Abstract
Background and Objectives: Identifying patients who may develop acute kidney injury (AKI) remains challenging, as clinical determinants explain only a portion of individual risk. Another factor that likely affects risk is intrinsic genetic variability. Therefore, a systematic review of studies was performed that related the development or prognosis of AKI to genetic variation.
Design, setting, participants, and measurements: MEDLINE, EMBASE, HuGEnet, SCOPUS, and Web of Science were searched for articles from 1950 to Dec 2007. Two independent researchers screened articles using predetermined criteria. Studies were assessed for methodological quality via an aggregate scoring system.
Results: The 16 included studies were of cohort or case-cohort design and investigated 35 polymorphisms in 21 genes in association with AKI. Fifteen gene-gene interactions were also investigated in four separate studies. Study populations were primarily premature infants or adults who were critically ill or postcardiac bypass patients. Among the studies, five different definitions of AKI were used. Only one polymorphism, APO E e2/e3/e4, had greater than one study showing a significant impact (P < 0.05) on AKI incidence. The mean quality score of 5.8/10 (range four to nine), heterogeneity in the studies, and the dearth of studies precluded additional meta-analysis of the results.
Conclusions: Current association studies are unable to provide definitive evidence linking genetic variation to AKI. Future success will require a narrow consensus definition of AKI, rigorous epidemiologic techniques, and a shift from a priori hypothesis-driven to genome-wide association studies.
Acute kidney injury (AKI) is a complex disorder manifested by a rapid loss of renal function that results in retention of metabolic waste products (1). The incidence of AKI has steadily increased over the past decade, and while mortality has fallen with advances in renal replacement therapy (RRT), AKI still confers significant morbidity and mortality (2–4). In-hospital mortality for patients who develop AKI ranges from 20% to 28%; for those requiring RRT, mortality ranges from 28% to 33% (3,4). As such, the ability to identify high-risk patients and potentially prevent AKI becomes crucial.
The current literature maintains that a patient's risk for AKI depends on a combination of acute insults and chronic co-morbidities. Acute risk factors include volume depletion, exposure to nephrotoxic agents, surgery, and sepsis (5–8). Chronic risk factors include age, chronic kidney disease (CKD), diabetes, and congestive heart failure (8,9). However, models using these traditional risk factors remain inadequate (5,6,10–12). Two patients with identical clinical risk factors often react differently to the same insult; one may suffer no harm while the other may require RRT. Furthermore, we remain unable to predict who will progress to chronic dialysis and who will recover.
Consequently, there are likely to be clinically unobservable risk factors that contribute to one's susceptibility to AKI. Our understanding of epithelial, vascular, and immune responses in kidney injury makes it likely that genetic variability in regulatory elements of these responses plays a major role in determining one's risk of AKI. This has been seen in other complex diseases, such as the discovery of a single nucleotide polymorphism (SNP) in complement factor H as a risk factor for macular degeneration (13). In AKI, genetic variation in inflammatory cytokines such as Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-6 (IL-6) have recently been proposed as risk factors (11,14–17). This is due to the role of inflammatory mediators in the pathophysiology of AKI, especially with ischemia and sepsis (1,18,19). Genetic variations of vasomotor regulatory proteins, such as angiotensin converting enzyme (ACE) and endothelial nitric oxide synthase (eNOS), have also been investigated given the importance of vascular reactivity in the pathogenesis of AKI (11,18,20–22).
However, it remains difficult to ascertain which polymorphisms are truly associated with AKI (23). False positive reports are common in genetic association studies, and the plausibility of an association is highly dependent on the quality of the studies involved (24). Therefore, we conducted this systematic review to evaluate the quality of published studies on genetic associations with AKI and to ascertain if the current evidence demonstrates any polymorphism to be conclusively associated with AKI.
Materials and Methods
Literature Selection
Studies were selected by searching MEDLINE, EMBASE, HuGEnet, SCOPUS, and Web of Science for articles listed from 1950 until December 2007. Terms such as “acute kidney injury” and “acute renal failure” were used in combination with “genetic variation” and “polymorphism” as the search criteria. The exact criteria are listed in Table 1.
Table 1.
Search criteria
| MEDLINE |
| Anuria, acute kidney failure, acute renal insufficiency, uremia, oliguria, creatinine, acute nephropathy, or acute kidney injury |
| AND |
| Gene frequency, genotype, phenotype, variation (genetics), gene expression, gene expression regulation, genes or nucleic acid regulatory sequences |
| EMBASE |
| Oliguria, creatinine blood level, creatinine, creatinine clearance, anuria, uremia, kidney tubule necrosis, acute kidney failure, acute kidney tubule necrosis, kidney dysfunction, acute nephropathy or acute kidney injury |
| AND |
| Allelism, genetic heterogeneity, genotype, human genetics, mutation, phenotype, population genetics, population genetic parameters, gene expression or gene |
| Web of Science, SCOPUS and HuGEnet |
| Acute renal insufficiency, acute nephropathy, acute kidney injury, acute tubular necrosis, acute kidney failure, kidney dysfunction, or acute renal failure |
| AND |
| Gene frequency, genetic frequency, genotype, phenotype, gene variation, genetic variation, gene expression, genetic expression, polymorphism, nucleic acid regulatory sequences, allelism, genetic heterogeneity, mutation or population genetics |
Any original study that pertained to associations between AKI and human gene polymorphisms or variability was included. Exclusion criteria included the following: (1) studies with less than 20 subjects; (2) case reports and series; (3) animal studies; (4) outpatient studies; (5) studies where the etiology of AKI was hemolytic uremic syndrome/thrombotic thrombocytopenic purpura (HUS/TTP), ischemia/reperfusion of a kidney allograft, IgA nephropathy, or glomerulonephritis. The final criterion was included as we hoped to capture studies of AKI primarily from ischemia or nephrotoxic injury, which are the most common causes of AKI in hospitalized settings.
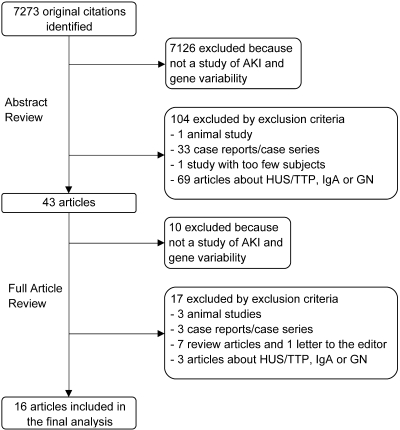
Titles and abstracts of articles found were screened, and articles of interest were selected for full article evaluation (Figure 1); bibliographies of relevant articles were also searched. Full articles were then reviewed by two researchers (JL and UP) independently and a final selection was made; disagreements were discussed and a consensus was reached. On one article pertaining to AKI in preeclampsia (25), discussion was inadequate to achieve consensus and a third researcher (CP) evaluated the study, which resulted in its exclusion.
Figure 1.
Selection of studies. AKI, acute kidney injury; HUS/TTP, hemolytic uremic syndrome/thrombotic thrombocytopenic purpura; IgA, immunoglobulin A nephropathy; GN, glomerulonephritis.
Data Abstraction
The following data was extracted from each study: first author, journal, year of publication, number of cases/controls, ethnicity, and the clinical setting in which AKI occurred. The gene polymorphisms or combinations thereof investigated by each study were recorded, and conclusions of the authors noted. Primary and secondary endpoints of each study were recorded as well. Study quality parameters were also collected. Each study then received an aggregate quality score using a system adapted from Clark et al. (26). Studies were scored in 10 categories (Table 2). Cohort studies were automatically given one point in the control group category to facilitate comparison; this was deemed acceptable, as cohort studies are better for studying high prevalence conditions such as AKI. Studies were scored as “good” if the score was 8 to 10, “fair” if the score was 5 to 7 and “poor” if the score was <4.
Table 2.
Scoring system for study quality used in this systematic review
| Quality Criterion | Explanation | Scoring |
|---|---|---|
| Control Group | Was the control group equal or larger than the case group, and can it be replicated from the description given? Cohort studies were automatically given a point. | Yes = 1No = 0 |
| Hardy-Weinberg Equilibrium | Were the case and control groups assessed for Hardy-Weinberg Equilibrium? | Yes = 1No = 0 |
| Case Group | Can the case group be replicated from the description given, and was the disease state of interest adequately defined? | Yes = 1No = 0 |
| Primer | Was the primer sequence used for genotyping or a reference to one provided? | Yes = 1No = 0 |
| Reproducibility | Can the genotyping method be reproduced from the description given, and was the method validated via a second technique? | Yes = 1No = 0 |
| Blinding | Were the researchers performing the genotyping blinded to the clinical status of the patient? | Blinded = 1Not blinded = 0 |
| Power Calculation | Was a power calculation performed? | Yes = 1No = 0 |
| Statistics | Were the major findings presented with well described tests of significance? | Yes = 1No = 0 |
| Corrected Statistics | If a study examined two or more polymorphisms, were the statistics corrected for the increased risk of a false-positive finding? | Corrected = 1No correction = 0 |
| Independent Replication | Was a second, confirmatory study performed? | Yes = 1No = 0 |
Results
The authors’ search returned 7273 unique articles, of which 43 were retrieved for full article review based upon titles and abstracts. Of these, 16 articles met the established eligibility criteria and were included in the analysis; initial inter-reviewer agreement on article selection was excellent (40/43). Overall, these 16 articles investigated 21 candidate genes and 35 separate polymorphisms in association with AKI. Four studies investigated the role of gene-gene interactions describing 15 different combinations and their association with AKI. Of the 21 genes studied, seven were involved in inflammatory pathways, five in oxidative stress or ischemia, four in vasomotor regulation, two in drug metabolism and one each in angiogenesis, coagulation regulation, and cholesterol metabolism.
Of the 16 included studies, 14 described genetic associations with AKI incidence (Table 3a) while two analyzed AKI outcomes (Table 3b). All studies had a cohort or case-cohort design; 11 were prospective studies. All study populations were of mixed gender; 12 studies investigated adults, while four studied neonates. Only nine of 16 studies reported the ethnicity of their populations. The clinical setting where AKI was studied fell primarily into two groups: postcardiopulmonary bypass (seven studies), and critically ill with or without sepsis (six studies). Of the remaining studies, two investigated all hospitalized patients with AKI, while one study investigated patients after hematopoietic cell transplant. AKI in all studies was caused by ischemia/reperfusion, sepsis, or nephrotoxic agents.
Table 3a.
Characteristics of studies examining genetic risk factors for acute kidney injury (AKI) incidence
| Article (author, year) | Type of study | Site | Gene Polymorphism(s) studied | # of patients | # of AKI cases | Study Population | Clinical Setting | Ethnicity | % Caucasian | Definition of AKI or clinical variable studied | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Banyasz 2006 | Retrospective case cohort | SC | VEGF −2578 C/A, +405 G/C, −460 T/C | 128 | 41 | VLBW infants | Critically ill | NR | Modi 1999b | 5 | |
| Chew 2000 | Prospective cohort | SC | APO E e2/e3/e4 | 564 |
|
Adults | Post-CPB | NR | Delta Cr | 7 | |
| Fekete 2003 | Retrospective case cohort | SC | HSP72 + 1267 A/G HSP73 + 190 G/C | 120 | 37 | VLBW infants | Critically ill | Hungarian Ethnicity | 100 | Modi 1999 | 5 |
| Gordon 2004 | Prospective cohort | MC | TNF-a −238 G/A, −308 G/A LTA + 365 C/G, +249G/A TNFRSF1A + 1135 C/T, +36 A/G,−609 G/T TNFRSF1B + 1663 A/G, +676 T/G | 213 | Adults | Critically ill with sepsis | Caucasian | 100 | SOFA renal score | 9 | |
| Guadino 2002 | Prospective cohort | SC | IL-6 −174 G/C | 111 | Adults | Post-CPB | NR | Delta Cr | 6 | ||
| Isbir 2007 | Prospective case cohort | NR | ACE I/D APO E e2/e3/e4 AGTR1 + 1166 A/C | 248 | 54 | Adults | Post-CPB | NR | Bellomo 2004 (RIFLE)c | 4 | |
| Luo 2004 | Prospective cohort | SC | Haptoglobin phenotype (Hp2-2 vs (Hp2−1 and Hp1−1)) | 148 | 27 | Adults | Post-CPB | Chinese, Malay Indian | 0 | Nash 2002d | 4 |
| MacKensen 2004 | Prospective cohort | NR | APO E e2/e3/e4 | 130 | Adults | Post-CPB | Mixed | NR | Delta Cr | 7 | |
| Nobilis 2001 | Retrospective case cohort | SC | ACE I/D AGTR1 + 1166 A/C | 110 | 42 | VLBW infants | Critically ill | NR | Modi 1999 | 4 | |
| Stafford-Smith 2005 | Prospective cohort | SC | ACE I/D Angiotensinogen + 842 T/C AGTR 1 + 1166 A/C eNOS + 894 G/T IL-6 −174 G/C, −572 G/C, −597 G/A TNF-a + 488 G/A, +376 G/A, −308 G/A APO E + 448 T/C (APOE e4) APO E + 586 C/T (APOE e2) | 1671 | Adults | Post-CPB | Caucasian, African American | 88 | Delta Cr | 8 | |
| Sirgo 2004 | Prospective cohort | SC | PAI-1 4G/5G | 150 | 11 | Adults | Post-CPB | Caucasian | 100 | Doubling of Cr | 4 |
| Treszl 2002 | Retrospective case cohort | SC | TNF-a −308 G/A IL-1b + 3954 C/T IL-6 −174 G/C IL-10 −1082 G/A | 92 | 36 | VLBW infants | Critically ill with sepsis | NR | Modi 1999 | 7 | |
| Wattanathum 2005 | Prospective cohort | SC | IL-10 haplotype (−592 C/A, −1082 A/G,+3367 G/A) | 158 | Adults | Critically ill with sepsis | Caucasian | 100 | # of days free of renal dysfunction | 5 | |
| Woodahl 2007 | Retrospective cohort | SC | ABCB1 + 1236C/T, +2677 G/T/A +3435 C/T,+1199 G/A | 121 | 48 | Adults | Post-HCT | NR | Doubling of Cr | 6 |
Studies that looked at continuous outcome variables did not define cases.
Modi 1999 definition of AKI: serum Cr > 120 umol and/or serum urea >9 mmol/L, and diuresis of 1.0 ml urine/kg/h.
Bellomo 2004 (RIFLE) definition of AKI: increase in serum creatinine of 50% or greater.
Nash 2002 definition of AKI: delta Cr > 0.5 mg/dl from baseline of 1.9 mg/dl or less, 1.0 mg/dl from baseline of 2.0 to 4.9 mg/dl and 1.5 mg/dl for patients with baseline >5.0 mg/dl. NR, not reported; SC, single center; MC, multicenter; VLBW, very low birth weight; CPB, cardiopulmonary bypass; Delta Cr, change in serum creatinine in mg/dl; HCT, hematopoetic cell transplantation.
Table 3b.
Characteristics of studies examining genetic risk factors for AKI outcome
| Article (author, year) | Type of study | Site | Gene Polymorphism(s) studied | # of patients | # of AKI cases | Study Population | Clinical Setting | Ethnicity | % Caucasian | Definition of AKI or clinical variable studied | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Perianayagam 2007 | Prospective cohort | MC | NADPH Oxidase p22phox + 242C/T Catalase −262 C/T | 200 | 200 | Adults | Hospitalized | Mixed | 90 | Dialysis or mortality | 7 |
| Jaber 2004 | Prospective cohort | MC | TNF-a −308 G/A IL-10 −1082 A/G | 61 | 61 | Adults | Hospitalized | Mixed | 93 | Mortality and recovery of renal function | 5 |
MC, multicenter.
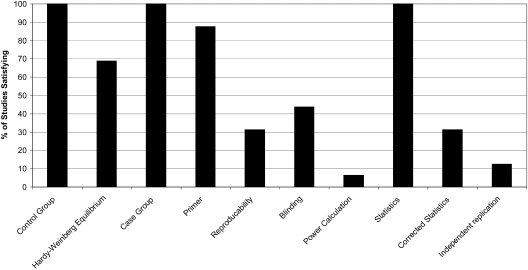
The definition of AKI varied greatly in the included studies, as five different definitions of AKI were used (9,27,28). In addition, four studies opted to report only the change in creatinine. Two studies used hard endpoints of dialysis or mortality. The quality of studies was generally mediocre; mean quality score was 5.8/10 (range 4 to 9). Four studies were scored as “poor,” 10 were “mediocre,” while only two were of “good” quality. Primarily, studies were lacking in the areas of reproducibility, blinding, power calculations, corrected statistics, and independent replication (Figure 2) (26).
Figure 2.
Percentage of all studies reviewed that achieved each of the 10 quality criteria.* *Based on Clark MF, Baudouin SV: A systematic of the quality of genetic association studies in human sepsis (26). Please see Table 2 for full descriptions of each criteria.
Table 4 describes the specific polymorphisms studied and the authors’ conclusions. In summary, nine polymorphisms (NADPH Oxidase p22phox + 242C/T; Haptoglobin Hp2-2, Hp2-1 or HP1-1; Heat Shock Protein 72 (HSP72) +1267A/G; Apolipoprotein E (APO E) e2/e3/e4; Angiotensin Converting Enzyme (ACE) I/D; TNF-α −308G/A; IL-6 −174G/C; Interleukin-10 (IL-10) −1082G/A; Vascular Endothelial Growth Factor (VEGF) −2578C/A) were found to have significant associations in individual studies (14,16,20,29–33). Only one polymorphism, APO E e2/e3/e4, had an association with AKI demonstrated in multiple studies.
Table 4.
Gene characteristics
| Gene | Polymorphism | Chromosome | Functional significance | Type of polymorphism | Study | Significant association with AKI | Comparison | Association Variable | Magnitude of association | Significance of association |
|---|---|---|---|---|---|---|---|---|---|---|
| APO E | e2/e3/e4 | 19q13 | Cholesterol metabolism | SNP | Isbir 2007 | Yes | e4 vs. non-e4 | AKI | OR = 0.18b | P = 0.002 |
| e2/e3/e4 | MacKensen 2004 | No | e4 vs. non-e4 | Delta Cr | P = 0.82 | |||||
| e2/e3/e4 | Chew 2000 | Yes | e4 vs. e2, e3 | Delta Cr | P = 0.038, P = 0.015 | |||||
| e2/e3/e4 | Stafford-Smith 2005 | No | e4 vs. non-e4 | Delta Cr | P = 0.009a,c | |||||
| e2/e3/e4 | Stafford-Smith 2005 | No | e2 vs. non-e2 | Delta Cr | P = 0.32d | |||||
| NADPH Oxidase p22phox | +242C/T | 16q24 | Oxidative stress | SNP | Perianayagam 2007 | Yes | CT/TT versus CC | Dialysis or mortality | OR = 2.11 | P = 0.01 |
| Catalase | −262 C/T | 11p13 | Oxidative stress | SNP | Perianayagam 2007 | No | CT/TT vs. CC | Dialysis or mortality | OR = 1.05 | P = 0.86 |
| ACE | I/D | 17q23 | Vasomotor regulation | Intron deletion | Isbir 2007 | Yes | ID/DD vs. II | AKI | OR = 2.37b | P = 0.021 |
| I/D | Stafford-Smith 2005 | No | II/ID vs. DD | Delta Cr | P = 0.004a | |||||
| I/D | Nobilis 2001 | No | I vs. D allele | AKI | NS | |||||
| AGT | +842 T/C | 1q42 | Vasomotor regulation | SNP | Stafford-Smith 2005 | No | CC/CT vs. TT | Delta Cr | P < 0.0001a,c; 0.99d | |
| AGTR1 | +1166 A/C | 3q21-25 | Vasomotor regulation | SNP | Isbir 2007 | No | AA vs. AC/CC | AKI | OR = 1.09b | P > 0.05 |
| +1166 A/C | Stafford-Smith 2005 | No | CC/CA vs. AA | Delta Cr | P = 0.84c | |||||
| +1166 A/C | Nobilis 2001 | No | C vs. A allele | AKI | NS | |||||
| +1166 A/C | Li 2007 | No | AA vs. AC/CC | AKI | OR = 0.34b | P = 0.116 | ||||
| eNOS | +894 G/T | 7q36 | Vasomotor regulation | SNP | Stafford-Smith 2005 | No | TT/TG vs. GG | Delta Cr | P = 0.17c; 0.04a,d | |
| ABCB1 | +1236 C/T | 7q21 | Drug metabolism | SNP | Woodahl 2007 | No | TT vs. CC/CT | AKI | OR = 1.9 | P = 0.21 |
| +2677 G/T/A | Woodahl 2007 | No | TT vs. GG/GT | AKI | OR = 1.6 | P = 0.34 | ||||
| +2677 G/T/A | Woodahl 2007 | No | TT vs. TA/GA | AKI | OR = 2.5 | P = 0.33 | ||||
| +3435 C/T | Woodahl 2007 | No | TT vs. CT/CC | AKI | OR = 1.1 | P = 0.82 | ||||
| +1199 G/A | Woodahl 2007 | No | GG vs. GA/AA | AKI | OR = 3.2 | P = 0.14 | ||||
| CYP3A5 | a1/a3 | 7q21 | Drug metabolism | Splice variant | Woodahl 2007 | No | a3/a3 vs. a1/a3 + a1/a1 | AKI | OR = 0.8 | P = 0.68 |
| HSP72 | +1267 A/G | 6p21 | Ischemia tolerance | SNP | Fekete 2003 | Yes | GG vs. GA/AA | AKI | OR = 3.17 | P < 0.01 |
| HSP73 | +190 G/C | 11q24 | Ischemia tolerance | SNP | Fekete 2003 | No | G vs. C allele | AKI | NS | |
| Haptoglobin | Hp2-2, Hp2-1, or Hp1-1 | 16q22 | Fe metabolism, Anti-oxidant | Phenotype | Luo 2004 | Yes | Hp2-2 vs. Hp1-1/2-1 | AKI | OR = 5.4 | P = 0.03 |
| TNF-α | −308 G/A | 6p21 | Proinflammatory | SNP | Jaber 2004 | Yes | AA/AG vs. GG | Mortality | HR = 2.47 | P = 0.04 |
| −308 G/A | Stafford-Smith 2005 | No | AA/AG vs. GG | Delta Cr | P = 0.17c | |||||
| −308 G/A | Gordon 2004 | No | A vs. G allele | Renal SOFA score | NS | |||||
| −308 G/A | Treszl 2002 | No | A vs. G allele | AKI | NS | |||||
| −238 G/A | Gordon 2004 | No | A vs. G allele | Renal SOFA score | NS | |||||
| +376 G/A | Stafford-Smith 2005 | No | AA/AG vs. GG | Delta Cr | NS | |||||
| +488 G/A | Stafford-Smith 2005 | No | AA/AG vs. GG | Delta Cr | NS | |||||
| LTA | +365 C/G | 6p21 | Proinflammatory | SNP | Gordon 2004 | No | C vs. G allele | Renal SOFA score | NS | |
| +249 G/A | Gordon 2004 | No | G vs. A allele | Renal SOFA score | NS | |||||
| IL-1b | +3954 C/T | 2q14 | Proinflammatory | SNP | Treszl 2002 | No | T vs. C allele | AKI | NS | |
| IL-6 | −174 G/C | 7p21 | Inflammation modulator | SNP | Stafford-Smith 2005 | No | GG vs. CC/CG | Delta Cr | NS | |
| −174 G/C | Guadino 2002 | Yes | GG vs. CC/CG | Delta Cr | P < 0.0001 | |||||
| −174 G/C | Treszl 2002 | No | C vs. G allele | AKI | NS | |||||
| −572 G/C | Stafford-Smith 2005 | No | GG vs. CC/CG | Delta Cr | P < 0.0001a,c | |||||
| −597 G/A | Stafford-Smith 2005 | No | AA/AG vs. GG | Delta Cr | NS | |||||
| TNFRSF1A | +1135 C/T | 12p13 | Anti-inflammatory | SNP | Gordon 2004 | No | C vs. T allele | Renal SOFA score | NS | |
| +36 A/G | Gordon 2004 | No | A vs. G allele | Renal SOFA score | NS | |||||
| -609 G/T | Gordon 2004 | No | G vs. T allele | Renal SOFA score | NS | |||||
| TNFRSF1B | +1663 A/G | 1p36 | Anti-inflammatory | SNP | Gordon 2004 | No | A vs. G allele | Renal SOFA score | NS | |
| +676 T/G | Gordon 2004 | No | T vs. G allele | Renal SOFA score | NS | |||||
| IL-10 | −1082 G/A | 1q31-32 | Anti-inflammatory | SNP | Jaber 2004 | Yes | GG/GA vs. AA | Mortality | HR = 0.36 | P = 0.03 |
| −1082 G/A | Treszl 2002 | No | G vs. A allele | AKI | NS | |||||
| VEGF | −2578 C/A | 6p12 | Angiogenesis | SNP | Banyasz 2006 | Yes | AA vs. AC/CC | AKI | OR = 0.2 | P = 0.021 |
| −460 T/C | Banyasz 2006 | No | CC vs. CT/TT | AKI | NS | |||||
| +405 G/C | Banyasz 2006 | No | CC vs. GC/GG | AKI | NS | |||||
| PAI-1 | -675 4G/5G | 7q21 | Coagulation activation | SNP | Sirgo 2004 | No | 4G/4G vs. 4G/5G + 5G/5G | AKI | NS |
Not significant after adjustment for multiple comparisons;
calculated from published data;
for Caucasian subgroup;
for African-American subgroup.
NR, not reported; SC, single centre; MC, multicenter; VLBW, very low birth weight; HCT, hematopoietic cell transplantation; Cr, creatinine; NS, not significant. Italics represent results from multivariable analysis.
Apolipoprotein E
Isbir et al. (20) found that in patients undergoing coronary artery bypass grafting (CABG), carriers of the APO E e4 allele had a decreased risk of AKI compared with non-APO E e4 patients (unadjusted odds ratio [OR] = 0.18, P = 0.002). Chew et al. (30) found the same result in a similar population, as those with the e4 allele had a smaller postoperative change in creatinine compared with those with e3 and e2 alleles (P = 0.015 versus e3, P = 0.038 versus e2), even after adjustment for preoperative creatinine, age, bypass time, hypertension, diabetes, and ejection fraction. Mackensen et al. (34), examining a similar group of 130 CABG patients, also found this allele to have a protective effect but only after adjustment for ascending aortic atheroma burden; without adjustment, no association was found. Finally, a large study of 1671 cardiac surgical patients by Stafford-Smith et al. (11) found no significant association between APO E alleles and the degree of change of postoperative creatinine in Caucasian or African American populations.
Oxidative Stress Genes
In a study of 200 patients with mixed-cause AKI, Perianayagam et al. (33) examined the association of polymorphisms in NADPH Oxidase p22phox and Catalase, two enzymes involved with the regulation of reactive oxygen species, with dialysis and mortality. Individuals with the T allele in the NADPH Oxidase p22phox gene possessed a greater risk of dialysis or mortality (unadjusted OR = 2.11). This remained true after adjusting for race, gender, age, APACHE II score and CKD. No association was demonstrated between the Catalase gene and AKI. This study was unique in that it is one of two studies that examined associations with firm outcomes, i.e., dialysis or mortality (16,33).
Vasomotor Regulation Genes
For the ACE I/D polymorphism, three studies were identified, of which only one found significant associations with AKI. Isbir et al. found that patients with the ACE D allele exhibited increased risk of AKI following CABG (unadjusted OR = 2.37, P = 0.021). However, Stafford-Smith and colleagues also examined the ACE I/D polymorphism and found no association with AKI (11). Another study of 110 very low birth weight (VLBW) infants also found no association (21).
No significant associations were found with other genes involved with vasomotor regulation, including Angiotensinogen (AGT), Angiotensin Receptor 1 (AGTR1), and eNOS.
Inflammatory and Anti-Inflammatory Genes
Six different studies examined a total of 15 polymorphisms in seven genes involved in inflammatory and anti-inflammatory pathways. Jaber et al. (16), in a study of 61 patients with AKI requiring hemodialysis, found that high producers of TNF-α (−308 A-allele carriers) possessed an increased risk of death after adjustment for APACHE II score (adjusted hazard ratio [HR] = 2.5, P = 0.04). However, three other studies searched for an association between this polymorphism and AKI incidence, but found none (11,15,17).
The same study by Jaber et al. (16) also found that IL-10 intermediate/high producers (−1082 G-allele carriers) had a decreased risk of death after adjustment for the multiple organ failure score (adjusted HR = 0.36, P = 0.03). Treszl et al. also investigated this polymorphism in VLBW infants but found no association with AKI incidence (17).
The IL-6 −174G/C polymorphism was investigated in three studies, of which only Guadino et al. (14) found a significant association. This study found that in patients undergoing CABG, IL-6 −174GG, carriers had significantly higher elevations in perioperative creatinine versus non-GG carriers (P < 0.0001). However, Stafford-Smith et al. (11) found no such association in a similar population, nor did Treszl et al. (17) in VLBW infants.
Other Genes
Luo et al. (32), in a population of 148 CABG patients, found that Haptoglobin 2-2 phenotype was associated with an increased risk of AKI (OR = 5.4, P = 0.03). In the HSP72 gene, Fekete et al. (31) found that VLBW infants homozygous for the G allele were at increased risk for AKI (OR = 3.17, P < 0.01). This same group also found that for the VEGF −2578 C/A polymorphism, VLBW infants homozygous for the A allele were protected against AKI (OR = 0.2, P = 0.021) (29).
None of the remaining polymorphisms investigated were found to be significantly associated with AKI (35,36).
Gene-Gene Interactions
There were four studies that investigated gene-gene interactions and their association with AKI. The combinations studied were mostly those that augmented inflammatory or down-regulated anti-inflammatory pathways (Table 5). Wattanathum et al. (37) found that the CGG haplotype involving three separate polymorphisms in the IL-10 gene (−592 C/A, +734 A/G and + 3367 G/A) was associated with a greater degree of AKI in patients with sepsis from pneumonia. They postulated that this genotype is associated with lower anti-inflammatory IL-10 production, thus causing increased renal dysfunction.
Jaber et al. (16) investigated whether the combination of pro-inflammatory alleles from the TNF-α −308G/A and IL-10 −1082 G/A polymorphisms, namely the TNF-α −308 AA and IL-10 −1082 AA/AG genotypes, were associated with an increased risk for dialysis or death. They found that patients with these genotypes had an elevated risk for dialysis or death after adjustment for APACHE II score (adjusted HR = 5.17, P = 0.005). Treszl et al. (17) also investigated the TNF-α −308G/A polymorphism, but in combination with the IL-6 −174G/C polymorphism. VLBW infants who had a combination of the IL-6 −74C allele and the TNF-α −308A allele were at increased risk for developing AKI (OR = 6.07, P < 0.01).
Finally, Stafford-Smith et al. (11) investigated the interaction between AGT + 842T/C and IL-6 −572G/C polymorphisms and found the combination of C-alleles in both genes to be significantly associated with AKI (P < 0.0001) in Caucasians. This study also found many interactions that trended toward significance (Table 5); however, only this association remained significant after adjustment for multiple comparisons (adjusted P = 0.032).
Table 5.
Gene combinations
| Study | Gene polymorphism combinations | Significant association with AKI | Magnitude of association | Significance of association |
|---|---|---|---|---|
| Wattanathum 2005 | IL-10 −592 C/A, +734 A/G and + 3367 G/A | Yes | P = 0.024 | |
| Stafford-Smith 2005 | eNOS + 894 G/T and AGTR1 + 1166 A/C | No | P = 0.006a,c | |
| IL-6 −572 G/C and TNF-α −308 G/A | No | P = 0.05a,c | ||
| AGT + 842 T/C and IL-6 −572 G/C | Yes | P ≤ 0.0001c | ||
| APO E e4 and AGT + 842 T/C | No | P = 0.03a,c | ||
| AGTR1 + 1166 A/C and APO E e4 | No | P = 0.02a,c | ||
| eNOS + 894 G/T and ACE I/D | No | P = 0.006a,d | ||
| AGT + 842 T/C and APO E e2 | No | P = 0.03a,d | ||
| Jaber 2004 | IL-10 −1082 G/A and TNF-α −308 G/A | Yes | HR = 5.72 | P = 0.004 |
| Treszl 2002 | TNF-α −308 G/A and IL-6 −174 G/C | Yes | OR = 6.07b | P < 0.01 |
| TNF-α −308 G/A and IL-1b + 3954 C/T | No | NS | ||
| TNF-α −308 G/A and IL-10 −1082 G/A | No | NS | ||
| IL-1b + 3954 C/T and IL-6 −174 G/C | No | NS | ||
| IL-1b + 3954 C/T and IL-10 −1082 G/A | No | NS | ||
| IL-10 −1082 G/A and IL-6 −174 G/C | No | NS |
Not significant after adjustment for multiple comparisons;
calculated from published data;
for Caucasian subgroup;
for African-American subgroup.
Italics represent results of multivariable analysis.
Discussion
While there have been several descriptive reviews on this topic (38,39), to the authors’ knowledge this is the first systematic review of genetic determinants of AKI. In summary, it was found that there is no single polymorphism that can be conclusively described as a risk factor in AKI. The general dearth of studies, the lack of confirmatory studies, and their overall mediocre quality led to this conclusion.
Of the 35 individual polymorphisms whose association with AKI has been studied, only APO E e2/e3/e4 had a significant association in more than one study (20,30). Recent evidence points to a regulatory role of APO E in inflammatory responses and may thus be affecting a given patient's susceptibility to AKI (18,40). However, the positive association found in two studies (20,30) was contradicted by the results from the largest, highest quality study by Stafford-Smith et al. (11). In this case, having to correct for multiple comparisons decreased this study's power to find an effect, for while the p-values found by this study were <0.05, they were not statistically significant after adjustment. While a future, higher-powered study may find a role of APO E in AKI, current evidence is inadequate to make such a claim.
Of the gene-gene interactions studied, the association between the TNF-α −308 AA and IL-10 −1082 AA/AG genotypes with dialysis or mortality demonstrated by Jaber et al. is the most promising (16). While this association has yet to be replicated, the individual polymorphisms had small but significant associations with poor outcomes, and the combined gene-gene interaction was associated with a much higher risk than either polymorphism individually. This group also demonstrated phenotypic differences in ex vivo production of TNF-α and IL-10 between genotypic groups, which lend biologic plausibility to this linkage.
These results however do not belie the major finding of this review that, thus far, findings in this field have been inconsistent and contradictory. There is significant interstudy heterogeneity of results, as seen here with APO E. Causes for such heterogeneity include the large number of comparisons performed, which significantly increases the number of associations found by chance (41). The case of APO E clearly shows this, for while Stafford-Smith et al. demonstrated p-values <0.05 in a number of genes, statistical significance was not achieved due to the large number of comparisons performed (11). Population stratification from ethnic admixture, variable linkage disequilibrium, and population specific gene-gene or gene-environment interactions are also potential sources of heterogeneity. Therefore, it is recommended for authors to independently verify their results before publication (42). This is especially necessary given that initial studies tend to show more impressive associations than subsequent research, a problem that is particularly endemic in genetic association research (24).
The lack of a gold standard outcome has also contributed to study heterogeneity (43). The authors found five different definitions of AKI in the studies found by their search; such variability increases the number of spurious associations and makes interstudy comparison difficult. Use of well-defined criteria, such as the RIFLE or AKIN criteria, will decrease heterogeneity and facilitate comparisons in future investigations; (27,44) specifically, using stage 2 of the AKIN criteria would allow for the creation of case groups that are both highly specific and have a well-defined phenotype. Also, as evidence accumulates for the role of novel biomarkers such as Neutrophil Gelatinase-associated Lipocalin (NGAL) in AKI, these biomarkers can be used to further increase specificity (45). Studying outcomes such as dialysis or mortality would also alleviate this problem; however, due to the rarity of such events, an adequately powered study examining such outcomes may not be feasible.
The authors’ study has limitations in several regards. This systematic review is vulnerable to several types of bias, the first being publication bias (46). The literature has an inherent bias toward the publication of studies that find positive results (47). No attempt was made to retrieve unpublished data, as it is unknown whether this would have corrected the bias or worsened it. Therefore, despite using systematic methods, this may be a nonrepresentative sample of existing genetic association studies of AKI.
Another potential limitation is analysis reporting bias caused by researchers who report only a portion of their analyses (48). This is especially salient in the reporting of gene-gene interactions, where many combinations may have been analyzed for association but never reported.
In conclusion, the present understanding of AKI suggests that genetic heterogeneity in pathways that regulate vascular and inflammatory responses to injury provide a plausible explanation for individual variability in susceptibility to AKI. Continued efforts in this field are important, as finding genetic risk factors will allow clinicians to identify patients at risk and implement preventive therapies. Identification of culprit genes may also elucidate the true pathophysiology of AKI. Although some genes show promise, existing candidate gene studies have thus far been unable to find conclusive evidence to confirm any association.
An alternative approach will be to use genome-wide association (GWA) studies. This technique has been harnessed to successfully investigate genetic vulnerabilities in complex diseases such as macular degeneration and diabetes (13,49). GWA studies have the advantages of searching for candidate polymorphisms unhindered by previous hypotheses and greater power in demonstrating the effects of gene-gene interactions or high-risk haplotypes (50). This is especially important for complex disorders, where increased genetic risk may be from several different polymorphisms acting together. A pitfall is that GWA studies have unprecedented potential for false-positive results given the immense number of statistical tests performed; this problem can be somewhat alleviated by multistage designs and stringent requirements for statistical significance (50). Nevertheless, the success of future work will likely depend on harnessing this revolutionary technique and the application of a true consensus definition of AKI.
Disclosures
None.
Acknowledgments
The authors would like to thank Dr. H. Dean Hosgood, III, Yale University Department of Public Health. The authors would also like to thank the following TRIBE-AKI members: Dr. Charles Edelstein, Dr. Prasad Devarajan, Dr. Amit Garg, Dr. Mike Shlipak, Dr. Mike Zappatelli, Dr. Patrick Murray, Dr. Jay Koyner, Dr. Madhav Swaminathan, Dr. Catherine Dent, and Dr. Zhu Wang.
Jonathan Lu was supported by a Yale University School of Medicine Medical Student Research Fellowship. Dr. Parikh was supported by NIH grants (RO1-HL 85757), Dr. Patel grant K23 DK075929-01, Dr. Coca grant F32 DK076318-01A1
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UptoDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Lameire N, Van Biesen W, Vanholder R: Acute renal failure. Lancet 365: 417–430, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS: Community-based incidence of acute renal failure. Kidney Int 72: 208–212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ: Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chawla LS, Abell L, Mazhari R, Egan M, Kadambi N, Burke HB, Junker C, Seneff MG, Kimmel PL: Identifying critically ill patients at high risk for developing acute renal failure: A pilot study. Kidney Int 68: 2274–2280, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, Landolfo K: Acute renal failure following cardiac surgery. Nephrol Dial Transplant 14: 1158–1162, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Lameire N, Van Biesen W, Vanholder R: The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol 2: 364–377, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Shusterman N, Strom BL, Murray TG, Morrison G, West SL, Maislin G: Risk factors and outcome of hospital-acquired acute renal failure. Clinical epidemiologic study. Am J Med 83: 65–71, 1987 [DOI] [PubMed] [Google Scholar]
- 9.Nash K, Hafeez A, Hou S: Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Hoste EA, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JM, Colardyn FA: Acute renal failure in patients with sepsis in a surgical ICU: Predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol 14: 1022–1030, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Stafford-Smith M, Podgoreanu M, Swaminathan M, Phillips-Bute B, Mathew JP, Hauser EH, Winn MP, Milano C, Nielsen DM, Smith M, Morris R, Newman MF, Schwinn DA; Perioperative Genetics and Safety Outcomes Study (PEGASUS) Investigative Team: Association of genetic polymorphisms with risk of renal injury after coronary bypass graft surgery. Am J Kidney Dis 45: 519–530, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16: 162–168, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J: Complement factor H polymorphism in age-related macular degeneration. Science 308: 385–389, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudino M, Di Castelnuovo A, Zamparelli R, Andreotti F, Burzotta F, Iacoviello L, Glieca F, Alessandrini F, Nasso G, Donati MB, Maseri A, Schiavello R, Possati G: Genetic control of postoperative systemic inflammatory reaction and pulmonary and renal complications after coronary artery surgery. J Thorac Cardiovasc Surg 126: 1107–1112, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Gordon AC, Lagan AL, Aganna E, Cheung L, Peters CJ, McDermott MF, Millo JL, Welsh KI, Holloway P, Hitman GA, Piper RD, Garrard CS, Hinds CJ: TNF and TNFR polymorphisms in severe sepsis and septic shock: A prospective multicentre study. Genes Immun 5: 631–640, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Jaber BL, Rao M, Guo D, Balakrishnan VS, Perianayagam MC, Freeman RB, Pereira BJ: Cytokine gene promoter polymorphisms and mortality in acute renal failure. Cytokine 25: 212–219, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Treszl A, Tóth-Heyn P, Kocsis I, Nobilis A, Schuler A, Tulassay T, Vásárhelyi B.: Interleukin genetic variants and the risk of renal failure in infants with infection. Pediatr Nephrol 17: 713–717, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Bonventre JV, Weinberg JM: Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ: Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol 168: 5817–5823, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Isbir SC, Tekeli A, Ergen A, Yilmaz H, Ak K, Civelek A, Zeybek U, Arsan S: Genetic polymorphisms contribute to acute kidney injury after coronary artery bypass grafting. Heart Surg Forum 10: E439–E444, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Nobilis A, Kocsis I, Tóth-Heyn P, Treszl A, Schuler A, Tulassay T, Vásárhelyi B: Variance of ACE and AT1 receptor gene does not influence the risk of neonatal acute renal failure. Pediatr Nephrol 16: 1063–1066, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Oken DE: Hemodynamic basis for human acute renal failure (vasomotor nephropathy). Am J Med 76: 702–710, 1984 [DOI] [PubMed] [Google Scholar]
- 23.Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N: Assessing the probability that a positive report is false: An approach for molecular epidemiology studies. J Natl Cancer Inst 96: 434–442, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NCI-NHGRI Working Group on Replication in Association Studies, Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, Hirschhorn JN, Abecasis G, Altshuler D, Bailey-Wilson JE, Brooks LD, Cardon LR, Daly M, Donnelly P, Fraumeni JF Jr, Freimer NB, Gerhard DS, Gunter C, Guttmacher AE, Guyer MS, Harris EL, Hoh J, Hoover R, Kong CA, Merikangas KR, Morton CC, Palmer LJ, Phimister EG, Rice JP, Roberts J, Rotimi C, Tucker MA, Vogan KJ, Wacholder S, Wijsman EM, Winn DM, Collins FS: Replicating genotype-phenotype associations. Nature 447: 655–660, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Li H, Ma Y, Fu Q, Wang L: Angiotensin-converting enzyme insertion/deletion (ACE I/D) and angiotensin II type 1 receptor (AT1R) gene polymorphism and its association with preeclampsia in Chinese women. Hypertension in Pregnancy 26: 293–301, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Clark MF, Baudouin SV: A systematic review of the quality of genetic association studies in human sepsis. Intensive Care Med 32: 1706–1712, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P: Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modi N: Disorders of the kidney and urinary tract. In: Textbook of Neonatology. edited by Rennie JM, Roberton NR, Edinburgh, Churchill-Livingston, 1999, pp 1009–1037
- 29.Bányász I, Bokodi G, Vásárhelyi B, Treszl A, Derzbach L, Szabó A, Tulassay T, Vannay A: Genetic polymorphisms for vascular endothelial growth factor in perinatal complications. Eur Cytokine Netw 17: 266–270, 2006 [PubMed] [Google Scholar]
- 30.Chew ST, Newman MF, White WD, Conlon PJ, Saunders AM, Strittmatter WJ, Landolfo K, Grocott HP, Stafford-Smith M: Preliminary report on the association of apolipoprotein E polymorphisms, with postoperative peak serum creatinine concentrations in cardiac surgical patients. Anesthesiology 93: 325–331, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Fekete A, Treszl A, Tóth-Heyn P, Vannay A, Tordai A, Tulassay T, Vásárhelyi B: Association between heat shock protein 72 gene polymorphism and acute renal failure in premature neonates. Pediatr Res 54: 452–455, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Luo HD, Ramirez SP, Costa MD, Tan CT, Oakley RE, Lee CN, Hsu SI: Preoperative microalbuminuria, haptoglobin phenotype 2-2, and age are independent predictors for acute renal failure following coronary artery bypass graft. Ann Acad Med Singapore 33: S15–S16, 2004 [PubMed] [Google Scholar]
- 33.Perianayagam MC, Liangos O, Kolyada AY, Wald R, MacKinnon RW, Li L, Rao M, Balakrishnan VS, Bonventre JV, Pereira BJ, Jaber BL: NADPH oxidase p22phox and catalase gene variants are associated with biomarkers of oxidative stress and adverse outcomes in acute renal failure. J Am Soc Nephrol 18: 255–263, 2007 [DOI] [PubMed] [Google Scholar]
- 34.MacKensen GB, Swaminathan M, Ti LK, Grocott HP, Phillips-Bute BG, Mathew JP, Newman MF, Milano CA, Stafford-Smith M; Perioperative Outcomes Research Group; Cardiothoracic Anesthesiology Research Endeavors (C.A.R.E.) Investigators of the Duke Heart Center: Preliminary report on the interaction of apolipoprotein E polymorphism with aortic atherosclerosis and acute nephropathy after CABG. Ann Thorac Surg 78: 520–526, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Sirgo G, Perez JL, Renes E, Rubio M, Paredes S, Garcia A, Hernandez E, Morales P, Del Rey MJ, Perales N: Role of plasminogen activator inhibitor-1 (PAI-1) 4G/5G promoter polymorphism in cardiac surgery outcome: Ventricular dysfunction, mortality, and postoperative complications and functional recovery. Investigacion Cardiovascular 7: 116–130, 2004 [Google Scholar]
- 36.Woodahl EL, Hingorani SR, Wang J, Guthrie KA, McDonald GB, Batchelder A, Li M, Schoch HG, McCune JS: Pharmacogenomic associations in ABCB1 and CYP3A5 with acute kidney injury and chronic kidney disease after myeloablative hematopoietic cell transplantation. Pharmacogenomics J 8: 248–255, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Wattanathum A, Manocha S, Groshaus H, Russell JA, Walley KR: Interleukin-10 haplotype associated with increased mortality in critically ill patients with sepsis from pneumonia but not in patients with extrapulmonary sepsis. Chest 128: 1690–1698, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Haase-Fielitz A, Haase M, Bellomo R, Dragun D: Genetic polymorphisms in sepsis- and cardiopulmonary bypass-associated acute kidney injury. Contrib Nephrol 156: 75–91, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Jaber BL, Pereira BJ, Bonventre JV, Balakrishnan VS: Polymorphism of host response genes: Implications in the pathogenesis and treatment of acute renal failure. Kidney Int 67: 14–33, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Brown CM, Wright E, Colton CA, Sullivan PM, Laskowitz DT, Vitek MP: Apolipoprotein E isoform mediated regulation of nitric oxide release. Free Radic Biol Med 32: 1071–1075, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Ioannidis JP: Molecular evidence-based medicine: Evolution and integration of information in the genomic era. Eur J Clin Invest 37: 340–349, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Anonymous: Freely associating. Nat Genet 22: 1–2, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Bellomo R: Defining, quantifying, and classifying acute renal failure. Crit Care Clin 21: 223–237, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coca SG, Yalavarthy R, Concato J, Parikh CR: Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int 73: 1008–1016, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Kavvoura FK, Ioannidis JP: Methods for meta-analysis in genetic association studies: A review of their potential and pitfalls. Hum Genet 123: 1–14, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Ioannidis JP: Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA 279: 281–286, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG: Empirical evidence for selective reporting of outcomes in randomized trials: Comparison of protocols to published articles. JAMA 291: 2457–2465, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P: A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445: 881–885, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Pearson TA, Manolio TA: How to interpret a genome-wide association study. JAMA 299: 1335–1344, 2008 [DOI] [PubMed] [Google Scholar]