Abstract
The T cell receptor alpha constant gene (TRAC) encodes the constant region of the α chain for the T cell receptor, and the association of its gene variants with IgA nephropathy remains controversial. The authors resequenced the gene in 100 patients with IgA nephropathy and 100 controls, tested its linkage disequilibrium pattern, constructed haplotypes, and performed association and functional studies. First, the association between TRAC variants and IgA nephropathy was tested in 704 patients and 704 controls. Next, these 704 patients were divided into two independent datasets—310 with family member(s) and 394 single patients—to test the association separately. Results showed that the gene is located in a recombination hot spot, with nine linkage disequilibrium blocks within a 6.9-kb region. There is a hypervariable region with six single-nucleotide polymorphisms (SNPs) in an 85-bp stretch in intron 1. We identified multiple SNPs and two haplotypes that associate with IgA nephropathy (P = 0.0000013–0.0096 by logistic regression for SNPs; P = 0.0003 and P = 0.0398 for haplotype associations). The family-based study replicated both haplotype findings, and the 394 single-patient case-control study replicated the association with haplotype 1 (P = 0.0033). The overtransmitted/observed haplotypes demonstrated reduced transcription activity compared with the undertransmitted/observed haplotypes. In conclusion, this study suggests an association between TRAC variants and susceptibility to IgA nephropathy.
IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis worldwide.1 The reported incidence among the Chinese population is 35% to 45% of all primary glomerulonephritides.1–3 Approximately 20% to 50% patients who presented when older than 30 yr subsequently developed end stage renal disease within the next 20 yr.4 The disease is characterized by prominent polymeric IgA deposition within the glomerular mesangium, leading to mesangial proliferation and sclerosis and, finally, tubulointerstitial damage and fibrosis.5
Despite the perception of IgAN as a humoral immune-mediated disease, as evidenced by the glomerular IgA deposition and elevated IgA in circulation,6 abnormalities in cellular immunity contributory to the pathogenesis have been implicated. These include hyperactivity of T helper cell,7 defective suppressor T cell function,8–10 abnormal modulation of IgA synthesis by T cell subsets,10,11 enhanced production of cytokine such as IL-2 and IFN-γ,12 and T cell infiltration in affected kidneys.13
T cell receptors (TCRs) are responsible for recognizing antigens bound to MHC molecules. Activated T cells then provide help for the production of IgA. The receptors are heterodimers consisting of either α and β, or γ and δ chains on the cell surface linked by disulphide bonds. T cell receptor alpha constant gene (TRAC, Entrez GeneID: 28755, alias: T cell receptor alpha chain C region) encodes the constant region of the TCR α chain, which composes the V, J, and C segments for the variable, joining, and constant regions of the peptide, respectively. These regions are joined by somatic rearrangement during T cell maturation in the thymus.14,15 The association between genetic variation of the TRAC gene and IgAN remains controversial. Li et al.16 reported a 7-kb TaqI fragment in the TRAC gene was associated with susceptibility to, but not progression of, IgAN. However, opposite results with the TaqI-based fragment had been reported by a Japanese group.17
Here, we present evidence that TRAC contributes to the susceptibility to IgAN. We confirmed a significant association between IgAN and multiple single nucleotide polymorphisms (SNPs) and haplotypes in the TRAC locus in both case-control and family-based investigations. Functional differences were also detected in the associated haplotypes in the tested individuals.
RESULTS
Clinical Characteristics of Patients
The study comprises 704 biopsy-proven IgAN patients and equal number of age-, gender- and ethnicity-matched controls recruited from Southern China and Hong Kong. Included in the 704 cases are 310 patients who also had family member(s) (patient-parent(s) trios or patient-siblings). The clinical characteristics of the patients and the detailed family data are shown in supplementary Tables 1 and 2.
Sequence Variations in the TRAC Gene
For identification of genetic variations and exclusion of coding and splice site mutations of the TRAC gene, we first resequenced the gene in 100 patients with IgAN and 100 controls randomly selected from our collection. Fifty-eight SNPs were identified (supplementary Table 3). Among them two were coding SNPs (cSNPs), all located in exon 1. Exons 2 and 3 were highly conserved. Exon 4 was untranslated. We then further sequenced exon 1 in all 704 patients with IgAN and 704 age- and gender-matched controls. Four additional cSNPs were detected (supplementary Table 3). Of the six cSNPs, three were nonsynonymous. The introns, UTR, and the 5′ and 3′ flanking regions were rich in sequence variations. There was a hypervariable region in intron 1, where six SNPs (SNP 3: rs56131962, SNP 4: rs55766725, SNP 5: rs1263648, SNP 6: rs1263649, SNP 7: rs1263650, SNP 8: rs1263651) were concentrated within an 85-bp DNA stretch. The Hardy-Weinberg equilibrium was achieved in the control subjects.
Linkage Disequilibrium (LD) Structure and Haplotype Constitution
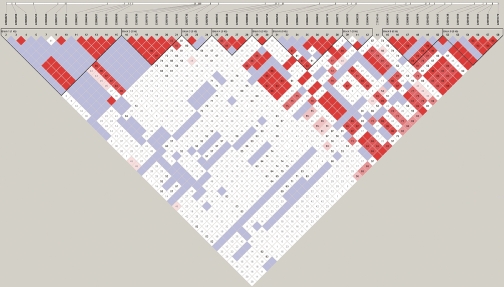
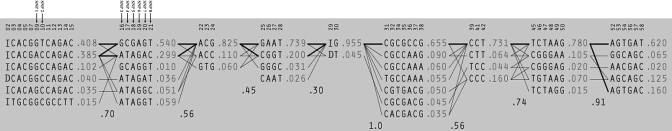
Our results showed that the gene was indeed in a recombination hot spot. There were nine LD blocks within just a 6.9-kb genomic region (Figure 1). There were two to seven haplotypes in each of the LD blocks (Figure 2). This is consistent with the findings of the International HapMap project: that genes involved in immune responses are more often located in low-LD regions with a high recombination rate.18,19
Figure 1.
Linkage disequilibrium (LD) pattern of the TRAC gene from the 100 healthy subjects. There are nine LD blocks in the 6.9 kb sequenced genomic region of the gene. Single nucleotide polymorphism (SNP) 1 (rs943887, chromosome 14:22085701) and SNP 2 (rs943888, chromosome 14:22085714) are located at the first block, and SNPs 3 to 8 (rs56131962-chromosome 14:22087521, rs55766725-chromosome 14:22087530, rs1263648-chromosome 14:22087570, rs1263649-chromosome 14: 22087579, rs1263650-chromosome 14:22087594, rs1263651-chromosome 14:22087605 respectively) are located at the second block.
Figure 2.
Haplotypes of the TRAC gene constructed from the 100 healthy subjects. There are two to seven haplotypes with frequencies ≥ 1% in each of the blocks. Arrows indicate the positions of the eight single nucleotide polymorphisms (SNPs) selected for the association tests in the haplotypes. SNPs 1 and 2 are located at the first linkage disequilibrium block, and SNPs 3 to 8 at the second linkage disequilibrium block.
Association Analyses
cSNP Association Test
cSNPs resulting in nonsynonymous changes in amino acids are more likely to have potential functional implication. Hence, we first exploited the significance of the nonsynonymous cSNPs in all 704 patients and 704 control subjects. All of the three nonsynonymous cSNPs were rare with minor allele frequencies from 0.0021 to 0.0036. None showed significant difference between the patient and control groups (supplementary Table 4).
Single SNP Association Tests
Because no significant results were obtained from the cSNP association test, we selected eight noncoding SNPs (SNP 1 to 8) with the highest statistical power for further analyses. The power estimation showed that SNP 1 (rs943887) and SNP 2 (rs943888) located in the 5′ flanking region, LD block 1, and SNPs 3 to 8 (SNP 3: rs56131962, SNP 4: rs55766725, SNP 5: rs1263648, SNP 6: rs1263649, SNP 7: rs1263650, SNP 8: rs1263651) located in the hypervariable region of intron 1, LD block 2, demonstrated the highest statistical power (supplementary Table 3). Among these, SNP 1 (previously reported −575 A/G) was the locus that previous controversial restriction fragment length polymorphism results were based on.16,17 Logistic regression analysis with adjustment for age and gender demonstrated all eight SNPs (SNPs 1 to 8) were significantly associated with IgAN (Table 1). After Bonferroni correction for multiple tests (corrected for eight tests), SNPs 1 to 6 remained significant (Table 1). All patients with IgAN in this study were unrelated. None of the members in the 310 families was included in the control group in the study. The detailed age- and gender-matched data are shown in supplementary Table 5.
Table 1.
Single SNP analysis of SNP 1–8 in 704 cases and 704 controlsa
| SNP | Genotype | OR (95% CI)b | Global Pc | Corrected Pd |
|---|---|---|---|---|
| SNP 1 | GG | 1.37 (0.94–2.00) | 1.3 × 10−6 | 1.1 × 10−5 |
| (rs943887) | AG | 0.88 (0.60–1.29) | ||
| AA | Reference | |||
| SNP 2 | TT | 1.60 (1.18–2.17) | 2.0 × 10−5 | 1.6 × 10−4 |
| (rs943888) | CT | 1.17 (0.90–1.52) | ||
| CC | Reference | |||
| SNP 3 | GG | 1.36 (0.97–1.91) | 0.0014 | 0.0110 |
| (rs56131962) | AG | 0.97 (0.70–1.36) | ||
| AA | Reference | |||
| SNP 4 | CC | 1.37 (0.97–1.93) | 0.0009 | 0.0069 |
| (rs55766725) | CT | 0.97 (0.69–1.35) | ||
| TT | Reference | |||
| SNP 5 | GG | 1.40 (1.00–1.96) | 0.0008 | 0.0067 |
| (rs1263648) | AG | 0.99 (0.71–1.37) | ||
| AA | Reference | |||
| SNP 6 | AA | 1.34 (0.96–1.88) | 0.0011 | 0.0089 |
| (rs1263649) | AG | 0.95 (0.69–1.32) | ||
| GG | Reference | |||
| SNP 7 | GG | 0.89 (0.58–1.37) | 0.0096 | 0.0745 |
| (rs1263650) | AG | 0.71 (0.46–1.10) | ||
| AA | Reference | |||
| SNP 8 | TT | 1.02 (0.67–1.55) | 0.0088 | 0.0680 |
| (rs1263651) | CT | 0.79 (0.52–1.21) | ||
| CC | Reference |
Data for single nucleotide polymorphisms (SNPs) 1 and 2 were missing in eight individuals, and SNP 3–8 data (located in the same PCR product) were missing in three individuals in the patient group. There were no missing genotype data in the control group.
Odds ratio (OR) values after adjusting for age and gender by logistic regression.
After adjusting for age and gender by logistic regression, calculated by the likelihood ratio chi-square test (using genotype data), which compares the model with the marker and the model without the marker.
After Bonferroni correction for multiple testing.
Haplotype Association Tests
Haplotype 1-GT (constructed from SNPs 1 and 2, at LD block 1) and haplotype 3-GCGAGT (constructed from SNPs 3 to 8, at LD block 2) were present more frequently in the patient group, whereas haplotype 2-AC and haplotype 4-ATAGAC were present less frequently (Table 2). The corresponding odds ratios (ORs) were 1.37 (95% confidence interval [CI] 1.15 to 1.63) and 1.21 (95% CI 1.01 to 1.46) with P values of 0.0003 and 0.0398, respectively (Table 2). Logistic regression produced global P values of 3.8 × 10−6 and 0.0286 for haplotypes at block 1 and block 2, respectively (Table 2).
Table 2.
Haplotype association in the 704 cases and 704 controlsa
| Haplotype | Haplotype Count (Frequency)
|
OR (95% CI)b | P for ORc | Global Pd | |
|---|---|---|---|---|---|
| Patient | Control | ||||
| Block 1 | |||||
| GT (haplotype 1) | 746 (0.53) | 675 (0.48) | 1.37 (1.15–1.63) | 0.0003 | 3.8 × 10−6 |
| AC (haplotype 2) | 369 (0.26) | 458 (0.33) | Reference | — | |
| GC | 269 (0.19) | 273 (0.19) | 1.22 (0.98–1.52) | 0.0692 | |
| Block 2 | |||||
| GCGAGT (haplotype 3) | 897 (0.64) | 840 (0.60) | 1.21 (1.01–1.46) | 0.0398 | 0.0286 |
| ATAGAC (haplotype 4) | 285 (0.20) | 324 (0.23) | Reference | — | |
| ATAGGT | 65 (0.05) | 94 (0.07) | 0.79 (0.55–1.12) | 0.1827 | |
| ATAGGC | 55 (0.04) | 64 (0.05) | 0.98 (0.66–1.45) | 0.9077 | |
| ATAGAT | 47 (0.03) | 47 (0.03) | 1.14 (0.74–1.76) | 0.5629 | |
| Others | 53 (0.04) | 39 (0.03) | 1.55 (0.99–2.41) | 0.0543 | |
Haplotypes with frequencies ≥ 3% were tested.
Odds ratio (OR) calculated by logistic regression.
By Wald χ2 test in logistic regression.
For likelihood ratio chi-square test (using haplotype data).
Association Tests in Independent Family and Case-Control Data Sets
To confirm the above results, we next divided the 704 patients into two independent sets: 310 with available family member(s) and 394 single cases, to test their association separately. The results were compared with the 704 case-control study. Comparison was also made between the two independent datasets of IgAN.
The family-based studies confirmed the associations of the two haplotypes shown in the 704 case-control study, and also demonstrated the reduced transmission of haplotype 2 (AC) and haplotype 4 (ATAGAC) (Table 3).
Table 3.
Family-based association analysis
| Haplotype | Frequency | Informative Families | Za | P |
|---|---|---|---|---|
| Block 1 | ||||
| GT (haplotype 1) | 0.53 | 156 | 3.27 | 0.0011 |
| AC (haplotype 2) | 0.26 | 129 | −1.93 | 0.0531 |
| GC | 0.20 | 98 | −1.39 | 0.1650 |
| Block 2 | ||||
| GCGAGT (haplotype 3) | 0.67 | 144 | 2.73 | 0.0063 |
| ATAGAC (haplotype 4) | 0.20 | 97 | −2.88 | 0.0040 |
| ATAGGT | 0.04 | 34 | −0.43 | 0.6643 |
| ATAGAT | 0.04 | 27 | −0.98 | 0.3271 |
| ATAGGC | 0.03 | 31 | −0.59 | 0.5525 |
Z: the overobserved (Z > 0), or underobserved (Z < 0) haplotypes in families calculated by the FBAT (Family Based Association Testing Software).
The 394 single cases and the equal number control studies confirmed the association with SNPs 1 to 6 and haplotypes at block 1 and block 2 (Table 4 and 5) obtained from the 704 case-control study. After Bonferroni correction (corrected for eight tests), SNPs 1 to 5 remained significant (Table 4). The overall haplotype association at block 1 and block 2 produced global P values of 7.3 × 10−5 and 0.0280, respectively, and a P value of 0.0033 with haplotype 1 alone (Table 5).
Table 4.
Single SNP association analyses of SNPs 1–8 in 394 cases and 394 controlsa
| SNP | Genotype | OR (95% CI)b | Global Pc | Corrected Pd |
|---|---|---|---|---|
| SNP 1 | GG | 1.46 (0.91–2.35) | 6.0 × 10−5 | 4.8 × 10−4 |
| (rs943887) | AG | 0.96 (0.59–1.56) | ||
| AA | Reference | |||
| SNP 2 | TT | 1.60 (1.09–2.36) | 0.0002 | 0.0017 |
| (rs943888) | CT | 1.24 (0.88–1.75) | ||
| CC | Reference | |||
| SNP 3 | GG | 1.66 (1.08–2.53) | 0.0061 | 0.0476 |
| (rs56131962) | AG | 1.27 (0.84–1.92) | ||
| AA | Reference | |||
| SNP 4 | CC | 1.72 (1.12–2.64) | 0.0036 | 0.0284 |
| (rs55766725) | CT | 1.29 (0.85–1.96) | ||
| TT | Reference | |||
| SNP 5 | GG | 1.61 (1.06–2.45) | 0.0064 | 0.0499 |
| (rs1263648) | AG | 1.22 (0.81–1.82) | ||
| AA | Reference | |||
| SNP 6 | AA | 1.58 (1.04–2.39) | 0.0080 | 0.0623 |
| (rs1263649) | AG | 1.20 (0.80–1.79) | ||
| GG | Reference | |||
| SNP 7 | GG | 1.07 (0.63–1.80) | 0.1028 | 0.5803 |
| (rs1263650) | AG | 0.97 (0.57–1.66) | ||
| AA | Reference | |||
| SNP 8 | TT | 1.20 (0.72–2.01) | 0.0668 | 0.4249 |
| (rs1263651) | CT | 1.03 (0.61–1.74) | ||
| CC | Reference |
Data for single nucleotide polymorphisms (SNPs) 1 and 2 were missing in eight individuals, and SNP 3–8 data were missing in three individuals in the patient group. No missing data in the control group.
Odds ratio (OR) values after adjusting for age and gender by logistic regression.
Adjusting for age and gender by logistic regression, calculated by the likelihood ratio chi-square test (using genotype data) which compares the model with the marker and the model without the marker.
After Bonferroni correction for multiple testing.
Table 5.
Haplotype association analysis in 394 cases and 394 controlsa
| Haplotype | Haplotype Count (Frequency)
|
OR (95% CI)b | P for ORc | Global Pd | |
|---|---|---|---|---|---|
| Case | Control | ||||
| Block 1 | |||||
| GT (haplotype 1) | 412 (0.52) | 372 (0.47) | 1.41 (1.12–1.77) | 0.0033 | 7.3 × 10−5 |
| AC (haplotype 2) | 208 (0.26) | 265 (0.33) | Reference | — | |
| GC | 154 (0.19) | 159 (0.20) | 1.23 (0.93–1.64) | 0.1503 | |
| Block 2 | |||||
| GCGAGT (haplotype 3) | 478 (0.60) | 443 (0.56) | 1.18 (0.92–1.51) | 0.1849 | 0.0280 |
| ATAGAC (haplotype 4) | 172 (0.22) | 188 (0.24) | Reference | — | |
| ATAGGT | 40 (0.05) | 62 (0.08) | 0.71 (0.45–1.10) | 0.1265 | |
| ATAGGC | 34 (0.04) | 47 (0.06) | 0.79 (0.49–1.29) | 0.3449 | |
| ATAGAT | 26 (0.03) | 31 (0.04) | 0.92 (0.52–1.61) | 0.7612 | |
| Others | 42 (0.05) | 27 (0.03) | 1.70 (1.00–2.88) | 0.0479 | |
Haplotypes with frequencies ≥ 3%.
Odds ratio (OR) calculated by logistic regression.
By Wald χ2 test in logistic regression.
For likelihood ratio chi-square test (using haplotype data).
Patients in the 310 family-based and the 394 case-control studies were the subsets of the 704 patients. However, there was no overlap between these two groups. Also, there was no overlap between the control subjects and any of the family members. Hence, neither the patients nor the controls in the family-based study and in the 394 case-control study were overlapped. The two datasets were completely independent of each other and were analyzed by different statistical tools. Because both showed association with haplotype 1 (P = 0.0011 in the family-based study and P = 0.0033 in the case-control study) (Table 3 and 5), the 394 case-control data were the replication/confirmation of the family-based association data.
Transcription Assays and TRAC mRNA Abundance of the Over- and Undertransmitted/Observed Haplotypes
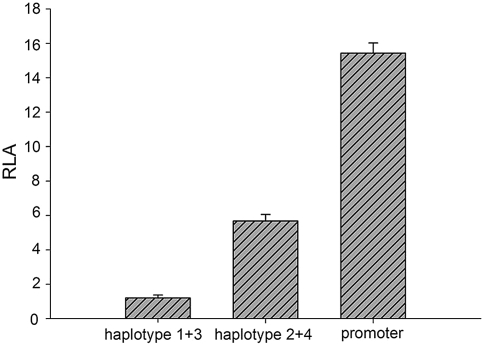
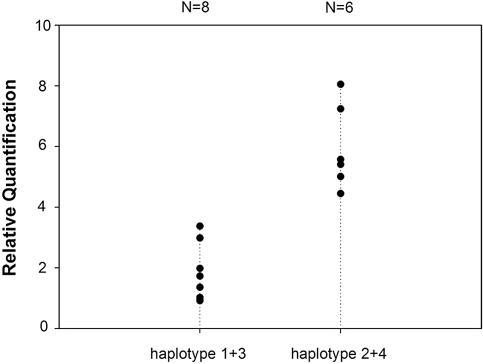
Because haplotypes and multiple SNPs over- and undertransmitted/observed in IgAN were located at the 5′ of the gene, we further examined their impact on transcription. As a result of allelic exclusion during maturation of the gene,20 only samples homozygous for over- and undertransmitted/observed haplotypes were tested. Luciferase activity assays were performed with constructs containing either the overtransmitted/observed haplotypes 1 (GT) and 3 (GCGAGT) or the undertransmitted/observed haplotypes 2 (AC) and 4 (ATAGAC). We found a 3.7-fold reduction in the transcription activity by the overtransmitted/observed haplotypes in comparison with the undertransmitted/observed haplotypes (Figure 3). We also quantified the TRAC gene mRNA abundance by real-time quantitative PCR in peripheral blood mononuclear cells. A two-fold reduction of the mRNA abundance was observed in individuals carrying the over-transmitted/observed haplotypes compared with those carrying the undertransmitted/observed haplotypes (P < 0.001) (Figure 4).
Figure 3.
Luciferase activity assay. Haplotype 1 + 3 denotes the construct containing the overtransmitted/observed haplotypes 1 (GT) and 3 (GCGAGT), and haplotype 2 + 4 denotes the construct containing the undertransmitted/observed haplotypes 2 (AC) and 4 (ATAGAC). Promoter indicates the negative control with empty pGL-3 promoter vector. Bars represent relative luciferase activity (RLA) for different constructs, with values expressed as mean (± SEM). There is a 3.7-fold reduction in the luciferase activity by the haplotype 1 + 3 construct in comparison with the haplotype 2 + 4 construct (P < 0.001, by t test).
Figure 4.
mRNA abundance of individuals with over- or under- transmitted/observed haplotypes. mRNA levels of the TRAC are measured by the real-time quantitative PCR. Haplotype 1 + 3 denotes individuals homozygous for the overtransmitted/observed haplotypes 1 (GT) and 3 (GCGAGT), and haplotype 2 + 4 denotes individuals homozygous for the undertransmitted/observed haplotypes 2 (AC) and 4 (ATAGAC). Individuals (n = 8) with overtransmitted/observed haplotypes demonstrate a lower level of the TRAC mRNA than those (n = 6) with undertransmitted/observed haplotypes (P < 0.001, by t test).
DISCUSSION
IgAN is generally considered a complex disease with both genetic and environmental contribution to its pathogenesis and progression.21 It is believed that genes responsible for or predisposing for the disease are heterogeneous with polygenic contribution in most sporadic cases22 and monogenic/oligogenic contribution in some extended/multiplex IgAN families (MIM 161950).4,23,24 So far, some genetic variations have been reported to be associated with IgAN in the nonfamilial cases with no single gene cloned in the linked regions from the extended/multiplex families.
T cell receptors play a vital role in Ig synthesis and recognition of MHC-bound antigens presented by B cells. The vast majority of T cell receptors are composed of α and β dimers. The entire TCR α chain gene is located on 14q11–12 spanning a 1-Mb genomic region.25 There are approximately 50 V and 60 J gene segments dispersed over a 920-kb region coding for variable and joining regions of the α chain. In contrast, there is only one Cα gene segment that codes for the constant region of the chain. The Cα gene segment is relatively small with only 4 exons spanning a 4.7-kb genomic region.14,26–29 Our resequencing revealed that all cSNPs are located at the first exon and are very rare. In contrast, the intron and flanking regions are densely compacted with polymorphisms within the sequenced regions, mostly at the 5′. The 5′ concentration of the SNPs seems to concur with the hypothesis that the hypervariability in the V-J segment extends into the Cα gene segment and the “imprecise joining” of the TCR gene segments.30,31 More intriguing is the finding of six SNPs concentrated within a short 85-bp stretch in the intron 1. This may reflect frequent mutational events during evolution in this short stretch. The International HapMap project shows that genes involved in immune responses are more often located in regions of low LD.18,19 Our LD tests produce a striking result: that there are nine LD blocks in the 6.9 kb resequenced and analyzed region of the gene. Clearly the gene is in a highly active hot spot of recombination. Because of this, in addition to haplotype association, we also tested associations with each of the eight SNPs.
Previous restriction fragment length polymorphism-based single locus (SNP 1) association studies produced controversial results on association of the gene with IgAN.16,17 This prompted us to resequence and further analyze the gene. Our case-control and family-based analyses with haplotypes and multiple SNPs clearly support the notion that the overtransmitted/observed haplotypes and multiple SNPs confer risk to IgAN, and, conversely, that the undertransmitted/observed haplotypes and multiple SNPs protect from IgAN. The risk alleles are common in the Chinese population, which may explain the relatively higher prevalence of IgAN in that group. Whether the reported negative association in the Japanese population was due to insufficient sample size or the population difference remains to be investigated. These associated haplotypes and SNPs are located at the 5′ of the gene. Our transcription assays and real-time quantitative PCR show reduction in transcription activities and the mRNA abundance by the overtransmitted/observed haplotypes in comparison with the undertransmitted/observed haplotypes. Therefore, it is apparent that the altered transcription may be responsible for the risk or protective effects conferred by those over- and under transmitted/observed haplotypes.
T cell receptor genes, like Ig genes, undergo somatic rearrangement. Different V and J gene segments are first joined by somatic rearrangement before the joined V-J segment is brought to the nearest C transcription unit for the entire α chain. The joining of the V-J and C is controlled by chromatin remodeling,32 and the transcription is also regulated by enhancers and transcription factors.33 A 1.1-kb BamHI-HindIII fragment immediately upstream to the Cα in the intron between Jα and Cα has been reported specifically for T and B cells.31 This fragment has a similar location as the Ig gene enhancer, but no cis-regulatory element has yet been demonstrated in this region. Instead, a T cell-specific enhancer at the region 3 kb to the 3′ of the Cα gene has been reported.34 This is not located in the region we studied, and our present study cannot determine whether there is a cis-acting regulatory element in the studied region.
Our functional tests demonstrated reduction in transcription activities and the mRNA abundance by overtransmitted/observed haplotypes in comparison with undertransmitted/observed haplotypes, suggesting that these may affect the expression of TRAC gene. There has been strong convergent evidence indicating that reduced suppressor T cells and impaired suppressor T cell function may correlate with enhanced IgA production and immunoregulatory abnormalities in the pathogenesis of IgAN.8–10 It is now well established that regulatory T cells play a crucial role in self-tolerance and autoimmunity and are potentially important in the prevention and treatment of immune-mediated disease.35,36 A recent study suggests that higher avidity thymic stromal interactions between TCRs and self- peptide/MHC as well as Foxp3 (forkhead/winged-helix family transcriptional repressor p3) play a crucial role in the development of regulatory T cells.37 The activation of CD4+CD25- T cells through TCRs can lead to an increased expression of Foxp3 mRNA and CD25.37 In turn, Foxp3 acts as a master control gene for the development and function of regulatory T cells.38 Furthermore, TCR and IL-2 stimulation are essential to achieve regulatory T cell suppressive function.39 Therefore, reduced expression of TRAC may be associated with defective regulatory T cell function. These findings suggest that TCR may be the primary defect that could influence the frequency and function of regulatory T cells, which in turn results in IgA hyperproduction and renal injury.
In conclusion, the present study provides evidence of association between haplotypes/multiple SNPs of the TRAC gene and the susceptibility to IgAN in a Chinese population. Better knowledge of genetic factor contributing to the pathogenesis of IgAN should lead to improved medical care of this disorder in the future.
CONCISE METHODS
Study Subjects
We recruited 704 patients from three renal units in Southern China and Hong Kong (the First and the Third Affiliated Hospitals of Sun Yat-Sen University and Queen Mary Hospital, University of Hong Kong), according to the criteria described previously.40 IgAN was diagnosed by renal biopsy according to the World Health Organization criteria. Systemic lupus erythematosis, Henoch-Schonlein purpura, and hepatic diseases were excluded by detailed clinical history, examination, and negative laboratory findings for hypocomplementemia, anti-DNA antibody or hepatitis B virus surface antigen. All patients were unrelated. Blood samples of the parent(s) and/or available sibling(s) of the patients were also collected. Detailed family data are depicted in supplementary Table 2. Seven hundred and four healthy control subjects were recruited in parallel with the cases. These subjects were healthy volunteers, as confirmed by detailed clinical and laboratory examinations. Detailed age- and gender-matched data are shown in supplementary Table 5. None of the members in the 310 families was recruited as control subjects. All patients and controls are of the Chinese Han ethnicity. All subjects gave their informed consent for blood or tissue collection. The study was conducted in accordance with the Declaration of Helsinki and was approved by ethics committees for human studies of both institutions.
Resequencing of the TRAC Gene and SNP Identification
Genomic DNA was extracted with QiAamp Maxi kit (Qiagen, Germany). We first resequenced 100 patients and 100 healthy controls randomly selected from the collection. Primers for coding, intron-exon boundaries with extensions into intronic regions from 200 to 1000 bp, 5′ and 3′ flanking regions (2 kb at each end), were designed. The PCR products were sequenced using ABI Prism 3730XL (Applied Biosystems, Foster City, CA). Results were analyzed against sequences retrieved from the UCSC Genome Browser (http://genome.ucsc.edu).
LD Test and Haplotype Construction
LD of the genomic region and haplotypes of the TRAC gene was analyzed by Haploview 3.32 (http://www.broad.mit.edu/mpg/haploview/41) with SNPs with allele frequencies ≥1% obtained from the 100 resequenced healthy subjects.
Association Studies
The genotypes of all patients, controls and family members of the patients for the SNPs used in the association study were determined by direct sequencing as described in the resequencing. Considering the gene is located in a recombination hotspot, we analyzed the single SNP association as well as the haplotype association. The following association tests were performed.
cSNPs.
We first tested the significance of the cSNPs. Because all of the cSNPs identified in the resequencing were located in exon 1, we further sequenced exon 1 in all 704 patients and 704 matched controls. The allele frequencies of the nonsynonymous cSNPs in the patient and control population were compared.
Noncoding SNPs and Haplotypes.
Second, we selected eight SNPs (SNPs 1 to 8), all noncoding, with the highest statistical power at type I error rate of 0.05 by the Power Procedure in SAS version 9.1 (SAS Institute, Cary, NC). The associations with each of the eight selected SNPs and the haplotypes were analyzed in 704 patients and 704 matched controls.
Independent Family-Based and Case-Control Data Sets.
Third, we divided the 704 patients into two independent datasets: 310 with family members (mostly patient-parents trios) and 394 single patients (no other available family members). The transmission of the haplotypes was examined in the families. The association of the SNPs and haplotypes in 394 single patients and the matched equal number controls were also tested.
Functional Tests of Haplotypes
Transient Transfection and Luciferase Activity Assay.
A 2.45-kb DNA fragment of either the over- (haplotype 1 and 3), or under- (haplotype 2 and 4) transmitted/observed haplotypes was amplified from genomic DNA and cloned into the pGL3-promoter vector (Promega, Madison, WI) upstream of the firefly luciferase gene in the 5′ to 3′ orientation by using KpnI and Xhol I. Because the gene undergoes allelic exclusion, only homozygotes were constructed and analyzed. The constructs were examined and confirmed by sequencing. For transient transfection, the Jurkat T cells were co-transfected with 0.8 μg/well constructs and 0.02 μg/well pRL-TK (Renilla luciferase) vector (Promega) as the internal control, by using 2 μl/well Lipofectamin 2000 (Invitrogen, Carlsbad, CA). Empty pGL3-promoter vector was used as the negative control. The luciferase activity assays were performed with the Dual-Luciferase Reporter Assay System (Promega). The relative luciferase activity was expressed as the ratio of firefly luciferase activity/Renilla luciferase activities (RLA = LAF / LAR). Each experiment was performed in triplicate and repeated three times.
Real-time Quantitative PCR to Measure the Abundance of TRAC mRNA.
Total RNA was isolated from peripheral blood mononuclear cells collected from individual homozygous of the over- or undertransmitted/observed haplotypes using Trizol Reagent (Invitrogen). cDNA was synthesized by the Super-Script III First-Strand Synthesis Systems for RT-PCR (Invitrogen). The relative expression levels of TRAC were quantified using the Taqman real-time quantitative PCR assay on the ABI 7500 Real Time PCR System (Applied Biosystems) with β actin as the internal control. The relative mRNA levels were normalized to the β actin abundance by using the comparative Ct method.42 Each experiment was repeated nine times.
Statistical Analyses
Haploview41 was used to test for Hardy-Weinberg equilibrium and LD pattern, and to construct haplotypes. The significance of the case-control data in each of the selected SNPs was analyzed by logistic regression in SAS version 9.1. The global P value for each SNP in the logistic model was derived from likelihood ratio chi-square test by comparing the model including the SNP to that without the SNP. Bonferroni correction was applied to multiple testing. Haplotype data were analyzed by logistic regression and likelihood ratio chi-square test. The family-based data were analyzed by the transmission disequilibrium test (TDT) using FBAT version 1.5.3 (http://www.biostat.harvard.edu/∼fbat/43). For the measurement of relative luciferase activity and the TRAC mRNA, unpaired two-tailed t test was used.
DISCLOSURES
None.
Acknowledgments
The project was supported by the second phase of the State 985 Project of China, The National Natural Science Foundation of China (30570869 and 30771013), China Medical Board of New York (05-827), and the Guangdong Provincial Natural Science Foundation (07001511). We thank Professor P. C. Sham, Department of Psychiatry, University of Hong Kong, for his advice. We also thank Dr. Yong Du for recruiting patients and families.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.D'Amico G: The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 64: 709–727, 1987 [PubMed] [Google Scholar]
- 2.Lai KN, Mac-Moune, Lai F, Li PK, Chan KW, Au TC, Tong KL: The clinicopathological characteristics of IgA nephropathy in Hong Kong. Pathology 20: 15–19, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Li LS, Liu ZH: Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int 66: 920–923, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bisceglia L, Cerullo G, Forabosco P, Torres DD, Scolari F, Di Perna M, Foramitti M, Amoroso A, Bertok S, Floege J, Mertens PR, Zerres K, Alexopoulos E, Kirmizis D, Ermelinda M, Zelante L, Schena FP, European IgAN Consortium: Genetic heterogeneity in Italian families with IgA nephropathy: Suggestive linkage for two novel IgA nephropathy loci. Am J Hum Genet 79: 1130–1134, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Amico G: Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Schena FP: For further investigations in IgA nephropathy the approach from phenotype to genotype is welcome. Clin Exp Immunol 127: 399–401, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai KN, Ho RT, Lai CK, Li PK: Increase of both circulating Th1 and Th2 T lymphocyte subsets in IgA nephropathy. Clin Exp Immunol 96: 116–121, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai H, Nomoto Y, Arimori S: Decrease of IgA-specific suppressor T cell activity in patients with IgA nephropathy. Clin Exp Immunol 38: 243–248, 1979 [PMC free article] [PubMed] [Google Scholar]
- 9.Casanueva B, Rodriguez-Valverde V, Fariñas MC, Vallo A, Rodriguez-Soriano J: Autologous mixed lymphocyte reaction and T-cell suppressor activity in patients with Henoch-Schönlein purpura and IgA nephropathy. Nephron 54: 224–228, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Egido J, Blasco R, Sancho J, Lozano L: T-cell dysfunctions in IgA nephropathy: Specific abnormalities in the regulation of IgA synthesis. Clin Immunol Immunopathol 26: 201–212, 1983 [DOI] [PubMed] [Google Scholar]
- 11.Rothschild E, Chatenoud L: T cell subset modulation of immunoglobulin production in IgA nephropathy and membranous glomerulonephritis. Kidney Int 25: 557–564, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Lai KN, Leung JC, Li PK, Lui SF: Cytokine production by peripheral blood mononuclear cells in IgA nephropathy. Clin Exp Immunol 85: 240–245, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falk MC, Ng G, Zhang GY, Fanning GC, Roy LP, Bannister KM, Thomas AC, Clarkson AR, Woodroffe AJ, Knight JF: Infiltration of the kidney by alpha beta and gamma delta T cells: Effect on progression in IgA nephropathy. Kidney Int 47: 177–185, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Sim GK, Yague J, Nelson J, Marrack P, Palmer E, Augustin A, Kappler J: Primary structure of human T-cell receptor alpha-chain. Nature 312: 771–775, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Kronenberg M, Siu G, Hood LE, Shastri N: The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol 4: 529–591, 1986 [DOI] [PubMed] [Google Scholar]
- 16.Li PK, Poon P, Phil M, Poon AS, Szeto CC, Yu AW, Lai KN: Association of IgA nephropathy with T-cell receptor constant alpha chain gene polymorphism. Am J Kidney Dis 30: 260–264, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Deenitchina SS, Shinozaki M, Hirano T, Ando T, Hirakata H, Kiyohara Y, Katafuchi R, Fujishima M: Association of a T-cell receptor constant alpha chain gene polymorphism with progression of IgA nephropathy in Japanese patients. Am J Kidney Dis 34: 279–288, 1999 [DOI] [PubMed] [Google Scholar]
- 18.International HapMap Consortium: A haplotype map of the human genome. Nature 437: 1299–1320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International HapMap Consortium: A second generation human haplotype map of over 3.1 million SNPs. Nature 449: 851–861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldmit M, Bergman Y: Monoallelic gene expression: A repertoire of recurrent themes. Immunol Rev 200: 197–214, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hsu SI, Ramirez SB, Winn MP, Bonventre JV, Owen WF: Evidence for genetic factors in the development and progression of IgA nephropathy. Kidney Int 57: 1818–1835, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Barratt J, Feehally J: IgA nephropathy. J Am Soc Nephrol 16: 2088–2097, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Gharavi AG, Yan Y, Scolari F, Schena FP, Frasca GM, Ghiggeri GM, Cooper K, Amoroso A, Viola BF, Battini G, Caridi G, Canova C, Farhi A, Subramanian V, Nelson-Williams C, Woodford S, Julian BA, Wyatt RJ, Lifton RP: IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22–23. Nat Genet 26: 354–357, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Paterson AD, Liu XQ, Wang K, Magistroni R, Song X, Kappel J, Klassen J, Cattran D, St George-Hyslop P, Pei Y: Genome-wide linkage scan of a large family with IgA nephropathy localizes a novel susceptibility locus to chromosome 2q36. J Am Soc Nephrol 18: 2408–2415, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Caccia N, Bruns GA, Kirsch IR, Hollis GS, Bertness V, Mak TW: T cell receptor alpha chain genes are located on chromosome 14 at 14q11–14q12 in humans. J Exp Med 161: 1255–1260, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mak TW, Yanagi Y: Genes encoding the human T cell antigen receptor. Immunol Rev 81: 221–333, 1984 [DOI] [PubMed] [Google Scholar]
- 27.Davis MM: Molecular genetics of the T cell-receptor beta chain. Annu Rev Immunol 3: 537–560, 1985 [DOI] [PubMed] [Google Scholar]
- 28.Yoshikai Y, Clark SP, Taylor S, Sohn U, Wilson BI, Minden MD, Mak TW: Organization and sequences of the variable, joining and constant region genes of the human T-cell receptor alpha-chain. Nature 316: 837–840, 1985 [DOI] [PubMed] [Google Scholar]
- 29.Winoto A, Mjolsness S, Hood L: Genomic organization of the genes encoding mouse T-cell receptor alpha-chain. Nature 316: 832–836, 1985 [DOI] [PubMed] [Google Scholar]
- 30.Nagasawa R, Matsumura O, Maruyama N, Mitarai T, Isoda K: T-cell receptor beta-chain gene polymorphism and the prognosis of IgA nephropathy in Japanese patients. Nephron 70: 502–503, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Luria S, Gross G, Horowitz M, Givol D: Promoter and enhancer elements in the rearranged alpha chain gene of the human T cell receptor. EMBO J 6: 3307–3312, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strachan T, Read AP: Human gene expression. In: Human Molecular Genetics, 3rd Ed., London and New York, Garland Science, 2004, pp 309
- 33.Leiden JM: Transcriptional regulation of T cell receptor genes. Annu Rev Immunol 11: 539–570, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Winoto A, Baltimore D: A novel, inducible and T cell-specific enhancer located at the 3′ end of the T cell receptor alpha locus. EMBO J 8: 729–733, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, Chess L: An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest 114: 1198–1208, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lan RY, Ansari AA, Lian ZX, Gershwin ME: Regulatory T cells: Development, function and role in autoimmunity. Autoimmun Rev 4: 351–363, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der Zanden L, de Vries RR, Huizinga TW, Ottenhoff TH, Toes RE: Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol 66: 13–20, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Fehérvari Z, Sakaguchi S: CD4+ Tregs and immune control. J Clin Invest 114: 1209–1217, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piccirillo CA, Thornton AM: Cornerstone of peripheral tolerance: Naturally occurring CD4+CD25+ regulatory T cells. Trends Immunol 25: 374–380, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Li YJ, Du Y, Li CX, Guo H, Leung JC, Lam MF, Yang N, Huang F, Chen Y, Fang JQ, Maxwell PH, Lai KN, Wang Y: Family-based association study showing that immunoglobulin A nephropathy is associated with the polymorphisms 2093C and 2180T in the 3′ untranslated region of the Megsin gene. J Am Soc Nephrol 15: 1739–1743, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Barrett JC, Fry B, Maller J, Daly MJ: Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laird NM, Horvath S, Xu X: Implementing a unified approach to family-based tests of association. Genet Epidemiol 19[Suppl 1]: S36–S42, 2000 [DOI] [PubMed] [Google Scholar]