Abstract
Invariant natural killer T (iNKT) cells represent a particular subset of T lymphocytes capable of producing several cytokines, which exert regulatory or effector functions, following stimulation of the T cell receptor. In this study, we investigated the influence of iNKT cells on the development of experimental anti-glomerular basement membrane glomerulonephritis (anti-GBM GN). After injection of anti-GBM serum, the number of kidney iNKT cells rapidly increased. iNKT cell-deficient mice (Jα18−/−) injected with anti-GBM serum demonstrated worse renal function, increased proteinuria, and greater glomerular and tubular injury compared with similarly treated wild-type mice. We did not detect significant differences in Th1/Th2 polarization in renal tissue that might have explained the severity of disease in Jα18−/− mice. Interestingly, expression of both TGF-β and TGF-β-induced (TGFBI) mRNA was higher in wild-type kidneys compared with Jα18−/− kidneys, suggesting a possible protective role for TGF-β in anti-GBM GN. Administration of an anti-TGF-β neutralizing antibody significantly enhanced the severity of disease in wild-type, but not Jα18−/−, mice. In conclusion, in experimental anti-GBM GN, iNKT cells attenuate disease severity and TGF-β has a renoprotective role.
The experimental anti-glomerular basement membrane (anti-GBM) model was first described in dogs by Jean Redman Oliver and was adapted for rats by Matazo Masugi. Today, this model is used most often in mice,1–5 and it remains a useful and robust tool for studying inflammatory renal injury.6,7 Briefly, this method involves the injection of a heterologous serum rich in immunoglobulins against antigens from the GBM; this results in an immediate inflammatory response, characterized by the infiltration of cells of the innate immune system, including polymorphonuclear cells, into the kidney. This first wave of the innate immune response is followed by T and B cell activation, resulting in the progressive infiltration of CD4+ T cells and macrophages into the site of inflammation.7 Although the triggering factor is an alloantigen, this model mimics several human glomerular inflammatory diseases in which immune deposits induce endocapillary wounds, with or without extracapillary proliferation, with direct or collateral podocyte injury.
The mechanisms involved in the development of experimental anti-GBM glomerulonephritis (anti-GBM GN) are still unclear. Some authors have implicated a type-1 (Th1) pattern of immune response in the development of anti-GBM GN in C57BL/6 (B6) mice.3,8,9 Therefore, it was reasonable to hypothesize that an efficient type 2 (Th2) counter response could play an important role in resistance to anti-GBM pathogenesis.3,10 Along these lines, we decided to study the role of a particular immunoregulatory T cell population, the invariant natural killer T (iNKT) cells, in the development of this disease. iNKT cells constitute a distinct population of mature T lymphocytes positively selected by the non-polymorphic MHC class-I-like molecule CD1d. In contrast to variant NKT cells, iNKT cells are defined by a highly restricted T cell receptor (TCR) repertoire, composed of a single invariant Vα14Jα18 chain in mice and a Vα24Jα18 chain in humans, preferentially paired with a limited TCR Vβ chain repertoire that specifically recognizes glycolipids. These cells can be specifically recognized by the use of CD1d/α-galactosylceramide (α-GalCer) tetramers.11 Interest in iNKT cells arose first from their unique capacity to simultaneously produce large amounts of Th1 (IFN-γ) and Th2 (IL-4) cytokines, conferring the ability to influence the outcome and development of several inflammatory diseases depending on the immunological context.12 iNKT cells have been effectively implicated in inflammatory immune responses, namely tumor immunity, infections, autoimmune diseases and allergic asthma. In most of these pathologies, iNKT cells play a protective role; in some cases, however, they can become deleterious. It is likely that these contrasting effects result from the cytokine profile generated by iNKT cells in each situation. In fact, IFN-γ production by iNKT cells is required for their protection against a variety of pathogens. Administration of α-GalCer, a glycolipid capable of specifically stimulating iNKT cells, inhibits hepatitis B virus and cytomegalovirus replication by activation of NK cells by an IFN-γ-dependent mechanism.13,14 Others reports demonstrate that IL-12 associated with IL-18 promotes the secretion of IFN-γ by iNKT cells without TCR crosslinking and can enhance the antiviral response mediated by NK cells, conferring protection during murine cytomegalovirus infection.15 Others authors have reported that IFN-γ produced by iNKT cells in response to cytokines can mediate protection against Mycobacterium bovis or Leishmania major infections.16 On the other hand, IL-4-producing iNKT cells protect the host against cerebral malaria.17 In contrast to these protective roles, a deleterious effect has been ascribed to both IL-4 and IL-13 secreted by iNKT cells in the development of airway hyper-reactivity, a cardinal feature of asthma.18,19
We have recently demonstrated iNKT plasticity using an ovalbumin (OVA)-induced asthma model. iNKT cell activation during the sensitization phase increases Th2 responses, whereas α-GalCer treatment administered during the effector phase decreases Th2-associated inflammatory responses.19,20 This dichotomous effect of iNKT cells was also demonstrated in pristane-induced lupus, where its specific activation by α-GalCer suppressed or promoted lupus-like autoimmunity in a strain-dependent manner.21 Other authors associated the regulatory effect of iNKT lymphocytes in lupus-like disease with Th1/Th2 bias and B lymphocyte activation.21–25 In opposition to the regulatory role of iNKT cells in autoimmunity, there is other evidence suggesting that iNKT cells are the major early-acting CD4+ T cell type in renal ischemia-reperfusion injury through IFN-γ-mediated neutrophil accumulation.26,27 Despite these studies, the regulatory role of iNKT in kidney physiology remains unclear.
Recently, it was shown that CD1d-deficient mice, which are deficient for all CD1d-restricted T cells including iNKT cells, developed an accelerated course of GN compared with controls.28 In that study, however, the precise contribution of the iNKT subset was not evaluated since the use of CD1d−/− mice did not exclude the possible effects of other CD1d-restricted T cell subpopulations. In the study presented here, the use of Jα18−/− mice,29 which are exclusively deficient for iNKT cells, allowed us to demonstrate that these cells confer resistance to the development of experimental anti-GBM GN and that TGF-β plays a major role in the protection against anti-GBM GN.
RESULTS
Development of Anti-GBM GN Is Associated with a Local Influx of iNKT Cells
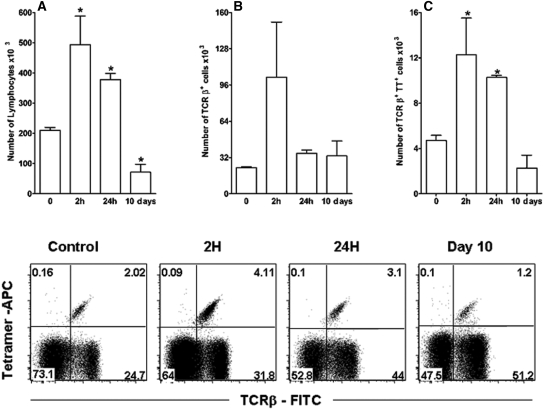
It has been shown that during the development of an inflammatory response, iNKT cells can migrate to the site of inflammation, where they can contribute to immune responses.30,31 This effect was also seen in the kidneys of anti-GBM-treated mice. We found a 3-fold increase in the number of iNKT cells in the renal tissue of such mice; this number peaked 2 h after injection of the anti-GBM serum and started to decrease 24 h after serum administration (Figure 1).
Figure 1.
The development of anti-GBM GN is associated with local influx of iNKT cells. C57BL/6 mice were injected with anti-GBM serum on day 0. At 0, 2, and 24 h and on day 10, kidneys were collected, treated with collagenase and DNase, and stained for TCR-β antigens (TCRβ-FITC) and Vα14-Jα18 TCR (TT, tetramer-CD1d+αGalCer-APC) to detect iNKT cells at different time points. FACS analysis showed the time-dependent influx of lymphocytes (A), TCRβ+ cells (B), and iNKT lymphocytes (C). Results are reported as absolute numbers of infiltrating cells per kidney (mean ± SEM) from five to eight animals for each time point. *P < 0.05 versus 0 h. Data are representative of three separate experiments.
Lack of iNKT Cells Aggravates Renal Injury
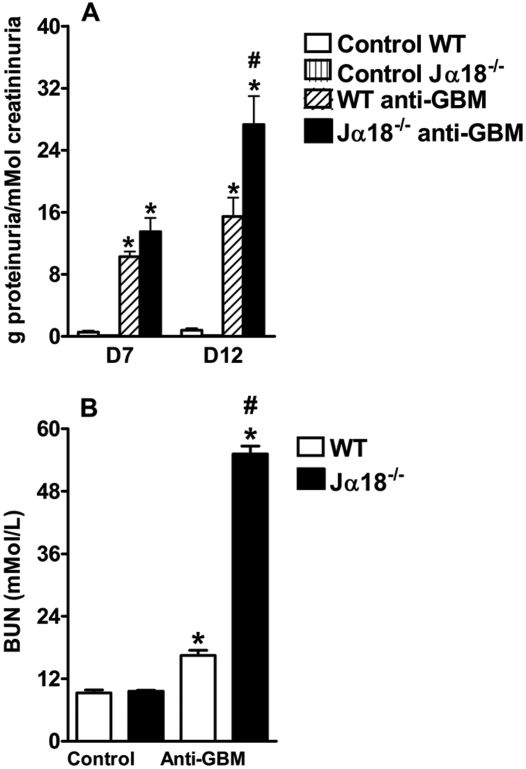
To determine the role of iNKT cells in the pathogenesis of anti-GBM GN, we used C57Bl/6 Jα18−/− animals, which are exclusively deficient in these lymphocytes. After the administration of anti-GBM serum, the C57Bl/6 Jα18−/− mice demonstrated higher levels of proteinuria and blood urea nitrogen (BUN) than wild-type (WT) mice (Figure 2, A and B, respectively), suggesting a protective role of iNKT cells in the development of anti-GBM GN.
Figure 2.
iNKT deficiency results in more significant alterations in renal function after anti-GBM serum administration. C57BL/6J WT and Jα18−/− mice were injected with anti-GBM serum, and control mice were injected with PBS. Urine and blood were collected at selected time points to assess renal function. C57BL/6J Jα18−/− mice, which lack iNKT lymphocytes, had higher levels of proteinuria on days 7 and 12 (A) and a higher BUN level on day 14 (B) compared with C57BL/6 WT mice. Results are expressed as mean ± SEM (n = 5). *P < 0.05 versus control; #P < 0.05 versus WT. This is representative of three isolated experiments.
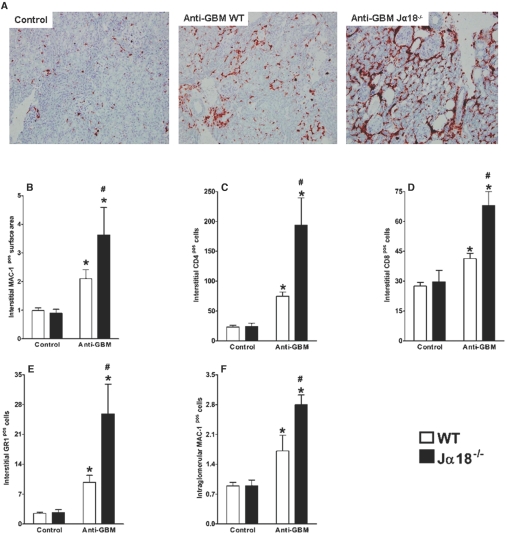
The development of anti-GBM GN is associated with infiltration of T cells, macrophages, and polymorphonuclear cells (PMNs) into the kidney interstitium.32 Indeed, immunohistological analysis of the renal tissue demonstrated that the administration of nephrotoxic sera resulted in a significant influx of inflammatory cells into the kidney tissue. In parallel with the increased proteinuria and renal failure described above, Jα18−/− mice showed increased infiltration of inflammatory cells into the kidneys compared with WT animals (Figure 3). The infiltrating cells were essentially macrophages (MAC-1+ cells), followed by CD4+ T cells, CD8+ T cells, and PMNs (GR1+ cells) (Figure 3, A to E). Figure 3F demonstrates that the development of anti-GBM GN was associated with glomerular infiltration of MAC-1+ cells. This infiltration was significantly increased in iNKT cell-deficient mice compared with the WT group, suggesting that the protective role of iNKT cells during the development of anti-GBM GN may be, at least in part, mediated by decreased glomerular macrophage accumulation.
Figure 3.
Increased infiltration of inflammatory cells into renal tissue in mice lacking iNKT cells. C57BL/6 WT and Jα18−/− mice were injected with anti-GBM serum, and control mice were injected with PBS. After 14 d, kidneys were collected. (A) Immunohistochemical analysis showed greater influx of inflammatory cells into renal tissue of Jα18−/− mice, which lack iNKT cells, than into renal tissue from WT mice. MAC-1+ cells (A, B, and F) made up the largest proportion of infiltrating cells, followed by CD4+ T cells (C), CD8+ T cells (D), and GR1+ cells (E). Jα18−/− mice also had more intraglomerular MAC-1+ cells than WT mice (F). Results are expressed as mean ± SEM (n = 5). *P < 0.05 versus control; #P < 0.05 versus WT. This is representative of three isolated experiments.
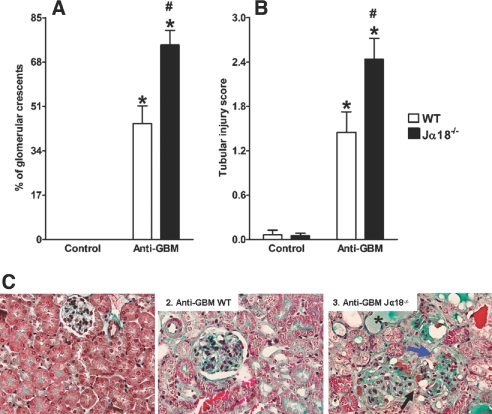
In accordance with the results concerning renal function and inflammatory infiltrates, the Jα18−/− mice demonstrated more significant and severe tubular and glomerular injury than the WT mice. Figure 4, A and C3 show that iNKT cell-deficient mice exhibited a higher number of glomerular crescents at day 14 in comparison to WT animals. With regard to tubular injury, we found that Jα18−/− mice presented significant and diffuse tubular dilation and necrosis with hyaline casts (Figure 4, B and C3). In WT mice, only occasional tubulointerstitial lesions were noted.
Figure 4.
Increased glomerular crescent formation and tubular injury in mice lacking iNKT cells. C57BL/6J WT and Jα18−/− mice were injected with anti-GBM serum, and control mice were injected with PBS. After 14 d, kidneys were collected to determine the extent of glomerular crescent formation (A) and tubular injury (B). Sections were stained with Masson's trichrome. More glomerular crescents were observed in Jα18−/− mice (blue arrow in C3), which lack iNKT cells, than in WT mice. These mice also lacked a capsular urinary space (black arrows in C3), whereas this was observed less frequently in WT mice (black arrow in C2). There were significantly more hyaline casts and tubular dilation in Jα18-/- mice than in WT mice (asterisk [*] in C3). Original magnification, ×600. Results are expressed as mean ± SEM (n = 5). *P < 0.05 versus control; #P < 0.05 versus WT. This is representative of three isolated experiments.
Th1/Th2 Polarization Does Not Differ Between WT and Jα18−/− Mice During the Development of Anti-GBM GN
To investigate the possibility that Th1 polarization contributed to the increased severity of anti-GBM GN in iNKT cell-deficient mice, we analyzed the production of several cytokines and subclasses of mouse anti-sheep immunoglobulins. We failed to detect any significant difference with regard to the expression of mRNA for IL-4 or IFN-γ in the renal tissue of WT versus Jα18−/− mice. Indeed, in anti-GBM-treated mice, IL-4 and IFN-γ mRNA were at the lower limit of detection by quantitative PCR (Q-PCR) at day 14 (data not shown). Similarly, comparable plasma levels of IFN-γ and TNF-α were measured in both groups, and IL-4, IL-5, and IL-2 levels were below the limit of detection in all groups (supplemental data Figure S1). Furthermore, WT and Jα18−/− mice showed no significant differences in the level of mouse anti-sheep IgG1 or IgG2a (supplemental data Figure S2, A and B) or in the renal deposition of nephrotoxic sheep IgG or mouse anti-sheep IgG (supplemental data Figure S2C). These data indicate that mechanisms other than disequilibrium in the Th1/Th2 balance might explain the increased disease severity observed in the absence of iNKT cells.
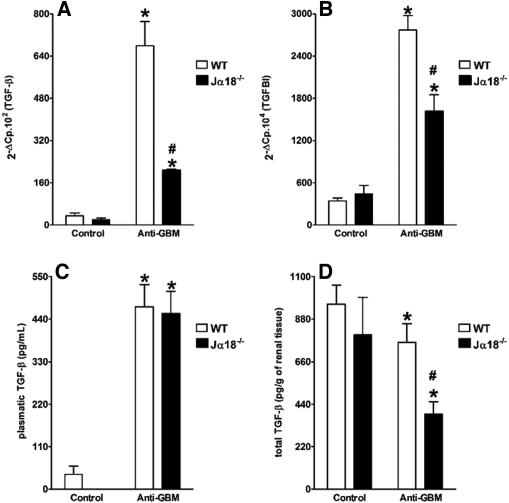
WT Mice Express More TGF-β and TGFBI (BIGH3) than Jα18−/− Mice During the Development of Anti-GBM GN
One potential factor that might explain the differences observed between WT and Jα18−/− mice is TGF-β1. This factor has been implicated in the development of anti-GBM GN, although its exact role is not entirely clear.33,34 Q-PCR analysis demonstrated that WT mice injected with anti-GBM nephrotoxic serum developed increased expression of TGF-β1 mRNA in the kidney compared with Jα18−/− mice (Figure 5A). To determine the biologic activity of TGF-β in our model, we used Q-PCR to quantify the expression of TGFBI (BIGH3) mRNA, which is strictly related to TGF-β1 activity in renal tissue and can reflect the levels of the active form of TGF-β.35–37 In accordance with the higher expression of TGF-β mRNA in WT mice, the relative expression of TGFBI-associated genes was significantly higher in WT animals (Figure 5B) compared with Jα18-/- mice. Plasma levels of TGF-β were significantly increased in both WT and Jα18−/− mice after the administration of anti-GBM serum, with no significant difference between the two strains (Figure 5C). In contrast, renal TGF-β protein levels were significantly lower in Jα18−/− mice than in WT mice (Figure 5D). Taken together, these results suggest that iNKT cells could provide a protective effect in the anti-GBM GN model through the renal, but not systemic, production of TGF-β.
Figure 5.
Anti-GBM serum administration increases the expression of renal TGF-β and TGFBI mRNA in WT but not iNKT-deficient mice, despite induction of systemic TGF-β. C57BL/6J WT and Jα18−/− mice were injected with anti-GBM serum, and control mice were injected with PBS. After 14 d, kidneys were collected, and Q-PCR was carried out to assess the expression of TGF-β (A) and TGFBI (B) mRNA. Both plasma (C) and kidney (D) TGF-β levels were measured at day 14 after anti-GBM serum injection. Results are expressed as mean ± SEM (n = 5). *P < 0.05 versus control; #P < 0.05 versus WT. This is representative of two isolated experiments.
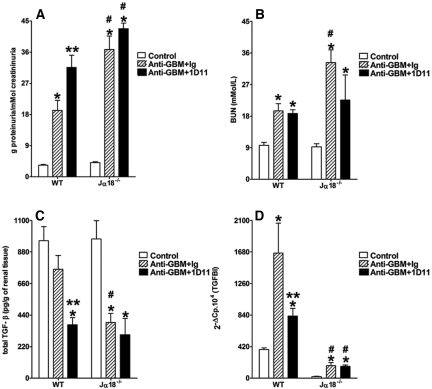
In Vivo Administration of TGF-β Neutralizing Antibody Impaired Resistance to the Development of Anti-GBM GN
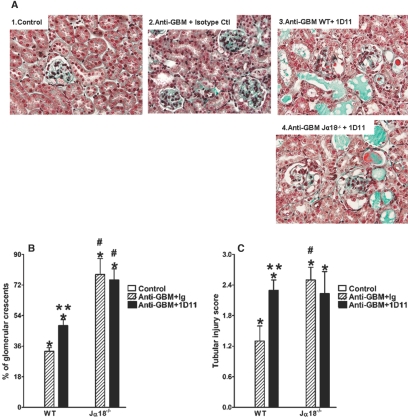
Finally, to confirm the renoprotective role of TGF-β in the development of anti-GBM GN, we treated WT mice with the 1D11 antibody, a mouse monoclonal anti-TGF-β neutralizing antibody.38,39 Treatment with 1D11 2 d after serum administration impaired the resistance to anti-GBM GN previously seen in WT mice when evaluated at day 14. The 1D11-treated WT mice demonstrated higher proteinuria in comparison to animals receiving an irrelevant matched isotype antibody (Figure 6A), although we did not find any significant alteration in the BUN (Figure 6B). 1D11 administration decreased TGF-β protein levels in the renal tissue of WT mice (Figure 6C). In accordance with this, renal TGFBI mRNA expression was also reduced after anti-TGF-β treatment, confirming the effective in vivo neutralization of TGF-β (Figure 6D). The pathologic analysis showed that, along with the increased proteinuria, the 1D11-treated WT mice exhibited a higher number of glomerular crescents (Figure 7, A and B) and more pronounced tubular injury (Figure 7, A and C) in comparison to animals that received control Ig. Moreover, the administration of 1D11 did not affect the outcome of anti-GBM GN in Jα18−/− mice in terms of proteinuria and renal function, suggesting that TGF-β production, which confers renoprotection to WT mice in this model, is dependent on iNKT cells. Taken together, these data support the idea that, in our model, TGF-β contributes to the renoprotective role of iNKT cells in the development of anti-GBM GN.
Figure 6.
TGF-β neutralization by 1D11 mAb impairs renal function in WT mice but not in Jα18−/− mice. Animals were injected with nephrotoxic serum and treated with 1D11, a TGF-β-neutralizing mouse mAb, 2 days after administration of the anti-GBM serum. The control group received only PBS, whereas the anti-GBM plus Ig group was injected with nephrotoxic serum and treated with a nonrelevant IgG1. At days 7 and 12, urinary protein (A) and BUN (B) were measured. The levels of renal TGF-β protein (C) and the expression of the TGFBI mRNA (D) were evaluated at day 14 after the induction of anti-GBM GN. Results are expressed as mean ± SEM (n = 8). *P < 0.05 versus control; #P < 0.05 versus WT; **P < 0.05 versus irrelevant IgG1-treated mice; ***P < 0.05 1D11 Jα18−/− versus 1D11 WT. This is representative of two isolated experiments.
Figure 7.
1D11 treatment increases the level of glomerular crescent formation and tubular injury in WT mice but not in Jα18−/− mice. Animals were injected with nephrotoxic serum at day 0 and treated with 1D11, a TGF-β-neutralizing mouse mAb, 2 days after administration of the anti-GBM serum. The control group received only PBS, whereas the anti-GBM plus Ig group was injected with nephrotoxic serum and treated with a nonrelevant IgG1. The percentage of glomerular crescents was analyzed at day 14 (B). We also found increased hyaline casts and tubular dilation in 1D11-treated mice (A and C). Sections were stained with Masson's trichrome. Original magnification, ×600. Results are expressed as mean ± SEM (n = 8). *P < 0.05 versus control; #P < 0.05 versus WT; **P < 0.05 versus irrelevant IgG1-treated mice. This is representative of two isolated experiments.
DISCUSSION
Our finding that iNKT-deficient mice exhibit a more severe form of GN agrees with previous results showing that CD1d-deficient mice, deficient for all CD1d-restricted T cells, develop more severe disease than WT mice after anti-GBM antiserum administration.28
In agreement with our results, iNKT cell-deficient mice have been shown to develop spontaneous lupus-like glomerular lesions with age.16 Collectively, our results suggest that iNKT cells may attenuate auto- or alloimmune-mediated renal injury. The anti-GBM GN model in mice has been shown to be mainly a Th1-driven disease, while Th2 cytokines are thought to exert a protective role. Given the Th2-like response induced by iNKT cells in airway hypersensitivity reactions,11 it would have been logical to find a Th1-like profile in iNKT cell-deficient mice, which might, in turn, explain the more severe form of GN seen in these animals. Using various cytokine and Ig subclass analyses, however, we failed to detect any Th1 polarization in Jα18−/− mice.
When examining the immune process of anti-GBM GN at day 14, we found a striking relationship between iNKT cell deficiency and impaired production of TGF-β. Unlike previous studies that described the harmful role of TGF-β during the development of renal fibrosis or nephritis,40–43 we have demonstrated that the absence of TGF-β exacerbates the loss of renal function and the influx of inflammatory cells into renal tissue after acute kidney injury. TGF-β plays different roles in the immune response, depending on several factors such as the nature of the cell targets, the location of the immune response, and the timing of disease evaluation (chronic versus acute).44–49 In kidney tissue, TGF-β plays an important but ambiguous role; it is probably harmful in chronic inflammatory processes50 and beneficial in acute kidney injury, as suggested by our work and several other reports.34,51,52 It seems that iNKT cells play a protective role in our model by modulating the bioactivity of intrarenal TGF-β, since circulating levels of this protein were similar in both WT and Jα18−/− mice. Wild-type mice injected with nephrotoxic serum demonstrated significant expression of TGF-β and TGFBI mRNA, indicating that iNKT cells were directly or indirectly involved in the local production of bioactive TGF-β. The expression of TGFBI is a reliable marker of active TGF-β signaling in renal tissue, as described previously.35,36 In contrast to TGF-β mRNA levels, which increase during renal disease, the total renal TGF-β protein content was found to decrease during disease in both strains of mice. This result suggests that during the acute phase of GN, latent TGF-β stored in the extracellular matrix is released and activated. Recent work by Huang et al. demonstrated that systemic overexpression of latent TGF-β, which may accumulate locally in the extracellular matrix and be released during inflammatory processes, is associated with protection in crescentic GN, corroborating our findings and reinforcing the idea of a protective role for intrarenal TGF-β in anti-GBM GN.34
Therefore, to confirm our hypothesis that TGF-β is an important agent in the control of anti-GBM GN, possibly related to iNKT cells, we treated both C57Bl/6 WT and Jα18−/− mice with the TGF-β 1, 2, and 3 neutralizing antibody 1D11. We found that TGF-β neutralization exacerbated the anti-GBM GN seen in WT mice but not in Jα18−/− mice. The neutralization of TGF-β in WT mice was associated with decreased expression of TGFBI mRNA and exacerbation of disease. Here, we demonstrate a strict relationship between iNKT cells and intrarenal production of TGF-β during crescentic GN. Our results suggest that iNKT cells can, in the early stages of acute glomerular inflammation, contribute to an anti-inflammatory response via intrarenal expression of TGF-β. In addition, our results suggest that both TGF-β and iNKT cells are required to observe a full protective effect in anti-GBM GN, since in the absence of iNKT cells, the blockade of TGF-β had no influence on GN severity.
A recent report using CD1d−/− mice suggested that NKT cells exert a renoprotective role during the development of anti-GBM GN.28 It is noteworthy that CD1d−/− animals lack different populations of CD1d-dependent cells, including the invariant Vα14Jα18 and noninvariant NKT lymphocytes, whereas Jα18−/− mice are deficient only in iNKT cells.29 We and others have demonstrated that in immune responses against helmintic infections as well as tumors, both cellular populations can be implicated and play distinct roles.53–55 The use of Jα18−/− mice, however, clearly demonstrated the renoprotective role of iNKT cells, since these mice exhibited more severe disease despite the presence of the noninvariant NKT lymphocytes. Our findings demonstrated that bioactive TGF-β1 production was associated with protection, while Yang et al. reported a decreased level of TGF-β1 protein in the kidneys of CD1d−/− mice transferred with TCR+NK1.1+ cells and concluded that the decrease in TGF-β was associated with a better prognosis.28 These authors, however, adoptively transferred TCR+NK1.1+ cells to CD1d−/− mice. These cells, in addition to containing cells others than iNKT cells, cannot be activated in a CD1d-dependent manner when transferred to CD1d−/− mice. Thus, after transfer, and to induce the protection described, these NKT cells are likely activated by an alternative pathway that could modify the pattern of cytokines produced and potentially induce mechanisms independent of TGF-β that could contribute to the renoprotection. Further experiments are necessary to test this possibility.
In conclusion, our data show that, during the development of experimental anti-GBM GN, iNKT lymphocytes play a major role in controlling the inflammatory process and glomerular injury. Additionally, we have shown that this regulatory role either directly or indirectly involves the production of bioactive TGF-β. Although further analysis is required to dissect the precise relationship between TGF-β and iNKT cells in our model, our data support the idea that both factors are essential in the regulation of acute kidney injury.
CONCISE METHODS
Animals and Anti-GBM GN
C57BL/6 mice (9 to 14 wk old) were purchased from Janvier (Centre d’Elevage R. Janvier, Le Genest-St-Isle, France). Jα18−/− mice29 were bred in our own facilities onto a pure C57BL/6-background (>B12). All mice were kept in well-controlled animal housing facilities and had free access to tap water and pellet food. All animal experiments were performed according to the French Institutional Committee. Decomplemented sheep anti-rat GBM sera were prepared as described previously.33 We induced passive anti-GBM GN by intravenous administration of a total of 1.5 mg total protein/g body weight, administered over three consecutive days (days 0, 1, and 2). This protocol has been popularized by D.J. Salant and coworkers, according to the seminal work of A.R. Morley and J. Wheeler in mice.6,56,57 We evaluated renal injury and mRNA expression on day 14. Control mice were injected with PBS.
FACS Analysis of iNKT Cells in Kidney Tissue
Mice were deeply anesthetized with urethane (Sigma-Aldrich, St Quentin Fallavier, France), and kidneys were perfused via injection of 10 ml PBS into the left ventricle. We collected one kidney per animal and minced and incubated them for 15 min at 37°C in 1 ml RPMI 1640 containing 1 mg/ml DNase (Roche Diagnostics, Meylan, France) and 2 mg/ml collagenase (Sigma). Next, we teased the kidney homogenate through a 100-μM cell strainer (BD Biosciences, San Diego, CA), and then we further separated the mononuclear cells by gradient centrifugation on 35% Percoll (Amersham Biosciences, Piscataway, NJ). We stained the collected cells (2 × 106 cells) with FITC-conjugated anti-TCRβ and allophycocyanin (APC)-conjugated CD1d/α-GalCer tetramers (plasmids containing CD1d and β2m genes were provided by M. Kronenberg, LA Jolla Institute for Allergy and Immunology, San Diego, CA) and analyzed on a FACS Canto II using DIVA software (Becton Dickinson). Further analyses of FACS data were performed using FLOWJO 7.2 software (Tree Star, Ashland, OR).
Histopathological Analysis of Glomerular and Tubular Injury
We fixed the kidneys in alcohol-formalin-acetic acid, embedded them in paraffin, cut them into 4-μm sections, and stained them with Masson's trichrome. We defined crescent formation as glomeruli exhibiting two or more layers of cells in Bowman's space, with or without podocyte injury, as indicated by ballooning, necrosis, or cyst formation. The proportion of affected glomeruli per mouse was determined in 100 randomly selected glomeruli, as described previously.33 We scored tubulointerstitial injury on a scale of 0 to 4, as follows: 0, no tubulointerstitial injury; 1, less than 25% injury; 2, 25% to 50% injury; 3, 51% to 75% injury; 4, more than 75% injury. Tubulointerstitial injury was defined as tubular dilation or atrophy, denudation of the tubular basement membrane, or tubular necrosis, as described previously.33
Immunohistochemical Analysis of Renal Inflammation
Kidneys were snap-frozen in liquid nitrogen, and 5-μm acetone-fixed cryostat sections were used for conventional immunohistochemistry with a two-layer immunoperoxidase method. We identified T lymphocytes using rat monoclonal antibodies against CD3, CD4, and CD8; macrophages, using a monoclonal antibody against MAC-1; and polymorphonuclear cells, with an antibody against GR1.1. We purchased all primary antibodies from Serotec (Oxford, UK) or Becton Dickinson, and secondary antibodies were purchased from Nichirei (Tokyo, Japan). We analyzed a minimum of 25 glomeruli, and the total number of T lymphocytes (CD3+/CD4+/CD8+), macrophages (MAC-1+), or polymorphonuclear cells (GR1.1+) was determined at ×20 magnification. Results are expressed as cells per glomerular cross-section or cells per high-power field, as described previously.33
Proteinuria and Plasma BUN Levels
Urine was manually collected at selected times. We assessed proteinuria using the Pyrogallol Red method, utilizing a KONELAB automater (Thermo Scientific, Waltham, MA), and we expressed proteinuria as gram protein/mmol creatininuria. We assessed the BUN level in blood plasma obtained on the day of sacrifice, using an enzymatic-spectrophotometric method with an automater, and it was expressed in mmol/L.
TGF-β Measurement in Renal Tissue and Plasma
We determined active and total TGF-β1 levels by ELISA (Duo-Set; R&D Systems, Minneapolis, MN) on whole protein extracts isolated from 25 mg renal cortex homogenized in 100 μl RIPA buffer, pH 7.4. We centrifuged the homogenate at 12,000 × g for 20 min at 4°C and collected the supernatant. We first determined active TGF-β1 levels without acid treatment of samples. We determined total (active plus latent) TGF-β1 levels after acid activation of samples (1 N HCl for 10 min, then 1.2 N Hepes NaOH to correct the pH to 7.4). Acidification of the sample was checked for each sample, according to the manufacturer's protocol. Concerning renal tissue, we observed a large and unreliable interassay variation in spontaneously active TGF-β depending on the duration of renal tissue processing, temperature, extract buffer, and freeze-thaw cycles. Thus, we report the total active TGF-β concentration after acid activation, which gave more consistent results. In parallel, we evaluated the in vivo TGF-β activity by measuring TGFBI mRNA expression, as described.35–37
Q-PCR to Measure Renal Expression of IFN-γ, IL-4, TGF-β, and TGFBI (BIGH3)
We obtained kidneys from Jα18-/- and WT B6 mice 14 d after the first injection of sheep anti-rat GBM serum. We extracted RNA from the kidneys using TRIzol solution (Life Technologies BRL, Gaithersburg, MD). RNA quality was checked by measuring the ratio of optical densities at 260 and 280 nm and by electrophoresis. We used reverse transcription with Superscript II (Life Technologies BRL) to convert 1 μg RNA into cDNA, which was then amplified by PCR using a LightCycler 480 (Roche Diagnostic) using SYBR Green (Fast Start DNA Master SYBR Green I; Roche Applied Science, Roche Diagnostic), specific primers for IL-4 and INF-γ, and a corresponding hydrolysis probe designed by Roche (Universal Probe Library) (Table 1) under the following conditions: 95°C for 5 min, and 45 cycles at 95°C for 15 s and 60°C for 15 s, then 72°C for 15 s. PCR was also carried out for several housekeeping genes; namely, 18s ribosomal RNA, RPL32, and hypoxanthine-guanine phosphoribosyltransferase (HPRT). We used the geometric mean of these three reference genes to normalize the Q-PCR results, using Roche LightCycler 2.0 software (Roche Diagnostic). The use of a multiple housekeeping gene-based normalization approach provides the most conservative and reliable method of data normalization and greatly improves the accuracy of unbiased gene expression levels.58–60 We expressed results as 2-deltaCp, where Cp is the cycle threshold number. We analyzed dissociation curves after each run for each amplicon to access the specificity of quantification when using SYBR Green. Q-PCR for TGFBI mRNA was also carried out as a measure of active TGF-β protein in renal tissues of rodents, as described previously in several articles.35–37
Table 1.
| Gene Name | Forward Sequence | Reverse Sequence |
|---|---|---|
| IFNγ | TCAAGTGGCATAGATGTGGAAGAA | TGGCTCTGCAGGATTTTCATG |
| IL-4 | GAGAGATCATCGGCATTTTGA | TCTGTGGTGTTCTTCGTTGC |
| TGF-β1 | TGGAGCAACATGTGGAACTC | GTCAGCAGCCGGTTACCA |
| TGFBI | TCCTTGCCTGCGGAAGTG | GGAGAGCATTGAGCAGTTCGA |
| 18s | GCATGCACTCTCCCGTTC | AGCGCGAGAGAGGAGGAG |
| rpl32 | GCTGCCATCTGTTTTACGG | TGACTGGTGCCTGATGAACT |
| hprt1 | TCCTCCTCAGACCGCTTTT | CCTGGTTCATCATCGCTAATC |
Cytokine Levels
We measured plasma cytokine levels using mouse Th1/Th2 cytometric bead arrays (CBA; BD Biosciences, San Diego, CA), according to the manufacturer's protocol.
In Vivo Blocking of TGF-β
We blocked the in vivo activity of TGF-β using the mouse monoclonal antibody 1D11 (R&D Systems), as described previously.38,39 Briefly, mice received a single intravenous injection of 150 μg 1D11 2 d after injection of the anti-GBM serum, and the development of anti-GBM GN was evaluated on day 14. Control mice received a nonrelevant mouse IgG1.
Statistical Analysis
Quantitative analyses of histology and immunostaining were carried out using blinded coded slices. We analyzed a total of three to eight mice per group in two or three separate experiments, and the results are expressed as means ± SEM. We compared differences using an unpaired t test with Welch correction, performed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). Tubulointerstitial grades were compared using a two-sided Fischer exact t test. We considered differences to be significant when P was less than 0.05.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was financially supported by the Institut National de la Santé et de la Recherche Médicale and by the Faculté de Médecine Pierre et Marie Curie. L.M. is a research fellow of INSERM. A.C.K. is recipient of grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP no. 2007/07120-0) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq no. 470716/2007), Brazil.
Published online ahead of print. Publication date available at www.jasn.org.
L.M. and A.C.K. contributed equally to this work
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Wilson GW, Oliver J: Experiments on the production of specific antisera for infections of unknown cause: III. Nephrotoxins: Their specificity as demonstrated by the method of selective absorption. J Exp Med 32: 183–198, 1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masugi M: Uber die spezifischen zytotoxischen veranderungen der niere und der leber durch das spezifische antiserum (nephrotoxin and hepatotoxin). Zugleich ein beitrag zur pathogenese der glomerulonephritis. Jap Path Soc 21: 329–341, 1932 [Google Scholar]
- 3.Tipping PG, Kitching AR: Glomerulonephritis, Th1 and Th2: What's new? Clin Exp Immunol 142: 207–215, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheeler J, Morley AR, Appleton DR: Anti-glomerular basement membrane (GBM) glomerulonephritis in the mouse: Development of disease and cell proliferation. J Exp Pathol (Oxford) 71: 411–422, 1990 [PMC free article] [PubMed] [Google Scholar]
- 5.Tipping PG, Huang XR, Qi M, Van GY, Tang WW: Crescentic glomerulonephritis in CD4- and CD8-deficient mice. Requirement for CD4 but not CD8 cells. Am J Pathol 152: 1541–1548, 1998 [PMC free article] [PubMed] [Google Scholar]
- 6.Salant DJ, Cybulsky AV: Experimental glomerulonephritis. Methods Enzymol 162: 421–461, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Tipping PG, Holdsworth SR: T cells in glomerulonephritis. Springer Semin Immunopathol 24: 377–393, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Ruth AJ, Kitching AR, Semple TJ, Tipping PG, Holdsworth SR: Intrinsic renal cell expression of CD40 directs Th1 effectors inducing experimental crescentic glomerulonephritis. J Am Soc Nephrol 14: 2813–2822, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Timoshanko JR, Kitching AR, Holdsworth SR, Tipping PG: Interleukin-12 from intrinsic cells is an effector of renal injury in crescentic glomerulonephritis. J Am Soc Nephrol 12: 464–471, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Kitching AR, Tipping PG, Mutch DA, Huang XR, Holdsworth SR: Interleukin-4 deficiency enhances Th1 responses and crescentic glomerulonephritis in mice. Kidney Int 53: 112–118, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M: Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med 192: 741–754, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC: Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med 204: 995–1001, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dommelen SL, Tabarias HA, Smyth MJ, Degli-Esposti MA: Activation of natural killer (NK) T cells during murine cytomegalovirus infection enhances the antiviral response mediated by NK cells. J Virol 77: 1877–1884, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV: Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med 192: 921–930, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leite-De-Moraes MC, Hameg A, Arnould A, Machavoine F, Koezuka Y, Schneider E, Herbelin A, Dy M: A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J Immunol 163: 5871–5876, 1999 [PubMed] [Google Scholar]
- 16.Tupin E, Kinjo Y, Kronenberg M: The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol 5: 405–417, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hansen DS, Siomos MA, Buckingham L, Scalzo AA, Schofield L: Regulation of murine cerebral malaria pathogenesis by CD1d-restricted NKT cells and the natural killer complex. Immunity 18: 391–402, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT: Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med 9: 582–588, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, Fourneau JM, Sidobre S, Kronenberg M, Taniguchi M, Van Endert P, Dy M, Askenase P, Russo M, Vargaftig BB, Herbelin A, Leite-de-Moraes MC: Cutting edge: Invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol 171: 1637–1641, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hachem P, Lisbonne M, Michel ML, Diem S, Roongapinun S, Lefort J, Marchal G, Herbelin A, Askenase PW, Dy M, Leite-de-Moraes MC: Alpha-galactosylceramide-induced iNKT cells suppress experimental allergic asthma in sensitized mice: Role of IFN-gamma. Eur J Immunol 35: 2793–2802, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Singh AK, Yang JQ, Parekh VV, Wei J, Wang CR, Joyce S, Singh RR, Van Kaer L: The natural killer T cell ligand alpha-galactosylceramide prevents or promotes pristane-induced lupus in mice. Eur J Immunol 35: 1143–1154, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sireci G, Russo D, Dieli F, Porcelli SA, Taniguchi M, La Manna MP, Di Liberto D, Scarpa F, Salerno A: Immunoregulatory role of Jalpha281 T cells in aged mice developing lupus-like nephritis. Eur J Immunol 37: 425–433, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Yang JQ, Wen X, Liu H, Folayan G, Dong X, Zhou M, Van Kaer L, Singh RR: Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum 56: 1219–1233, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J-Q, Singh AK, Wilson MT, Satoh M, Stanic AK, Park J-J, Hong S, Gadola SD, Mizutani A, Kakumanu SR, Reeves WH, Cerundolo V, Joyce S, Van Kaer L, Singh RR: Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J Immunol 171: 2142–2153, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Oishi Y, Sumida T, Sakamoto A, Kita Y, Kurasawa K, Nawata Y, Takabayashi K, Takahashi H, Yoshida S, Taniguchi M, Saito Y, Iwamoto I: Selective reduction and recovery of invariant Valpha24JalphaQ T cell receptor T cells in correlation with disease activity in patients with systemic lupus erythematosus. J Rheumatol 28: 275–283, 2001 [PubMed] [Google Scholar]
- 26.Kinsey GR, Li L, Okusa MD: Inflammation in acute kidney injury. Nephron Exp Nephrol 109: e102–e107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD: NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Yang SH, Kim SJ, Kim N, Oh JE, Lee JG, Chung NH, Kim S, Kim YS: NKT cells inhibit the development of experimental crescentic glomerulonephritis. J Am Soc Nephrol 19: 1663–1671, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M: Requirement for V{alpha}14 NKT cells in IL-12-mediated rejection of tumors. Science 278: 1623–1626, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Askenase PW, Szczepanik M, Itakura A, Kiener C, Campos RA: Extravascular T-cell recruitment requires initiation begun by Valpha14+ NKT cells and B-1 B cells. Trends Immunol 25: 441–449, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Stein-Streilein J: Invariant NKT cells as initiators, licensors, and facilitators of the adaptive immune response. J Exp Med 198: 1779–1783, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tipping PG, Kitching AR, Cunningham MA, Holdsworth SR: Immunopathogenesis of crescentic glomerulonephritis. Curr Opin Nephrol Hypertens 8: 281–286, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Hertig A, Berrou J, Allory Y, Breton L, Commo F, Costa De Beauregard MA, Carmeliet P, Rondeau E: Type 1 plasminogen activator inhibitor deficiency aggravates the course of experimental glomerulonephritis through overactivation of transforming growth factor beta. FASEB J 17: 1904–1906, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Huang XR, Chung AC, Zhou L, Wang XJ, Lan HY: Latent TGF-beta1 protects against crescentic glomerulonephritis. J Am Soc Nephrol 19: 233–242, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert RE, Wilkinson-Berka JL, Johnson DW, Cox A, Soulis T, Wu LL, Kelly DJ, Jerums G, Pollock CA, Cooper ME: Renal expression of transforming growth factor-beta inducible gene-h3 (beta ig-h3) in normal and diabetic rats. Kidney Int 54: 1052–1062, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Edgtton KL, Gow RM, Kelly DJ, Carmeliet P, Kitching AR: Plasmin is not protective in experimental renal interstitial fibrosis. Kidney Int 66: 68–76, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Li C, Lim SW, Choi BS, Lee SH, Cha JH, Kim IS, Kim J, Yang CW: Inhibitory effect of pravastatin on transforming growth factor beta1-inducible gene h3 expression in a rat model of chronic cyclosporine nephropathy. Am J Nephrol 25: 611–620, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Esplugues E, Sancho D, Vega-Ramos J, Martinez-A C, Syrbe U, Hamann A, Engel P, Sanchez-Madrid F, Lauzurica P: Enhanced antitumor immunity in mice deficient in CD69. J Exp Med 197: 1093–1106, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sancho D, Gomez M, Viedma F, Esplugues E, Gordon-Alonso M, Garcia-Lopez MA, de la Fuente H, Martinez AC, Lauzurica P, Sanchez-Madrid F: CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis. J Clin Invest 112: 872–882, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ka S-M, Huang X-R, Lan H-Y, Tsai P-Y, Yang S-M, Shui H-A, Chen A: Smad7 gene therapy ameliorates an autoimmune crescentic glomerulonephritis in mice. J Am Soc Nephrol 18: 1777–1788, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Kanamaru Y, Nakao A, Mamura M, Suzuki Y, Shirato I, Okumura K, Tomino Y, Ra C: Blockade of TGF-beta signaling in T cells prevents the development of experimental glomerulonephritis. J Immunol 166: 2818–2823, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Hou C-C, Wang W, Huang XR, Fu P, Chen T-H, Sheikh-Hamad D, Lan HY: Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-{beta} signaling and fibrosis in rat remnant kidney. Am J Pathol 166: 761–771, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akagi Y, Isaka Y, Arai M, Kaneko T, Takenaka M, Moriyama T, Kaneda Y, Ando A, Orita Y, Kamada T, Ueda N, Imai E: Inhibition of TGF-beta 1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int 50: 148–155, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Ebert EC: Inhibitory effects of transforming growth factor-beta (TGF-beta) on certain functions of intraepithelial lymphocytes. Clin Exp Immunol 115: 415–420, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S: Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 90: 770–774, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Yang P, Zhou H, Meng Q, Huang X: Involvement of Foxp3-expressing CD4(+) CD25(+) regulatory T cells in the development of tolerance induced by transforming growth factor-beta(2)-treated antigen-presenting cells. Immunology 124: 304–314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christ M, McCartney-Francis NL, Kulkarni AB, Ward JM, Mizel DE, Mackall CL, Gress RE, Hines KL, Tian H, Karlsson S: Immune dysregulation in TGF-beta 1-deficient mice. J Immunol 153: 1936–1946, 1994 [PubMed] [Google Scholar]
- 48.Bottinger EP, Kopp JB: Lessons from TGF-beta transgenic mice. Miner Electrolyte Metab 24: 154–160, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Li MO, Sanjabi S, Flavell RA: Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25: 455–471, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Yu L, Border WA, Huang Y, Noble NA: TGF-beta isoforms in renal fibrogenesis. Kidney Int 64: 844–856, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Huang XR, Li AG, Liu F, Li J-H, Truong LD, Wang XJ, Lan HY: Signaling mechanism of TGF-{beta}1 in prevention of renal inflammation: Role of Smad7. J Am Soc Nephrol 16: 1371–1383, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Raz E, Dudler J, Lotz M, Baird SM, Berry CC, Eisenberg RA, Carson DA: Modulation of disease activity in murine systemic lupus erythematosus by cytokine gene delivery. Lupus 4: 286–292, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Arrenberg P, Halder R, Kumar V: Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol 218: 246–250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, Platt F, Trottein F: Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27: 597–609, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Terabe M, Berzofsky JA: NKT cells in immunoregulation of tumor immunity: A new immunoregulatory axis. Trends Immunol 28: 491–496, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC: RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med 185: 1371–1380, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morley AR, Wheeler J: Cell proliferation within Bowman's capsule in mice. J Pathol 145: 315–327, 1985 [DOI] [PubMed] [Google Scholar]
- 58.Wong ML, Medrano JF: Real-time PCR for mRNA quantitation. Biotechniques 39: 75–85, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F: Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elberg G, Elberg D, Logan CJ, Chen L, Turman MA: Limitations of commonly used internal controls for real-time RT-PCR analysis of renal epithelial-mesenchymal cell transition. Nephron Exp Nephrol 102: e113–e122, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.