Abstract
Intracerebroventricular (ICV) infusion of NaCl mimics the effects of a high-salt diet in salt-sensitive hypertension, raising the sodium concentration in the cerebrospinal fluid (CSF [Na]) and subsequently increasing the concentration of an endogenous ouabain-like substance (OLS) in the brain. The OLS, in turn, inhibits the brain Na+-K+-ATPase, causing increases in the activity of the brain renin-angiotensin system (RAS) and blood pressure. The Na+-K+-ATPase α (catalytic)-isoform(s) that mediates the pressor response to increased CSF [Na] is unknown, but it is likely that one or more isoforms that bind ouabain with high affinity are involved (e.g., the Na+-K+-ATPase α2- and/or α3-subunits). We hypothesize that OLS-induced inhibition of the α2-subunit mediates this response. Therefore, a chronic reduction in α2 expression via a heterozygous gene knockout (α2 +/−) should enhance the pressor response to increased CSF [Na]. Intracerebroventricular (ICV) infusion of artificial CSF containing 0.225 M NaCl increased mean arterial pressure (MAP) in both wild-type (+/+) and α2 +/− mice, but to a greater extent in α2 +/−. Likewise, the pressor response to ICV ouabain was enhanced in α2 +/− mice, demonstrating enhanced sensitivity to brain Na+-K+-ATPase inhibition per se. The pressor response to ICV ANG I but not ANG II was also enhanced in α2 +/− vs. α2+/+ mice, suggesting an enhanced brain RAS activity that may be mediated by increased brain angiotensin converting enzyme (ACE). The latter hypothesis is supported by enhanced ACE ligand binding in the organum vasculosum laminae terminalis. These studies demonstrate that chronic downregulation of Na+-K+-ATPase α2-isoform expression by heterozygous knockout increases the pressor response to increased CSF [Na] and activates the brain RAS. Since these changes mimic those produced by the endogenous brain OLS, the brain α2-isoform may be a target for the brain OLS during increases in CSF [Na], such as in salt-dependent hypertension.
Keywords: α2-isoform, gene knockout, sodium chloride, brain ouabain-like substance, brain renin-angiotensin system, intracerebroventricular infusion
na+-k+-atpase is critically important for cardiovascular function since it is crucial in the production of electrochemical gradients for sodium and potassium across the plasma membranes of cells in the heart, kidney, blood vessels, and brain. The functional enzyme complex consists of at least one α- and one β-protein (e.g., αβ or 2α2β32), and it mediates the transport of sodium (against its gradient) from the cell cytosol to the extracellular fluid in exchange for potassium in the opposite direction (also against its gradient). In each catalytic cycle there are three Na+ ions expelled from the cell and two K+ ions internalized, with the impetus provided by ATP. The α-protein is the catalytic domain (2, 10, 40, 44) and also contains critical determinants of ouabain binding. The β-subunit is required for processing the α-protein into the plasma membrane. Another protein (γ-subunit) may play a regulatory role (1, 4, 63).
Multiple versions of both the α- and β-subunits exist (α1-4 and β1-3, respectively) (3, 45, 48, 49). Each α- and β-isoform has a separate gene and a separate pattern of tissue expression. However, the reason(s) for the existence of the different isoforms and their exact roles has not been established. The α1-protein is found in all cells of higher eukaryotes, while the pattern of expression of the α2-isoform is more limited, i.e., the central nervous system (CNS), muscle [including skeletal and vascular smooth muscle (43, 46, 61)], and heart. The α3-isoform is most abundant in the brain (8, 18, 19, 34, 58) with expression in neurons. The α4-protein is testis specific (45). In rodents, the α1-subunit is highly resistant to ouabain (38). In contrast, the affinities of the α2- and α3-isoforms for ouabain are much greater than that of the α1-protein.
Brain Na+-K+-ATPase inhibition plays a critical role in salt-dependent hypertension [e.g., Dahl salt-sensitive (Dahl-S) and spontaneously hypertensive rats (SHR) on a high-salt diet]. Fab fragments of an anti-ouabain antibody (Fab) given intracerebroventricularly (ICV) completely abolish/reverse the sympathetic hyperactivity and hypertension that are associated with a high-salt diet in both Dahl-S and SHR (24, 25, 26). The Fab presumably bind an endogenous brain ouabain-like substance (OLS) and prevent it from inhibiting the brain Na+-K+-ATPase. No effects are seen when the Fab are given intravenously (IV) instead of ICV, ruling out peripheral effects that could be due to leakage/diffusion of the Fab from the CNS.
It has been previously shown that increases in cerebrospinal fluid sodium concentration (CSF [Na]) precede hypertension in both Dahl-S and SHR on a high-salt diet, suggesting that increased CSF sodium levels are important in the etiology of salt-dependent hypertension (28). In further support of this concept, raising CSF [Na] by directly administering Na-rich artificial CSF (aCSF-HiNa), ICV has effects that are identical to a high-salt diet in Dahl-S and SHR, increasing the brain OLS concentration, activating the brain renin-angiotensin system (RAS) and increasing sympathetic activity and blood pressure (22, 29, 30).
We have shown that ICV aCSF-HiNa infusion in mice causes elevations in blood pressure and heart rate that are mediated by an endogenous brain OLS and RAS, also mimicking the effects of a high-salt diet in salt-sensitive rat strains (51). The development of a mouse model with pressor responses to CSF sodium permits a new experimental approach, using gene-targeted mice, to further examine the mechanisms of the CNS response to sodium in the CSF. For example, due to the lack of an available isoform-specific antagonist, it is not known which isoform of the Na+-K+-ATPase mediates the brain RAS activation and the increase in blood pressure in response to increased CSF [Na]. However, partial or complete downregulation of a specific isoform can be achieved by gene knockout. We hypothesize that inhibition of the brain Na+-K+-ATPase α2-isoform (by the OLS) causes the increases in brain RAS activity and blood pressure that occur in response to increased CSF [Na]. Because the inhibition of the brain Na+-K+-ATPase (by the brain OLS or ICV ouabain) stimulates the brain RAS and increases blood pressure, we further postulate that a chronic reduction in the expression of the α2-isoform should also stimulate the brain RAS and enhance the pressor response. These hypotheses are tested in α2 heterozygous knockout mice in the present study.
MATERIALS AND METHODS
Maintenance of the α2 Na+-K+-ATPase Knockout Mouse Line and Genotyping
All animal studies were carried out in agreement with guidelines established by the University of Ottawa Animal Care Committee and were approved by this Committee. Mice were housed in group cages in a temperature-controlled environment with a 12:12-h light-dark cycle and were given standard mouse chow and water ad libitum. A full description of the development of the α2 Na+-K+-ATPase knockout mouse line has been published previously (31). The homozygous knockout (−/−) is lethal in the late embryonic/early neonatal period, while the heterozygous (α2 +/−) animals are viable, with normal baseline cardiovascular function (17, 31). Thus, in the present study α2 +/− mice were mated with wild-type mice (+/+) to produce +/+ controls and α2 +/− offspring in a 1-to-1 ratio.
Genotyping was performed by PCR of DNA isolated from a tail sample that was obtained at age 3 wk, using a previously described method (14). DNA was extracted from each sample via a DNeasy kit from Qiagen (Mississauga, ON, Canada). The PCR assay took advantage of the exogenous hypoxanthine phosphoribosyltransferase (HPRT) gene that had been inserted into the α2 gene to create the null allele of the heterozygous mice. To identify the null allele, a sense primer (5′-CCTCTACCACGCGTCCTAG-3′) that binds to its complementary sequence in the α2 gene and an antisense primer (5′-CCTACCCGCTTCCATTGCTC-3′) that anneals to the HPRT cassette were used. Since no band is produced by this assay in +/+ mice (no HPRT gene), a second set of primers (5′-TCCTCAAAGATGCTCATTAG-3′ and 5′-GTAACTCACTCATGCAAAGT-3′) was included to amplify part of the coding sequence for the endogenous thyroid-stimulating hormone-β chain (TSHβ), to ensure that the absence of a PCR product from the +/+ mice in the first reaction was the result of the absence of the null allele and was not due to the lack of DNA in the tail sample or poor DNA quality. The PCR reaction was carried out as follows: an initial denaturation at 94°C was performed for 4 min, followed by 40 cycles of 94°C, 57°C, and 72°C (45 s at each temperature), and a final extension at 72°C for 5 min.
Surgery for Cardiovascular Studies
Studies were carried out according to procedures that have been previously described in detail elsewhere (51). Briefly, mice aged 8–10 wk were anesthetized with isoflurane, and a line of antithrombogenic tubing (ID 0.025, OD 0.040 in.; Braintree Scientific, Braintree, MA) was implanted in a carotid artery, anchored with sutures, and exteriorized at the nape of the neck. In some mice, the same type of cannula was also placed into an internal jugular vein for IV infusions. The animals were placed in a stereotaxic frame, the head was leveled, and a guide cannula was implanted (stereotaxic coordinates: 0.1 mm anterior, 1.0 mm lateral, and 1.5 mm ventral to lambda) and cemented in place with dental acrylic. Animals were allowed to recover for 26 h after the completion of surgery, during which time the guide cannula was kept plugged. Following the recovery period, mice were randomized to one of the two study protocols described below. ICV infusions were performed using an injection pipette that was constructed to protrude 1.0 mm beyond the end of the guide cannula. Blood pressure and heart rate were monitored in each animal via the carotid cannula both before and during infusions in conscious mice, as described below.
Protocol 1: Blood Pressure and Heart Rate Responses to ICV Infusions of Na and Ouabain
Blood pressure and heart rate responses to ICV infusions of artificial CSF (aCSF) with increased [NaCl] or with exogenous ouabain were determined. aCSF containing each substance was given in separate mice at a flow rate of 0.4 μl/min for 60 min. The composition of aCSF (pH 7.0) was (in mM/l): 117 NaCl, 2.5 KCl, 0.65 NaH2PO4·2H2O, 2.27 NaH2PO4·7H2O, 0.5 Na2SO4, 2.14 MgCl2·6H2O, 1.0 CaCl2, and 27 NaHCO3. When given at this rate, aCSF per se produces no effects on blood pressure or heart rate (51). Baseline blood pressures and heart rates were recorded for 10 min before each infusion.
In one group of animals, aCSF containing 0.225 M NaCl (aCSF-HiNa) was infused ICV for 60 min. In a separate group of mice, aCSF containing ouabain (aCSF-ouabain, 5 ng/min for 60 min) was given ICV at the same flow rate. To confirm that the cardiovascular responses to ICV ouabain were not due to peripheral effects caused by leakage/diffusion of ouabain from the CNS, the same dose was also infused IV instead of ICV in separate mice. Amounts of ICV sodium that are over 2.6-fold greater than that used in the present study were previously shown to be without effect on blood pressure and heart rate when they are given IV instead of ICV (51). In other animals, norepineprhine (NE) was dissolved in 0.9% saline containing 1 mM ascorbic acid and was given IV at 256 ng/min for 30 min.
Protocol 2: Biochemical Measurements
For baseline biochemical analyses, 8- to 10-wk-old unoperated/untreated mice were euthanized with CO2 and the brains were immediately removed, frozen in liquid nitrogen, and stored at −80°C. The frozen tissue was partially thawed at a later date and dissected to isolate the hypothalamus and combined pons/medulla. Tissue from a separate group of mice treated with ICV aCSF-HiNa was processed in the identical fashion for postinfusion OLS analysis.
Na+-K+-ATPase Isoform Expression
Microsomal membrane preparation.
All steps were performed at 4°C. Tissue samples were homogenized by sonication in 1 ml of ice-cold SH buffer (0.32 M sucrose, 5 mM histidine, pH 7.4) per 100 mg of tissue. Each sample was centrifuged at 1,000 g for 10 min. The supernatant was transferred to a new tube, and the pellet was resuspended in SH buffer and recentrifuged at 1,000 g for another 10 min. Both supernatants were combined and centrifuged at 10,000 g for 20 min. The supernatants were then transferred to new tubes and centrifuged at 100,000 g for 1 h. Final pellets were resuspended in SH buffer and the resuspension was aliquoted and frozen at −80°C until the time of assay.
Western blot analysis.
The above microsomal preparations were used to quantify the protein expression levels of α1, α2, and α3 Na+-K+-ATPase subunits. Samples were diluted in Laemmli buffer containing β-mercaptoethanol and loaded onto an 8% denaturing polyacrylamide gel. Two micrograms of total protein was loaded for the α1- and α2 assays and 0.2 μg protein was used for α3. After electrophoresis and transfer of proteins to a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA), each α-isoform was probed with an appropriate antibody. For α1, α6F (stock diluted 1:3,000, University of Iowa Developmental Studies Hybridoma Bank, Iowa City, IA) was used; for α2, McB2 (stock diluted 1:3,000, obtained from Dr. Kathleen Sweadner) was used; and for α3, MA3-915 (1 mg/ml diluted 1:30,000; Affinity Bioreagents, Golden, CO) was used. As a loading control, brain tissue was also probed for α-tubulin, using a 1:20,000 dilution of stock B-1-2-5 mAb (Sigma). For all primary antibodies used, biotinylated sheep anti-mouse Ig (0.5 mg/ml diluted 1:5,000, RPN1001; Amersham, Piscataway, NJ) was used as a secondary antibody, and the membranes were subsequently incubated in horseradish peroxidase-streptavidin (0.5 mg/ml diluted 1:5,000, RPN1001; Amersham). In all blots, three different concentrations of the same sample were used to make a standard curve to which all other samples in the same blot were standardized. To normalize for loading, all samples were expressed as a ratio of α2-isoform/α-tubulin density.
ELISA for Brain Concentration of OLS
Extraction of OLS from brain tissue.
Samples for OLS assays were protected from light at all times. Briefly, brain tissue samples from separate mice were homogenized in methanol (1 ml/100 mg of tissue) containing 2 mM l-ascorbic acid. The samples were centrifuged and the supernatants were transferred to a new tube and dried overnight by vacuum centrifugation. The next day, samples were reconstituted and deproteinized in 1 ml of 0.1% trifluroacetic acid for 3 h at room temperature. After centrifugation, OLS was collected by passing the supernatants through C18 columns (Sep-Pak Vac; Waters, Milford, MA) and eluting with 3 ml of 25% acetonitrile, after washing three times with 3 ml of H2O and once with 3 ml 2.5% acetonitrile. The samples were again dried overnight by vacuum centrifugation. On the day of the assay, samples were resuspended in PBS (150 mM NaCl, 10 mM NaH2PO4-H2O, 10 mM Na2HPO4, pH 7.4) with 0.05% Tween-20 (PBS-T).
OLS ELISA.
All samples were assayed with the same basic ELISA procedure and each assay was performed with an approximately equal number of α2 +/− and wild-type samples ( ± one extra sample in either group). For baseline samples (from unoperated mice), the wells were coated with a digoxin-BSA conjugate, the primary antibody was Digibind (GlaxoSmithKline) and the secondary antibody was a horseradish peroxidase-conjugated F(ab′)2-specific anti-sheep IgG secondary antibody (cat. no. 313-036-047; Jackson ImmunoResearch, West Grove, PA). For assays of samples that were obtained post-ICV aCSF-HiNa infusion, the wells were coated with a ouabain-ovalbumin conjugate, a specific rabbit anti-ouabain antibody was the primary antibody, and the secondary antibody was anti-rabbit IgG secondary antibody, as previously described (30, 55, 57). Specific concentrations of antibodies and conjugates and/or amounts used are given below.
Three days prior to the assay, the wells of a 96-well microplate were coated with 200 μl of either a ouabain-ovalbumin (0.6 ng/ml) or a digoxin-BSA (0.1 μg/μl) conjugate that had been diluted in a sodium carbonate buffer (35 mM NaHCO3, 15 mM Na2CO3, pH 9.6). After the addition of conjugates to wells, plates were stored up to 3 days at 4°C. The day before the assay, the coating solution was removed, and the wells were washed five times with 400 μl of PBS-T (each wash was incubated for 10 min at 25°C on a horizontal shaker) and then blocked overnight with 5% milk in PBS-T. On the day of the assay, the plate was again washed, and 100 μl of sample or ouabain standard was added to separate wells. The ouabain standard curve consisted of serial twofold dilutions ranging from 128 to 0.125 ng/ml. Also, 100 μl of either a rabbit anti-ouabain primary antibody (55, 57) (1:160,000) or Digibind (0.001 mg/ml) was added to each well. After a 1-h incubation at 25°C and subsequent washing, 200 μl of either an anti-rabbit IgG (0.0008 mg/ml = 1:1,000 dilution) or of an anti-sheep IgG Fab fragment secondary antibody (0.002 mg/ml = 1:250) was added to each well. Following an additional 1-h incubation at 25°C and subsequent washing, 200 μl of 3,3′,5,5′-tetramethylbenzidine (Sigma) was added as a colorimetric substrate, and the reaction was incubated for 15 min at 25°C. The reaction was stopped by adding 50 μl of 2 M H2SO4; thereafter, absorbances were measured at an emission wavelength of 450 nm.
Since ouabain is a steroid, the reactivities of various endogenous steroids were determined for the version of the assay that used Digibind (cross-reactivities for the specific anti-ouabain antibody that was also used here, have been previously reported) (55, 57). For these determinations, a concentration of 0.55 μmol/l (which is near the middle of the standard curve for ouabain) of each substance was assayed in duplicate, and the change in absorbance was recorded and extrapolated to a ouabain standard curve. The calculated concentration, based on the change in absorbance for each compound was (% of ouabain): ouabain (100%), cortisol (0.6%), corticosterone (1.7%), aldosterone (3.4%), testosterone (0.8%), progesterone (3.3%), estradiol (2.2%), cholesterol (6.5%), and marinobufagenin (24%). In addition, the affinities of Digibind per se for various cardenolides and bufodienolides have been previously reported (41).
Brain RAS Function
Crude homogenate preparation for angiotensin converting enzyme assays.
Hypothalami and pons/medullae from unoperated/untreated mice were sonicated in Tris-NaCl buffer (50 mM Tris, 150 mM NaCl, pH 7.4). Samples were then centrifuged at 16,000 g for 15 min at 4°C. The supernatants were transferred to new tubes and were frozen at −80°C until the time of assay.
Angiotensin converting enzyme activity assay.
An angiotensin converting enzyme (ACE) activity assay was performed as previously described, with slight modifications (38). Briefly, ACE activity in the crude homogenates was measured as the amount of captopril-inhibitable histidine-leucine produced from the cleavage of hippuryl-histidine-leucine (cat. no. H-4884; Sigma) at 37°C. The amount of histidine-leucine produced was determined by fluorimetry (480-nm emission wavelength) after conjugation of the dipeptide to o-phthaldialdehyde (cat. no. P-0657; Sigma) and excitation at 360-nm wavelength. Histidine-leucine (0–15 μM, cat. no. H-2504; Sigma) was used as a standard. All samples were incubated either with or without 0.1 mM captopril to distinguish total proteolytic activity from captopril-inhibitable activity (i.e., ACE activity). Finally, ACE activity was expressed as nmol histidine-leucine produced per minute per gram protein (equivalent to mU/g protein).
Response to ICV ANG I and II.
Additional studies examined the function of the brain RAS as relates to central cardiovascular regulation. This was done by determining the pressor responses to ICV ANG I both before and after captopril. Pressor/depressor doses for sustained ICV infusion of ANG I/captopril have not been reported previously in mice and were determined experimentally in C57Bl/6 mice prior to carrying out these studies (data not shown). ANG I was administered ICV over a 35-min period on each occasion (275 ng/min). Fifteen minutes after the end of the first ANG I infusion, an ICV captopril infusion (150 ng/min) was given over a 10-min period. Next, a 15-min recovery period ensued, followed by a second ICV infusion of the same dose of ANG I. Baseline data were collected for 10 min immediately prior to the start of each ANG I infusion.
ANG II was given as a single infusion in separate mice at a rate of 150 ng/min for 30 min. This rate was predetermined to cause an increase in blood pressure that was similar in magnitude (∼20 mmHg) to that for ANG I in α2 +/− mice.
ACE ligand binding.
These methods have been described in detail previously (50). Briefly, brains were frozen in 2-methylbutane at −40°C and were subsequently stored in a freezer at −80°C. Serial frozen brain sections (20-μm-thick) were cut and mounted onto microscope slides and were stored at −80°C. At the time of the binding assay, the slides were incubated in a 10-mM phosphate buffer (pH 7.4) containing 0.3 μCi/ml (30 pM) of 125I-labeled 351A (125I-351A, a derivative of lisinopril) and 0.2% BSA. The ligand concentration was calculated to cause maximal binding for assessment of actual binding densities. Nonspecific binding was determined by including 100 mM EDTA in the incubation buffer, which abolishes the 125I-351A binding.
After four washes, the slides were dried and subsequently exposed to autoradiography film (Eastman Kodak, Rochester, NY) for 48 h, along with a set of methylacrylate 125I standards (Washington State University Peptide Radioiodination Service Center). The film was processed in a Kodak X-OMAT automatic developer. 125I-351A binding densities were quantified using a computerized image analysis system (model AIS/C; Imaging Research, St. Catharines, ON, Canada) and converted to femtomoles per gram by comparison with the 125I standards. Specific binding density was calculated as total binding minus nonspecific binding, which was < 5% of specific binding.
Confirmation of lateral ventricular cannula position.
Mice were euthanized with pentobarbital sodium (90 mg/kg) at the termination of each study. After an ICV injection of a bolus of 1% India ink (500 nl), each mouse was perfused via the thoracic aorta with 20 ml of cold isotonic saline and subsequently with 20 ml of 4% paraformaldehyde in normal saline. Perfusions were not performed in animals for which brain tissue was required for biochemical analyses. Brains were then removed and soaked in the same paraformaldehyde solution overnight. The brains were allowed to equilibrate in 30% sucrose at 4°C, and the staining of the ventricular system was examined.
Data Analysis
Comparisons of multiple repeat data points over time between two groups were carried out by one-way ANOVA for repeated measures, e.g., consecutive blood pressure or heart rate changes from baseline in Na-, ouabain- or ANG I-treated mice. Once a difference was detected by the ANOVA, pairwise a posteriori comparisons were performed using a Bonferroni t-test (54). Comparisons among groups of single measurements (e.g., biochemical data and baseline blood pressures or baseline heart rates) were made by ANOVA. Post hoc pairwise comparisons (e.g., male +/− vs. male +/+) were carried out by Student-Newman-Keuls tests.
RESULTS
Na+-K+-ATPase α-Isoform Expression
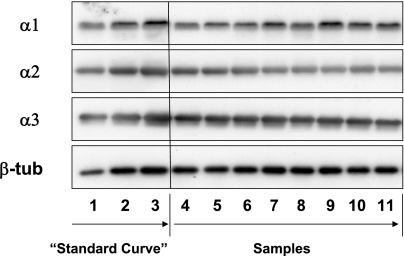
Western blot analyses of the Na+-K+-ATPase α-isoforms were performed, to determine the expression levels of the gene-targeted (α2) and off-target (α1 and α3) Na+-K+-ATPase α-subunits in the brains of adult α2 +/− and +/+ mice. Representative Western blots of each isoform are shown in Fig. 1. To standardize the results from one sample blot to another, a three-point standard curve of the same sample was loaded in the first three lanes of all blots.
Fig. 1.
Representative Western blots of hypothalamic homogenates are shown for each brain Na+-K+-ATPase α-isoform and β-tubulin (β-tub). The first 3 lanes of all Western blots were loaded with 1, 2, and 3 μg (α1 and α2 blots) or 0.1, 0.2, and 0.3 μg (α3 blots) of a reference sample. Starting with lane 4, samples were loaded in the order: male wild-type (+/+), male α2 +/−, female +/+, female α2 +/−, male +/+, male α2 +/−, etc. The staggering of group samples was performed to minimize bias due to potential variations from one region of a blot to another. A total of 20 samples from either hypothalamus or pons/medulla were loaded on each blot.
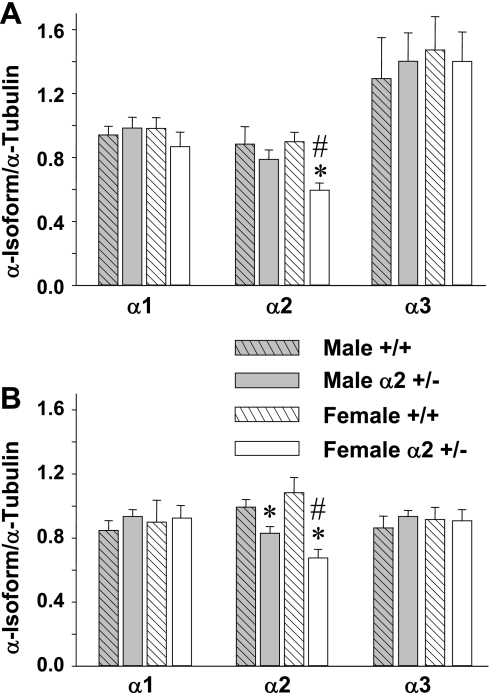
Na+-K+-ATPase α-isoform expression was examined by Western blot analysis of tissue homogenates from two major brain regions that contain critical cardiovascular regulatory centers, the hypothalamus and the pons/medulla. In the hypothalamus (Fig. 2A), female α2 +/− mice had a 34% decrease in the α2 level vs. female +/+ (P < 0.001), whereas expression in male +/− tended to decrease only slightly (−10% vs. male +/+, P = NS). A similar pattern was found in the pons/medulla (Fig. 2B), where α2 expression in +/− mice was decreased by 38% in females (P < 0.001 vs. +/+) and by 16% in males (P < 0.05). In both the hypothalamus and pons/medulla, the percent decrease in α2 expression from wild-type in female +/− mice was significantly greater than that in male +/− (P < 0.05). There were no gender differences in wild-type α2 expression. In both brain regions, there were no significant differences between α2 +/− and +/+ mice in the expression of either the α1- or α3-isoforms, and there were no gender differences in the expression of these two subunits (Figs. 2, A and B).
Fig. 2.
Summary of results from Western blot analyses of α-isoforms of Na+-K+-ATPase in homogenates from the hypothalamus (A) and pons/medulla (B) of 8- to 10-wk-old mice. The relative protein level for each sample is expressed as the ratio of α-isoform: α-tubulin band densities. Data from two different blots were pooled for each group, using 3 different concentrations of an identical standard on each blot for standardization. Values are means ± SE, n = 8–12 animals per group. *P < 0.05 male α2 +/− vs. male +/+ or female α2 +/− vs. female +/+. #P < 0.05 for female α2 +/− vs. male α2 +/−.
ICV infusion of aCSF-HiNa
We have previously shown that the pressor response to increased CSF [Na] in mice is mediated by a brain OLS that inhibits the Na+-K+-ATPase (51). Therefore, we reasoned that a decrease in the expression of the same Na+-K+-ATPase α-isoform that is involved in the pressor response to sodium in the CSF should result in an enhanced response to ICV Na infusion. We further postulated that the α2-isoform mediates this response and compared the pressor effect of ICV aCSF-HiNa in α2 +/− vs. +/+ mice.
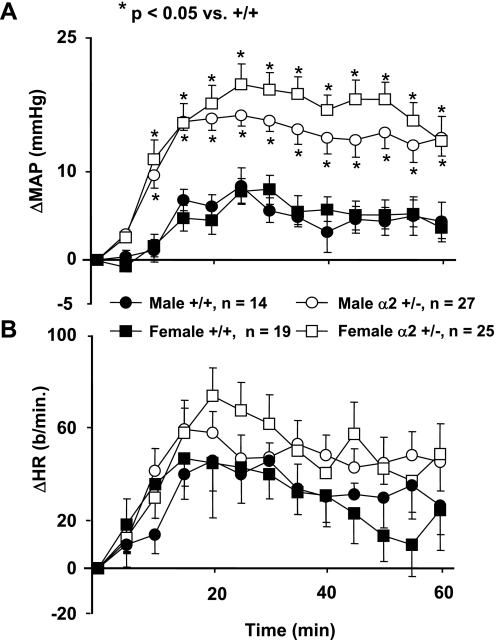
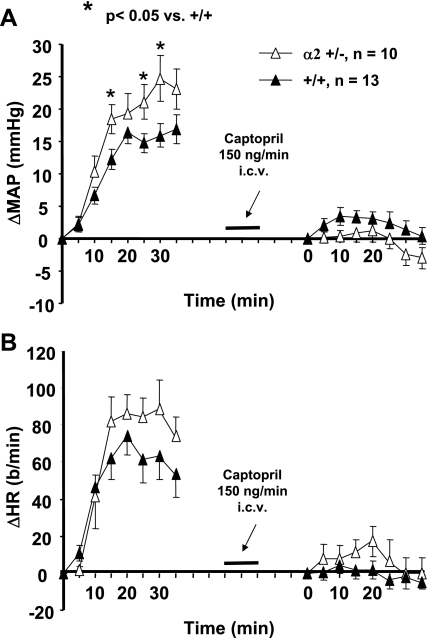
Baseline blood pressures (obtained before ICV aCSF-HiNa infusion) were not significantly different between α2 +/− and +/+ mice (Fig. 3), and there were no differences in baseline heart rate between male +/− and +/+ mice. However, heart rate was significantly elevated in female α2 +/− vs. female +/+ groups (P < 0.05, Fig. 3). Since this is an isolated difference (i.e., it did not occur in baseline heart rates for other studies), and since heart rate is inherently more variable than blood pressure, the increased baseline heart rate in female +/− mice before ICV aCSF-HiNa infusion may be due to random variation.
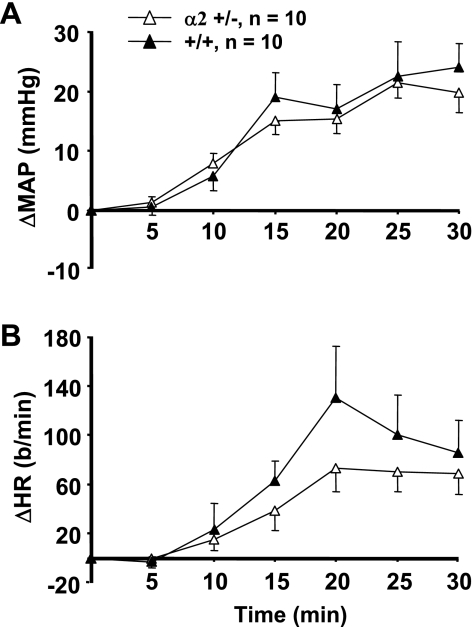
Fig. 3.
Changes in mean arterial pressure (ΔMAP) (A) and heart rate (ΔHR) (B) from baseline values in mice in response to the intracerebroventricular (ICV) infusion of 0.225 M NaCl in artificial cerebrospinal fluid (aCSF-HiNa) at 0.4 μl/min for 60 min. α2 +/− = mice heterozygous for the α2 Na+-K+-ATPase gene knockout; +/+ = wild-type mice. Values represent means ± SE of ΔMAP or ΔHR (MAP or HR averaged over 5-min intervals minus baseline) during the 60-min infusion. Baseline blood pressures (male +/+: 123 ± 2 mmHg; male α2 +/−: 122 ± 2 mmHg; female +/+: 118 ± 3 mmHg; female α2 +/−: 118 ± 2 mmHg) and heart rates (male +/+: 500 ± 24 beats/min; male α2 +/−: 507 ± 13 beats/min; female +/+: 483 ± 20 beats/min; female α2 +/−: 557 ± 18 beats/min) were recorded over a 10-min interval immediately preceding the start of aCSF-HiNa. *P < 0.05 vs. female +/+.
Because the percent decrease from controls in brain α2-isoform expression was greater in α2 +/− females than in α2 +/− males (Fig. 2), the blood pressure responses to ICV aCSF-HiNa were sorted by gender (Fig. 3A). Both male and female α2 +/− mice had significantly greater blood pressure responses to ICV aCSF-HiNa than their +/+ counterparts (Fig. 3A). In addition, the α2 +/− females almost always had a greater mean pressor response than α2 +/− males (Fig. 3A). However, the differences in the blood pressure responses between the two genders did not reach the level of statistical significance (P = 0.24 for repeated-measures ANOVA), despite the large numbers of mice studied. Heart rate responses to ICV aCSF-HiNa infusion were not significantly different between α2 +/− mice and their +/+ counterparts (Fig. 3B), nor were there any gender differences in the heart rate response within the +/+ and +/− groups.
ICV/IV Ouabain Infusion
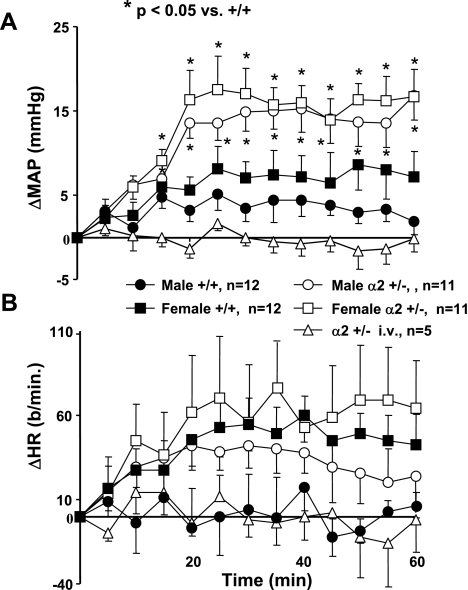
To determine whether the enhanced pressor response to ICV aCSF-HiNa in α2 +/− mice might be related to enhanced sensitivity to brain Na+-K+-ATPase inhibition per se and not to a change in a non-Na+-K+-ATPase protein, the specific Na pump inhibitor ouabain was infused ICV, and the blood pressure and heart rate responses were examined. There was a slight pressor response in +/+ mice with the dose used (Fig. 4A). However, in α2 +/− mice of both genders, there were significant increases in blood pressure from baseline values that were significantly greater than the respective +/+ responses (Fig. 4A). There were no gender differences in the magnitude of the pressor responses, within either +/+ (P = 0.3) or α2 +/− groups (P = 0.57). There were no differences in the heart rate response to ICV ouabain between the two genotypes, and there were no gender differences (Fig. 4B).
Fig. 4.
Effect of ICV or intravenous (IV, i.v.) ouabain (5 ng/min) on blood pressure (A) and HR (B) in Na+-K+-ATPase α2 +/− vs. +/+ mice. Values represent means ± SE of the change from baseline in MAP or HR averaged over the preceding 5-min interval. Baseline values were determined as described in Fig. 3. Baseline MAP was male +/+: 123 ± 4 mmHg; male α2 +/−: 122 ± 4 mmHg; female +/+: 119 ± 3 mmHg; female α2 +/−: 114 ± 2 mmHg. Baseline HR was male +/+: 490 ± 29 beats/min; male α2 +/−: 513 ± 28 beats/min; female +/+: 560 ± 46 beats/min; female α2 +/−: 480 ± 39 beats/min.
When the same dose of ouabain was given to +/− mice IV instead of ICV, there was no effect on blood pressure or heart rate (Figs. 4, A and B). This suggests that there is no peripheral effect on blood pressure or heart rate of the ICV ouabain treatment used in the present study. Doses of ICV sodium that are >2.5-fold higher than those used in the present study have previously been given IV and produced no effects on blood pressure or heart rate in wild-type mice (51).
OLS Concentration in the Hypothalamus
Studies of brain OLS levels were focused on the hypothalamus for several reasons. First both high-salt diet (33, 55) and ICV Na-rich aCSF infusion (56) increase OLS levels within this brain region in rats [OLS levels are also increased in the pituitary (33, 55, 56), but in the mouse, the pituitary is too small to permit detection of OLS]. In addition, the injection of an anti-OLS antibody specifically into the median preoptic nucleus (MnPO) of the hypothalamus abolishes the hypertension that is caused by both a high-salt diet and acute ICV infusion of aCSF-HiNa in SHR (5, 52), demonstrating the critical importance of the OLS in this brain region.
To determine whether chronic downregulation of the α2-isoform influences baseline OLS levels in the hypothalamus, OLS concentrations were assessed in unoperated mice. There were no significant differences between +/− and +/+ mice (n = 5–7 in each group) in hypothalamic [OLS] (male +/+, +/−: 0.64 ± 0.34, 0.66 ± 0.52 ng/g; female +/+, +/−: 0.89 ± 0.68, 0.98 ± 0.27 ng/g). In addition, similar measurements were made in hypothalamic homogenates from separate animals that had received ICV aCSF-HiNa infusions for 60 min, but there was again no difference between +/− (n = 7) and +/+ (n = 9) mice (0.85 ± 0.12 vs. 0.86 ± 0.10 ng/g tissue, respectively).
Brain RAS Function
Forms of hypertension that are mediated by increases in brain concentration of OLS or ouabain are abolished by ICV losartan [e.g., a high-salt diet in Dahl-S rats (62); Wistar rats and mice given ICV sodium-rich aCSF (20, 51); rats and mice given ICV ouabain (7, 51)]. Thus, chronic inhibition/downregulation of the brain Na+-K+-ATPase by the brain OLS or by ICV ouabain increases blood pressure by activating the brain RAS. In the present study, brain RAS function was assessed in unoperated α2 +/− mice to determine whether the chronic decrease in brain α2 expression in these mice may have chronically elevated baseline RAS activity.
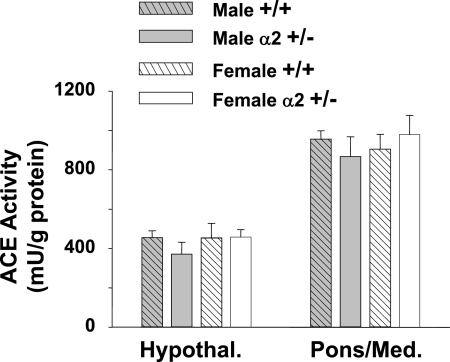
ACE activity was initially measured in the hypothalamus and pons/medulla, since changes in brain ACE expression (62) or in the binding of ACE ligands (20) have been reported in these regions in Dahl-S on high salt and in Wistar rats given ICV sodium, respectively. However, there were no significant differences between α2 +/− and +/+ mice in ACE activity in either the hypothalamus or pons/medulla (Fig. 5).
Fig. 5.
Angiotensin converting enzyme (ACE) activity in the hypothalamus and pons/medulla of 8- to 10-wk-old mice. For hypothalamus (Hypothal, n = 3–4) and pons/medulla (Pons/Med, n = 5–6), ACE activity was measured in 40 μg of total protein from crude homogenate preparations. Proteolytic activity was measured by monitoring the fluorescence emission (360 nm emission, 480 nm excitation) of o-phthaldialdehyde coupled to histidine-leucine produced from the cleavage of hippuryl-histidine-leucine. ACE activity was determined specifically as captopril-inhibitable proteolytic activity. Values are means ± SE.
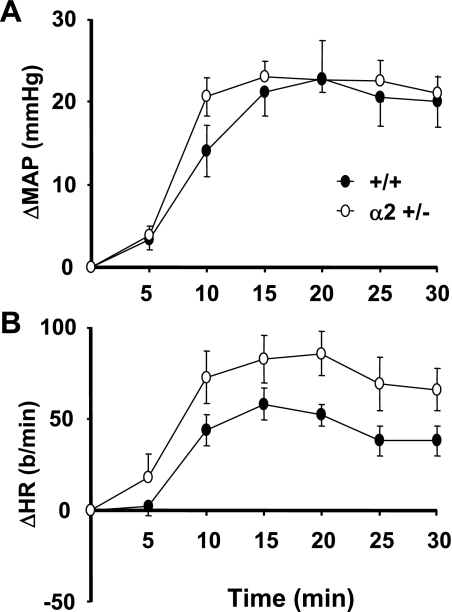
A second approach was then undertaken to determine whether there are chronic differences in brain RAS function between α2 +/− and +/+ mice. Initially, an ICV dose of the ACE substrate ANG I (275 ng/min) was established that causes a small pressor response in wild-type C57Bl/6 mice (data not shown). When the blood pressure response to ICV ANG I was compared in α2 +/− and +/+ mice, there was a significantly greater response in the +/− mice (Fig. 6A). The heart rate response also tended to be greater in the +/− genotype (Fig. 6B), but the differences did not reach the level of statistical significance. The pressor and tachycardic responses to ICV ANG I were abolished by ICV captopril in both +/+ and α2 +/− mice (Fig. 6A), confirming that they were mediated by ACE. The latter treatment had no effect on baseline blood pressure and heart rate, as the baseline blood pressures and heart rates measured just prior to the first and second ANG I infusions (pre- and postcaptopril) were not significantly different from each other.
Fig. 6.
Changes from baseline MAP or HR following ICV infusions of the ACE substrate ANG I (275 ng/min). ANG I was given both before and after an ICV infusion of captopril (1.5 μg) as described in materials and methods. There was a 15-min gap between the end of the first ANG I infusion and the start of captopril infusion. Another 15-min recovery period ensued after captopril before the start of the second ANG I infusion. Baseline MAP and HR values were determined over the 10-min period immediately preceding each ANG I infusion and were subtracted from the absolute MAP and HR to calculate ΔMAP or ΔHR. Precaptopril baseline MAP: α2 +/− = 113 ± 4, +/+ = 116 ± 3 mmHg; HR: α2 +/− = 491 ± 17, +/+ = 457 ± 28 beats/min. Postcaptopril baseline MAP: α2 +/− = 120 ± 4, +/+ = 118 ± 3 mmHg; HR: α2 +/− = 502 ± 20, +/+ = 450 ± 25 beats/min.
ANG II infusion (ICV) caused a sustained increase in blood pressure in both +/+ and +/− mice (Fig. 7A) that was of similar magnitude to that produced by ICV ANG I in α2 +/− mice. However, there were no significant differences between the two genotypes in the blood pressure responses to ANG II (P = 0.66). Heart rate also increased significantly from baseline in both genotypes, and there was a tendency for the increase be greater in the α2 +/− genotype vs. +/+ (P = 0.061).
Fig. 7.
Changes from baseline in MAP or HR following the ICV infusion of ANG II (150 ng/min). Baseline MAP and HR values were averaged over the 10-min period immediately preceding each ANG II infusion, and this value was subtracted from the absolute MAP and HR at each time point to calculate ΔMAP or ΔHR. Baseline MAP before ICV infusions was: α2 +/− = 113 ± 5, +/+ = 113 ± 4 mmHg; HR: α2 +/− = 455 ± 33, +/+ = 474 ± 29 beats/min.
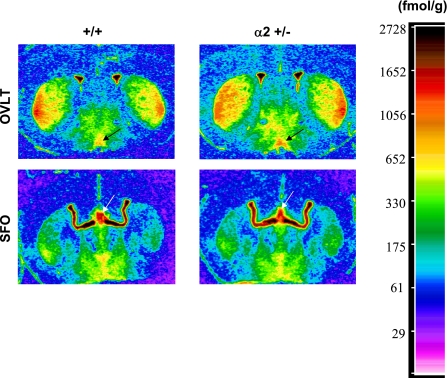
We also assessed ACE ligand binding in cardiovascular regulatory nuclei in which this binding was previously shown to be augmented by increases in CSF [Na] (20) (an effect that is abolished by ICV Fab, indicating that it is mediated by an OLS and downregulation of Na+-K+-ATPase activity). These nuclei included the organum vasculosum laminae terminalis (OVLT), the subfornical organ (SFO), the MnPO, and paraventricular nucleus (PVN) (20). While there was no significant difference between the two genotypes in the PVN and MnPO (P = 0.20 and 0.45), 125I-labeled 351A binding was increased in the OVLT (P < 0.035) and tended to be increased in the SFO and PVN (P = 0.056) of α2 +/− mice vs. +/+ (Table 1 and Fig. 8).
Table 1.
Densities for 125I-labeled 351A (ACE ligand) binding in various hypothalamic cardiovascular regulatory nuclei of α2 +/− (n = 4) and +/+ (n = 3) mice
| Nucleus |
Genotype |
|
|---|---|---|
| α2+/+ | α2+/− | |
| OVLT | 1386±50 | 1596±51* |
| SFO | 3099±110 | 3411±72 |
| MnPO | 723±32 | 771±45 |
| PVN | 843±51 | 964±59 |
Values represent the means ± SE for the amounts of 125I-labeled 351A bound (fmol/g wet tissue). Data were compiled from densitometric analyses of autoradiographs, such as those shown in Fig. 8. OVLT, organum vasculosum laminae terminalis; SFO, subfornical organ; MnPO, median preoptic nucleus; PVN, paraventricular nucleus.
P < 0.05 vs. +/+.
Fig. 8.
Representative densitometric scans of autoradiographs produced from the binding of a labeled ligand (125I-351A) for ACE. Shown are images from coronal sections of the brain containing the organum vasculosum laminae terminalis (OVLT) and subfornical organ (SFO) in +/+ or +/− mice, as indicated. Scale indicates standards for assessing the relative intensity of the signal. Arrows indicate the OVLT and SFO nuclei.
To compare the responses of blood pressure and heart rate to circulating catecholamines in +/− vs. +/+ mice, NE was given IV in separate animals. Despite the fact that the magnitude of the pressor response to IV NE was similar to that for ICV Na, ouabain, and ANG I in the present study, there were no significant differences between α2 +/− and +/+ mice in the blood pressure and heart rate responses to NE (Fig. 9).
Fig. 9.
Changes from baseline in MAP or HR following IV infusion of norepinephrine (NE; 256 ng/min). Baseline MAP and HR values were averaged over the 10-min period immediately preceding each NE infusion, and this value was subtracted from the absolute MAP and HR at each time point to calculate ΔMAP or ΔHR. Baseline MAP before ICV infusions was α2 +/− = 122 ± 3, +/+ = 114 ± 10 mmHg; HR: α2 +/− = 463 ± 28, +/+ = 517 ± 28 beats/min. There were no significant differences between α2 +/− and +/+.
DISCUSSION
The present study demonstrates several findings for the first time in adult mice that are heterozygous for an α2 Na+-K+-ATPase gene disruption (α2 +/−). First, the pressor response to ICV Na-rich artificial CSF infusion (aCSF-HiNa), which was previously shown to be mediated in mice by an endogenous brain inhibitor of the Na+-K+-ATPase (51), is enhanced in α2 +/− vs. wild-type (+/+) mice. Second, ouabain itself given ICV also produced an enhanced pressor response in α2 +/− mice vs. +/+. Since ouabain is a specific ligand for the Na+-K+-ATPase, this finding suggests that the augmented pressor response to ICV aCSF-HiNa (and subsequently to the brain OLS) in α2 +/− mice is not due to changes in non-Na+-K+-ATPase proteins that may occur in response to the α2 gene knockout. Third, in both the pons/medulla and hypothalamus, baseline α2 protein expression was chronically reduced in α2 +/− mice without changes in the nontarget isoforms (α1 and α3). Fourth, the pressor response to ICV ANG I was also enhanced in the α2 +/− genotype, demonstrating that the chronic downregulation of the α2-isoform is associated with activation of the brain RAS. Finally, ACE expression (125I-351A binding) is increased in the OVLT of the α2 +/− mice.
The continuous ICV infusion of aCSF-HiNa was undertaken in the present study because it closely mimics the effects of a high-salt diet in experimental salt-sensitive hypertension, such as Dahl-S and SHR on high salt. Both high-salt diet in Dahl-S/SHR and ICV sodium-rich aCSF infusion increase CSF [Na] (28, 30), increase the concentration of an endogenous OLS in the hypothalamus and pituitary (33, 55, 56), activate the brain RAS (20, 26, 51, 62), augment sympathetic activity (22–30), and raise blood pressure (22–30). In addition, it has been shown that increases in CSF [Na] precede increases in blood pressure when Dahl-S and SHR are switched to a high-salt diet from regular salt intake (28). This suggests a role for elevated CSF sodium levels in the etiology of the hypertension (27, 28). Besides causing the same downstream effects as a high-salt diet, the direct ICV infusion of sodium-rich aCSF can increase CSF [Na] to a similar extent as increasing dietary sodium levels (28–30).
Although our knockout was not tissue-specific, the effects of the heterozygous knockout on the brain RAS and on blood pressure responses to increased CSF [Na] may be due to the reduction in the expression of the α2-isoform in the brain. The pressor responses to ICV sodium and ouabain in our study likely originate in the CNS, since we have established that ICV doses of either sodium (51) or ouabain (present study) that are effective in increasing blood pressure are completely ineffective when given IV instead. Also, ICV doses of anti-OLS antibody Fab fragments and losartan that abolish this pressor response have no effect on the response when given IV in mice (51), indicating that the activation of the OLS system and RAS occur centrally as well.
However, vascular smooth muscle (VSM) in adult α2 +/− mice has enhanced contractile responses to exogenous agents (ouabain, phenylephrine) vs. +/+ mice (46, 61), and thus a contribution of intrinsic vascular hyperreactivity to the current augmentation of pressor responses in the +/− genotype could not initially be ruled out. Since previous studies in rats have shown that increased CSF [Na] augments sympathetic nervous system (SNS) activity (27, 29, 30) and this also appears to be the case in mice (51), the enhanced pressor response to ICV sodium in α2 +/− mice in the present study may be due to one or more mechanisms, including 1) an enhanced SNS outflow from the CNS in α2 +/− vs. +/+ mice and/or 2) an augmented intrinsic contractile response of α2 +/− VSM to SNS activity that is equal in both genotypes.
We attempted to address this issue by giving peripheral (IV) infusions of NE and comparing the pressor responses in α2 +/− vs. +/+ mice. Both the blood pressure and heart rate responses to IV NE infusions were similar in α2 +/− and +/+ mice. These results would favor hypothesis 1 over 2. This notion is supported by the current lack of a difference between these two genotypes in the pressor response to ICV ANG II. If the present differences between +/− and +/+ mice in the responses to ICV Na, ouabain, and ANG I were due to inherent VSM hyperreactivity alone, then a significant difference in the response to ICV ANG II would also be expected. The reasons why α2 +/− VSM has augmented contractile responses to ouabain and phenylephrine in vitro (46, 61) but no enhancement of the in vivo blood pressure and heart rate responses to IV NE in the present study cannot be deduced from the present data, but may relate to differences in agents used (responses to NE have not been previously assessed in +/− mice in vitro or in vivo), different biodistributions, concentrations at the site of action, etc.
Although the Western blot analysis was repeated several times in both hypothalamus and pons/medulla, each time the female +/− mice showed a greater decrease in α2 expression than male +/−. This dimorphism between male and female α2 +/− mice may be related to previously reported gender and/or sex hormone effects on Na+-K+-ATPase expression in the brain. Both estrogen and progesterone have been previously reported to downregulate Na+-K+-ATPase expression in cultured brain cells as well as in the in vivo brain, while androgens increase expression (11, 12, 16, 42). The reasons why the present gender differences were seen in +/− but not +/+ mice are unknown, but gender/sex hormone effects on the Na+-K+-ATPase may operate maximally when endogenous Na+-K+-ATPase expression is low (e.g., α2 +/− mice). No statistically significant gender differences were seen within the +/− genotype in the pressor and tachycardic responses to ICV aCSF-HiNa or ouabain.
The α2-isoform of the Na+-K+-ATPase is present at low levels in some neurons, but it is the predominant α-isoform of the Na+-K+-ATPase in astrocytes and other glial cells. Astrocytes not only surround axons, but also encase synaptic terminals, and thus they are positioned to directly affect synaptic transmission (15, 53). The relevance of glial/astrocyte activity to cardiovascular regulation is suggested by studies demonstrating the effects of up- or downregulation of the RAS in glia. Glia-specific renin overexpression in mice increases blood pressure vs. control mice, and the hypertension associated with glial renin overexpression is at least as great as that produced when renin is overexpressed in neurons (37). As well, the upregulation of angiotensinogen mRNA in glia causes hypertension, while angiotensinogen downregulation in this cell type lowers blood presssure (21, 36).
The hypothalamus is a brain region in which chronic treatments with either a high-salt diet or ICV sodium infusion increase OLS levels (33, 55, 56). Therefore, in the present study, we examined OLS levels in the hypothalamus both at baseline (unoperated mice) and immediately after ICV aCSF-HiNa infusion. The present lack of a difference between the +/− and +/+ genotypes in hypothalamic concentration of OLS is consistent with the notion that the enhanced pressor response to increased CSF [Na] in the +/− mice is due to the reduced expression of the α2-isoform per se and is not due to a possible increase in hypothalamic OLS content caused by the knockout.
Despite the fact that a link between the brain RAS and brain OLS/ Na+-K+-ATPase has been known to exist for over a decade, few details have emerged about the brain mechanisms by which the two systems interact. In the present study, we tested an additional hypothesis that chronic downregulation of α2-isoform expression activates the brain RAS as a downstream effect. If this were the case, then the brain RAS activity should be upregulated by chronic downregulation of the α2 protein itself in α2 +/− mice, much like chronic increases in brain Na+-K+-ATPase inhibitors (ouabain or OLS) are associated with brain RAS activation. The pressor response to ICV ANG I was indeed increased in α2 +/− mice, supporting this hypothesis. Since it has been previously shown that the pressor response to an elevation in CSF [Na] is mediated by a brain inhibitor of the Na+-K+-ATPase (brain OLS), which in turn activates the brain RAS, it is attractive to hypothesize from the present results that the RAS activation by the OLS is mediated by specific inhibition of the α2-isoform.
The enhanced pressor response to ICV ANG I but not ANG II in α2 +/− mice seen in the present study would be more consistent with an increased activity of brain ACE in these mice than of upregulation of brain AT1 receptors. Despite the present lack of an alteration in regional (hypothalamus and pons/medulla) ACE activity in α2 +/− mice, there was still the possibility of alterations in individual nuclei. The expression of brain ACE has previously been shown to be markedly increased in central cardiovascular regulatory nuclei [the OVLT, the SFO, the MnPO, and PVN] under conditions where brain OLS or ouabain levels are chronically increased (20).
Therefore, we examined the binding of the ACE-specific ligand 125I-351A to the same nuclei. 125I-351A binding was increased in α2 +/− mice in the OVLT only. This indicates that ACE expression was elevated at least in the OVLT. Although the OVLT is a circumventricular organ and is well known to mediate CNS responses to substances in the systemic circulation, this nucleus contains ventricular ependymal cells that are also exposed to substances in the CSF. Sympathetic hyperactivity mediates the increase in blood pressure after increases in CSF [Na] (22, 24–26, 29), and the OVLT is known to regulate SNS responses to increased brain [Na] (39, 47). Thus, it is conceivable that upregulation of ACE expression in the OVLT in the α2 +/− mice mediates the present enhanced pressor responses, i.e., to ICV Na-rich aCSF, ouabain, and ANG I. Alternatively, enhanced ACE and/or RAS activity in other cardiovascular regulatory nuclei that were not assayed here could also contribute to the present augmented responses or could mediate them entirely.
The precise mechanisms by which increases in CSF [Na] upregulate the OLS in the brain, activate the brain RAS, increase SNS activity, and raise blood pressure are presently understudied and ill-defined. The increase in brain concentrations of OLS after a high-salt diet or ICV sodium infusion appears to require brain Na channels as an early step (55, 56). OLS immunoreactivity and/or levels appear to be the greatest in the posterior pituitary, PVN, and supraoptic nucleus (likely magnocellular neurons), but are also high in the OVLT, MnPO, SFO, median eminence, and other areas of the hypothalamus vs. extra-hypothalamic nuclei. In addition, previous studies have shed light on key brain nuclei that are involved in the brain RAS activation and sympathoexcitation.
The MnPO
This nucleus appears to be a critical site of OLS action in the scenario of increased CSF [Na]. The specific microinjection of ouabain-binding Fab fragments into the MnPO abolishes the increases in blood pressure that are caused by both a high-salt diet in SHR and ICV sodium infusion in Wistar rats (both treatments raise CSF [Na]) (5).
The PVN
A recent study has pointed to the importance of AT1 receptors (AT1R) in the PVN in the pressor responses to increased CSF [Na]. The bilateral microinjection of the AT1R blocker telmisartan into the PVN abolishes the blood pressure and heart rate responses to an ICV co-infusion of sodium and a subpressor dose of aldosterone (the latter treatment augments the response to ICV sodium) (13). Similar injections of losartan into the PVN also attenuate the increase in renal sympathetic nerve activity produced by intracarotid artery infusion of hypertonic saline (6) (the latter is another method designed to increase brain [Na]).
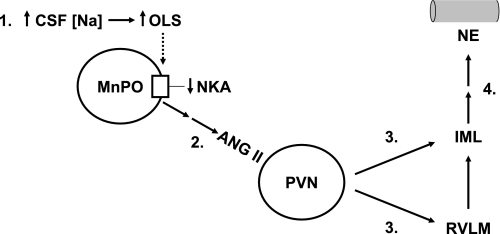
Fig. 10 is based on the above literature and on a recent study involving the MnPO and PVN. It has previously been postulated that increases in CSF [Na] activate sodium sensors along the lamina terminalis (which includes the SFO, MnPO, and OVLT), leading to AT1 receptor stimulation in the parvocellular subnucleus of the PVN (13). According to this model, information from the resulting activation of neurons in the parvocellular PVN would be relayed through the intermediolateral (IML) cell column of the spinal cord either directly or via the rostral ventrolateral medulla. This putative system would ultimately culminate in the release of NE at peripheral SNS postganglionic terminals, such as those on blood vessels and renal tubules.
Fig. 10.
Hypothetical sequence of events following increases in CSF [Na]. Step 1: increased production/release of OLS caused by increased CSF [Na] produces inhibition of the Na+-K+-ATPase (NKA) within the median preoptic nucleus (MnPO). Possible sensors of CSF [Na] are ventricular ependymal cells in the OVLT, SFO, and MnPO. The NKA inhibition in the MnPO results in: Step 2: increased activity of neuronal projections (either mono- or polysynaptic) from the MnPO to the parvocellular division of the paraventricular nucleus (PVN). This causes an increased AT1 receptor stimulation in this region of the PVN, presumably via the release of ANG II. Step 3: AT1 receptor stimulation in the parvocellular PVN causes activation of direct projections to the intermediolateral cell column (IML) of the spinal cord; or alternatively, these PVN neurons project to the rostral ventrolateral medulla (RVLM), which in turn projects sympathetic preganglionic fibers that pass through the IML. Step 4: Activation of the sympathetic preganglionic neurons in the IML tract ultimately culminates in enhanced release of NE at nerve terminals on vascular smooth muscle and other tissues. See discussion for further explanation, background, and references.
Since inhibition of the Na+-K+-ATPase has a neuroexcitatory effect (9, 35), activation of the baseline activity (i.e., prior to increases in CSF [Na]) of such a pathway may occur in α2 +/− mice. This may include hyperresponsiveness of step 1 in Fig. 10, in which the OLS-induced inhibition of the Na+-K+-ATPase on somata or dendrites within the MnPO appears to serve a critical regulatory function (5). It is also possible that any or all neurons/glia along this pathway in which the α2-isoform is expressed may be hyperexcitable in α2 +/− mice, due the effects of reduced Na+-K+-ATPase activity, which in turn is known to decrease cell membrane potential and/or increase cell Ca2+ stores (9, 35).
It is not presently possible to pinpoint exactly at what point in this system, if any, an alteration in the OVLT ACE expression, such as that seen presently, might interact to contribute to the hyperresponsiveness of blood pressure and heart rate to increased CSF [Na]. The MnPO appears to play a central role in the OLS-mediated response to increases in CSF [Na] as discussed above (5). It is possible that ACE-containing neurons from the OVLT participate in the regulation of the release/stores of the OLS in the MnPO in response to increased CSF [Na] via mono- or polysynaptic connections. There are dense reciprocal connections between the OVLT and MnPO, and OVLT-MnPO efferents appear to be involved in SNS regulation (59, 60). Hypothetical ACE-containing OVLT neurons influencing OLS release or stores in the MnPO would not necessarily be angiotensinergic, since the brain RAS activation produced by increased CSF [Na] appears to follow the OLS effect, not precede it (51) (ACE regulates other brain peptides besides angiotensins). An absolute determination of the pathways leading from the brain OLS/ Na+-K+-ATPase to sympathoexcitation and increased blood pressure would require additional neuroanatomical and physiological studies.
Perspectives and Significance
Previous studies have demonstrated that increases in CSF [Na] caused by a high-salt diet in salt-sensitive rats or by ICV sodium infusions lead to increased levels of an OLS in the brain. The OLS in turn activates the brain RAS, which augments sympathetic activity and raises blood pressure. This cascade has been shown to be critical in the genesis of salt-dependent hypertension (20, 24, 25, 28, 31, 56). Since the OLS is an inhibitor of the Na pump and binds to the Na+-K+-ATPase α-subunit, an early step in this process appears to be a decrease in the activity of one or more brain α-isoforms of the Na+-K+-ATPase by the OLS. However, the isoforms that mediate this response had not been investigated prior to the present study. The present findings that the chronic downregulation of the expression of the brain Na+-K+-ATPase α2-isoform in α2 +/− mice is associated with augmented pressor responses to ICV sodium-rich aCSF suggests that the blood pressure responses to increased CSF [Na], and subsequently to the brain OLS, are mediated by decreased activity of the α2-isoform. The fact that these mice also have an enhanced pressor response to ICV ANG I suggests that the brain RAS is activated in the heterozygous mice as well. The latter notion is supported by upregulation of ACE activity in the OVLT in the α2 +/− mice. If the hypotheses generated by the present results are ultimately verified, they would be consistent with a role of the brain α2-isoform in the control of blood pressure mediated via regulation of the brain RAS.
GRANTS
This work was supported by Heart and Stroke Foundation of Ontario Grants-in-Aid NA-5102 and NA-6324 (to J. W. Van Huysse), by a Canadian Institutes of Health Research Operating Grant FRN-74572 (to J. W. Van Huysse), and by National Heart, Lung, and Blood Institute Grants R01-HL-28573 and R01-HL-66062 (to J. B Lingrel).
Acknowledgments
The authors are grateful to Dr. Kathleen Sweadner for providing the monoclonal antibody McK2 that was used to quantify the protein expression of the Na+-K+-ATPase α2-isoform, to Dr. Peter Doris for providing marinobufagenin, and to Junhui Tan in the laboratory of Dr. Frans Leenen for measuring ACE ligand binding densities.
Present address of S. F. Theriault and S. Dean: Natural Health Products Directorate, Health Canada, Ottawa, Ontario. Present address of A. E. Moseley: Monsanto Company, St. Louis, MO.
REFERENCES
- 1.Arystarkhova E, Wetzel RK, Asinovski NK, Sweadner KJ. The γ-subunit modulates Na+ and K+ affinity of the renal Na,K-ATPase. J Biol Chem 274: 33183–33185, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Bastide F, Meissner G, Fleischer S, Post RL. Similarity of the active site of phosphorylation of the adenosine triphosphatase from transport of sodium and potassium ions in kidney to that for transport of calcium ions in the sarcoplasmic reticulum of muscle. J Biol Chem 248: 8385–8391, 1973. [PubMed] [Google Scholar]
- 3.Baxter-Lowe LA, Hokin LE. The Red Cell Membrane: A Model for Solute Transport, edited by Raess BU and Tunnicliff G. Totowa, NJ: Humana, 1989, p. 185–280.
- 4.Beguin P, Wang X, Firsov D, Puoti A, Claeys D, Horisberger JD, Geering K. The γ-subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J 16: 4250–4260, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budzikowski AS, Leenen FH. Brain “ouabain” in the median preoptic nucleus mediates sodium-sensitive hypertension in spontaneously hypertensive rats. Hypertension 29: 599–605, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Chen QH, Toney GM. AT1-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol 281: R1844–R1853, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Cheung WJ, Kent MA, El-Shahat E, Wang H, Tan J, White R, Leenen FH. Central and peripheral renin-angiotensin systems in ouabain-induced hypertension. Am J Physiol Heart Circ Physiol 291: H624–H630, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Corthesy-Theulaz I, Merillat AM, Honegger P, Rossier BC. Na+-K+-ATPase gene expression during in vitro development of the rat fetal forebrain. Am J Physiol Cell Physiol 258: C1062–C1069, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Doll CJ, Hochachka PW, Reiner PB. Effects of anoxia and metabolic arrest on turtle and rat cortical neurons. Am J Physiol Regul Integr Comp Physiol 260: R747–R755, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB. The α2-isoform of Na+-K+-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 288: H477–H485, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Fraser CL, Sarnacki P. Na+-K+-ATPase pump function in rat brain synaptosomes is different in males and females. Am J Physiol Endocrinol Metab 257: E284–E289, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Fraser CL, Swanson RA. Female sex hormones inhibit volume regulation in rat brain astrocyte culture. Am J Physiol Cell Physiol 267: C909–C914, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Gabor A, Leenen FH. Mechanisms in the PVN mediating local and central sodium-induced hypertension in Wistar rats. Am J Physiol Regul Integr Comp Physiol 296: R618–R630, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Golovina V, Song H, James P, Lingrel J, Blaustein M. Regulation of Ca2+ signaling by Na+ pump α2-subunit expression. Ann NY Acad Sci 986: 509–513, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Bruckner G. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci 2: 139–143, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Guerra M, Rodriguez del Castillo A, Battaner E, Mas M. Androgens stimulate preoptic area Na+,K+-ATPase activity in male rats. Neurosci Lett 78: 97–100, 1987. [DOI] [PubMed] [Google Scholar]
- 17.He S, Shelly DA, Moseley AE, James PF, James JH, Paul RJ, Lingrel JB. The α1- and α2-isoforms of Na+-K+-ATPase play different roles in skeletal muscle contractility. Am J Physiol Regul Integr Comp Physiol 281: R917–R925, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Herrera VL, Cova T, Sassoon D, Ruiz-Opazo N. Development and cell-specific regulation of Na+-K+-ATPase α1-, α2-, and α3-isoform gene expression. Am J Physiol Cell Physiol 266: C1301–C1312, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Hieber V, Siegel GJ, Fink DJ, Beaty MW, Mata M. Differential distribution of Na+K+-ATPase α-isoforms in the central nervous system. Cell Mol Neurobiol 11: 253–262, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Huang BS, Cheung WJ, Wang H, Tan J, White RA, Leenen FH. Activation of brain renin-angiotensin-aldosterone system by central sodium in Wistar rats. Am J Physiol Heart Circ Physiol 291: H1109–H1117, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Huang BS, Ganten D, Leenen FHH. Responses to central Na+ and ouabain are attenuated in transgenic rats deficient in brain angiotensinogen. Hypertension 37: 683–686, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Huang BS, Harmsen E, Yu H, Leenen FHH. Brain ouabain-like activity and the sympathoexcitatory and pressor effects of central sodium in rats. Circ Res 71: 1059–1066, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Huang BS, Huang X, Harmsen E, Leenen FH. Chronic central versus peripheral ouabain, blood pressure, and sympathetic activity in rats. Hypertension 23: 1087–1090, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Huang BS, Leenen FHH. Brain “ouabain” mediates the sympathoexcitatory and hypertensive effects of high sodium intake in Dahl salt-sensitive rats. Circ Res 74: 586–595, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Huang BS, Leenen FHH. Blockade of brain “ouabain” prevents sympathoexcitatory and pressor responses to high sodium in SHR. Am J Physiol Heart Circ Physiol 271: H103–H108, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Huang BS, Leenen FHH. Both brain angiotensin II and “ouabain” contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension 32: 1028–1033, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Huang BS, Leenen FH. Sympathoexcitatory and pressor responses to increased brain sodium and ouabain are mediated via brain ANG II. Am J Physiol Heart Circ Physiol 270: H275–H280, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Huang BS, Van Vliet BN, Leenen FHH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on high salt diet. Am J Physiol Heart Circ Physiol 287: H1160–H1166, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Huang BS, Veerasingham SJ, Leenen FHH. Brain “ouabain,” ANG II, and sympathoexcitation by chronic central sodium loading in rats. Am J Physiol Heart Circ Physiol 274: H1269–H1276, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Huang BS, Wang H, Leenen FHH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs. -resistant rats. Am J Physiol Heart Circ Physiol 281: H1881–H1889, 2001. [DOI] [PubMed] [Google Scholar]
- 31.James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB. Identification of a specific role for the Na,K-ATPase α2-isoform as a regulator of calcium in heart. Mol Cell 3: 555–563, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Lane LK, Copenhaver JH Jr, Lindenmayer GE, Schwartz A. Purification and characterization of and [3H]ouabain binding to the transport adenosine triphosphatase from outer medulla of canine kidney. J Biol Chem 248: 7197–7200, 1973. [PubMed] [Google Scholar]
- 33.Leenen FH, Harmsen E, Yu H, Ou C. Effects of dietary sodium on central and peripheral ouabain-like activity in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 264: H2051–H2055, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Mata M, Siegel GJ, Hieber V, Beaty MW, Fink DJ. Differential distribution of (Na,K)-ATPase α-isoform mRNAs in the peripheral nervous system. Brain Res 546: 47–54, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Malek SA, Adorante JS, Stys PK. Differential effects of Na-K-ATPase pump inhibition, chemical anoxia, and glycolytic blockade on membrane potential of rat optic nerve. Brain Res 1037: 171–179, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Morimoto S, Cassell MD, Beltz TG, Johnson AK, Davisson RL, Sigmund CD. Elevated blood pressure in transgenic mice with brain-specific expression of human angiotensinogen driven by the glial fibrillary acidic protein promoter. Circ Res 89: 365–72, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Morimoto S, Cassell MD, Sigmund CD. Glial- and neuronal-specific expression of the renin-angiotensin system in brain alters blood pressure, water intake, and salt preference. J Biol Chem 277: 33235–33241, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Morrell NW, Danilov SM, Satyan KB, Morris KG, Stenmark KR. Right ventricular angiotensin converting enzyme activity and expression is increased during hypoxic pulmonary hypertension. Cardiovasc Res 34: 393–403, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Peng N, Wei CC, Oparil S, Wyss JM. The organum vasculosum of the lamina terminalis regulates noradrenaline release in the anterior hypothalamic nucleus. Neuroscience 99: 149–156, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Price EM, Rice DA, Lingrel JB. Site-directed mutagenesis of a conserved, extracellular aspartic acid residue affects the ouabain sensitivity of sheep Na,K-ATPase. J Biol Chem 264: 21902–21906, 1989. [PubMed] [Google Scholar]
- 41.Pullen MA, Brooks DP, Edwards RM. Characterization of the neutralizing activity of digoxin-specific Fab toward ouabain-like steroids. J Pharmacol Exp Ther 310: 319–325, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez del Castillo A, Battaner E, Guerra M, Alonso T, Mas M. Regional changes of brain Na+,K+-transporting adenosine triphosphatase related to ovarian function. Brain Res 416: 113–118, 1987. [DOI] [PubMed] [Google Scholar]
- 43.Sahin-Erdemli I, Rashed SM, Songu-Mize E. Rat vascular tissues express all three α-isoforms of Na+-K+-ATPase. Am J Physiol Heart Circ Physiol 266: H350–H353, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz A, Lendenmayer GE, Allen JC. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol Rev 27: 3–134, 1975. [PubMed] [Google Scholar]
- 45.Shamraj OI, Lingrel JB. A putative fourth Na+,K+-ATPase α-subunit gene is expressed in testis. Proc Natl Acad Sci USA 91: 12952–12956, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shelly DA, He S, Moseley A, Weber C, Stegemeyer M, Lynch RM, Lingrel J, Paul RJ. Na+ pump α2-isoform specifically couples to contractility in vascular smooth muscle: evidence from gene-targeted neonatal mice. Am J Physiol Cell Physiol 286: C813–C820, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am J Physiol Regul Integr Comp Physiol 293: R2279–R2289, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skou JC The Na,K-pump. Methods Enzymol 156: 1–25, 1988. [DOI] [PubMed] [Google Scholar]
- 49.Sweadner KJ Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta 988: 185–220, 1989. [DOI] [PubMed] [Google Scholar]
- 50.Tan J, Wang H, Leenen FH. Increases in brain and cardiac AT1 receptor and ACE densities after myocardial infarct in rats. Am J Physiol Heart Circ Physiol 286: H1665–H1671, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Van Huysse JW, Hou XH. Pressor response to CSF sodium in mice: mediation by a ouabain-like substance and renin-angiotensin system in the brain. Brain Res 1021: 219–223, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Veerasingham SJ, Leenen FH. Ouabain- and central sodium-induced hypertension depend on the ventral anteroventral third ventricle region. Am J Physiol Heart Circ Physiol 276: H63–H70, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci 19: 6897–6906, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res 47: 1–9, 1980. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Leenen FHH. Brain sodium channels mediate increases in brain “ouabain” and blood pressure in Dahl S rats. Hypertension 40: 96–100, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Leenen FHH. Brain sodium channels and central sodium-induced increases in brain ouabain-like compound and blood pressure. J Hypertens 21: 1519–24, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Wang H, Lu Z, Yuan YK. Measurement of endogenous ouabain with a new method of ELISA. Chin J Med Lab Sci 21: 85–87, 1998. [Google Scholar]
- 58.Watts AG, Sanchez-Watts G, Emanuel JR, Levenson R. Cell-specific expression of mRNAs encoding Na+,K+-ATPase α- and β-subunit isoforms within the rat central nervous system. Proc Natl Acad Sci USA 88: 7425–7430, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Westerhaus MJ, Loewy AD. Sympathetic-related neurons in the preoptic region of the rat identified by viral transneuronal labeling. J Comp Neurol 414: 361–378, 1999. [PubMed] [Google Scholar]
- 60.Zardetto-Smith AM, Thunhorst RL, Cicha MZ, Johnson AK. Afferent signaling and forebrain mechanisms in the behavioral control of extracellular fluid volume. Ann NY Acad Sci 689: 161–176, 1993. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, Lee MY, Cavalli M, Chen L, Berra-Romani R, Balke CW, Bianchi G, Ferrari P, Hamlyn JM, Iwamoto T, Lingrel JB, Matteson DR, Wier WG, Blaustein MP. Sodium pump α2-subunits control myogenic tone and blood pressure in mice. J Physiol 569: 243–256, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao X, White R, Huang BS, Van Huysse J, Leenen FH. High salt intake and the brain renin-angiotensin system in Dahl salt-sensitive rats. J Hypertens 19: 89–98, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Zouzoulas A, Therien AG, Scanzano R, Deber CM, Blostein R. Modulation of Na,K-ATPase by the γ-subunit: studies with transfected cells and transmembrane mimetic peptides. J Biol Chem 278: 40437–40441, 2004. [DOI] [PubMed] [Google Scholar]