Abstract
Background and objectives: In the general population, an early invasive strategy of routine coronary angiography is superior to a conservative strategy of selective angiography in patients who are admitted with unstable angina or non–ST segment elevation myocardial infarction (MI), but the effectiveness of this strategy in individuals with chronic kidney disease (CKD) is uncertain.
Design, setting, participants, & measurements: We conducted a collaborative meta-analysis with data provided by the main authors of identified trials to estimate the effectiveness of early angiography in patients with CKD. The Cochrane, Medline, and EMBASE databases were searched to identify randomized trials that compared invasive and conservative strategies in patients with unstable angina or non-ST MI. Pooled risks ratios were estimated using data from enrolled patients with estimated GFR <60 ml/min per 1.73 m2.
Results: Five randomized trials that enrolled 1453 patients with CKD were included. An early invasive strategy was associated with nonsignificant reductions in all-cause mortality, nonfatal MI, and a composite of death or nonfatal MI. The invasive strategy significantly reduced rehospitalization.
Conclusions: This collaborative study suggests that the benefits of an early invasive strategy are preserved in patients with CKD and that an early invasive approach reduces the risk for rehospitalization and is associated with trends of reduction in the risk for death and nonfatal re-infarction in patients with CKD. Coronary angiography should be considered for patients who have CKD and are admitted with non–ST elevation acute coronary syndromes.
An invasive strategy of routine coronary angiography with revascularization when indicated anatomically after non-ST elevation myocardial infarction (MI) or unstable angina may be more efficacious than a conservative strategy of selective angiography limited to patients whose medical therapy fails. Recent meta-analyses of randomized, controlled trials found that an early invasive strategy was associated with an 18% lower risk for death or nonfatal MI and a 25% lower risk for MI than a conservative strategy (1,2). Accordingly, clinical practice guidelines for the management of non-ST acute coronary syndromes (ACS) recommend an invasive strategy for patients with hemodynamic instability, refractory angina, electrical instability, or an elevated risk for clinical events (3).
Although guidelines do not recommend treatment modification on the basis of renal function (3,4), the risk-to-benefit tradeoff may be different in the 11% of adults with chronic kidney disease (CKD) (5). The risk for developing de novo coronary artery disease or death after an initial MI increases markedly with even minor reductions in GFR (4,6–8). Given this high risk, patients with CKD could derive greater absolute benefits from an invasive strategy than patients without CKD; however, the risk for adverse outcomes is also high in patients with CKD. Coronary angiography is more likely to cause cholesterol embolism or acute kidney injury—an event associated with a high risk for death (9,10)—in patients with CKD (9). In addition, both percutaneous and surgical coronary revascularization may provide less durable results in patients with CKD (11–13).
It is therefore unclear how clinical trials that compare conservative and invasive strategies apply to patients with CKD. Accordingly, we performed a collaborative meta-analysis in which the authors of randomized trials that compared invasive and conservative strategies in non-ST ACS prepared data on enrolled patients with CKD.
Materials and Methods
Search Strategy
Trial investigators were identified by literature search and provided data on enrolled patients with CKD. We searched Medline, EMBASE, and Cochrane databases (Ovid Technologies, 1966 through September 2007; English language) for keywords related to ACS (e.g., coronary artery disease, myocardial infarction, unstable angina), medical or interventional therapies (platelet aggregation inhibitor, antithrombotic, thrombolysis, medical therapy, angioplasty, percutaneous transluminal coronary angioplasty, coronary angiography, stent), and therapeutic strategy (invasive, conservative, risk stratification). The following Ovid limits were used: “Adult,” “human,” and “randomized clinical trial.”
Because the Ovid limit “randomized clinical trial” is not valid within EMBASE, both randomized and nonrandomized investigations were retrieved by this strategy. After the computerized search, two investigators independently reviewed citations to identify randomized, controlled trials. In addition, the reference lists of included articles were manually reviewed for studies not identified electronically.
Trials were selected when they randomly allocated patients with non-ST ACS to routine, predischarge coronary angiography followed by revascularization when appropriate or to selective coronary angiography in patients with inducible ischemia or recurrent, spontaneous ischemia. Trials were required to measure mortality, re-infarction, or rehospitalization as outcomes and to have at least 3 mo of follow-up. Trials that enrolled patients with ST-segment elevation MI or stable coronary disease were excluded. The manuscript reporting the principal end points was used to identify the principal investigators of each trial and for extraction of data on overall design and trial characteristics.
Data Extraction
Two investigators independently extracted data on overall trial design, conduct, and baseline characteristics. Trial quality was assessed with respect to blinding, loss to follow-up, and use of intention-to-treat analysis. Disagreements were resolved by consensus.
Because outcome data on patients with CKD were not publicly available, investigators from each trial were contacted and asked to prepare data on randomized individuals with stage 3a (estimated GFR [eGFR] 45 to <60 ml/min per 1.73 m2) 3b (30 to <45 ml/min per 1.73 m2), and stages 4 to 5 CKD (<30 ml/min per 1.73 m2). GFR was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation and preprocedure serum creatinine (14).
Statistical Analysis
The risk for death at 6 mo to 1 yr was the primary outcome (1-yr outcomes). Secondary outcomes included in-hospital death, MI, and combined death and MI as well the 1-yr risk for MI, rehospitalization, and combined death and MI. Risk ratios (RR) were calculated for each study, and publication bias was explored with funnel plots and according to the methods of Begg (15,16) and Egger et al. (16). A DerSimonian and Laird random effects model (17) was used to calculate summary estimates, and sensitivity to model choice and outcome measure was explored in secondary analyses using fixed-effects models or the odds ratio instead of the RR (data not shown). Heterogeneity was assessed by inspection of individual RR, forest plots, the Q-statistic (18), and the I2 statistic (19). Heterogeneity was further explored by eliminating outliers and with meta-regression. Because of the small numbers of trials and modest numbers of patients with CKD, variability in patient characteristics was modest. We nevertheless explored the effects of several characteristics on study outcomes: (1) The proportion of patients with ST-segment depression on admission, (2) the proportion of patients with positive cardiac biomarkers, and (3) the percentage of patients with diabetes in each trial. Statistical analysis was performed using Stata 9.2 (Stata Corp., College Station, TX). P < 0.05 was considered significant.
Results
Search Results
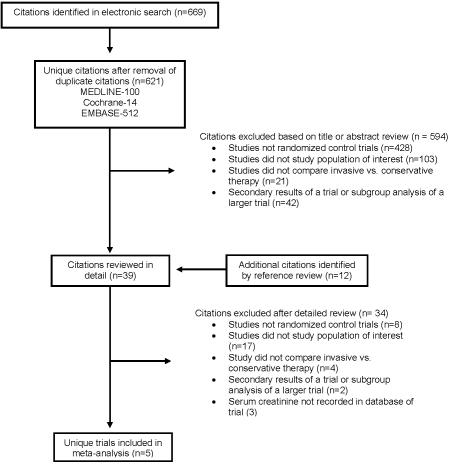
The electronic search identified 621 unique articles, and 594 were excluded after abstract or title review (Figure 1) (20). The remaining 27 references and 12 identified by manual search were reviewed in detail. We excluded 31 articles for the following reasons (Appendix): Not a randomized controlled trial (n = 8), studied patients without non-ST elevation ACS (n = 17), did not study routine versus selective coronary angiography (n = 4), or presented secondary findings of a trial (n = 2). After this review, eight reports representing the primary outcomes of eight unique trials remained (21–28). Investigators from all trials were contacted, but serum creatinine had not been recorded in three of them (23,26,27). The remaining five trials (Table 1) served as the basis of our analysis.
Figure 1.
Results of search strategy.
Table 1.
Study design of included trialsa
| Parameter | Trial Name
|
||||
|---|---|---|---|---|---|
| VINO | FRISC II | TIMI IIIB | TACTICS-TIMI 18 | ICTUS | |
| Year | 2002 | 2001 | 1994 | 2001 | 2005 |
| Interventions | First-day angiography versussymptom/stress test–driven angiography | First-week angiography versussymptom/stress test–driven angiography Dalteparin versusplacebo | Angiography within 18 to 48 h versussymptom/stress test–driven angiography TPA versusplacebo | Angiography within 4 to 48 h versussymptom/stress test–driven angiography | Angiography within 24 to 48 h versussymptom/stress test–driven angiography |
| Type of ACS | Non-STEMI | Non-STEMI and UA | Non-Q wave MI and UA | Non-STEMI and UA | Non-STEMI |
| Major exclusions | Cardiogenic shock, Q-wave MI, thrombolysis within 30 d, disease with effect on 1-yr prognosis | Age >75, previous CABG, waiting list for revascularization, thrombolysis or PTCA within 6 mo, renal or hepatic insufficiency, severe illness | Treatable cause of ischemia, MI within 21 d, angiography within 30 d, PTCA within 6 mo, previous CABG, pulmonary edema, uncontrolled hypertension, contraindication to anticoagulation, severe illness | ST elevation, CABG or PTCA within 6 mo, severe CHF, severe shock, serious systemic disease | Age >80, STEMI within 48 h, indication for primary PTCA or thrombolysis, pulmonary edema, hemodynamic instability, fibrinolytics within 96 h, PTCA within 14 d |
| Renal exclusion | NA | >1.8 mg/dl | >3 mg/dl | Creatinine >2.5 mg/dl | NA |
| Primary end point | Composite of death and nonfatal MI | Composite of death and MI | Composite of death, MI, or “unsatisfactory” stress test | Composite of death, MI, and rehospitalization for acute coronary syndrome | Composite of death, MI, or hospitalization for angina |
| Interval for assessment of primary end point | 6 mo | 6 mo | 6 wk | 6 mo | 1 yr |
| GIIb/IIIa inhibitors | None | Abciximab | None | Tirofiban | Abciximab |
| Anticoagulants | UFH | UFH, Dalteparin | UFH | UFH | Enoxaparin |
| Procedural MI | Not diagnosed within 72 h | At least two of the following: (1) CK-MB >1.5× ULN in one sample or CK, CK-B, or CK-MB activity >1.5× ULN in two samples or CK, CK-B, or CK-MB activity >3× ULN in one sample; (2) chest pain; or (3) diagnostic ECG changes | Post-CABG: CK or CK-MB >5× ULN; otherwise, CK-MB>ULN or CK >2× ULN | CK-MB >3× ULN and if preprocedure CK elevated a ≥50% over preprocedure and documentation that decreasing before procedure or new Q waves and/or pathologic findings distinct from index MI | Within first 48 h in patients with elevated CK-MB at randomization: CK-MB >ULN after a 50% decrease from peak value; otherwise, CK-MB >ULN. Post-CABG: New Q waves |
| Nonprocedural MI | Ischemic ECG changes with CK-MB >1.5× ULN or positive troponin I | At least two of the following: (1) CK-MB >ULN in one sample or CK, CK-B, or CK-MB activity >ULN in two samples; (2) chest pain; or (3) diagnostic ECG changes | CK-MB> ULN OR CK >2× ULN | (1) New Q waves distinct from index MI or both (b) Troponin >ULN or CK-MB >ULN or CK >2× ULN and ST/T wave changes or ischemic chest pain; or (3) pathologic findings of acute MI | CK-MB >ULN |
| Intention-to-treat analysis | Yes | Yes | Yes | Yes | Yes |
| Loss to follow-up (%) | 0 | <5 | <5 | <5 | <1 |
| Outcomes blinded? | Yes | Yes | Yes | Yes | Yes |
ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CK, creatinine kinase; ECG, electrocardiogram; GIIb/IIIa, glycoprotein IIb/IIIa; MI, myocardial infarction; NA, not applicable; PTCA, percutaneous transluminal coronary angioplasty; STEMI, ST elevation MI; TIMI IIIB, Thrombolysis in Myocardial Infarction IIIB; TPA, tissue plasminogen activator; UA, unstable angina; UFH, unfractionated heparin; ULN, upper limit of normal; TIMI IIIB, Thrombolysis in Myocardial Infarction IIIb Trial; VINO, Value of first day angiography/angioplasty in evolving non-ST segment elevation myocardial infarction: An open multicenter randomized trial; FRISC II, FRagmin and Fast Revascularisation during InStability in Coronary artery disease II trial; ICTUS, Invasive versus Conservative Treatment in Unstable Coronary Syndromes; TACTICS-TIMI 18, Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy—Thrombolysis in Myocardial Infarction 18 Trial.
Trial Characteristics
Between 1989 and 2003, these trials randomly assigned 7481 patients. Trial size ranged from 131 to 2457 patients. Mean age ranged from 59 to 66 yr; 14 to 28% of patients had diabetes, 33 to 48% had ST-segment depression, and mortality rates in the conservative arm were 2.5 to 10.1%. In all trials, outcomes assessors were blinded, the primary analyses were based on intention-to-treat, and loss to follow-up was low.
Patients with stages 3 to 5 CKD accounted for 19.4% (1453 of 7481) of patients. The majority (81.6%) had stage 3 CKD, with 218 patients having an eGFR between 30 and 44 ml/min per 1.73 m2. A total of 267 (18.4%) patients, principally from Thrombolysis in Myocardial Infarction (TIMI) IIIB (21), had stages 4 to 5 CKD. Patients with CKD were older and more likely to have diabetes and ST-segment depression on admission or to die to during follow-up (Tables 2 and 3).
Table 2.
Characteristics of randomly assigned patients in included trials
| Characteristic | TIMI IIIB | FRISC II | TACTICS-TIMI 18 | VINO | ICTUS |
|---|---|---|---|---|---|
| No. randomly assigned | 1473 | 2457 | 2220 | 131 | 1200 |
| Age (yr; mean) | 59 | 66a | 62 | 66 | 62a |
| Men (n [%]) | 972 (66) | 1708 (70) | 1463 (66) | 80 (61) | 880 (73) |
| White race (n [%]) | 1178 (80) | NA | 1722 (78) | NA | NA |
| Diabetes (n [%])† | 114 (8) | 299 (12) | 613 (28) | 33 (25) | 166 (14) |
| Previous MI (n [%]) | 604 (41) | 546 (22) | 866 (39) | 34 (26) | 278 (23) |
| MI at randomization (n [%]) | 471 (32) | 1348 (58) | 826 (37) | 131 (100) | 1200 (100) |
| ST-segment changes (n [%]) | 486 (33) | 1114 (46) | 852 (39) | 61 (47) | 474 (48) |
| T-wave inversion (n [%]) | 678 (46) | 871 (36) | 203 (9) | NA | NA |
| Thrombolytic therapy (n [%]) | 729 (49) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Percutaneous revascularization during initial hospitalization (routine angiography/selective angiography; %) | 37/23 | 42/7 | 37/23 | 47/3 | 60/28 |
| CABG during initial hospitalization (routine angiography/selective angiography; %) | 24/18 | 34/7 | 20/13 | 25/3 | 16/11 |
| Percutaneous revascularization at end of follow-up (routine angiography/selective angiography; %) | 38/26 | 43/18 | 42/29 | 52/13 | 61/40 |
| CABG at end of follow-up (routine angiography/selective angiography; %) | 25/24 | 35/19 | 22/16 | 35/30 | 18/14 |
| Mortality, (routine angiography/selective angiography; %) | 2.4/2.5 | 7.8/10.1 | 3.3/3.5 | 3.1/13.4 | 2.5/2.5 |
Median. Information on diabetes was available for only 782 of 1473 patients in TIMI IIIB.
Table 3.
Baseline characteristics of randomly assigned patients with CKDa
| Characteristic Invasive/Conservative Strategies | TIMI IIIB
|
FRISC II
|
TACTICS-TIMI 18
|
VINO
|
ICTUS
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| GFR <30 | GFR 30 to 60 | GFR <30 | GFR 30 to 60 | GFR <30 | GFR 30 to 60 | GFR <30 | GFR 30 to 60 | GFR <30 | GFR 30 to 60 | |
| No. randomly assigned | 102/114 | 119/114 | 2/2 | 209/216 | 17/12 | 199/201 | 4/6 | 8/11 | 5/3 | 53/56 |
| Age (yr; mean) | 60/61 | 65/65 | 74/71 | 70/69 | 69/67 | 68/68 | 68/65 | 65/65 | 73/78 | 72/72 |
| Men (n [%]) | 72/81 (71/71) | 61/49 (51/43) | 0/1 (0/50) | 115/104 (55/48) | 6/6 (35/50) | 106/101 (53/50) | 2/2 (50/33) | 4/6 (50/55) | 3/3 (60/100) | 31/29 (58/52) |
| White race (n [%]) | 102/112 (100/98) | 100/89 (84/78) | 2/2 (100/100) | 208/216 (100/100) | 12/12 (71/100) | 159/157 (80/78) | 4/6 (100/100) | 8/11 (100/100) | NA | NA |
| Diabetes (n [%]) | 8/10 (8/9) | 9/12 (8/11) | 0/0 (0/0) | 39/36 (19/17) | 12/4 (71/33) | 67/63 (34/31) | 3/4 (75/67) | 5/6 (63/55) | 2/1 (40/33) | 13/13 (25/23) |
| Previous MI (n [%]) | 47/52 (46/46) | 52/37 (44/32) | 1/1 (50/50) | 79/65 (38/30) | 7/6 (41/50) | 86/89 (43/44) | 1/3 (25/50) | 5/6 (63/55) | 2/1 (40/33) | 24/16 (45/29) |
| ST deviations (n [%]) | 48/40 (47/35) | 41/47 (34/41) | 1/1 (50/50) | 111/124 (53/57) | 7/5 (41/42) | 82/76 (41/38) | 3/5 (75/83) | 5/7 (63/64) | 5/1 (100/33) | 27/19 (51/34) |
| T-wave inversion (n [%]) | 36/43 (35/38) | 51/64 (43/56) | 2/2 (100/100) | 144/162 (69/75) | 6/7 (35/58) | 74/78 (37/39) | 1/1 (25/17) | 3/4 (38/36) | 1/0 (20/0) | 9/8 (17/14) |
| Thrombolytic therapy (n [%]) | 51/58 (50/51) | 58/52 (49/46) | 0/0 (0/0) | 0/0 (0/0) | 0/0 (0/0) | 0/0 (0/0) | 0/0 (0/0) | 0/0 (0/0) | 0/0 (0/0) | 0/0 (0/0) |
| Coronary revascularization during follow-up (n [%]) | 68/73 (67/64) | 79/71 (66/62) | 1/1 (50/50) | 157/100 (75/46) | 10/7 (59/58) | 113/83 (57/41) | 1/2 (25/33) | 5/5 (63/45) | 1/0 (20/0) | 37/27 (70/48) |
| PCI during initial hospitalization (n [%]) | 63/52 (62/46) | 75/49 (63/43) | 1/1 (50/50) | 154/38 (74/18) | 8/2 (47/17) | 72/36 (36/18) | 1/2 (25/33) | 5/5 (63/45) | 1/0 (20/0) | 35/17 (66/30) |
| CABG at initial hospitalization (n [%]) | 26/22 (25/19) | 33/25 (28/22) | 1/1 (50/50) | 89/23 (43/11) | 2/2 (12/17) | 35/26 (18/13) | 1/1 (25/17) | 4/4 (50/36) | 0/0 (0/0) | 11/7 (21/13) |
| 1-yr mortality (n [%]) | 5/6 (5/5) | 6/11 (5/10) | 0/0 (0/0) | 10/19 (5/9) | 3/1 (18/8) | 9/11 (5/5) | 1/3 (25/50) | 0/3 (0/27) | 0/2 (0/67) | 8/3 (15/5) |
Patients with stages 4 to 5 chronic kidney disease (CKD) were primarily enrolled in the TIMI IIIB trial. Approximately half of the patients with CKD in the TIMI IIIB trial underwent thrombolysis. Thrombolysis was not performed in the other trials. PCI, percutaneous revascularization.
Effect of Treatment Strategy on Outcomes
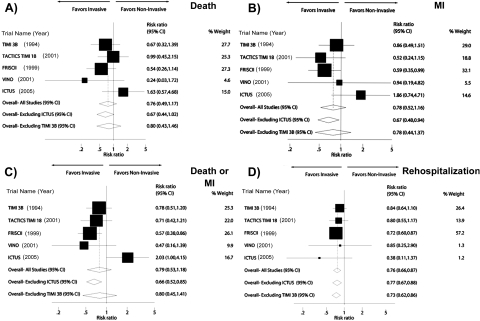
Study-specific and pooled results for 1-yr outcomes for patients with stages 3 to 5 CKD are presented in Figure 2 and Table 4. An invasive strategy was associated with a nonsignificant reduction in all-cause mortality (RR 0.76; 95% confidence interval [CI] 0.49 to 1.17; P = 0.21), nonfatal MI (RR 0.78; 95% CI 0.52 to 1.16; P = 0.22), and a composite of death or nonfatal MI (RR 0.79; 95% CI 0.53 to 1.18; P = 0.24). The invasive strategy significantly reduced rehospitalization (RR 0.76; 95% CI 0.66 to 0.87; P < 0.001). In-hospital re-infarction was not reduced by an invasive compared with a conservative strategy (RR 1.06; 95% CI 0.51 to 2.20), but the RR for death was similar in-hospital and at 1 yr.
Figure 2.
(A through D) Comparison of invasive and noninvasive strategies with respect to the likelihood of death at 1 yr (A), myocardial infarction (MI) at 1 yr (B), the composite end point of death or nonfatal MI (C), or rehospitalization during the year after randomization (D). For the ICTUS trial, only cardiovascular hospitalizations were recorded. Data presented as the study-specific and composite risk ratio (RR) estimates comparing early invasive and conservative therapy groups. RR <1 indicates that the routine angiography strategy was superior to selective strategy. The filled boxes represent the RR from the individual trials, with the size of the box reflecting the sample sizes of the trials. The horizontal bars extending from the box represent the 95% confidence intervals (CI) for the RR. The open diamonds represent the cumulative RR with or without the inclusion. The size of the diamond represents the 95% CI for the RR.
Table 4.
Summary of treatment effects of early invasive versus conservative therapy in patients with CKD and non–ST elevation ACS overall and according to CKD classa
| Outcome | All-Cause Mortality (RR [95% CI]) | Nonfatal MI (RR [95% CI]) | Death or Nonfatal MI (RR [95% CI]) | Rehospitalization (RR [95% CI]) |
|---|---|---|---|---|
| Overall, stages 3 to 5 CKD | ||||
| in-hospital | 0.77 (0.42 to 1.44) | 1.06 (0.51 to 2.20) | 1.00 (0.64 to 1.56) | NA |
| 1 yr | 0.76 (0.49 to 1.17) | 0.78 (0.52 to 1.16) | 0.79 (0.53 to 1.18) | 0.76 (0.66 to 0.87) |
| Stage 3 CKD | ||||
| in-hospital | 0.89 (0.45 to 1.76) | 0.95 (0.41 to 2.22) | 0.88 (0.43 to 1.79) | NA |
| 1 yr | 0.75 (0.41 to 1.37) | 0.72 (0.47 to 1.11) | 0.76 (0.45 to 1.27) | 0.72 (0.62 to 0.84) |
| Stage 3a CKD (GFR 45 to 59 ml/min per 1.73 m2) | ||||
| in-hospital | 1.07 (0.45 to 2.58)b | 0.90 (0.38 to 2.12) | 0.97 (0.45 to 2.05) | NA |
| 1 yr | 0.75 (0.40 to 1.40) | 0.72 (0.47 to 1.10) | 0.84 (0.50 to 1.42) | 0.73 (0.62 to 0.86) |
| Stage 3b CKD (GFR 30 to 44 ml/min per 1.73 m2) | ||||
| in-hospital | 0.69 (0.24 to 1.97) | 0.52 (0.18 to 1.54)b | 0.63 (0.30 to 1.35) | NA |
| 1 yr | 0.63 (0.30 to 1.32) | 0.58 (0.24 to 1.42) | 0.57 (0.32 to 1.00) | 0.85 (0.51 to 1.41) |
| Stages 4 to 5 CKD (GFR <30 ml/min per 1.73 m2) | ||||
| in-hospital | 0.41 (0.11 to 1.55)b | 1.37 (0.58 to 3.24)c | 0.94 (0.47 to 1.90)c | NA |
| 1 yr | 0.78 (0.33 to 1.82) | 1.11 (0.49 to 2.52) | 0.94 (0.55 to 1.60) | 1.01 (0.70 to 1.46) |
Only cardiovascular hospitalizations were recorded in the ICTUS trial. CI, confidence interval; RR, relative risk.
Because of low number of events, based on
four or
three trials only.
Inspection of study-specific RR and forest plots suggested qualitative differences between Invasive versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) and the remaining trials, but tests of heterogeneity were significant or consistent with a notable degree of heterogeneity only for combined death and MI at 1 yr (P = 0.04, I2 = 59.9%) and for in-hospital MI (P = 0.04, I2 = 60.3%). Conversely, heterogeneity was minimal for rehospitalization and in-hospital death and low to moderate for the other outcomes according to standard criteria (19): I2 ranged from 0.0% for in-hospital death and for rehospitalization to 45.2% for combined in-hospital death or nonfatal MI (Table 5).
Table 5.
Estimates of heterogeneity for fatal and nonfatal outcomes in patients with stages 3 to 5 CKDa
| Outcome | All-Cause Mortality
|
Nonfatal MI
|
Death or Nonfatal MI
|
Rehospitalization
|
||||
|---|---|---|---|---|---|---|---|---|
| I2 (%) | P χ2 | I2 (%) | P χ2 | I2 (%) | P χ2 | I2 (%) | P χ2 | |
| All Trials | ||||||||
| in-hospital | 0.0 | 0.77 | 60.3 | 0.04 | 45.2 | 0.12 | NA | NA |
| 1 yr | 19.3 | 0.29 | 29.3 | 0.23 | 59.9 | 0.04 | 0.0 | 0.83 |
| Without ICTUS | ||||||||
| in-hospital | 0.0 | 0.63 | 42.6 | 0.16 | 8.1 | 0.35 | NA | NA |
| 1 yr | 0.0 | 0.51 | 0.0 | 0.67 | 0.0 | 0.68 | 0.0 | 0.83 |
| Without TIMI 3b | ||||||||
| in-hospital | 0.0 | 0.66 | 69.5 | 0.02 | 53.9 | 0.08 | NA | NA |
| 1 yr | 33.7 | 0.21 | 44.2 | 0.15 | 69.8 | 0.02 | 0.0 | 0.73 |
Only cardiovascular hospitalizations were recorded in the ICTUS trial.
Excluding ICTUS decreased heterogeneity, yielding lower I2 values and more beneficial effect estimates (Figure 2, Table 5). After exclusion of ICTUS, an invasive strategy significantly reduced the risk for nonfatal MI as well as combined death and MI and had a borderline significant effect on all-cause mortality (RR 0.67; 95% CI 0.44 to 1.02; P = 0.06).
TIMI IIIB was the oldest trial and randomized to thrombolysis versus placebo as well as to invasive therapy versus conservative therapy. To explore these factors, we recalculated summary estimates after excluding TIMI IIIB. Heterogeneity increased slightly, but effect estimates for all-cause mortality, nonfatal MI, death or MI, and hospitalization did not change appreciably.
Meta-regression exploring the percentage of patients with diabetes, ST-segment depression, or elevated cardiac enzymes did not identify any significant effects on between-trial heterogeneity (P ≥ 0.20 for all comparisons). There was no evidence of publication bias.
Findings in patients with stage 3 CKD were qualitatively similar to the overall findings demonstrating nonsignificant reductions in in-hospital and 1-yr mortality as well as the combined outcome of death and nonfatal MI. The number of patients with late stage 3 CKD (eGFR <45 ml/min per 1.73 m2) was small, but the RR reduction of death, MI, or combined death and MI seemed equal or greater within this group compared with early stage 3 CKD (Table 4). Only a small number of patients had stages 4 to 5 CKD, with the majority enrolled in TIMI IIIB (21). Within the context of these limitations, the combined estimates for stages 4 to 5 CKD were consistent with similar reductions in 1-yr mortality as in less advanced CKD (RR 0.78; 95% CI 0.33 to 1.82; P = 0.56) but an increased risk for recurrent MI (RR 1.11; 95% CI 0.49 to 2.52; P = 0.66).
Discussion
In this collaborative meta-analysis, we compared outcomes of invasive and conservative strategies in patients who had at least moderate CKD and were admitted with non–ST segment elevation ACS. We found that an invasive strategy significantly reduced the risk for rehospitalization and resulted in nonsignificant reductions in the risks for death and MI compared with a conservative management strategy.
Although the CI (with the exception of rehospitalization) were broad, the point estimates were consistent with clinically meaningful effects (RR between 21 and 24% for 1-yr outcomes). The magnitude of the reductions in death and recurrent MI that we observed in the CKD population were comparable to or greater than those found in two previous meta-analyses that compared invasive with conservative strategies in the overall trial populations (up to 8% reduction in the risk for death and 25% reduction in the risk for nonfatal MI [1,2]).
Furthermore, because patients with CKD are at substantially higher risk for death than patients without significant CKD—with a mortality rate of 8.0% in patients with CKD compared with only 3.1% in patients without CKD randomly assigned to conservative therapy in these trials—the observed relative risk reductions likely mean substantially higher absolute benefits from an invasive strategy for this group of patients. Quantitatively, this suggests that an invasive strategy could prevent up to 20 deaths for every 1000 patients compared with only six deaths prevented in patients without CKD. Additional studies are needed to elucidate the degree to which comorbidities such as diabetes or previous MI modify the relationship between CKD and the choice of post-ACS therapy. Unfortunately, this will be difficult if cardiovascular trials continue routinely to exclude patients with overt CKD (29). Not only do these exclusions limit understanding of how to treat individuals with CKD, but also by removing patients who are likely to derive a greater absolute benefit from effective therapy, they may result in underestimation of overall treatment efficacy. Broader enrollment of patients with all ranges of CKD in future trials should be strongly encouraged.
Coronary angiography is underused in patients with CKD (30,31). It is uncertain whether this low use represents an appropriate regard for the risk for contrast nephropathy in this population, an overly conservative approach to therapy in patients with CKD, or a combination of both. Retrospective studies demonstrating higher mortality among patients who have CKD and do not undergo angiography after MI have raised concerns that the low use of post-ACS coronary angiography in patients with CKD is inappropriate and may partly underlie the high mortality in patients with CKD (30–32). Whether the association is causal or the result of greater degrees of comorbidity among patients who do not undergo angiography, however, has remained unanswered because of the lack of randomized studies. Our findings support the possibility of a causal link between the low use of angiography in patients with CKD and the high mortality and suggest that broader use of an invasive strategy in patients with CKD and non–ST elevation ACS could significantly affect outcomes.
Although additional studies are needed, we believe that our results suggest that an invasive strategy of routine angiography should be the preferred approach to the treatment of non-ST ACS in CKD; however, the absence of statistical significance for the death and MI end points in our analysis must be acknowledged. It may be due to low statistical power for these end points given the relatively small number of trials, moderate within-trial power for these end points, the modest number of patients with stage 3 or higher CKD in the analysis, and the modest number of fatalities. We cannot exclude a true lack of benefit or even harm from a routine invasive compared with a conservative strategy in patients with CKD. Well-powered, randomized studies of this question are needed for clarification.
Alternatively, the lack of a statistically significant benefit could reflect the heterogeneity of the trials that we studied. The changes in the I2 statistic and the narrowing of CI after the removal of the ICTUS trial from our analysis suggest the possibility of a moderate discrepancy between this trial and the others in our analysis. Meta-regression suggested that between-trial differences in the proportion of patients with diabetes, ST-segment depression, or positive biomarkers of ischemia do not account for the observed differences in outcomes, and other factors are likely to be responsible. ICTUS is the most contemporary of the trials that we analyzed, and it is possible that the benefits of an invasive strategy have diminished with more contemporary approaches to the medical therapy of ischemic heart disease; however, the overall mortality in the ICTUS trial was low, and the frequency of coronary revascularization in the conservative arm of this trial was similar to the frequency in the early invasive arm of other trials (Tables 1 and 2). Thus, an alternative is that the high rate of revascularization in the control arm or the inclusion of lower risk patients in the ICTUS trial may have diluted the benefit of an early invasive strategy seen in the higher risk populations enrolled in the other trials.
These considerations suggest the interpretation that early invasive therapy after MI may be most beneficial for patients who have CKD and are otherwise at high risk for cardiovascular morbidity and mortality and that it is of less benefit in other cases. Further studies are required to determine whether the ICTUS results are more or less informative for contemporary practice than the older trials.
This study had several strengths. The collaborative nature of the analysis resulted in a sample of randomly assigned, post-ACS patients with CKD several times larger than those in any previous investigation (33). In addition, the use of randomized data yielded a sample free from the significant imbalances in baseline conditions and the potential for indication bias that may confound retrospective analyses of nonrandomized data (30–32). Finally, the trials included in this analysis randomly assigned patients internationally, used a variety of concomitant medical treatments, and had slightly different entry criteria. Thus, our aggregate results are likely to be more broadly generalizable than the results of any single trial
In addition to the potential for type II statistical error, there are several limitations to this study. First, the nature of the interventions prevented blinding, and this could have influenced clinical outcomes. Second, patients who met entry criteria for these trials may have differed in important but unmeasured ways from patients in general clinical practice, and this could limit generalizability. Third, we cannot exclude the possibility that acute kidney injury was misclassified as CKD; however, the use of preangiography creatinine to estimate GFR and the strict exclusion criteria (Table 1) make it unlikely that large numbers of patients were misclassified. Finally, the trials were conducted during a period of >10 yr, during which treatment of coronary disease evolved. There was substantial variation in the use of coronary stents and background medical therapies. In addition, it is worth noting that in TIMI IIIB (21), roughly 50% of patients underwent thrombolysis—a therapy no longer used for non-ST ACS, largely as a consequence of this trial. The risks and benefits of coronary angiography may be different in contemporary practice than in the context of the therapies used in TIMI IIIB. The absence of qualitative changes in our analysis after exclusion of TIMI IIIB as well as the lack of statistical evidence for heterogeneity is reassuring that this trial did not unduly influence our findings.
Although we did not find evidence of publication bias, the small number of trials in our analysis limited our power to assess formally for unpublished studies; however, we are unaware of any published trials that specifically assessed these interventions in the CKD population, and we therefore believe that it is unlikely that trials that enrolled substantial numbers of such patients would remain unpublished. Finally, we studied a very small number of patients who had stages 4 to 5 CKD and were primarily enrolled in a single trial. To our knowledge, no other study has examined the effects of an invasive compared with a conservative post-ACS management strategy in a randomly allocated population of patients with stages 4 to 5 CKD. In this regard, it is interesting that our analysis suggests that reduction in mortality with early invasive therapy is similar across all three stages of CKD; however, because the CI on this estimate were broad, this finding should be considered hypothesis generating and extrapolated cautiously.
Conclusions
We found a significantly lower risk for rehospitalization and trends toward a lower risk for death and re-infarction in patients who had CKD and were randomly assigned to an early invasive strategy after admission for non–ST elevation ACS. Additional studies to refine the effect estimates in patients with advanced CKD and to determine longer term outcomes are needed. Our results suggest that an early invasive strategy of routine post-ACS coronary angiography with revascularization when anatomically indicated may be beneficial in patients with CKD and suggest that this strategy should be considered in all non-ST elevation ACS-patients regardless of renal function.
Disclosures
None.
Acknowledgments
Dr. Charytan was funded by a Norman S. Coplon Grant from Satellite Health Care and a Scientist Development Grant from the American Heart Association. Preliminary findings were presented at the American Society of Nephrology in Philadelphia, PA in November 2008.
Appendix: Reasons for Exclusion of Citations Reviewed in Detail
Study Not a Randomized Controlled Trial (n = 8)
King SB 3rd: Do elderly patients with non-ST-segment elevation acute coronary syndromes benefit from early invasive treatment? Nat Clin Pract Cardiovasc Med 3: 188–189, 2006
Schuhlen H, Kastrati A, Pache J, Dirschinger J, Schomig A: Incidence of thrombotic occlusion and major adverse cardiac events between two and four weeks after coronary stent placement: Analysis of 5678 patients with a four-week ticlopidine regimen. J Am Coll Cardiol 37: 2066–2073, 2001
Yip HK, Wu CJ, Chang HW, Chen MC, Hang CL, Fang CY, Hsieh YK, Yang CH, Yeh KH, Fu M: Comparison of impact of primary percutaneous transluminal coronary angioplasty and primary stenting on short-term mortality in patients with cardiogenic shock and evaluation of prognostic determinants. Am J Cardiol 87: 1184–1188, A4, 2001
Gould KL, Casscells SW, Buja LM, Goff DC: Noninvasive management of coronary artery disease: Report of a meeting at the University of Texas Medical School at Houston. Lancet 346: 750–753, 1995
Meijer A, Verheugt FW: Optimal treatment after thrombolysis: Aggressive or conservative? Ned Tijdschr Geneeskd 133: 349–354, 1989
Bertrand ME, Simoons ML, Fox KA, Wallentin LC, Hamm CW, McFadden E, De Feyter PJ, Specchia G, Ruzyllo W: Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 23: 1809–1840, 2002
Boden WE: “Routine invasive” versus “selective invasive” approaches to non-ST-segment elevation acute coronary syndromes management in the poststent/platelet inhibition era. J Am Coll Cardiol 41: 113S–122S, 2003
Perez de Arenaza D, Bakhai A, Flather M: Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes. N Engl J Med 345: 1573, author reply 1574–1575, 2001
Study Did not Study Population of Interest (n = 17)
Pfisterer M, Buser P, Osswald S, Allemann U, Amann W, Angehrn W, Eeckhout E, Erne P, Estlinbaum W, Kuster G, Moccetti T, Naegeli B, Rickenbacher P: Outcome of elderly patients with chronic symptomatic coronary artery disease with an invasive versus optimized medical treatment strategy: One-year results of the randomized TIME trial. JAMA 289: 1117–1123, 2003
Zhao MZ, Hu DY, Jiang LQ, Wu Y, Zhu TG, Hao HJ, Zhang LJ, Huo Y, Wang MS: The effect of early invasive strategy on early and late outcomes in high-risk non-ST-segment elevation acute coronary syndromes [in Chinese]. Zhonghua Nei Ke Za Zhi 44: 737–740, 2005
Minai K, Horie H, Takahashi M, Nozawa M, Kinoshita M: Long-term outcome of primary percutaneous transluminal coronary angioplasty for low-risk acute myocardial infarction in patients older than 80 yr: A single-center, open, randomized trial. Am Heart J 143: 497–505, 2002
Michalis LK, Stroumbis CS, Pappas K, Sourla E, Niokou D, Goudevenos JA, Siogas C, Sideris DA: Treatment of refractory unstable angina in geographically isolated areas without cardiac surgery: Invasive versus conservative strategy (TRUCS study). Eur Heart J 21: 1954–1959, 2000
Chen JS, Hwang CL, Lee DY, Chen YT: Regression of left ventricular aneurysm after delayed percutaneous transluminal coronary angioplasty (PTCA) in patients with acute myocardial infarction. Int J Cardiol 48: 39–47, 1995
Chaitman BR, McMahon RP, Terrin M, Younis LT, Shaw LJ, Weiner DA, Frederick MM, Knatterud GL, Sopko G, Braunwald E: Impact of treatment strategy on predischarge exercise test in the Thrombolysis in Myocardial Infarction (TIMI) II Trial. Am J Cardiol 71: 131–138, 1993
Kim JW, Lim DS, Sun K, Shim WJ, Rho YM: Stenting or MIDCAB using ministernotomy for revascularization of proximal left anterior descending artery? Int J Cardiol 99: 437–441, 2005
Germing A, Lindstaedt M, Ulrich S, Grewe P, Bojara W, Lawo T, von Dryander S, Jager D, Machraoui A, Mugge A, Lemke B: Normal angiogram in acute coronary syndrome: Preangiographic risk stratification, angiographic findings and follow-up. Int J Cardiol 99: 19–23, 2005
Chiamvimonvat V, Goodman SG, Langer A, Barr A, Freeman MR: Prognostic value of dipyridamole SPECT imaging in low-risk patients after myocardial infarction. J Nucl Cardiol 8: 136–143, 2001
Schachinger V, Kasper W, Zeiher AM: Adjunctive intracoronary urokinase therapy during percutaneous transluminal coronary angioplasty. Am J Cardiol 77: 1174–1178, 1996
Gibbons RJ, Holmes DR, Reeder GS, Bailey KR, Hopfenspirger MR, Gersh BJ: Immediate angioplasty compared with the administration of a thrombolytic agent followed by conservative treatment for myocardial infarction. The Mayo Coronary Care Unit and Catheterization Laboratory Groups. N Engl J Med 328: 685–691, 1993
Grines CL, Browne KF, Marco J, Rothbaum D, Stone GW, O'Keefe J, Overlie P, Donohue B, Chelliah N, Timmis GC, et al.: A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 328: 673–679, 1993
Coronary angioplasty versus medical therapy for angina: The second Randomized Intervention Treatment of Angina (RITA-2) trial. RITA-2 trial participants. Lancet 350: 461–468, 1997
Widimsky P, Groch L, Zelizko M, Aschermann M, Bednar F, Suryapranata H: Multicenter randomized trial comparing transport to primary angioplasty versus immediate thrombolysis versus combined strategy for patients with acute myocardial infarction presenting to a community hospital without a catheterization laboratory. The PRAGUE study. Eur Heart J 21: 823–831, 2000
O'Neill WW, Weintraub R, Grines CL, Meany TB, Brodie BR, Friedman HZ, Ramos RG, Gangadharan V, Levin RN, Choksi N, et al.: A prospective, placebo-controlled, randomized trial of intravenous streptokinase and angioplasty versus lone angioplasty therapy of acute myocardial infarction. Circulation 86: 1710–1717, 1992
Zijlstra F, de Boer MJ, Hoorntje JC, Reiffers S, Reiber JH, Suryapranata H: A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med 328: 680–684, 1993
Topol EJ, Califf RM, George BS, Kereiakes DJ, Abbottsmith CW, Candela RJ, Lee KL, Pitt B, Stack RS, O'Neill WW: A randomized trial of immediate versus delayed elective angioplasty after intravenous tissue plasminogen activator in acute myocardial infarction. N Engl J Med 317: 581–588, 1987
Study Did not Compare Invasive with Conservative Therapy (n = 4)
Lawrence JR, Shepherd JT, Bone I, Rogen AS, Fulton WF: Fibrinolytic therapy in unstable angina pectoris: A controlled clinical trial. Thromb Res 17: 767–777, 1980
Luchi RJ, Scott SM, Deupree RH: Comparison of medical and surgical treatment for unstable angina pectoris: Results of a Veterans Administration Cooperative Study. N Engl J Med 316: 977–984, 1987
Neumann FJ, Kastrati A, Pogatsa-Murray G, Mehilli J, Bollwein H, Bestehorn HP, Schmitt C, Seyfarth M, Dirschinger J, Schomig A: Evaluation of prolonged antithrombotic pretreatment (“cooling-off” strategy) before intervention in patients with unstable coronary syndromes: A randomized controlled trial. JAMA 290: 1593–1599, 2003
An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med 329: 673–682, 1993
Study Reported Secondary Results of Trial or Subgroup Analysis of Trial (n = 2)
Anderson HV, Cannon CP, Stone PH, Williams DO, McCabe CH, Knatterud GL, Thompson B, Willerson JT, Braunwald E: One-year results of the Thrombolysis in Myocardial Infarction (TIMI) IIIB clinical trial: A randomized comparison of tissue-type plasminogen activator versus placebo and early invasive versus early conservative strategies in unstable angina and non-Q wave myocardial infarction. J Am Coll Cardiol 26: 1643–1650, 1995
Early coronary angiography and revascularization significantly reduces death and heart attacks in elderly people with acute coronary syndromes. Evidence-Based Healthcare & Public Health 9: 38–39, 2005
Serum Creatinine not Recorded in the Trial's Database (n = 3)
Boden WE, O'Rourke RA, Crawford MH, Blaustein AS, Deedwania PC, Zoble RG, Wexler LF, Kleiger RE, Pepine CJ, Ferry DR, Chow BK, Lavori PW: Outcomes in patients with acute non-Q-wave myocardial infarction randomly assigned to an invasive as compared with a conservative management strategy. Veterans Affairs Non-Q-Wave Infarction Strategies in Hospital (VANQWISH) Trial Investigators. N Engl J Med 338: 1785–1792, 1998
Fox KA, Poole-Wilson PA, Henderson RA, Clayton TC, Chamberlain DA, Shaw TR, Wheatley DJ, Pocock SJ: Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: The British Heart Foundation RITA 3 randomized trial. Randomized Intervention Trial of unstable Angina. Lancet 360: 743–751, 2002
McCullough PA, O'Neill WW, Graham M, Stomel RJ, Rogers F, David S, Farhat A, Kazlauskaite R, Al-Zagoum M, Grines CL: A prospective randomized trial of triage angiography in acute coronary syndromes ineligible for thrombolytic therapy: Results of the medicine versus angiography in thrombolytic exclusion (MATE) trial. J Am Coll Cardiol 32: 596–605, 1998
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at http://www.cjasn.org/.
References
- 1.Choudhry NK, Singh JM, Barolet A, Tomlinson GA, Detsky AS: How should patients with unstable angina and non-ST-segment elevation myocardial infarction be managed? A meta-analysis of randomized trials. Am J Med 118: 465–474, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Mehta SR, Cannon CP, Fox KA, Wallentin L, Boden WE, Spacek R, Widimsky P, McCullough PA, Hunt D, Braunwald E, Yusuf S: Routine vs selective invasive strategies in patients with acute coronary syndromes: A collaborative meta-analysis of randomized trials. JAMA 293: 2908–2917, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B: ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 50: e1–e157, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Murphy SW: Management of heart failure and coronary artery disease in patients with chronic kidney disease. Semin Dial 16: 165–172, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA: Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 351: 1285–1295, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Al Suwaidi J, Reddan DN, Williams K, Pieper KS, Harrington RA, Califf RM, Granger CB, Ohman EM, Holmes DR Jr: Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation 106: 974–980, 2002 [DOI] [PubMed] [Google Scholar]
- 9.McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW: Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med 103: 368–375, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr: Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105: 2259–2264, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Attallah N, Yassine L, Fisher K, Yee J: Risk of bleeding and restenosis among chronic kidney disease patients undergoing percutaneous coronary intervention. Clin Nephrol 64: 412–418, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Best PJ, Berger PB, Davis BR, Grines CL, Sadeghi HM, Williams BA, Willerson JT, Granett JR, Holmes DR Jr: Impact of mild or moderate chronic kidney disease on the frequency of restenosis: Results from the PRESTO trial. J Am Coll Cardiol 44: 1786–1791, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Szczech LA, Best PJ, Crowley E, Brooks MM, Berger PB, Bittner V, Gersh BJ, Jones R, Califf RM, Ting HH, Whitlow PJ, Detre KM, Holmes D: Outcomes of patients with chronic renal insufficiency in the bypass angioplasty revascularization investigation. Circulation 105: 2253–2258, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Begg CB: A comparison of methods to detect publication bias in meta-analysis by P. Macaskill, S. D. Walter and L. Irwig, Statistics in Medicine, 2001; 20:641–654. Stat Med 21: 1803, author reply 1804, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Smith GD, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7: 177–188, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Cochran WG: The combination of estimates from different experiments. Biometrics 10: 101–129, 1956 [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557–560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF: Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 354: 1896–1900, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q-wave myocardial infarction: Results of the TIMI IIIB Trial. Thrombolysis in Myocardial Ischemia. Circulation 89: 1545–1556, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Lancet 354: 708–715, 1999 [PubMed] [Google Scholar]
- 23.Boden WE, O'Rourke RA, Crawford MH, Blaustein AS, Deedwania PC, Zoble RG, Wexler LF, Kleiger RE, Pepine CJ, Ferry DR, Chow BK, Lavori PW: Outcomes in patients with acute non-Q-wave myocardial infarction randomly assigned to an invasive as compared with a conservative management strategy. Veterans Affairs Non-Q-Wave Infarction Strategies in Hospital (VANQWISH) Trial Investigators. N Engl J Med 338: 1785–1792, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM, Braunwald E: Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 344: 1879–1887, 2001 [DOI] [PubMed] [Google Scholar]
- 25.de Winter RJ, Windhausen F, Cornel JH, Dunselman PH, Janus CL, Bendermacher PE, Michels HR, Sanders GT, Tijssen JG, Verheugt FW: Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med 353: 1095–1104, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Fox KA, Poole-Wilson PA, Henderson RA, Clayton TC, Chamberlain DA, Shaw TR, Wheatley DJ, Pocock SJ: Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet 360: 743–751, 2002 [DOI] [PubMed] [Google Scholar]
- 27.McCullough PA, O'Neill WW, Graham M, Stomel RJ, Rogers F, David S, Farhat A, Kazlauskaite R, Al-Zagoum M, Grines CL: A prospective randomized trial of triage angiography in acute coronary syndromes ineligible for thrombolytic therapy: Results of the medicine versus angiography in thrombolytic exclusion (MATE) trial. J Am Coll Cardiol 32: 596–605, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Spacek R, Widimsky P, Straka Z, Jiresova E, Dvorak J, Polasek R, Karel I, Jirmar R, Lisa L, Budesinsky T, Malek F, Stanka P: Value of first day angiography/angioplasty in evolving non-ST segment elevation myocardial infarction: An open multicenter randomized trial. The VINO Study. Eur Heart J 23: 230–238, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Charytan D, Kuntz RE: The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int 70: 2021–2030, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charytan D, Mauri L, Agarwal A, Servoss S, Scirica B, Kuntz RE: The use of invasive cardiac procedures after acute myocardial infarction in long-term dialysis patients. Am Heart J 152: 558–564, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chertow GM, Normand SL, McNeil BJ: “Renalism”: Inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol 15: 2462–2468, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Keeley EC, Kadakia R, Soman S, Borzak S, McCullough PA: Analysis of long-term survival after revascularization in patients with chronic kidney disease presenting with acute coronary syndromes. Am J Cardiol 92: 509–514, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Johnston N, Jernberg T, Lagerqvist B, Wallentin L: Early invasive treatment benefits patients with renal dysfunction in unstable coronary artery disease. Am Heart J 152: 1052–10528, 2006 [DOI] [PubMed] [Google Scholar]