Abstract
The balance of matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMPs) determines the integrity of the extracellular matrix. TIMP3 is the most highly expressed tissue inhibitor of metalloproteinase (TIMP) in the kidney, but its function in renal disease is incompletely understood. In this study, TIMP3−/− mice demonstrated an age-dependent chronic tubulointerstitial fibrosis. After unilateral ureteral obstruction (UUO), young TIMP3−/− mice exhibited increased renal injury (tubular atrophy, cortical and medullary thinning, and vascular damage) compared with wild-type mice. In addition, TIMP3−/− mice had greater interstitial fibrosis; increased synthesis and deposition of type I collagen; increased activation of fibroblasts; enhanced apoptosis; and greater activation of MMP2, but not MMP9, after UUO. TIMP3 deficiency also led to accelerated processing of TNFα, demonstrated by significantly higher TACE activity and greater soluble TNFα levels by 3 d after UUO. The additional deletion of TNFα markedly reduced inflammation, apoptosis, and induction of a number of MMPs. Moreover, inhibition of MMPs in TIMP3−/−/TNFα−/− mice further abrogated postobstructive injury and prevented tubulointerestitial fibrosis. In humans, TIMP3 expression increased in the renal arteries and proximal tubules of subjects with diabetic nephropathy or chronic allograft nephropathy. Taken together, these results provide evidence that TIMP3 is an important mediator of kidney injury, and regulating its activity may have therapeutic benefit for patients with kidney disease.
Renal interstitial fibrosis is a progressive and potentially lethal disease caused by diverse clinical entities including urinary tract obstruction, chronic inflammation and allograft injury, chemotherapy-induced renal injury, proteinuria, and diabetes mellitus.1–3 Acute unilateral ureteral obstruction due to renal stones is a frequent event affecting 5% to 15% of the population worldwide.4 During obstruction, functional and biochemical alterations occur in the kidney, with partial chronic obstruction leading to chronic renal insufficiency, whereas an immediate onset of acute obstruction can result in acute renal failure. Increased tubulointerstitial fibrosis is a common feature of kidney injury and results from accumulation of extracellular matrix (ECM) structural proteins and is maintained by a continuous remodeling through the proteolytic action of matrix metalloproteinases (MMPs) and synthesis of new proteins. Matrix metalloproteinases are inhibited by tissue inhibitors of matrix metalloproteinases (TIMPs); therefore, a balance in the function of TIMPs and MMPs determines the ECM integrity. Among the four members of the TIMP family, TIMP3 is unique in that it is ECM bound; is the most highly expressed TIMP in the kidney;5 and has a very broad protease inhibition profile that extends to members of the ADAM (a disintegrin and metalloproteinase domain) and ADAM-TS families, proteases that control the bioactivity of many growth factors and cytokines.6–8
Loss of TIMP3 in mice leads to pulmonary alveolar enlargement,9 enhanced susceptibility to cardiomyopathy,10 and hepatic injury.11 In this study, we examined the role of TIMP3 in age-dependent kidney disease as well as in response to an experimental model of renal injury. We used a well established model of tubulointerstitial injury, unilateral ureteral obstruction (UUO),12–14 and characterized the mechanism of renal injury progression in mice lacking TIMP3 (TIMP3−/−) compared with wild-type (WT) control mice. Here we demonstrate that early activation of the TNFα signaling pathway in the absence of TIMP3 is accompanied by enhanced MMP activation, apoptosis, and neutrophil infiltration, which collectively contribute to the accelerated and severe tubulointerstitial injury. We further confirm the key role of TNFα and MMPs by demonstrating that TIMP3−/−/TNFα−/− mice exhibit attenuated tubulointerstitial injury, while inhibition of the residual MMP activities in these mice markedly resolved the interstitial nephritis at 2 wk post-UUO. In human biopsies, we have found that TIMP3 levels are up-regulated in patients with diabetes and chronic allograph nephropathy. These results provide strong evidence for a dynamic and important role of TIMP3 in kidney disease.
RESULTS
Loss of TIMP3 Is Associated with Age-Dependent Renal Fibrosis and Tubulointerstitial Injury
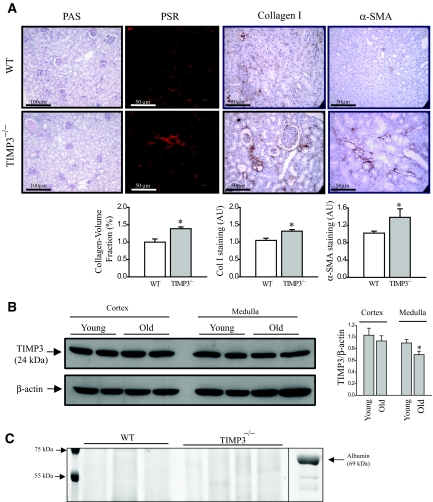
MMPs and their physiologic inhibitors (TIMPs) play significant roles in renal morphogenesis15 and tubulointerstitial injury.16,17 TIMP3 is the most highly expressed TIMP in the kidney,5 thus we examined the role of TIMP3 in the development and progression of renal disease. Light microscopy examination of PAS and Masson Trichrome-stained longitudinal mouse kidney sections from 2-yr-old male TIMP3-deficient mice showed small but significant chronic glomerular and tubulointerstitial abnormalities compared with sections from age-matched WT mice. Specifically, increased interstitial fibrosis and tubular atrophy with shrunken glomerular tufts and collapsed segmental tufts were found in 2-yr-old TIMP3−/− mice but not in age-matched WT mice (Figure 1A). These areas correspond to a strong staining for collagen I, the main component of fibrotic lesions, and α-smooth muscle actin (α-SMA), marker of activated fibroblasts which are the main source of collagen production (Figure 1A). Western blotting for TIMP3 in the cortex and medulla of old (2-yr-old) compared with young (12-wk-old) WT kidneys shows a significant age-dependent reduction in TIMP3 levels primarily in the medulla (Figure 1B). This age-dependent tubulointerstitial injury in TIMP3−/− mice occurs despite comparable systemic BP (determined by tail-cuff measurements, 138.3 ± 11.2 mmHg in TIMP3−/− versus 132.5 ± 12.9 mmHg in WT, n = 5), similar serum creatinine levels (92.9 ± 13.1 μM in TIMP3−/− versus 87.5 ± 10.2 μM in WT, n = 5), and no proteinuria in either genotype at 2 yr of age (Figure 1C).
Figure 1.
Loss of tissue inhibitor of matrix metalloproteinases 3 (TIMP3) enhances age-dependent tubulointerstitial fibrosis. (A) Histologic analyses of 2-yr-old TIMP3−/− show enhanced tubulointerstitial injury, shrunken glomerular tufts, and increased fibrosis compared with wild-type (WT) mice. Collagen/volume fraction (Picrosirius red [PSR] staining), collagen type-I, and α-smooth muscle actin (αSMA) quantification are shown for WT (white) and TIMP3−/− kidneys (gray) (n = 5 per genotype; *P < 0.05 compared with WT group). (B) TIMP3 protein levels in young and old mice show a significant reduction in the medullar of the old kidneys (n = 4 per group; *P < 0.05 compared with young group). (C) No protein was detected in the urine of old TIMP3−/− compared with old WT mice. Chemical-grade bovine serum albumin was used as positive control. AU, arbitrary units; PAS, periodic acid-Schiff.
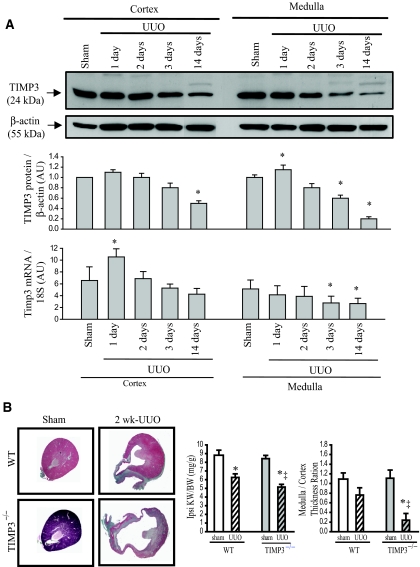
Given the age-dependent morphologic alterations in TIMP3−/− mice, we investigated whether TIMP3 deficiency affects susceptibility of young mice to renal injury. We subjected 10- to 12-wk-old mice of either genotype to unilateral ureteral obstruction (UUO), a validated model of tubulointerstitial injury.12,14,18 We found that in WT mice, at 1 d post-UUO, TIMP3 protein and mRNA levels show a small but significant increase, followed by a significant reduction at 3 d of UUO in the medulla, whereas the reduction in the cortex does not reach statistical significance until 14 d post-UUO (Figure 2A). The initial increase in TIMP3 levels could suggest a possible protective role for TIMP3 during the early stages of disease followed by disruption in its synthesis in the later stages of disease, which will further promote renal injury. Interestingly, UUO-induced renal injury was strikingly exacerbated in TIMP3−/− mice, as demonstrated by tubular atrophy and widespread cortical and medullary thinning, most markedly in the deep cortex and medulla (Figure 2B). In contrast, WT kidneys showed mild interstitial fibrosis, with only mild cortical and medullar thinning and tubular atrophy at 2 wk post-UUO. Ipsilateral kidney-to-body weight ratio and medulla-to-cortex thickness ratio are significantly reduced in TIMP3−/−-UUO compared with WT-UUO kidneys (Figure 2B). These data demonstrate that TIMP3 deficiency exacerbates renal injury in response to UUO.
Figure 2.
Tissue inhibitor of matrix metalloproteinases 3 (TIMP3) deficiency exacerbates tubulointerstitial injury after unilateral ureteral obstruction (UUO) in young mice. (A) In wild-type (WT) mice, TIMP3 protein and mRNA levels are increased early after UUO, followed by a reduction at 3d and 14 d post-UUO. Protein values are normalized to sham levels. (B) Young TIMP3−/− mice show exacerbated renal injury after UUO, as shown by severe tubular atrophy, and cortical and medullary thinning compared with WT kidneys at 2 wk post-UUO. Ipsilateral kidney-to-body weight ratio (Ipsi KW/BW) and medulla-to-cortex thickness ratio are significantly reduced in TIMP3−/−-UUO compared with WT-UUO kidneys (n = 6 per group). *P < 0.05 compared with corresponding sham-operated group; ‡P < 0.05 compared with corresponding WT group. AU, arbitrary units.
TIMP3 Deficiency Results in Adverse Extracellular Matrix (ECM) Remodeling and Interstitial Fibrosis After UUO
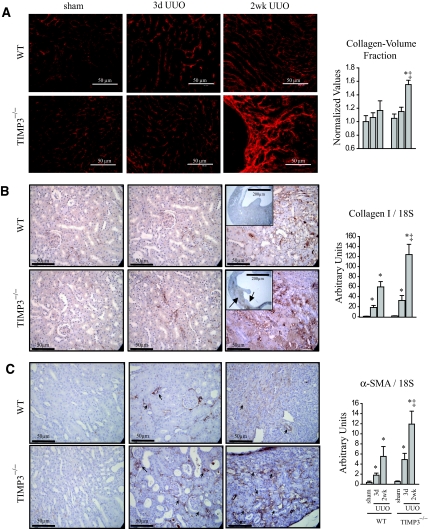
Integrity of the ECM is essential for optimal functioning of any organ and is maintained by a balance in the functions of matrix metalloproteinases (MMPs) that degrade ECM components, and their inhibitors, TIMPs. We used Picrosirius red (PSR) staining and confocal microscopy to visualize the fibrillar collagen component of the ECM structure in the kidneys. We found that at 3 d post-UUO there is no significant difference in the extent of fibrosis between the two genotypes, but by 2 wks post-UUO, TIMP3−/− kidneys exhibit a strikingly greater peritubular interstitial fibrosis (Figure 3A). Consistent with this finding, staining for collagen type I, the main structural component of the renal ECM, shows markedly stronger staining in TIMP3−/−-UUO kidneys in the interstitial tubules compared with the WT group (Figure 3B). We further confirmed the enhanced production of collagen type I by TaqMan real-time PCR, which demonstrates a significantly higher induction of collagen synthesis in TIMP3−/−-UUO compared with WT-UUO kidneys (Figure 3B). Collagen is produced primarily by fibroblasts that express α-smooth muscle actin (αSMA) in association with a phenotypic transformation to the myofibroblast state.2,18 Immunostaining for αSMA reveals an abundance of fibroblasts in the fibrotic regions of TIMP3−/−-UUO kidneys coupled with enhanced synthesis of αSMA mRNA (Figure 3C), suggesting enhanced migration and/or amplification of the fibroblasts in the TIMP3-deficient kidneys.
Figure 3.
Increased interstitial fibrosis, enhanced collagen synthesis, and α-smooth muscle actin (αSMA) expression in response to tubulointerstitial injury in tissue inhibitor of matrix metalloproteinases 3 (TIMP3)−/− mice. (A) Picrosirius red staining and confocal microscopy shows marked thickening and disorganization of the peritubular fibrosis in TIMP3-deficient kidneys compared with wild-type (WT) kidneys at 2 wk after unilateral ureteral obstruction (UUO). (B) Enhanced collagen synthesis and deposition as shown by immunostaining for collagen type I (arrows indicate areas of collagen deposition), as well as TaqMan RNA analysis for collagen type I normalized to 18S. (C) αSMA shows a significantly higher degree of staining and mRNA expression in TIMP3−/− kidneys at 3 d and 2 wk post-UUO. Arrows indicate regions of interstitial staining for αSMA. *P < 0.05 compared with sham-operated group; ‡P < 0.05 compared with corresponding WT group.
Differential Alterations in MMPs and Mitogen-Activated Protein Kinase (MAPK) Pathways, and Accelerated Apoptosis in TIMP3−/− Mice After Tubulointerstitial Injury
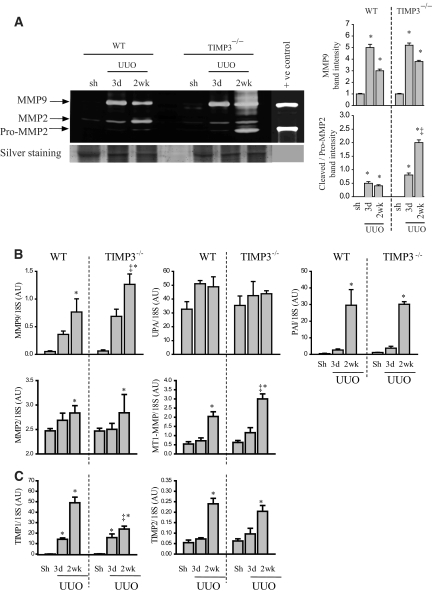
Given the adversely remodeled peritubular ECM in TIMP3−/− kidneys, we examined the expression and activity of key MMPs in the absence of TIMP3. MMP2 and MMP9 are both gelatinases with additional collagenolytic activities and have been shown to contribute to kidney disease.19,20 Gelatin zymography shows that activation of MMP2 and MMP9 is induced within 3 d of UUO in both genotypes; however, by 2 wk TIMP3−/− kidneys showed significantly higher levels of MMP9 and activated (cleaved) MMP2 compared with WT kidneys (Figure 4A). TaqMan expression analysis confirms increased production of MMP9 in the TIMP3−/−-UUO kidneys (Figure 4B). The urokinase plasminogen activator (UPA) mediates activation of MMP9 from the 92 kD to the 86 kD cleaved form, while its inhibitor, plasminogen activator inhibitor (PAI), inhibits this process. UPA and PAI have been shown to be important mediators of kidney injury.14,21 TaqMan mRNA analysis shows similar expression of UPA and PAI in both genotypes post-UUO (Figure 4B), which may account, at least in part, for the increase in pro-MMP9 but not cleaved MMP9 levels. Other factors, including other MMPs, may play an important role in the activation of pro-MMP9. Cell-surface activation of MMP2 is brought about by formation of a trimolecular complex involving membrane-bound MMP (MT1-MMP), TIMP2, and pro-MMP2. Through a two-step proteolytic cleavage, pro-MMP2 (68 kD) is converted to the 62-kD active MMP2.22 Although the increase in MMP2 mRNA production did not reach statistical significance compared with WT-UUO, the marked increase in MT1-MMP in TIMP3−/−-UUO (Figure 4B) could explain the strong 62-kD active MMP2 band in these kidneys. TIMP2 levels are increased similarly between the two genotypes; however, TIMP1 levels are significantly higher in the WT compared with TIMP3−/− kidneys at 2 wk post-UUO (Figure 4C). Hence, the increased MMP2 activation in TIMP3-deficient mice is driven by loss of TIMP3, while the lower TIMP1 levels in the late-stage UUO in TIMP3−/− kidneys could further contribute to increased overall MMP activity and disease severity in these mice.
Figure 4.
Altered activation and expression of matrix metalloproteinases (MMP) in tissue inhibitor of MMPs 3 (TIMP3)-deficient mice in response to tubulointerstitial injury. (A) Gelatin zymography shows that pro-MMP9 (92 kD) levels are significantly increased after unilateral ureteral obstruction (UUO) in both genotypes, while cleavage of pro-MMP2 (68 kD) to its cleaved form (62 kD) is markedly higher in TIMP3−/−-UUO kidneys. Averaged band densities for MMP9 and cleaved/pro-MMP2 from 5 zymograms are shown on the right. (B) TaqMan real-time PCR shows a significantly greater increase in MMP9 mRNA at 2 wk post-UUO in TIMP3−/− kidneys, whereas expression of urokinase plasminogen activator was not altered, and plasminogen activator inhibitor was significantly increased in both genotypes (n = 10 per group). MMP2 expression increased similarly in both genotypes, but MT1-MMP expression increased more significantly in TIMP3−/− kidneys at 2 wk post-UUO. (C) TIMP1 levels increased significantly at 3 d and 2 wk post-UUO in both genotypes, with a markedly greater increase in the wild-type (WT) group at 2 wk post-UUO. TIMP2 levels increased similarly in WT and TIMP3−/− mice, reaching statistical significance at 2 wk post-UUO (n = 10 per group). AU, arbitrary unit. *P < 0.05 compared with sham (Sh)-operated group; ‡P < 0.05 compared with corresponding WT group.
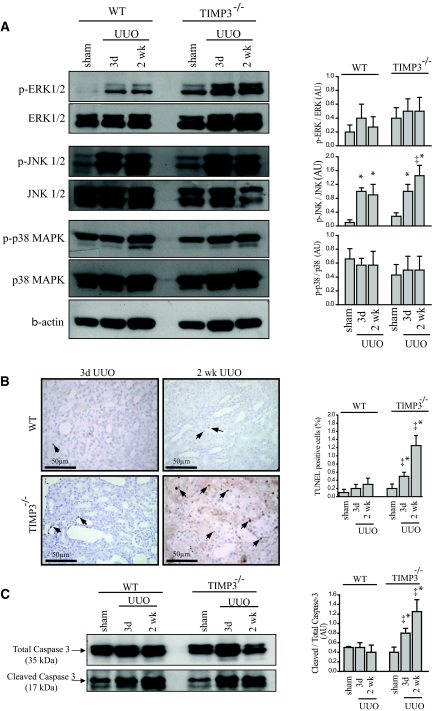
We then sought to determine the signaling pathway(s) underlying the exacerbated renal injury in TIMP3−/− kidneys. We examined the activation of the MAPKs, which have been shown to be involved in cellular events such as apoptosis, cell amplification, and survival in different tissues and disease models. For instance, activation of the JNK pathway is generally linked to triggered apoptosis,23,24, whereas extracellular activated kinase (ERK) activation has been associated with cell survival and amplification.25 Western blot analysis shows that the phospho-to-total ratio of c-Jun activated kinase (JNK)1/2 is increased at 3 d post-UUO in both genotypes, but continues to rise in the TIMP3−/− group to a significantly higher level than the WT group at 2 wk post-UUO (Figure 5A). Although there was an increase in phosphorylation of ERK1/2 in TIMP3−/−-UUO kidneys, the phospho-to-total ratio for ERK1/2 and p38 were comparable between genotypes (Figure 5A). Given the MAPK activation profile and the reported antiapoptotic role of TIMP3,26 we examined whether TIMP3-deficient kidneys exhibit an altered apoptotic response after UUO. TUNEL staining revealed a significantly larger population of apoptotic cells in the TIMP3−/− kidneys within 3 d of UUO, which increase further by 2 wk (Figure 5B). Consistently, cleavage of caspase 3, a molecular marker for apoptosis, was enhanced in TIMP3−/− kidneys, as evident from the higher levels of cleaved-to-total caspase 3 ratio at 3 d and 2 wks post-UUO (Figure 5C). These data suggest that TIMP3 deficiency accelerates apoptosis in response to tubulointerstitial injury.
Figure 5.
Increased activation of the maladaptive mitogen-activated protein kinase (MAPK) and enhanced apoptosis in tissue inhibitor of matrix metalloproteinases 3 (TIMP3)−/− mice after tubulointerstitial injury. (A) Western blots for phosphorylated and total extracellular-activated kinase (ERK) 1 and 2, c-Jun activated kinase (JNK) 1 and 2, p38, and phospho-to-total ratio for each MAPK are shown on the right. Phospho-to-total ratio for JNK1/2 shows a significant increase in TIMP3−/− compared with that of the wild-type (WT) group, but similar changes for ERK1/2 and p38 between the two genotypes after unilateral ureteral obstruction (UUO) (n = 5 per group). Phospho-to-total ratios for ERK1/2 and p38 show similar changes between the two genotypes after UUO. The right panels show the quantitative analysis for the western blots. (B) TUNEL staining shows a larger population of apoptotic cells in the TIMP3−/− kidneys within 3 d of UUO that amplifies by 2 wk. Arrows indicate TUNEL positive cells. (C) Western blots show cleaved and total caspase 3, a molecular marker for apoptosis. Cleaved-to-total ratio for caspase 3 is enhanced in TIMP3−/− kidneys, as evident from the higher levels of cleaved-to-total caspase 3 ratio at 3 d and 2 wks post-UUO (n = 5). AU, arbitrary unit; p, phosphorylated. *P < 0.05 compared with sham-operated group; ‡P < 0.05 compared with corresponding WT group.
TNFα Is a Key Player in the Enhanced Tubulointerstitial Injury in TIMP3-Deficient Mice
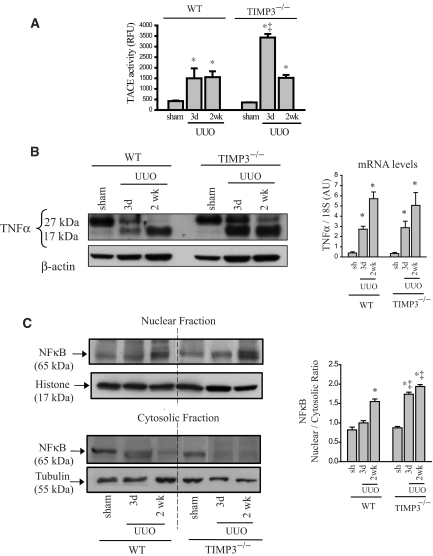
TIMP3 is the only physiologic inhibitor of TNFα-converting enzyme (TACE), the enzyme responsible for shedding of membrane-bound TNFα.27 Loss of TIMP3 can amplify TACE-mediated processing of membrane-bound TNFα into its soluble form,10,11,28 which then can stimulate its receptors, TNFR1/p55 and TNFR2/p75, triggering various tissue responses including apoptosis, which has been shown to result in increased tubulointerstitial injury.29–34 We found significantly higher TACE activity at 3 d post-UUO in TIMP3−/− kidneys (Figure 6A), which closely corresponds to markedly greater levels of soluble TNFα (17 kD) in TIMP3−/− compared with WT kidneys post-UUO, whereas TNFα mRNA levels increased similarly in both genotypes (Figure 6B). As a marker of TNFα pathway activation, we examined nuclear translocation of NFκB and found a significantly higher nuclear-to-cytosolic ratio of NFκB in TIMP3−/− at 3 d post-UUO (Figure 6C). To verify purity of our subcellular protein extraction, we blotted for histone as a marker of nuclear protein fraction, and tubulin as a marker of cytosolic fraction. To further demonstrate that increased TNFα levels can trigger cell death associated with activation of the JNK pathway, we treated cultured human renal proximal tubule epithelial cells with human recombinant TNF to demonstrate that elevated TNFα levels lead to early activation of the JNK pathway and trigger cell death as measured by cleaved caspase 3 (Supplemental Figures A and B). The increased activation of the TNFα pathway at the very early stage of disease suggests a key role for TNFα in mediating the severe tubulointerstitial injury in TIMP3-deficient mice.
Figure 6.
TNF-α is a key molecule mediating the severe renal injury in tissue inhibitor of matrix metalloproteinases 3 (TIMP3)−/− mice in response to unilateral ureteral obstruction (UUO). (A) Activity of TNFα-converting enzyme (TACE) is significantly increased in (TIMP3)−/− kidneys at 3 d post-UUO but comparable to WT-UUO at 2 wk post-UUO (n = 5). (B) Western blot for TNFα shows the membrane-bound (27 kD) and soluble TNFα (17 kD). Strikingly higher levels of soluble TNFα are detected in TIMP3−/− kidneys at 3 d post-UUO, suggesting enhanced processing of TNFα protein by TACE early after disease in these mice compared with WT (n = 5). TNFα mRNA production is increased similarly in TIMP3−/− and WT kidneys (n = 10 per group). (C) Western blots for NFκB (nuclear factor Kappa-β) on the nuclear and cytosolic fractions show greater nuclear translocation of NFκB in TIMP3−/−-UUO kidneys, as indicated by a higher nuclear-to-cytosolic ratio shown on the right (n = 5). Histone was used as a loading control for the nuclear fraction and tubulin for the cytosolic fraction. Averaged nuclear-to-cytosolic ratio from four blots is shown on the right. *P < 0.05 compared with corresponding sham group. RFU, relative fluorescence units.
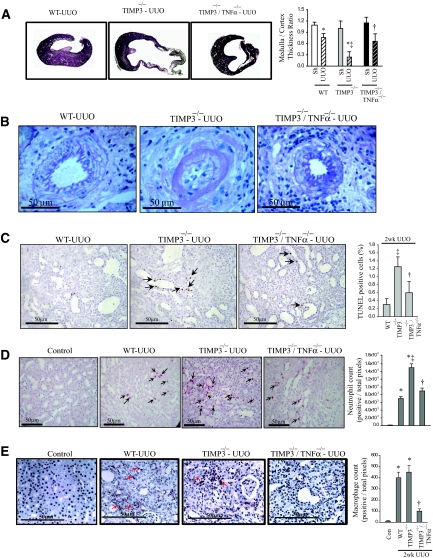
To determine the causal role of TNFα in this disease process, we generated double-knock-out mice lacking both TIMP3 and TNFα (TIMP3−/−/TNFα−/−) and subjected them to UUO. Additional deletion of TNFα in TIMP3−/− mice significantly attenuated the tubulointerestitial renal injury as TIMP3−/−/TNFα−/− mice exhibit a mild cortical and medullary thinning, and non-necrotic papillae post-UUO compared with TIMP3−/−-UUO mice (Figure 7A). In addition, marked vascular changes seen in the TIMP3−/−-UUO mice, such as diffuse arterial mural necrosis with focally associated luminal thrombi, were drastically improved in the TIMP3−/−/TNFα−/− mice, with only minimal changes noted, and without necrotizing lesions or thrombi (Figure 7B). Consistent with the proapoptotic and proinflammatory role of TNFα and the marked morphologic improvements seen in the double mutant mice, loss of TNFα also resulted in reduced apoptosis, neutrophil, and macrophage infiltration post-UUO (Figure 7, C–E).
Figure 7.
Loss of TNFα in tissue inhibitor of matrix metalloproteinases 3 (TIMP3)-deficient mice markedly improves renal injury, apoptosis, and inflammation after unilateral ureteral obstruction (UUO). (A) Marked improvement in cortical and medullary thinning in TIMP3−/−/TNFα−/− compared with TIMP3−/− kidneys after UUO (n = 6 per genotype). (B) Interlobular arteries show post-UUO disruption of normal vasculature in TIMP3−/− mice, which was largely prevented by the loss of TNFα. (C) TUNEL staining shows a drastic reduction in the degree of apoptosis in TIMP3−/−/TNFα−/− kidneys. (D) Immunostaining for neutrophils shows that the significantly greater infiltration in TIMP3−/−-UUO kidneys is markedly reduced in the TIMP3−/−/TNFα−/− kidneys, to levels comparable to WT-UUO. Neutrophil counts per heart are shown on the right. (E) Immunostaining for macrophages (F4/80) shows a similar post-UUO increase in macrophage population in wild-type (WT) and TIMP3−/− groups, which is clearly reduced in the TIMP3−/−/TNFα−/− kidneys. Averaged macrophage numbers per heart are shown on the right. Arrows indicate positive stainings. *P < 0.05 compared with sham-operated group; ‡P < 0.05 compared with corresponding WT-UUO group; †P < 0.05 compared with TIMP3−/− group.
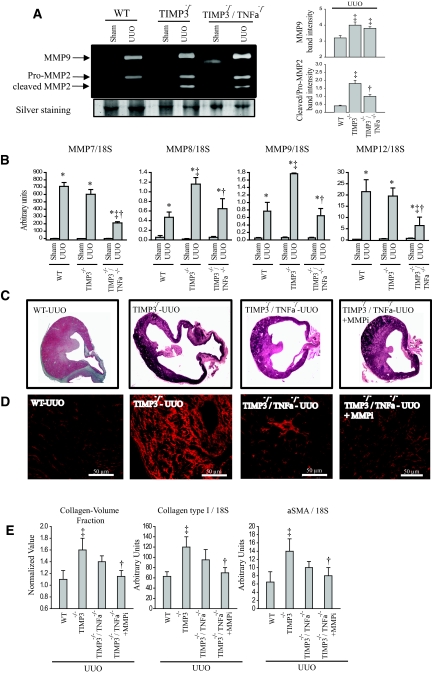
Molecular analysis of the double knock-out kidneys reveals reduced active MMP2 (62 kD) compared with TIMP3−/−, although it remains higher than in the WT-UUO group (Figure 8A). Expression analyses further revealed significant reductions in a number of MMPs such as MMP7, MMP8, MMP9, and MMP12 in the TIMP3−/−/TNFα−/− compared with TIMP3−/− kidneys (Figure 8B). MMP8 and MMP9 are produced by neutrophils35 and MMP12 by macrophages.36 Hence, the reduced levels of these MMPs are consistent with our finding of reduced neutrophil and macrophage infiltration TIMP3−/−/TNFα−/− mice.
Figure 8.
Inhibition of residual matrix metalloproteinases (MMP) activities in tissue inhibitor of MMP 3 (TIMP3)−/−/TNFα−/− mice further improves after unilateral ureteral obstruction (UUO) injury and fibrosis. (A) Gelatin zymography shows comparable levels of MMP9 and pro-MMP2, but reduced cleaved MMP2 in TIMP3−/−/TNFα−/−-UUO kidneys compared with TIMP3−/−-UUO kidneys, although still higher than WT-UUO group. Averaged band densities for five zymograms are shown on the right. (B) TaqMan real-time PCR analysis of candidate MMPs shows significant reductions in expression of MMP7, MMP8, MMP9, and MMP12 in the TIMP3−/−/TNFα−/− compared with TIMP3−/−-UUO. (C) Representative cross-sectional images of wild-type (WT), TIMP3−/−, TIMP3−/−/TNFα−/−, and TIMP3−/−/TNFα−/− + MMPi (PD166793, a broad-spectrum MMP inhibitor) show a clear improvement in the medullary thickness in the last group. (D) Picrosirius red (PSR) staining and confocal imaging show that tubulointerstitial fibrosis is significantly improved in the double-knock out kidneys and completely blocked with additional MMP inhibition. Collagen-volume fraction (calculated on the basis of the PSR-stained section), production of collagen type I and α-SMA, and markers of fibrosis at 2wk post-UUO are significantly reduced in TIMP3−/−/TNFα−/− + MMPi compared with TIMP3−/− kidneys. *P < 0.05 compared with sham-operated group; ‡P < 0.05 compared with corresponding WT group. †P < 0.05 compared with TIMP3−/− group.
Because TIMP3−/−/TNFα−/−-UUO kidneys exhibited higher MMP2 activation compared with the WT-UUO, and greater levels of other MMPs compared with the sham groups, we examined whether inhibition of MMPs could further improve the kidney injury in TIMP3−/−/TNFα−/− mice. TIMP3−/−/TNFα−/− mice received PD166793, a broad-spectrum MMP inhibitor that does not inhibit TACE, by daily gavage as before.37 We found that MMP inhibition further improves post-UUO renal injury, as shown by gross morphology (Figure 8C), and also restores renal fibrosis to the WT levels, with reduced levels of collagen type I and αSMA, two indicators of tissue fibrosis (Figure 8D). These data collectively indicate that the severe tubulointerstitial injury in the absence of TIMP3 is brought about by a combination of elevated MMPs with enhanced and accelerated activity of the TNFα pathway, which together modulate cellular responses such as apoptosis, inflammation, and fibrosis.
Increased TIMP3 Levels in Human Kidney Disease
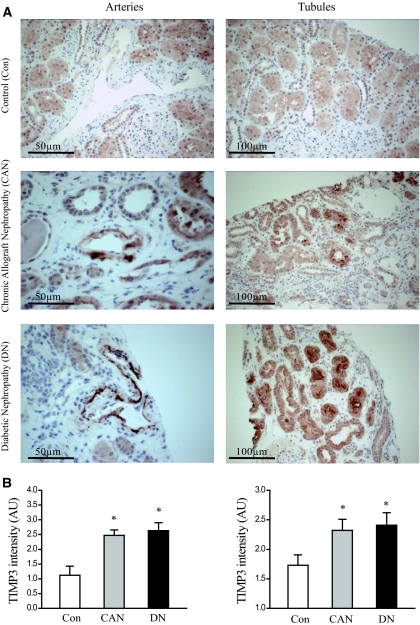
To determine the role of TIMP3 in patients with different types of nephropathies, we examined TIMP3 levels in patients with kidney diseases. We compared the core transplant biopsies from control patients with healthy kidneys and no morphologic abnormalities (Con; n = 9; six men/three women, age = 57 ± 5 yr), patients with diabetic nephropathy that showed glomerulosclerosis (DN; n = 10; six men/four women, age = 61 ± 7 yr), and patients with chronic allographt nephropathy (CAN; n = 10; six men/four women, age = 53 ± 6 yr). Immunohistochemical staining for TIMP3 protein was graded by an experienced renal pathologist (A.M.H.) in a blinded manner. Staining was graded from 0 to 3+ in proximal tubules and arteries. In all biopsies examined, TIMP3 was most prominently expressed in the proximal tubules, with accentuation along the luminal brush border of the proximal tubular epithelial cells (Figure 9A). Proximal tubular brush border staining was significantly more intense in DN biopsies (2.41 ± 0.83) and CAN biopsies (2.32 ± 0.68) compared with Con biopsies (1.73 ± 0.65, P < 0.05) (Figure 7B). Vascular staining was present primarily along the endothelium, in the intima, and along the internal elastic lamina (Figure 7A). Arteriolar and arterial staining was identified primarily in DN (2.63 ± 0.54) and CAN (2.47 ± 0.38) biopsies, and identified only focally in few cases of Con biopsies (1.12 ± 0.82, P < 0.05) (Figure 7B). These results show that in human kidney disease TIMP3 is up-regulated primarily in the proximal tubular and vascular compartments, possibly as a protective mechanism aimed at minimizing renal damage.
Figure 9.
Increased tissue inhibitor of matrix metalloproteinases 3 (TIMP3) expression in human kidney disease based on immunohistochemical staining in human kidney biopsies. (A) Increased TIMP3 staining in kidney biopsy samples from patients with diabetic nephropathy (DN) (n = 10) and chronic allograft nephropathy (CAN) (n = 10) compared with controls (Con) (n = 9) particularly confined to the proximal tubular and vascular compartments. (B) Averaged semiquantitative analysis shows significantly increased TIMP3 staining in kidney biopsies from patients with type 2 diabetes mellitus and chronic allograft nephropathy. *P < 0.05 compared with the control group.
DISCUSSION
Whereas obstruction of the urinary tract results primarily in alterations in the tubulointerstitial components of the kidney such as tubular dilation, apoptotic tubular cell deletion, and fibrosis,38 tubulointerstitial injury occurs in a wide variety of diseases including diabetic nephropathy, chronic allograft nephropathy, and inflammatory kidney diseases. Tubulointerstitial inflammation and fibrosis are important prognostic markers of an adverse outcome in patients with a wide variety of kidney diseases.39,40 Interstitial fibrosis involves inflammatory cell infiltration; fibroblast proliferation; and an imbalance in synthesis, deposition, and degradation of ECM components. The key enzymes in regulating ECM integrity are MMPs and their endogenous inhibitors, TIMPs, which have been reported to play key roles in renal pathophysiology.16,17,41
In this study, we show that TIMP3 deficiency influences the UUO-induced alterations in induction and activation of MMPs. For instance, activation of MMP2 occurs via the formation of a trimolecular complex composed of MT1-MMP, TIMP2, and pro-MMP-2. We have shown that TIMP3 exerts an inhibitory effect on this process,42 which explains the greater levels of cleaved 62 kD MMP2 in TIMP3-deficient kidneys. Indeed, this augmented MMP2 activation in TIMP3−/− kidneys occurs in the absence of an increase in MMP2 mRNA levels. Enhanced MMP activation has been linked to progressive chronic kidney damage and tubulointerstitial fibrosis.42 Although increased fibrosis in the face of enhanced MMP activity may seem counterintuitive, it has been reported that MMPs can activate the TGFβ1 complex, which subsequently activates fibroblasts and triggers collagen synthesis.44–46 The causal role of MMPs in tubulointerstitial fibrosis in our model is clearly demonstrated, because treatment with MMPi completely blocked renal fibrosis in TIMP3−/−/TNF−/−-UUO mice.
In the absence of TIMP3, UUO results in a significantly greater fibroblast amplification and collagen synthesis, leading to markedly more severe interstitial fibrosis. We further determined that early up-regulation of TNFα signaling and activation of a number of MMPs after induction of UUO in TIMP3−/− kidneys trigger interstitial inflammatory infiltration that further contributes to apoptosis and interstitial fibrosis. This is consistent with previous reports linking inflammatory infiltration to cytokine release, ECM imbalance, and fibrosis.2,47,48 TNFα plays an important role in mediating renal fibrosis and dysfunction in response to UUO31,33 and chemotherapy-induced nephropathy.49 TNFα-induced injury is mediated via both its receptors, TNFR1 and TNFR2, with clear evidence for a proapoptotic role in renal tissue.33,49,50 In this study we identify TIMP3 as a critical negative regulator of renal TNFα activity. In the absence of the negative regulatory function of TIMP3, TNFα further triggers apoptosis and inflammation, as also reported in a wide range of pathophysiological conditions.49 In addition, the renal vasculature showed prominent pathologic changes in the TIMP3-deficient mice in a TNFα-dependent manner. TIMP3 has previously been shown to be involved in kidney injury by regulating tissue fibrosis.41 In this study we demonstrate that increased apoptosis in TIMP3 deficient kidneys preceded the development of marked interstitial fibrosis, suggesting that the latter may in part be a secondary process. Although we have shown a clear role of TNFα in mediating the renal injury, we cannot rule out a contributing role of other renal injury-inducing agonists such as TGFα.32,51
Collectively, our data reveal a mechanistic dual role of TIMP3 in kidney disease, as a negative regulator of TACE/ADAM-17 and MMP axes. The pathophysiological importance of TIMP3 is further supported by the observation that TIMP1-null mice showed no differential response to unilateral ureteral obstruction12 and overload proteinuria16 compared with WT mice. This could be due to the early and marked up-regulation of TIMP3 coupled with high constitutive expression of TIMP3 in these mice.12,16 A role for TIMP3 is supported by our observations in human kidney biopsies, in which TIMP3 protein levels are elevated in patients with diabetic and chronic allographt nephropathy. The initial rise in TIMP3 levels followed by its marked reduction in our mouse UUO model is clearly associated with tubulointerstitial and vascular damage in the murine kidney, and so it is tempting to speculate that the up-regulation of TIMP3 in the proximal tubules and renal arteries in human kidney disease may be a compensatory change required to minimize renal damage and disease progression. Our results demonstrate an important role for TIMP3 in renal injury, thus enhancing TIMP3 effects could serve as a potential therapeutic strategy for the amelioration of renal tubulointerstitial injury.
CONCISE METHODS
Experimental Animals and Human Kidney Biopsies
TIMP3−/− mice were generated as described previously,52 and TNFα−/− mice were purchased from Jackson Laboratories. The TIMP3−/−/TNFα−/− double knock-out mice were generated in our laboratory. All mice are from a pure C57BL/6 background. All animal experiments were performed in accordance with the Institutional Guidelines of University of Alberta and the Canadian Council on Animal Care.
Renal biopsies were performed as part of routine clinical diagnostic investigation. Informed consent was obtained from patients for use of archived human biopsy material and chart review in accord with institutional research ethics board review.
Unilateral Ureteral Obstruction
Ten- to 12-week-old male wild-type (WT), TIMP3−/− (TIMP3 deficient) and TIMP3−/−/TNFα−/− (double knockout) mice were subjected to UUO. Mice were anesthetized, an incision was made in the right side of the back, and the right proximal ureter was exposed and double-ligated, while renal nerves and vascular supply remained intact. After UUO, a group of TIMP3−/−/TNFα−/− mice received 25mg/kg/d of a broad-spectrum MMP inhibitor (MMPi, PD166793) by daily gavage as before.10 Sham-operated control mice from each genotype underwent the exact same surgical procedure without ligation of the ureter. Measurement of tail-cuff systolic BP plasma, creatinine, and urine protein electrophoresis for albuminuria were carried out as described previously.10,53 Ipsilateral kidneys were fixed in 10% formalin for immunohistochemical analysis, or flash-frozen in liquid nitrogen for further RNA and DNA analyses. No morphologic differences were observed in the contralateral kidneys between genotypes.
Immunohistochemistry, Light and Confocal Microscopy
Periodic acid Schiff (PAS) staining, immunostaining for collagen I and α-smooth muscle actin, and quantification using Image Pro Plus (Media Cybernetics, Crofton, MA) computer image analysis software were carried out as described previously.54 Ten-micrometer sections from fixed kidneys were stained with Picrosirius red (PSR) and visualized using two-photon confocal microscopy (Zeiss LSM 510 META NLO). Neutrophils were stained using rat anti-neutrophil antibody (AbD Serotec, Raleigh, NC), and macrophages were stained using F4/80 staining, and quantified using Image-Pro plus software. Apoptosis was assessed by terminal deoxynucleotidyl transferase mediated end-labeling (TUNEL) using the ApopTag Plus kit (Intergen, Purchase, NY). TUNEL staining was quantified by counting positive nuclei per mouse kidney section. Immunohistochemical staining for human TIMP3 in kidney biopsy samples was carried out as described previously using an anti-human TIMP3 antibody from Chemicon (Temecula, CA).15 For each staining, four fields per section, two sections per kidney, and five kidneys per group were examined unless otherwise stated.
Zymography, Activity Assay, Western Blot Analysis, and TaqMan RT-PCR Analysis
Latent and cleaved MMP2 and MMP9 were detected by gelatin zymography. TACE activity was measured using a fluorometric assay kit (Innozyme from Calbiochem) according to the manufacturer's instructions. Western blot analysis for TIMP3 was performed on cortex and medulla tissue after microdissection of WT kidneys, using anti-TIMP3 antibody (Chemicon), and blotting for membrane-bound and soluble TNFα was performed as before.10 Nuclear and cytosolic protein was extracted using an extraction kit from PIERCE. Urine protein electrophoresis for albuminuria was performed as before.53 TaqMan real-time analysis was used to measure alterations in mRNA levels for the indicated genes, values were normalized by 18S levels as the internal control as before.10
Statistical Analysis
Comparisons between two groups were performed using the t test while comparisons involving more than two groups were performed by ANOVA followed by multiple comparison testing (Student-Neuman Keuls). Values are reported as mean ± SEM. Statistical significance is recognized at P < 0.05.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by Canadian Institute of Health Research Grant84279 to Z.K. and by a Canadian Diabetes Association grant to J.W.S., G.Y.O., and A.M.H. Z.K. is a New Investigator of Heart and Stroke Foundation of Canada, and G.Y.O. is a Clinician-Investigator of the Alberta Heritage Foundation for Medical Research.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Remuzzi G, Bertani T: Pathophysiology of progressive nephropathies. N Engl J Med 339: 1448–1456, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Eddy AA: Molecular basis of renal fibrosis. Pediatr Nephrol 15: 290–301, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Schrier RW: Cancer therapy and renal injury. J Clin Invest 110: 743–745, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moe OW: Kidney stones: Pathophysiology and medical management. Lancet 367: 333–344, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR: Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett 563: 129–134, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knauper V, Docherty AJ, Murphy G: TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett 435: 39–44, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Loechel F, Fox JW, Murphy G, Albrechtsen R, Wewer UM: ADAM 12-S cleaves IGFBP-3 and IGFBP-5 and is inhibited by TIMP-3. Biochem Biophys Res Commun 278: 511–515, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Kashiwagi M, Tortorella M, Nagase H, Brew K: TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J Biol Chem 276: 12501–12504, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Leco KJ, Waterhouse P, Sanchez OH, Gowing KL, Poole AR, Wakeham A, Mak TW, Khokha R: Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3). J Clin Invest 108: 817–829, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassiri Z, Oudit GY, Sanchez O, Dawood F, Mohammed FF, Nuttall RK, Edwards DR, Liu PP, Backx PH, Khokha R: Combination of tumor necrosis factor-alpha ablation and matrix metalloproteinase inhibition prevents heart failure after pressure overload in tissue inhibitor of metalloproteinase-3 knock-out mice. Circ Res 97: 380–390, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Mohammed FF, Smookler DS, Taylor SE, Fingleton B, Kassiri Z, Sanchez OH, English JL, Matrisian LM, Au B, Yeh WC, Khokha R: Abnormal TNF activity in Timp3-/- mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet 36: 969–977, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Oda T, Lopez-Guisa J, Wing D, Edwards DR, Soloway PD, Eddy AA: TIMP-1 deficiency does not attenuate interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 12: 736–748, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Butler DG, Zhang DH, Villadiego R, Oudit GY, Youson JH, Cadinouche MZ: Response by the corpuscles of Stannius to hypotensive stimuli in three divergent ray-finned fishes (Amia calva, Anguilla rostrata, and Catastomus commersoni): Cardiovascular and morphological changes. Gen Comp Endocrinol 132: 198–208, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Kernan KA, Collins SJ, Cai X, Lopez-Guisa JM, Degen JL, Shvil Y, Eddy AA: Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: Role of plasmin-activated signals. J Am Soc Nephrol 18: 846–859, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Legallicier B, Trugnan G, Murphy G, Lelongt B, Ronco P: Expression of the type IV collagenase system during mouse kidney development and tubule segmentation. J Am Soc Nephrol 12: 2358–2369, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Eddy AA, Kim H, Lopez-Guisa J, Oda T, Soloway PD: Interstitial fibrosis in mice with overload proteinuria: Deficiency of TIMP-1 is not protective. Kidney Int 58: 618–628, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Catania JM, Chen G, Parrish AR: Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Renal Physiol 292: F905–F911, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A: Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112: 1486–1494, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rysz J, Banach M, Stolarek RA, Pasnik J, Cialkowska-Rysz A, Koktysz R, Piechota M, Baj Z: Serum matrix metalloproteinases MMP-2 and MMP-9 and metalloproteinase tissue inhibitors TIMP-1 and TIMP-2 in diabetic nephropathy. J Nephrol 20: 444–452, 2007 [PubMed] [Google Scholar]
- 20.Ronco P, Lelongt B, Piedagnel R, Chatziantoniou C: Matrix metalloproteinases in kidney disease progression and repair: A case of flipping the coin. Semin Nephrol 27: 352–362, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Kim H, Cai X, Lopez-Guisa JM, Alpers CE, Liu Y, Carmeliet P, Eddy AA: Urokinase receptor deficiency accelerates renal fibrosis in obstructive nephropathy. J Am Soc Nephrol 14: 1254–1271, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Atkinson SJ, Crabbe T, Cowell S, Ward RV, Butler MJ, Sato H, Seiki M, Reynolds JJ, Murphy G: Intermolecular autolytic cleavage can contribute to the activation of progelatinase A by cell membranes. J Biol Chem 270: 30479–30485, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Hauser P, Oberbauer R: Tubular apoptosis in the pathophysiology of renal disease. Wien Klin Wochenschr 114: 671–677, 2002 [PubMed] [Google Scholar]
- 24.Shastry S, Ingram AJ, Scholey JW, James LR: Homocysteine induces mesangial cell apoptosis via activation of p38-mitogen-activated protein kinase. Kidney Int 71: 304–311, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Baba M, Furihata M, Hong SB, Tessarollo L, Haines DC, Southon E, Patel V, Igarashi P, Alvord WG, Leighty R, Yao M, Bernardo M, Ileva L, Choyke P, Warren MB, Zbar B, Linehan WM, Schmidt LS: Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J Natl Cancer Inst 100: 140–154, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fata JE, Chaudhary V, Khokha R: Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta-estradiol during the estrous cycle. Biol Reprod 65: 680–688, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Zheng Y, Saftig P, Hartmann D, Blobel C: Evaluation of the contribution of different ADAMs to tumor necrosis factor alpha (TNFalpha) shedding and of the function of the TNFalpha ectodomain in ensuring selective stimulated shedding by the TNFalpha convertase (TACE/ADAM17). J Biol Chem 279: 42898–42906, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Federici M, Hribal ML, Menghini R, Kanno H, Marchetti V, Porzio O, Sunnarborg SW, Rizza S, Serino M, Cunsolo V, Lauro D, Mauriello A, Smookler DS, Sbraccia P, Sesti G, Lee DC, Khokha R, Accili D, Lauro R: Timp3 deficiency in insulin receptor-haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-alpha. J Clin Invest 115: 3494–35505, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo G, Morrissey J, McCracken R, Tolley T, Liapis H, Klahr S: Contributions of angiotensin II and tumor necrosis factor-alpha to the development of renal fibrosis. Am J Physiol Renal Physiol 280: F777–F785, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Misseri R, Meldrum DR, Dinarello CA, Dagher P, Hile KL, Rink RC, Meldrum KK: TNF-alpha mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am J Physiol Renal Physiol 288: F406–F411, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Meldrum KK, Misseri R, Metcalfe P, Dinarello CA, Hile KL, Meldrum DR: TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am J Physiol Regul Integr Comp Physiol 292: R1456–R1464, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F: Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Guo G, Morrissey J, McCracken R, Tolley T, Klahr S: Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. Am J Physiol 277: F766–F772, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Klahr S, Morrissey J: Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Owen CA, Hu Z, Lopez-Otin C, Shapiro SD: Membrane-bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metal loproteinase-resistant collagenase and serpinase. J Immunol 172: 7791–7803, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Nenan S, Boichot E, Lagente V, Bertrand CP: Macrophage elastase (MMP-12): A pro-inflammatory mediator? Mem Inst Oswaldo Cruz 100 [Suppl 1]: 167–172, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Peterson JT, Hallak H, Johnson L, Li H, O'Brien PM, Sliskovic DR, Bocan TM, Coker ML, Etoh T, Spinale FG: Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation 103: 2303–2309, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Misseri R, Rink RC, Meldrum DR, Meldrum KK: Inflammatory mediators and growth factors in obstructive renal injury. J Surg Res 119: 149–159, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Henger A, Schmid H, Kretzler M: Gene expression analysis of human renal biopsies: Recent developments towards molecular diagnosis of kidney disease. Curr Opin Nephrol Hypertens 13: 313–318, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Nangaku M: Mechanisms of tubulointerstitial injury in the kidney: Final common pathways to end-stage renal failure. Intern Med 43: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Kawamoto H, Yasuda O, Suzuki T, Ozaki T, Yotsui T, Higuchi M, Rakugi H, Fukuo K, Ogihara T, Maeda N: Tissue inhibitor of metalloproteinase-3 plays important roles in the kidney following unilateral ureteral obstruction. Hypertens Res 29: 285–294, 2006 [DOI] [PubMed] [Google Scholar]
- 42.English JL, Kassiri Z, Koskivirta I, Atkinson SJ, Di Grappa M, Soloway PD, Nagase H, Vuorio E, Murphy G, Khokha R: Individual Timp deficiencies differentially impact pro-MMP-2 activation. J Biol Chem 281: 10337–10346, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH: Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J 20: 1898–1900, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Karsdal MA, Larsen L, Engsig MT, Lou H, Ferreras M, Lochter A, Delaisse JM, Foged NT: Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem 277: 44061–44067, 2002 [DOI] [PubMed] [Google Scholar]
- 46.George SJ, Johnson JL, Smith MA, Angelini GD, Jackson CL: Transforming growth factor-beta is activated by plasmin and inhibits smooth muscle cell death in human saphenous vein. J Vasc Res 42: 247–254, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Diamond JR, Kees-Folts D, Ding G, Frye JE, Restrepo NC: Macrophages, monocyte chemoattractant peptide-1, and TGF-beta 1 in experimental hydronephrosis. Am J Physiol 266: F926–F933, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Diamond JR, Ricardo SD, Klahr S: Mechanisms of interstitial fibrosis in obstructive nephropathy. Semin Nephrol 18: 594–602, 1998 [PubMed] [Google Scholar]
- 49.Ramesh G, Reeves WB: TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Messmer UK, Winkel G, Briner VA, Pfeilschifter J: Suppression of apoptosis by glucocorticoids in glomerular endothelial cells: Effects on proapoptotic pathways. Br J Pharmacol 129: 1673–1683, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dell KM, Nemo R, Sweeney WE, Jr., Levin JI, Frost P, Avner ED: A novel inhibitor of tumor necrosis factor-alpha converting enzyme ameliorates polycystic kidney disease. Kidney Int 60: 1240–1248, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Higuchi M, Yasuda O, Kawamoto H, Yotsui T, Baba Y, Ozaki T, Maeda N, Fukuo K, Rakugi H, Ogihara T: Tissue inhibitor of metalloproteinase-3 deficiency inhibits blood pressure elevation and myocardial microvascular remodeling induced by chronic administration of Nomega-nitro-L-arginine methyl ester in mice. Hypertens Res 30: 563–571, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, Crackower MA, Backx PH, Penninger JM, Scholey JW: Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol 168: 1808–1820, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW: Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol 171: 438–451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.