Abstract
Cyclosporine A (CsA) is a substrate of P-glycoprotein, an efflux transporter encoded by the ABCB1 gene. Compared with carriers of the wild-type gene, carriers of T allelic variants in exons 21 or 26 have reduced P-glycoprotein activity and, secondarily, increased intracellular concentration of CsA; therefore, carriers of T variants might be at increased risk for CsA-related adverse events. We evaluated the associations between ABCB1 genotypes (in exons 12, 21, and 26) and CsA-related outcomes in 147 renal transplant recipients who were receiving CsA-based immunosuppression and were included in the Mycophenolate Steroids Sparing study. During a median of 65.5 mo follow-up, carriers of T allelic variants in exons 21 or 26 had a three-fold risk for delayed graft function (DGF), a trend to slower recovery of renal function and lower GFR at study end, and significantly higher incidences of new-onset diabetes and cytomegalovirus reactivation compared with carriers of the wild-type genotype. T variants in both exons 21 and 26 were independently associated with 3.8- and 3.5-fold higher risk for DGF, respectively (P = 0.022 and P = 0.034). The incidence of acute rejection and the mean CsA dose and blood levels were comparable in genotype groups. In conclusion, renal transplant recipients with T allelic variants in ABCB1 exons 21 or 26 are at increased risk for CsA-related adverse events. Genetic evaluation may help to identify patients at risk and to modulate CsA therapy to optimize graft and patient outcomes.
The introduction of cyclosporine A (CsA) therapy in the early 1980s opened a new era in organ transplantation. Compared with steroid- and azathioprine-based regimens, immunosuppressive protocols including this inhibitor of calcineurin—a key enzyme involved in T cell activation1—decreased the incidence of acute rejections from 40% to 50% to 20% to 30% and increased one-year survival rates of the grafts from 60% to between 80% and 90%.2 Thirty years later, CsA remains a cornerstone of immunosuppressive therapy for recipients of both renal and nonrenal transplants worldwide. However, standard recommended doses are associated with nephrotoxicity, resulting in delayed graft function (DGF) and progressive renal function deterioration in the long term.3,4 Moreover, CsA worsens glucose tolerance and lipid profile; increases systemic BP; and, similarly to other immunosuppressants, enhances the risk of opportunistic infections, lymphoproliferative disorders, and cancer.1,5
To minimize side effects without increasing the risk of rejection, treatment is titrated to target CsA blood levels according to well established guidelines.6 However, the therapeutic index remains narrow, whereas the frequency and severity of CsA-related adverse effects are considerably variable among patients, even at comparable CsA levels.1,6 This suggests the possibility that a heterogeneous individual susceptibility may result in increased risk in some patients despite exposure to CsA levels that in the majority of cases are devoid of significant toxicity.6 Thus, identifying markers or predictors of individual response to CsA therapy might help to tailor CsA therapy and optimize the risk/benefit profile of CsA-based immunosuppression.
Drug efficacy and tolerability are influenced by several factors, including the activity of proteins and enzymes involved in drug transport and metabolism.7 CsA is a substrate of an efflux transporter—the P-glycoprotein (P-gp) encoded by the multidrug resistance-1 gene (now referred as ABCB1)—which actively transports lipophilic drugs and other xenobiotics from the intracellular to the extracellular domain.8 This transporter is expressed in lymphocytes,9 and in other leukocytes,10 as well as in hepatocytes and on the brush border of enterocytes and proximal tubular cells.8 Reduced expression or functional inhibition of this efflux-pump invariably results in increased intracellular and tissue drug concentrations,8,9 but may have unpredictable effects on CsA blood levels. Indeed, CsA blood levels increased, decreased, or did not change in different settings, likely because of different balances between enhanced distribution into the tissue compartment and decreased excretion into the gastrointestinal lumen or urinary tract.7 Increased CsA concentrations in lymphocytes,11 polymorphonuclear cells,12 and other circulating leukocytes13 have been associated with increased production and release of reactive oxygen species (ROS). ROS production by leukocytes is the primary defense against invading micro-organisms, but has also been involved in the pathogenesis of ischemia-reperfusion damage of engrafted tissues or organs.3,14 Thus, increased intracellular CsA disposition with enhanced ROS production might amplify oxidative stress and tissue damage to the graft after reperfusion.15 Increased intralymphocyte drug concentration is also associated with more effective inhibition of lymphocyte proliferation by CsA in vitro,16 an effect that, in vivo, might translate into more effective protection against graft rejection,17 but also into excess risk of opportunistic infections, lymphoproliferative disorders, or cancer.
P-glycoprotein expression and activity are reduced in carriers of one or two T allelic variants in exons 12, 21, and 26 as compared with carriers of the wild-type ABCB1 gene.8,18,19 Thus, at comparable CsA exposure, carriers of the allelic variants are expected to have higher intracellular and tissue CsA levels than wild-type carriers and, conceivably, should be exposed to more, and more severe, CsA-related events. We formally tested this hypothesis in a large cohort of renal transplant recipients prospectively monitored in the setting of a randomized, controlled, clinical trial, the Mycophenolate Steroids Sparing (MYSS) study, which aimed to compare the risk/benefit profile of mycophenolate mofetil and azathioprine therapy in immunosuppressive regimens including the CsA microemulsion Neoral.20
RESULTS
Donor and Recipient Characteristics at Inclusion
One hundred forty-seven of the 336 MYSS patients consented to enter the present study. Gender distribution and weight of recipients and corresponding donors at the time of transplantation in different considered ABCB1 genotype groups were similar, as was the distribution of primary renal diseases in recipients, the percentage of B cell negative cross-matches, the number of HLA mismatches, allocation to mycophenolate mofetil or azathioprine treatment, and cold/warm ischemia times (Table 1). However, recipients carrying the allelic “T” variants in exons 21 and 26 happened to be significantly older and to receive the graft from older donors compared with those with the wild-type genotypes, whereas no differences were observed between the exon 12 genotypes (Table 1). Baseline characteristics of patients considered in the present study were similar to those observed in the general population of patients included in the MYSS trial. Moreover, all of the considered safety and efficacy outcome variables were independent of patient randomization to MMF or azathioprine therapy, as well as of steroid discontinuation (Table 2).
Table 1.
Patient/donor characteristics according to SNPs in the exon 12, 21 and 26 of the ABCB1 gene
| Characteristic | Exon 12
|
Exon 21a
|
Exon 26
|
|||
|---|---|---|---|---|---|---|
| CC (n = 54) | CT/TT(n = 93) | GG (n = 54) | GT/TT(n = 92) | CC(n = 52) | CT/TT(n = 95) | |
| Recipients | ||||||
| men | 33 (61.1) | 61 (65.6) | 33 (61.1) | 61 (66.3) | 31 (59.6) | 63 (66.3) |
| age (years) | 41.6 (12.3) | 45.1 (11.8) | 40.6 (12.4) | 45.6 (11.5)b | 39.0 (11.9) | 46.5 (11.3)c |
| weight (kg) | 65.6 (11.7) | 67.2 (11.5) | 65.6 (11.9) | 67.3 (11.4) | 64.7 (12.8) | 67.7 (10.7) |
| height (cm) | 168 (9.0) | 168.4 (8.9) | 167.9 (8.9) | 168.4 (9.0) | 167.4 (9.2) | 168.7 (8.9) |
| Serology | ||||||
| CMV positive | 37 (68.5) | 75 (81.5) | 40 (74.4) | 71 (78.0) | 37 (72.5) | 75 (78.9) |
| hepatitis B positive | 4 (7.5) | 7 (7.6) | 4 (7.5) | 7 (7.7) | 3 (5.9) | 8 (8.5) |
| hepatitis C positive | 5 (9.4) | 6 (6.5) | 5 (9.4) | 6 (6.6) | 4 (7.8) | 7 (7.4) |
| Primary disease | ||||||
| diabetes mellitus | 1 (1.8) | 0 (0) | 1 (1.8) | 0 (0) | 1 (1.9) | 0 (0) |
| hypertension | 2 (3.7) | 5 (5.4) | 2 (3.7) | 5 (5.4) | 1 (1.9) | 6 (6.3) |
| glomerulonephritis | 23 (45.6) | 43 (46.2) | 24 (44.4) | 42 (45.7) | 24 (46.1) | 42 (44.2) |
| polycystic kidney disease | 3 (5.6) | 10 (10.7) | 3 (5.6) | 9 (9.8) | 3 (5.8) | 10 (10.5) |
| pyelonphritis | 9 (16.7) | 10 (10.7) | 8 (14.8) | 11 (12.0) | 9 (17.3) | 10 (10.5) |
| other | 5 (9.3) | 5 (5.4) | 5 (9.3) | 5 (5.4) | 5 (9.6) | 5 (5.3) |
| uncertain | 11 (20.4) | 20 (21.5) | 11 (20.4) | 20 (21.7) | 9 (17.3) | 22 (23.2) |
| Donors | ||||||
| men | 31 (57.4) | 49 (52.7) | 35 (64.8) | 45 (48.9) | 33 (63.5) | 47 (49.5) |
| age (years) | 40.0 (16.7) | 43.6 (15.0) | 37.8 (16.6) | 44.8 (14.7)c | 36.7 (15.7) | 45.4 (14.9)c |
| weight (kg) | 68.3 (9.8) | 69.9 (11.6) | 68.3 (9.4) | 69.7 (11.8) | 67.8 (8.9) | 70.2 (11.9) |
| height (cm) | 169.8 (8.4) | 168.7 (8.2) | 170.3 (8.3) | 168.4 (8.3) | 169.4 (8.7) | 168.9 (8.0) |
| Serology | ||||||
| CMV positive | 38 (70.4) | 62 (66.7) | 35 (64.8) | 64 (69.6) | 35 (67.3) | 65 (68.4) |
| hepatitis B positive | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| hepatitis C positive | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| B-cell crossmatch | ||||||
| negative | 54 (100) | 91 (98.9) | 54 (100) | 90 (98.9) | 52 (100) | 93 (98.9) |
| positive | 0 (0) | 1 (1.1) | 0 (0) | 1 (1.1) | 0 (0) | 1 (1.1) |
| not done | 0 (0) | 1 (1.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HLA mismatches | ||||||
| 0 | 1 (1.8) | 4 (4.3) | 1 (1.8) | 4 (4.3) | 0 (0) | 5 (5.3) |
| 1 | 13 (24.1) | 23 (24.7) | 11 (20.4) | 24 (26.1) | 11 (21.1) | 25 (26.3) |
| 2 | 27 (50.0) | 45 (48.4) | 27 (50.0) | 45 (48.9) | 28 (53.9) | 44 (46.3) |
| 3 | 13 (24.1) | 21 (22.6) | 15 (27.8) | 19 (20.7) | 13 (25.0) | 21 (22.1) |
| Treatment | ||||||
| mycophenolate mofetil | 30 (55.6) | 42 (45.2) | 31 (57.4) | 41 (44.6) | 28 (53.9) | 44 (46.3) |
| azathioprine | 24 (44.4) | 51 (54.8) | 23 (42.6) | 51 (55.4) | 24 (46.1) | 51 (53.7) |
| Cold ischemia (h) | 18.4 (6.9) | 17.5 (7.3) | 18.1 (6.9) | 17.7 (7.3) | 18.1 (7.0) | 17.7 (7.2) |
| Warm ischemia (min) | 40.6 (16.9) | 40.7 (16.7) | 40.4 (16.7) | 40.8 (16.9) | 41.9 (17.0) | 39.8 (16.6) |
Data are shown as number (percentage), except for ischemia time, which is mean (SE).
One patient was not characterized for exon 21.
P < 0.05,
P < 0.01 vs GG (exon 21) or CC (exon 26) genotype.
Table 2.
Patients with events according to immunosuppressive regimen concomitant with cyclosporine A
| Overall
|
Patients who completed steroid withdrawal
|
Patients who did not complete steroid withdrawal
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Azathioprine (n = 75) | MMF (n = 72) | P | Azathioprine (n = 50) | MMF (n = 47) | P | Azathioprine (n = 25) | MMF (n = 25) | P | |
| Delayed graft function | 17 (22.7) | 16 (22.2) | 0.95 | 12 (24.0) | 9 (19.2) | 0.56 | 5 (20.0) | 7 (28.0) | 0.51 |
| Proteinuria | 19 (25.3) | 19 (26.4) | 0.88 | 14 (28.0) | 11 (23.4) | 0.60 | 5 (20.0) | 8 (32.0) | 0.33 |
| Diabetes | 14 (18.7) | 10 (13.9) | 0.43 | 11 (22.0) | 4 (8.5) | 0.07 | 3 (12.0) | 6 (24.0) | 0.46 |
| Rejection | 43 (57.3) | 30 (41.7) | 0.06 | 27 (54.0) | 17 (36.2) | 0.08 | 16 (64.0) | 13 (52.0) | 0.39 |
| Graft loss | 3 (4.0) | 3 (4.2) | 0.99 | 1 (2.0) | 0 (0) | 0.99 | 2 (8.0) | 3 (12.0) | 0.99 |
| Cardio/cerebro-vasculara | 11 (14.5) | 14 (19.4) | 0.44 | 8 (16.0) | 8 (17.0) | 0.89 | 3 (12.0) | 6 (24.0) | 0.46 |
| Infectionsa | 54 (72.0) | 43 (59.7) | 0.12 | 37 (74.0) | 27 (57.4) | 0.09 | 17 (68.0) | 16 (64.0) | 0.77 |
| cytomegalovirus | 27 (36.0) | 23 (31.9) | 0.60 | 20 (40.0) | 15 (31.9) | 0.41 | 7 (28.0) | 8 (32.0) | 0.76 |
| urinary tract infection | 23 (30.7) | 26 (36.1) | 0.49 | 18 (36.0) | 16 (34.0) | 0.84 | 5 (20.0) | 10 (40.0) | 0.12 |
| other causes | 38 (50.7) | 29 (40.2) | 0.21 | 24 (48.0) | 16 (34.0) | 0.16 | 14 (56.0) | 13 (52.0) | 0.78 |
| Cancersa | 9 (12.0) | 5 (6.9) | 0.30 | 7 (14.0) | 2 (4.3) | 0.16 | 2 (8.0) | 3 (12.0) | 0.99 |
Data are n (%). MMF, mycophenolate mofetil.
Some patients have recorded >1 event.
Thirty-seven percent of the patients had the wild-type ABCB1 genotype in exon 12 or 21 and 36% in exon 26. The other patients carried at least one allelic variant. These frequencies were in agreement with those reported in the literature.8,21,22 All of the single nucleotide polymorphisms (SNPs) in the ABCB1 gene were in linkage disequilibrium (r2 ≥ 0.44). No deviation from the expected proportions of ABCB1 genotypes predicted by the Hardy-Weinberg equilibrium was detected.
ABCB1 Genotypes and Frequency of DGF
The incidence of DGF was almost three-fold higher in carriers of the T variant in exon 21 and 26 of the ABCB1 gene compared with those with the wild-type genotype, and the difference between genotype groups was statistically significant (Table 3). Conversely, there was no significant association between exon 12 genotype and incidence of DGF. Mean CsA C0 values during the first week after transplantation in patients with or without delayed graft function (262 ± 61 versus 273 ± 119 ng/ml, respectively; P = 0.92), as well as in those with the wild-type or mutated ABCB1 in exon 21 (274 ± 103 versus 276 ± 86 ng/ml, respectively; P = 0.94) or in exon 26 (263 ± 85 versus 280 ± 96 ng/ml, respectively; P = 0.60) were similar. C2 levels over the same observation period were also similar between those with or without DGF (1120 ± 319 versus 1345 ± 433 ng/ml respectively, P = 0.22). Multiple logistic regressions considering donor and recipient variables that were significantly associated with DGF at univariate analyses showed that the presence of one or two T alleles in exon 21 (odds ratio [OR] and 95% confidence interval [CI]: 3.8 [1.2 to 11.9], P = 0.022) or 26 (3.4 [1.1 to 10.8], P = 0.034) significantly predicted the risk of DGF, in addition to cold ischemia time and donor age (Tables 4 and 5).
Table 3.
Patients with events according to different genotypes in the exons 12, 21, and 26 of ABCB1
| Event | Exon 12
|
Exon 21a
|
Exon 26
|
|||
|---|---|---|---|---|---|---|
| CC(n = 54) | CT/TT(n = 93) | GG(n = 54) | GT/TT(n = 92) | CC(n = 52) | CT/TT(n = 95) | |
| Delayed graft function | 9 (17%) | 24 (26%) | 6 (11%) | 27 (29%)e | 6 (11%) | 27 (28%)e |
| Proteinuria | 14 (26%) | 24 (26%) | 14 (26%) | 24 (26%) | 10 (21%) | 28 (29%) |
| Diabetes | 7 (13%) | 17 (18%) | 7 (13%) | 17 (18%) | 4 (8%) | 20 (21%)e |
| Rejection | 30 (56%) | 43 (46%) | 29 (54%) | 43 (47%) | 27 (52%) | 46 (48%) |
| Time of the onset of AR (days) | ||||||
| median (IQ) | 24 (10–330) | 30 (16–282) | 23 (10–330) | 30 (17–282) | 22 (10–288) | 33 (15–308) |
| Graft loss | 2 (4%) | 4 (4%) | 1 (2%) | 5 (5%) | 1 (2%) | 5 (5%) |
| Cardio/cerebrovascularb | 9 (17%) | 16 (17%) | 7 (13%) | 18 (20%) | 8 (15%) | 17 (18%) |
| CMV disease | 13 (24%) | 37 (40%) | 9 (17%) | 40 (43%)f | 11 (21%) | 39 (41%)e |
| ganciclovir-treated | 13 (24%) | 33 (35%) | 9 (17%) | 36 (39%)f | 11 (21%) | 35 (37%) |
| primary CMV infectionc | 6 (11%) | 5 (5%) | 3 (6%) | 8 (9%) | 4 (8%) | 7 (7%) |
| reactivationd | 6 (11%) | 32 (34%)f | 5 (9%) | 32 (35%)f | 6 (11%) | 32 (34%)f |
| Other infectionsb | ||||||
| urinary tract | 16 (30%) | 35 (38%) | 16 (30%) | 35 (38%) | 18 (35%) | 33 (35%) |
| viral | 5 (9%) | 10 (11%) | 7 (13%) | 8 (9%) | 6 (11%) | 9 (9%) |
| pneumonias | 7 (13%) | 9 (10%) | 5 (9%) | 11 (12%) | 6 (11%) | 10 (10%) |
| other | 7 (13%) | 27 (29%)e | 6 (11%) | 28 (30%)f | 6 (11%) | 28 (29%)e |
| Cancerb | 2 (4%) | 12 (13%) | 2 (4%) | 12 (13%) | 2 (4%) | 12 (13%) |
AR, acute rejection; IQ, interquartile range; CMV, cytomegalovirus.
One patient was not characterized for exon 21.
Some patients with more than one event.
Primary CMV infection was defined by the presence of viral antigenemia in recipients without evidence of previous viral exposure (negative serology) at transplantation who received a graft from a seropositive donor.
CMV reactivation was defined by the presence of viral antigenemia in recipients with evidence of previous viral exposure (positive serology) at transplantation.
P < 0.05,
P < 0.01 versus GG (exon 21) or CC (exon 26) genotype.
Table 4.
Univariate logistic regressions using DGF as the dependent variable
| Univariate Analyses | Estimate | Standard Error | Odds Ratio | CI | P |
|---|---|---|---|---|---|
| Cold ischemia time | 0.0015 | 0.0001 | 1.002 | 1.000–1.003 | 0.0037 |
| Age recipient | 0.0276 | 0.0172 | 1.028 | 0.994–1.063 | 0.1085 |
| Weight recipient | 0.0222 | 0.0172 | 1.022 | 0.989–1.057 | 0.1948 |
| Sex recipient | −0.0771 | 0.2086 | 0.857 | 0.378–1.942 | 0.7118 |
| Age donor | 0.0371 | 0.0140 | 1.038 | 1.010–1.067 | 0.0079 |
| Weight donor | −0.0221 | 0.0187 | 0.978 | 0.943–1.015 | 0.2369 |
| Sex donor | 0.1537 | 0.1981 | 1.360 | 0.626–2.956 | 0.4377 |
| MMF or azathioprine | 0.0128 | 0.1977 | 1.026 | 0.473–2.227 | 0.9485 |
| B-cell crossmatch | 7.7594 | 623.6 | Not estimable | - | 0.9901 |
| Primary disease | 0.8117 | ||||
| diabete mellitus vs uncertain | −8.2308 | 176.2 | Not estimable | - | 0.9627 |
| hypertension vs uncertain | 1.5041 | 29.3718 | 1.371 | 0.217–8.664 | 0.9592 |
| glomerulonephritis vs uncertain | 0.9163 | 29.3645 | 0.762 | 0.267–2.175 | 0.9751 |
| polycystic kidney disease vs uncertain | 1.9504 | 29.3672 | 2.143 | 0.529–8.681 | 0.9470 |
| pyelonphritis vs uncertain | 1.0986 | 29.3671 | 0.914 | 0.228–3.662 | 0.9702 |
| other vs uncertain | 1.5731 | 29.3691 | 1.469 | 0.299–7.228 | 0.9573 |
| HLA mismatches | 0.9103 | ||||
| 1 vs 0 | −0.0351 | 1.1947 | 0.966 | 0.093–10.039 | 0.9766 |
| 2 vs 0 | 0.2877 | 1.1507 | 1.333 | 0.140–12.717 | 0.8026 |
| 3 vs 0 | 0.0364 | 1.1958 | 1.037 | 0.100–10.805 | 0.9757 |
CI, 95% confidence interval.
Table 5.
Multivariate logistic regressions using DGF as the dependent variable
| Multivariate Analyses | Estimate | Standard Error | Odds Ratio | CI | P |
|---|---|---|---|---|---|
| Exon 12 | |||||
| CC vs CT/TT | 0.7633 | 0.5255 | 2.145 | 0.766–6.010 | 0.1464 |
| cold ischemia time | 0.0002 | 0.0001 | 1.002 | 1.001–1.003 | 0.0028 |
| age donor | 0.0416 | 0.0172 | 1.042 | 1.008–1.078 | 0.0157 |
| Exon 21 | |||||
| GG vsGT/TT | 1.3307 | 0.5836 | 3.784 | 1.205–11.876 | 0.0226 |
| cold ischemia time | 0.0017 | 0.0006 | 1.002 | 1.001–1.003 | 0.0026 |
| age donor | 0.0375 | 0.0174 | 1.038 | 1.003–1.074 | 0.0314 |
| Exon 26 | |||||
| CC vs CT/TT | 1.2320 | 0.5841 | 3.428 | 1.091–10.771 | 0.0349 |
| cold ischemia time | 0.0017 | 0.0006 | 1.002 | 1.001–1.003 | 0.0026 |
| age donor | 0.0378 | 0.0174 | 1.039 | 1.004–1.074 | 0.0293 |
CI, 95% confidence interval.
ABCB1 Genotypes and Post-Transplant Estimated GFR
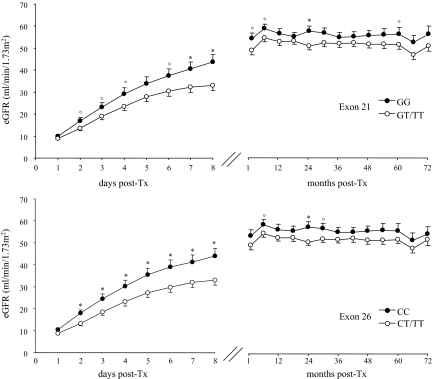
At each considered time point after transplantation up to study end, estimated GFR (eGFR) was lower in carriers of the allelic T variants in exon 21 or 26 than in carriers of the ABCB1 wild genotype (Figure 1), whereas no differences were observed in eGFRs among different exon 12 genotypes (data not shown). During the first week after transplantation, there was a trend to slower eGFR increase in carriers of the T allelic variants compared with carriers of the wild-type genotype in exon 21 (ΔeGFR: 3.7 ± 0.3 ml/min per day versus 5.1 ± 0.4 ml/min per day, respectively; P = 0.07) as well as in exon 26 (ΔeGFR: 3.7 ± 0.3 ml/min per day versus 5.1 ± 0.4 ml/min per day, respectively, P = 0.08). At final visits carriers of the T variants tended also to have lower eGFRs than carriers of the wild-type genotype in exon 21 (eGFR: 54.8 ± 15.5 ml/min/1.73 m2 versus 49.4 ± 16.3 ml/min/1.73 m2, respectively; P = 0.05) and in exon 26 21 (eGFR: 54.3 ± 16.5 ml/min/1.73 m2 versus 49.8 ± 15.7 ml/min/1.73 m2, respectively; P = 0.11).
Figure 1.
Time-course of estimated GFR in 147 kidney transplant recipients during the first 8 d post-transplant (left panels) and up to the last available follow-up (right panels), divided according to ABCB1 genotypes in exon 21 (upper panels) and exon 26 (lower panels). *P < 0.01 and °P < 0.05 versus GT/TT (exon 21) or CT/TT (exon 26).
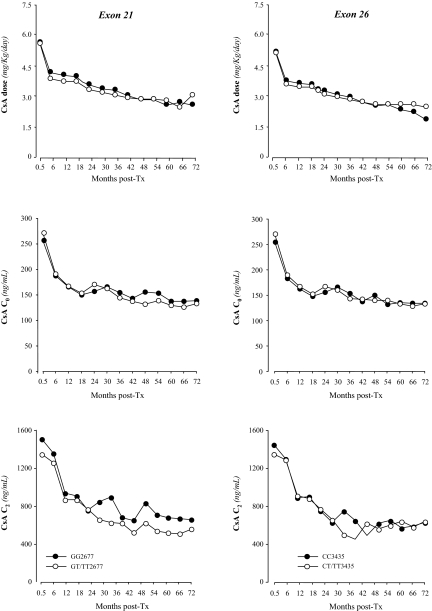
Mean CsA C0 and C2 values and daily drug doses during the whole follow-up period in patients with mutated or wild-type ABCB1 in exon 21 or exon 26 were similar (Figure 2).
Figure 2.
Median daily cyclosporine A (CsA) C0 doses and C2 blood CsA concentrations at different time points throughout the study period according to different genotypes in exon 21 and 26 of the ABCB1 gene.
Other Safety Outcomes
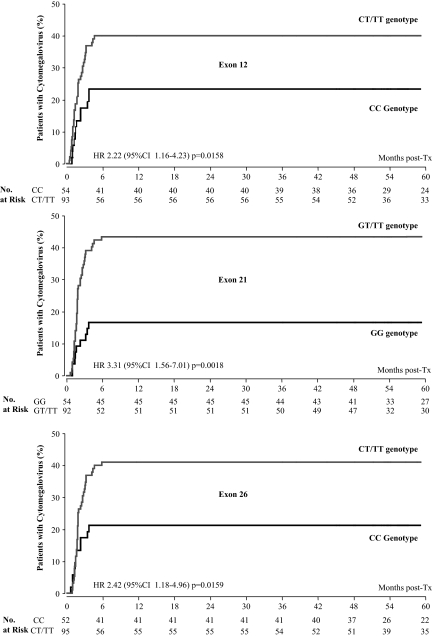
Over a median follow-up of 65.5 months (interquartile range 52.7 to 73.6 months), incidence of new onset diabetes was significantly higher in carriers of T variants in exon 26 than in wild-type recipients (Table 3). Carriers of T variants in both exons also had a significantly higher incidence of infections compared with wild-type recipients (Table 3). The difference was largely explained by a two- to three-fold excess in CMV reactivations in mutated compared with wild-type recipients (Table 3 and Figure 3). The excess risk was significant even after adjustment for predefined baseline covariates, including CMV serostatus at the time of transplant and CsA C0 and C2 levels. Incidence of neoplasms followed a similar trend, but numbers were small and differences between genotypes were nonsignificant.
Figure 3.
Kaplan-Meier survival curve showing the probability to experience cytomegalovirus antigenemia after transplantation according to different genotypes in exon 21 (upper panel) and exon 26 (lower panels) of the ABCB1 gene in 147 kidney transplant recipients treated with cyclosporine. HR, hazard ratio; CI, confidence interval.
Efficacy Outcomes
Carriers of the allelic T variants in exons 12, 21, or 26 showed a similar incidence of rejection episodes and time to the onset of the event compared with the carriers of the wild-type ABCB1 gene (Table 3). Similarly, no significant differences in the frequency of persistent proteinuria and cardiovascular events were observed between ABCB1 genotypes (Table 3). The number of graft losses (n = 6) was too limited for meaningful comparisons. The frequency of patients completing steroid withdrawal was comparable between T variant or wild-type carriers in exon 21 (67% versus 65%, respectively; P = 0.86) or in exon 26 (62% versus 68%, respectively; P = 0.40).
Concomitant Antihypertensive and Lipid-Lowering Therapy
At baseline evaluation, the proportion of patients on different concomitant medications was similar across genotype groups (Table 6). Conversely, at final visit, a trend to increased need for antihypertensive treatment with sympatholytic drugs was observed in patients with the T variants.
Table 6.
Patients on concomitant medications at the time of transplantation and at last visit
| Concomitant medication | Baseline
|
Final visit
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon 12
|
Exon 21a
|
Exon 26
|
Exon 12
|
Exon 21a
|
Exon 26
|
|||||||
| CC (n = 54) | CT/TT (n = 93) | GG(n = 54) | GT/TT(n = 92) | CC (n = 52) | CT/TT (n = 95) | CC (n = 54) | CT/TT (n = 93) | GG (n = 54) | GT/T (n = 92) | CC (n = 52) | CT/TT (n = 95) | |
| Antihypertensive agents | ||||||||||||
| any | 38 (70.4) | 60 (64.5) | 35 (64.8) | 62 (67.4) | 34 (65.4) | 64 (67.4) | 53 (98.1) | 93 (100) | 53 (98.2) | 92 (100) | 51 (98.1) | 95 (100) |
| diuretic | 25 (46.3) | 39 (41.9) | 26 (48.2) | 38 (41.3) | 23 (44.2) | 41 (43.2) | 38 (70.4) | 68 (73.1) | 36 (66.7) | 70 (76.1) | 33 (63.5) | 73 (76.8) |
| beta blocker | 1 (1.8) | 2 (2.1) | 0 | 3 (3.3) | 0 | 3 (3.2) | 24 (44.4) | 50 (53.8) | 25 (46.3) | 48 (52.2) | 25 (48.1) | 49 (51.6) |
| calcium channel blocker | 22 (40.7) | 30 (32.3) | 18 (33.3) | 33 (35.9) | 20 (38.5) | 32 (33.7) | 53 (98.1) | 85 (91.4) | 53 (98.2) | 84 (91.3) | 51 (98.1) | 87 (91.6) |
| Nifedipine | 17 (31.5) | 18 (19.4) | 14 (25.9) | 20 (21.7) | 15 (28.8) | 20 (21.0) | 46 (85.2) | 65 (69.9)b | 45 (83.3) | 65 (70.6) | 43 (82.7) | 68 (71.6) |
| Diltiazem | 5 (9.3) | 9 (9.7) | 4 (7.4) | 10 (10.9) | 4 (7.7) | 10 (10.5) | 5 (9.3) | 11 (11.8) | 4 (7.4) | 12 (13.0) | 5 (9.6) | 11 (11.6) |
| Verapamil | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.8) | 0 | 0 | 1 (1.1) | 0 | 1 (1.0) |
| Amlodipine | 0 | 3 (3.2) | 0 | 3 (3.3) | 1 (1.9) | 2 (2.1) | 13 (24.1) | 27 (29.0) | 15 (27.8) | 25 (27.2) | 14 (26.9) | 26 (27.4) |
| Felodipine | 0 | 0 | 0 | 0 | 0 | 0 | 3 (5.6) | 3 (3.2) | 4 (7.4) | 2 (2.2) | 4 (7.7) | 2 (2.1) |
| Lacipidine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.1) | 0 | 1 (1.1) | 0 | 1 (1.0) |
| Sympatholytic agent | 5 (9.3) | 10 (10.7) | 5 (9.3) | 10 (10.9) | 3 (5.8) | 12 (12.6) | 19 (35.2) | 43 (46.2) | 17 (31.5) | 44 (47.8) | 16 (30.8) | 46 (48.4)b |
| ACE inhibitor/ARBs | 0 | 0 | 0 | 0 | 0 | 0 | 10 (18.5) | 16 (17.2) | 9 (16.7) | 16 (17.4) | 8 (15.4) | 18 (19.0) |
| Lipid-lowering agents | 0 | 0 | 0 | 0 | 0 | 0 | 10 (18.5) | 19 (20.4) | 11 (20.4) | 18 (19.6) | 11 (21.2) | 18 (19.0) |
| Antiplatelet agent | 15 (27.8) | 28 (30.1) | 15 (27.8) | 27 (29.4) | 15 (28.9) | 28 (29.5) | 38 (70.4) | 64 (68.8) | 37 (68.5) | 64 (69.6) | 35 (67.3) | 67 (70.5) |
Data are shown as number (percentage). ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blockers.
One patient was not characterized for exon 21.
P < 0.05 vs the CC/GG genotype.
Haplotype Analyses
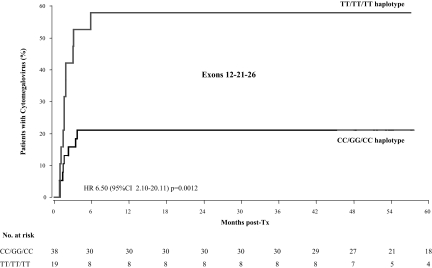
Haplotype analyses comparing CC-GG-CC (n = 38) and TT-TT-TT (n = 19) groups showed that the “T” haplotype was associated with a 6.5-fold excess CMV infection compared with fully wild-type genotype (Figure 4). The excess risk was significant even after adjustment for predefined baseline covariates. Incidence of DGF followed a similar trend (26.3% versus 10.5%), but the number of events was small and the differences between the two haplotypes failed to achieve the statistical significance (P = 0.201).
Figure 4.
Kaplan-Meier survival curve showing the probability to experience cytomegalovirus antigenemia after transplantation according to TT-TT-TT or CC-GG-CC haplotypes of the ABCB1 gene. HR, hazard ratio; CI, confidence interval.
DISCUSSION
Here we found that in renal transplant recipients who received CsA-based maintenance immunosuppressive therapy, the allelic T variants of exons 21 and 26 of the ABCB1 gene predicted increased incidence of DGF, slower post-transplant renal function recovery, lower final eGFRs, a significant increase in new cases of diabetes, CMV infections, and a trend to more neoplasms. These findings were not explained by different CsA exposure in different genotype groups, as the CsA dose and blood levels throughout the whole observation period were similar in carriers of the T variant or wild-type ABCB1 gene. The older age of donors of the T allele carriers did not explain the observed associations between ABCB1 genotypes and DGF or other considered outcomes, because at multiregression analyses different genotypes in exons 21 and 26 retained their predictive value even after adjustments for this potential confounding factor. This is consistent with evidence that age has an appreciable effect on the rate of renal function recovery among recipients of grafts from donors aged more than 50 to 55 yr.23,24 On the same line, a recent analysis from the USRDS registry involving more than 64,000 renal transplant recipients found that the risk of opportunistic infections increased only among recipients who were more than 50 yr old, whereas there was no relationship between outcome and age among recipients 18 to 50 yr old.36 That the large majority of donors and recipients in the present study were younger than 50 yr at transplantation provides additional evidence that age did not affect the study findings to any appreciable extent. All considered outcomes were also independent of patient randomization to mycophenolate mofetil or azathioprine therapy (see Table 2).
We suggest that delayed and impaired kidney function recovery in carriers of T allelic variants in ABCB1 exons 21 or 26 might be the consequence of a more severe reperfusion injury. Conceivably, oxidative stress in grafts given to carriers of the T allelic variants could have been amplified by ROS generated and released in increased amounts by infiltrating lymphocytes, polymorphonuclear cells, and other host leukocytes.11–13 This effect might be sustained by increased intracellular CsA concentration secondary to depressed CsA efflux via P-glycoprotein transport in this population.9,10 Although this is consistent with findings that both acute and chronic CsA nephrotoxicity are associated with oxidative stress and can be limited by free radical scavengers acting to block ROS,25–26 ad hoc studies using mass spectrometry technology to measure intracellular CsA concentration are needed to specifically address this working hypothesis.
Increased intracellular CsA concentration is also associated with excess inhibition of lymphocyte function in vivo,17 which is likely to underlie the significant increase in infections and CMV reactivations as well as the six-fold increase in neoplasms we observed during the study period in T variant compared with wild-type carriers. Differences in the incidence of neoplasms, however, were only marginally significant, most probably because the small number of events reduced the statistical power of comparative analyses. Whether the excess incidence of diabetes in carriers of the T allelic variant may reflect more severe CsA toxicity to the pancreatic β cells27 or, rather, increased tissue resistance to insulin28 is matter of investigation.
In our population the excess risk observed in carriers of the T alleles largely exceeded the potential benefits. This finding can be taken to suggest that CsA therapy might be optimized in this population by titrating CsA dose to lower than the usually recommended target blood levels. Further investigation is needed to address this hypothesis. Ideally, treatment should be titrated to intracellular CsA levels.17 More pragmatically, CsA dose could be titrated by considering the ABCB1genotype. Because delayed or partial renal function recovery after kidney transplantation strongly predict decreased graft survival in the long term,3,29 this approach might help to improve long-term allograft survival in patients at increased risk of CsA nephrotoxicity because of their genotype. Again, prospective ad hoc designed trials are needed to address whether analysis of the ABCB1 genotype may serve to guide CsA therapy to optimize renal graft outcomes.
All previous studies evaluating the relationships between ABCB1 genotypes and graft outcomes were observational and retrospective,30,31 and the results were often confounded by heterogeneity in patient characteristics and follow-up duration, and by changes in monitoring and treatment strategies introduced during the observation period.31 Here data were prospectively retrieved over a long follow-up period in the setting of a randomized, controlled trial with standardized treatments and predefined serial evaluations of patient and graft outcomes. A limitation is that we did not evaluate the ABCB1 genotype in kidney donors of our present patients. On the basis of recent evidence, donor ABCB1 genotype may also predict chronic CsA nephrotoxicity on the graft.32 Indeed, P-glycoprotein is also localized on the brush border of proximal tubular cells, and its reduced activity in grafts from donors with the T allelic variants would result in increased intracellular CsA concentration and toxicity. Thus, different interactions between donor and recipient genotypes might also affect graft susceptibility to CsA nephrotoxicity. Moreover, ad hoc prospective studies are needed to definitively establish the predictive role of ABCB1 genotype in kidney transplantation.
In conclusion, findings that mutant alleles for exons 21 or 26 of the ABCB1 gene predict increased susceptibility to CsA-related adverse effects can be taken to suggest that pretransplant genetic evaluation to identify subjects at risk might serve to modulate CsA therapy in this population. Whether our present findings mighty apply also to other calcineurin inhibitors is worth investigating.
CONCISE METHODS
Study Design
This prespecified analysis included renal transplant recipients randomized into the MYSS study who had provided a written informed consent to the evaluation of ABCB1 gene polymorphisms in exons 12, 21, and 26. MYSS was a prospective, randomized, clinical trial aimed to evaluate whether mycophenolate mofetil offers advantages over azathioprine in the opportunity to reduce or stop steroids in patients receiving maintenance immunosuppressive therapy with the CsA microemulsion Neoral.20 At completion of the first 6-mo treatment period, steroid dosage was progressively tapered and eventually discontinued and patients were then followed for up to month 72 post-transplantation, with clinical and laboratory evaluations every 6 mo.20,33 Genetic data and any other relevant information were retrieved from the study population without interfering with patient management and were handled according to standard regulations for data registration, use, and preservation of patient anonymity and privacy. The study protocol was approved by the Ethical Committee of the Coordinating Center and of all of the Transplant Centers involved in the study.
Outcomes
The main outcome variable was the incidence of DGF, defined as the need for dialysis therapy within the first week post-transplantation in patients without evidence of renal artery or vein thrombosis, urinary tract obstruction, or acute/hyperacute biopsy-proven graft rejection. Additional outcomes were the rate of renal function recovery over the first week post transplant; achieved GFR on follow-up; new-onset proteinuria or diabetes; incidence of cardiovascular events, infections, CMV reactivations, or neoplasms; and freedom from acute allograft rejection before and after steroid withdrawal.
The GFR was estimated by using the abbreviated Modification of Diet in Renal Disease formula based on gender, age, and serum creatinine values.34 Diabetes was diagnosed by World Health Organization criteria.
Previous exposure of recipients and corresponding donors to CMV infection was assessed by evaluating CMV serology at the time of transplantation. Primary CMV infection was diagnosed in recipients with evidence of viral antigens in their peripheral blood leukocytes who had no evidence (negative serology) for previous CMV exposure. CMV reactivation was diagnosed when viral antigenemia was detected in recipients with evidence (positive serology) of previous exposure to the virus. CMV disease was diagnosed when CMV antigenemia was associated with at least one clinical or laboratory sign such as neutropenia, leukopenia, or pneumonia-like symptoms (i.e., fever, cough, or dyspnea).35
Acute rejection episodes were diagnosed when patients presented with three or more of the following: temperature 38 °C or higher without obvious signs of infection, graft swelling or tenderness, a rise of 0.30 mg/dl or more in serum creatinine concentration in the presence of low or therapeutic CsA trough concentration; oliguria, increased resistive index on Doppler ultrasonography, and clinical response to steroid treatment consistent with rejection.
Genotyping
Genomic DNA was collected from EDTA-anticoagulated whole blood using the Nucleon BACC2 kit (Amersham, Biosciences, Buckingamshire, UK). The primer sequences (Sigma-Aldrich, UK) used for PCR are shown in Table 7 (together with detailed information on the analysis). Subsequently, the PCR product was subjected to denaturing HPLC (dHPLC) analysis or to direct sequencing. Moreover, according to currently common practice in pharmacogenomics research, we have confirmed 20% of the genotyping results obtained with dHPLC with direct sequencing.
Table 7.
Primer sequences and analytical conditions for ABCB1 SNPs detection by dHPLC
| Primer Name | Sequence (5′ to 3′) | Product Size (bp) | Temperature (°C) | Gradient (%B) |
|---|---|---|---|---|
| ABCB1 exon 12 | (F) AGTCAGTTCCTATATCCTGTGTCTGTGA | 251 | 60.6 | 54.1–64.1 |
| (R) GCAGTCACATTGCACATCTTTCT | ||||
| ABCB1exon 21 | (F) ATAGGTTCCAGGCTTGCTGTAATT | 279 | 54.6 | 56.1–66.1 |
| (R) AATCAGTGTTATTTTGTTACTCCCTACTG | ||||
| ABCB1 exon 26 | (F) GACTGCAGCATTGCTGAGAACA | 221 | 60.8 | 52.8–62.8 |
| (R) GGTCTCCCAGAAGTGAAGAGAAATT |
DHPLC analysis was performed using the WAVE Systems (WAVE DNA Fragment Analysis System, model MD4000plus; Transgenomics, Cedex, France), equipped with a DNA-Sep column (Transgenomics). Solvent gradient was obtained with buffer A 100 mM triethylammonium acetate pH 7.0 (TEAA, Transgenomics) and buffer B 100 mM TEAA pH 7.0 and 25% acetonitrile pumped at a flow rate of 1.5 ml/min. UV detector was set at 260 nm. SNP, single nucleotide polymorphism. F, forward; R, reverse; bp, base pair.
Haplotypes analyses were based on exons 12, 21, and 26 genotypes, as described previously.37,38 Of the different possible haplotype combinations derived from the homozygous genotypes of ABCB1, the most frequent ones were CC-GG-CC (n = 38) and TT-TT-TT (n = 19), the two haplotypes previously associated with highest and lowest P-gp activity, respectively.37,38 Therefore, comparative analyses of the main study outcomes (DGF and CMV infection) were focused on these two haplotypes.
Whole-Blood CsA Measurements
CsA levels were measured in whole-blood samples collected immediately before CsA administration (C0) or 2 h later (C2). C0 and C2 were measured daily for the first 15 d after transplantation, monthly from month 1 to month 21, and then as per clinical practice in each Transplant Center until study end.
Statistical Analyses
Genotype frequencies for the various SNPs were assessed for deviation from Hardy-Weinberg equilibrium using chi-square test. Pairwise D′ and r2 values for linkage disequilibrium (LD) were calculated using LD plotter (http://innateimmunity.net//IIPAG2/Bioinformatics). Statistical analyses were performed by comparing wild-type patients (CC for exon 12, GG for exon 21, and CC for exon 26) with those carrying at least one allelic “T” variant of ABCB1 (CT+TT, GT+TT, and CT+TT for exons 12, 21, and 26, respectively). Two out of the 147 patients who carried the rare GA and TA allelic variants in the exon 21 were categorized as heterozygous and included in the GT group.
Dichotomous and polychotomous baseline characteristics of the patients were compared with the use of the chi-square test or Fisher's exact test, and continuous characteristics were compared with the use of unpaired t test or Wilcoxon rank-sum as appropriate. Because of a skewed distribution, a nonparametric Wilcoxon rank-sum test was used to compare GFR levels at various time points (e.g., for the first 8 d and every months since transplantation, between the ABCB1 SNPs). An “acute” GFR slope (ΔGFR) was calculated on the basis of available eGFRs from day 0 to day 8 to describe the rate of renal function recovery after kidney transplant. Univariate and multivariable logistic regression models were used to investigate the effects of polymorphisms on the safety and efficacy outcomes. Multiple logistic regressions included the independent variables that were significantly (P < 0.05) associated with the outcome in the univariate approach.
Kaplan-Meier analysis was used to investigate whether cumulative incidence of new episodes of CMV antigenemia after transplantation differed between ABCB1 SNPs. The difference between the survival curves was assessed by means of the log-rank test. Cox proportional hazards models, with adjustment for main demographic and clinical covariates that were significantly associated with the outcome in the univariate approach, were used to detect the effect of polymorphisms on CMV episodes.
The data were presented as numbers and percentages, means ± SD, or medians and interquartile ranges, as appropriate. All of the statistical analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC). A p value of less than 0.05 was considered as statistically significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
Portions of these results were presented at the American Transplant Congress 2008, Toronto, May 30 to June 5, 2008. We are grateful to the Foundation ART for Research on Transplantation Onlus for the continuous support. We thank Monica Cortinovis for her contribution to data management.
APPENDIX
The Mycophenolate Steroids Sparing (MYSS) Genetics Study Group Organization*
Principal Investigator
Giuseppe Remuzzi
Study Coordinator
Dario Cattaneo
Participating Centers
E Gotti, P Ruggenenti, N Perico, G Remuzzi, (Azienda Ospedaliera, Ospedali Riuniti, Bergamo; n = 57)
S Sandrini, G Setti, R Maiorca (Azienda Ospedaliera Spedali Civili, Brescia; n = 30)
R Piperno, E Bertoni, M Salvadori (Azienda Ospedaliera Careggi Monna Tessa, Firenze; n = 27)
G Segoloni, M Messina, M Rocchietti, G Piccoli (Azienda Ospedaliera S. G. Battista, Torino; n = 19)
P Rigotti, N Baldan, L Liberati, L Rigoni, E Ancona (Ospedale Giustinianeo, Padova; n = 8)
D Donati, L Gastaldi (Ospedale Regionale di Circolo e Fondazione Macchi, Varese; n = 6)
Laboratory Measurements
D Cattaneo, S Baldelli, M Cortinovis, A Bitto (Istituto di Ricerche Farmacologiche Mario Negri)
Statistical Analysis
A Perna, N Motterlini (Istituto di Ricerche Farmacologiche Mario Negri)
* In brackets the Institution and the number of patients included
Published online ahead of print. Publication date available at www.jasn.org.
D.C. and P.R. contributed equally to the work.
REFERENCES
- 1.Halloran PF: Immunosuppressive drugs for kidney transplantation. N Engl J Med 351: 2715–2729, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D: Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605–612, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Perico N, Cattaneo D, Sayegh MH, Remuzzi G: Delayed graft function in kidney transplantation. Lancet 364: 1814–1827, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Marcén R, Pascual J, Teruel JL, Villafruela JJ, Rivera ME, Mampaso F, Burgos FJ, Ortuño J: Outcome of cadaveric renal transplant patients treated for 10 years with cyclosporine: Is chronic allograft nephropathy the major cause of late graft loss? Transplantation 72: 57–62, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Andoh TF, Bennett WM: Chronic cyclosporine nephrotoxicity. Curr Opin Nephrol Hypertens 7: 265–270, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Schiff J, Cole E, Cantarovich M: Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol 2: 374–384, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Fahr A: Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet 24: 472–495, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Marzolini C, Paus E, Buclin T, Kim RB: Polymorphisms in human MDR1 (P-glycoprotein): Recent advances and clinical relevance. Clin Pharmacol Ther 75: 13–33, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Crettol S, Venetz JP, Fontana M, Aubert JD, Ansermot N, Fathi M, Pascual M, Eap CB: Influence of MDR1 genetic polymorphisms on cyclosporine intracellular concentration in transplant recipients. Pharmacogenet Genomics 18: 307–315, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Klimecki WT, Futscher BW, Grogan TM, Dalton WS: P-glycoprotein expression and function in circulating blood cells from normal volunteers. Blood 83: 2451–2458, 1994 [PubMed] [Google Scholar]
- 11.Tamer S, Ademoglu E, Gokkusu C, Albeniz I: Effects of cyclosporine A on deformability and oxidative stress in the peripheral lymphocytes of rats. Clin Hemorheol Microcirc 36: 75–81, 2007 [PubMed] [Google Scholar]
- 12.Nguyen NS, Pulido SM, Rüegg UT: Biphasic effects of cyclosporin A on formyl-methionyl-leucyl-phenylalanine stimulated responses in HL-60 cells differentiated into neutrophils. Br J Pharmacol 124: 1774–1780, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Johnston TD, Reddy KS, Merrick JC, Mastrangelo M, Ranjan D: Cyclosporine directly causes oxidative stress and promotes Epstein-Barr virus transformation of human B cells. J Surg Res 100: 166–170, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Toledo-Pereyra LH, Lopez-Neblina F, Toledo AH: Reactive oxygen species and molecular biology of ischemia/reperfusion. Ann Transplant 9: 81–83, 2004 [PubMed] [Google Scholar]
- 15.Thurman RG, Zhong Z, von Frankenberg M, Stachlewitz RF, Bunzendahl H: Prevention of cyclosporine-induced nephrotoxicity with dietary glycine. Transplantation 63: 1661–1667, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Singh D, Alexander J, Owen A, Rustom R, Bone M, Hammad A, Roberts N, Park K, Pirmohamed M: Whole-blood cultures from renal-transplant patients stimulated ex vivo show that the effects of cyclosporine on lymphocyte proliferation are related to P-glycoprotein expression. Transplantation 77: 557–561, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Falck P, Asberg A, Guldseth H, Bremer S, Akhlaghi F, Reubsaet JL, Pfeffer P, Hartmann A, Midtvedt K: Declining intracellular T-lymphocyte concentration of cyclosporine a precedes acute rejection in kidney transplant recipients. Transplantation 85: 179–184, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Hoffmeyer S, Burk O, von Richter O, rnold HP, Brockmöller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U: Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 97: 3473–3478, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM: A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 315: 525–528, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Remuzzi G, Lesti M, Gotti E, Ganeva M, dimitrov BD, Ene-Iordache B, Gherardi G, Donati D, Salvadori M, Sandrini S, Valente U, Segoloni G, Mourad G, Federico S, Rigotti P, Sparacino V, Bosmans JL, Perico N, Ruggenenti P: Mycophenolate mofetil versus azathioprine for prevention of acute rejection in renal transplantation (MYSS): A randomised trial. Lancet 364: 503–512, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Anglicheau D, Verstuyft C, Laurent-Puig P, Becquemont L, Schlageter MH, Cassinat B, Beaune P, Legendre C, Thervet E: Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol 14: 1889–1896, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Cascorbi I, Gerloff T, Johne A, et al: Meisel C, Hoffmeyer S, Schwab M, Schaeffeler E, Eichelbaum M, Brinkmann U, Roots I: Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther 69: 169–174, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Humar A, Ramcharan T, Kandaswamy R, Gillingham K, Payne WD, Matas AJ: Risk factors for slow graft function after kidney transplants: A multivariate analysis. Clin Transplant 16: 425–429, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Asderakis A, Dyer P, Augustine T, Worthington J, Campbell B, Johnson RW: Effect of cold ischemic time and HLA matching in kidneys coming from “young” and “old” donors: Do not leave for tomorrow what you can do tonight. Transplantation 72: 674–678, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Cattaneo D, Perico N, Gaspari F, Remuzzi G: Nephrotoxic aspects of cyclosporine. Transplant Proc 36: 234S–239S, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Baliga R, Ueda N, Walker PD, Shah SV: Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev 31: 971–997, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Plaumann S, Blume R, Börchers S, Steinfelder HJ, Knepel W, Oetjen E: Activation of the dual-leucine-zipper-bearing kinase and induction of beta-cell apoptosis by the immunosuppressive drug cyclosporin A. Mol Pharmacol 73: 652–659, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Kutkuhn B, Hollenbeck M, Heering P, Koch M, Voiculescu A, Reinhard T, Grabensee B: Development of insulin resistance and elevated blood pressure during therapy with cyclosporine A. Blood Press 6: 13–17, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Yarlagadda SG, Klein CL, Jani A: Long-term renal outcomes after delayed graft function. Adv Chronic Kidney Dis 15: 248–256, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Hesselink DA, van Gelder T, van Schaik RH: The pharmacogenetics of calcineurin inhibitors: One step closer toward individualized immunosuppression? Pharmacogenomics 6: 323–337, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Cattaneo D, Baldelli S, Perico N: Pharmacogenetics of immunosuppressants: Progress, pitfalls and promises. Am J Transplant 8: 1–10, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Hauser IA, Schaeffeler E, Gauer S, Scheuermann EH, Wegner B, Gossmann J, Ackermann H, Seidl C, Hocher B, Zanger UM, Geiger H, Eichelbaum M, Schwab M: ABCB1 genotype of the donor but not of the recipient is a major risk factor for cyclosporine-related nephrotoxicity after renal transplantation. J Am Soc Nephrol 16: 1501–1511, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Remuzzi G, Cravedi P, Costantini M, Lesti M, Ganeva M, Gherardi G, Ene-Iordache B, Gotti E, Donati D, Salvadori M, Sandrini S, Segoloni G, Federico S, Rigotti P, Sparacino V, Ruggenti P: Mycophenolate mofetil versus azathioprine for prevention of chronic allograft dysfunction in renal transplantation: The MYSS follow-up randomized, controlled clinical trial. J Am Soc Nephrol 18: 1973–1985, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Gaspari F, Ferrari S, Stucchi N, Centemeri E, Carrara F, Pellegrino M, Gherardi G, Gotti E, Segoloni G, Salvadori M, Rigotti P, Valente U, Donati D, Sandrini S, Sparacino V, Remuzzi G, Perico N: Performance of different prediction equations for estimating renal function in kidney transplantation. Am J Transplant 4: 1826–1835, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Gotti E, Suter F, Baruzzo S, Perani V, Moioli F, Remuzzi G: Early ganciclovir therapy effectively controls viremia and avoids the need for cytomegalovirus (CMV) prophylaxis in renal transplant patients with cytomegalovirus antigenemia. Clin Transplant 10: 550–555, 1996 [PubMed] [Google Scholar]
- 36.Dharnidharka VR, Caillard S, Agodoa LY, Abbott KC: Infection frequency and profile in different age groups of kidney transplant recipients. Transplantation 81: 1662–1672, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Kim RB: MDR1 single nucleotide polymorphisms: Multiplicity of haplotypes and functional consequences. Pharmacogenetics 12: 425–427, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Fredericks S, Moreton M, Reboux S, Carter ND, Goldberg L, Holt DW, MacPhee IA: Multidrug resistance gene-1 (MDR-1) haplotypes have a minor influence on tacrolimus dose requirements. Transplantation 82: 705–708, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.