Nondepleting anti-CD25 monoclonal antibodies (daclizumab) and depleting polyclonal antithymocyte globulin (Thymoglobulin) both prevent acute rejection, but these therapies have not been directly compared in a high-risk, HLA-sensitized renal transplant population. We randomly assigned 227 patients, who were about to receive a kidney graft from a deceased donor, to either Thymoglobulin or daclizumab if they met one of the following risk factors: current panel reactive antibodies (PRA) >30%; peak PRA >50%; loss of a first kidney graft from rejection within 2 yr of transplantation; or two or three previous grafts. Maintenance immunosuppression comprised tacrolimus, mycophenolate mofetil, and steroids. Compared with the daclizumab group, patients treated with Thymoglobulin had a lower incidence of both biopsy-proven acute rejection (15.0% versus 27.2%; P = 0.016) and steroid-resistant rejection (2.7% versus 14.9%; P = 0.002) at one year. One-year graft and patient survival rates were similar between the two groups. In a comparison of rejectors and nonrejectors, overall graft survival was significantly higher in the rejection-free group (87.2% versus 75.0%; P = 0.037). In conclusion, among high-immunological-risk renal transplant recipients, Thymoglobulin is superior to daclizumab for the prevention of biopsy-proven acute rejection, but there is no significant benefit to one-year graft or patient survival.
Acute rejection (AR) after renal transplantation can lead to rapid graft loss from irreversible rejection or to the onset of chronic graft rejection with ultimate graft failure. Moreover, despite the availability of potent immunosuppressive drugs such as tacrolimus and mycophenolate mofetil (MMF), the negative impact of AR episodes on graft survival has remained important.1 Acute rejection typically occurs during the first weeks after transplantation, and consequently, to suppress lymphocyte function, many kidney transplant recipients receive induction therapy with either lymphocyte-depleting rabbit antithymocyte globulin (ATG) or nondepleting IL-2 receptor-antagonizing monoclonal antibodies (IL2RA mAbs).2
Both types of anti-lymphocytes are equally effective in low-risk recipients (i.e., patients with no previous exposure to HLA antigens).3 Previous studies suggest that in this patient population induction therapy significantly decreases the incidence of one-year AR from 20 to 25% to 10 to 15%,3–5 with a corresponding incremental increase in both graft and patient survival.3–6
However, the patient population most likely to experience AR episodes are high-risk HLA-sensitized patients or those receiving a third or fourth kidney graft.7–10 This cohort represents 15 to 20% of waitlisted renal transplant recipients in the United States and Europe,11–13 and because of their broad HLA sensitization, these patients are often forced to spend prolonged periods on dialysis before a suitable graft can be allocated. It is also interesting to note that, because these patients experience more frequent and more severe AR that results in poorer graft survival than that of low-risk recipients,7–10 they are usually excluded from clinical trials that are designed to examine the efficacy of immunosuppressive agents.14–16
As a further rationale for our present trial, data from a decade ago has shown that in HLA-sensitized recipients treated with a combination of the older cyclosporine A formulation (Sandimmun, Novartis, Basel, Switzerland) and azathioprine (Imuran, GlaxoSmithKline, Greenford, UK) induction therapy with either ATG or OKT3 (Muromonab-CD3, Ortho Biotech, Bridgewater, NJ) mAb reduced rejection rates and improved graft survival.17
Despite these promising early data however, modern-day induction therapy with either ATG or IL2RA mAbs has only been compared in a single study of patients at high risk for delayed graft function or AR.18 Here, we report the results of a prospective, multicenter, randomized trial that compared biopsy-proven acute rejection (BPAR) incidence in high-immunological-risk renal transplant patients receiving induction therapy with either ATG or daclizumab.
RESULTS
Patient Characteristics and Demographics
Figure 1 depicts the patient flow throughout the trial. A total of 240 patients were enrolled from 19 sites in France and Belgium between May 2001 and November 2005. One-hundred-thirteen patients in the ATG arm and 114 in the daclizumab arm subsequently entered the trial and are reported here. The groups were well balanced with respect to patient demographics and baseline disease characteristics (Table 1), and patients were broadly sensitized against HLA antigens, as reflected by a mean current panel reactive antibodies (PRA) of 35% and a peak PRA of 72%. Approximately 10% of patients were highly HLA sensitized, confirmed by a peak PRA above 80%.
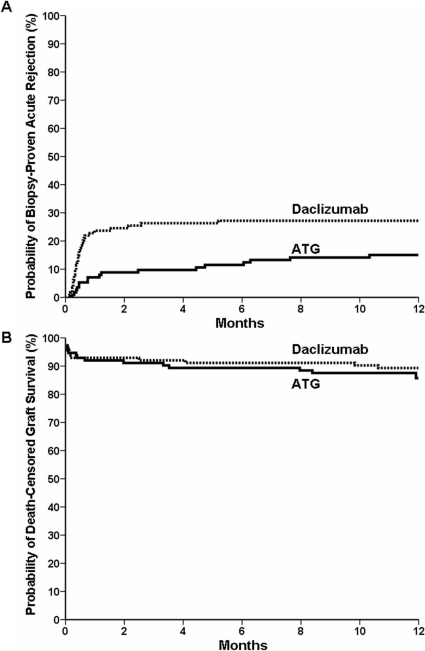
Figure 1.
Diagram depicting enrollment, randomization, and follow-up of study patients.
Table 1.
Baseline characteristics of organ recipients and donors
| ATG | Daclizumab | |
|---|---|---|
| Characteristics | n = 113 | n = 114 |
| Males, n (%) | 52 (46.0) | 59 (51.8) |
| Age, yr (mean ± SD) | 45.4 ± 10.3 | 46.9 ± 9.0 |
| Cause of ESRD, n (%) | ||
| Glomerulonephritis | 51 (45.2) | 45 (39.4) |
| Uropathy | 11 (9.7) | 15 (13.2) |
| Autosomal dominant polycystic kidney disease | 10 (8.9) | 10 (8.8) |
| Diabetes | 4 (3.5) | 2 (1.7) |
| Other | 26 (23.0) | 25 (21.9) |
| Unknown | 11 (9.7) | 17 (15.0) |
| Number of HLA mismatches | ||
| HLA A | 0.9 ± 0.7 | 0.9 ± 0.7 |
| HLA B | 1.1 ± 0.7 | 1.1 ± 0.8 |
| HLA DR | 0.9 ± 0.7 | 0.9 ± 0.8 |
| First graft, n (%): | 30 (26.5%) | 34 (29.8%) |
| Current PRA (mean ± SD, %) | 35 ± 32 | 39 ± 33 |
| Peak PRA (mean ± SD, %) | 77 ± 20 | 79 ± 20 |
| Second graft, n (%): | 59 (52.2%) | 58 (50.9%) |
| Current PRA (mean ± SD, %) | 35 ± 29 | 39 ± 31 |
| Peak PRA (mean ± SD, %) | 69 ± 23 | 75 ± 18 |
| Third and fourth graft, n (%): | 24 (21.2%) | 22 (19.3%) |
| Current PRA (mean ± SD, %) | 26 ± 30 | 27 ± 31 |
| Peak PRA (mean ± SD, %) | 60 ± 30 | 61 ± 27 |
| All patients | n = 113 | n = 114 |
| Current PRA (mean ± SD, %) | 33 ± 30 | 37 ± 32 |
| Peak PRA (mean ± SD, %) | 69 ± 25 | 74 ± 22 |
| % with PRA >80% | 8.8% | 11.4% |
| Cold ischemia time, h (mean ± SD) | 24.0 ± 7.9 | 22.7 ± 6.8 |
| Donor | ||
| Males, n (%) | 76 (67.3) | 65 (57.0) |
| Age, yr (mean ± SD) | 44.3 ± 13.8 | 44.6 ± 14.8 |
| Death from stroke, n (%) | 56 (49.6) | 46 (40.4) |
| Cytomegalovirus serologic status, n (%) | ||
| D+R+ | 37 (32.7) | 45 (39.5) |
| D+R− | 16 (14.2) | 12 (10.5) |
| D−R+ | 46 (40.7) | 42 (36.8) |
| D−R− | 14 (12.4) | 15 (13.2) |
With regard to graft history, 64 patients (28.4%) were recipients of a first graft, 117 (51.5%) of a second graft, 43 (18.9%) of a third graft, and 3 (1.3%) of a fourth graft. Among the 227 patients analyzed in the study, only 19 (8.4%) had a current PRA <30% and a peak PRA <50%. However, these 19 patients were also considered at high immunological risk, because 12 were recipients of a third graft and seven were recipients of a second graft after rejection of their previous graft within the first 2 years.
An intergroup comparison of the mean doses of the immunosuppressant agents administered during the study (i.e., tacrolimus, MMF, and steroids) showed that doses were similar in both groups at all times. Mean MMF doses in both groups were 1.8, 1.5, and 1.3 g/d at months 1, 3, and 12, respectively; mean methylprednisolone doses were approximately 19, 11, and 7 mg/d at months 1, 3, and 12, respectively. A similar intergroup comparison showed that tacrolimus trough levels were higher in daclizumab patients at month one (12.5 versus 11.1 ng/ml; P = 0.026) but that no intergroup differences occurred at any other time point (11.2 versus 11.2 ng/ml at month 3 (P = NS) and 9.1 versus 8.6 ng/ml at month 12 (P = NS)).
Efficacy Endpoints
The primary endpoint, BPAR, was observed in 17 (15.0%) ATG patients and 31 (27.2%) daclizumab patients (P = 0.016) (Figure 2 and Table 2). The median time between transplantation and rejection occurrence was significantly shorter in the daclizumab arm than that in the ATG arm (13 versus 35 d; P = 0.007). Rejection gradings are shown in Table 2.
Figure 2.
Cumulative probability of biopsy-proven acute rejection (A) and death-censored allograft survival (B), according to study group.
Table 2.
Intergroup comparison of key efficacy endpoints at 1 yr
| ATG (n = 113) | Daclizumab (n = 114) | P value | |
|---|---|---|---|
| BPAR | 17 (15.0%) | 31 (27.2%) | 0.016 |
| Borderline changes | 2 | 3 | |
| Grade I | 8 | 5 | |
| Grade IIa | 3 | 13 | |
| Grade IIb | 1 | 5 | |
| Grade III | 2 | 4 | |
| Pure AMR | 1 | 1 | |
| Steroid-resistant rejection | 3 (2.7%) | 17 (14.9%) | 0.002 |
| Median time to rejection (d) [interquartile range 25 to 75%] | 35 [13 to 164] | 13 [9 to 19] | 0.007 |
| Recurrent rejection | 4 (3.6%) | 7 (6.1%) | 0.54 |
| Delayed graft function | 35 (31.5%) | 50 (44.6%) | 0.044 |
| Graft loss | 20 (17.7%) | 16 (14.0%) | 0.47 |
| From death with functioning graft | 3 (2.7%) | 4 (3.5%) | |
| From primary nonfunction | 3 (2.7%) | 2 (1.8%) | |
| From vascular thrombosis | 2 (1.8%) | 1 (0.9%) | |
| From uncontrolled rejection | 3 (2.7%) | 3 (2.6%) | |
| From hemolytic uremic syndrome | 2 (1.8%) | 1 (0.9%) | |
| From technical failure | 2 (1.8%) | 1 (0.9%) | |
| From BK virus | 2 (1.8%) | 0 | |
| From other causes | 3 (2.7%) | 4 (3.5%) | |
| Death | 5 (4.4%) | 4 (3.5%) | 0.75 |
| From stroke | 2 (1.8%) | 0 | |
| From sepsis | 2 (1.8%) | 1 (0.9%) | |
| From bleeding | 0 | 1 (0.9%) | |
| From cancer | 1 (0.9%) | 0 | |
| From cardiac arrest | 0 | 1 (0.9%) | |
| Unknown cause | 0 | 1 (0.9%) |
The severity of rejection, as scored by the Banff criteria, was higher among the daclizumab patients than the ATG patients, but this difference did not reach statistical significance (P = 0.10). One patient in each arm experienced a rejection episode that was histologically described as antibody mediated, and only one patient (in the ATG group) experienced an episode of BPAR (grade borderline) that was left untreated. All patients received steroids boluses as a first-line treatment for rejection. Additional therapy was administered to 17 daclizumab patients (14.9%) and 3 ATG patients (2.7%) (P = 0.002). In the ATG group, one patient received OKT3 and intravenous immunoglobulin (IVIg), one patient received IVIg and plasmapheresis, and one patient was treated using plamapheresis alone. Of the daclizumab patients, seven received ATG, three received IVIg, three were treated using plasmapheresis, and one was treated with rituximab. Eleven patients experienced recurrent rejection, four in the ATG arm and seven in the daclizumab arm.
At one year, overall graft survival in the ATG and daclizumab groups were 82.3% and 86.0%, respectively (P = 0.47), death-censored graft survival were 85.0% and 89.5%, respectively (P = 0.42), and patient survival were 95.6% and 96.5%, respectively (P = 0.75) (Figure 2). One-year renal function as assessed by serum creatinine (mg/dl) and Modified Diet in Renal Disease GFR (ml/min/1.73 m2, mean ± SD) were 1.7 ± 1.2 versus 1.5 ± 0.6, respectively (P = 0.27), and 49.3 ± 17.9 versus 50.9 ± 17.2, respectively (P = 0.38). Proteinuria (g/d) at one year was 0.53 ± 1.40 (n = 69) and 0.30 ± 0.74 (n = 71) (P = 0.22). Delayed graft function occurred in 31.5% of ATG patients and 44.6% of daclizumab patients (P = 0.044).
To elucidate whether rejection had had a negative impact on the overall cohort of 227 patients, we performed a post hoc analysis to compare those patients who had experienced rejection (n = 48) with those who remained rejection-free (n = 179) at one year. Overall graft survival at one year was 87.2% among rejection-free patients, compared with 75.0% among patients with rejection episodes (P = 0.037).
Safety Endpoints
The proportion of patients who experienced bacterial infectious episodes was identical in both groups (46%; Table 3). However, the number of bacterial infectious events among patients who experienced at least one infection was higher in the ATG arm than that in the daclizumab arm (2.5 ± 1.8 versus 1.7 ± 1.2; P = 0.014). The percentage of cytomegalovirus infections requiring therapy was numerically more frequent in the ATG group when compared with the daclizumab group (18.6 versus 10.5%; P = 0.093). No patients developed Pneumocystis jiroveci infections or posttransplant lymphoproliferative disorder.
Table 3.
Intergroup comparison of key safety endpoints at 1 yr
| ATG (n = 113) | Daclizumab (n = 114) | P value | |
|---|---|---|---|
| Bacterial infection, n (%) | 53 (46.9%) | 53 (46.5%) | NS |
| No. of bacterial infections/patient (mean ± SD) | 2.5 ± 1,8 | 1.8 ± 1,2 | 0.014 |
| Treated cytomegalovirus infection, n (%) | 21 (18.6%) | 12 (10.5%) | 0.093 |
| Sepsis (n, %) | 8 (7.1%) | 8 (7.0%) | NS |
| Posttransplant lymphoproliferative disorder | 0 | 0 | NS |
| Pneumocystis jiroveci | 0 | 0 | NS |
| Fungal infections | 7 (6.2%) | 6 (5.3%) | NS |
| Cancer | 1 (0.9%) | 0 | NS |
| White blood cell count (×103/mm3) (mean ± SD) | |||
| Day 0 | 7.2 ± 2.5 | 7.1 ± 2.9 | 0,7 |
| 1 wk | 5.3 ± 4.2 | 7.8 ± 3.2 | <0.001 |
| 1 month | 5.8 ± 2.3 | 6.7 ± 2.8 | 0.007 |
| 1 yr | 5.8 ± 2.6 | 6.6 ± 2.5 | 0.056 |
| Lymphocyte count (×103/mm3) (mean ± SD) | |||
| Day 0 | 1.41 ± 0.65 | 1.54 ± 0.72 | 0.2 |
| 1 wk | 0.26 ± 0.23 | 1.29 ± 0.82 | <0.001 |
| 1 month | 0.54 ± 0.40 | 1.39 ± 0.97 | <0.001 |
| 1 yr | 0.97 ± 0.40 | 1.65 ± 0.87 | <0.001 |
| Neutrophil count (×103/mm3) (mean ± SD) | |||
| Day 0 | 4.76 ± 2.29 | 4.48 ± 2.10 | 0.40 |
| 1 wk | 4.61 ± 4.23 | 5.46 ± 2.42 | 0.09 |
| 1 month | 9.54 ± 4.86 | 4.79 ± 2.02 | 0.36 |
| 1 yr | 4.11 ± 2.52 | 4.25 ± 1.93 | 0.66 |
| Platelet count (×106/mm3) (mean ± SD) | |||
| Day 0 | 0.23 ± 0.09 | 0.24 ± 0.07 | 0.4 |
| 1 wk | 0.18 ± 0.07 | 0.23 ± 0.08 | <0.001 |
| 1 month | 0.26 ± 0.10 | 0.27 ± 0.10 | 0.70 |
| 1 yr | 0.23 ± 0.07 | 0.23 ± 0.07 | 0.75 |
Patients in the ATG group exhibited lower leukocyte counts throughout the study (Table 3). This was mainly due to a sharp drop in lymphocyte levels at week one, which remained significant after one year. Platelets were transiently lower among the ATG patients at week one but normalized thereafter.
DISCUSSION
The main result from this trial is that ATG was more effective than daclizumab in protecting high-immunological-risk renal-graft recipients from first-year AR. Not only were rejections less frequent, they were also less severe, as indicated by both lower histologic rejection grades and by the reduced incidence of steroid-resistant rejection episodes requiring additional therapy. It was also interesting to note that the lower rejection rates observed in the ATG arm occurred despite the delayed introduction of tacrolimus.
The rejection incidence that we observed in the daclizumab arm was approximately 30%. Although this is much higher than the 10 to 15% rejection incidence reported with a comparable quadruple immunosuppressive regimen in low-risk patients,1,16 it is similar to recent data from other studies involving HLA-sensitized patients.7–10
Our study was not powered to reveal significant differences in graft survival between the ATG and daclizumab arms, but post hoc analysis of the total patient population revealed that, when compared with patients who remained rejection-free, both graft function and graft survival were significantly lower among patients who experienced rejection. It is therefore likely that, as has been repeatedly observed in low-risk patients, first-year AR is an important surrogate endpoint for graft survival in patients with high immunological risk.
Longer-term follow-up of the present cohort, meta-analyses of similarly designed trials involving high-risk patients,18 and analysis of registry data could all contribute to an improved understanding of the long-term merits of ATG induction on graft survival in high-risk patients.
To date, only one prospective, randomized study has compared ATG with an anti-IL2RA mAb in patients at high risk for delayed graft function or AR.18 As in our study, maintenance immunosuppression comprised MMF and steroids, but ATG was given for 4 d instead of 8 d in our trial, cyclosporine A was given instead of tacrolimus, and basiliximab (Simulect, Novartis) was administered whereas we have been using daclizumab. Furthermore, unlike our study, only 10% of patients enrolled in Brennan's trial were retransplants (versus more than 70% in the present trial), and peak and current PRA values were 14% and 6%, respectively (versus 72% and 35%, respectively, in our cohort).
Despite these differences in immunosuppression and demography, the results of Brennan's study closely mirrored our findings. When compared with the basiliximab group, the ATG group reported a lower incidence of both AR (15.6% versus 25.5%; P = 0.02) and AR that required antibody treatment (1.4% versus 8.0%; P = 0.005). It can therefore be concluded that, in terms of rejection prevention, all high-risk patients benefit from ATG rather than IL2RA mAb induction therapy.
Exactly why ATG prevents AR more successfully than an IL2RA mAb in these high-risk patients remains unclear. It is possible that HLA-specific, memory T cells are able to use non-IL-2 cytokines for activation, while remaining susceptible to ATG-depleting effects. In addition, the B cell depleting actions of thymoglobulin may also be operative in HLA-sensitized patients.19
With regard to safety, ATG patients had lower lymphocyte counts that persisted at one year. They experienced more bacterial infections and showed a trend to more frequent cytomegalovirus infection. This, however, had no impact on patient survival. In addition, although the risk of posttransplant lymphoproliferative disorder has been reported to reach 1% in patients receiving ATG6 (a significantly higher level than that in patients given anti-IL2RA mAbs), no patients in either of our study groups developed the disorder.
There are several noticeable limitations to our study. First, it was an unblinded trial. Blinding the two arms would have been logistically complex and ethically compromising, because it would have required the maintenance of a central intravenous line in all patients. In reality, of course, a central intravenous line is needed only for ATG administration. Furthermore, clinicians would have easily identified the resultant antibody via a simple examination of leukocyte count.
Second, during the four years (2001–2005) required to recruit these relatively uncommon patients, donor-specific antibodies were not reported in the database, and our biopsies were still scored according to the Banff 97 criteria and not by the more recent classification that takes into account humoral rejection and C4d staining. The reason is that these techniques were not already widely available when the protocol was designed. Although reporting humoral rejection as a distinct entity would certainly have been of value, it is unlikely to have changed the results. This is because even although humoral rejections were not reported as such in the database they were increasingly recognized by the transplant teams and treated accordingly.
Today, the transplantation of HLA-sensitized patients relies on several complementary approaches, such as desensitization with IVIg,9,10,20 avoidance of immunostimulatory HLA antigens,21 and the use of paired-donor-exchange programs.22 Our results suggest that the use of ATG induction therapy rather than the IL2RA mAb, daclizumab, could also help to prevent AR episodes in these problematic, high-risk patients.
CONCISE METHODS
Study Design
The objective of this one-year, prospective, randomized study was to compare the efficacy and safety of ATG and daclizumab in patients at high risk of AR.
This investigator-driven study was undertaken at 16 French and three Belgian centers and was approved by the institutional review board at each site in Belgium and by the Comité de Protection des Personnes dans la Recherche Biomédicale in France. The design, data collection, analysis, and writing were performed by the investigators. Written informed consent was obtained from all patients. The study was registered at the Cochrane Renal Group database (CRG020600038).
Patients were assigned to receive either ATG or daclizumab before transplantation, according to a 1:1 central randomization procedure. A stratification was performed for patients with current PRA >80% to ensure an even distribution between groups. Each patient also received maintenance therapy comprising tacrolimus, MMF, and steroids.
Inclusion and Exclusion Criteria
Adult renal transplant recipients (age range, 18 to 70 yr), each assigned to receive an isolated kidney graft from a deceased donor, were eligible for the study if one or more of the following risk factors were present:
a current anti-HLA PRA ≥30%;
a peak PRA ≥50%;
patients scheduled for a second transplantation, in case the first graft was lost to rejection within two years;
a third or a fourth kidney graft, irrespective of HLA sensitization.
The main exclusion criteria were the receipt of a multiorgan or a previous nonrenal transplant or transplantation from a donor after cardiac death. Transplantations were performed only if the cytotoxic-dependent crossmatch performed with serum sampled on the day of transplantation was negative. Possible additional crossmatching techniques and HLA matching selection policy were left to center practices.
Immunosuppression and Concomitant Medications
Antithymocyte globulin (Thymoglobulin, Genzyme, Cambridge, MA) was administered daily between day 0 and day 7 at a dose of 1.25 mg/kg per d. It was initiated intraoperatively, before graft reperfusion, and in cases of significant leucopenia (<3,000/mm3) or thrombocytopenia (<80.000/mm3), the dose was either reduced or temporarily discontinued.
Five injections of daclizumab (Zenapax, Roche, Basel, Switzerland) were administered at a dose of 1 mg/kg on days 0, 14, 28, 42, and 56, with the first dose being administered before graft reperfusion. Daclizumab was selected over basiliximab because its administration schedule permits a longer duration of IL-2 receptor blockade,4 which we regarded as a potential benefit in this group of high-risk patients.
Methylprednisolone was administered on days 0 (500 mg intravenously) and 1 (250 mg intravenously), followed by oral doses of 16 mg/d from days 2 to 15, 12 mg/d from days 16 to 30, 10 mg/d from days 31 to 60, 8 mg/d from days 61 to 90, and then 0.1 mg/kg up to 1 yr. Mycophenolate mofetil (CellCept, Roche, Basel, Switzerland) was administered at a dose of 2 g/d during months 1 and 2. Thereafter, the MMF dose could subsequently be reduced to 1.5 g/d during month 3 and to 1 g/d thereafter according to individual center practices.
Tacrolimus (Prograf, Astellas, Tokyo, Japan) was preferred over cyclosporine A because it was thought to be more effective in rejection prophylaxis.23 In the daclizumab group, it was initiated at a dose of 0.2 mg/kg before transplantation. However, because registry data have shown that coadministration of a calcineurin inhibitor together with ATG on day 0 can be deleterious to graft survival,24 tacrolimus was initiated on day 2 in the ATG group and delayed until up to day 5 if there was no spontaneous decrease in serum creatinine. Target trough levels were 10 to 15 ng/ml for the initial 3 mo and then 8 to 12 ng/ml up to one year.
Rejection episodes were treated with steroid boluses as by center practice. Banff grade III rejections were treated with either ATG or OKT3. Cytomegalovirus prophylaxis with oral acyclovir or ganciclovir, according to center practices, was administered to all patients for 3 mo, unless both donor and recipient tested negative for cytomegalovirus. Pneumocystis jiroveci prophylaxis with trimethoprim/sulfamethoxazole was administered to all patients for a total of 3 mo.
Study Endpoints
The primary endpoint of the study was the proportion of patients with BPAR up to one year. Rejection severity was scored via biopsy, according to the Banff 1997 criteria.25 Patients with borderline changes suspicious for AR (mild tubulitis) were considered as having BPAR when they received a full AR treatment.
Secondary endpoints were:
the proportion of patients with biopsy-proven and clinical (non-biopsy-proven) rejection;
the proportion of patients with recurrent AR episodes;
the proportion of patients who needed treatments in addition to steroids for rejection (ATG, OKT3, IVIgs, plasmapheresis, or rituximab);
the comparison of the histologic severity and the time of occurrence of the first AR;
the proportion of patients with delayed graft function, defined as the need for dialysis within the first week after transplantation;
renal function at 12 mo, as evaluated by plasma creatinine levels and glomerular filtration rates estimated by abbreviated Modified Diet in Renal Disease;
patient and graft survival at 12 mo.
Safety Assessments
Safety assessments included occurrence of infections, adverse events, serious adverse events, and malignancies. Hematologic and biochemical laboratory evaluations were undertaken at baseline, on days 7, 14, 28, 42, and 56, and at months 3, 6, 9, and 12.
Statistical Analysis
The working hypothesis was the equivalence of both therapies in the prevention of the first BPAR episode. We predicted that AR incidence would approximate 30% and calculated that 115 patients were needed in each group with a noninferiority threshold set at 15%, an alpha risk set at 5%, and a power of 80%. All study endpoints were analyzed according to the intention-to-treat principle. Categorical data were compared with the use of Fisher's exact test, and continuous variables were compared with the use of the t test or the Wilcoxon–Mann–Whitney test as appropriate. The incidences of graft rejection, graft loss, and death were calculated by Kaplan–Meier analysis and compared with the log-rank test. A P value of less than 0.05 was considered to indicate statistical significance.
DISCLOSURES
None.
Acknowledgments
The authors would like to thank the following coinvestigators for their contribution to this study: Lille, F. Provôt, M.D., F. Glowacky, M.D., F.-R. Pruvot, M.D., D. Buop, M.D., Bruxelles-Erasme, N. Broeders, M.D., L. Ghisdal, M.D., A.D. Hoang, M.D., M. Depierreux, M.D., Toulouse, L. Rostaing, M.D., Ph.D., N. Kamar, M.D., Ph.D.; Bicêtre, R. Snanoudj, M.D.; Poitiers, F. Bridoux, M.D., Ph.D.; Saint-Etienne, E. Alamartine, M.D., Ph.D.; Bordeaux, K. Moreau, M.D.; Tenon, E. Rondeau, M.D., Ph.D.; Bruxelles-Saint Luc, C. Eddour, M.D.; Grenoble, T. Romanet, M.D.; Dijon, G. Rifle, M.D., Ph.D.; Y. Tanter, M.D.; C. Mousson, M.D., Ph.D.; Saint Denis, LA Réunion, B. Bourgeon, M.D.; Suresnes, M. Delahousse, M.D., Ph.D., M. Pastural-Thaunat, M.D., A. Karras, M.D.; L. Tricot, M.D.; Gand, P. Peeters, M.D.; Saint-Louis, C. Legendre, M.D., Ph.D.; Brest, B. Boubigot, M.D., Ph.D.; M.C. Moal, M.D. We thank the many research nurses, nurses, assistants, and physicians who facilitated the conduct of this study and helped in the care of the patients.
Published online ahead of print. Publication date available at www.jasn.org.
C.N. and D.A. contributed equally to this article.
See related editorial, “Anti–IL-2 Receptor Antibodies versus Anti-Thymocyte Globulin for Induction Therapy in Kidney Transplantation,” on pages 1170–1171.
REFERENCES
- 1.Wissing KM, Fomegne G, Broeders N, Ghisdal L, Hoang AD, Mikhalski D, Donckier V, Vereerstraeten P, Abramowicz D: HLA mismatches remain risk factors for acute kidney allograft rejection in patients receiving quadruple immunosuppression with anti-interleukin-2 receptor antibodies. Transplantation 85: 411–416, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Andreoni KA, Brayman KL, Guidinger MK, Sommers CM, Sung RS: Kidney and pancreas transplantation in the United States, 1996–2005. Am J Transplant 7: 1359–1375, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Webster AC, Playford EG, Higgins G, Chapman JR, Craig JC: Interleukin 2 receptor antagonists for renal transplant recipients: A meta-analysis of randomized trials. Transplantation 77: 166–176, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Vincenti F, de Andrés A, Becker T, Choukroun G, Cole E, Gonzalez-Posada JM, Kumar MA, Moore R, Nadalin S, Nashan B, Rostaing L, Saito K, Yoshimura N: Interleukin-2 receptor antagonist induction in modern immunosuppression regimens for renal transplant recipients. Transpl Int 19: 446–457, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Patlolla V, Zhong X, Reed GW, Mandelbrot DA: Efficacy of anti-IL-2 receptor antibodies compared to no induction and to antilymphocyte antibodies in renal transplantation. Am J Transplant 7: 1832–1842, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Opelz G, Naujokat C, Daniel V, Terness P, Dohler B: Disassociation between risk of graft loss and risk of non-Hodgkin lymphoma with induction agents in renal transplant recipients. Transplantation 81: 1227–1233, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Zaltzman JS, Boucher A, Busque S, Halloran PF, Landsberg DN, McAlister VC, Russell D, Shoker A, Shapiro J, Tchervenkov JI, Ferguson R: A prospective 3-yr evaluation of tacrolimus-based immunosuppressive therapy in immunological high risk renal allograft recipients. Clin Transplant 19: 26–32, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Vo AA, Toyoda M, Peng A, Bunnapradist S, Lukovsky M, Jordan SC: Effect of induction therapy protocols on transplant outcomes in crossmatch positive renal allograft recipients desensitized with IVIG. Am J Transplant 6: 2384–2390, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Stegall MD, Gloor J, Winters JL, Moore SB, Degoey S: A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant 6: 346–351, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Anglicheau D, Loupy A, Suberbielle C, Zuber J, Patey N, Noel LH, Cavalcanti R, Le Quintrec M, Audat F, Mejean A, Martinez F, Mamzer-Bruneel MF, Thervet E, Legendre C: Posttransplant prophylactic intravenous immunoglobulin in kidney transplant patients at high immunological risk: a pilot study. Am J Transplant 7: 1185–1192, 2007 [DOI] [PubMed] [Google Scholar]
- 11.US Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients. 2006 Annual Report: Transplant Data 1996–2005. http://www.optn.org/AR2006/. 2006 Ref Type: Electronic Citation
- 12.Agence de la Biomedecine. Assistance medicale a la procreation 2002–2003-2004. http://www.agence-biomedecine.fr/fr/rapport_2005/som/som_assist_1.htm. 2005 Ref Type: Electronic Citation
- 13.Eurotransplant International Foundation. 2006 Annual Report. http://www.eurotransplant.nl/files/annual_report/AR2006. 2006 Ref Type: Electronic Citation
- 14.Vitko S, Wlodarczyk Z, Kyllonen L, Czajkowski Z, Margreiter R, Backman L, Perner F, Rigotti P, Jaques B, Abramowicz D, Kessler M, Sanchez-Plumed J, Rostaing L, Rodger RS, Donati D, Vanrenterghem Y: Tacrolimus combined with two different dosages of sirolimus in kidney transplantation: results of a multicenter study. Am J Transplant 6: 531–538, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, Citterio F, Wiecek A, Chadban S, El-Shahawy M, Budde K, Goto N: Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 7: 1506–1514, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, Margreiter R, Hugo C, Grinyo JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF: Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Szczech LA, Berlin JA, Feldman HI: The effect of antilymphocyte induction therapy on renal allograft survival. A meta-analysis of individual patient-level data. Anti-Lymphocyte Antibody Induction Therapy Study Group. Ann Intern Med 128: 817–826, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Brennan DC, Daller JA, Lake KD, Cibrik D, Del CD: Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 355: 1967–1977, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Zand MS, Vo T, Huggins J, Felgar R, Liesveld J, Pellegrin T, Bozorgzadeh A, Sanz I, Briggs BJ: Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation 79: 1507–1515, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, Peng A, Villicana R, Jordan SC: Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 359: 242–251, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Claas FH, Witvliet MD, Duquesnoy RJ, Persijn GG, Doxiadis II: The acceptable mismatch program as a fast tool for highly sensitized patients awaiting a cadaveric kidney transplantation: short waiting time and excellent graft outcome. Transplantation 78: 190–193, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Gentry SE, Segev DL, Simmerling M, Montgomery RA: Expanding kidney paired donation through participation by compatible pairs. Am J Transplant 7: 2361–2370, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC: Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: Meta-analysis and meta-regression of randomised trial data. BMJ 331: 810, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opelz G: Efficacy of rejection prophylaxis with OKT3 in renal transplantation. Collaborative Transplant Study. Transplantation 60: 1220–1224, 1995 [PubMed] [Google Scholar]
- 25.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Marcussen N, Mihatsch MJ, Nadasdy T, Nickerson P, Olsen TS, Papadimitriou JC, Randhawa PS, Rayner DC, Roberts I, Rose S, Rush D, Salinas-Madrigal L, Salomon DR, Sund S, Taskinen E, Trpkov K, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]