Abstract
People with ESRD are at increased risk for cancer, but it is uncertain when this increased risk begins in the spectrum of chronic kidney disease (CKD). The aim of our study was to determine whether moderate CKD increases the risk for cancer among older people. We linked the Blue Mountains Eye Study, a prospective population-based cohort study of 3654 residents aged 49 to 97 yr, and the New South Wales Cancer Registry. During a mean follow-up of 10.1 yr, 711 (19.5%) cancers occurred in 3654 participants. Men but not women with at least stage 3 CKD had a significantly increased risk for cancer (test of interaction for gender P = 0.004). For men, the excess risk began at an estimated GFR (eGFR) of 55 ml/min per 1.73 m2 (adjusted hazard ratio [HR] 1.39; 95% confidence interval [CI] 1.00 to 1.92) and increased linearly as GFR declined. For every 10-ml/min decrement in eGFR, the risk for cancer increased by 29% (adjusted HR 1.29; 95% CI 1.10 to 1.53), with the greatest risk at an eGFR <40 ml/min per 1.73 m2 (adjusted HR 3.01; 95% CI 1.72 to 5.27). The risk for lung and urinary tract cancers but not prostate was higher among men with CKD. In conclusion, moderate CKD (stage 3) may be an independent risk factor for the development of cancer among older men but not women, and the effect of CKD on risk may vary for different types of cancer.
Chronic kidney disease (CKD) is common in older people. Among those aged ≥50 yr, the prevalence of moderate (stage 3) CKD or worse, defined as estimated GFR (eGFR) <60 ml/min per 1.73 m2, is >20% in the United States and Australia.1,2 CKD is associated with significant morbidity and premature death. Cardiovascular complications and deaths are increased in the CKD population independent of traditional risk factors such as diabetes, hypertension, and dyslipidemia.3–5 Increased cancer risk is also well defined in the end-stage kidney disease (ESKD) and kidney transplant populations.6–8 The overall cancer incidence after transplantation is approximately three-fold greater than in the general population.
Observational studies have suggested an increased cancer risk in people with early-stage CKD, before requiring dialysis or transplantation.9,10 An excess risk of 1.2 times for all cancers was reported during the 5 yr before renal replacement therapy in a population-based cohort study of dialysis and transplant patients, but inclusion was limited to those who progressed to ESKD, and comorbidity data were limited.6 Recently, an association between elevated albumin-to-creatinine ratio and cancer incidence was reported in a longitudinal population-based study of older individuals.11 Previous studies have not evaluated the threshold of CKD that is associated with an increased risk for cancer, adjusted for measurement error in estimating the severity of CKD, or determined the independent effect of CKD after accounting for known risk factors for cancer. The aim of our study was to estimate the independent effect of mild to moderately reduced kidney function on the risk for incident cancers among older people and to identify the threshold at which any excess risk begins.
RESULTS
There were 3448 participants after excluding all prevalent cancers, and 399 (11.6%) of the 3448 participants were subsequently excluded because of incomplete blood serum creatinine profiles, leaving 3049 participants in our study. There were no statistically significant differences in demographic features and comorbidities among those included and excluded. Of those included, 42.5% were men (P = 0.60), 57.9% received tertiary education (P = 0.96), 6.04% had diabetes (P = 0.95), and 51% were current and/or ex-smokers (P = 0.20). Of those excluded, 43.9% were men, 58.1% received tertiary education, 6.1% had diabetes, and 54.8% were current and/or ex-smokers. The mean follow-up period was 10.1 yr. The mean age of those included was 65.8 yr and among those excluded was 67.4 yr.
Baseline Characteristics of Study Participants
Baseline characteristics for participants stratified by gender and CKD status are shown in Table 1. The eGFRs ranged from a maximum of 113 ml/min per 1.73 m2 to a minimum of 14.5 ml/min per 1.73 m2. Only 50 (1.6%) had an eGFR >90 ml/min per 1.73 m2, 2153 (70.6%) had an eGFR of 60 to 90 ml/min per 1.73 m2, and 851 (27.9%) had an eGFR <60 ml/min per 1.73 m2. Of those with moderate CKD (eGFR <60 ml/min), 838 (27.5%) had an eGFR 30 to 60 ml/min per 1.73 m2 and 12 (0.4%) had an eGFR 15 to 30 ml/min per 1.73 m2. No participants were on dialysis or had had a kidney transplant. All participants with CKD were older, had higher systolic BP (SBP), had a history of myocardial infarction, and had sun-related skin damage. In addition, women with CKD had higher levels of fasting blood glucose and cholesterol but were less likely to have received tertiary education.
Table 1.
Baseline characteristics of participants stratified by gender and GFR
| Characteristic | CKD Status (eGFR Measured in ml/min per 1.73 m2) | |||||
|---|---|---|---|---|---|---|
| Men (n = 1295)
|
Women (n = 1752)
|
|||||
| <60 (n = 399) | ≥60 (n = 896) | P for Difference | <60 (n = 457) | ≥60 (n = 1297) | P for Difference | |
| Age (yr; mean ± SD) | 70.8 ± 9.2 | 63.6 ± 8.6 | <0.001 | 72.5 ± 9.0 | 63.6 ± 8.7 | <0.001 |
| SBP (mmHg; mean ± SD) | 148.1 ± 21.1 | 146.0 ± 21.1 | <0.001 | 152.7 ± 24.3 | 146.1 ± 21.1 | <0.001 |
| DBP (mmHg; mean ± SD) | 84.3 ± 10.9 | 83.1 ± 9.6 | 0.240 | 83.7 ± 10.0 | 83.1 ± 9.6 | 0.280 |
| Alcohol consumption (standard drinks/d; n [%]) | <0.001 | <0.001 | ||||
| 1 to 2 | 206 (51.6) | 409 (45.6) | 216 (47.8) | 663 (50.9) | ||
| 3 to 4 | 49 (12.3) | 178 (19.9) | 23 (5.1) | 100 (7.7) | ||
| 5 to 8 | 25 (6.3) | 81 (9.0) | 1 (0.2) | 16 (1.2) | ||
| ≥9 | 7 (1.8) | 34 (3.8) | 2 (0.4) | 8 (0.6) | ||
| Previous or current smoking (n [%]) | 282 (70.7) | 576 (64.3) | 0.080 | 171 (37.8) | 501 (38.5) | 0.120 |
| Body mass index (kg/m2; mean ± SD) | 26.7 ± 4.0 | 26.0 ± 4.9 | 0.004 | 26.2 ± 4.9 | 26.1 ± 4.8 | 0.670 |
| Diabetes (n [%]) | 36 (9.0) | 55 (6.1) | 0.140 | 32 (7.1) | 60 (4.6) | 0.120 |
| Acute coronary syndrome (n [%]) | 75 (18.8) | 82 (9.2) | <0.001 | 51 (11.3) | 57 (4.4) | <0.001 |
| Sun-related skin damage (n [%]) | 177 (44.4) | 266 (29.7) | <0.001 | 116 (25.7) | 201 (15.4) | <0.001 |
| Fasting blood glucose (mmol/L; mean ± SD) | 5.5 ± 1.7 | 5.4 ± 1.4 | 0.380 | 5.3 ± 1.8 | 5.0 ± 1.4 | 0.003 |
| Total cholesterol (mmol/L; mean ± SD) | 5.8 ± 1.1 | 5.7 ± 1.1 | 0.820 | 6.3 ± 1.1 | 6.1 ± 1.0 | 0.008 |
| Tertiary education | 237 (59.4) | 564 (63.8) | 0.440 | 204 (45.1) | 691 (53.1) | 0.007 |
| eGFR (mmol/min per 1.73 m2; n [%]) | ||||||
| <20 | 2 (0.15) | – | 1 (0.06) | – | ||
| 20 to 39 | 33 (2.6) | – | 45 (2.6) | – | ||
| 40 to 59 | 364 (28.1) | – | 406 (23.2) | – | ||
| 60 to 79 | – | 789 (60.9) | – | 1130 (64.4) | ||
| 80 to 89 | – | 101 (7.8) | – | 168 (9.6) | ||
| ≥90 | – | 6 (0.5) | – | 4 (0.2) | ||
| Serum creatinine concentration (mmol/L; n [%]) | ||||||
| 40 to 89 | – | 124 (13.8) | – | 870 (66.8) | ||
| 90 to 104 | – | 480 (53.6) | 179 (39.6) | 432 (33.2) | ||
| 105 to 119 | 160 (40.1) | 292 (32.6) | 173 (38.3) | – | ||
| 120 to 134 | 150 (37.6) | – | 53 (11.7) | – | ||
| 135 to 375 | 89 (22.3) | – | 47 (10.4) | – | ||
Cancer Frequency and Incidence Rate
Table 2 shows the incidence and frequency of the six most frequent cancers stratified by gender and CKD status. Breast cancer was the most frequent cancer in women, followed by colorectal cancer, melanoma, lung cancer, and urinary tract cancers. In men, prostate cancer was the most frequent, followed by colorectal, melanoma, lung, and cancers of the urinary tract system.
Table 2.
Cancer incidence rate and frequency by selected sites stratified by gender and eGFRa
| eGFR (ml/min per 1.73 m2) | Men (Incidence Rate [n]; n = 1295)
|
Women (Incidence Rate [n]; n = 1752)
|
||
|---|---|---|---|---|
| <60 (n = 399) | ≥60 (n = 896) | <60 (n = 457) | ≥60 (n = 1297) | |
| All sites | 23.10 (93) | 17.00 (152) | 11.90 (55) | 13.9 (182) |
| Breast | – | – | 2.25 (11) | 3.97 (52) |
| Prostate | 4.71 (19) | 4.75 (43) | – | – |
| Colorectal | 3.72 (15) | 2.21 (20) | 2.60 (12) | 2.21 (29) |
| Melanoma | 2.48 (10) | 2.76 (25) | 1.08 (5) | 0.92 (12) |
| Lung | 2.48 (10) | 1.88 (17) | 0.27 (1) | 1.00 (17) |
| Urinary tract | 2.23 (9) | 0.77 (7) | – | 0.45 (6) |
Incidence rate calculated as per 10−3 person-years. There were no urinary tract cancers in women with eGFR <60 ml/min per 1.73 m2.
Univariate Analyses
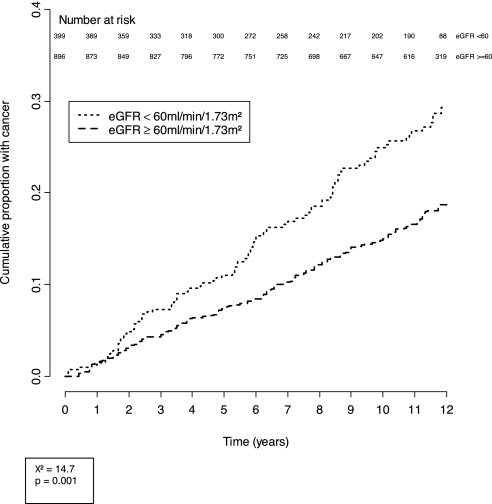
Cumulative incidence curves of cancers in the study cohort during the course of follow-up are shown in Figures 1 and 2, stratified by eGFR and gender. The cumulative incidence of cancer in men with CKD was 23.1 per 1000 person-years compared with 16.8 per 1000 person-years among those without CKD. The cumulative incidence curves of cancers in men stratified by eGFR (<60 ml/min per 1.73 m2 and ≥60 ml/min per 1.73 m2) progressively diverged after 2 yr of follow-up and was significantly different (χ2 = 14.7; P = 0.001). After a mean follow-up period of 10.1 yr, 23.3% of those with an eGFR <60 ml/min per 1.73 m2 had incident cancers compared with 16.9% of those with an eGFR >60 ml/min per 1.73 m2.
Figure 1.
Cumulative incidence of cancers in men, stratified by eGFR.
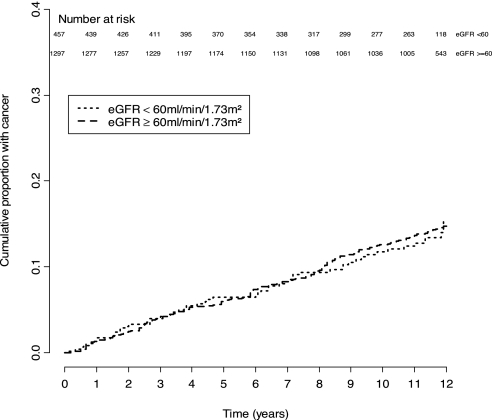
Figure 2.
Cumulative incidence of cancers in women, stratified by eGFR.
In women, the cumulative incidence of cancers for those with CKD was 11.9 per 1000 person-years compared with 13.8 per 1000 persons-years among those without CKD. There was no significant difference between the two strata of eGFR (χ2 = 0.015; P = 0.90). A total of 12.0% of those with eGFR <60 ml/min per 1.73 m2 developed cancers compared with 14.0% of those with an eGFR ≥60 ml/min per 1.73 m2.
Cancer Risk
Table 3 shows the unadjusted and adjusted hazard ratios (HRs) for all cancers by demographic and other risk factors, stratified by gender. Age and history of sun-related skin damage were independent risk factors for cancers in both men and women. After adjustment for age as well as variables significant in the univariate analysis (age group, smoking status, degree of sun-related skin damage, and diastolic BP [DBP]), men with reduced kidney function were at increased risk for cancer. The fully adjusted HR of incident cancer attenuated to 1.17 (95% confidence interval [CI] 1.03 to 1.32; P = 0.015) for every 10-ml/min per 1.73 m2 decrease in eGFR. In women, the association between cancer risk and reduced kidney function was not demonstrated. For women, the adjusted HR for cancer was 0.94 (95% CI 0.0.83 to 1.06; P = 0.32) for every 10-ml/min per 1.73 m2 decrease in eGFR.
Table 3.
Unadjusted and adjusted HRs for all cancers by demographic and other risk factors, stratified by gender
| Variables | Men
|
Women
|
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis
|
Multivariate Analysis
|
Univariate Analysis
|
Multivariate Analysis
|
|||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age groups (yr) | ||||||||
| 45 to 54 | Referent | Referent | Referent | Referent | ||||
| 55 to 64 | 1.98 (1.10 to 3.53) | 0.0200 | 1.65 (0.92 to 2.98) | 0.0900 | 1.37 (0.86 to 2.19) | 0.1800 | 1.52 (0.94 to 2.46) | 0.0900 |
| 65 to 74 | 3.36 (2.07 to 6.36) | <0.0010 | 2.61 (1.46 to 4.69) | 0.0010 | 1.56 (0.99 to 2.47) | 0.0600 | 1.68 (1.04 to 2.73) | 0.0400 |
| ≥75 | 6.41 (3.60 to 11.40) | <0.0010 | 3.78 (2.01 to 7.13) | <0.0001 | 2.10 (1.28 to 3.43) | 0.0030 | 2.36 (1.38 to 4.04) | 0.0020 |
| Coffee intake | 0.71 (0.54 to 0.93) | 0.0120 | 0.75 (0.58 to 1.00) | 0.0600 | 0.90 (0.68 to 1.18) | 0.4600 | – | |
| History of skin damage | 1.81 (1.40 to 2.34) | <0.0010 | 1.38 (1.06 to 1.81) | 0.0200 | 1.43 (1.05 to 1.94) | 0.0200 | 1.35 (0.99 to 1.85) | 0.0600 |
| History of and/or currently smoking | 1.12 (0.85 to 1.47) | 0.4300 | – | – | 1.27 (0.98 to 1.64) | 0.0700 | 1.31 (1.02 to 1.71) | 0.0400 |
| History of anterior myocardial infarction | 0.98 (0.68 to 1.47) | 0.9100 | – | – | 0.67 (0.35 to 1.31) | 0.2600 | – | – |
| History of diabetes | 1.05 (0.63 to 1.75) | 0.8400 | – | – | 1.22 (0.71 to 2.10) | 0.4600 | – | – |
| Alcohol consumption (standard drinks/d) | ||||||||
| 1 to 2 | Referent | Referent | ||||||
| 3 to 4 | 0.96 (0.66 to 1.40) | 0.8300 | – | – | 1.33 (0.84 to 2.09) | 0.2200 | – | – |
| 5 to 8 | 1.44 (0.93 to 2.24) | 0.1000 | – | – | 0.42 (0.06 to 3.00) | 0.3900 | – | – |
| ≥9 | 1.06 (0.52 to 2.18) | 0.8400 | – | – | 0.70 (0.10 to 5.00) | 0.7200 | – | – |
| History of oral contraceptive use | – | – | – | – | 0.98 (0.75 to 1.29) | 0.9000 | – | – |
| History of hormone replacement therapy use | – | – | – | – | 0.96 (0.72 to 1.27) | 0.7600 | – | – |
| DBP (per 10-mmHg increase) | 0.88 (0.77 to 0.99) | 0.0400 | 0.98 (0.97 to 0.99) | 0.0200 | 0.92 (0.81 to 1.05) | 0.2300 | 0.99 (0.98 to 1.01) | 0.2400 |
| SBP (per 10-mmHg increase) | 1.09 (1.02 to 1.16) | 0.0080 | 1.01 (0.98 to 1.01) | 0.1400 | 1.02 (0.96 to 1.08) | 0.5400 | – | – |
| Age of menarche (per 1-yr increase) | – | – | – | – | 0.97 (0.92 to 1.03) | 0.2900 | – | – |
| Age of menopause (per 1-yr increase) | – | – | – | – | 0.99 (0.98 to 1.01) | 0.4400 | – | – |
| eGFR (per 10-ml/min per 1.73 m2 decrease) | 1.30 (1.15 to 1.46) | <0.0010 | 1.17 (1.03 to 1.32) | 0.0200 | 1.02 (0.91 to 1.15) | 0.7100 | 0.94 (0.83 to 1.06) | 0.3200 |
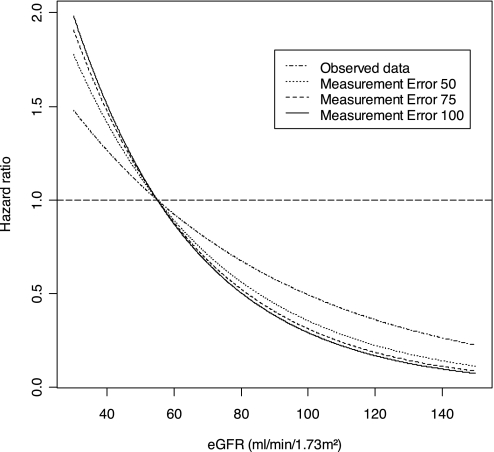
Adjustment for Measurement Error.
Figure 3 shows the multivariate HR for incident cancer across continuous measures of eGFR before and after adjustment for measurement error in men. The effect of adjusting for potential measurement error in estimating the GFR resulted in stronger association between reduced GFR and cancer in men. The HR increased by 7.7% to 1.26 (95% CI 1.08 to 1.47) per 10-ml/min per 1.73 m2 decrease in eGFR using a measurement error variance of 50 (ml/min per 1.73 m2)2, increased by 10.3% to 1.29 (95% CI 1.10 to 1.53) per 10-ml/min per 1.73 m2 decrease in eGFR using a measurement error variance of 77.56 (ml/min per 1.73 m2)2, and increased by 12.8% to 1.32 (95% CI 1.11 to 1.55) per 10-ml/min per 1.73 m2 decrease in eGFR with a measurement error variance of 100 (ml/min per 1.73 m2)2.
Figure 3.
Adjusted HRs for incident cancers across continuous measures of eGFR before and after adjustment for measurement errors in men.
In women, there was no demonstrable association between GFR and cancer after adjustment for potential measurement error in estimating GFR. For measurement error variance of 50 (ml/min per 1.73m2)2, 77.56 (ml/min per 1.73m2)2, and 100 (ml/min per 1.73m2)2, the adjusted HRs were 0.92 (95% CI 0.78 to 1.08), 0.90 (95% CI 0.77 to 1.06), and 0.90 (95% CI 0.76 to 1.05) per 10 ml/min per 1.73 m2, respectively.
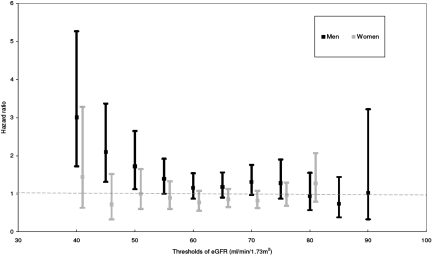
Threshold Analyses.
To identify the threshold of reduced kidney function at which the excess risk for cancer began, we performed a series of sensitivity analyses with various thresholds of eGFR using the multivariate Cox proportional hazard models: 35, 40, 45, 55, 65, 75, 80, and >90 ml/min per 1.73 m2. Figure 4 shows results of the threshold analyses assessing the relationship between reduced kidney function and cancer risk in both men and women. Cancer risk increased significantly in men with reduced kidney function, beginning with a threshold GFR of 55 ml/min per 1.73 m2. The adjusted HR for cancer was 1.39 (95% CI 1.00 to 1.92; P = 0.04) for men with an eGFR <55 ml/min per 1.73 m2 compared with those with an eGFR ≥55 ml/min per 1.73 m2. Within the range of eGFR in our study cohort, the threshold below which the greatest cancer risk occurred was 40 ml/min per 1.73 m2. The adjusted HR for incident cancer was 3.01 (95% CI 1.72 to 5.27; P = 0.0001) for men with an eGFR <40 ml/min per 1.73 m2 compared with those with an eGFR ≥40 ml/min per 1.73 m2. Estimates below a threshold of 35 ml/min per 1.73 m2 were less precise because only 35 participants had an eGFR between 15 and 35 ml/min per 1.73 m2. There was no significant association between reduced kidney function and cancer risk at any of the thresholds of eGFR in women.
Figure 4.
Adjusted HRs for incident cancers across various thresholds of eGFR in both men and women.
Cancer Site–Specific Analyses.
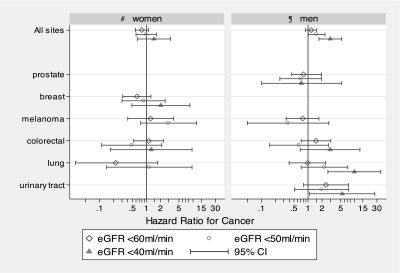
Figure 5 shows the selected site-specific HR by gender and at different thresholds of eGFR. Reduced kidney function to the lowest threshold of eGFR (<40 ml/min) was significantly associated with lung (P < 0.001) and urinary tract cancers (P = 0.04) in men but showed no significant association for colorectal, breast, and prostate cancers after adjustment for the effect of age, smoking status, sun-related skin damage, and DBP. There was no significant association between reduced kidney function (eGFR <40 ml/min) and site-specific cancers (breast cancer, P = 0.33; colorectal cancer, P = 0.81) in women. There were insufficient numbers of lung and urinary tract cancers below the eGFR <40 ml/min per 1.73 m2 in women to perform any conclusive analyses.
Figure 5.
Adjusted HR for all sites and site-specific cancers by gender. ¶Adjusted for the effect of age, smoking status, sun-related skin damage, and DBP in men. #Adjusted for the effect of age, smoking status, and sun-related skin damage in women. *Unable to calculate HRs for urinary tract cancers in women with CKD and for melanoma in women and men with eGFR <40 ml/min per 1.73 m2. There were insufficient numbers of cancers at the threshold below the designated eGFR.
Sensitivity Analyses.
Exclusion of incident cancers diagnosed in the first 4 yr after baseline examination did not affect the association between reduced kidney function and overall cancer risk in men; 87 such cancers were excluded from the analysis, and the fully adjusted HR of incident cancer in men was 1.17 (95% CI 1.00 to 1.37; P = 0.06) per 10-ml/min per 1.73 m2 decrease in eGFR. In addition, the overall adjusted HR of incident cancers in men after excluding very elderly participants (age >85 yr at baseline) was 1.16 (95% CI 1.02 to 1.32; P = 0.02) per 10-ml/min per 1.73 m2 decrease in eGFR, showing no impact on the overall association between reduced kidney function and cancer risk.
DISCUSSION
These data suggest that the excess risk for cancer in men with kidney disease may not be limited to those on dialysis or with a transplant but also those with moderate CKD, which is present in approximately one third of the population aged ≥50 yr. Moderate CKD increases the risk for all cancers in men by approximately 40%, independent of other known risk factors such as age and smoking. The threshold of kidney dysfunction at which this risk begins is approximately an eGFR of 55 ml/min, and risk increases linearly as the eGFR falls, reaching a maximum three-fold increased risk with GFR ≤40 ml/min per 1.73 m2, which is similar to the risk increase seen in dialysis and kidney transplant patients.6,12–14 The association between reduced kidney function (at a threshold of eGFR <40 ml/min per 1.73 m2) also seems to be site specific for lung and urinary tract cancers.
Our findings are consistent with emerging literature showing that kidney disease is an independent risk factor for diseases in other organ systems and that this increased risk is not limited to severe kidney disease or ESKD but begins with early or mild to moderate kidney disease. Previously, excess risk associated with CKD was shown for increased all-cause mortality, primarily driven by cardiovascular outcomes for which very similar estimates of excess risk (approximately 40%) at similar thresholds (eGFR <60 ml/min per 1.73 m2) for GFR were demonstrated.15
Our observed link between cancer and CKD was limited to men only, and we did not show any relationship between cancer and CKD in women. There are no comparable studies in the pre-ESKD population, but a similar but less extreme finding has been reported in older kidney transplant recipients. Male recipients of kidney transplants had the highest overall cancer risk, compared with women who had received a transplant and the general population.14,16 Compared with women who had received a transplant, men who had received a transplant had a relative risk of 1.2 for nonskin and 2.2 for skin cancers.8 A three-fold increase in the overall cancer risk was observed in men who had received a transplant compared with the general population. In contrast to our finding of no increased cancer risk in women with mildly to moderately reduced kidney function, there was a two-fold increase in cancer incidence in women who had received a transplant compared with the general population.8
A significant association was detected between urinary tract and lung cancers in men below the lowest threshold of eGFR defined in our study cohort. A similar observation was reported in a recent study showing a significant relationship between increasing levels of albumin-creatinine ratio and increased risk for bladder, renal, and lung cancers.11 Given the consistent evidence of a two- to three-fold increased risk for virus-related neoplasms such as cervical cancers and non-Hodgkin lymphomas in transplant recipients,6,14 assessing the relationship between these cancers and reduced kidney function in our study cohort may have been a more robust approach, but there was insufficient statistical power to elucidate this further.
The precise biological explanation for the association between reduced kidney function and increased cancer risk in men is not clear and may be multifactorial. Some have suggested an association between vitamin D deficiency, which is highly prevalent among people with moderately reduced kidney function,17,18 and risk for prostate, breast, and colon cancer in the general population19–21; however, the results of our study are comparable to the findings in the transplant and dialysis populations14 and show no site-specific increased risk for breast and prostate cancers in both men and women. CKD is also a proinflammatory state,22–24 and there is now emerging evidence linking an association between chronic inflammation and risk for cancer.25 Markers of inflammation, such as white blood cell count, have also been associated with an increased risk for cancer mortality in the general population.26–28 Our findings could represent a CKD threshold below which potential uremic factors associated with reduced kidney function increase the risk for cancer independent of other, well-established risk factors, such as iatrogenic immunosuppression used after transplantation. Future studies with greater statistical power assessing potential underlying mechanisms and site-specific cancer risk factors are warranted.
The lack of positive association between cancer and moderate CKD in women was unexpected. There are a number of plausible explanations. With >1700 women followed for >10 yr, during which 240 developed cancer, we believe our study was sufficiently powered overall to detect an association between moderate CKD and overall cancer risk in women; however, there are important gender-related differences in the risk for organ-specific cancers that may explain why no association was found. First, exposure factors such as ethyl benzenes and phenacetin that caused the primary renal disease may also be responsible for cancers of the urinary tract. If this exposure occurred more frequently in men than women, then there may be resultant differential confounding effect on the associations of reduced kidney function with cancer risk between genders.29 Second, it seems that the overall increased risk in men was driven mainly by lung and urinary tract cancers, so it is possible that meaningful analyses assessing the relationship between urinary tract cancers and reduced kidney function in women were not feasible as a result of the limited numbers available in our study cohort. In addition, serum creatinine concentration (or eGFR) is an imperfect measure of kidney function because serum creatinine, a breakdown product of muscle, is influenced not only by kidney function but also by age, gender, ethnicity, and body mass.30,31 In particular, it is less sensitive to detect reduced kidney function in women with mildly to moderately reduced kidney function. A serum creatinine–based estimated equation for kidney function in women may underestimate the true level of kidney function32 and thus could have attenuated the true influence of CKD on cancer risk in women. Alternatively, there may indeed be true gender-based effect modification, with a lower threshold of <40 ml/min in women before the risk for cancer increased in women, for which our study has insufficient data to be conclusive.
Our study has a number of strengths. It is population based with very few missing baseline data and 31,620 person-years of follow-up. Detailed data on confounders were also available. Using linked data from a mandatory statewide cancer registry, we accurately ascertained all cancer diagnoses since inception of the cohort. It is possible that some cancers were not reported to this register because of interstate movement, but there is unlikely to be differential classification according to CKD stage, and this number is likely to be very small, because, since 1992, only 5% of the cohort have moved outside the district.33 We excluded all prevalent cancers before the first survey for several reasons. Cancer, in particular cancer of the urinary tract and hematologic systems such as renal cell carcinoma and multiple myeloma, are known to result in CKD and reduced kidney function. Moreover, chemotherapeutic agents such as platinum-based chemotherapy can affect renal function adversely. In our analyses, >44% of the participants with prevalent cancer (excluded from the analyses) had an eGFR <60 ml/min per 1.73 m2. This was comparable to the findings from a recent study that showed that >30% of all prevalent cancer patients had an eGFR <80%.34 By removing from the analyses all individuals with prevalent cancer, our observations showed that reduced kidney function may be an independent risk factor for cancer in men after adjusting for the effect of age, smoking, and sun-related skin damage.
Our study has a number of potential limitations. We excluded 11% of the original sample because of incomplete blood and serum profile, but this number is relatively small and those who were excluded were similar to the overall study population. Regrettably, testing for urinary protein was not undertaken, which may be regarded as a study weakness. Microalbuminuria is a marker of CKD and end organ damage. Post hoc analyses of long-term clinical trials and large prospective cohort studies had shown an association between microalbuminuria and cardiovascular risk.35–38 Analysis using albuminuria/proteinuria as another marker of CKD may have been useful as a potential independent risk factor for cancer.11 The progression of CKD over time and the relationship with cancer was not evaluated because we had only measurements of serum creatinine at baseline. Findings from our study may not be generalizable to other populations such as blacks or indigenous Australians because the majority of our study participants were white. Early censoring of people who had CKD and died prematurely from causes other than cancer, such as cardiovascular disease, may have attenuated the true effect of reduced kidney function and cancer risk; however, if the effect were differential between men and women, then it would bias the effect toward the null in men but not in women, because the overall mortality is significantly greater in men than in women in our study cohort (results available upon request). Finally, although we had fully adjusted for all known risk factors for cancer available in the data set, residual confounding by other, unmeasured socioeconomic and epidemiologic factors such as the finer details of smoking (amount of cigarettes smoked per year or years of smoking) may also have occurred.
Understanding an individual's cancer risk is important for long-term clinical management strategies. Much emphasis has been placed on assessing and modifying cardiovascular risk in CKD via lipid lowering and dietary and exercise modification. Similarly, fruitful areas for future research should be to evaluate potentially modifiable risk factors for the prevention and early detection of cancer. The impact of CKD on the treatment, prognosis, and survival of cancers should therefore be explored.
In conclusion, we report that moderately reduced kidney function may be an independent risk factor for cancer in older men and that this risk begins when GFR falls to approximately 55 ml/min and progressively increases as GFR declines further to 40 ml/min when a similar risk for patients on dialysis or transplant recipients is evident. Given that CKD of this severity affects 20% of the male population, our finding suggests that CKD prevention may be a novel and worthwhile strategy for reducing cancer risk in the general population.
CONCISE METHODS
Study Population
The Blue Mountains Eye Study (BMES) is a well-established population-based cohort study of predominantly white Australians. The main aim of the original study was to assess the prevalence, incidence, and risk factors of common eye disease but has since expanded to include other important health outcomes in older people.33 Full details of recruitment methods are reported elsewhere.33,39 In brief, during 1992 through 1993, all noninstitutionalized permanent residents of two postal code districts in the Blue Mountains area, west of Sydney, who were born before January 1, 1943, were invited to participate in the study. The number of eligible individuals found in the first census differed from the Australian Census, conducted 3 mo earlier, by only six people. This study was conducted according to the recommendations of the Declaration of Helsinki and was approved by the Western Sydney Area Human Research Ethics Committee, with written informed consent obtained from all participants. Of the 4433 eligible individuals identified at the original census, 3654 (82.4%) participated in the study, which involved a detailed interview, medical examination including body weight and SBP and DBP (measured with a single mercury sphygmomanometer), and fasting blood samples (blood samples were collected from 3222 [88%] of participants at baseline). The Institute of Clinical Pathology and Medical Research at Westmead hospital performed the laboratory tests within 4 h of blood collection. Serum creatinine was measured within 4 h of collection using a Hitachi 747 Biochemistry analyzer (Roche reagents, modified kinetic Jaffe). In accordance with the National Kidney Foundation, we defined participants as having moderate CKD, with an eGFR of <60 ml/min per 1.73 m2, calculated using the four-variable abbreviated Modification of Diet in Renal Disease (MDRD) equation: eGFR (ml/min per 1.73 m2 = 186 * (serum creatinine in ml/min * 0.0113)−1.154 * age−0.203 * 0.742 (if female) * 1.210 (if black). Body mass index was calculated as weight/height2 (kg/m2).40 Self-reported history of smoking (yes or no), sun-related skin damage (mild, moderate, and severe), previous heart attack, alcohol consumption (categorized into 1 to 2, 3 to 4, 5 to 9, and >9 standard drinks per day), diabetes, coffee drinking (yes or no), oral contraceptive intake (yes or no), and age at menarche and menopause were recorded.
New South Wales Central Cancer Registry
The Central Cancer Registry (CCR) was established under the Public Heath Act of New South Wales 1991 to receive notifications of incident cancer in New South Wales. Notification of cancer is a statutory requirement for all public and private hospitals, radiotherapy, outpatient, and pathology departments. The CCR maintains a case-based register of all cancer diagnoses in individuals resident in New South Wales, except for nonmelanocytic skin cancers, since 1972. The rate of interstate movement is small and accounts for <3.2% of the total populations in New South Wales.41 The CCR holds data on date and age at cancer diagnosis, site, histologic type, topography, and morphology.
Record Linkage
Ethics approval for this study was obtained from the Cancer Institute of New South Wales and the University of Sydney. The linkage process, using specialized probabilistic linkage software, was carried out by the Centre for Health Record Linkage (CheReL) at the Cancer Institute of New South Wales. Using key identifiers such as name, age, gender, address, and postcode from the BMES data set, CheReL created a unique data-linkage key that linked to records to the same person in the CCR database. The linkage process was repeated using six passes. Personnel at CheReL estimated the probabilities and set error thresholds for each pass. The probabilities then were aggregated into a score and checked against a threshold to determine whether a match was made. Manual clerical review was undertaken to improve the accuracy of the linked record.
Assessment of Incident Cancers
Incident cancer was defined as the first cancer diagnosed after the initial survey in 1992 through 1993. All incident cancers diagnosed before December 31, 2004, were included. Diagnoses of incident cancers were coded using the International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision for cancers (C010 through C809). Nonmelanocytic skin cancers were excluded from the analysis because CCR New South Wales does not hold any information on skin cancers except for melanomas. Participants with cancers diagnosed before the initial survey (all prevalent cancers) were excluded from the analysis.
Statistical Analysis
Statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC) and R 2.6.1. (R Foundation for Statistical Computing, Auckland, Australia). Baseline characteristics of participants with and without CKD were compared using t test, χ2 tests, and ANOVA. For survival analyses, the follow-up period was defined from the time of first survey (1992 through 1993) to time of cancer diagnosis. Those who did not develop incident cancers were censored at the end of the follow-up period (December 31, 2004). Participants who died during the follow-up period were censored at the time of death. The proportion of participants free from incident cancer was calculated using the Kaplan-Meier method.
We applied univariate and multivariable-adjusted Cox proportional hazard models to assess the relationship between incident cancer and kidney function while controlling for age, age at menarche and menopause, SBP and DBP, body mass index, fasting serum cholesterol and glucose, smoking status, history of oral contraceptive use, hormone replacement therapy, cardiovascular events, and sun-related skin damage. HRs of all incident cancers with reduced kidney function were then compared with those with normal kidney function in the unadjusted and multivariable-adjusted models. eGFR, as a measure of kidney function, was modeled as a continuous and categorical (<60 and ≥60 ml/min per 1.7 3m2) variables. All explanatory variables that had an association with cancer at P < 0.25 in the unadjusted analyses were included in the multivariable-adjusted analyses. The least significant variables were then removed from the base model using a step-wise backward elimination process until only variables with P < 0.05 remained in the final parsimonious model. In all models, however, we adjusted for smoking status, on the basis of the prior knowledge that smoking is an independent risk factor for cancers.42–44 We checked for any deviations from linearity in serum creatinine and eGFR using linear spline models and other continuous variables such as age, SBP and DBP, and serum blood cholesterol by categorizing each of these variables into quartiles and plotting the estimated regression coefficients against the midpoints of each of the groups. No deviations from linearity were identified. Potential effect modification was also tested between the study factor (eGFR and serum creatinine) and all other covariates using two-way interaction terms. Effect modification was found between gender and eGFR (test of interaction for gender P = 0.004), so two models with different covariates were developed for men and women to assess the relationship between reduced kidney function and cancer risk. The proportional hazards assumptions of all Cox models was assessed by fitting log(time)-dependent covariates in the multivariate models and checking graphically by plotting the Schoenfeld residuals, but no variables showed evidence of departure from the proportional assumption. To assess the robustness of our results, we performed threshold analyses at the various cut points of eGFR for the prediction of cancer risk. Site-specific analyses were performed to assess the relationship between reduced kidney function and risk for common cancers.
To account for any potential measurement error that might occur when estimating GFR using a single creatinine test, we used SIMEX (simulation and extrapolation) methods.45 SIMEX is a simulation-based method of inference that allows correction for measurement error. Using data extrapolated from published literature, we applied a value, 77.56 (ml/min per 1.73 m2)2, for the variance of measurement error in the base case analysis.46 We also performed sensitivity analyses using measurement error variances of 50 (ml/min per 1.73 m2)2 and 100 (ml/min per 1.73 m2)2.
To ensure that undiagnosed cancers at the time of first survey did not affect the outcomes of our study, we performed separate analyses excluding participants who had incident cancers diagnosed up to 4 yr after baseline examination. We also conducted a sensitivity analysis excluding very elderly participants (age ≥85 yr at baseline) in the study cohort to eliminate the effect of competing risk as a result of mortality.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
G.W. is the recipient of a Kidney Health Australia Biomedical Scholarship and the Centre for Clinical Research Excellence in Renal Medicine Postgraduate Medical Scholarship.
This report was presented, in part, at the 40th annual meeting of the American Society of Nephrology (San Francisco, CA; October 31 through November 1, 2007) and the 44th Annual Scientific Meeting of the Australia and New Zealand Society of Nephrology (ANZSN) (New Castle N.S.W., September 8 through 10, 2008).
G.W. conceived and designed the study, analyzed and interpreted the results, undertook the statistical modeling, and wrote the manuscript; A.H. contributed to the analysis and interpretation of results, undertook the statistical modeling, and revised the manuscript; J.R.C. contributed to the design of the study, advised on the presentation of results, and revised the manuscript; A.C.W. contributed to the analysis and interpretation of results and revised the manuscript; J.J.W. contributed to the Blue Mountains Eye Study data set and to the interpretation and presentation of findings; P.M. was principal investigator of the Blue Mountains Eye Study, designed and obtained funding for the study, and contributed to the interpretation and revision of the manuscript; J.C.C. conceived and designed the study, analyzed and interpreted the results, advised on the presentation of results and revised the manuscript. G.W. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.The burden of chronic kidney disease. In: Chronic Kidney Disease in Australia 2005, Canberra, Australian Institute of Health and Welfare, 2005
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB: Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol 41: 1364–1372, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Shlipak MG, Simon JA, Grady D, Lin F, Wenger NK, Furberg CD, Heart and Estrogen/progestin Replacement Study (HERS) Investigators: Renal insufficiency and cardiovascular events in postmenopausal women with coronary heart disease. J Am Coll Cardiol 38: 705–711, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S: Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med 134: 629–636, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE: Cancer incidence before and after kidney transplantation. JAMA 296: 2823–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Brunner FP, Landais P, Selwood NH: Malignancies after renal transplantation: The EDTA-ERA registry experience. European Dialysis and Transplantation Association-European Renal Association. Nephrol Dial Transplant 10 [Suppl 1]: 74–80, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C: Cancer after kidney transplantation in the United States. Am J Transplant 4: 905–913, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Matas AJ, Simmons RL, Kjellstrand CM, Buselmeier TJ, Najarian JS: Increased incidence of malignancy during chronic renal failure. Lancet 1: 883–886, 1975 [DOI] [PubMed] [Google Scholar]
- 10.Cengiz K: Increased incidence of neoplasia in chronic renal failure (20-year experience). Int Urol Nephrol 33: 121–126, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen L, Heuch I, Jenssen T, Jacobsen BK: Association of albuminuria and cancer incidence. J Am Soc Nephrol 19: 992–998, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adami J, Gabel H, Lindelof B, Ekstrom K, Rydh B, Glimelius B, Ekbom A, Adami HO, Granath F: Cancer risk following organ transplantation: A nationwide cohort study in Sweden. Br J Cancer 89: 1221–1227, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birkeland SA, Lokkegaard H, Storm HH: Cancer risk in patients on dialysis and after renal transplantation. Lancet 355: 1886–1887, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR: Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: A cohort study of 15,183 recipients. Am J Transplant 7: 2140–2151, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, Graeb C, Jauch KW, Guba M: The janus face of immunosuppression: De novo malignancy after renal transplantation—The experience of the Transplantation Center Munich. Kidney Int 71: 1271–1278, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ: Vitamin D insufficiency and deficiency in chronic kidney disease: A single center observational study. Am J Nephrol 24: 503–510, 2004 [DOI] [PubMed] [Google Scholar]
- 18.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM: Prevalence of calcidiol deficiency in CKD: A cross-sectional study across latitudes in the United States. Am J Kidney Dis 45: 1026–1033, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P: Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control 11: 847–852, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Mawer EB, Walls J, Howell A, Davies M, Ratcliffe WA, Bundred NJ: Serum 1,25-dihydroxyvitamin D may be related inversely to disease activity in breast cancer patients with bone metastases. J Clin Endocrinol Metab 82: 118–122, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Vandewalle B, Adenis A, Hornez L, Revillion F, Lefebvre J: 1,25-Dihydroxyvitamin D3 receptors in normal and malignant human colorectal tissues. Cancer Lett 86: 67–73, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Keller CR, Odden MC, Fried LF, Newman AB, Angleman S, Green CA, Cummings SR, Harris TB, Shlipak MG: Kidney function and markers of inflammation in elderly persons without chronic kidney disease: The health, aging, and body composition study. Kidney Int 71: 239–244, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G, Cholesterol and Recurrent Events (CARE) Trial Investigators: Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int 68: 237–245, 2005 [DOI] [PubMed] [Google Scholar]
- 24.de Vinuesa SG, Goicoechea M, Kanter J, Puerta M, Cachofeiro V, Lahera V, Gomez-Campdera F, Luno J: Insulin resistance, inflammatory biomarkers, and adipokines in patients with chronic kidney disease: Effects of angiotensin II blockade. J Am Soc Nephrol 17[Suppl 3]: S206–S212, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Coussens LM, Werb Z: Inflammation and cancer. Nature 420: 860–867, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimm RH Jr, Neaton JD, Ludwig W: Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA 254: 1932–1937, 1985 [PubMed] [Google Scholar]
- 27.Friedman GD, Fireman BH: The leukocyte count and cancer mortality. Am J Epidemiol 133: 376–380, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Shankar A, Wang JJ, Rochtchina E, Yu MC, Kefford R, Mitchell P: Association between circulating white blood cell count and cancer mortality: A population-based cohort study [published erratum appears in Arch Intern Med 166: 681, 2006]. Arch Intern Med 166: 188–194, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Stewart JH, Buccianti G, Agodoa L, Gellert R, McCredie MR, Lowenfels AB, Disney AP, Wolfe RA, Boyle P, Maisonneuve P: Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: Analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol 14: 197–207, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Stevens LA, Levey AS: Measurement of kidney function. Med Clin North Am 89: 457–473, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Levey AS: Measurement of renal function in chronic renal disease. Kidney Int 38: 167–184, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Rule AD, Rodeheffer RJ, Larson TS, Burnett JC Jr, Cosio FG, Turner ST, Jacobsen SJ: Limitations of estimating glomerular filtration rate from serum creatinine in the general population [published erratum appears in Mayo Clin Proc 81: 1639. 2006]. Mayo Clin Proc 81: 1427–1434, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Mitchell P, Smith W, Attebo K, Wang JJ: Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology 102: 1450–1460, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Janus N: Renal insufficiency in bone metastasis cancer patients: Prevalence and implications on anticancer drugs management, subgroup analysis of the IRMA study [Abstract]. J Clin Oncol ASCO Annu Meet Proc I 2007, in press
- 35.Yudkin JS, Forrest RD, Jackson CA: Microalbuminuria as predictor of vascular disease in non-diabetic subjects. Islington Diabetes Survey. Lancet 2: 530–533, 1988 [DOI] [PubMed] [Google Scholar]
- 36.Dinneen SF, Gerstein HC: The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus: A systematic overview of the literature. Arch Intern Med 157: 1413–1418, 1997 [PubMed] [Google Scholar]
- 37.Agewall S, Wikstrand J, Ljungman S, Fagerberg B: Usefulness of microalbuminuria in predicting cardiovascular mortality in treated hypertensive men with and without diabetes mellitus. Risk Factor Intervention Study Group. Am J Cardiol 80: 164–169, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S, HOPE Study Investigators: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Cumming RG, Mitchell P, Leeder SR: Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med 337: 8–14, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G, National Kidney Foundation: National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification [published erratum appears in Ann Intern Med 139: 605, 2003]. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Cancer Institute New South Wales: NSW Cancer Registry Statistical Reporting Module, New South Wales, Australia, Cancer Institute of N.S.W., 2008
- 42.Nagata C, Mizoue T, Tanaka K, Tsuji I, Wakai K, Inoue M, Tsugane S, Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan: Tobacco smoking and breast cancer risk: An evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J Clin Oncol 36: 387–394, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Inoue M, Tsuji I, Wakai K, Nagata C, Mizoue T, Tanaka K, Tsugane S, Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan: Evaluation based on systematic review of epidemiological evidence among Japanese populations: Tobacco smoking and total cancer risk. Jpn J Clin Oncol 35: 404–411, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Alberg AJ, Kouzis A, Genkinger JM, Gallicchio L, Burke AE, Hoffman SC, Diener-West M, Helzlsouer KJ, Comstock GW: A prospective cohort study of bladder cancer risk in relation to active cigarette smoking and household exposure to secondhand cigarette smoke. Am J Epidemiol 165: 660–666, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Carrol RJ, Ruppert D, Stefanski LA, Crainiceanu C: Measurement Error in Nonlinear Models: A Modern Perspective, CRC Press, Taylor and Francis Group, 2nd Ed., 2006
- 46.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE, Coresh J: Reduced kidney function as a risk factor for incident heart failure: The Atherosclerosis Risk in Communities (ARIC) study. J Am Soc Nephrol 18: 1307–1315, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.