Abstract
Nephrotoxicity is common with the use of the chemotherapeutic agent cisplatin, but the cellular mechanisms that modulate the extent of injury are unknown. Cisplatin downregulates expression of the taurine transporter gene (TauT) in LLC-PK1 proximal tubular renal cells, and forced overexpression of TauT protects against cisplatin-induced apoptosis in vitro. Because the S3 segments of proximal tubules are the sites of both cisplatin-induced injury and adaptive regulation of the taurine transporter, we hypothesized that TauT functions as an anti-apoptotic gene and protects renal cells from cisplatin-induced nephrotoxicity in vivo. Here, we studied the regulation of TauT in cisplatin nephrotoxicity in a human embryonic kidney cell line and in LLC-PK1 cells, as well as in TauT transgenic mice. Cisplatin-induced activation of p53 repressed TauT and overexpression of TauT prevented the progression of cisplatin-induced apoptosis and renal dysfunction in TauT transgenic mice. Although cisplatin activated p53 and PUMA (a p53-responsive proapoptotic Bcl-2 family protein) in the kidneys of both wildtype and TauT transgenic mice, only wildtype animals demonstrated acute kidney injury. These data suggest that functional TauT plays a critical role in protecting against cisplatin-induced nephrotoxicity, possibly by attenuating a p53-dependent pathway.
Acute kidney injury due to ischemic or toxic renal damage is a common disorder with mortality of approximately 50%.1,2 As a highly effective chemotherapeutic agent, cisplatin has been used to treat a wide variety of solid tumors.3 However, 25% to 35% of patients experience a significant decline in renal function after the administration of a single dose of cisplatin.4 Several mechanisms, including oxidation, inflammation, genotoxic damage, and cell cycle arrest, have been implicated in cisplatin nephrotoxicity.5–10
Elevated levels of the tumor suppressor gene p53 have been found in the kidneys of animal models of acute kidney injury induced by cisplatin administration.11 Jiang et al.12 have demonstrated that p53 activation is an early signal in cisplatin-induced apoptosis in renal tubular cells. The Varmus group13 has found that transgenic mice overexpressing p53 undergo progressive renal failure through a novel mechanism by which p53 appears to alter cellular differentiation, rather than by growth arrest or the direct induction of apoptosis. These findings suggest that altered expression of certain p53 target gene(s) involved in renal development may be responsible for p53-induced progressive renal injury in p53 transgenic mice.
Our studies have shown that TauT is negatively regulated by p53 in renal cells.14 Interestingly, the progressive renal injury seen in p53 transgenic mice is similar to that previously observed in the offspring of taurine-deficient cats, which showed ongoing kidney damage and abnormal renal and retinal development,15 suggesting that the taurine transporter gene is an important target of p53 during kidney development and renal injury. It is worth noting that cisplatin accumulates in cells from all nephron segments, but is preferentially taken up by the highly susceptible proximal tubule cells within the S3 segment, which is the site for renal adaptive regulation of TauT.16,17 A recent study showed that taurine was able to attenuate cisplatin-induced nephrotoxicity and protect renal tubular cells from tubular atrophy and apoptosis.18 Therefore, downregulation of TauT by p53 may play an important role in cisplatin-induced nephrotoxicity.
RESULTS
Cisplatin Down-Regulates Expression of TauT through a p53-dependent Pathway
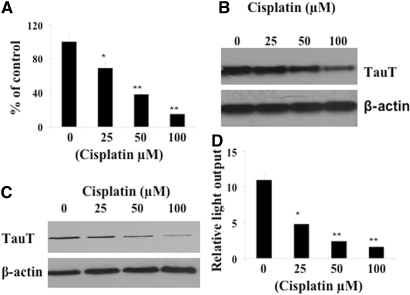
To determine whether cisplatin impairs the function of the taurine transporter, we treated LLC-PK1 cells with cisplatin (0 to 100 μM) for 24 h and measured taurine transport activity as described previously.19 As shown in Figure 1A, introduction of cisplatin results in a dose-dependent decrease of taurine transport activity by LLC-PK1 cells. Consistent with this result, levels of TauT mRNA and TauT protein were also repressed by cisplatin in a dose-dependent manner (Figure 1, B and C).
Figure 1.
Downregulation of TauT by cisplatin in LLC-PK1 renal proximal tubule cells. LLC-PK1 cells (1 × 106) were cultured in DMEM/F12 medium containing cisplatin as indicated for 24 h. (A) Taurine uptake data are presented as percentage of control in response to cisplatin. (B) Northern blot analysis of TauT mRNA upon cisplatin treatment. (C) Western blot analysis of TauT protein after cisplatin treatment. (D) Downregulation of TauT promoter activity by cisplatin. Taurine uptake and luciferase assays were measured in triplicate samples (n = 3), and all data represent three independent experiments. *P < 0.05 versus control, and **P < 0.01 versus control.
To examine whether downregulation of TauT by cisplatin occurs at the transcriptional level, a 963-bp DNA of the 5′-flanking region of the TauT promoter was constructed into a pGL-3 basic vector. Then the reporter construct pGL-963 was transiently transfected into LLC-PK1 cells, and regulation of TauT promoter activity by cisplatin was determined. As shown in Figure 1D, cisplatin decreased TauT promoter activity in a manner similar to that observed in Figures 1 A through C, suggesting that cisplatin represses expression of TauT at the transcriptional level.
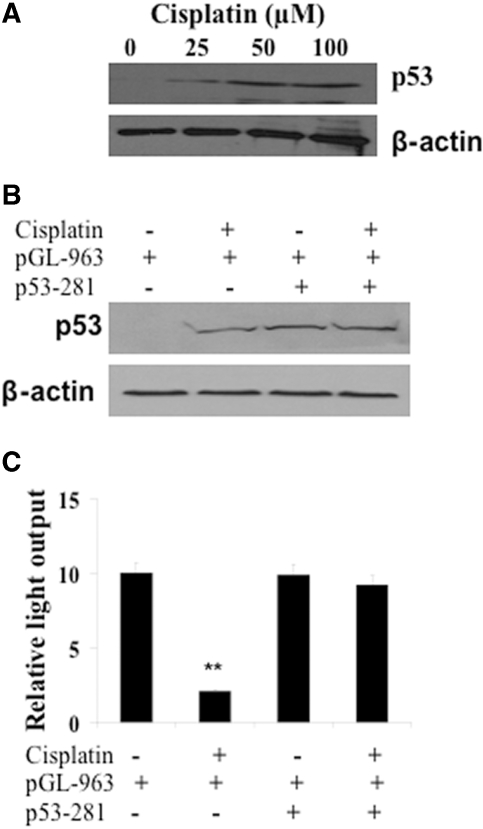
Interestingly, the effect of cisplatin on TauT is likely to be mediated by p53, because the level of nuclear p53 was induced by cisplatin in a dose-dependent manner in LLC-PK1 cells (Figure 2A). To test whether the downregulation of TauT occurs through a p53-dependent pathway, we co-transfected a mutant p53 (p53–281) with pGL-963 into LLC-PK1 cells in the presence or absence of cisplatin as indicated in Figures 2 B and C. As shown in Figure 2B, cells treated with cisplatin or co-transfected with mutant p53–281 induced p53 expression. Again, cisplatin decreased the promoter activity of TauT, while transient co-transfection of mutant p53 alone had no effect on pGL-963 reporter gene activity (Figure 2C). However, cisplatin-mediated downregulation of TauT promoter activity was abolished by mutant p53, suggesting that cisplatin repressed TauT in LLC-PK1 cells through, at least in part, a p53-dependent pathway.
Figure 2.
Cisplatin downregulated TauT in LLC-PK1 cell through a p53-dependent pathway. LLC-PK1 cells (1 × 106) were cultured in DMEM/F12 medium containing cisplatin as indicated for 24 h. (A) Western blot analysis of p53 after cisplatin treatment. (B) Western blot analysis of the level of p53 in the presence of cisplatin and/or co-transfection of mutant p53–281. (C) Regulation of TauT promoter activity by cisplatin and/or co-transfection of mutant p53–281. The promoter activity (mean ± SD of three samples) is represented by relative light output normalized to pRL-CMV control. The graph represents typical results of three separate experiments. *P < 0.05 versus control.
TauT Is a Specific Target of the p53 Tumor Suppressor Gene
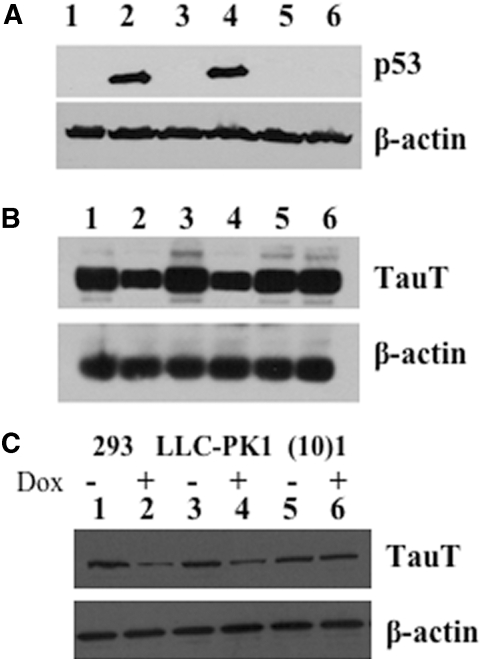
We first examined whether doxorubicin (DOX), a DNA-damaging drug, would induce endogenous p53 expression in human embryonic kidney 293 and renal proximal tubule LLC-PK1 cells. A p53-negative cell line, murine fibroblast (10)1 was used as control. Expression of p53 was induced in both 293 and LLC-PK1 cells after treatment with doxorubicin (500 ng/ml) for 48 h (Figure 3A, lanes 2 and 4), as compared with untreated cells (Figure 3A, lanes 1 and 3). As expected, p53 was not detected in p53-negative (10)1 cells before or after doxorubicin treatment (Figure 3A, lanes 5 and 6). In renal cells, activation of p53 resulted in a decrease in TauT expression at the levels of TauT mRNA (Figure 3B, lanes 2 and 4) and TauT protein (Figure 3C, lanes 2 and 4). Doxorubicin had no effect on TauT and p53 expression in the p53-negative (10)1 cells (Figure 3, B and C, lanes 5 and 6).
Figure 3.
TauT is downregulated following induction of endogenous WT p53 by doxorubicin. (A) Western blot analysis of p53 gene expression in cells treated with or without doxorubicin for 48 h. (B) Northern blot analysis of TauT expression in cells treated with or without doxorubicin for 48 h. (C) Western blot analysis of TauT protein in cells treated with or without doxorubicin for 48 h. β-actin was used as an internal control for both northern and western loadings. The result represents three independent experiments.
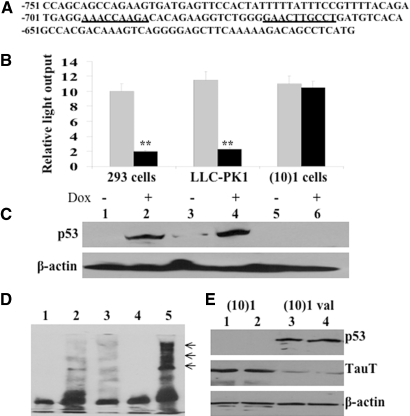
To further determine whether TauT is a target gene of p53, the reporter construct pGL-963, containing a putative p53-binding site from −663 to −695 (Figure 4A), was transiently transfected into 293, LLC-PK1, and p53-negative (10)1 cells. We examined the regulation of TauT promoter function by p53 in cells treated with or without doxorubicin. Treatment with doxorubicin decreased TauT promoter activity in both 293 and LLC-PK1 cells, but had no effect in the p53-negative (10)1 cells (Figure 4B). Deletion or mutation of this putative p53-binding site effectively abolished the negative effect of p53 on TauT promoter activity (data not shown), which was consistent with our previous study.14 As shown in Figure 4C, doxorubicin induced p53 expression in renal cells (lanes 2 and 4) but not in p53-negative (10)1 cells (lanes 5 and 6).
Figure 4.
TauT is specifically repressed by WT p53 in renal cells. (A) Sequence of TauT promoter and construct of the pGL-963 reporter gene. A consensus p53-binding site located at −663 to −695 relative to the transcription start site is shown. (B) pGL-963 was transiently expressed in 293, LLC-PK1, or (10)1 cells and TauT promoter activity was measured 48 h after cells were treated with (dark box) or without (light box) Dox. (C) 293, LLC-PK1, or (10)1 cells were cultured in the presence or absence of doxorubicin (Dox) for 48 h, and expression of p53 was measured by Western blot analysis. (D) Electrophoretic mobility shift assays were done using radiolabeled TauT oligonucleotides with nuclear extracts from (10)1 val cells expressing p53. Lane 1, negative control using nuclear extracts from p53-negative (10)1 cells; lane 2, nuclear extracts from p53-expressing (10)1 cells; lane 3, WT p53 with excess unlabeled probe; lane 4, oligonucleotide with mutant p53-binding site; and lane 5, super-shift yielded by adding p53 antibody (arrows). (E) Western blot analysis of TauT in p53-negative (10)1 (lanes 1 and 2) and p53-expressing (10)1 val cells (lanes 3 and 4). Data represent three independent experiments. **P < 0.01 versus control.
To define whether p53 binds to this putative p53-binding site in the TauT promoter, we carried out electrophoretic mobility shift assays using a synthetic oligonucleotide corresponding to this site. As shown in Figure 4D, nuclear extracts from p53-expressing (10)1 val cells, which produce wildtype p53, yielded a band (lane 2) that could be competed out by excess unlabeled probe (lane 3, 1:100). Nuclear extracts from p53-negative (10)1 cells did not yield a band (lane 1). A mutated oligonucleotide (the p53-binding core sequence CTTG was mutated to TTTT) resulted in the loss of p53 binding to this site (lane 4). A polyclonal antibody to p53 (FL-393, 1 μg) resulted in super-shifted bands (lane 5). Expression of TauT was downregulated in (10)1 val cells as compared with that in (10)1 cells (Figure 4E), suggesting that p53 specifically repressed TauT expression by binding to the putative p53-binding site in the TauT promoter region.
TauT Transgenic Mice Are Resistant to Cisplatin-induced AKI
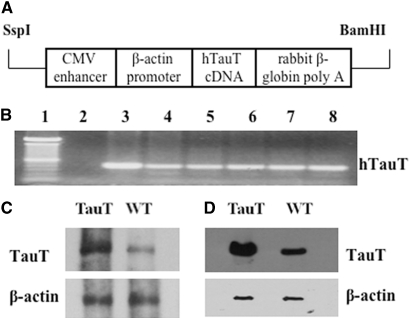
Based on the results from in vitro studies that overexpression of TauT attenuates cisplatin-induced apoptosis of renal proximal tubular cells,20 we should be able to infer the function of TauT in vivo in transgenic mice. We created a line of TauT-overexpressing mice by fusing a human TauT cDNA to the EcoR I site of the pCAGGS vector (Figure 5A) as described in the Concise Methods and used in this study. There were no visible differences between the TauT transgenic mice (heterozygotes and homozygotes) and wildtype (WT) animals regarding body hair, birth weight, organ weight, or growth curve (data not shown). Expression of TauT was analyzed by RT-PCR using RNA extracted from organs, including brain, lung, heart, liver, spleen, and kidney (Figure 5B). Northern and Western blot analysis showed that expression of TauT was elevated by 2.5-fold in the kidneys of TauT transgenic mice as compared with WT control mice (Figure 5, C and D). The expression pattern of the transgene is consistently steady after F6 of breeding (data not shown). Thus, this line of TauT transgenic mice was used for the following study.
Figure 5.
Expression of TauT in TauT transgenic mice. (A) Diagram of transgene construct. (B) RT-PCR analysis of hTauT expression in various tissues of hTauT transgenic mice. Lane 1, DNA standard; lane 2, negative control using H2O instead of sample DNA; lane 3, brain; lane 4, lung; lane 5, heart; lane 6, liver; lane 7, spleen; and lane 8, kidney. (C) Northern blot analysis of TauT mRNA using a TauT-specific probe recognizing both human and mouse TauT mRNA. (D) Western blot analysis of TauT expression in kidney. Total kidney protein (50 μg) was analyzed by Western blot using an antibody recognizing both human and mouse taurine transporters, as described in the Concise Methods section. β-actin was used as an internal control for both Northern and Western blots. Data represent three independent experiments.
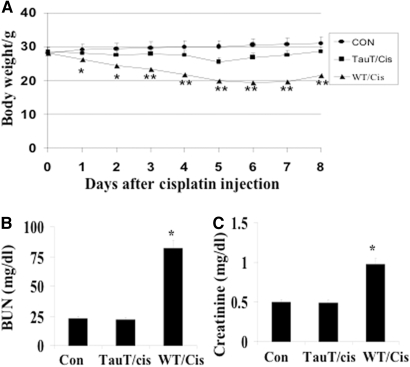
To determine whether overexpression of TauT protects against cisplatin-induced AKI, 12-wk-old male FVB/N mice or TauT transgenic mice (heterozygous) weighing 28 to 30 g were injected with a single intraperitoneal dose of cisplatin (15 mg/kg body wt). We used WT or TauT transgenic mice injected with saline as negative controls, and we recorded body weight changes and animal deaths. Cisplatin administration resulted in a significant weight loss in WT mice, but not in the TauT transgenic mice, during the first 4 d after injection. Significant weight loss was first observed in TauT transgenic mice at day 5 after cisplatin injection (from 28.5 ± 0.5 g to 25.8 ± 0.8 g), while the weight loss reached maximum in cisplatin-injected WT mice (from 28.4 ± 0.6 g to 20.0 ± 0.9 g). All TauT transgenic mice survived cisplatin injection, began to regain weight on day 6, and had recovered to their initial weight by day 8. In contrast, the first cisplatin-induced death was observed on day 4 in WT mice, and the mortality rate was 75% during the 8-d experimental period. The cisplatin-injected WT mice who survived started to regain weight after day 7. By day 8, the average weight of cisplatin-injected WT mice was still 23.5% less than their initial weight (Figure 6A).
Figure 6.
Overexpression of TauT protects against cisplatin-induced AKI. (A) Eight male 12-wk-old WT (WT/cis) or TauT transgenic mice (heterozygotes, TauT/cis), weighing 28 to 30 g each, were injected with a single intraperitoneal dose of cisplatin (15 mg/kg body wt). WT mice injected with saline were used as a negative control. Cisplatin-induced body weight changes and mortality rates were recorded in both WT and TauT transgenic mice. (B) Levels of BUN in saline-injected WT mice, cisplatin-injected WT, or cisplatin-injected TauT transgenic mice. (C) Levels of serum creatinine in WT or TauT transgenic mice injected with saline or cisplatin. Data are mean ± SEM; n = 8 mice in each group. The graph represents three separate experiments. *P < 0.05 versus saline control. **P < 0.01 versus saline control.
Kidney function was evaluated in animals (n = 8) 3 d after cisplatin injection by determining blood urea nitrogen (BUN) and serum creatinine levels. Injection of cisplatin (15 mg/kg body wt) resulted in a significant increase of both BUN and serum creatinine in the WT mice, but not in the TauT transgenic mice, as compared with the WT mice injected with saline (Figures 6, B and C).
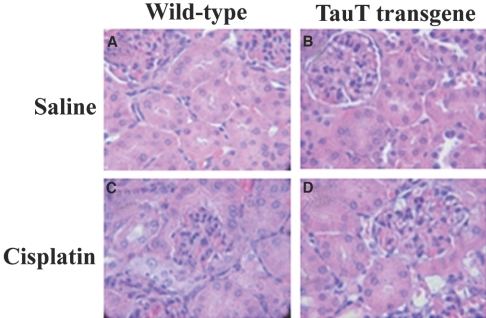
We performed histologic examination of kidneys from saline- and cisplatin-treated WT and cisplatin-treated TauT transgenic mice (Figures 7, A through D). We observed cisplatin-induced pathologic changes predominantly in the cortical and outer medullary regions of the kidney. Changes included interstitial edema, tubular dilation, sloughing of individual epithelial cells, glomerulus distruction, and tubular cell death. However, these pathologic changes were mainly observed in the kidneys of cisplain-treated WT mice (Figure 7C), and significant differences between TauT transgenic (Figure 7D) and WT mice were evident by post-treatment day 3. Tubular injury was rated on a scale of 0 to 4, where 0 = normal, 1 = <10%, 2 = 10 to 25%, 3 = 26 to 75%, and 4 = >75% injury. The tubular injury index was 1 for cisplatin-treated TauT transgenic mice and 3 for cisplatin-treated WT mice.
Figure 7.
Histologic renal lesions in cisplatin-induced AKI. Histologic examination (H&E, Magnification, ×200) of kidney tissue from cisplatin-treated WT and TauT transgenic mice. Data represent three (n = 8) independent experiments.
We further examined kidney tissues by immunohistochemistry. We found TauT protein and taurine mainly in the S3 segment of proximal tubule cells in normal WT animals, while TauT protein and taurine were also detected in the outer cortex stripe of TauT transgenic mice (data not shown).
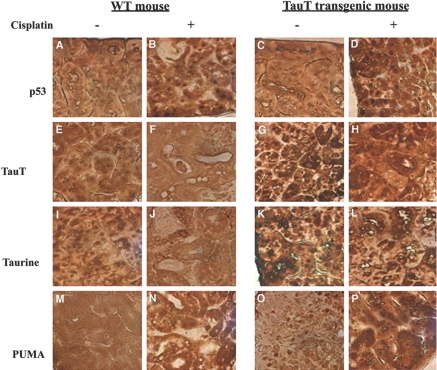
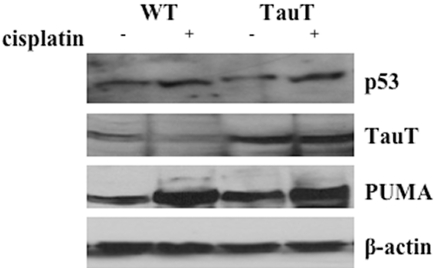
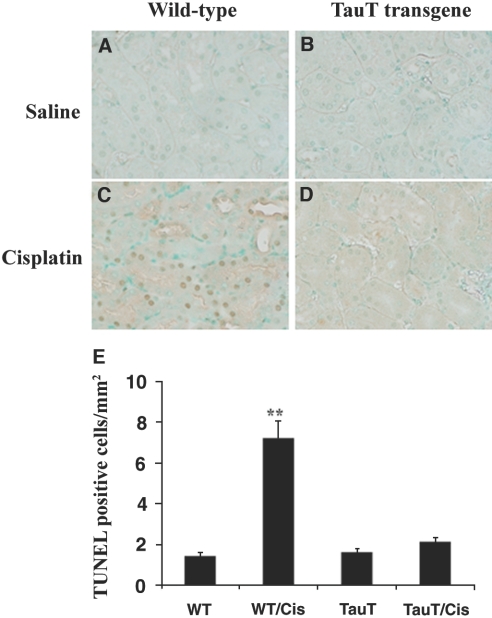
In saline-treated mice, p53 was detected in both proximal and distal tubular cells (Figure 8, A and C, light brown dots). Treatment of cisplatin increased expression of p53 to a similar degree in kidneys of both WT and transgenic animals (Figure 8, B and D, dark brown dots). Cisplatin-induced activation of p53 down-regulated expression of TauT in the kidney from both WT and TauT transgenic mice (Figure 8, E through H, brown color immunostaining), which in turn reduced the amount of intracellular taurine in the kidneys of both WT (Figure 8, I and J) and TauT transgenic mice (Figure 8, K and L), respectively. However, both TauT protein and taurine were undetectable in cisplatin-treated WT mouse kidney (Figures 8, F and J), while signals of immunostaining for TauT protein and taurine in cisplatin-treated transgenic mouse kidney were still relatively high (Figure 8, H and L). Cisplain also increased expression of PUMA protein in kidneys of both WT and TauT transgenic mice (Figure 8, M through P). Regulation of p53, PUMA, and TauT by cisplatin was confirmed by western blot analysis (Figure 9), which showed that cisplatin treatment elevated the levels of p53 and PUMA in the kidneys of both WT and TauT transgenic mice. Cisplatin treatment repressed expression of TauT, especially in the kidneys of WT mice. However, cisplatin treatment caused little change in the level of TauT protein, largely because expression of TauT in the kidneys of transgenic mice is driven by an β-actin gene promoter. Moreover, terminal deoxynucleotidyl transferase (TdT)-mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay revealed widespread apoptosis among proximal tubule cells within the S3 segment and clear evidence of cell death in proximal tubules of the cortex outer stripe (Figure 10C, brown color) of kidneys from cisplatin-treated WT mice, whereas few numbers of TUNEL-positive tubular epithelial cells were detected in the kidneys of cisplatin-treated TauT transgenic mice (Figure 10D), as compared with saline controls (Figure 10, A and B). Interestingly, strong signals of immunostaining for PUMA were observed in the proximal tubules of the ourter cortex stripe (Figure 8, N and P), where vast apoptosis was observed in cisplatin-treated WT mice (Figure 10C), but much less apoptosis was found in cisplatin-treated TauT transgenic mice (Figure 10D) as determined by apoptotic index (Figure 10E). Taken together, these results strongly suggest that TauT plays a critical role in protecting against cisplatin-induced AKI, possibly through blocking the p53-dependent pathway.
Figure 8.
Immunohistochemistry study of kidney tissues from WT and TauT transgenic mice with or without cisplatin treatment. Groups of mice (n = 8) were injected with saline or cisplatin as indicated and were killed 3 d later. Immunostaining for p53 (A through D), TauT (E through H), taurine (I through L), and PUMA (M through P) was performed on kidney tissue slides using specific antibodies against each protein or taurine, respectively. Data represent three independent experiments. Magnification, ×400 in A through P.
Figure 9.
Regulation of p53, PUMA, and TauT by cisplatin in vivo. Kidney tissues from mice (n = 8) injected with saline or cisplatin were prepared, and 50 mg of each protein sample were loaded for Western blot analysis of p53, PUMA, and TauT protein. β-actin was used as an internal control. The data represent three separate experiments.
Figure 10.
TUNEL assay analysis of apoptosis in kidneys of mice treated with cisplatin. Groups of mice (n = 8) were injected with saline or cisplatin as indicated and were killed 3 d later. (A through D) Kidney tissues were prepared and TUNEL assay was performed using a TACS TdT DAB in situ apoptosis detection kit (R&D Systems). Magnification, ×400 in A through D. (E) The apoptotic index values for the four groups of mice. The apoptotic index of cisplatin-treated WT mice was significantly higher than that found in WT and TauT transgenic saline control mice, as well as cisplatin-treated TauT transgenic mice. Data are means ± SEM of cell counts per mm2 per kidney (n = 8). **P < 0.01 versus WT control and TauT transgenic mice. The results represent three independent experiments.
DISCUSSION
Recently, the mechanisms of renal cell repair and regeneration have garnered much attention.7 Unfortunately, the development of therapeutic strategies that are efficacious in humans with acute renal failure has proven problematic. This suggests that the development of more successful therapies requires approaching the problem from a different vantage point.1 The regenerative capacity of the kidney is well documented,2 and the responses of surviving renal proximal tubule cells are thought to be crucial to the restoration of renal function following AKI. Consequently, identifying genes that are involved in renal proximal tubule cell protection, repair, and regeneration may uncover new therapeutic targets that promote renal recovery and decrease the severity of AKI.
In this study, we showed that TauT was downregulated by cisplatin in renal cells. Regulation of TauT by cisplatin appears to be p53-dependent, since such action can be blocked by a dominate-negative p53. Furthermore, we have also demonstrated that induction of p53 by doxorubicin represses TauT expression through a p53-binding site located in the TauT promoter region. These results suggest that TauT is a specific target of p53 in cisplatin nephrotoxicity. Our previous study showed that forced overexpression of TauT is able to attenuate cisplatin-induced apoptosis in LLC-PK1 renal cells,20 suggesting that functional TauT might be important in protecting against cisplatin-induced AKI. Downregulation of TauT by p53 may make kidney cells more susceptible to cisplatin nephrotoxicity.
Unlike most amino acids, taurine is not metabolized or incorporated into protein but remains free in the intracellular compartment, where it plays an important role in cell volume regulation.21–23 Studies have demonstrated that taurine and the taurine transporter play an important role in kidney development.24,25 In the F1 generation of inbred taurine-deficient cats, taurine deficiency results in renal malformation, with significantly diminished renal size and progressive kidney damage.15 In mammals, the taurine total body pool is controlled at the site of the S3 segment of the renal proximal tubule through renal adaptive regulation of the taurine transporter gene.17 Studies have shown that TauT is regulated by many systems, such as transcriptional factors (p53, WT1, and Sp1), osmolarity, and dietary taurine.14,26–28 Regulation of TauT by WT1 and Sp1 mainly occurs during the process of renal development, whereas p53 represses TauT expression in injured renal cells. Furthermore, overexpression of p53 results in progressive renal failure in p53 transgenic mice,13 similar to observations made in taurine-deficient kittens.15 Consistent with our finding that TauT may play a role in renal development, Heller-Stilb et al.24 demonstrated that knockout of TauT results in severe and progressive retinal degeneration, a small brain, and shrunken kidneys in a TauT−/− mouse model.
To further examine the role of TauT in protecting against cisplatin-induced AKI, we created a TauT transgenic mice model and evaluated cisplatin nephrotoxicity in both WT and TauT transgenic mice. Cisplatin treatment resulted in a typical AKI in WT mice, which was evidenced by the significant increase in BUN and serum creatinine levels associated with severe tubular injury. In contrast, cisplatin-treated TauT transgenic mice showed a normal BUN and serum creatinine levels consistent with minor tubular injury. It is worth noting that treatment with cisplatin increased expression of p53 to a similar degree in kidneys of both WT and transgenic mice. Cisplatin-induced p53 activation repressed TauT protein to an undetectable level, which in turn resulted in the depletion of taurine in the kidneys of WT mice. However, the levels of immunostaining of TauT and taurine in the kidneys of cisplatin-treated TauT transgenic mice are similar to that in the kidneys of normal control animals. These findings suggest that relatively normal levels of TauT and/or taurine are able to protect against cisplatin-induced AKI.
The mechanisms by which functional TauT protects animals from cisplatin-induced AKI are unknown. However, results from this study suggest that overexpression of TauT protects against cisplatin-induced AKI, possibly through modulation of a p53-dependent pathway rather than changing the transport of cisplatin by renal cells. This speculation was supported by the observation that cisplatin induced p53 to a similar degree in the kidneys of both WT and TauT transgenic mice. Furthermore, we have shown that PUMA, a p53 downstream target gene, is upregulated in the kidneys of both WT and TauT transgenic mice after cisplatin treatment. Interestingly, the vast apoptosis observed in the proximal tubules of cisplatin-treated WT mice was where the strong signals of immunostaining for PUMA were found. Jiang et al.29 have recently demonstrated that PUMA is involved in cisplatin-induced injury, which could be attenuated in p53-deficient animals and PUMA knockout cells, suggesting that the p53/PUMA pathway plays an important role in cisplatin-induced AKI. Therapeutically, our findings suggest the possibility of protecting the kidney by inducing TauT expression during cisplatin treatment. Overexpression of TauT may not limit cisplatin-mediated killing of cancer cells, because cisplatin therapy may not strictly depend on the p53/PUMA pathway, especially in the cancers (more than 50%) carrying p53 mutations.30–32
Taken together, our findings strongly support the argument that functional TauT plays an essential role in maintaining normal kidney functions. Activation of p53 represses TauT expression, which in turn renders animals more sensitive to cisplatin-induced AKI. Forced overexpression of TauT is capable of protecting against cisplatin-induced AKI, possibly through attenuating p53-dependent pathway.
CONCISE METHODS
Cell Culture
We cultured human embryonic kidney 293 cells, pig renal proximal tubule (LLC-PK1) cells, and murine fibroblast (10)1 val cells according to AATC (American Type Culture Collection, Rockville, MD) guidelines. Briefly, cells were grown as confluent monolayers in 10-cm diameter tissue culture plates in media specific for each cell line with 10% fetal calf serum at 37°C in the presence of 5% CO2 in a humidified incubator. Cells were plated 18 h before transfection and fed with fresh medium 4 h before transfection.
Construction of the Reporter Gene
The promoter region of TauT was identified in previous studies,33 and a p53-binding consensus site was found in the TauT promoter sequence, located at −663 to −695. In this study, approximately 1.1 kb of the TauT promoter region DNA was used as the template for PCR (GenBank™/EBI accession number AR151716), and the PCR fragment was cloned into the promoter-less luciferase vector pGL3-Basic (Promega, Madison, WI) to generate the plasmid p963 for use in transfections and luciferase assays. The conditions used were 30 cycles of 1 min of denaturation at 94°C, 1 min of annealing at 58°C, and 1 min of elongation at 72°C. The sense primer (5′-GGGGTACCTTACTGAAGGTCACACAGC-3′) designed for PCR contained a unique site for KpnI, and the antisense primer (5′-AAGATCTTGGCACGGGAGTTCA-3′) contained a unique site for BglII. PCR products were digested with KpnI and BglII, and re-ligated into KpnI and BglII sites of pGL3-Basic to generate plasmids containing segments of the TauT promoter sequence extending from the + 48 nucleotide corresponding to the transcriptional start site. We verified the constructs by DNA sequencing. We generated the p53-binding site deletion (del pGL-563) and p53 mutation (mt pGL-963) constructs from the p963 plasmid by using sense primers 5′-GGGGTACCGAGTTGGGGAGGGA-3′, and 5′-GGGGTACCAGATGAGG-AAACCCCCACACAGAAGGTCTGGGGCTTGCCTGATGTCA-3′, respectively. The antisense primer used for these constructs was the same as described above.
Transient Transfection
We introduced plasmid DNA into cultured LLC-PK1 cells using cationic liposomes (LipofectAMINE; Invitrogen, Carlsbad, CA). Transfection was carried out for 16 to 18 h, and then cells were washed twice with PBS and incubated in fresh medium for 24 to 48 h before harvesting. pGL-control, which contains a luciferase gene driven by the SV40 early region promoter/enhancer, and empty pGL-Basic vectors were used as positive and negative controls, respectively. To standardize the transfection efficiency, 0.1 μg of pRL-CMV vector (pRL Renilla reniformis luciferase control reporter vector; Promega) was cotransfected in all experiments. Cells were harvested 48 h after transfection and lysed in 200 μl of reporter lysis buffer (Promega). A luciferase assay was performed using a dual luciferase assay kit (Promega), and activity was measured with an Optocomp 1 luminometer (MGM Instruments, Inc., Hamden, CT). Promoter activity (mean ± SD of four samples in relative light units) of each construct is represented by relative light output normalized to pRL-CMV control. Graphs represent typical results of four separate experiments. We determined the concentrations of protein in the cell extracts using the Bradford method (Bio-Rad, Hercules, CA).
Measurement of Taurine Transport
We performed taurine transport studies on confluent monolayers 3 d after cell seeding. Briefly, we washed cells with Earle's Balanced Salt Solution (EBSS) at 37°C and initiated uptake by the addition of uptake buffer (2 mM KCl, 1 mM MgCl2, 96 mM NaCl, 1.8 mM CaCl2, 5 mM Hepes, pH 7.6) to which 50 μM unlabeled taurine and 0.5 μCi/ml 14C-taurine (Perkin Elmer, Boston, MA) were added. After incubation for 30 min at room temperature, we terminated uptake by the removal of uptake buffer followed by three rapid washes with cold EBSS. We solubilized cells in 1% SDS in 0.2 N NaOH and counted radioactivity in a Packard 2000-CA Liquid Scintillation Analyzer (Packard Instrument Co. Inc., Meriden, CT).
Model of TauT Transgenic Mice
For generation of TauT transgenic mice, we used a pCAGGS expression vector (a kind gift from Dr. Jun-ichi Miyazaki at Osaka University Medical School),34 which has been widely used for creating transgenic mice.35 Human TauT cDNA was fused to the EcoR I site of the pCAGGS vector tailed with a rabbit ß-globin poly A, which is driven by a chicken β-actin promoter (Figure 5A). The transgene was purified from vector sequences and 2 ng/ml of the DNA was injected into fertilized FVB/N mouse eggs to establish lines of transgenic animals using standard methodologies at the University of Tennessee Health Science Center transgenic facility. The transgenic nature of these animals was tested by PCR using flanking primers. Primer 1 (a specific human TauT forward primer 5′-AACCCCATCTTTGGCAGGCA-3′ residues 3691–3710 of GenBank Z18956), primer 2 (a specific rabbit β-globin reverse primer 5′-AGCCAGAA-GTCAGATGCTCAA-3′ residues 1486–1466 of GenBank V00882), primer 3 (a specific human and mouse TauT forward primer 5′-GGCCTGCCTGTGTTTTTCTT-3′ residues 501 to 520 of GenBank L03292), and primer 4 (a specific mouse TauT reverse primer 5′-GGTGAAGTTGGCAGTGCTAAGG-3′ residues 807 to 785 of GenBankL03292) were used for PCR. Amplification of heterozygous transgenic DNA resulted in two bands, WT DNA yielded only band I (1.3 kb) and homozygous transgenic DNA yielded only band II (406 bp). Band I contains an approximate 1.0-kb intron of mouse TauT. Two lines of TauT overexpressing mice were created and used in this study. There were no visible differences between the TauT transgenic mice (heterozygotes and homozygotes) and WT animals regarding body hair, birth weight, organ weight, or growth curve (data not shown). Expression of TauT was analyzed by RT-PCR using RNA extracted from organs, including brain, lung, heart, liver, spleen, and kidney (Figure 5B). Western blot analysis showed that expression of TauT was elevated by 2.5-fold in the kidney of TauT transgenic mice as compared with WT control mice (Figure 5D). The expression pattern of the transgene has been consistently steady after six generations of breeding.
In vivo Model of Cisplatin-induced AKI
Male mice (WT and TauT transgenic), 10 to 12 wk old and weighing 28 to 30 g, were assigned to treatment groups (n = 8 per group). For the experiment, eight TauT transgenic mice and eight WT mice received a single dose of cisplatin (15 mg/kg body wt) by intraperitoneal injection. Eight saline-injected mice were used as controls. To determine cisplatin-induced nephrotoxicity, mice were killed 3 d after cisplatin injection. Blood and urine samples were collected. The levels of BUN, serum creatinine, and urinary creatinine were measured by an enzymatic colorimetric assay kit (Sigma, St. Louis, MO).
Northern Blot Analysis
Thirty micrograms of total RNA were separated in an agarose gel and transferred to a nylon membrane by overnight capillary blotting in 10× SSC (sodium chloride/sodium citrate buffer). The northern blot was hybridized overnight at 42°C with a 32P-labeled riboprobe of the taurine transporter cDNA. The blot was washed successively in standard decreasing concentrations of SSC/0.1% SDS at 65°C for 30 min each and exposed to Kodak film with one intensifying screen at −80°C for 24 h.
Western Blot Analysis
We lysed cells in 50 μl M-PER mammalian protein extraction reagent (Pierce, Rockford, IL) supplemented with a protease inhibitor cocktail for use with mammalian cell and tissue extracts (Sigma). We cleared the lysates by centrifugation at 14,000 × g for 2 min and transferred the supernatants to clean tubes. We separated equal amounts of protein (50 μg) by electrophoresis on a 12% SDS-polyacrylamide gel and transferred protein to a nitrocellulose membrane (Millipore, Bedford, MA) using a semidry electrophoretic transfer system (Bio-Rad). We incubated membranes in 5% nonfat dry milk in Tris base/sodium chloride (TBS) buffer with 0.2% Tween 20 (TBST) at 4°C overnight. We incubated the membranes with primary antibodies for 1 h at room temperature. Blots were washed with TBST and incubated with horseradish peroxidase-linked secondary antibody (Sigma) for another hour, and then proteins of interest were detected using a chemiluminescence detection kit (Pierce).
Immunohistochemistry
We cultured cells directly on sterile cover slips (Sigma) that were placed into a 6-well tissue culture plates for 2 d. Media was removed, and cells were rinsed once with 1× PBS at room temperature. Cells were fixed for 10 min at room temperature in 3.7% buffered formaldehyde and then rinsed again with 1× PBS. Fixed cells were dehydrated by immersing in 70%, 95%, and 100% ethanol for 5 min each followed by air drying for 10 min each. Immunohistochemistry was performed by following the manufacturer's instructions (Pierce). Briefly, samples were rehydrated in decreasing ethanol series (100%, 95%, and 70%) for 5 min each. Samples were immersed in 1× PBS for 5 min at room temperature, then immersed in quenching solution (3% H2O2 in methanol) for 5 min, and then washed twice in dH2O for 10 min. We blocked slides for 20 min with the blocking buffer. Primary antibodies (antibody against taurine transporter protein) were applied to slides and incubated for 1 h. Slides were washed for 10 min with PBS, and then the biotinylated secondary antibody was applied and incubated for 1 h. After washing for 10 min with PBS, ABC reagent was applied for 30 min. Finally, we detected immunostaining using a Metal Enhanced DAB Substrate Kit (Pierce).
Assessment of Apoptosis
We detected apoptotic cells in kidney sections using the TUNEL method following the manufacturer's instructions (R&D Systems, Minneapolis, MN). We defined the apoptotic index as the number of TUNEL-positive cells per mm2 section in 15 sequentially selected nonoverlapping fields (magnification, ×200) of renal cortex and medulla.
Statistical Analysis
We performed all experiments in triplicate. Luciferase assays are expressed in units of relative light output. The data represent the mean ± standard error of three or four experiments. We made statistical comparisons using one-way ANOVA and t test to determine significant differences in the means.
DISCLOSURES
None.
Acknowledgments
We thank Andrea Patters for insightful comments and suggestions. We also thank Drs. Ioannis Dragatsis of the University of Tennessee Health Science Center transgene core facility for generation of hTauT transgenic mice. This work was supported by grants from the National Kidney Foundation, Le Bonheur Children's Medical Center, and the Le Bonheur Chair of Excellence in Pediatrics.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Kelly KJ, Molitoris BA: Acute renal failure in the new millennium: Time to consider combination therapy. Semin Nephrol 20: 4–19, 2000 [PubMed] [Google Scholar]
- 2.Thadhani R, Pascual M, Bonventre JV: Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Lebwohl D, Canetta R: Clinical development of platinum complexes in cancer therapy: An historical perspective and an update. Eur J Cancer 34: 1522–1534, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Ries F, Klastersky J: Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis 8: 368–379, 1986 [DOI] [PubMed] [Google Scholar]
- 5.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV: In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int 53: 394–401, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Megyesi J, Safirstein RL, Price PM: Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest 101: 777–782, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price PM, Megyesi J, Saf Irstein RL: Cell cycle regulation: Repair and regeneration in acute renal failure. Kidney Int 66: 509–514, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Ramesh G, Reeves WB: TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramesh G, Reeves WB: TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 285: F610–F618, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Ramesh G, Reeves WB: Inflammatory cytokines in acute renal failure. Kidney Int Suppl: S56–61, 2004 [DOI] [PubMed]
- 11.Miyaji T, Kato A, Yasuda H, Fujigaki Y, Hishida A: Role of the increase in p21 in cisplatin-induced acute renal failure in rats. J Am Soc Nephrol 12: 900–908, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Jiang M, Yi X, Hsu S, Wang CY, Dong Z: Role of p53 in cisplatin-induced tubular cell apoptosis: Dependence on p53 transcriptional activity. Am J Physiol Renal Physiol 287: F1140–F1147, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Godley LA, Kopp JB, Eckhaus M, Paglino JJ, Owens J, Varmus HE: Wild-type p53 transgenic mice exhibit altered differentiation of the ureteric bud and possess small kidneys. Genes Dev 10: 836–850, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Han X, Patters AB, Chesney RW: Transcriptional repression of taurine transporter gene (TauT) by p53 in renal cells. J Biol Chem 277: 39266–39273, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Han X, Budreau AM, Chesney RW: The taurine transporter gene and its role in renal development. Amino Acids 19: 499–507, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Leibbrandt ME, Wolfgang GH, Metz AL, Ozobia AA, Haskins JR: Critical subcellular targets of cisplatin and related platinum analogs in rat renal proximal tubule cells. Kidney Int 48: 761–770, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Matsell DG, Bennett T, Han X, Budreau AM, Chesney RW: Regulation of the taurine transporter gene in the S3 segment of the proximal tubule. Kidney Int 52: 748–754, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Saad SY, Al-Rikabi AC: Protection effects of taurine supplementation against cisplatin-induced nephrotoxicity in rats. Chemotherapy 48: 42–48, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Jones DP, Miller LA, Chesney RW: The relative roles of external taurine concentration and medium osmolality in the regulation of taurine transport in LLC-PK1 and MDCK cells. Pediatr Res 37: 227–232, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Han X, Chesney RW: Regulation of TauT by cisplatin in LLC-PK1 renal cells. Pediatr Nephrol 20: 1067–1072, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Falktoft B, Lambert IH: Ca2+-mediated potentiation of the swelling-induced taurine efflux from HeLa cells: On the role of calmodulin and novel protein kinase C isoforms. J Membr Biol 201: 59–75, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Huang Z, Hou Q, Cheung NS, Li QT: Neuronal cell death caused by inhibition of intracellular cholesterol trafficking is caspase dependent and associated with activation of the mitochondrial apoptosis pathway. J Neurochem 97: 280–291, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Molchanova S, Oja SS, Saransaari P: Characteristics of basal taurine release in the rat striatum measured by microdialysis. Amino Acids 27: 261–268, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, Warskulat U, Haussinger D: Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. Faseb J 16: 231–233, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Sturman JA, Moretz RC, French JH, Wisniewski HM: Taurine deficiency in the developing cat: persistence of the cerebellar external granule cell layer. Prog Clin Biol Res 179: 43–52, 1985 [PubMed] [Google Scholar]
- 26.Han X, Budreau AM, Chesney RW: Identification of promoter elements involved in adaptive regulation of the taurine transporter gene: role of cytosolic Ca2+ signaling. Adv Exp Med Biol 483: 535–544, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Han X, Chesney RW: Regulation of taurine transporter gene (TauT) by WT1. FEBS Lett 540: 71–76, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Han X, Patters AB, Jones DP, Zelikovic I, Chesney RW: The taurine transporter: Mechanisms of regulation. Acta Physiol (Oxf) 187: 61–73, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Jiang M, Wei Q, Wang j, Du Q, Yu J, Zhang L, Dong Z: Regulation of PUMA-α by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25: 4055–4066, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Siddik ZH: Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 22: 7265–7279, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Gudkov AV and Konarova EA: Prospective therapeutic applications of p53 inhibitors. Biochem Biophys Res Commun 331: 726–736, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Hollstein M, Shomer B, Greenblatt M, Soussi T, Hovig E, Montesano R, Harris C: Somatic point mutations in the p53 gene of human tumors and cell lines: Updated compilation. Nucl Acids Res 24: 141–146, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han X, Budreau AM, Chesney RW: Cloning and characterization of the promoter region of the rat taurine transporter (TauT) gene. Adv Exp Med Biol 483: 97–108, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Maruyama H, Higuchi N, Nishikawa Y, Hirahara H, Iino N, Kameda S, Kawachi H, Yaoita E, Gejyo F, Miyazaki J: Kidney-targeted naked DNA transfer by retrograde renal vein injection in rats. Hum Gene Ther 13: 455–468, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Gawlik A, Quaggin SE: Deciphering the renal code: Advances in conditional gene targeting. Physiology (Bethesda) 19: 245–252, 2004 [DOI] [PubMed] [Google Scholar]