Abstract
Mutations in the WNK kinases WNK1 and WNK4 cause a rare familial form of hypertension (Gordon syndrome) by increasing expression of the thiazide-sensitive co-transporter NCCT in the kidney. Regulation of NCCT expression involves a scaffold of proteins composed of several kinases, including the third member of the WNK kinase family, WNK3. This protein, expressed in several tissues including kidney and brain, displays splice variation around exons 18 and 22. We expressed these proteins in Xenopus oocytes and found that the renal isoform of WNK3 increased but the brain isoform decreased NCCT expression and activity. Introduction of a kinase-inactivating mutation into renal WNK3 reversed its action on NCCT, and the same mutation in the brain isoforms led to loss of function. We also studied the effect of phosphorylation of a key NCCT threonine (T58) on the effects of WNK3/4 coexpression; NCCT mutants with a T58A or T58D substitution had the same surface expression as T58 but had significantly altered transporter activity; however, both isoforms of WNK3 as well as WNK4 still modulated expression of these NCCT mutants. Finally, experiments using kinase-dead STE20/SPS1-related proline/alanine-rich kinase (SPAK), a putative downstream target for WNKs, revealed that brain WNK3 acts in tandem with SPAK, whereas renal WNK3 seems to upregulate NCCT through a SPAK-independent pathway. Taken together, these results suggest that the C-terminal motifs contributed by exons 18 and 22 play an important role in the actions of WNK3 isoforms on NCCT.
The Na-Cl transporter NCCT (SLC12A3) is expressed in the distal convoluted tubule and targeted by thiazide diuretics, one of the most widely used classes of antihypertensive therapy.1,2 In the past decade, the importance of NCCT in regulating BP has come from studying two rare familial BP syndromes. The first of these, Gitelman syndrome, is associated with low BP as a result of mutations in NCCT itself that reduce either its function or its expression in the distal convoluted tubule. In contrast, patients with the much rarer Gordon syndrome (pseudohypoaldosteronism type II) have high BP and overexpress NCCT. The mutations in Gordon syndrome are not in NCCT itself but are located in genes encoding two members of a novel family of serine-threonine kinases called WNK kinases (WNK13 and WNK43,4), which seem to regulate the trafficking of NCCT.4,5 The WNK kinases are a very small family within the kinome containing just four members and share an N-terminal catalytic domain and a regulatory C-terminal that includes a highly conserved acidic motif and two coil-coil domains6 (Figure 1).
Figure 1.
Structural differences between the brain and renal WNK3 isoforms. WNK3 in the brain exists as two isoforms. Isoform 1 contains a short version of exon 18 (18a), and isoform 2 contains a long version of exon 18 (18b) that has an additional 47 amino acids. Both isoforms contain exon 22. Renal WNK3 (isoform 3) contains only the shorter exon 18 (18a) and lacks exon 22.
Initially, it was thought that WNK4 inhibited forward trafficking of NCCT and that WNK1 interacted with it to suppress WNK4 function and restore NCCT expression at the cell surface5,7; however, it is now clear that SLC12A transporter regulation involves a complicated network of proteins that incorporates diverse kinases, phosphatases, and scaffolding proteins.8–11 One additional regulatory kinase is WNK3, the third member of the WNK family and a protein of approximately 1800 amino acids.6 It shows significant homology with the other WNK kinases and is expressed widely in human and mouse tissues.12,13 Human WNK3 has splice variation based around exons 18 and 22 that affects tissue distribution.12 In the brain, two isoforms of WNK3 exist. One contains a short version of exon 18 (exon 18a; isoform 1); the other contains a longer exon 18 with an additional 47 amino acids (exon 18b; isoform 2), and both contain exon 22. WNK3 in the kidney contains exon 18a but not exon 22 (isoform 3; Figure 1). For the rest of this article, brain WNK3 refers to the brain-specific isoform 2 and renal WNK3 refers to the renal-specific isoform 3. In contrast to the inhibitory effects of WNK4 on NCCT expression, renal WNK3 has been shown to increase membrane expression of NCCT, NKCC1, and NKCC2 in oocytes and also to inhibit the basolateral K-Cl transporters KCC1 through 4.13,14 Kinase-dead (KD) renal WNK3 mutants produce opposite effects. The function of brain WNK3 is unknown,15 but reports that a C-terminal fragment of renal WNK3 is able to stimulate NCCT expression points to key motifs within it being responsible for the stimulatory actions of WNK3.8
Although WNK kinases can affect the density of NCCT transporters in the cell membrane through an effect on NCCT trafficking,6 it is clear that NCCT function can also be affected by the phosphorylation state of key serine/threonine residues in the N-terminal of NCCT (especially T58 in rodent sequence or T60 in human). For example, in Xenopus oocytes, increased NCCT phosphorylation in response to chloride depletion has been observed to increase 22Na+ flux through NCCT without any change in surface membrane expression of the transporter.16 Nevertheless, there is no evidence that WNK kinases actually phosphorylate NCCT directly. This is thought to involve WNK proteins phosphorylating an intermediary kinase, STE20/SPS1-related proline/alanine-rich kinase (SPAK), which is responsible for the actual phosphorylation of the T58 residue of NCCT.17 Indeed, SPAK is phosphorylated and activated by both WNK4 and WNK117,18 and can phosphorylate human NCCT at three conserved N-terminal residues, including T60.9 In HEK 293 cells, site mutation of T60 to alanine not only abrogates phosphorylation by SPAK at this residue but also reduces phosphorylation at the nearby T46 and T55 sites and markedly attenuates NCCT activation by a low-chloride medium.9 SPAK also seems necessary for (renal) WNK3 activation of the related sodium co-transporter NKCC2.19
Because splice variation within the C-terminal region of WNK3 is likely to affect protein–protein interactions with the complex scaffold of interacting proteins regulating NCCT, we hypothesized that the WNK3 splice variants may differentially affect NCCT expression. In addition, we investigated how the WNK3–NCCT interaction might be affected by mutation of the key T residue in the N-terminal of NCCT (T58) to mimic different phosphorylation states of the transporter. We have also looked at the involvement of SPAK in the effects of WNK3 by coexpressing a dominant-negative KD mutant (SPAK KD). We report here that renal and brain isoforms of WNK3 produce opposite actions on NCCT expression and that SPAK KD differentially blocks the actions of the WNK3 isoforms. Mutation of the T58 residue in NCCT also affects transporter activity but does not affect basal membrane expression or the ability of WNK3 isoforms or WNK4 to alter transporter density in oocyte membranes.
RESULTS
Alternative Splicing of WNK3 Exons 18 and 22 in Mouse Kidney and Brain
PCR using exon 18–specific primers in the human renal and brain WNK3 clones used for the subsequent oocyte experiments confirmed that a shorter product (291 bp) was present in the renal clone and a longer product in brain WNK3 (432 bp) corresponding to exons 18a and 18b, respectively (Figure 2A). PCR of mouse tissue cDNA showed that brain contains both exon 18b and exon 18a, but the kidney contains only the shorter exon 18a.
Figure 2.
Agarose gel electrophoresis of WNK3 exons 18 and 22. (A) PCR of exon 18 of human WNK3 containing plasmid (renal and brain) showing exon 18a (291 bp) and exon 18b (432 bp), respectively. Reverse transcription–PCR (RT-PCR) using mouse tissue shows that the brain contains both exon 18a and exon 18b, whereas the mouse kidney contains only exon 18a. (B) PCR for exon 22 of the human WNK3 containing plasmid shows the shorter product attributable to exon 22 deletion from the renal isoform. RT-PCR of mouse tissue confirms that only the brain expresses exon 22. W and M are water and 100-bp ladders respectively. K, kidney; B, brain.
Similarly, using primers to amplify exon 22, the human clones showed a difference of 33 bp corresponding to the expected additional 11 amino acids in the brain form of WNK3 contributed by exon 22 (Figure 2B). Using the mouse cDNAs, only the brain contains a transcript corresponding to exon 22. Thus, as in humans, murine brain expresses both exon 18a and exon 18b and also contains exon 22, whereas WNK3 in the kidney contains only exon 18a and lacks exon 22.
Brain WNK3 Produces Opposite Effects to Renal WNK3
For comparison of the effects of the two isoforms of WNK3, the clones were first coexpressed with wild-type (wt) NCCT by injection of the respective cRNAs into Xenopus oocytes. The 22Na+ flux measurable in NCCT-injected oocytes was completely inhibited by hydrochlorothiazide (100 μM; Figure 3A). Renal WNK3 co-injection with NCCT increased 22Na+ uptake by 2.5-fold (6.96 ± 1.29 to 16.88 ± 1.60 nmol/oocyte per h; P < 10−4), in keeping with previous reports; however, brain WNK3 produced the opposite effect, reducing 22Na+ uptake through NCCT by almost one half (6.96 ± 1.29 to 3.51 ± 0.21 nmol/oocyte per h; P < 10−4). The effects of both isoforms were kinase dependent, but the loss of kinase activity affected the isoforms differently. Hence, KD renal WNK3 (D294A) reduced 22Na+ flux compared with NCCT injected alone, and KD brain WNK3 (D294A) lost its ability to inhibit NCCT. The action of brain WNK3 was reminiscent of WNK4 in this system in that both were able to suppress NCCT expression and the KD mutant was not functional (Figure 3A).
Figure 3.
Effect of WNK3 isoforms versus WNK4. (A) 22Na+ flux in oocytes injected with NCCT and either WNK3 (R, renal; B, brain) or WNK4 as indicated. Significant differences from NCCT alone are indicated. *P < 0.001. (B) ECFP-NCCT fluorescence signal from oocytes injected as in A. Significant differences are indicated. *P < 0.001; **P < 0.05. (C) Representative confocal microscopy images for each injection condition in B. HCTZ, hydrochlorothiazide.
Confocal microscopy of enhanced cyan flourescent protein (ECFP)-NCCT injected oocytes showed that WNK3 effects on NCCT flux activity were accompanied by parallel changes in surface membrane expression of the transporter (Figure 3). Figure 3B shows that renal WNK3 increased membrane expression of ECFP-NCCT approximately two-fold (31.2 ± 4.1 to 64.2 ± 5.8 arbitrary fluorescence units [AFU]; P < 10−3), whereas brain WNK3 reduced it by approximately 50% (31.2 ± 4.1 to 15.2 ± 1.6 AFU; P < 10−3). The KD forms of WNK3 also produced changes in expression that paralleled their effects on 22Na+ flux.
NCCT T58 Mutants Display Altered Activity but not Surface Expression
Xenopus oocytes were injected with cDNA for wt ECFP-NCCT or the T58A or T58D mutants of ECFP-NCCT. Figure 4A shows that 22Na+ uptake by NCCT T58A, which mimics the nonphosphorylated form of the transporter, was reduced by one half compared with wt. Conversely, 22Na+ flux through NCCT T58D, a mimic of the phosphorylated transporter, was increased two-fold over wt. Despite these changes in activity, the membrane expression of NCCT was not altered in either of the T58 mutants on the basis of their membrane fluorescence (Figure 4B).
Figure 4.
Effect of T58 substitution on NCCT. (A) 22Na+ flux in oocytes injected with NCCT wt, T58A NCCT, or T58D NCCT mutants. Significant difference from NCCT wt is indicated. *P < 0.001. (B) ECFP-NCCT fluorescence signal from oocytes injected as in A.
Effect of WNK3 Isoforms on NCCT Are not Altered by NCCT T58 Mutation
The brain isoform of WNK3 inhibited 22Na+ flux through the T58D and T58A mutants of NCCT in the same qualitative manner as wt NCCT, and the converse held for coexpression of the renal isoform of WNK3 that stimulated 22Na+ flux through NCCT T58D mutant (Figures 5A and 6A). The KD forms of brain and renal WNK3 (D294A) affected 22Na+ flux through the NCCT T58D mutant in the same way as wt NCCT: The brain isoform was nonfunctional and the renal isoform reversed its function, inhibiting flux. Confocal microscopy of the same oocytes showed that the changes in activity when WNK3 isoforms were coexpressed with the NCCT T58 mutants was accompanied by exactly parallel changes in surface expression (Figures 5B and 6B).
Figure 5.
Effect of brain WNK3 on T58 NCCT mutants. (A) 22Na+ flux in oocytes injected with NCCT wt or T58 mutants and brain WNK3 isoform as indicated. Significant difference from NCCT clone used is indicated. *P < 0.001; ***P < 0.01. (B) ECFP-NCCT fluorescence signal from oocytes injected as in A. Significant difference from NCCT is indicated. *P < 0.001; ***P < 0.01; **P < 0.05.
Figure 6.
Effect of renal WNK3 on T58 NCCT mutants. (A) 22Na+ flux in oocytes injected with NCCT wt or T58 mutants and renal WNK3 isoform as indicated. Significant difference from NCCT clone is indicated. *P < 0.001; ***P < 0.01. (B) eCFP-NCCT fluorescence signal from oocytes injected as in A. Significant difference from NCCT is indicated. *P < 0.001.
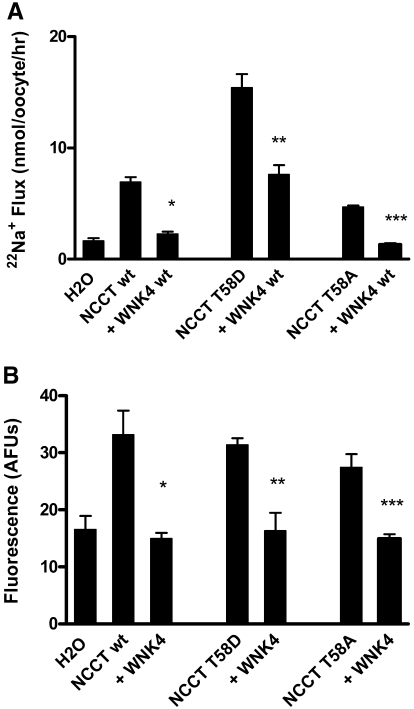
WNK4 Effect on NCCT Is not Influenced by NCCT Threonine 58 Mutation
For determination of whether WNK4 behaved in a similar manner to WNK3 when coexpressed with the T58 mutants, wt WNK4 cRNA was injected into Xenopus oocytes along with either wt T58D or T58A NCCT. The 22Na+ flux was reduced by WNK4 for all three NCCT transporters (Figure 7A), and confocal microscopy showed that this reflected a parallel reduction in surface membrane expression of ECFP-NCCT (Figure 7B).
Figure 7.
Effect of WNK4 on T58 NCCT mutants. (A) 22Na+ flux in oocytes injected with NCCT wt or T58 mutants and WNK4 as indicated. Significant difference from NCCT clone is indicated. *P < 0.001; ***P < 0.01. (B) ECFP-NCCT fluorescence signal from oocytes injected as in A. Significant difference from NCCT is indicated. *P < 0.001; ***P < 0.01; **P < 0.05.
Role of SPAK in the Regulation of NCCT by WNK3 Isoforms
Coexpression of SPAK wt with NCCT did not affect 22Na+ flux through NCCT; however, coexpression of the dominant-negative SPAK KD (D212A) reduced 22Na+ flux through NCCT by at least 55% (7.99 ± 1.79 versus 3.55 ± 0.46 nmol/oocyte per h; P < 10−4; Figure 8A). Similarly, SPAK wt did not affect regulation of NCCT by either WNK3 isoform (Figure 8, B and C). The coexpression of SPAK KD did not prevent NCCT regulation by renal WNK3 or brain WNK3 wt, but it did abolish the NCCT flux with brain WNK3 KD (13.60 ± 2.44 versus 3.13 ± 0.72 nmol/oocyte per h; P < 10−4; Figure 8C).
Figure 8.
Effect of SPAK wt and SPAK KD on NCCT and its regulation by WNK3 isoforms. (A) SPAK KD but not SPAK wt inhibits 22Na+ flux through wt NCCT. Significance from NCCT is indicated. *P < 0.001. (B) Neither SPAK wt nor SPAK KD affects regulation by renal WNK3. (C) SPAK wt does not affect regulation of NCCT by brain WNK3 wt or KD; however, SPAK KD inhibits NCCT when coexpressed with brain WNK3 KD but not brain WNK3 wt. Significance from NCCT + WNK3 KD is indicated. *P < 0.001.
NCCT, WNK3, and SPAK Do not Directly Interact
Immunoprecipitation of 35S-Methionine–labeled proteins with anti-NCCT antibody failed to show an interaction between NCCT and either WNK3 isoform (expected approximately 190 kD; Supplemental Figures S1 and S2). Using anti-HA antibody to precipitate SPAK, there was also no demonstrable interaction between SPAK and either WNK3 isoform or with NCCT (Supplemental Figures S1 and S2).
DISCUSSION
The control of distal tubular sodium handling through NCCT is regulated by diverse kinases, phosphatases, and scaffolding proteins.8–11 In addition to WNK1 and WNK4, WNK3 is now recognized as an important regulator of NCCT activity,15 although there are no reports of mutations within this protein among families with Gordon syndrome. WNK4 seems to reduce NCCT expression by reducing forward trafficking, but WNK1 does not affect NCCT expression in Xenopus oocytes unless coexpressed with WNK4.5 This is in marked contrast to the interaction of WNK proteins with the ROMK ion channel, where WNK1 and WNK4 both independently regulate internalization.20
Our findings with WNK3 confirm previous reports that renal WNK3 increases NCCT Na+ flux by increasing surface membrane expression.10,13 This effect requires intact WNK3 kinase activity; however, the KD mutation does not simply lose its activity but actually reverses it. In fact, this behavior is seen in other members of the WNK kinase family in which intermolecular protein–protein interactions are as important as the catalytically active kinase domain to the functionality of the WNK protein. Wang et al.21 also highlighted the importance of intramolecular salt bridge formation in the functioning of WNK1. Our novel finding is that the splice variant of WNK3 found in the brain behaves substantially differently from the renal isoform. The brain isoform behaves, in fact, like WNK4 in that it inhibits NCCT by reducing its surface expression, and it does this in a kinase-dependent manner,5 so the KD mutants of WNK4 and brain WNK3 are nonfunctional and do not show the reversal of function seen with renal WNK3. This splice variation in WNK3 is not confined to the human genome; we have demonstrated that tissue-specific WNK3 splice variation also occurs in the mouse.
We investigated the role of the putative downstream kinase SPAK in regulating NCCT function, because it was previously reported to be involved in the regulation of the related CC co-transporter NKCC2.19 Expression of SPAK wt does not significantly alter NCCT function in our hands; neither does it affect NCCT regulation by either wt or KD WNK3 isoforms. In contrast, the dominant-negative SPAK KD (D212A) reduces basal NCCT activity but does not affect either the upregulation of NCCT caused by renal WNK3 wt or downregulation by renal WNK3 KD. This result differs from NKCC2, for which SPAK KD reduced basal NKCC2 activity but also antagonized the upregulation of NKCC2 by renal WNK319; however, the interaction of SPAK KD and brain WNK3 is strikingly different, with SPAK KD suppressing NCCT function when coexpressed with brain WNK3 KD. This suggests that brain WNK3 acts in tandem with SPAK, whereas the renal isoform seems to upregulate NCCT through a pathway that is not dependent on SPAK. This could explain the divergent effects of the two WNK3 isoforms with the brain form operating purely in tandem with SPAK; however, the different effects of the two isoforms do not seem to be explained by differences in the physical interaction of SPAK with either isoform and/or NCCT. In fact, under the conditions we used, we cannot show any evidence that either WNK3 isoform interacts with NCCT or SPAK.
It has been suggested that WNK3 might have a pivotal role in regulating neuronal excitability by stimulating Cl− entry through NKCC1 and inhibit Cl− exit through the K/Cl co-transporter KCC2.13 The resulting increase in intracellular [Cl−] would affect the neuronal response to GABAA/chloride ionophore activation by pushing its response in a depolarizing direction; however, this work was based on expression of the renal isoform of WNK3 in oocytes and not the brain isoform. Because the brain isoform clearly behaves very differently against NCCT, its effects on KCC2 and NKCC1 need to be formally explored before a clear role for WNK3 in membrane excitability within the central nervous system can be made.
The difference between the two WNK3 isoforms amounts to two small peptide insertions into the C-terminal encoded by exon 18b and exon 22. The C-terminal is thought to be important for the renal isoform of WNK3 because C-terminal fragments but not an N-terminal fragment encompassing the kinase domain are sufficient to reproduce the activating effect on NCCT in oocytes.8 Coil–coil domains are important for protein interaction, and deletion of the second coil–coil domain of WNK4 disrupts its interaction with WNK3.8 Because the peptides encoded by exons 18 and 22 in WNK3 occur immediately distal to C-terminal coil–coil domains, it is conceivable that the difference between the two isoforms reflects alteration in protein–protein interactions through the coil–coil regions, for example, in the recruitment of a phosphatase that may occur in the interaction of renal WNK3 with KCC1 through 4.10 The exon 18b sequence also contains two predicted myristoylation sites and three protein kinase C phosphorylation sites, but their role in regulating membrane targeting or response to signal transduction is uncertain.
Phosphorylation of three conserved N-terminal residues in members of the SLC12A transporter family was previously reported to alter transporter activity without affecting expression of the transporter at the cell surface. This has been reported for NKCC1 phosphorylation by SPAK/OSR117,22 and for NCCT after hypotonic low-chloride stimulation.16 Here we focused on the T58 residue because it seemed in the Xenopus oocyte system to be the most important of the three residues in NCCT.16 The Alessi group9 recently confirmed this in HEK cells and showed that alanine substitution of this residue blocked phosphorylation of the adjacent threonine residues (T46 and T55). This suggests that T58 phosphorylation may force a conformational change in the NCCT protein that facilitates further phosphorylation. In our hands, the nonphosphorylatable T58A NCCT mutant also shows reduced transporter activity, and the constitutively phosphorylated T58D NCCT has substantially increased activity. Interestingly, the same aspartate substitution in NKCC1 produces an inactivating effect like T58A in NCCT.23 The reason for this difference is not clear; however, despite high sequence homology within the phosphorylation motif, the N-terminal of NKCC1 is approximately 130 residues longer. Differences in three dimensional structure of their N-terminal domains may explain the divergent impact of T58 mutation in the two CC co-transporter proteins. For both T58 mutants, the level of expression of NCCT at the cell surface is the same, suggesting that this phospho-threonine activates intrinsic transporter kinetics. More important, neither NCCT mutation affects the ability of WNK4 or either isoform of WNK3 to affect transporter trafficking as measured by expression at the cell surface. This suggests that in oocytes at least, the effect of WNK3/4 on NCCT trafficking is not dependent on phosphorylation of T58. In HEK cells, it has been suggested that the WNK1–SPAK pathway regulates NCCT activity by T58 phosphorylation.9 There are no reports that either WNK3 or WNK4 uses the same signaling pathway in HEK cells. In fact, our results suggest they do not, and it is worth remembering that WNK1 is able to affect NCCT expression in oocytes only when coexpressed with WNK4.
Our in vitro findings confirm that there are two distinct methods for regulating NCCT function: One by modulating NCCT trafficking to the cell surface and the other by N-terminal phosphorylation affecting the intrinsic activity of individual transporters within the membrane. This model reflects the mutations seen in patients with Gitelman syndrome, which either block trafficking of NCCT (through impaired synthesis or glycosylation) or reduce its intrinsic transporter activity. The T60M mutation in particular24 (homologous to the mouse T58) emphasizes that the inability to phosphorylate this key threonine has pathophysiologic consequences. Nevertheless, the ability of WNK3/4 to alter NCCT expression regardless of the phosphorylation state of T58 suggests the two mechanisms operate relatively independently.
CONCISE METHODS
Cloning and cRNA Synthesis
Wt DNA for human full-length renal WNK3 in pcDNA3 and brain WNK3 in pT7TS plasmid were gifts of Dr. Shmuel Muallem (University of Texas, Dallas, TX) and Dr. Lucy Raymond (University of Cambridge, Cambridge, United Kingdom), respectively. Human HA-SPAK wt and HA-SPAK KD (212D→A) in pCMV5 plasmid from Dr. Hilary McLauchlan (University of Dundee, Dundee, United Kingdom) were subcloned into a modified pTNT vector. Wt mouse NCCT in ECFP-TNT with cyan fluorescence protein at its N-terminal and wt WNK4 in pcDNA3 were as described previously.5
Both WNK3 isoforms were mutated to produce KD (294D→A) proteins, and wt NCCT was mutated to 58T→D or 58T→A, respectively, using site-directed mutagenesis (Stratagene, Amsterdam, The Netherlands). All sequences were verified using an ABI 377 and Big Dye fluorescence chemistry (Applied Biosystems, Foster City, CA). Copy RNA was transcribed in vitro from linearized plasmids using T7 and SP6 mMESSAGE mMACHINE kits (Ambion, Austin, TX) and quantified using ultraviolet absorption spectroscopy (Nanodrop, Wilmington, DE).
Expression in Xenopus Oocytes
Xenopus laevis oocytes were harvested and defolliculated as detailed previously.4,5,20 Briefly, 10 ng of NCCT cRNA was injected in a total volume of 50 nl per oocyte, and for co-injections involving WNK3, WNK4, SPAK, or one of the mutants, an additional 10 ng of cRNA was added to the injectate. RNAase and DNAase-free water-injected oocytes were used as controls throughout. Oocytes were then incubated in ND96 containing 2 mM sodium pyruvate and 0.1 mg/ml gentamicin at 18°C for 5 d before use.
For 22Na+ flux studies, oocytes were placed for 24 h in Cl−-free ND96 solution containing 96 mM sodium isethionate, 2 mM potassium gluconate, 1.8 mM calcium gluconate, 1 mM magnesium gluconate, 5 mM HEPES, 2.5 mM sodium pyruvate, and 50 μg/ml gentamicin. Thirty minutes before the addition of uptake medium, oocytes were added to Cl−-free ND96 with inhibitors (1 mM ouabain, 100 μM amiloride, and 100 μM bumetanide) according to the protocol of Gamba et al.25 Oocytes were then transferred to isotonic uptake medium (58 mM NaCl, 38 mM M-methyl-d-glucamine, 2 mM KCl, 1.8 mM CaCl2, and 5 mM HEPES with inhibitors [pH 7.4]) containing 22Na+ (2.5 μCi/ml) and incubated in shaking incubator at 30°C for 1 h. Oocytes were then washed five times with 5 ml of ice-cold aliquots of isotonic medium and counted individually in a gamma counter (Perkin-Elmer Cobra 5003, Perkin-Elmer, Waltham, MA). Thiazide sensitivity was shown using 100 μM hydrochlorothiazide (data not shown). Membrane expression measurements were performed 5 d after injection by laser-scanning confocal microscopy with a Leica DMRXA confocal microscope as described previously5,20 and presented as mean total fluorescence intensity in AFUs.
Reverse Transcription–PCR of Alternatively Spliced Murine WNK3
RNA was extracted from mouse renal and brain tissue using Triazol (Invitrogen) reagent and reverse-transcribed using Superscript III (Invitrogen). PCR amplification of the splice variants of exon 18 (producing 291- or 432-bp products) used a forward primer 5′-ATTCAAGATAGCCCTGCACAAT-3′ in exon 17 and reverse primer 5′-GTCAGAGGAATGGATCAGAAG-3′ in exon 19. Similarly, alternatively spliced transcripts of exon 22 were amplified with a forward primer in exon 21 (5′-GGTGGTCAGTCTTCAAACACAA-3′) and reverse primer in exon 23 (5′-GTCAACATCCCCTTCTTACTGG-3′). Exon 22 deletion reduced the product size by 33 bp. As a control, the same primers were used to amplify human WNK3 renal and brain clones used in the oocyte experiments.
Immunoprecipitation Experiments
Xenopus oocytes were injected with cRNA as already described and incubated at 18°C for 4 d in 1 mCi/ml 35S-Methionine containing MBS medium (88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.82 mM MgSO4, 0.33 mM CaCl2, 10 mM HEPES-NaOH, and 5 mg/dl gentamicin). Proteins were extracted using Digitonin homogenization buffer (100 mM NaCl, 0.5% Digitonin, 20 mM Tris-HCl [pH 7.6], 1 mM PMSF, and protease inhibitor cocktail). Protein extract was then incubated overnight at 4°C with primary antibody (polyclonal anti-NCCT antibody [Chemicon, Billerica, MA] or monoclonal anti-HA antibody [Sigma, St. Louis, MI]) and then incubated with Protein A Sepharose (Amersham) and Protein G agarose (Calbiochem) beads for 1 h. The beads were washed, and proteins were eluted with loading buffer. Samples were separated on an 8% SDS-PAGE. The gel was dried after fixation, and imaging was performed using x-ray film (Fuji, Düsseldorf, Germany).
Statistical Analysis
For all oocyte experiments, 10 to 15 oocytes were injected for each cRNA used, and the results are presented as means ± SEM. Differences between groups were compared by one-way ANOVA with post hoc analyses using Tukey or Scheffe test. Figures show representative experiments that were replicated using at least four different batches of oocytes from different donor animals and cRNA batches. SPSS 12 (SPSS, Chicago, IL) was used throughout with significance defined as P < 0.05.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
M.G. is supported by a British Heart Foundation Clinical PhD studentship award and the Sackler Foundation. A.M.Z. is supported by the Swiss National Science Foundation.
Published online ahead of print. Publication date available at www.jasn.org.
M.G. and A.M.Z. contributed equally to this work.
See related editorial, “Splicing a Kinase and the Regulation of Salt Transport,” on pages 1166–1168.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial: Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [published erratum appears in JAMA 289: 178, 2003 and JAMA 291: 2196, 2004]. JAMA 288: 2981–2997, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, McInnes GT, Thom S: Guidelines for management of hypertension: Report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens 18: 139–185, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Wilson FH, Disse-Nicodeme S, Chate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunal M, Milford DV, Lipkin GW, Achard JM, Feeley MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP: Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Golbang AP, Murthy M, Hamad A, Liu C-H, Cope G, Van’t Hoff W, Cuthbert AW, O’Shaughnessy KM: A new kindred with pseudohypoaldosteronism type II and a novel mutation (564D>H) in the acidic motif of the WNK4 gene. Hypertension 46: 295–300, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Golbang AP, Cope G, Hamad A, Murthy M, Liu C-H, Cuthbert AW, O’Shaughnessy KM: Regulation of the expression of the Na/Cl cotransporter by WNK4 and WNK1: Evidence that accelerated dynamin-dependent endocytosis is not involved. Am J Physiol Renal Physiol 291: F1369–F1376, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cope G, Golbang AP, O’Shaughnessy KM: WNK kinases and the control of blood pressure. Pharmacol Ther 106: 221–231, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Subramanya AR, Wade JB, Ellison DH, Welling PA: The carboxyl terminus of WNK4 suppresses forward trafficking of the thiazide-sensitive cotransporter. FASEB J 21: 938.14–2007. www.fasebj.org/cgi/content/meeting/abstract/21/6/A1337-a. Accessed April 9, 2009.
- 8.Yang CL, Zhu X, Ellison DH: The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest 117: 3403–3411, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson C, Rafiq FH, Karlsson HKR, Molelek N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR: Activation of the thiazide-sensitive Na-Cl cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 10.De los Heros P, Kahle KT, Rinehart J, Bobadilla NA, Vazquez N, San Cristobal P, Mount DB, Lifton RP, Hebert SC, Gamba G: WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc Natl Acad Sci U S A 103: 1976–1981, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagnon KB, England R, Diehl L, Delpire E: Apoptosis-associated tyrosine kinase scaffolding of protein phosphatase 1 and SPAK reveals a novel pathway for Na-K-Cl cotransporter regulation. Am J Physiol Cell Physiol 292: C1809–C1815, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Holden S, Cox J, Raymond FL: Cloning, genomic organization, alternative splicing and expression analysis of the human gene WNK3 (PRKWNK3). Gene 335: 109–119, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Kahle KT, Rinehart J, De los Heros P, Louvi A, Meade P, Vazquez N, Hebert SC, Gamba G, Gimenez I, Lifton RP: WNK3 modulates transport of Cl− in and out of cells: Implications for control of cell volume and neuronal excitability. Proc Natl Acad Sci U S A 102: 16783–16788, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rinehart J, Kahle KT, De los Heros P, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP: WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci U S A 102: 16777–16782, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahle KT, Ring AM, Lifton RP: Molecular physiology of the WNK kinases. Annu Rev Physiol 70: 329–355, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Pacheco-Alvarez D, Cristobal PS, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G: The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H: WNK-1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE related kinases, SPAK and OSR-1. J Biol Chem 280: 42685–42693, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Vitari AC, Deak M, Morrice NA, Alessi DR: The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391: 17–24, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponce-Coria J, San Cristobal P, Kahle KT, Vasquez N, Pacheco-Alvarez D, De los Heros P, Juarez P Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G: Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci U S A 105: 8458–8463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cope G, Murthy M, Golbang AP, Hamad A, Liu CH, Cuthbert AW, O’Shaughnessy KM: WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J Am Soc Nephrol 17: 1867–1874, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Wang HR, Liu Z, Huang CL: Domains of WNK1 kinase in the regulation of ROMK1. Am J Physiol Renal Physiol 295: F438–F445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagnon KB, England R, Delpire E: Volume sensitivity of cation Cl− cotransporters is modulated by the interaction of two kinases: Ste-related proline-alanine-rich kinase and WNK4. Am J Cell Physiol 290: C134–C142, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Darman RB, Forbush B: A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J Biol Chem 277: 37542–37550, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Lin S-H, Shiang J-C, Huang C-C, Yang S-S, Hsu Y-J, Cheng C-J: Phenotype and genotype analysis in Chinese Patients with Gitelman’s syndrome. J Clin Endocrinol Metab 90: 2500–2507, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC: Molecular cloning, primary structure and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.