ratio of mean labeling intensity of bladder afferent neurons vs. unlabeled afferent neurons was obtained in each DRG section. The staining density ratio (dye-labeled vs. unlabeled cells) in each section was then averaged in randomly selected DRG sections in each animal, and thereafter the mean ratio in each animal was averaged again in either normal or HCl-induced cystitis group of animals. These analytical methods for Kv α-subunit staining were used to avoid comparing labeling intensity of dye-labeled afferent neurons between different DRG sections, which can be affected by different staining conditions and nonlinear fluorescent signal decay among sections.
Statistical analysis.
Data are presented as means ± SE. Statistical comparisons between two groups were performed by Wilcoxon test or two-tailed unpaired Student's t-test. Two-way ANOVA followed by two-tailed unpaired Student's t-test was used for statistical comparisons in K+ currents obtained during whole cell patch-clamp experiments. For all comparisons, a value of P < 0.05 was considered to be significant differences.
RESULTS
Intravesical application of HCl induces bladder inflammation with urinary frequency and bladder hyperalgesia.
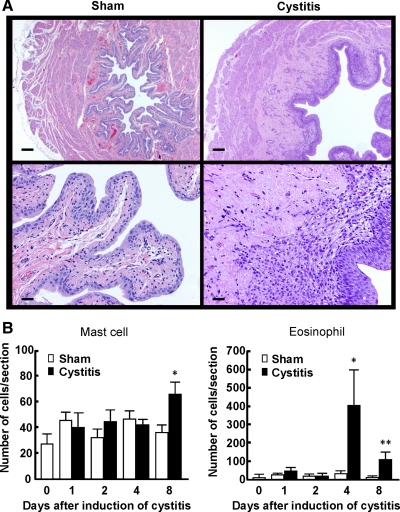
Fig. 1A shows photomicrographs of hematoxylin-eosin-stained bladder tissues from sham and cystitis rats 8 days after intravesical application with HCl. Edema and infiltration of inflammatory cells were observed predominantly in the suburothelial layer of the bladder from HCl-treated rats compared with sham-treated rats. Infiltrated mast cells and eosinophils, which were respectively identified by Alcian blue-safranin and Luna staining, were counted in the entire field of a randomly selected transverse section of the bladder, which contained urothelial, suburothelial, and muscle layers, from sham and cystitis rats. The number of eosinophils reached a peak at 4 days after intravesical HCl application and was still significantly increased at 8 days (Fig. 1B). The number of mast cells in the bladder was also significantly increased in cystitis rats compared with sham rats at 8 days after intravesical HCl application (Fig. 1B). These results indicate that intravesical application of HCl induces sustained bladder inflammation over 1 wk, characterized by infiltration of inflammatory cells mainly in the suburothelial layer of the bladder.
Fig. 1.
Bladder inflammation induced by intravesical application of HCl (0.4 N, 0.2 ml) in the rat. A: photomicrographs of hematoxylin-eosin-stained bladder sections from sham and cystitis rats (8 days after HCl injection). Magnification (upper: ×20, lower: ×100). Scale bars (upper: 200 μm, lower: 40 μm). B: time course (1–8 days after the treatment) of the changes in the number of mast cells and eosinophils, which were identified by Alcian blue-safranin and Luna staining, respectively, per entire transverse section of the bladder wall in sham and cystitis rats. Data are means ± SE in sham (n = 4–7) and cystitis groups (n = 4–9). *P < 0.05, **P < 0.01 compared with the sham group at each time point.
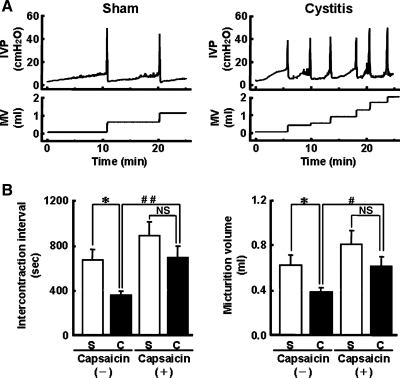
Cystometry was performed by continuous saline infusion (3.0 ml/h) into the bladder under urethane anesthesia to evaluate bladder function in sham and cystitis rats (8 days after HCl treatment). HCl-induced cystitis rats showed urinary frequency, which was characterized by shortened intercontraction intervals (cystitis rats: 353 ± 41 s vs. sham rats: 668 ± 95 s, P < 0.05) and decreased micturition volume (cystitis rats: 0.38 ± 0.04 ml vs. sham rats: 0.62 ± 0.10 ml, P < 0.05) (Fig. 2). To examine the contribution of capsaicin-sensitive C-fiber afferent pathways to urinary frequency in HCl-induced cystitis rats, C-fiber desensitization was induced by pretreatment with capsaicin (125 mg/kg sc) 5 days before the experiments. In capsaicin-pretreated groups, no significant changes in intercontraction intervals (883 ± 133 vs. 687 ± 114 s) and micturition volume (0.72 ± 0.11 vs. 0.61 ± 0.08 ml) were observed between HCl-untreated and HCl-treated rats (Fig. 2B). These results indicate that activation of capsaicin-sensitive C-fiber afferent pathways contributes to urinary frequency in HCl-induced cystitis rats. Other cystometric parameters, such as maximal voiding pressure and threshold pressure, were not different among any groups.
Fig. 2.
Urinary frequency in rats with HCl-induced cystitis (8 days). A: representative recordings of intravesical pressure (IVP) and micturition volume (MV) during continuous cystometry in sham and cystitis rats. Continuous cystometry was performed at infusion rate of 3.0 ml/h under urethane anesthesia (1.0 g/kg sc). B: summarized data of intercontraction intervals and micturition volume in sham (S; white bars) and cystitis (C; black bars) rats with or without capsaicin-pretreatment [capsaicin (+) and (−), respectively]. Capsaicin-untreated sham rats (n = 5), capsaicin-untreated cystitis rats (n = 7), capsaicin-pretreated sham rats (n = 5), capsaicin-pretreated cystitis rats (n = 6). Data are means ± SE. *P < 0.05 compared with the capsaicin-untreated sham group. #P < 0.05, ##P < 0.01 compared with the capsaicin-untreated cystitis group. NS, not significant.
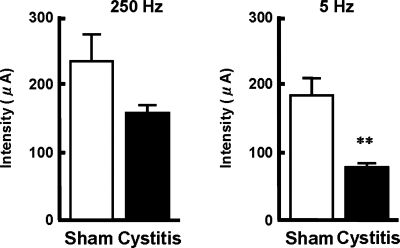
The contributions of two types of bladder sensory pathways (i.e., Aδ- and C-fiber afferents) were evaluated by measuring CPTs in μA using a Neurometer electrodiagnostic device (Neurotron) (Fig. 3). This device utilizes neuroselective electrical stimuli with different frequencies (5, 250, and 2,000 Hz) to perform quantitative assessments of subpopulations (C-, Aδ- and Aβ-fibers) of sensory nerve fibers (4). When stimuli at 250 Hz, which reportedly activate Aβ- and Aδ-fiber afferent fibers (29), were applied to the inner surface of the rat bladder using an electrode-mounted balloon catheter, the CPT values, which were the minimal current intensities to induce vocalization and/or sudden body movements of animals, tended to be decreased in cystitis rats (n = 8) compared with sham rats (n = 8); however, the differences were not statistically significant. When the stimulation was applied at 5 Hz to activate C-fiber afferents in addition to A-fiber populations (2, 29), the CPT was significantly reduced in cystitis rats compared with sham rats. These results indicate that C-fiber afferents more significantly mediate bladder hyperalgesia following HCl-induced cystitis than Aδ-fiber afferents.
Fig. 3.
Enhanced bladder sensation in HCl-induced cystitis rats. The minimal current intensity of electrical stimuli, which induced vocalization and/or startling behavior, with different frequencies (5 or 250 Hz) applied to the luminal surface of the bladder was evaluated as current perception thresholds (CPT) for bladder sensation. In somatic nerves, 5 Hz stimulation is shown to activate C-fiber axons in addition to A-fiber axons, which were selectively activated by 250 Hz stimulation. White and black bars represent the sham (n = 8) and cystitis rat group (n = 8), respectively. Data are means ± SE. **P < 0.01 compared with the sham rats group.
Increased excitability of afferent neurons innervating the bladder in cystitis.
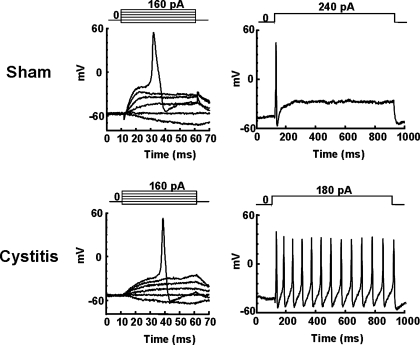
Because all of the above results indicate that rats with HCl-induced cystitis exhibited bladder hyperactivity/hyperalgesia mainly due to hyperexcitability of C-fiber bladder afferent pathways, the changes in electrophysiological properties of bladder afferent neurons in cystitis rats 8 days after HCl treatment were examined using a whole cell patch-clamp recording. Since the majority (80%) of C-fiber bladder afferent neurons are known to be sensitive to capsaicin, while only a small population (5%) of Aδ-fiber bladder neurons are capsaicin-sensitive (58), bladder afferent neurons that exhibited capsaicin sensitivity were investigated. Capsaicin sensitivity was confirmed as demonstrated by inward currents elicited by application of capsaicin (500 nM) following the evaluation of action potential characteristics and K+ channel properties in each cell. Table 1 shows a summary of the electrophysiological properties of capsaicin-sensitive bladder afferent neurons from sham and cystitis rats. Fig. 4 shows representative recordings of action potentials in capsaicin-sensitive bladder afferent neurons from the two groups. The resting membrane potential of capsaicin-sensitive bladder afferent neurons did not differ between the two groups (Table 1). However, the mean threshold for eliciting action potentials was significantly lower (10 mV less) than those from sham rats. Also, the firing pattern during a sustained membrane depolarization (800 ms duration) in these neurons differed between cystitis and sham groups. Capsaicin-sensitive bladder afferent neurons from sham rats exhibited a phasic pattern of firing during membrane depolarization when the current intensity was set to the value just above the threshold for inducing spike activation with a 50-ms pulse, whereas those from cystitis rats showed repetitive firings (tonic firing pattern) (Fig. 4). The number of action potentials during 800-ms membrane depolarization in capsaicin-sensitive bladder afferent neurons from cystitis rats was significantly greater (∼8-fold increase) than that in neurons from sham animals. (Table 1). These results indicate that capsaicin-sensitive bladder afferent neurons became hyperexcitable in rats with HCl-induced cystitis. In addition, the diameter and the cell input capacitance of capsaicin-sensitive bladder afferent neurons from cystitis rats were significantly greater than in neurons from sham rats, indicating that bladder inflammation lasting over 1 wk induced somal hypertrophy of these neurons as noted in previous studies (59).
Table 1.
Electrophysiological properties of capsaicin-sensitive bladder afferent neurons from sham and cystitis rats
| Sham | Cystitis | |
|---|---|---|
| No. of cells | 19 | 28 |
| Diameter, μm | 23.5±1.5 | 28.0±1.1* |
| Input capacitance, pF | 29.8±2.1 | 40.5±2.5† |
| Membrane potentials, mV | ||
| Resting | −49.6±1.8 | −48.0±1.0 |
| Spike threshold | −18.8±1.3 | −28.6±1.1† |
| Peak | 42.9±2.9 | 45.4±2.4 |
| Spike duration, ms | 4.3±0.3 | 4.3±0.4 |
| No. of action potentials, 800-ms depolarization | 1.3±0.3 | 9.7±1.2† |
Data are means ± SE.
P < 0.05,
P < 0.01 vs. sham rats.
Fig. 4.
Changes in firing characteristics of capsaicin-sensitive bladder afferent dorsal root ganglion (DRG) neurons following HCl-induced cystitis of sham (A) and cystitis (B) rats. Left: action potentials evoked by 50-ms depolarizing current pulses injected through patch pipettes in a current-clamp condition. Right: firing patterns during membrane depolarization (800 ms of duration). Pulse protocols are shown in the insets. Note the lower threshold for spike activation and repetitive firing pattern in the bladder afferent neuron from a cystitis rat.
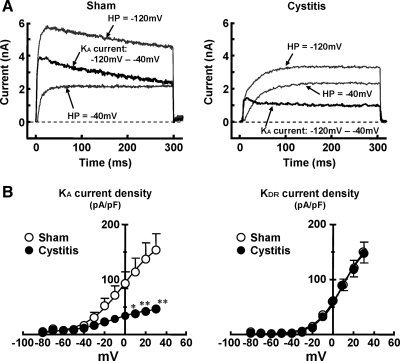
We have previously reported that slow-decaying, transient outward K+ currents (slow-decaying KA currents) are expressed in small-sized, capsaicin-sensitive bladder afferent neurons and that a reduction in slow KA currents by an application of 4-aminopyridine increased excitability of these neurons as evidenced by lower thresholds for spike activation and tonic firing (57, 59). Thus, in this study, we compared K+ current density in capsaicin-sensitive bladder afferent neurons from sham and cystitis rats (Fig. 5A). K+ currents were measured following replacement of external solution to the one that suppressed Na+ and Ca2+ currents after the evaluation of action potential characteristics in the same cell. Because in our previous studies, slow-decaying KA currents were activated by depolarizing voltage steps from hyperpolarized membrane potentials and were almost completely inactivated when the membrane potential was maintained at a depolarized level more than −40 mV in C-fiber bladder afferent neurons (57, 59), an estimate of the density of slow KA currents and sustained KDR currents was obtained by the difference in the currents activated by a depolarizing voltage pulse from two differential holding potentials (−120 and −40 mV). As shown in Fig. 5B, peak current densities of slow-decaying KA and sustained KDR currents were increased during membrane depolarization in capsaicin-sensitive bladder afferent neurons from both sham and cystitis rats. However, the peak current densities of slow KA were lower in neurons from cystitis rats than in those from sham rats. Significant differences in current density were detected at depolarizing pulses greater than +10 mV. However, the peak current density of sustained KDR currents in capsaicin-sensitive bladder afferent neurons was not different between sham and cystitis rats. These results indicate that a reduction in slow KA currents contributes to hyperexcitability of bladder afferent neurons in rats with HCl-induced cystitis.
Fig. 5.
Changes in K+ currents of capsaicin-sensitive bladder afferent DRG neurons following HCl-induced cystitis. Inward currents were suppressed by equimolar substitution of choline for Na+ and reduction of Ca2+ in the external solution. The A-type K+ (KA) currents were obtained by subtraction of the K+ currents evoked by depolarization to 0 mV from holding potentials of −40 and −120 mV. A: representative recordings of K+ currents in capsaicin-sensitive bladder afferent neurons from sham and cystitis rats, show superimposed outward K+ currents evoked by voltage steps to 0 mV from holding potentials of −120 and −40 mV. HP, holding potential. B: current-voltage relationships of slow-inactivating KA currents and sustained delayed rectifier-type K+ (KDR) currents in capsaicin-sensitive bladder afferent neurons (n = 16 and n = 17, respectively) from sham (open circles) and cystitis (closed circles) rats. Data are means ± SE. *P < 0.05; **P < 0.01. compared with the sham group at each voltage point. Note a reduction in KA current density in capsaicin-sensitive bladder afferent neurons from cystitis rats although KDR current density was not altered.
Reduction in Kv1.4 α-subunit expression in DRG neurons innervating the bladder of cystitis rats.
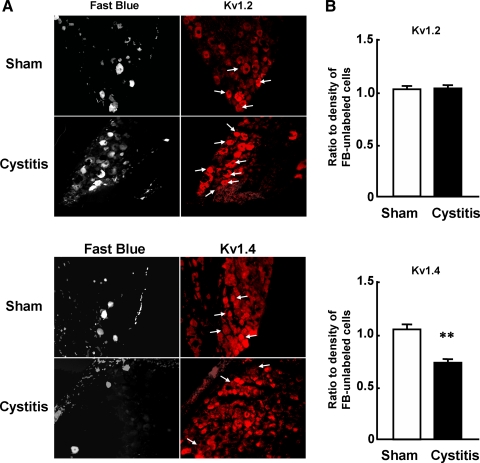
In small-sized DRG neurons, several Kv1-family subunits including Kv1.4 are abundantly expressed, and immunoreactivity of Kv1.4 and TRPV1 (capsaicin receptor) is highly colocalized (43, 56). Gating properties and toxin sensitivity of slow-decaying KA suggest that this current is carried by Kv1.4-containing channel complexes (42, 43, 52, 56). To examine whether a reduction in slow KA currents in bladder afferent neurons after cystitis is associated with decreased expression of these channels, the levels of Kv1.2 and 1.4 α-subunits were evaluated using immunohistochemical methods in sham and cystitis rats (8 days after HCl treatment). We randomly selected L6 DRG sections (3 rats from each group, 6 sections/animal) and compared staining densities of Kv1.2 or 1.4 α-subunits in Fast Blue-labeled DRG neurons innervating the bladder and unlabeled DRG neurons in the same section. The ratio of staining density of Kv1.4 α-subunit in bladder afferent neurons vs. unlabeled DRG neurons was significantly decreased in cystitis rats (n = 181 cells) when compared with sham rats (n = 153 cells) that exhibited similar levels of Kv1.4 α-subunit staining in Fast Blue-labeled and unlabeled neurons (Fig. 6). In contrast, in both groups of animals, similar levels of staining density of Kv1.2 α-subunits were observed in Fast Blue-labeled and unlabeled neurons (n = 144 and n = 135 cells for sham and cystitis groups, respectively) (Fig. 6). These results indicate that decreased expression of Kv1.4 α-subunit, but not Kv1.2 α-subunit, is associated with the reduction of slow-decaying KA currents in bladder afferent neurons from cystitis rats.
Fig. 6.
Changes in Kv α-subunit immunoreactivity after HCl-induced cystitis. A: photomicrographs of Fast Blue- (left) and Kv1.2- or 1.4-stained (right) L6 DRG sections from sham and cystitis rats. Left and right photomicrographs in each row show the same DRG section under fluorescence illumination with UV and Cy3 filters, respectively. Arrows indicate FB-labeled neuronal profiles identified in the corresponding left panel. Note that Kv1.4 α-subunit immunoreactivity of FB-labeled bladder neurons (3 cells with arrows) in a cystitis rat is weak or almost negative when compared with that in a sham rat (4 cells with arrows). B: expression levels of Kv1.4 or Kv1.2 α-subunits in L6 DRG cells from sham (white bars) and cystitis (black bars) rats. Levels are shown as the ratio of Cy3-intensity of Kv α-subunit immunoreactivity in FB-labeled cells (bladder afferent neurons) vs. FB-unlabeled cells from sham and cystitis groups. Data represent means ± SE. Kv1.2 α-subunit staining: sham (n = 144 cells) and cystitis groups (n = 135 cells). Kv1.4 α-subunit staining: sham (n = 153 cells) and cystitis groups (n = 181 cells). **P < 0.01 compared with the sham rats group. Note that a reduction in Kv1.4 α-subunit expression in FB-labeled bladder afferent neurons vs. FB-unlabeled neurons was observed in DRG neurons from cystitis and that the Kv1.2 α-subunit expression in FB-labeled bladder neurons vs. FB-unlabeled neurons was unchanged after cystitis because the ratio of ∼1.0 indicated an equal level of Kv1.2 α-subunit immunoreactivity in FB-labeled and FB-unlabeled cells.
DISCUSSION
The results of this study indicate that 1) HCl-induced cystitis resulting in bladder tissue inflammation lasting over 1 wk elicits urinary frequency due to an activation of capsaicin-sensitive C-fiber afferents pathways and is associated with decreased thresholds for C-fiber-mediated bladder sensation; 2) capsaicin-sensitive bladder afferent neurons obtained from L6-S1 DRG of HCl-induced cystitis rats shows hyperexcitability as evidenced by lower thresholds for spike activation, tonic firing pattern, and a reduction in slow-decaying KA current density; and 3) the expression of Kv1.4 α-subunits is reduced in bladder afferent neurons from HCl-induced cystitis rats. To our knowledge, this is the first report showing the association of functional and immunohistochemical changes in voltage-gated K+ channels that are responsible for hyperexcitability of afferent neurons innervating inflamed visceral organs such as the urinary bladder.
Afferent pathways innervating the urinary bladder consist of myelinated Aδ-fibers and unmyelinated C-fibers (15, 35, 54). In normal rats, conscious voiding is dependent on Aδ-fibers bladder afferents even though both Aδ-fibers and C-fibers bladder afferents pathways are mechanoceptive, whereas C-fibers afferents are responsible for bladder nociceptive responses (9, 10, 34, 48, 49). In the present study, urinary frequency observed in HCl-induced cystitis rats was suppressed by pretreatment with a high dose of capsaicin, a C-fiber neurotoxin that induces desensitization of capsaicin-sensitive C-fiber afferents, indicating that bladder inflammation over 1 wk can activate C-fiber afferent pathways to induce urinary frequency. This assumption is also supported by the present results using Neurometer CPT/C. HCl-induced cystitis rats had a significantly lowered threshold current intensity for electrical stimuli at 5 Hz applied onto the bladder luminal surface. This frequency is known to activate both C-fibers and A-fibers in somatic pathways (29). On the other hand, current thresholds for the behavioral responses induced by 250 Hz stimulation, which are known to activate Aβ- and Aδ-fiber afferents without significant excitation of C-fibers (29), were not significantly different in sham and cystitis rats. A recent study by Abouassaly et al. (2) also showed that the sensory perception thresholds at 250- and 5-Hz stimulation are useful to examine fiber-type selective responses (i.e., Aδ vs. C-fibers) in rat bladder afferent pathways. Thus, rats with HCl-induced cystitis seem to be an appropriate model of C-fiber-mediated bladder hyperactivity and hyperalgesia. In addition, although rats with repetitive administration of cyclophosphamide have frequently been used as another chronic cystitis model (28, 61), this animal model requires one-time application of HCl and exhibits histological changes including mastcytosis in the bladder. Although the robust inflammatory changes seen in rats with HCl-induced cystitis are not usually found in the human bladder of PBS/IC, the increased number of mast cells and their degranulation resulting in release of chemical mediators, such as histamine or nerve growth factor, are reportedly linked to neural changes in humans with PBS/IC (26, 51).
The present study also indicates that capsaicin-sensitive bladder afferent neurons become hyperexcitable when the bladder is irritated by intravesical HCl instillation. Our previous study showed that the majority (>80%) of bladder afferent neurons from rats that were not stained with antibody against a 200-kDa subunit of neurofilament, which is a marker of myelinated afferents, respond to capsaicin, whereas only a small proportion (5%) of neurofilament-rich Aδ-fiber bladder afferent neurons are sensitive to capsaicin (58). Thus, it is assumed that capsaicin-sensitive bladder afferent neurons mainly represent C-fiber afferent cells. The hyperexcitability of capsaicin-sensitive bladder afferent neurons characterized by lower thresholds for spike activation and tonic pattern of firing in HCl-induced cystitis rats is further evidence for enhanced C-fiber bladder afferent activity although hyperexcitability of Aδ-fiber bladder afferents cannot completely be excluded because of a tendency of a decline in Neurometer CPT values at 250-Hz stimulation, which can activate A-fiber afferent pathways without C-fiber activation, in cystitis rats.
Voltage-gated K+ currents in sensory neurons are divided into two major categories; i.e., sustained KDR currents and transient KA currents (19, 21, 56, 57, 59). Transient KA currents in sensory neurons including DRG cells can be further subdivided into at least two different subtypes based on their inactivation kinetics (i.e., fast- and slow-decaying KA currents) (3, 17–19, 37, 57). It has also been reported that the slow-decaying KA current, which was also termed the KD current by Everill et al. (17), is preferentially expressed in small-sized DRG neurons that exhibit tetrodotoxin-resistant action potentials with inflections and responded to capsaicin (19, 57, 59). We have previously reported that application of 4-aminopyridine, a blocker for the KA channel, increased excitability of bladder afferent neurons as evidenced by changes in the cell firing pattern from phasic to tonic during long-duration membrane depolarization (59). Thus, slow-decaying KA currents are likely to be involved in reducing excitability in small-sized, nociceptive C-fiber DRG neurons including bladder afferent neurons, although alterations in other mechanisms including sodium (Nav) channels (13) or TRP receptors (38) reportedly contribute to inflammation-induced afferent hyperexcitability. There is also the possibility that changes in Kv1.4 and other ion-gated channels might interact with each other to induce bladder afferent hyperexcitability.
In an earlier study, we discovered that bladder inflammation for 2 wk, which was induced by repeated systemic injections of cyclophosphamide, resulted in increased excitability of capsaicin-sensitive bladder afferent neurons due to a significant reduction in slow-decaying KA current density (59). The present study confirmed these changes in another rat model of cystitis, and further demonstrated that hyperexcitability of bladder afferent neurons was associated with bladder tissue inflammation, urinary frequency, and bladder hyperalgesia. Since we first reported the above findings of slow-decaying KA currents in bladder inflammation (59), similar results showing the close relationship between hyperexcitability of afferent pathways and a reduction in KA channel function following tissue inflammation have been reported in visceral afferent neurons innervating gastrointestinal organs, such as gastric afferent neurons from rats with gastric ulcers (14), intestinal afferent neurons from guinea pigs with ileitis (50), and pancreatic afferent neurons from rats with pancreatitis (55). Thus, the reduction in slow-decaying KA currents seems to be one of the key events resulting in hyperexcitability of C-fiber afferent neurons innervating visceral organs.
In this study, we also found that a reduction in slow-decaying KA channel activity was associated with decreased expression of Kv1.4 α-subunit in bladder afferent neurons from cystitis rats. Voltage-gated K+ channels are composed of homo- or heterotetramers of α-subunits that form K+ ion conducting pores (31, 33, 45). Over a dozen genes encoding the pore-forming α-subunits of voltage-gated K+ channels have been isolated from mammalian tissues, and have been divided into several subfamilies (20). Previous reports have indicated that Kv1 α-subunits including Kv1.1, Kv1.2, and Kv1.4 could be major components of voltage-gated K+ channels in DRG neurons. Kv1.1 and Kv1.2 α-subunits are expressed in both small- and medium/large-sized DRG neurons, whereas the expression of Kv1.4 α-subunits is predominantly seen in small-sized DRG neurons (43). In addition, Kv1.4 α-subunits and TRPV1 capsaicin receptors are highly colocalized in small-sized DRG neurons (5, 43). It is known that a homotetramer of Kv1.4 α-subunits exhibits rapid activation and prominent inactivation processes but is insensitive to α-dendrotoxin (DTX), a specific inhibitor for Kv1.1 and Kv1.2 (23). However, previous studies have demonstrated that heteromeric channels containing Kv1.4 and DTX-sensitive Kv1.1/Kv1.2 α-subunits are also toxin sensitive (12, 44) and that these heteromeric complexes exhibit inactivation much slower than the Kv1.4 homomeric channels, reminiscent of slow-decaying KA in DRG neurons (42). We have previously reported that DTX partially suppressed slow-decaying KA currents in capsaicin-sensitive, small-sized DRG neurons (56). Thus, it seems reasonable to assume that assembly with Kv1.4 and other DTX-sensitive Kv α-subunits, such as Kv1.1 and/or Kv1.2, contributes to formation of slow-decaying KA channels in capsaicin-sensitive C-fiber afferent neurons, including those innervating the urinary bladder. Although we did not examine whether reduced Kv1.4 expression in bladder afferent neurons occurs in C- or Aδ-fiber cells following bladder inflammation, high capsaicin sensitivity of C-fiber bladder afferent neurons (58) and the significant overlap of Kv1.4 and TRPV1 expression in small-sized DRG neurons (5, 43) suggest that changes in Kv1.4 expression is more likely to occur in capsaicin-sensitive C-fiber bladder afferent neurons than in Aδ-fiber bladder neurons, although there is a possibility that Kv1.4 expression in the small number of capsaicin-sensitive Aδ-fiber bladder afferent neurons is also decreased after bladder inflammation. Taken together, it seems reasonable to assume that reduced expression of Kv1.4 α-subunits in bladder afferent neurons following bladder inflammation is a molecular mechanism responsible for the reduction in slow-decaying KA currents and the resulting hyperexcitability of capsaicin-sensitive C-fiber bladder afferent neurons and C-fiber-mediated bladder hyperactivity/hyperalgesia in rats with HCl-induced cystitis.
PBS/IC is a debilitating chronic disease characterized by suprapubic pain related to bladder filling, coupled with urinary frequency, without proven urinary infection or other obvious pathology (8, 16, 41, 60). While the etiology is unknown, theories explaining the pathophysiology of PBS/IC include an altered urothelial barrier, neurogenic inflammation including mast cell infiltration and afferent sensitization (6, 7, 36, 61). A recent study using cats with naturally occurring feline-type IC has demonstrated that capsaicin-sensitive DRG neurons exhibited an increase in cell size and had increased firing rates to depolarizing current pulses due to a reduction in low-threshold K+ currents elicited by membrane depolarization between −50 to −30 mV (46), as similarly found in our present and previous studies using cystitis rats. Therefore, the reduction in KA currents due to decreased Kv1.4 α-subunit expression resulting in afferent hyperexcitability might be involved in the pathogenesis of visceral hypersensitive disorders, such as PBS/IC, although further studies are needed to clarify this point. In our preliminary results (data not shown), a reduction of Kv1.4 expression in L6-S1 DRG using intrathecal application of small-interfering RNA induces frequent voiding in normal rats (22). Overall, voltage-gated K+ channels composed of Kv1.4 α-subunits might represent an interesting target for the development of new antinociceptive drugs that can activate or facilitate slow-decaying KA channels and thereby reduce the symptoms of PBS/IC.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK-057267, R01-DK-068557, P01-HD-039768, and P01-DK-044935.
REFERENCES
- 1.Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol 85: 644–658, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Abouassaly R, Liu G, Yamada Y, Ukimura O, Daneshgari F. Efficacy of a novel device for assessment of autonomic sensory function in the rat bladder. J Urol 179: 1167–1172, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akins PT, McCleskey EW. Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience 56: 759–769, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Bauis G Technology review: the Neurometer current perception threshold (CPT). AAEM Equipment and Computer Committee American Association of Electrodiagnostic Medicine. Muscle Nerve 22: 523–531, 1999. [PubMed] [Google Scholar]
- 5.Binzen U, Greffrath W, Hennessy S, Bausen M, Saaler-Reinhardt S, Treede RD. Co-expression of the voltage-gated potassium channel Kv1.4 with transient receptor potential channels (TRPV1 and TRPV2) and the cannabinoid receptor CB1 in rat dorsal root ganglion neurons. Neuroscience 142: 527–539, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Brookoff D, Bennett DS. Neuromodulation in intractable interstitial cystitis and related pelvic pain syndromes. Pain Med 7, Suppl 1: S166–S184, 2006. [Google Scholar]
- 7.Chai TC, Keay S. New theories in interstitial cystitis. Nat Clin Pract Urol 1: 85–89, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Chancellor MB, Yoshimura N. Treatment of interstitial cystitis. Urology 63: 85–92, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CL, Ma CP, de Groat WC. Effects of capsaicin on micturition and associated reflexes in rats. Am J Physiol Regul Integr Comp Physiol 265: R132–R138, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Chuang YC, Fraser MO, Yu Y, Beckel JM, Seki S, Nakanishi Y, Yokoyama H, Chancellor MB, Yoshimura N, de Groat WC. Analysis of the afferent limb of the vesicovascular reflex using neurotoxins, resiniferatoxin and capsaicin. Am J Physiol Regul Integr Comp Physiol 281: R1302–R1310, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Clemens JQ, Brown SO, Kozloff L, Calhoun EA. Predictors of symptom severity in patients with chronic prostatitis and interstitial cystitis. J Urol 175: 963–966, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Cooper EC, Milroy A, Jan YN, Jan LY, Lowenstein DH. Presynaptic localization of Kv 1.4-containing A-type potassium channels near excitatory synapses in the hippocampus. J Neurosci 18: 965–974, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain 131: 243–257, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang K, Bielefeldt K, Gebhart GF. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am J Physiol Gastrointest Liver Physiol 286: G573–G579, 2004. [DOI] [PubMed] [Google Scholar]
- 15.de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor KT. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst 3: 135–160, 1981. [DOI] [PubMed] [Google Scholar]
- 16.Evans RJ Treatment approaches for interstitial cystitis: multimodality therapy. Rev Urol, 4 Suppl 1: S16–S20, 2002. [PMC free article] [PubMed] [Google Scholar]
- 17.Everill B, Rizzo MA, Kocsis JD. Morphologically identified cutaneous afferent DRG neurons express three different potassium currents in varying proportions. J Neurophysiol 79: 1814–1824, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol 82: 700–708, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol 75: 2629–2646, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev 57: 473–508, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Hall A, Stow J, Sorensen R, Dolly JO, Owen D. Blockade by dendrotoxin homologues of voltage-dependent K+ currents in cultured sensory neurones from neonatal rats. Br J Pharmacol 113: 959–967, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi Y, Takimoto K, Sasatomi K, Chancellor MB, de Groat WC, Yoshimura N. Reduced expression of Kv1.4 potassium channel α-subunits by siRNA induces bladder overactivity in rats (Abstract). 35th Society for Neuroscience Annual Meeting, Washington, DC. Program No. 48.1, 2005.
- 23.Hopkins WF Toxin and subunit specificity of blocking affinity of three peptide toxins for heteromultimeric, voltage-gated potassium channels expressed in Xenopus oocytes. J Pharmacol Exp Ther 285: 1051–1060, 1998. [PubMed] [Google Scholar]
- 24.Ishikawa K, Tanaka M, Black JA, Waxman SG. Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve 22: 502–507, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Kim DS, Choi JO, Rim HD, Cho HJ. Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res Mol Brain Res 105: 146–152, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Kirimoto T, Nakano K, Irimura K, Hayashi Y, Matsuura N, Kiniwa M, Oka T, Yoshimura N. Beneficial effects of suplatast tosilate (IPD-1151T) in the rat cystitis model induced by intravesical hydrochloric acid. BJU Int 100: 935–939, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Kiso T, Nagakura Y, Toya T, Matsumoto N, Tamura S, Ito H, Okada M, Yamaguchi T. Neurometer measurement of current stimulus threshold in rats. J Pharmacol Exp Ther 297: 352–356, 2001. [PubMed] [Google Scholar]
- 28.Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F1778–F1789, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain 1: 13, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kostyuk PG, Veselovsky NS, Fedulova SA, Tsyndrenko AY. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons. III. Potassium currents. Neuroscience 6: 2439–2444, 1981. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Unwin N, Stauffer KA, Jan YN, Jan LY. Images of purified Shaker potassium channels. Curr Biol 4: 110–115, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Um SY, McDonald TV. Voltage-gated potassium channels: regulation by accessory subunits. Neuroscientist 12: 199–210, 2006. [DOI] [PubMed] [Google Scholar]
- 33.MacKinnon R Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature 350: 232–235, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Maggi CA, Conte B. Effect of urethane anesthesia on the micturition reflex in capsaicin-treated rats. J Auton Nerv Syst 30: 247–251, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Mallory B, Steers WD, de Groat WC. Electrophysiological study of micturition reflexes in rats. Am J Physiol Regul Integr Comp Physiol 257: R410–R421, 1989. [DOI] [PubMed] [Google Scholar]
- 36.Mayer R Interstitial cystitis pathogenesis and treatment. Curr Opin Infect Dis 20: 77–82, 2007. [DOI] [PubMed] [Google Scholar]
- 37.McFarlane S, Cooper E. Kinetics and voltage dependence of A-type currents on neonatal rat sensory neurons. J Neurophysiol 66: 1380–1391, 1991. [DOI] [PubMed] [Google Scholar]
- 38.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Oda M, Kitagawa N, Yang BX, Totoki T, Morimoto M. Quantitative and fiber-selective evaluation of dose-dependent nerve blockade by intrathecal lidocaine in rats. J Pharmacol Exp Ther 312: 1132–1137, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Pearce RJ, Duchen MR. Differential expression of membrane currents in dissociated mouse primary sensory neurons. Neuroscience 63: 1041–1056, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Phatak S, Foster HE Jr. The management of interstitial cystitis: an update. Nat Clin Pract Urol 3: 45–53, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Po S, Roberds S, Snyders DJ, Tamkun MM, Bennett PB. Heteromultimeric assembly of human potassium channels: molecular basis of a transient outward current? Circ Res 72: 1326–1336, 1993. [DOI] [PubMed] [Google Scholar]
- 43.Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci USA 98: 13373–13378, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhodes KJ, Kelibaugh SA, Barrezueta NX, Lopez KL, Trimmer JS. Association and colocalization of K+ channel α- and β-subunit polypeptides in rat brain. J Neurosci 15: 5360–5371, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salkoff L, Baker K, Butler A, Covarrubias M, Pak MD, Wei A. An essential “set” of K+ channels conserved in flies, mice and humans. Trends Neurosci 15: 161–166, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Sculptoreanu A, de Groat WC, Buffington CA, Birder LA. Abnormal excitability in capsaicin-responsive DRG neurons from cats with feline interstitial cystitis. Exp Neurol 193: 437–443, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol 171: 478–482, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 72: 2420–2430, 1994. [DOI] [PubMed] [Google Scholar]
- 49.Shea VK, Cai R, Crepps B, Mason JL, Perl ER. Sensory fibers of the pelvic nerve innervating the rat's urinary bladder. J Neurophysiol 84: 1924–1933, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Stewart T, Beyak MJ, Vanner S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J Physiol 552: 797–807, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology 57: 47–55, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol 66: 477–519, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Urban L, Campbell EA, Panesar M, Patel S, Chaudhry N, Kane S, Buchheit K, Sandells B, James IF. In vivo pharmacology of SDZ 249–665, a novel, non-pungent capsaicin analogue. Pain 89: 65–74, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Vera PL, Nadelhaft I. Conduction velocity distribution of afferent fibers innervating the rat urinary bladder. Brain Res 520: 83–89, 1990. [DOI] [PubMed] [Google Scholar]
- 55.Xu GY, Winston JH, Shenoy M, Yin H, Pasricha PJ. Enhanced excitability and suppression of A-type K+ current of pancreas-specific afferent neurons in a rat model of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 291: G424–G431, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Yang EK, Takimoto K, Hayashi Y, de Groat WC, Yoshimura N. Altered expression of potassium channel subunit mRNA and α-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neuroscience 123: 867–874, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura N, White G, Weight FF, de Groat WC. Different types of Na+ and A-type K+ currents in dorsal root ganglion neurones innervating the rat urinary bladder. J Physiol 494: 1–16, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshimura N, Erdman SL, Snider MW, de Groat WC. Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience 83: 633–643, 1998. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19: 4644–4653, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology 59: 61–67, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Yoshimuira N, Birder LA. Interstitial cystitis and related painful bladder syndromes: pathophysiology. In: Chronic Abdominal and Visceral Pain: Theory and Practice, edited by Pasricha PJ, Willis WD, and Gebhart GF. New York: Informa Healthcare USA, 2007, sect. V, chapt. 33, p. 495–520.