Abstract
Although diagnosis and staging of acute kidney injury uses serum creatinine, acute changes in creatinine lag behind both renal injury and recovery. The risk for mortality increases when acute kidney injury accompanies sepsis; therefore, we sought to explore the limitations of serum creatinine in this setting. In mice, induction of sepsis by cecal ligation and puncture in bilaterally nephrectomized mice increased markers of nonrenal organ injury and serum TNF-α. Serum creatinine, however, was significantly lower in septic animals than in animals subjected to bilateral nephrectomy and sham cecal ligation and puncture. Under these conditions treatment with chloroquine decreased nonrenal organ injury markers but paradoxically increased serum creatinine. Sepsis dramatically decreased production of creatinine in nephrectomized mice, without changes in body weight, hematocrit, or extracellular fluid volume. In conclusion, sepsis reduces production of creatinine, which blunts the increase in serum creatinine after sepsis, potentially limiting the early detection of acute kidney injury. This may partially explain why small absolute increases in serum creatinine levels are associated with poor clinical outcomes. These data support the need for new biomarkers that provide better measures of renal injury, especially in patients with sepsis.
Serum creatinine is used clinically to detect and evaluate acute kidney injury (AKI) and chronic kidney disease (CKD),1,2 although the limitations of serum creatinine for the early detection and accurate estimation of renal injury are widely known. In AKI, serum creatinine does not accurately reflect the GFR because the patient is not in steady state.3 Furthermore, serum creatinine is also influenced by tubular creatinine secretion and by nonrenal factors such as muscle mass, liver function, and nonrenal (gastrointestinal) elimination.
Sepsis remains a serious problem in critically ill patients, and mortality from sepsis is increased dramatically when complicated by AKI; therefore, early detection and accurate evaluation of AKI is important in patients with sepsis. We modified the cecal ligation and puncture (CLP) mouse sepsis model to make it more clinically relevant, and our model developed sepsis-induced AKI.4–6 We found that serum creatinine increased to a lesser extent in CLP (approximately 0.5 mg/dl) than ischemia/reperfusion or cisplatin administration (>1 mg/dl) within 24 h of injury. To explore nonrenal factors that might account for this disparity, we used bilateral nephrectomy (BNx), a technique used recently by others to study the contribution of the kidney to cytokine metabolism and acute lung injury.7
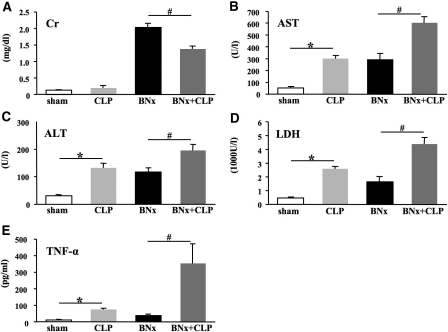
First, we evaluated sepsis in bilaterally nephrectomized mice. We induced sepsis by CLP surgery with 8-mm cecal ligation, which causes modest sublethal sepsis in normal outbred CD-1 mice.8 All animals survived until they were killed at 18 h after surgery. CLP surgery in non-nephrectomized mice caused a numerically small, but not significant increase of serum creatinine (shamBNx+CLP group). As expected, we found large increases of serum creatinine in the BNx+shamCLP group; however, induction of sepsis at the time of BNx significantly decreased serum creatinine (BNx+CLP group) compared with nonseptic BNx alone (BNx+shamCLP group; Figure 1A), raising doubt about whether serum creatinine accurately reflects impaired kidney function during sepsis. In contrast, nonrenal organ injury markers (aspartate aminotransferase [AST], alanine aminotransferase [ALT], and lactate dehydrogenase [LDH]) and serum TNF-α were higher in the BNx+CLP group than in the BNx+shamCLP group, confirming the presence of severe sepsis (Figure 1, B through E).
Figure 1.
BNx and subsequent sepsis induced by CLP. (A through E) Serum creatinine (A), AST (B), ALT (C), LDH (D), and serum TNF-α (E) were measured at 18 h after surgery (n = 5 to 6 per group). Data are means ± SEM. *P < 0.05 versus Sham BNx/Sham CLP; #P < 0.05 versus BNx.
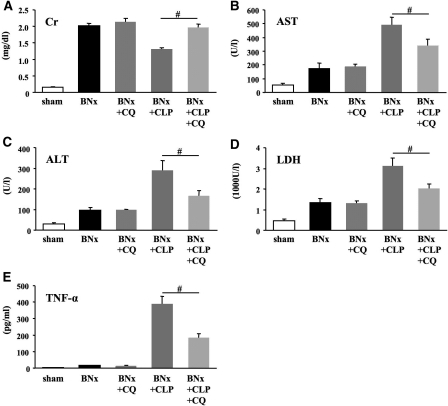
We next examined whether treatment of sepsis would affect this observation by using chloroquine, which decreases mortality and improves organ function in a model of CLP-induced sepsis.9 Chloroquine treatment of septic bilaterally nephrectomized mice (BNx+CLP+CQ) decreased nonrenal organ injury markers (AST, ALT, and LDH) and TNF-α compared with vehicle-treated mice (BNx+CLP); however, serum creatinine was paradoxically higher in treated animals (Figure 2). As a control, chloroquine did not cause any changes in the BNx+shamCLP group. We reported recently that chloroquine reduced mortality and sepsis-induced AKI, including renal pathologic damage.9 At first glance, these results seem contradictory: Serum creatinine is reduced by chloroquine when kidneys are intact yet increased by chloroquine in the absence of any kidneys. In the septic state, chloroquine alters nonrenal metabolism of creatinine, which is confirmed by completely removing urinary creatinine excretion by BNx; therefore, in both untreated and treated septic mice, serum creatinine changes in the opposite direction as markers of injury from other organs, challenging the underlying assumptions behind the use of serum creatinine as a biomarker for AKI.
Figure 2.
Effects of chloroquine treatment on BNx+CLP. (A through E) Serum creatinine (A), AST (B), ALT (C), LDH (D), and serum TNF-α (E) were measured at 18 h after surgery (n = 8 to 9 per each BNx group; n = 5 per sham group). Sham is Sham BNx/Sham CLP. Data are mean ± SEM. #P < 0.05 versus BNx+CLP.
To determine whether changes in fluid compartments could account for these observations, we measured body weight changes and hematocrit, as well as volume of distribution (Vd) for FITC-inulin and creatinine. There was no significant difference in body weight change or hematocrit (body weight change: BNx+shamCLP 2.3 ± 1.0% [n = 5], BNx+CLP 1.7 ± 0.6% [n = 5]; hematocrit: BNx+shamCLP 36.0 ± 0.9% [n = 6], BNx+CLP 34.6 ± 1.3% [n = 6]). Creatinine space, a marker of total body water, was not different between the BNx+CLP and the BNx+shamCLP groups (Figure 3A). FITC-inulin space, a marker of extracellular fluid volume, was only modestly decreased in the BNx+CLP group (Figure 3A). We then estimated creatinine production from the increase in serum creatinine between time 0 and 14 h and the creatinine Vd. We found that sepsis significantly decreased estimated creatinine production by 29.7 ± 4.3% (Figure 3B). Direct measurement of creatinine production10 would be necessary to confirm our result.
Figure 3.
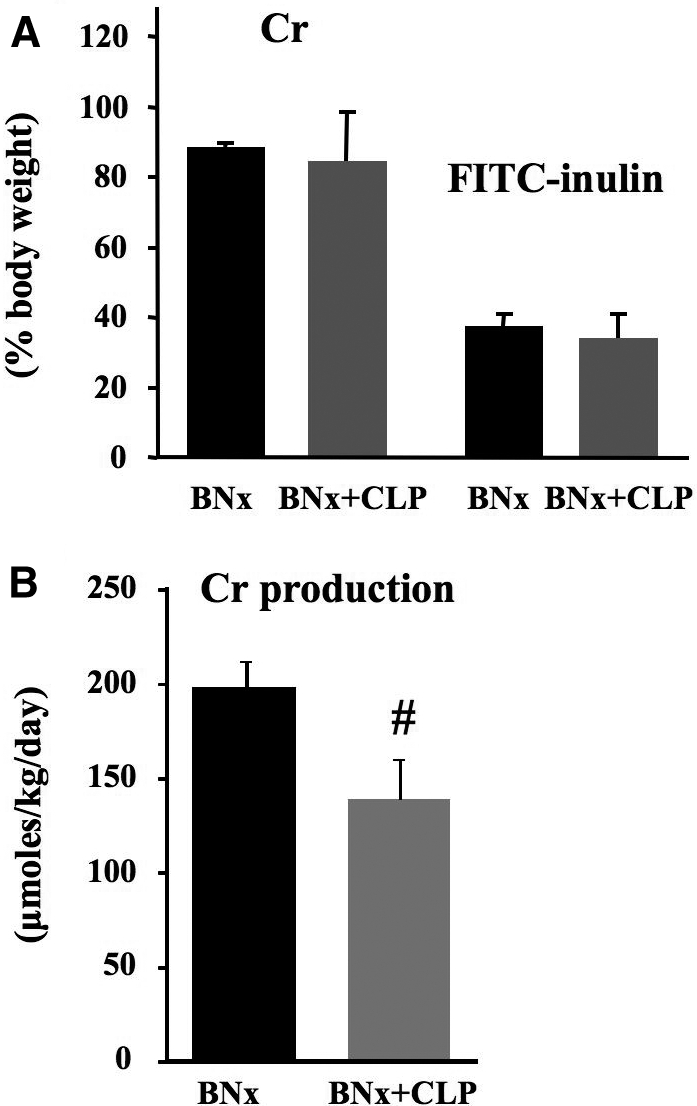
Volume of distribution (Vd) and estimated creatinine production. (A) Vd of creatinine and FITC-inulin were measured from 14 to 18 h after surgery. (B) Estimated creatinine production was measured at 0 to 14 h after surgery. Data are means ± SEM (n = 5 to 6 per group). #P < 0.05 versus BNx.
Creatinine is the end product of creatine metabolism. Alteration in creatinine production can alter serum creatinine levels. For example, N-acetylcysteine, which has been shown to protect from radiocontrast medium–induced nephropathy,11 decreases serum creatinine levels of healthy humans without changing serum cystatin C levels.12 This suggests that N-acetylcysteine directly reduces creatinine production. We suspected that creatinine production was reduced, on the basis of the small increase in serum creatinine after CLP sepsis versus renal ischemia-reperfusion. In this study, we found that sepsis reduced creatinine production, which would largely account for the slow rise in serum creatinine. Serum creatinine could fall as a result of reduced production or renal or extrarenal clearance (creatinine degradation or gastrointestinal excretion).10 Creatine is synthesized from guanidinoacetic acid primarily in the liver,13 enters skeletal muscle via a membrane transporter, and accumulates there because it is phosphorylated by creatine kinase. Creatine is converted into creatinine by a nonenzymatic cyclization throughout the body but especially in skeletal muscle as a result of the high abundance of creatine. Creatinine production can fall because of reductions in lean body mass, dietary intake of creatine, or liver disease.14 Indeed, the BNx+CLP group showed significantly higher liver enzymes and pathologic changes such as extensive loss of hepatocyte glycogen stores and bland cytoplasm (data not shown).4 Intensive care unit patients have a progressive decline in creatinine production as a result of a loss of muscle mass worsened by subclinical hepatic injury.15
Sepsis reduces energy production and metabolic rate because of hormonal and inflammatory mediators,16 which could reduce muscle production of creatinine. As we previously reported, CLP induced severe septic shock with hypodynamic circulation failure and reduced microvascular capillary perfusion,6,8,17 which could reduce muscle creatinine release, liver creatine-to-creatinine conversion, and/or release into the circulation. In this study, the numerical decrease in creatinine space in the BNx+CLP group is consistent with reduced cellular perfusion in sepsis. Because our CLP model has a prolonged period of hypodynamic shock,8 our results may not translate to patients with hyperdynamic shock. Examination of creatinine production in hyperdynamic animal sepsis models18–20 would be needed to determine the extent that microvascular perfusion may contribute to the decrease in creatinine production during hyperdynamic sepsis.
Sepsis-induced hypothermia may also decrease nonenzymatic conversion of creatine to creatinine. CLP sepsis causes profound hypothermia, which is a marker of reduced metabolism.21 In this study, we found that the severe hypothermia in the BNx+CLP animals was attenuated by chloroquine treatment (data not shown). Creatinine is converted from creatine and creatine phosphate nonenzymatically, and the rate of nonenzymatic conversion of creatine to creatinine depends on the pH and temperature; a 3°C decrease would reduce this conversion by 15 to 20%.22 In addition, decreasing metabolism by systemic injection of drugs such as 5′-adenosine monophosphate (AMP)23 or 2-deoxy-d-glucose (2-DG)24 instead of sepsis to bilaterally nephrectomized mice also caused hypothermia and decreases of serum creatinine 18 h after surgery (Supplemental Figure 1).
Our findings may have direct clinical significance. As a result of decreased creatinine production, serum creatinine is an even poorer indicator of renal damage in sepsis because its reduced production further magnifies the kinetic disparity between apparent and actual renal function; serum creatinine underestimates renal damage to a greater extent in sepsis than in other forms of AKI. The reduced creatinine production may also explain why such small increases in serum creatinine are associated with dramatic increases in morbidity and mortality of human patients.25,26 Higher serum creatinine was paradoxically associated with better survival in AKI in several clinical studies.27–29 Underestimation of renal function changes by “inappropriately low” serum creatinine will delay the early diagnosis of AKI, impede recognition of an additional organ injury for prognostic purposes, and suppress entry into clinical trials. Although the timing of renal replacement therapy initiation (including prophylactic dialysis)30 is controversial, renal replacement therapy may need to be started at relatively lower serum creatinine than other types of AKI.
The paradoxic effect of chloroquine on creatinine in bilaterally nephrectomized animals also has implications for clinical trial design. Chloroquine improved sepsis and sepsis-induced AKI by survival analysis and pathologic examination.9 We hypothesize that chloroquine restores creatinine metabolism indirectly by reducing the severity of sepsis, perhaps by improvement in muscle metabolism and/or liver function. Although serum creatinine is widely used as an end point for AKI clinical studies, the effects of treatment of sepsis and sepsis-induced AKI might be incorrectly ascertained if kidney damage in sepsis is evaluated only by serum creatinine. Confirmation with other end points such as injury biomarkers and mortality and morbidity rate should be required, especially for clinical studies on sepsis.
In conclusion, we demonstrated that sepsis reduced creatinine production, thereby blunting the expected increases of serum creatinine in bilaterally nephrectomized mice. Moreover, treatment with chloroquine reduced improved sepsis but paradoxically increased serum creatinine in bilaterally nephrectomized mice, suggesting a normalization of creatinine production. Our data indicate that evaluation of kidney injury by serum creatinine alone would cause a severe underestimation of renal injury, serious failure of early diagnosis of sepsis-induced AKI, and incorrect ascertainment of drug effects. Newer biomarkers that more accurately measure renal injury are needed, especially in patients with sepsis.
CONCISE METHODS
BNx and Subsequent CLP
All animal experiments were conducted in accordance with an animal study protocol approved by the National Institute of Diabetes and Digestive and Kidney Diseases animal care and use committee. Eight-week-old male CD-1 mice (Charles River Laboratories, Wilmington, MA) weighing 30 to 35 g were allowed food and water ad libitum. For each experiment, all of the animals received BNx or its sham operation (shamBNx), and CLP or its sham operation (shamCLP). Under isoflurane anesthesia, the kidneys were removed by flank approach, or kidneys were isolated in sham surgeries. Immediately after BNx, sepsis was induced by CLP surgery. A 4-0 silk ligature was placed 8 mm from the cecal tip, and the cecum was punctured twice with a 21-G needle and gently squeezed. At the end of surgery, 1 ml of prewarmed normal saline was injected intraperitoneally, and mice were subsequently kept at 29°C. Mice were treated with fluid and antibiotic at 6 h after surgery by subcutaneous injection of imipenem/cilastatin (14 mg/kg) in 1 ml of normal saline and killed at 18 h after for collecting specimens. Mice were not allowed food and water after the surgery.
Chloroquine, AMP, and 2-DG Administration
Chloroquine (Sigma-Aldrich, St. Louis, MO) dissolved in normal saline at the dosage of 50 mg/kg was administered orally at 3 h before surgery.9 In other animals, AMP (Sigma-Aldrich) at the dosage of 1.5 mmol/kg or 2-DG (Sigma-Aldrich) at the dosage of 400 mg/kg dissolved in 0.3 ml of normal saline was injected intraperitoneally at 0, 3, and 6 h after surgery. All of the animals in the other group received the same amount of vehicle at the indicated times.
Measurements of Blood Chemistries, Hematocrit, and Serum Cytokine
Serum creatinine was measured by HPLC, which has a coefficient of variation of 1.6 to 5.1%.31 AST, ALT, and LDH were measured using an autoanalyzer (Hitachi 917; Boehringer-Mannheim, Indianapolis, IN). Hematocrit was analyzed by an automated hematology analyzer (Abbott Cell-Dyn 3500; GMI, Ramsey, MN). Serum TNF-α was measured by ELISA (R&D Systems, Minneapolis, MN).
Measurement of Vd and Creatinine Production
Inulin-labeled FITC preparation and measurement of plasma concentration was performed as described previously.32 Inulin-labeled FITC was injected intravenously at 30 min after surgery, and its Vd was calculated from the amount injected and the plasma concentration at 18 h after injection. Creatinine Vd and production were measured using injection of a known amount of unlabeled creatinine, as previously reported in bilaterally nephrectomized dogs.33 Creatinine (30 mg/kg) was injected intravenously at 14 h after surgery, and blood samples were collected before injection and 4 h later. Vd of creatinine in nephrectomized mice was calculated from the 4-h increase in serum creatinine in the vehicle-injected mice (n = 6 to 8 per group). The creatinine production in each mouse was estimated from the time 0 to 14-h increase in serum creatinine multiplied by the creatinine Vd determined in that mouse. Creatinine at 0 h was assumed to be distributed in a Vd of 60% of body weight, which is approximately the same of total body water.34 Because the normal mouse creatinine is so low compared with the creatinine after BNx, the calculated creatinine production is not sensitive to the time 0 Vd. Nevertheless, a more precise value could be directly measured after equilibrating the mouse creatine compartment with radiolabel.
Statistical Analysis
Results are expressed as means ± SEM. Differences between groups were analyzed by ANOVA followed by Fisher least significant difference test. These calculations were done using SigmaStat 3.10 (Systat Software, Richmond, CA). The null hypothesis was rejected at P < 0.05.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. K.D. is a Japan Society for the Promotion of Science Postdoctoral Fellow for Research Abroad.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A: Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: S1–S201, 2003 [PubMed] [Google Scholar]
- 3.Star RA: Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Dear JW, Yasuda H, Hu X, Hieny S, Yuen PS, Hewitt SM, Sher A, Star RA: Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: Acute renal failure is dependent on myd88 but not renal cell apoptosis. Kidney Int 69: 832–836, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyaji T, Hu X, Yuen PS, Muramatsu Y, Iyer S, Hewitt SM, Star RA: Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int 64: 1620–1631, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Yasuda H, Yuen PS, Hu X, Zhou H, Star RA: Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 69: 1535–1542, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, Tao Y, Dursun B, Voelkel NF, Edelstein CL, Faubel S: Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol 18: 155–164, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Doi K, Hu X, Yuen PS, Leelahavanichkul A, Yasuda H, Kim SM, Schnermann J, Jonassen TE, Frokiaer J, Nielsen S, Star RA: AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int 73: 1266–1274, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasuda H, Leelahavanichkul A, Tsunoda S, Dear JW, Takahashi Y, Ito S, Hu X, Zhou H, Doi K, Childs R, Klinman DM, Yuen PS, Star RA: Chloroquine and inhibition of toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am J Physiol Renal Physiol 294: F1050–F1058, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitch WE, Collier VU, Walser M: Creatinine metabolism in chronic renal failure. Clin Sci (Lond) 58: 327–335, 1980 [DOI] [PubMed] [Google Scholar]
- 11.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W: Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 343: 180–184, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann U, Fischereder M, Kruger B, Drobnik W, Kramer BK: The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol 15: 407–410, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Walker JB: Creatine: Biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol 50: 177–242, 1979 [DOI] [PubMed] [Google Scholar]
- 14.Cocchetto DM, Tschanz C, Bjornsson TD: Decreased rate of creatinine production in patients with hepatic disease: Implications for estimation of creatinine clearance. Ther Drug Monit 5: 161–168, 1983 [DOI] [PubMed] [Google Scholar]
- 15.Wells M, Lipman J: Measurements of glomerular filtration in the intensive care unit are only a rough guide to renal function. S Afr J Surg 35: 20–23, 1997 [PubMed] [Google Scholar]
- 16.Singer M, De Santis V, Vitale D, Jeffcoate W: Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet 364: 545–548, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Piper RD, Pitt-Hyde M, Li F, Sibbald WJ, Potter RF: Microcirculatory changes in rat skeletal muscle in sepsis. Am J Respir Crit Care Med 154: 931–937, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Di Giantomasso D, Ma CN, Bellomo R: Vital organ blood flow during hyperdynamic sepsis. Chest 124: 1053–1059, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Fink MP, MacVittie TJ, Casey LC: Inhibition of prostaglandin synthesis restores normal hemodynamics in canine hyperdynamic sepsis. Ann Surg 200: 619–626, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langenberg C, Wan L, Egi M, May CN, Bellomo R: Renal blood flow and function during recovery from experimental septic acute kidney injury. Intensive Care Med 33: 1614–1618, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D: Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun 67: 6603–6610, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuller NJ, Elia M: Factors influencing the production of creatinine: Implications for the determination and interpretation of urinary creatinine and creatine in man. Clin Chim Acta 175: 199–210, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Kaasik K, Blackburn MR, Lee CC: Constant darkness is a circadian metabolic signal in mammals. Nature 439: 340–343, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Freinkel N, Metzger BE, Harris E, Robinson S, Mager M: The hypothermia of hypoglycemia: Studies with 2-deoxy-D-glucose in normal human subjects and mice. N Engl J Med 287: 841–845, 1972 [DOI] [PubMed] [Google Scholar]
- 25.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM: Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol 13: 1350–1357, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Paganini EP, Larive B, Kanagasundaram NS: Severity scores and outcomes with acute renal failure in the ICU setting. Contrib Nephrol 181–195, 2001 [DOI] [PubMed]
- 29.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Doig GS, Oudemans van Straaten H, Ronco C, Kellum JA: External validation of severity scoring systems for acute renal failure using a multinational database. Crit Care Med 33: 1961–1967, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Palevsky PM: Renal replacement therapy I: Indications and timing. Crit Care Clin 21: 347–356, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Yuen PS, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA: A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol 286: F1116–F1119, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD: Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Schloerb PR: Total body water distribution of creatinine and urea in nephrectomized dogs. Am J Physiol 199: 661–665, 1960 [DOI] [PubMed] [Google Scholar]
- 34.Canaud B, Garred LJ, Argiles A, Flavier JL, Bouloux C, Mion C: Creatinine kinetic modelling: A simple and reliable tool for the assessment of protein nutritional status in haemodialysis patients. Nephrol Dial Transplant 10: 1405–1410, 1995 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.