Abstract
Background and objectives: Mutations in nephrin (NPHS1) and podocin (NPHS2) genes represent a major cause of idiopathic nephrotic syndrome (NS) in children. It is not yet clear whether the presence of a single mutation acts as a modifier of the clinical course of NS.
Design, setting, participants, & measurements: We reviewed the clinical features of 40 patients with NS associated with heterozygous mutations or variants in NPHS1 (n = 7) or NPHS2 (n = 33). Long-term renal survival probabilities were compared with those of a concurrent cohort with idiopathic NS.
Results: Patients with a single mutation in NPHS1 received a diagnosis before those with potentially nongenetic NS and had a good response to therapies. Renal function was normal in all cases. For NPHS2, six patients had single heterozygous mutations, six had a p.P20L variant, and 21 had a p.R229Q variant. Age at diagnosis and the response to drugs were comparable in all NS subgroups. Overall, they had similar renal survival probabilities as non-NPHS1/NPHS2 cases (log-rank χ2 0.84, P = 0.656) that decreased in presence of resistance to therapy (P < 0.001) and in cases with renal lesions of glomerulosclerosis and IgM deposition (P < 0.001). Cox regression confirmed that the only significant predictor of dialysis was resistance to therapy.
Conclusions: Our data indicate that single mutation or variant in NPHS1 and NPHS2 does not modify the outcome of primary NS. These patients should be treated following consolidated schemes and have good chances for a good long-term outcome.
The discovery of podocyte genes that cause familial nephrotic syndrome (NS) allows a more appropriate approach to patients with NS, especially in children with a familial history of proteinuria (1,2). There is growing evidence that mutations in genes coding for slit diaphragm proteins frequently also occur in sporadic NS and are associated with potentially variable clinical outcome (3–9). Most reports have focused on monogenic diseases involving two major genes—nephrin (NPHS1) and podocin (NPHS2)—that together cause almost 60% of the cases of NS in those who are younger than 1 yr and in adolescents (10). A still unresolved issue is the clinical impact of heterozygous mutations and/or variants of NPHS1/NPHS2 because the contribution of heterozygous alleles to NS has received heterogeneous interpretation (3,9,11–16). In particular, it is unknown whether patients who carry a single mutation represent a separate clinical entity, because no data on response to drugs and on the long-term clinical outcome are available.
The reason for an association of NPHS1 and NPHS2 heterozygous mutations with NS remains elusive, because these are recessive conditions that require a molecular defect on both alleles to determine a pathologic effect. One explanation may be a digenic inheritance whereby a mutation of another gene affects the slit diaphragm assembly. Moreover, the presence of a heterozygous mutation or variant in NPHS1 or NPHS2 may act as a modifier of the phenotype and represent a clinical problem in terms of prognosis, drug response, and long-term outcome. Data on this aspect are scattered and offer no practical opportunity for discussion (3,9,11–16).
This article reports the clinical features of 40 patients with NS associated with heterozygous mutations/variants of NPHS1 or NPHS2. Renal survival probabilities of these children were compared with those of a concurrent cohort of children with classical idiopathic NS.
Materials and Methods
Patients
Forty patients who had sporadic NS with single mutations or functional variants in the NPHS1 (n = 7) or NPHS2 (n = 33) gene were retrospectively analyzed with respect to their major clinical features. They belong to a population of patients who were referred for NS to a second-level nephrology unit in Italy and underwent a detailed molecular screening to exclude genetic diseases that potentially are unresponsive to drugs. Relevant clinical features were compared with data from 28 patients with NS for homozygous or compound heterozygous mutations of NPHS1 (n = 11) and NPHS2 (n = 17) genes, respectively, and 221 children with idiopathic NS. Prerequisite for enrollment was the presence of nephrotic proteinuria (>40 mg/kg per d), absence of episodes of hematuria, and negativity of tests for autoimmunity (antibodies against native DNA, p/cANCA). Following our diagnostic flowchart, patients who presented with NS before 1 yr of age and/or those who were older than 16 yr had a renal biopsy. Between 1 and 16 yr, only those with resistance to steroids or to combined therapy with steroids and cyclosporin A (CsA) underwent the biopsy procedure. Patients who showed secondary and primary renal diseases other than congenital nephrosis of the Finnish type (17), diffuse mesangial sclerosis, minimal changes, mesangial proliferation with IgM deposition, or FSGS were excluded (18). As a rule, the therapeutic approach started with steroids 60 mg/m2 per d for 30 to 60 d (19,20), and in case of unresponsiveness (partial or global), three pulses of methylprednisolone were given (10 mg/kg) at successive days followed by another three with reduced dosages (5 mg/d) every other day. Steroids were then associated with CsA (5 mg/kg starting dosage, followed by tapering to reach the minimum dosage required to maintain CsA serum trough levels between 50 and 100 ng/ml). Steroid resistance was considered the failure to achieve remission of proteinuria after completion of the oral and pulse treatment. CsA resistance was the failure to modify proteinuria after 24 wk of CsA; intolerance was considered the worsening of renal function (creatinine clearance <70% of pre-CsA values) and/or the increment of mean arterial BP (≥50%) after the start of therapy. In case of CsA resistance, a few patients received tacrolimus (starting dosage 0.1 mg/kg followed by modification to maintain a trough level between 5 and 10 ng/ml).
The basic molecular approach included sequencing of NPHS1, NPHS2, and exons 8 and 9 of WT1. Adult patients and parents (for patients who were younger than 18 yr) were requested to give the informed consent for DNA analysis and for reviewing their clinical parameters. Patients who were enrolled in the study were numbered, and data were evaluated anonymously (access key numbers were in possession of the senior author [G.M.G.] only).
Mutation Analysis
Genomic DNA was extracted according to standard procedures. Molecular analyses of NPHS1, NPHS2, and WT1 were performed by direct sequencing as described previously (2,21–24). Primer sequences were selected on the basis of literature reports by different groups. Exons were amplified by PCR using flanking intronic primers and subjected to heteroduplex analysis (DHPLC) and/or automatic sequence analysis by dye-terminator reaction (automated sequencer ABI 3100; Applera, Milan, Italy).
Statistical Analysis
NPHS1 and NPHS2 were analyzed separately. Podocin data were compared with those from children with non-NPHS1/NPHS2 NS. Data are expressed as mean ± SD, median and interquartile range, or frequencies. Bivariate relationships were analyzed by t test or rank-sum test, and χ2 or Fisher exact test as appropriate. Renal survival times from disease onset to dialysis initiation were described using the Kaplan-Meier method. Cox regression was used to model the risk for dialysis as a function of the diagnosis considering age at disease onset, gender, clinical characteristics, and histologic patterns as covariates. Model specification, proportionality assumption, and overall fit were checked by reestimation and formal and graphical tests on the basis of residuals and testing of the interaction with time of the variables in the model. Analyses were performed using STATA 10 SE (Stata Corp., College Station, TX).
Results
A total of 289 patients with sporadic NS were screened for mutations of NPHS1, NPHS2, and exons 8 and 9 of WT1 starting from 2001. Most patients underwent renal biopsy for clinical reasons following our flowchart (see the Materials and Methods section). Overall, long-term clinical data were available for seven and six carriers a single NPHS1 and NPHS2 mutations, respectively. Data related to the outcome of NS in 27 patients who carried NPHS2 variants (p.P20L and p.R229Q) were also collected and reported in the following sections.
Heterozygous NPHS1 Mutations
NPHS1 mutations were heterozygous in seven and homozygous in 11 cases (Table 1). All of these mutations produced structural or functional problems in nephrin, were not observed in normal control subjects (n = 100) of comparable origin, and had never been reported in a large database of NPHS1 variants (>1000 normals). Gender characteristics were distributed equally between groups. Children with heterozygous NPHS1 received a diagnosis significantly later (mean age at onset 6.5 ± 5.8 versus 0.9 ± 2.7 yr; P = 0.0054). As opposed to children with homozygous mutations, all of whom were resistant to therapy, five children with heterozygous mutations responded to therapy. A pathology finding compatible with congenital nephrosis of the Finnish type was present in all homozygous cases and in none among heterozygous children.
Table 1.
Clinical characteristics of patients who had NS and presented NPHS1 mutationsa
| NPHS1 | Mutations and Variants Nt change; Aa Change (n) | N | Gender | Age at Onset (yr) | Histology | Response to Steroids | Response to CsA | ESRD (n) | Age at ESRD (yr) |
|---|---|---|---|---|---|---|---|---|---|
| Homozygous or compound heterozygous | c.3233; p.A1078D Homo (1) c.1707 C→A; p.S596R Homo (1) c.3312-1G→A; frameshift Homo (1) [c.658T→G; p.S220A + c.3230A→G; p.N1077S] (1) [c.468C→G; p.Y156X + c.3230A→G; p.N1077S] (1) [c.2491C→T; p.R831C + c.3250insG; p.G1083fsX] (2) [c.2491C→T; p.R831C + c.2131C→T; p.R711C] (1) [c.2614delG; p.N870fsX + c.2776C→T; p.L926F] (1) [c.456delT; p.G153fsX; + c.2131C→A; p.R711S] (1) [c.121delCT; p.N40fsX; + c.1135C→T; p.R379W] (1) | 11 | M: 6 F: 5 | 0.9 (0 to 3) | CNF: 11 | NA: 11 | NA: 11 | 9b | 4 (0 to 14) |
| Heterozygous | c.59-5C→G; frameshift (1) c.563A→T; p.N188I (2) c.1379G→A; p.R460Q (1)c.2491C→T; p.R831C (2) c.2746G→T; p.A916S (1) | 7 | M: 4 F: 3 | 6.5 (1 to 16) | None: 1 FSGS: 2MCN: 3 | Good: 5 Poor: 2 | NA: 2 Good: 4 Poor: 1 | 0 | |
| No NPHS1/NPHS2 mutations | 221 | M: 138F: 83 | 17 (0 to 68) | None: 51 FSGS: 129 IgM: 22 MCN: 19 | NA: 5 Good: 33 Poor: 183 | NA: 128 Good: 46 Poor: 47 | 92 | 21 (3 to 64) |
Continuous variables are mean (range). Aa, amino acid; CNF, congenital nephrosis of the Finnish type; Homo, homozygous; IgM, mesangial proliferation with IgM deposition; MCN, minimal-change nephropathy; NA, not administered; NS, nephrotic syndrome; Nt, nucleotide.
Two patients died before ESRD.
After an average follow-up of 44 mo, nine children started dialysis (incidence rate 0.010; range 0.005 to 0.019). These children all were carriers of homozygous mutation (log-rank χ2 4.58, P = 0.0324).
Heterozygous NPHS2 Mutations and Variants
NPHS2 mutations were heterozygous in six and homozygous in 17 cases (Table 2). Twenty-seven patients carried a single heterozygous variant (p.P20L in six cases, p.R229Q in 21 cases) that predicted alteration of the encoded protein. Mutations and p.P20L were not found in 300 Italian control subjects, whereas the p.R229Q allele frequency in the same population was 2%. Moreover, the mutations described here have never been reported in large databases and previous studies that overall include >1000 individuals (3,25,26). Gender characteristics were distributed equally between groups. Carriers of different variants or single mutations (see Table 2) had similar age at disease onset (mean 12.7 ± 16.0 yr) with little variation among groups. Overall, NS tended to be diagnosed later in children with heterozygous NPHS2 than in children with homozygous NPHS2 (4.7 ± 6.0 yr; P = 0.0157). Compared with children who had homozygous mutations, who were resistant to drugs, the response probability was 0.48 among heterozygous carriers. The proportion of FSGS or IgM deposits tended to be lower in the latter group (51 versus 71%; P = 0.196).
Table 2.
Clinical characteristics of patients who had NS and presented NPHS2 mutationsa
| NPHS2 | Mutations and Variants Nt change; Aa Change (n) | N | Gender | Age at Onset (yr) | Histology | Response to Steroids | Response to CsA | ESRD (n) | Age at ESRD (yr) |
|---|---|---|---|---|---|---|---|---|---|
| Homozygous or compound heterozygous | c.419delG; p.G140fsX Homo (5) c.413G→A; p.R138Q Homo (3) c.506T→C; p.L169P Homo (2) [c.412C→T; p.R138X + c.413G→A; p.R138Q] (2) [c.419delG; p.G140fsX + c.506T→C; p.L169P] (1) [c.413G→A; p.R138Q + c.538G→A; p.V180M] (1) [c.413G→A; p.R138Q + c.855_56delAA; p. Q285fsX] (1) [c.413G→A; p.R138Q + c.973C→T; p.H325Y] (1) [c.467_468insT; p.L156fsX + c.538G→A; p.V180M] (1) | 17 | M: 11 F: 6 | 4 (0 to 18) | None: 4 FSGS: 11 IgM: 1 MCN: 1 | Poor: 17 | NA: 9 Good: 1 Poor: 8 | 13 | 8 (3 to 20) |
| p.P20L heterozygous | c.59C→T; p.P20L (6) | 6 | M: 5F: 1 | 15 (2 to 64) | None: 2 FSGS: 3 IgM: 1 | Good: 2 Poor: 4 | NA: 4 Good: 1 Poor: 1 | 2 | 8 (7 to 9) |
| p.R229Q heterozygous | c.686G→A; p.R229Q (21) | 21 | M: 13 F: 8 | 12 (1 to 42) | None: 7 FSGS: 8 IgM: 3 MCN: 3 | Good: 6 Poor: 15 | NA: 12 Good: 8 Poor: 1 | 6 | 22 (1 to 50) |
| Others | c.419delG; p.L139fsX (1) c.451 + 3insA; frameshift (1) c.555delT; p.M184fsX (1)c.631T→A; p.S211T (1) c.872G→A; p.R291Q (2) | 6 | M: 4 F: 2 | 10 (1 to 34) | None: 2 FSGS: 2 MCN: 2 | Good: 3 Poor: 3 | NA: 4 Good: 1 Poor: 1 | 2 | 22 (6 to 38) |
| No NPHS1/NPHS2 mutations | 221 | M: 138 F: 83 | 17 (0 to 68) | None: 51 FSGS: 129 IgM: 22 MCN: 19 | NA: 5 Good: 33 Poor: 183 | NA: 128 Good: 46 Poor: 47 | 92 | 21 (3 to 64) |
Continuous variables are mean (range).
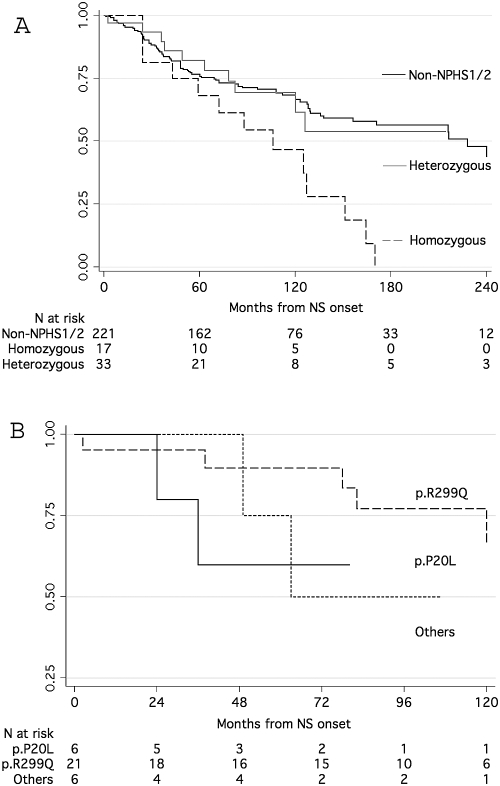
After an average follow-up of 93 mo, 23 children started dialysis (incidence rate 0.005; range 0.003 to 0.007). Children who had heterozygous mutations with different NPHS2 variants had similar renal survival probabilities (log-rank χ2 0.84, P = 0.656), which were significantly lower than those of children with homozygous mutations (log-rank χ2 5.27, P = 0.0217). Renal survival was significantly lower in the presence of resistance to therapy (P < 0.001) and FSGS or IgM deposits (P < 0.001) but was unaffected by gender or age categories. Children with heterozygous variants experienced similar risk for dialysis (Figure 1B) and also had the same risk as nongenetic cases of NS (Figure 1A). Cox regression showed that the only significant predictor of dialysis was resistance to therapy (hazard ratio 74.8; 95% confidence interval 10.2 to 548.0). When this covariate was excluded from the model, heterozygous and nongenetic forms were associated with similar risk for dialysis, and only homozygous podocin mutation remained a significant event predictor (hazard ratio 2.5; 95% confidence interval 1.4 to 4.5).
Figure 1.
Kaplan-Meier survival analysis of time interval to dialysis in different groups of patients with nephrotic syndrome. (A) Comparison of heterozygous carriers versus non-NPHS1/NPHS2 and homozygous carriers of NPHS2 mutation. (B) Three different groups of heterozygous carriers were split according to the presence of p.P20L, p.R229Q, or other mutations.
Discussion
In this article, we reviewed 40 cases of NS that were associated with a single mutation or a variant in NPHS1 or NPHS2. In both cases, the number of patients enrolled (seven for NPHS1 and 33 for NPHS2) and the length of follow-up were adequate to allow the definition of both clinical phenotypes and outcome. The direct implication of a single mutation in a recessive condition is behind the scope of our discussion because, as already pointed out, it may underscore other, undetectable mutations in noncoding regions of the same gene or in other interacting genes. We cannot exclude that the presence of a heterozygous mutation in one of the two major podocyte genes acts as a modifier of the clinical phenotype. We considered, however, that the clinical phenotype associated with heterozygous mutations could reveal specific features of the disease and be of importance for clinical purposes. Conversely, a good clinical phenotype would indirectly support the concept that a single mutation may be associated with NS with good prognosis. No data of the literature are available on this aspect of NS, thereby allowing no practical decision in terms of therapy and prognosis in these patients.
The main focus was therefore to review the clinical characteristics of these patients with NS in relation to therapeutic response and the outcome of renal function. A comparison was done with a cohort of patients who had NS and were referred to several Italian institutions and for whom a molecular workup including sequence analysis of NPHS1, NPHS2, and WT1 was available; therefore, these patients are not representative of the whole population of NS because they were followed in a second-level nephrology center and underwent a detailed molecular checkup. The results are for this reason unique and offer a practical support for clinicians.
NPHS1
NPHS1 heterozygous mutations have been occasionally associated with NS in adults with minimal changes (only three patients reported in the literature) (11,14). Several cases have instead been described by Patrakka et al. (12), who reviewed pregnancies that had been terminated because of raised concentration of α-protein and surprisingly found nine fetuses heterozygous for Fin-major or Fin-minor with the other allele devoid of any alteration. Renal pathology was reminiscent of minimal lesions with effacement of foot processes and reduced slit pores along the membrane; nephrin staining was maintained. Our cases, for which clinical details are available, are therefore unique. The main clinical features were an early onset of NS (between 1 and 16 yr), a histology of minimal lesions or FSGS, and in most cases a good response to steroids and CsA. Overall, these clinical features underlie a good outcome, and even though the follow-up is limited, the response to drugs suggests nonprogression to renal failure (27).
NPHS2
In the literature, the description of an association of heterozygous mutations in NPHS2 with NS is confined to cohorts of patients with limited follow-up (for review, see reference [13]). Recently, Hinkes et al. (9) reported an incidence of 2.0 and 5.5%, respectively, of single (unspecified) NPH2 mutations and p.R229Q variant in a large European cohort of pediatric patients with NS (Arbeitsgemeinschaft für Pädiatrische Nephrologie Study Group). More recently, Tonna et al. (16) screened a mixed population of 371 children and adults with NS (median age at onset 25 yr) and found 51 patients with a single heterozygous allele that predicted an altered protein. Most of these patients carried p.R229Q (n = 32) and a few p.R138Q (n = 3). Both studies limited the clinical observation to the age of onset of NS and found no difference compared with patients with two missense mutations and/or with classical NS. Our study confirms and further extends the description of clinical features in three cohorts of patients that included six patients who carried a heterozygous mutation with changes in the amino acid sequence or a frameshift, six patients who carried p.P20L, and 21 patients with heterozygous p.R229Q. On this basis, our study is the first to report clinical details (renal pathology, response to drugs, and evolution to end-stage renal failure) in a population that represents a significant part of all NS (2.2% considering only heterozygous mutations, 9.9% considering p.P20L and p.R229Q).
p.P20L is a variant of unclear functional significance that is not considered a mutation. Actually, in vitro experiments showed that the presence of p.P20L tended to reduce plasma membrane podocin localization (28). Data on the presence of p.P20L in normal cohorts are conflicting. We and others could not find p.P20L in 400 European control subjects (6,13,25), and the same was found in an unpublished database from the National Institutes of Health in 634 black individuals reported by Franceschini et al. (26). The null incidence of P20L was changed in an article from the same authors, which reported 1.3% of 621 black individuals with p.P20L (15). Ruf et al. (5) reported the presence of two homozygous p.P20L carriers out of 80 normal control subjects, an incidence that is out of the Hardy-Weinberg equilibrium as a result of absence of heterozygous carriers in the same cohort (theoretical incidence 16 patients instead of one). p.P20L is a part of a haplotype with a variant in the NPHS2 promoter (rs59301652) that is associated with marked downregulation of podocin expression (25). This haplotype that includes p.P20L and rs1079292 was found only in patients with renal disease, mostly in patients with nephrosis and less frequently in patients with IgA nephropathy (P < 0.035).
The p.R229Q variant is a common polymorphism that occurs at an increased frequency in association with late-onset FSGS. Pulldown experiments with antipodocin antibodies demonstrated a decreased binding to nephrin in the presence of this polymorphism (29). It was proposed that this common variant may predispose to microalbuminuria (30) or contribute to glomerulosclerosis in association with another mutant NPHS2 allele or acting in synergism with other factors. In our series of 271 patients with NS, p.R229Q had an allele frequency (3.8%) that is very close to the incidence reported by Hinkes et al. (9) in the Arbeitsgemeinschaft für Pädiatrische Nephrologie population (3.2%), whereas in our normal control subjects it was 2%. McKenzie et al. (15) reported a low allele frequency (1.3 and 1.0%) in 634 normal individuals and 247 black patients with NS, whereas they did find a notable 3.9 and 4.7% in 271 normal individuals and 129 white patients with NS. This variability makes it difficult to discern the association between p.R229Q and NS. Our results showing comparable clinical features in patients who carry the two variants reduce the problem to a mere pathophysiology aspect and conclusively show that they do not predispose to particular clinical features. In fact, the age of onset of NS was comparable between carriers of different variants (p.P20L and p.R229Q) and those with NPHS2 mutations. In general, steroid resistance was frequent (60 to 70%), and, with the exception of three cases, CsA reduced proteinuria when it was used. One single patient failed to respond to CsA but had a lasting remission with tacrolimus and steroids. Progression to end-stage renal failure occurred in 30 to 40% of cases and was comparable with other NS.
Conclusions
The main conclusion is that children who have heterozygous mutations with different variants have similar renal survival probabilities that are significantly better than those of children with homozygous mutations. They must be treated with the same therapeutic approaches used for patients without NPHS1/NPHS2 mutations and have good chances for a positive outcome. Resistance to therapy is a main characteristics that predicts a bad outcome. Overall, these patients should be treated following consolidated schemes with good chances for a long-term outcome similar to the other patients who do not carry NPHS1 or NPHS2 mutations.
Disclosures
None.
Acknowledgments
This work was done with the financial support of the E-RARE Project “PodoNet: EU Consortium for Clinical, Genetic and Experimental Research into Hereditary Diseases of the Podocyte.”
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Caridi G, Bertelli R, Di Duca M, Dagnino M, Emma F, Onetti Muda A, Scolari F, Miglietti N, Mazzucco G, Murer L, Carrea A, Massella L, Rizzoni G, Perfumo F, Ghiggeri GM: Broadening the spectrum of diseases related to podocin mutations. J Am Soc Nephrol 14: 1278–1286, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Caridi G, Bertelli R, Scolari F, Sanna-Cherchi S, Di Duca M, Ghiggeri GM: Podocin mutations in sporadic focal-segmental glomerulosclerosis occurring in adulthood. Kidney Int 64: 365, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, Zalewski I, Imm A, Ruf EM, Mucha B, Bagga A, Neuhaus T, Fuchshuber A, Bakkaloglu A, Hildebrandt F: Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol 15: 722–732, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Weber S, Gribouval O, Esquivel EL, Moriniere V, Tete MJ, Legendre C, Niaudet P, Antignac C: NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int 66: 571–579, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Beltcheva O, Martin P, Lenkkeri U, Tryggvason K: Mutation spectrum in the nephrin gene (NPHS1) in congenital nephrotic syndrome. Hum Mutat 17: 368–373, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Hinkes B, Vlangos C, Heeringa S, Mucha B, Gbadegesin R, Liu J, Hasselbacher K, Ozaltin F, Hildebrandt F: Specific podocin mutations correlate with age of onset in steroid-resistant nephrotic syndrome. J Am Soc Nephrol 19: 365–371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mucha B, Ozaltin F, Hinkes BG, Hasselbacher K, Ruf RG, Schultheiss M, Hangan D, Hoskins BE, Everding AS, Bogdanovic R, Seeman T, Hoppe B, Hildebrandt F: Mutations in the Wilms’ tumor 1 gene cause isolated steroid resistant nephrotic syndrome and occur in exons 8 and 9. Pediatr Res 59: 325–331, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Lahdenkari AT, Kestila M, Holmberg C, Koskimies O, Jalanko H: Nephrin gene (NPHS1) in patients with minimal change nephrotic syndrome (MCNS). Kidney Int 65: 1856–1863, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Patrakka J, Martin P, Salonen R, Kestila M, Ruotsalainen V, Mannikko M, Ryynanen M, Rapola J, Holmberg C, Tryggvason K, Jalanko H: Proteinuria and prenatal diagnosis of congenital nephrosis in fetal carriers of nephrin gene mutations. Lancet 359: 1575–1577, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Caridi G, Perfumo F, Ghiggeri GM: NPHS2 (Podocin) mutations in nephrotic syndrome: Clinical spectrum and fine mechanisms. Pediatr Res 57: 54R–61R, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Heeringa SF, Vlangos CN, Chernin G, Hinkes B, Gbadegesin R, Liu J, Hoskins BE, Ozaltin F, Hildebrandt F: Thirteen novel NPHS1 mutations in a large cohort of children with congenital nephrotic syndrome. Nephrol Dial Transplant 23: 1455–1460, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenzie LM, Hendrickson SL, Briggs WA, Dart RA, Korbet SM, Mokrzycki MH, Kimmel PL, Ahuja TS, Berns JS, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Cho M, Zhou YC, Binns-Roemer E, Kirk GD, Kopp JB, Winkler CA: NPHS2 variation in sporadic focal segmental glomerulosclerosis. J Am Soc Nephrol 18: 2987–2995, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonna SJ, Needham A, Polu K, Uscinski A, Appel GB, Falk RJ, Katz A, Al-Waheeb S, Kaplan BS, Jerums G, Savige J, Harmon J, Zhang K, Curhan GC, Pollak MR: NPHS2 variation in focal and segmental glomerulosclerosis. BMC Nephrol 9: 13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haltia A, Solin ML, Holmberg C, Reivinen J, Miettinen A, Holthofer H: Morphologic changes suggesting abnormal renal differentiation in congenital nephrotic syndrome. Pediatr Res 43: 410–414, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Liapis H: Molecular pathology of nephrotic syndrome in childhood: A contemporary approach to diagnosis. Pediatr Dev Pathol 11: 154–163, 2008 [DOI] [PubMed] [Google Scholar]
- 19.ISKDC: Prospective, controlled trial of cyclophosphamide therapy in children with nephrotic syndrome: Report of the International study of Kidney Disease in Children. Lancet 2: 423–427, 1974 [PubMed] [Google Scholar]
- 20.ISKDC: Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity: A report of the International Study of Kidney Disease in Children. Kidney Int 20: 765–771. 1981 [DOI] [PubMed] [Google Scholar]
- 21.Lenkkeri U, Mannikko M, McCready P, Lamerdin J, Gribouval O, Niaudet PM, Antignac CK, Kashtan CE, Homberg C, Olsen A, Kestila M, Tryggvason K: Structure of the gene for congenital nephrotic syndrome of the Finnish type (NPHS1) and characterization of mutations. Am J Hum Genet 64: 51–61, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caridi G, Bertelli R, Carrea A, Di Duca M, Catarsi P, Artero M, Carraro M, Zennaro C, Candiano G, Musante L, Seri M, Ginevri F, Perfumo F, Ghiggeri GM: Prevalence, genetics, and clinical features of patients carrying podocin mutations in steroid-resistant nonfamilial focal segmental glomerulosclerosis. J Am Soc Nephrol 12: 2742–2746, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Gigante M, Monno F, Roberto R, Laforgia N, Assael MB, Livolti S, Caringella A, La Manna A, Masella L, Iolascon A: Congenital nephrotic syndrome of the Finnish type in Italy: A molecular approach. J Nephrol 15: 696–702, 2002 [PubMed] [Google Scholar]
- 24.Aucella F, Bisceglia L, De Bonis P, Gigante M, Caridi G, Barbano G, Mattioli G, Perfumo F, Gesualdo L, Ghiggeri GM: WT1 mutations in nephrotic syndrome revisited: High prevalence in young girls, associations and renal phenotypes. Pediatr Nephrol 21: 1393–1398, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Di Duca M, Oleggini R, Sanna-Cherchi S, Pasquali L, Di Donato A, Parodi S, Bertelli R, Caridi G, Frasca G, Cerullo G, Amoroso A, Schena FP, Scolari F, Ghiggeri GM: Cis and trans regulatory elements in NPHS2 promoter: Implications in proteinuria and progression of renal diseases. Kidney Int 70: 1332–1341, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Franceschini N, North KE, Kopp JB, McKenzie L, Winkler C: NPHS2 gene, nephrotic syndrome and focal segmental glomerulosclerosis: A HuGE review. Genet Med 8: 63–75, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Ghiggeri GM, Catarsi P, Scolari F, Caridi G, Bertelli R, Carrea A, Sanna-Cherchi S, Emma F, Allegri L, Cancarini G, Rizzoni GF, Perfumo F: Cyclosporine in patients with steroid-resistant nephrotic syndrome: An open-label, nonrandomized, retrospective study. Clin Ther 26: 1411–1418, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Nishibori Y, Liu L, Hosoyamada M, Endou H, Kudo A, Takenaka H, Higashihara E, Bessho F, Takahashi S, Kershaw D, Ruotsalainen V, Tryggvason K, Khoshnoodi J, Yan K: Disease-causing missense mutations in NPHS2 gene alter normal nephrin trafficking to the plasma membrane. Kidney Int 66: 1755–1765, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Tsukaguchi H, Sudhakar A, Le TC, Nguyen T, Yao J, Schwimmer JA, Schachter AD, Poch E, Abreu PF, Appel GB, Pereira AB, Kalluri R, Pollak MR: NPHS2 mutations in late-onset focal segmental glomerulosclerosis: R229Q is a common disease-associated allele. J Clin Invest 110: 1659–1666, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira AC, Pereira AB, Mota GF, Cunha RS, Herkenhoff FL, Pollak MR, Mill JG, Krieger JE: NPHS2 R229Q functional variant is associated with microalbuminuria in the general population. Kidney Int 65: 1026–1030, 2004 [DOI] [PubMed] [Google Scholar]