Abstract
Racial disparities persist in the United States renal transplantation process. Previous studies suggest that the distance between a patient's residence and the transplant facility may associate with disparities in transplant waitlisting. We examined this possibility in a cohort study using data for incident, adult ESRD patients (1998 to 2002) from the ESRD Network 6, which includes Georgia, North Carolina, and South Carolina. We linked data with the United Network for Organ Sharing (UNOS) transplant registry through 2005 and with the 2000 U.S. Census geographic data. Of the 35,346 subjects included in the analysis, 12% were waitlisted, 57% were black, 50% were men, 20% were impoverished, 45% had diabetes as the primary etiology of ESRD, and 73% had two or more comorbidities. The median distance from patient residence to the nearest transplant center was 48 mi. After controlling for multiple covariates, distance from patient residence to transplant center did not predict placement on the transplant waitlist. In contrast, race, neighborhood poverty, gender, age, diabetes, hypertension, body mass index, albumin, and the use of erythropoietin at dialysis initiation was associated with waitlisting. As neighborhood poverty increased, the likelihood of waitlisting decreased for blacks compared with whites in each poverty category; in the poorest neighborhoods, blacks were 57% less likely to be waitlisted than whites. This study suggests that improving the allocation of kidneys may require a focus on poor communities.
Kidney transplantation is the preferred method of treatment for ESRD patients and is associated with increased quality of life and reduced morbidity and mortality compared with hemo- and peritoneal dialysis.1 Despite these advantages, substantial racial disparities exist in access to renal transplantation in the United States.2–4 Although the incidence of ESRD is higher among blacks than whites, the proportion of ESRD patients either transplanted or placed on the deceased donor waitlist within 1 yr of ESRD registration during 2003 was 14.5% for white patients versus 10.1% for black patients.5
Geographic variations in waitlist and kidney transplant rates among ESRD patients may contribute to these racial disparities.5–7 Blacks living in rural areas are less likely to be waitlisted and transplanted than those residing in urban areas.8 Access to transplantation may be impeded by greater distance from a transplant center, but the degree to which distance from a patient's residence to the nearest transplant center might contribute to racial disparities in the process of transplantation has not been described. The purpose of this report is to describe the association between the distance from a patient's residence to the nearest transplant facility and placement on a kidney transplant waitlist among incident ESRD patients.
RESULTS
Among the 35,346 subjects included in the analysis, the mean age was 61 yr (±15); 20,229 (57%) were black; 17,675 (50%) were men; 6873 (20%) lived in impoverished communities; 15,721 (45%) had diabetes as their primary etiology of ESRD; 25,847 (73%) had two or more comorbidities at the initiation of dialysis; and most had body mass indices >24.9 kg/m2 with 9555 (28%) overweight, 8089 (24%) obese, and 1497 (4%) morbidly obese according to World Health Organization guidelines. The median distance from a patient's residence to the nearest transplant center was 48 mi. This was similar to the median distance of 49 mi observed in the source population (i.e., the population from which any exclusion criteria were applied). Six percent of the study population lived in small remote rural areas versus 63% in major urban areas (Table 1).
Table 1.
Characteristics of waitlisted and not waitlisted incident ESRD patientsa
| Characteristic | Network 6 Incident ESRD Patients, n = 35,346 | Waitlisted, n = 4153 (11.8%)
|
Not Waitlisted, n = 31,193 (88.2%)
|
Crude OR for Waitlisting (95% CI) | P Valuee | |
|---|---|---|---|---|---|---|
| P Value for Waitlisting Difference | ||||||
| Distance to transplant facility (mi) | P <0.0006 | |||||
| <20 | 8585 (24.9%) | 1102 (27.0%) | 7483 (24.6%) | Reference | ||
| 20 to 39 | 6939 (20.1%) | 830 (20.4%) | 6109 (20.0%) | 0.92 (0.84 to 1.02) | 0.1006 | |
| 40 to 69 | 7089 (20.5%) | 814 (20.0%) | 6275 (20.6%) | 0.88 (0.80 to 0.97) | 0.0100 | |
| 70 to 99 | 6008 (17.4%) | 634 (15.5%) | 5374 (17.6%) | 0.80 (0.72 to 0.89) | <0.0001 | |
| 100 to 149 | 4124 (11.9%) | 532 (13.0%) | 3592 (11.8%) | 1.01 (0.90 to 1.12) | 0.9199 | |
| ≥150 | 1801 (5.2%) | 166 (4.1%) | 1635 (5.4%) | 0.69 (0.58 to 0.82) | <0.0001 | |
| Race | P = 0.0004 | |||||
| white | 15,117 (42.8%) | 1670 (40.2%) | 13,447 (43.1%) | Reference | 0.0004 | |
| black | 20,229 (57.2%) | 2483 (59.8%) | 17,746 (56.9%) | 1.13 (1.06 to 1.20) | ||
| Age (yr) | P <0.0001 | |||||
| 20 to 39 | 3624 (10.2%) | 1240 (19.9%) | 2384 (7.6%) | Reference | ||
| 40 to 49 | 4712 (13.3%) | 1070 (25.7%) | 3642 (11.7%) | 0.57 (0.51 to 0.62) | <0.0001 | |
| 50 to 59 | 7151 (20.2%) | 1174 (28.3%) | 5977 (19.2%) | 0.38 (0.34 to 0.41) | <0.0001 | |
| 60 to 69 | 8587 (24.3%) | 602 (14.5%) | 7985 (25.6%) | 0.15 (0.13 to 0.16) | <0.0001 | |
| ≥70 | 11,272 (32.0%) | 67 (1.6%) | 11,205 (35.9%) | 0.01 (0.01 to 0.02) | <0.0001 | |
| Gender | P <0.0001 | |||||
| male | 17,675 (50.1%) | 2429 (58.5%) | 15,246 (48.9%) | Reference | ||
| female | 17,671 (49.9%) | 1724 (41.5%) | 15,947 (51.1%) | 0.70 (0.64 to 0.73) | <0.0001 | |
| Primary Cause ESRD | P <0.0001 | |||||
| diabetes | 15,721 (44.5%) | 1521 (36.6%) | 14,200 (45.5%) | Reference | ||
| GN | 2833 (8.0%) | 756 (18.2%) | 2077 (6.7%) | 3.40 (3.08 to 3.75) | 0.0001 | |
| hypertension | 10,636 (30.1%) | 1063 (25.6%) | 9573 (30.7%) | 1.04 (0.96 to 1.13) | 0.3923 | |
| otherc | 6156 (17.4%) | 813 (19.6%) | 5343 (17.1%) | 1.42 (1.30 to 1.56) | 0.0001 | |
| Total comorbiditiesd | P <0.0001 | |||||
| 0 | 1478 (4.2%) | 297 (7.1%) | 1181 (3.8%) | Reference | ||
| 1 | 8021 (22.7%) | 1701 (41.0%) | 6320 (20.3%) | 1.07 (0.93 to 1.23) | 0.3351 | |
| 2 | 8387 (23.7%) | 969 (23.3%) | 7418 (23.8%) | 0.52 (0.45 to 0.60) | <0.0001 | |
| 3 | 7222 (20.4%) | 727 (17.5%) | 6495 (20.8%) | 0.45 (0.38 to 0.52) | <0.0001 | |
| 4 | 4743 (13.4%) | 307 (7.4%) | 4436 (14.2%) | 0.28 (0.23 to 0.33) | <0.0001 | |
| ≥5 | 5495 (15.6%) | 152 (3.7%) | 5343 (17.1%) | 0.11 (0.09 to 0.14) | <0.0001 | |
| Body mass index (kg/m2)b | P <0.0001 | |||||
| underweight (<18.5) | 2112 (6.2%) | 116 (2.9%) | 1996 (6.6%) | 0.51 (0.42 to 0.62) | <0.0001 | |
| normal (18.5 to 24.9) | 12,885 (37.7%) | 1320 (32.5%) | 11,565 (38.5%) | Reference | ||
| overweight (25 to 29.9) | 9555 (28.0%) | 1315 (32.4%) | 8240 (17.4%) | 1.40 (1.29 to 1.52) | <0.0001 | |
| obese (30.0 to 39.9) | 8089 (23.7%) | 1163 (28.7%) | 6926 (23%) | 1.47 (1.35 to 1.60) | <0.0001 | |
| morbidly obese (>40) | 1497 (4.4%) | 144 (3.5%) | 1353 (4.5%) | 0.93 (0.78 to 1.12) | 0.4490 | |
| Erythropoietin use (%) | P <0.0001 | |||||
| 9246 (26.2%) | 1262 (30.4%) | 7984 (25.6%) | 1.27 (1.18 to 1.36) | <0.0001 | ||
| Mean hemoglobin (±SD) (g/dl) | P = 0.1233 | |||||
| 9.4 ± 4.4 | 9.3 ± 3.9 | 9.4 ± 4.4 | 0.99 (0.98 to 1.01) | 0.1230 | ||
| Mean albumin (±SD) (g/dl) | P <0.0001 | |||||
| 2.56 ± 1.55 | 2.71 ± 01.51 | 2.54 ± 1.56 | 1.07 (1.05 to 1.09) | <0.0001 | ||
| Degree of rurality | P <0.0001 | |||||
| urban | 22,270 (63.0%) | 2761 (66.5%) | 19,509 (62.5%) | Reference | ||
| large rural | 6958 (19.7%) | 759 (18.3%) | 6199 (19.9%) | 0.87 (0.79 to 0.94) | 0.0009 | |
| small rural | 3910 (11.1%) | 391 (9.4%) | 3,519 (11.3%) | 0.79 (0.70 to 0.88) | <0.0001 | |
| small remote rural | 2208 (6.2%) | 242 (5.8%) | 1966 (6.3%) | 0.87 (0.76 to 1.00) | 0.0497 | |
| Neighborhood povertyb (% of census below poverty line) | P <0.0001 | |||||
| 0 to 4.9% (wealthiest) | 2850 (8.3%) | 477 (11.7%) | 2373 (7.8%) | 1.41 (1.25 to 1.60) | <0.0001 | |
| 5 to 9.9% | 7255 (21.1%) | 967 (23.8%) | 6258 (20.7%) | 1.09 (0.98 to 1.20) | 0.1083 | |
| 10 to 14.9% | 6594 (19.2%) | 822 (20.2%) | 5772 (19.1%) | Reference | ||
| 15 to 19.9% | 6045 (17.6%) | 716 (17.6%) | 5329 (17.6%) | 0.94 (0.85 to 1.05) | 0.2858 | |
| 20 to 24.9% | 4718 (13.8%) | 433 (10.7%) | 4285 (14.2%) | 0.71 (0.63 to 0.80) | <0.0001 | |
| ≥25% (poorest) | 6873 (20.0%) | 648 (16.0%) | 6225 (20.6%) | 0.73 (0.66 to 0.82) | <0.0001 | |
Clinical measurements were measured at dialysis initiation.
Columns do not add up to total population because of missing values (800 patients missing distance, 1208 patients missing body mass index measurements, 1041 missing neighborhood poverty, 3 missing erythropoietin status).
Other primary causes of ESRD (<5% of total) included secondary GN/vasculitis, interstitial nephritis/pyelonephritis, cystic/hereditary/congenital disease, neoplasms/tumors, and miscellaneous conditions.
Comorbid conditions included congestive heart failure, ischemic heart disease, myocardial infarction, cardiac arrest, cardiac dysrhythmia, pericarditis, cerebrovascular disease, peripheral vascular disease, history of hypertension, diabetes (primary or contributing), chronic obstructive pulmonary disease, tobacco use, malignant neoplasm (cancer), alcohol dependence, drug dependence, HIV positive status, AIDS, inability to ambulate, and inability to transfer.
P value associated with crude OR for waitlisting.
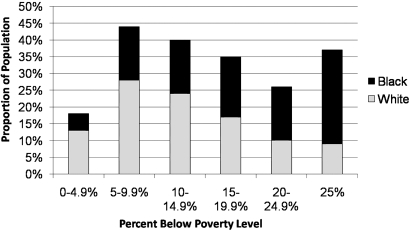
A greater proportion of black versus white patients lived <20 mi (28 versus 20%) from the nearest transplant center (Table 2). Blacks were younger (58 ± 15 yr versus 64 ± 14 yr) and less likely to have received erythropoietin before the initiation of dialysis (24 versus 29%, P < 0.0001) than white patients (Table 2). In addition, blacks were more likely to live in high poverty areas, with 28% of blacks (versus 9% of whites) residing in areas with >25% of the population living below the poverty line (Figure 1, Table 2).
Table 2.
Selected baseline characteristics by race
| Characteristic | Network 6 Patients, n = 35,346 | Black Patients, n = 20,229 (57.2%) | White Patients, n = 15,117 (42.8%) | P Value for Race Difference | |||
|---|---|---|---|---|---|---|---|
| Distance to transplant facility (mi)a | P < 0.0001 | ||||||
| <20 | 8585 (24.9%) | 5595 (28.3%) | 2990 (20.3%) | ||||
| 20 to 39 | 6939 (20.1%) | 3441 (17.4%) | 3498 (23.7%) | ||||
| 40 to 69 | 7089 (20.5%) | 3908 (19.7%) | 3181 (21.6%) | ||||
| 70 to 99 | 6008 (17.4%) | 3384 (17.1%) | 2624 (17.8%) | ||||
| 100 to 149 | 4124 (11.9%) | 2348 (11.9%) | 1776 (12.0%) | ||||
| ≥150 | 1801 (5.2%) | 1122 (5.7%) | 679 (4.6%) | ||||
| Age (yr) | P < 0.0001 | ||||||
| 20 to 39 | 3624 (10.2%) | 2549 (2.6%) | 1075 (7.1%) | ||||
| 40 to 49 | 4712 (13.3%) | 3274 (16.2%) | 1438 (9.5%) | ||||
| 50 to 59 | 7151 (20.2%) | 4554 (22.5%) | 2597 (17.2%) | ||||
| 60 to 69 | 8587 (24.3%) | 4787 (23.7%) | 3800 (25.1%) | ||||
| ≥70 | 11,272 (32.0%) | 5065 (25.0%) | 6207 (41.2%) | ||||
| Gender | P < 0.0001 | ||||||
| male | 17,675 (50.1%) | 9202 (45.5%) | 8473 (56.1%) | ||||
| female | 17,671 (49.9%) | 11,027 (54.5%) | 6644 (43.9%) | ||||
| State | P < 0.0001 | ||||||
| Georgia | 14,021 (39.7%) | 8276 (40.9%) | 5745 (38.0%) | ||||
| North Carolina | 13,665 (38.7%) | 7287 (36.0%) | 6378 (42.2%) | ||||
| South Carolina | 7,660 (21.7%) | 4666 (23.1%) | 2994 (19.8%) | ||||
| Degree of ruralityz | P < 0.0001 | ||||||
| urban | 22,270 (63.0%) | 12,764 (63.1%) | 9506 (62.9%) | ||||
| large rural | 6958 (19.7%) | 3803 (18.8%) | 3155 (20.9%) | ||||
| small rural | 3910 (11.1%) | 2480 (12.3%) | 1430 (9.5%) | ||||
| small remote rural | 2208 (6.2%) | 1182 (5.8%) | 1026 (6.8%) | ||||
| Neighborhood poverty (% of census tract below poverty line) | P < 0.0001 | ||||||
| 0 to 4.9% (wealthiest) | 2850 (8.3%) | 1032 (5.2%) | 1818 (12.5%) | ||||
| 5 to 9.9% | 7255 (21.1%) | 3233 (16.3%) | 3992 (27.6%) | ||||
| 10 to 14.9% | 6594 (19.2%) | 3147 (15.9%) | 3447 (23.8%) | ||||
| 15 to 19.9% | 6045 (17.6%) | 3623 (18.3%) | 2422 (16.7%) | ||||
| 20 to 24.9% | 4718 (13.8%) | 3261 (16.4%) | 1457 (10.1%) | ||||
| ≥25% (poorest) | 6873 (20.0%) | 5528 (27.9%) | 1345 (9.3%) | ||||
| Body mass index (kg/m2)a | P < 0.0001 | ||||||
| underweight (<18.5) | 2112 (6.2%) | 1253 (6.4%) | 859 (5.9%) | ||||
| normal (18.5 to 24.9) | 12,885 (37.7%) | 6887 (35.4%) | 5998 (40.9%) | ||||
| overweight (25.0 to 29.9) | 9555 (28.0%) | 5399 (27.7%) | 4156 (28.4%) | ||||
| obese (30.0 to 39.9) | 8089 (23.7%) | 4920 (25.3%) | 3169 (21.6%) | ||||
| morbidly obese (>40.0) | 1497 (4.4%) | 1021 (5.2%) | 476 (3.2%) | ||||
| Erythropoietin use (%) | 9246 (26.2%) | 4898 (24.2%) | 4348 (28.8%) | P < 0.0001 | |||
| Mean hemoglobin ± SD (g/dl) | 9.4 ± 4.4 | 9.1 ± 4.5 | 9.8 ± 4.2 | P < 0.0001 | |||
Columns do not add up to total population because of missing values (800 patients missing distance, 1208 patients missing body mass index measurements, and 1041 missing poverty information).
Figure 1.
Proportion of incident ESRD patients by neighborhood poverty. Neighborhood poverty was estimated by the proportion of individuals in a census tract residing below the federal poverty line.
A total of 4153 patients (11.8%) were waitlisted and 22,818 were censored by the end of the study period. Of those censored, 9598 (27.2%) transferred out of ESRD Network 6 before the end of the study, 12 (0.03%) were lost to follow-up, and 13,208 (37.4%) died. Patients who were placed on the transplant waiting list were significantly younger than those not waitlisted (47 ± 12 yr versus 63 ± 14 yr, P < 0.0001), and were less likely to have two or more comorbid conditions (52 versus 76%, respectively) (Table 1). Before adjustment, black patients were 13% more likely to be placed on the transplant waiting list than white patients [95% confidence interval (CI): 1.06 to 1.20] (Table 1).
After controlling for demographic and clinical factors, distance from a patient's residence was not associated with time to waitlisting. Compared with patients living <20 mi from the nearest transplant center, the likelihood of waitlisting was not significantly different for patients >20 mi away from the nearest transplant center (Table 3).
Table 3.
Cox models for time to waitlisting (model 1) and sensitivity analysis for best- (model 2) and worst- (model 3) case scenarios in waitlisting
| Effect | HR (95% CI), P Values
|
|||||
|---|---|---|---|---|---|---|
| Model 1 (Event = Waitlisting) | P Value | Model 2 (All Those Lost to Follow-Up Waitlisted) | P Value | Model 3 (All Those Lost to Follow-Up Not Waitlisted) | P Value | |
| Shortest distance to transplant facility (mi) | ||||||
| <20 | Reference | Reference | Reference | |||
| 20 to 39 | 0.92 (0.82 to 1.04) | P = 0.1896 | 0.87 (0.84 to 0.91) | P < 0.0001 | 0.93 (0.82 to 1.05) | P = 0.2422 |
| 40 to 69 | 0.97 (0.85 to 1.10) | P = 0.6008 | 0.86 (0.83 to 0.90) | P < 0.0001 | 0.98 (0.87 to 1.12) | P = 0.8102 |
| 70 to 99 | 0.93 (0.81 to 1.05) | P = 0.2331 | 0.82 (0.78 to 0.86) | P < 0.0001 | 0.94 (0.83 to 1.07) | P = 0.3797 |
| 100 to 149 | 1.13 (0.99 to 1.30) | P = 0.0793 | 0.88 (0.84 to 0.93) | P < 0.0001 | 1.16 (1.00 to 1.33) | P = 0.0405 |
| ≥150 | 0.81 (0.65 to 1.01) | P = 0.0567 | 0.85 (0.79 to 0.91) | P < 0.0001 | 0.81 (0.65 to 1.01) | P = 0.0598 |
| Age | ||||||
| 20 to 39 | Reference | Reference | Reference | |||
| 40 to 49 | 0.68 (0.61 to 0.76) | P < 0.0001 | 0.94 (0.89 to 0.99) | P = 0.0339 | 0.68 (0.61 to 0.76) | P < 0.0001 |
| 50 to 59 | 0.49 (0.44 to 0.54) | P < 0.0001 | 0.92 (0.87 to 0.97) | P = 0.0016 | 0.49 (0.44 to 0.54) | P < 0.0001 |
| 60 to 69 | 0.23 (0.21 to 0.26) | P < 0.0001 | 0.97 (0.92 to 1.02) | P = 0.2474 | 0.23 (0.21 to 0.26) | P < 0.0001 |
| ≥70 | 0.02 (0.01 to 0.03) | P < 0.0001 | 1.12 (1.06 to 1.17) | P < 0.0001 | 0.02 (0.01 to 0.03) | P < 0.0001 |
| Gender | ||||||
| male | Reference | Reference | Reference | |||
| female | 0.88 (0.82 to 0.96) | P = 0.0019 | 1.01 (0.98 to 1.04) | P = 0.5376 | 0.89 (0.82 to 0.96) | P = 0.0031 |
| Diabetes | ||||||
| no | Reference | Reference | Reference | |||
| yes | 0.78 (0.72 to 0.85) | P < 0.0001 | 1.02 (0.99 to 1.05) | P = 0.1328 | 0.81 (0.74 to 0.88) | P < 0.0001 |
| Hypertension | ||||||
| no | Reference | Reference | Reference | |||
| yes | 1.17 (1.05 to 1.32) | P = 0.0055 | 0.87 (0.84 to 0.91) | P < 0.0001 | 1.15 (1.03 to 1.29) | P = 0.0140 |
| Body mass index (kg/m2a | ||||||
| underweight (<18.5) | 0.77 (0.62 to 0.96) | P = 0.0207 | 1.16 (1.09 to 1.23) | P < 0.0001 | 0.78 (0.63 to 0.98) | P = 0.0300 |
| normal (18.5 to 24.9) | Reference | Reference | P < 0.0001 | Reference | ||
| overweight (25.0 to 29.9) | 1.29 (1.17 to 1.42) | P < 0.0001 | 0.93 (0.90 to 0.96) | P < 0.0001 | 1.27 (1.15 to 1.40) | P < 0.0001 |
| obese (30.0 to 39.9) | 1.10 (0.99 to 1.21) | P = 0.0729 | 0.85 (0.82 to 0.88) | P < 0.0001 | 1.09 (0.98 to 1.20) | P = 0.1065 |
| morbidly obese (>40.0) | 0.63 (0.51 to 0.78) | P < 0.0001 | 0.77 (0.72 to 0.83) | P < 0.0001 | 0.62 (0.50 to 0.77) | P < 0.0001 |
| Erythropoietin use | 1.28 (1.18 to 1.40) | P < 0.0001 | 0.82 (0.80 to 0.85) | P < 0.0001 | 1.26 (1.16 to 1.37) | P < 0.0001 |
| Albumin, mean (g/dl) | 1.06 (1.04 to 1.08) | P < 0.0001 | 0.96 (0.93 to 0.98) | P < 0.0001 | 1.40 (1.29 to 1.52) | P < 0.0001 |
| Degree of ruralitya | ||||||
| urban | Reference | Reference | Reference | |||
| large rural | 1.04 (0.93 to 1.15) | P = 0.4806 | 0.96 (0.92 to 0.99) | P = 0.0193 | 1.04 (0.93 to 1.15) | P = 0.5408 |
| small rural | 1.01 (0.88 to 1.18) | P = 0.8488 | 1.06 (1.01 to 1.11) | P = 0.0194 | 1.01 (0.87 to 1.17) | P = 0.9321 |
| small remote rural | 1.10 (0.93 to 1.32) | P = 0.2446 | 1.01 (0.95 to 1.07) | P = 0.7731 | 1.10 (0.92 to 1.31) | P = 0.3010 |
This model does not include race or neighborhood poverty because of interaction between these two variables.
Compared with normal weight patients, underweight [hazard ratio (HR): 0.77, 95% CI: 0.62 to 0.96] or morbidly obese patients (HR: 0.63, 95% CI: 0.51 to 0.78) were less likely to be waitlisted, as were those patients 40 yr and older (compared with patients 20 to 39 yr) (Table 3). In addition, female gender (HR: 0.88, 95% CI: 0.82 to 0.96) and the presence of diabetes (HR: 0.78, 95% CI: 0.72 to 0.85) decreased the likelihood of waitlisting.
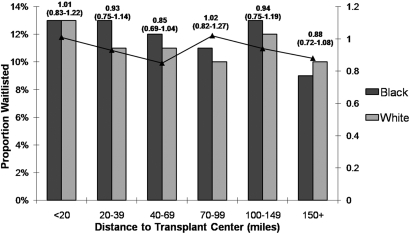
Hypertension (HR: 1.17, 95% CI: 1.05 to 1.32), overweight compared with normal weight (HR: 1.29, 95% CI: 1.17 to 1.42), erythropoietin use (HR: 1.28, 95% CI: 1.18 to 1.40), and higher mean albumin (HR: 1.06, 95% CI: 1.04 to 1.08) were associated with an increased likelihood of waitlisting (Table 3). The overall P value for trend for distance in our final model was not significant (P = 0.8874), nor was the effect of distance and waitlisting by race (Figure 2).
Figure 2.
Proportion waitlisted by race and distance to transplant center (miles). Hazard ratios are adjusted for covariates used in final model.
The relationship between race and waitlisting was modified by neighborhood poverty (two-way interaction term for race and neighborhood poverty, P = 0.0009). As neighborhood poverty increased, the likelihood of waitlisting decreased for blacks compared with whites in each poverty category. Compared with patients living in the wealthiest neighborhoods, blacks were 57% less likely than whites to be waitlisted in the poorest communities (Table 4).
Table 4.
Stratum-specific effects for poverty, race, and waitlistinga
| Neighborhood Poverty (% of Census below Poverty Line) | Crude Black:White HRs for Waitlisting (95% CI) | Adjustedb Black:White Hazard Ratios for Waitlisting (95% CI) |
|---|---|---|
| 0 to 4.9% (wealthiest) | Reference | Reference |
| 5 to 9.9% | 1.05 (0.88 to 1.22) | 0.65 (0.46 to 0.84) |
| 10 to 14.9% | 1.08 (0.92 to 1.28) | 0.63 (0.34 to 0.82) |
| 15 to 19.9% | 1.00 (0.83 to 1.21) | 0.55 (0.36 to 0.73) |
| 20 to 24.9% | 0.87 (0.67 to 1.13) | 0.42 (0.21 to 0.63) |
| ≥25% (poorest) | 0.75 (0.59 to 0.96) | 0.43 (0.22 to 0.64) |
These adjusted hazard ratios use white race as the reference group.
Adjusted for age, gender, degree of rurality, body mass index, albumin, erythropoietin, diabetes, and hypertension.
We examined the best- and worst-case scenarios of competing risks in a sensitivity analysis that treated all censored patients as if they were waitlisted (model 2) or not waitlisted (model 3). If all of the censored patients were waitlisted, those living >20 mi from the transplant center were significantly less likely to waitlist than those living <20 mi away (P < 0.0001). When we assumed that all censored patients were not waitlisted, our conclusions about the effect of distance on waitlisting did not change; distance did not affect waitlisting (Table 3).
In a sensitivity analysis exploring the effect of distance and waitlisting by age <65 yr versus age ≥65 yr, it was found that including the patients who died or were lost to follow-up did not meaningfully change the HR estimates (results not shown).
DISCUSSION
We found that black patients were less likely than whites to be placed on the kidney transplant waiting list, and this disparity was not associated with the distance to the nearest transplant center. Furthermore, we observed that neighborhood poverty was associated with waitlist placement and we report for the first time that racial disparities differ as neighborhood poverty increases.
Previous studies have reported that patients may have difficulty traveling to transplant centers to complete pretransplant referral and evaluation. In a Scottish cohort, ESRD patients were less likely to be waitlisted if they received dialysis treatment in a facility that did not have a transplant center, but patients living the farthest away (>100 km) were more likely to be waitlisted. In a random sample of Canadian dialysis patients, differences in the likelihood of transplantation occurred between provinces, but not within regions, indicating that proximity to a transplant facility was not predictive of waitlisting.9 Rodriguez et al.10 found that time to transplantation was longer among both black and white incident ESRD patients who lived in zip code areas with ≥75% of black residents. Volkova et al.11 recently reported that increasing poverty was associated with a greater disparity in ESRD incidence rates between blacks and whites. Socioeconomic status (SES) has been suggested as a risk factor for racial disparities in ESRD outcomes; however, there is little evidence to support or explain the association between SES and waitlisting outcomes.12,13
The few studies that have examined racial differences in waitlisting have shown that racial differences are not entirely explained by patient factors. Limited access to healthcare has been proposed as an explanation; however, as Karter et al.14 illustrated, racial differences in ESRD incidence are still observed in a fully insured population. In a national study of incident ESRD dialysis patients, Wolfe et al.15 found differences in waitlisting by race that were not explained by age, sex, ESRD cause, geographic region, or referral practices, but the effect of SES on waitlisting outcomes remains poorly understood.
We found that black patients were less likely to be waitlisted than whites in all neighborhood poverty levels, and that this disparity was most striking in the poorest communities. In areas with 20 to 24.9% of the census tract living below the poverty level, black patients were 58% less likely to be waitlisted than their white counterparts. In communities with >25% of the census tract living below the poverty level, blacks were 57% less likely to be waitlisted than whites (Table 4).
There are likely several explanations for this observed disparity. Race is often a surrogate for several social, behavioral, cultural, and biologic factors, whereas patient ethnicity may influence physician's beliefs about a patient's risky behaviors and likelihood of treatment adherence, resulting in referral bias.16,17 Additionally, patient preference may play a role: black patients have been shown to be less likely to want a transplant.18 Exploring the role of these factors in the observed racial differences in waitlisting outcomes is important in developing effective solutions for improving equality in access to healthcare.
This study, to our knowledge, is the first to examine residential distance to the nearest transplant center as a risk factor for kidney transplant waitlisting. This study extends the existing body of literature by incorporating neighborhood poverty as a proxy for SES, noting that poverty explains some of the racial disparities associated with waitlisting. Our study has several strengths. This study population more adequately represents the black ESRD population than prior studies (57% black) versus Wolfe et al. (35% black) and Furth et al. (32% black).15,19 The data used in this analysis were from a universal, population-based surveillance system from three southeastern states. The ESRD and UNOS data are complete and virtually all cases of waitlist placement in Network 6 were captured, thus the potential for misclassification bias of the outcome is limited.
Our study follow-up was complete (99%) and thus our results cannot be attributed to selection bias. Furthermore, a competing risks analysis was utilized to assess whether censoring due to deaths and loss to follow-up was informative.20 When we examined the best-case scenario that all censored patients were waitlisted, we found that distance could affect waitlisting (P < 0.0001), but there was no trend of dose response (Table 3, model 2). It is extremely unlikely that all censored patients were waitlisted because this would represent 75% of the study population; current reported waitlisting rates are generally between 10 and 15%.5 The more likely scenario is that none of the censored patients were waitlisted; results from model 3 assuming censored patients were not waitlisted were consistent with our original model, supporting our conclusion that distance is not associated with kidney transplant waitlisting (Table 3, model 3).
There are several limitations to this research. First, we were unable to ascertain health status at the time of waitlisting and comorbid risk factors may have changed over time. There is also possible misclassification bias of the distance from a patient's residence to the transplant center. The Euclidean distance we used may underestimate the mileage and/or time to a transplant center. In addition, our database did not contain information on the transplant center that each patient attended for their transplant referral appointment, thus we assumed that patients attended the nearest transplant center. We did include transplant facilities in the states surrounding ESRD Network 6 to control for those individuals who may travel outside of their home ESRD network for referral and evaluation at other transplant facilities. Another potential source of misclassification error is self-classification of race.
Although we adjusted for multiple potential confounders, we cannot exclude the possibility of residual confounding. Most importantly, we were unable to control for poverty at an individual level and future studies should aim to gather both individual- and neighborhood-level poverty data. There also may be a variety of unmeasured patient characteristics that precluded waitlisting (e.g., medical nonadherence or patient refusal). Finally, because our analyses were restricted only to Network 6, we may not be able to generalize these findings outside of Georgia, North Carolina, and South Carolina.
Racial disparities in access to kidney transplantation are complex and are likely explained by a combination of patient- and healthcare facility-related factors; however, neighborhood poverty appears to play a strong, previously under-recognized role.21–27 Although distance was not a significant predictor for placement on the kidney transplant waiting list, to our knowledge this is the first study to show that as neighborhood poverty increases, so does racial disparity in waitlisting, with black patients being 57% less likely than whites to be waitlisted in the poorest communities. Focusing on poor communities may be an effective way to improve fair allocation of kidney organs.
Few studies have examined access to waitlisting for kidney transplantation among patients living farther away from a transplant center, where patients must undergo a required referral and assessment. The results of this analysis show that distance from a patient's residence to the nearest transplant center does not explain the racial variation in placement on the kidney transplant waiting list. This may be interpreted as a relative success for transplant centers, because there appears to be no difference in waitlisting for patients who live far away compared with those who live close to the center. Although distance did not explain these racial disparities, we did find that neighborhood poverty had a large effect on waitlisting outcomes between blacks and whites. These findings suggest that ESRD networks may improve access to kidney transplantation and better address racial inequities by examining more closely the poorer communities within their respective networks.
CONCISE METHODS
Study Population and Data Sources
Incident adult patients (age >20 yr) between 1998 and 2002 from ESRD Network 6 (Georgia, North Carolina, and South Carolina) were included in this analysis.5 This study was restricted to black or non-Hispanic white patients. Demographic data were collected from the Centers for Medicare and Medicaid Services (CMS) Medical Evidence Form (CMS-2728), which is completed on all incident patients diagnosed with ESRD. Neighborhood poverty data were obtained from the Census 2000 by census tract. Data on placement on a transplant waiting list were obtained from the UNOS registry.
There were 36,617 eligible incident ESRD patients initiating hemodialysis between 1998 and 2002 in ESRD Network 6. A total of 913 patients were excluded because of missing information on race and 358 pediatric patients were omitted, leaving a total study population of 35,346.
Study Variables
The primary outcome variable was placement on the UNOS waiting list for a deceased donor kidney (waitlisted = yes or no). Study participants were identified at the initiation of dialysis and followed until placement on the transplant waiting list, loss to follow-up, death, or the end of the study.
Race and distance from a patient's residence to the nearest transplant center were the exposures in the analysis. Race was defined as non-Hispanic white versus black. Latitude and longitude coordinates of the patient's residence were entered into ArcView 9.1 software and the Euclidean distance between a patient's residence and the nearest transplant facility was calculated. Distance was then categorized by distribution (< 20, 20 to 39, 40 to 69, 70 to 99, 100 to 149, and ≥150 mi). All ten transplant centers within Network 6 as well as six adjacent transplant centers (in Chattanooga, Johnson City, and Knoxville, Tennessee; Gainesville and Jacksonville, Florida; and Birmingham, Alabama) were considered when calculating the distance to the nearest facility for each patient.
Other covariates examined included demographic and clinical characteristics potentially associated with transplantation, including patient age (mean ± SD); gender (male, female); state of residence (Georgia, North Carolina, or South Carolina); cause of ESRD (diabetes, hypertension, chronic GN, or other); presence of comorbid conditions at dialysis initiation (heart disease, vascular disease, hypertension, diabetes, pulmonary disease, tobacco use, cancer, alcohol dependence, drug dependence, and HIV status); body mass index (kg/m2); treatment facility; and clinical variables at dialysis initiation, including erythropoietin use, mean hemoglobin (g/dl), and mean albumin (g/dl).
Neighborhood poverty was estimated by the proportion of individuals residing below the federal poverty level in the census tract. Home addresses were linked to a specific census tract using 2000 U.S. Census Bureau data. The U.S. Census Bureau reports the proportion of households within each census tract below the federal poverty level on the basis of income, family size, and ages of individual family members. We defined a census tract as high poverty if 25% or more of the households were assigned to below the federal poverty level.
The degree of urbanity of a patient's residence and transplant waitlisting was examined. Residence data were classified into Rural Urban Commuting Area (RUCA) codes, which categorize U.S. census tracts by population density, degree of urbanization, and daily commuting distances. RUCA classifications include urbanized areas, large urban clusters, small urban clusters and rural areas (http://www.ers.usda.gov/Data/RuralUrbanCommutingAreaCodes/).
Data Analysis
χ2 tests and t test (or nonparametric equivalents of the t test) were used to examine the differences between baseline characteristics, including demographic and clinical characteristics of waitlisted individuals compared with those not waitlisted. Crude odds ratios were calculated using a logistic model with the outcome variable placement on the UNOS waiting list (yes or no). Results were considered significant at the P < 0.05 level.
We used a Cox proportional hazards model to examine the association between distance from a patient's residence to the nearest transplant center and time until waitlisting among incident ESRD patients. For time until waitlisting, patients were censored when they died (due to any cause), were lost to follow-up, or transferred out of Network 6. The proportional hazards model was identified by including potential confounders, followed by interaction assessment using the likelihood ratio test (P value for removal from the model was set at P < 0.05) and evaluating confounding by comparing meaningful changes in point estimates from a “gold standard” model to all other potential models.20,28
Because death and loss to follow-up may preclude a patient from waitlisting, we considered these events as competing risks and utilized a proportional hazards model that treated all of these events as censored observations. To examine whether censoring was informative or not, we conducted a sensitivity analysis that looked at the best- and worst-case scenarios; that is, all of those who were censored were placed on the waiting list and all of those censored were not placed on the waiting list, respectively.20 All analyses were performed with SAS software version 9.2. The Emory University Institutional Review Board approved this study.
DISCLOSURES
The analyses upon which this publication is based were performed under Contract Number 500-96-P704, entitled, “Operation Utilization and Quality Control Peer Review Organization (PRO) for the State of Georgia,” sponsored by the CMS, Department of Health and Human Services. The conclusions and opinions expressed and methods used herein are those of the author; they do not necessarily reflect CMS policy.
Supplementary Material
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “How Long Can We Afford to Wait for Equity in the Renal Transplant Waiting List?” on pages 1168–1170.
REFERENCES
- 1.Young CJ, Gaston RS: Renal transplantation in black Americans. N Engl J Med 343: 1545–1552, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Nzerue CM, Demissochew H, Tucker JK: Race and kidney disease: Role of social and environmental factors. J Natl Med Assoc 98(Suppl 8): 28S–38S, 2002 [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne C, Nedelman J, Luke RG: Race, socioeconomic status, and the development of end stage renal disease. Am J Kidney Dis 23: 16–22, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ: The excess incidence of diabetic end-stage renal disease among blacks: A population-based study of potential explanatory factors. JAMA 268: 379–384, 1992 [PubMed] [Google Scholar]
- 5. U.S. Renal Data System 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006
- 6.Rosansky SJ, Huntsberger TL, Jackson K, Eggers P: Comparative incidence rates of end-stage renal disease treatment by state. Am J Nephrol 10: 198–204, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB: Geographic variability in access to primary kidney transplantation in the United States, 1996–2005. Am J Transplant 7: 1412–1423, 2007 [DOI] [PubMed] [Google Scholar]
- 8.O'Hare A, Johansen KL, Rodriguez RA: Dialysis and kidney transplantation among patients ling in rural areas of the United States. Kidney Int 69: 343–349, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Tonelli M, Klarenbach S, Manns B, Culleton B, Hemmelgarn B, Bertazzon S, Wiebe N, Gill JS: Residence location and likelihood of kidney transplantation. CMAJ 175: 478–482, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O'Hare AM: Geography matters: Relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med 146: 493–501, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Volkova N, McClellan W, Klein M, Flanders D, Kleinbaum D, Soucie JM, Presley R: Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol 19: 356–364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perneger TV, Whelton PK, Klag MJ: Race and end-stage renal disease: Socioeconomic status and access to health care as mediating factors. Arch Intern Med 155: 1201–1208, 1995 [PubMed] [Google Scholar]
- 13.Young EW, Mauger EA, Jiang KH, Port FK, Wolfe RA: Socioeconomic status and end-stage renal disease in the United States. Kidney Int 45: 907–911, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV: Ethnic disparities in diabetic complications in an insured population. JAMA 287: 2519–2527, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Wolfe RA, Ashby VB, Milford EL, Bloembergen WE, Agodoa LY, Held PJ, Port FK: Differences in access to cadaveric renal transplantation in the United States. Am J Kid Dis 36: 1025–1033, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Krieger N, Smith GD: Seeking causal explanations in social epidemiology. Am J Epidemiol 151: 831–833, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Powe NR: To have and have not: Health and health care disparities in chronic kidney disease. Kidney Int 64: 763–772, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM: The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med 341: 1661–1669, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Furth SL, Garg PP, Neu AM, Hwang W, Fivush BA, Powe NR: Racial differences in access to the kidney transplant waiting list for children and adolescents with end-stage renal disease. Pediatrics 106: 756–761, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Kleinbaum DG, Klein M: Survival Analysis: A Self-Learning Text. 2nd ed., New York, NY, Springer, 2005
- 21.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, Weisman JS, David-Kasdan JA, Carlson D, Fuller J, Marsh D, Conti RM: Racial disparities in access to renal transplantation—Clinically appropriate or due to underuse or overuse? N Engl J Med 343: 1537–1544, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayanian JZ, Cleary PD, Keogh GH, Noonan SJ, David-Kasdan JA, Epstein AM: Physicians’ beliefs about racial differences in referral for renal transplantation. Am J Kidney Dis 43: 350–357, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Alexander G, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Navaneethan SD: A systematic review of barriers in access to renal transplantation among African Americans in the United States. Clin Transplant 20: 769–775, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Weng FL: Rates of completion of the medical evaluation for renal transplantation. Am J Kid Dis 46: 734–745, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Haas JS, Phillips KA, Sonneborn D, McCulloch CE, Baker LC, Kaplan CP, Perez-Stable EJ, Liang SY: Variation in access to health care for different racial/ethnic groups by the racial/ethnic composition of an individual's county of residence. Med Care 42: 707–714, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Boulware LE, Meoni LE, Fink NE, Parekh RS, Kao WH, Klag MJ, Powe NR, Preferences, knowledge, communication and patient-physician discussion of living kidney transplantation in African American families. Am J Transplant 5: 1503–1512, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Kleinbaum DG, Klein M: Logistic Regression: A Self-Learning Text. 2nd ed., Statistics for Biology and Health, New York, NY, Springer, 2002
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.