Abstract
Protein kinase C (PKC) is known to regulate ryanodine receptor (RyR)–mediated local Ca2+ signaling (Ca2+ spark) in airway and vascular smooth muscle cells (SMCs), but its specific molecular mechanisms and functions still remain elusive. In this study, we reveal that, in airway SMCs, specific PKCɛ peptide inhibitor and gene deletion significantly increased the frequency of Ca2+ sparks, and decreased the amplitude of Ca2+ sparks in the presence of xestospogin-C to eliminate functional inositol 1,4,5-triphosphate receptors. PKCɛ activation with phorbol-12-myristate-13-acetate significantly decreased Ca2+ spark frequency and increased Ca2+ spark amplitude. The effect of PKCɛ inhibition or activation on Ca2+ sparks was completely lost in PKCɛ−/− cells. PKCɛ inhibition or PKCɛ activation was unable to affect Ca2+ sparks in RyR1−/− and RyR1+/− cells. Modification of RyR2 activity by FK506-binding protein 12.6 homozygous or RyR2 heterozygous gene deletion did not prevent the effect of PKCɛ inhibition or activation. RyR3 homogenous gene deletion did not block the effect of PKCɛ inhibition and activation, either. PKCɛ inhibition promotes agonist-induced airway muscle contraction, whereas PKCɛ activation produces an opposite effect. Taken together, these results indicate that PKCɛ regulates Ca2+ sparks by specifically interacting with RyR1, which plays an important role in the control of contractile responses in airway SMCs.
Keywords: protein kinase C, local calcium signaling, ryanodine receptor, contraction, airway myocytes
CLINICAL RELEVANCE
This study reveals that protein kinase C (PKC)-ɛ regulates local Ca2+ signaling by specifically interacting with ryanodine receptor (RyR) 1, thereby modulating contractile responses in airway smooth muscle cells. Thus, PKC-ɛ and RyR1 may become new therapeutic targets for asthma and other lung diseases.
Local transient Ca2+ release events due to the opening of ryanodine receptors (RyRs) on the sarcoplasmic reticulum (SR), termed Ca2+ sparks (1), have been observed in a variety of cell types, including airway smooth muscle cells (SMCs). These local Ca2+ signals have been demonstrated to play an important role in various cellular responses, such as muscle contraction, neurotransmitter release, secretion, cell proliferation and migration, and gene expression. Interestingly, Ca2+ sparks can generate hyperpolarizing spontaneous transient outward currents (STOCs) in cerebral arterial SMCs, which results in the inhibition of voltage-dependent Ca2+ channels, prevention of Ca2+ influx, and cell relaxation, thus regulating cerebral vascular tone (2). On the other hand, Ca2+ sparks are also able to produce depolarizing spontaneous transient inward currents (STICs), causing Ca2+ influx and contraction in pulmonary arterial and airway SMCs (3, 4). Therefore, Ca2+ sparks can significantly regulate smooth muscle contractility by producing hyperpolarizing STOCs or depolarizing STICs.
Increasing attention has been paid to the study of the role of key regulators in the control of RyR-mediated Ca2+ sparks. Phosphorylation by protein kinases is a common and essential means of controlling the activity of RyRs and attendant Ca2+ sparks. In support of this view, previous studies have shown that Ca2+/calmodulin-dependent PKII affects Ca2+ sparks in cardiac cells (5–7). Moreover, protein kinase C (PKC), can also phosphorylate RyRs in cardiac and neural cells (8, 9), and reduce the activity of Ca2+ sparks in cerebral arterial myocytes (10, 11). Supplementary to these results, we have recently shown that PKCɛ is a major PKC isoform to regulate Ca2+ sparks in airway SMCs (12). However, the precise molecular mechanisms underlying the regulation of Ca2+ sparks by PKCɛ and the functional consequence of this regulation are largely unknown. In the present study, we sought to use distinct pharmacological agents and gene deletion mice to determine: (1) whether PKCɛ may regulate Ca2+ sparks through interaction with RyRs; (2) which subtype of RyRs mediates the regulatory effect of PKCɛ; and (3) whether PKCɛ can regulate contractile responses by affecting Ca2+ spark activity in SMCs.
MATERIALS AND METHODS
Cell Isolation
Freshly isolated mouse airway SMCs were prepared using the two-step enzymatic digestion method, as we described previously (12). In brief, adult (8–9 wk) male Swiss Webster mice (Taconic, Germantown, NY) were killed by intraperitoneal injection of sodium pentobarbital. Tracheae were removed from the connective tissue, cartilage, and epithelium, and then incubated for 20 minutes at 37°C in low-Ca2+ (100 μM) physiological saline solution (PSS) containing: 2.0 mg/ml papain, 0.5 mg/ml dithioerythritol, and 2.0 mg/ml BSA. After that, the tissues were continuously incubated for 25 to approximately 30 minutes in low-Ca2+ PSS containing: 1.0 mg/ml collagenase H, 1.0 mg/ml collagenase II, 1.0 mg/ml dithiothreitol, and 2.0 mg/ml BSA. The digested tissues were washed three or four times with ice-cold, low-Ca2+ PSS. Single SMCs were released by gentle trituration and stored on ice for daily use. PSS consisted of: 125 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 10 mM HEPES, and 10 mM glucose (pH 7.4).
PKCɛ gene deletion (PKCɛ−/−) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The generation of RyR1−/−, FK506-binding protein 12.6 (FKBP12.6−/−), RyR2−/−, and RyR3−/− mice were reported previously (13–16). Because RyR1−/− mice die just before or at birth, heterozygous (RyR1+/−) animals were bred as timed pregnancy. At Day 17, pregnant mothers were killed to obtain RyR1−/−, RyR1+/−, and wild-type (RyR1+/+) mice. Single airway SMCs from adult PKCɛ−/−, RyR1+/−, FKBP12.6−/−, RyR2+/−, and RyR3−/− mice, embryonic RyR1−/− and RyR1+/− mice, and their matching control (wild-type) mice with the same background, sex, and age were prepared using the same procedure as described above.
The activity of Ca2+ sparks may vary, to a greater or lesser extent, in cells and animals. As such, in pharmacological experiments, Ca2+ sparks before and after application of specific agents were measured in the same cells from the same mice. To determine effects of specific gene deletion, cells from wild-type and gene-deleted mice were prepared in parallel.
Measurement of Ca2+ Sparks
Ca2+ sparks were measured and analyzed, as we have reported previously (12). Isolated cells were incubated in PSS containing 2.5 μM fluo-4/AM for 30 minutes and then superfused with dye-free PSS for 10 minutes. Line-scan images were acquired using an LSM-510 laser scanning confocal system (Carl Zeiss, Göttingen, Germany). Spatiotemporal characteristics of Ca2+ sparks were analyzed using the Physiology Analysis software package (Carl Zeiss) and the modified Interactive Data Language software package (Research Systems, Boulder, CO).
Measurement of Intracellular Ca2+ Concentration
Intracellular Ca2+ concentration ([Ca2+]i) was measured using a dual excitation wavelength fluorescence method, with an IonOptix imaging system (Milton, MA) and a Nikon inverted microscope (Melville, NY), as described previously (17). Cells were loaded with 5 μM fura-2/AM at room temperature for 30 minutes followed by perfusion of dye-free bath solution for 20 minutes. [Ca2+]i was determined from the ratio of dye fluorescence intensity at 340 and 380 nm, with an emission wavelength at 510 nm.
Immunofluorescent Staining
Expression of smooth muscle–specific actin and RyR1 were determined using immunofluorescent staining (18). Single cells were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and then incubated with anti-RyR1 or anti-actin (smooth muscle) antibody, followed by Alexa Fluor 488–conjugated anti-mouse antibody. Staining was examined using a Zeiss LSM510 laser scanning confocal microscope with a 40× oil immersion objective (numerical aperture 1.3). Alexa Fluor 488 was excited at 488 nm using a krypton–argon laser, and the emitted fluorescence was detected at 510 nm.
Measurement of PKCɛ Activity
Immunoprecipitation of PKCɛ was performed, as we reported previously (19). Tracheal muscle tissues were treated with and without PKCɛ peptide inhibitor for 8 minutes, and then underwent lysis and centrifugation. Samples were incubated with monoclonal anti-PKCɛ antibody. Immune complexes were collected on protein G beads and then eluted with SDS buffer. The activity of immunoprecipitated PKCɛ was determined by measuring the absorbance intensity of tetramethylbenzidine at 450 nm, which represents a function of PKCɛ activity, using nonradioactive PKC Kinase Activity Assay Kit (Assay Designs, Ann Arbor, MI), according to the manufacturer's instructions. Samples were assayed in duplicate.
Measurement of Muscle Contraction
Muscle contraction in isolated tracheal rings was measured using the organ bath technique, as described previously (17), with isometric transducers (Harvard Apparatus, South Natick, MA) and a PowerLab/4SP recording system (AD Instruments, Colorado Springs, CO). Tracheae were quickly removed from mice and transferred into ice-cold PSS containing: 135 mM NaCl, 5 mM KCl, 1 mM MgCI2, 2 mM CaCl2, 10 mM HEPES, and 10 mM glucose (equilibrated with 20% O2, 5% CO2, and 75% N2 [pH 7.4]). After the connective tissue and epithelia were removed, tracheal rings were mounted vertically in 2-ml organ bath chambers containing PSS at 37°C. The resting tension was set at 200 mg. During a 60-minute equilibration period, rings were washed every 20 minutes. The muscarinic receptor agonist, methacholine (0.5 μM), was given to induce muscle contraction.
Reagents
Alexa Fluor 488–conjugated anti-mouse antibody and fluo-4/AM were purchased from Molecular Probes (Eugene, OR); anti-actin (smooth muscle) antibody, phorbol-12-myristate-13-acetate (PMA), and ryanodine from Sigma (St. Louis, MO); anti-RyR1 antibody from Upstate Biotech (Lake Placid, NY); and PKCɛ translocation peptide inhibitor and xestospongin-C were purchased from Calbiochem (San Diego, CA). PKCɛ peptide inhibitor was dissolved in H2O, and all other chemicals in DMSO at a final DMSO concentration of less than 0.01%. Drugs were delivered onto the cell through a glass pipette connected to a Picospritzer III pressure controller (Parker Instrumentation, Chicago, IL). All experiments were conducted at room temperature (∼22°C).
Statistical Analysis
Statistical comparisons between groups were performed with the Student's t test or one-way ANOVA with an appropriate post hoc test. Differences with a P value less than 0.05 were considered significant; data are presented as means (±SEM).
RESULTS
Specific PKCɛ Inhibition Increases the Activity of Ca2+ Sparks in the Absence of Functional Inositol 1,4,5-Triphosphate Receptors
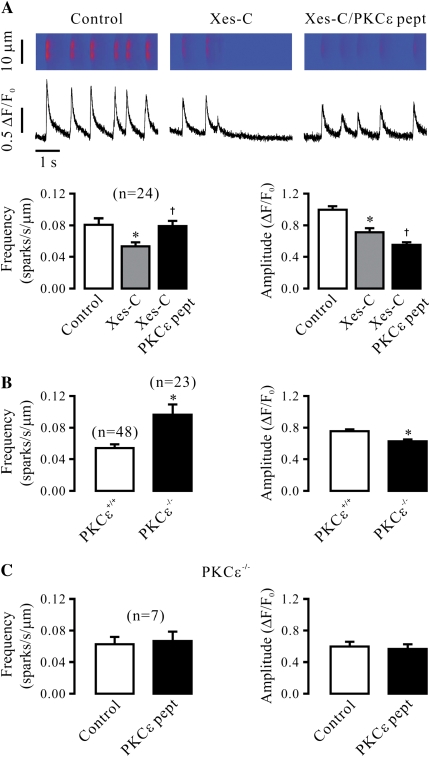
Our recent study has shown that PKCɛ significantly regulates Ca2+ sparks, mainly manifesting as a decrease in the frequency of Ca2+ sparks and an increase in the amplitude of Ca2+ sparks in airway SMCs (12). Because PKC can phosphorylate RyRs, we reasoned that the effect of PKCɛ on Ca2+ sparks might occur due to its direct interaction with RyRs. To prove this possibility, we examined the effect of specific PKCɛ inhibition on Ca2+ sparks after treatment with xestospongin-C to effectively inhibit inositol 1,4,5-triphosphate receptors (IP3Rs). Consistent with our previous report (12), application of xestospongin-C (10 μM) for 8 minutes significantly decreased the frequency and amplitude of Ca2+ sparks (Figure 1A). However, in the continued presence of xestospongin-C, application of PKCɛ peptide inhibitor (100 μM) for 8 minutes could still result in a significant increase in the frequency of Ca2+ sparks, and a decrease in the amplitude of Ca2+ sparks. The mean frequency was increased from 0.053 (±0.005) to 0.079 (±0.006) sparks/second/μm, and the mean amplitude decreased from 0.71 (±0.05) to 0.55 (±0.03) ΔF/F0 (n = 24; P < 0.05).
Figure 1.
Specific inhibition of protein kinase C (PKC)-ɛ reduces Ca2+ spark activity in airway smooth muscle cells (SMCs) in the absence of functional inositol 1,4,5-triphosphate receptors (IP3Rs). (A) Effect of specific PKCɛ peptide inhibitor on Ca2+ sparks after treatment with xestospogin-C. Line-scanning images show Ca2+ sparks in a cell before control and after treatment with 10 μM xestospongin-C (xes-C) for 8 minutes to block IP3Rs as well as after application of specific PKCɛ peptide inhibitor (100 μM) for 8 minutes in the continued presence of xestospongin-C. Bar graphs summarize the effects of xestospongin-C and PKCɛ peptide inhibitor in the presence of xestospongin-C on the frequency and amplitude of Ca2+ sparks. Numbers in parentheses indicate the number of cells examined. (B) Effect of PKCɛ gene deletion on Ca2+ sparks after treatment with xestospogin-C. Quantification data show the mean frequency and amplitude of Ca2+ sparks in 48 PKCɛ+/+ and 23 PKCɛ−/− cells after treatment with xestospongin-C (10 μM) for 8 minutes. (C) Effect of PKCɛ peptide inhibitor (100 μM) on Ca2+ sparks in PKCɛ−/− cells after treatment with xestospogin-C (10 μM) for 8 minutes. (D) Effect of PKCɛ peptide inhibitor (100 μM) on Ca2+ sparks in PKCɛ+/+ cells after treatment with xestospogin-C (10 μM) for 8 minutes. Data are presented as means (±SEM). *P < 0.05 compared with control (before application of xestospongin-C or PKCɛ pept) or PKCɛ+/+; †P < 0.05 compared with after treatment with xestospongin-C.
To further test the effect of specific PKCɛ inhibition on Ca2+ sparks in the absence of functional IP3Rs, we measured Ca2+ sparks in airway SMCs from PKCɛ−/− and PKCɛ+/+ (control) mice in the presence of xestospongin-C. As shown in Figure 1B, the frequency of Ca2+ sparks was much higher, whereas the amplitude was lower, in PKCɛ−/− than in PKCɛ+/+ cells.
We also examined the effect of PKCɛ peptide inhibitor on Ca2+ sparks in PKCɛ+/+ and PKCɛ−/− airway SMCs. Consistent with its specific effect, application of PKCɛ peptide inhibitor (100 μM) for 8 minutes failed to affect the frequency and amplitude of Ca2+ sparks in PKCɛ−/− cells in the presence of xestospongin-C (Figure 1C). In control (PKCɛ+/+) cells, however, PKCɛ peptide inhibitor greatly increased Ca2+ spark frequency, and decreased Ca2+ spark amplitude (Figure 1D).
As the activity of Ca2+ sparks can be affected by a change in [Ca2+]i, we examined and compared the resting level of [Ca2+]i before and after application of PKCɛ peptide inhibitor (100 μM) for 8 minutes. The results indicate that the peptide inhibitor had no significant effect on the resting [Ca2+]i. The mean resting levels were 143 (±14) and 141 (±15) nM (n = 58). To test whether a change in the SR Ca2+ load might be involved in the role of PKCɛ inhibition in the regulation of Ca2+ sparks, we next investigated the effect of PKCɛ peptide inhibitor on caffeine (30 mM)-induced SR Ca2+ release. The mean increase in [Ca2+]i in cells untreated (n = 42) and treated with 100 μM PKCɛ peptide inhibitor for 8 minutes (n = 44) was 450 (±29) and 435 (±31) nM, respectively. Thus, specific PKCɛ inhibition does not alter Ca2+ load in the SR.
PKCɛ Activation Decreases the Activity of Ca2+ Sparks in the Absence of Functional IP3Rs
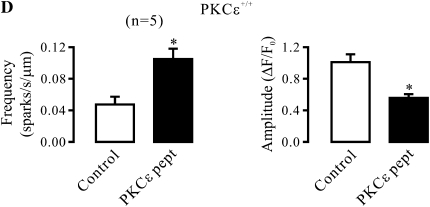
In contrast to PKCɛ inhibition, application of PMA to stimulate PKCɛ resulted in a significant decrease in Ca2+ spark activity and an increase in Ca2+ spark amplitude in control (PKCɛ+/+) airway SMCs in the presence of xestospongin-C (Figure 2A). However, PMA failed to produce an effect in PKCɛ−/− cells in the presence of xestospongin-C (Figure 2B).
Figure 2.
PKCɛ is a major PKC isoform to regulate Ca2+ sparks in airway SMCs in the absence of functional IP3Rs. (A) Effect of the PKC activator phorbol-12-myristate-13-acetate (PMA) on Ca2+ sparks in PKCɛ+/+ cells after treatment with xestospogin-C. Original recordings of Ca2+ sparks in a PKCɛ+/+ cell before and after application of PMA (50 nM) for 8 minutes after treatment with xestospongin-C (10 μM) for 8 minutes. Bar graphs summarize the effect of PMA on the frequency and amplitude of Ca2+ sparks in PKCɛ+/+ cells. (B) Quantification of the effect of 50 nM PMA on the frequency and amplitude of Ca2+ sparks in PKCɛ−/− cells after treatment with xestospogin-C (10 μM) for 8 minutes. Data are presented as means (±SEM). *P < 0.05 compared with control.
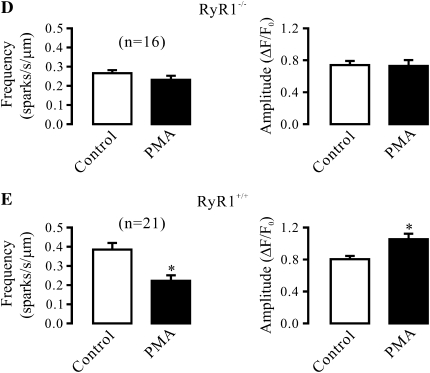
RyR1 Gene Deletion Abolishes the Effect of PKCɛ Inhibition and Activation on Ca2+ Sparks
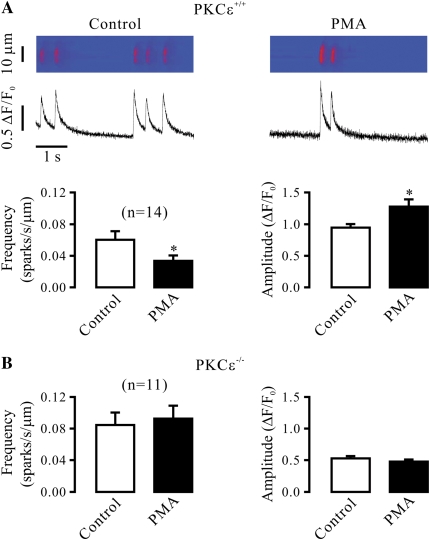
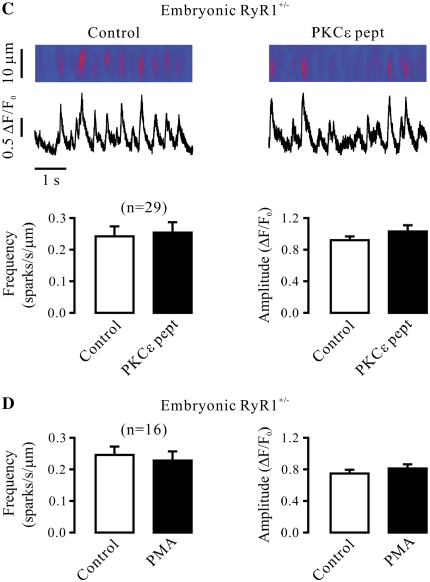
It is well known that all three subtypes of RyRs (RyR1, -2, and -3) are expressed in SMCs; thus, we first sought to use RyR1−/− mice to determine whether this Ca2+ release channel was involved in the role of PKCɛ in the regulation of Ca2+ sparks in airway SMCs. Spindle cells isolated from tracheal muscle tissues of embryonic RyR1+/+ (control) mice were found to be stained by an antibody specific for smooth muscle–specific actin and RyR1 using immunofluorescent staining (Figure 3A), indicating that these isolated cells are SMCs, and express RyR1. Application of PKCɛ peptide inhibitor also resulted in a significant increase in Ca2+ spark frequency and a decrease in Ca2+ spark amplitude in embryonic mouse airway SMCs (Figure 3B). Compared with embryonic RyR1+/+ cells, the activity of Ca2+ sparks was much lower in embryonic RyR1−/− cells. Moreover, application of PKCɛ peptide inhibitor failed to increase the frequency of Ca2+ sparks and to decrease the amplitude of Ca2+ sparks in RyR1−/− cells (Figure 3C).
Figure 3.
Ryanodine receptor (RyR)-1 gene deletion abolishes the effect of specific PKCɛ inhibition on Ca2+ sparks in airway SMCs in the absence of functional IP3Rs. (A) Expression of smooth muscle-specific actin and RyR1 in cells isolated from embryonic RyR1+/+ mice. Cells were initially incubated with anti-RyR1 antibody, anti-actin (smooth muscle) antibody or without either antibody (control), and then with Alexa Fluor 488–conjugated anti-mouse antibody. Immunofluorescent staining was examined using a Zeiss laser scanning confocal microscope. (B) Effect of PKCɛ peptide inhibitor on Ca2+ sparks in embryonic RyR1+/+ cells after treatment with xestospogin-C. Original recordings of Ca2+ sparks were made in an RyR1+/+ cell before and after application of 100 μM PKCɛ peptide inhibitor (PKCɛ pept) for 8 minutes in the presence of xestospongin-C (10 μM). Bar graphs summarize the effects of PKCɛ peptide inhibitor on the frequency and amplitude of Ca2+ sparks in RyR1+/+ cells. (C) Effect of PKCɛ peptide inhibitor (100 μM) on Ca2+ sparks in embryonic RyR1−/− cells after treatment with xestospogin-C (10 μM) for 8 minutes. (D) Effect of PMA (50 nM) on Ca2+ spark frequency and amplitude in embryonic RyR1−/− cells after treatment with xestospogin-C (10 μM) for 8 minutes. (E) Effect of PMA (50 nM) on Ca2+ sparks in embryonic RyR1+/+ cells after treatment with xestospogin-C (10 μM) for 8 minutes. Data are presented as means (±SEM). *P < 0.05 compared with control.
RyR1 gene deletion abolished the effect of PKC activation on Ca2+ sparks as well. Application of PMA had no effect on either Ca2+ spark frequency or amplitude in RyR1−/− cells (Figure 3D), but significantly decreased Ca2+ spark frequency and increased Ca2+ spark amplitude in RyR1+/+ cells (Figure 3E).
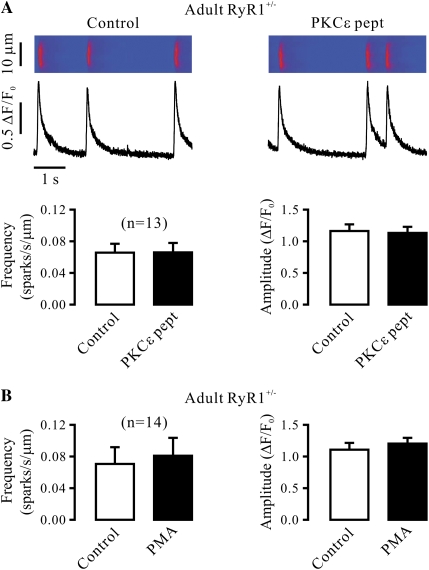
We noted that the activity of Ca2+ sparks was much higher in embryonic than adult mouse airway SMCs. In addition, it is known that heterozygous animals may show an impaired activity of targeted molecules. As such, we next examined and compared the effect of PKCɛ inhibition and activation in adult RyR1+/+ and RyR1+/− mouse airway SMCs. The results show that the frequency of Ca2+ sparks was remarkably lower in cells from RyR1+/− than from RyR1+/+ mice. In parallel to this finding, RyR1 protein expression was decreased by approximately 50% in RyR1+/− cells (data not shown). However, neither PKCɛ peptide inhibitor nor PMA could affect the frequency or amplitude of Ca2+ sparks in RyR1+/− cells (Figures 4A and 4B). Similar to adult RyR1+/− cells, the effect of PKCɛ peptide inhibitor and PMA were both abolished in embryonic RyR1+/− cells (Figures 4C and 4D). These results further demonstrate the involvement of RyR1 in PKCɛ-mediated regulation of Ca2+ sparks in airway SMCs.
Figure 4.
PKCɛ inhibition and activation are unable to regulate Ca2+ sparks in RyR1 heterozygous gene deletion airway SMCs in the absence of functional IP3Rs. (A) Effect of PKCɛ peptide inhibitor on Ca2+ sparks in RyR1+/− cells in the presence of xestospogin-C. Recordings show Ca2+ sparks in an RyR1+/− cell before and after application of 100 μM PKCɛ peptide inhibitor (PKCɛ pept) for 8 minutes after treatment with 10 μM xestospogin-C for 8 minutes. Bar graph summarizes the effect of PKCɛ peptide inhibitor on the frequency and amplitude of Ca2+ sparks in RyR1+/− cells. (B) Effect of PMA (50 nM) on Ca2+ sparks in RyR1+/− cells after treatment with 10 μM xestospogin-C for 8 minutes. (C) Effect of 10 μM PKCɛ peptide inhibitor on Ca2+ sparks in RyR1+/− cells after treatment with 10 μM xestospogin-C for 8 minutes. (D) Effect of PMA (50 nM) on Ca2+ sparks in RyR1+/− cells after treatment with 10 μM xestospogin-C for 8 minutes. Data are presented as means (±SEM).
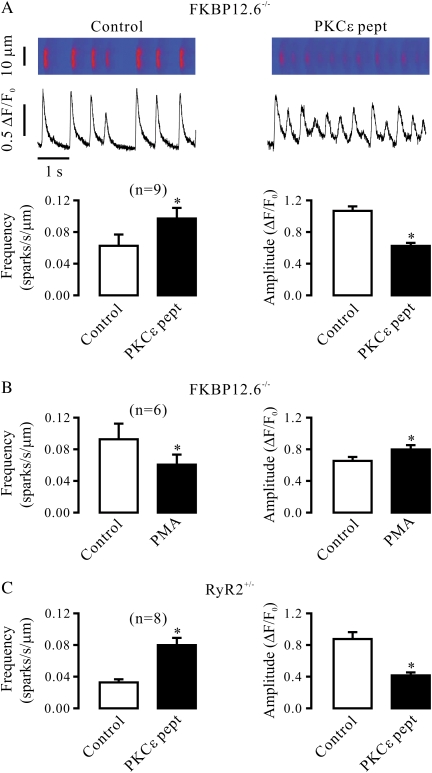
Modification of RyR2 Activity by FKBP12.6 Gene Deletion or RyR2 Heterozygous Gene Deletion Does Not Block the Effect of PKCɛ Inhibition and Activation on Ca2+ Sparks
PKA can phosphorylate RyR2 and then cause its disassociation with FKBP12.6, leading to the channel opening in cardiac cells (20). Accordingly, we wondered whether a similar mechanism could function in PKCɛ-mediated regulation of Ca2+ sparks in airway SMCs. To address this question, we sought to examine and compare the effect of PKCɛ inhibition and activation on Ca2+ sparks in airway SMCs from adult FKBP12.6−/− and FKBP12.6+/+ mice. Our data indicate that FKBP12.6 gene deletion did not prevent the effect of PKCɛ peptide inhibitor on the frequency and amplitude of Ca2+ sparks (Figure 5A). Similarly, FKBP12.6 gene deletion was unable to block the effect of PKCɛ activation with PMA on Ca2+ sparks (Figure 5B).
Figure 5.
Modification of RyR2 activity by FKBP12.6 homozygous gene deletion or RyR2 heterozygous gene deletion fails to prevent the effect of PKCɛ inhibition and activation on Ca2+ sparks in airway SMCs in the absence of functional IP3Rs. (A) Effect of PKCɛ peptide inhibitor on Ca2+ sparks in FKBP12.6−/− cells in the presence of xestospogin-C. Line-scanning recordings of Ca2+ sparks were recorded in an FKBP12.6−/− cell before and after application of 100 μM PKCɛ peptide inhibitor (PKCɛ pept) for 8 minutes after treatment with 10 μM xestospogin-C for 8 minutes. Bar graphs summarize the effect of PKCɛ peptide inhibitor on the frequency and amplitude of Ca2+ sparks in FKBP12.6−/− cells. (B) Effect of 50 nM PMA on Ca2+ sparks in FKBP12.6−/− cells after treatment with 10 μM xestospogin-C for 8 minutes. (C) Effect of 100 μM PKCɛ peptide inhibitor on Ca2+ sparks in RyR2+/− cells after treatment with 10 μM xestospogin-C for 8 minutes. Data are presented as means (±SEM). *P < 0.05 compared with control.
Because RyR2−/− mice die in the early embryonic stage (15), we investigated whether RyR2 heterozygous gene deletion could block the effect of PKCɛ inhibition on Ca2+ sparks. As shown in Figure 5C, application of PKCɛ peptide inhibitor was still able to significantly increase the frequency of Ca2+ sparks, and to reduce the amplitude of Ca2+ sparks in adult RyR2+/− mouse airway SMCs.
RyR3 Gene Deletion Does Not Prevent the Effect of PKCɛ Inhibition and Activation on Ca2+ Sparks
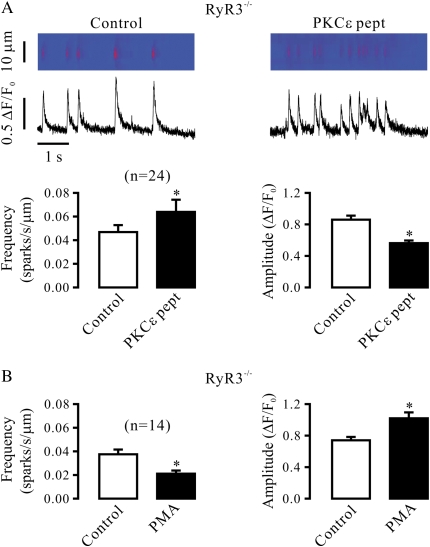
The effects of PKCɛ inhibition and activation on Ca2+ sparks were not prevented in RyR3−/− airway SMCs, either. In cells from adult RyR3−/− mice, application of PKCɛ peptide inhibitor produced significant effects on both Ca2+ spark frequency and amplitude (Figure 6A), whereas PMA was able to cause a significant decrease in the frequency of Ca2+ sparks and an increase in the amplitude of Ca2+ sparks as well (Figure 6B).
Figure 6.
RyR3 gene deletion does not block the effect of PKCɛ inhibition and activation on Ca2+ sparks in airway SMCs in the absence of functional IP3Rs. (A) Effect of PKCɛ peptide inhibitor on Ca2+ sparks in RyR3−/− cells in the presence of xestospogin-C. Original recordings show Ca2+ sparks recorded in an RyR3−/− cell before and after application of 100 μM PKCɛ peptide inhibitor (PKCɛ pept) for 8 minutes after treatment with 10 μM xestospogin-C for 8 minutes. Bar graphs illustrate the effect of PKCɛ peptide inhibitor in RyR3−/− cells. (B) Effect of 50 nM PMA on Ca2+ sparks in RyR3−/− cells after treatment with 10 μM xestospogin-C for 8 minutes. Data are presented as means (±SEM). *P < 0.05 compared with control.
Modifications of Ca2+ Sparks by PKCɛ Inhibition and Activation Affect Contractile Responses in Airway Smooth Muscle
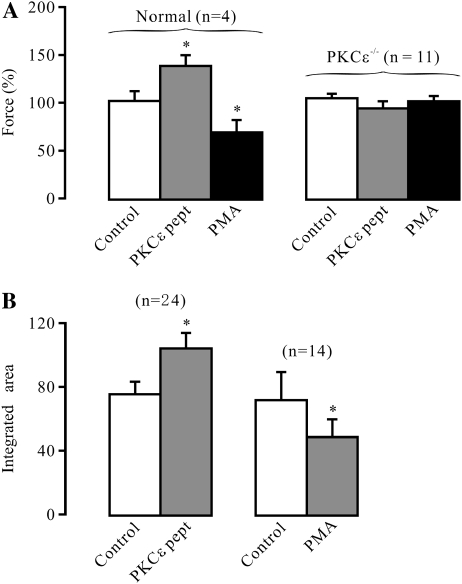
Because Ca2+ sparks preferentially cause depolarizing STICs in airway SMCs (3), we wondered whether PKCɛ inhibition to increase Ca2+ spark activity could promote airway muscle contraction, whereas PKCɛ activation to decrease Ca2+ spark activity could inhibit airway muscle contraction. PKCɛ peptide inhibitor is known to be cell permeable, but it is unknown whether this peptide can actually penetrate into cells of airway muscle tissues to produce an effect. As such, we examined the effect of PKCɛ peptide inhibitor on the activity of PKCɛ in isolated tracheal muscle tissues. The results indicate that the absorbance intensity of tetramethylbenzidine at 450 nm as a function of PKCɛ activity was 0.74 (±0.17) and 0.04 (±0.01) optical density in tracheal muscle tissues untreated and treated with the peptide inhibitor for 8 minutes, respectively (n = 4; P < 0.05), indicating that PKCɛ peptide inhibitor blocks the activity of PKCɛ in airway muscle tissues. As shown in Figure 7A, treatment with 100 μM PKCɛ peptide inhibitor for 8 minutes could significantly augment muscle contraction induced by the muscarinic receptor agonist, methacholine (0.5 μM), in normal tracheal rings, whereas application of 50 nM PMA for 8 minutes produced an opposite effect. In contrast, neither PKCɛ peptide inhibitor nor PMA affected methacholine-induced muscle contraction in PKCɛ−/− tracheal rings.
Figure 7.
PKCɛ inhibition and activation regulate muscarinic contraction in airway muscle. (A) Muscle contraction induced by the muscarinic receptor agonist, methacholine, was measured in isolated tracheal rings from normal and PKCɛ−/− mice before and after treatment with 100 μM PKCɛ peptide inhibitor (PKCɛ pept) or 50 nM PMA for 8 minutes. The results are expressed as the percentage of methacholine-induced contraction before and after the treatment of PKCɛ peptide inhibitor or PMA. In control experiments, isolated tracheal rings were treated identically, but without either PKCɛ peptide inhibitor or PMA. (B) Effect of 100 μM PKCɛ peptide inhibitor and 50 nM PMA on the integrated local Ca2+ signals, determined by integrating the areas of Ca2+ sparks. Data analysis was performed in cells before and after application of PKCɛ peptide inhibitor (Figure 1A) and PMA (Figure 2A). Data are presented as means (±SEM). *P < 0.05 compared with control.
To define whether the role of PKCɛ inhibition and activation in the regulation of airway muscle contraction were correlated with their effects on local Ca2+ signals, we analyzed and compared the integrated areas of Ca2+ sparks in cells before and after application of PKCɛ peptide inhibitor and PMA. The results indicate that the integrated local Ca2+ signals were significantly increased after treatment with PKCɛ peptide inhibitor, and decreased after treatment with PMA (Figure 7B). Thus, PKCɛ inhibition may enhance the integrated local Ca2+ signals to promote neurotransmitter-induced airway muscle contraction, whereas PKCɛ activation may lessen the integrated local Ca2+ signals to reduce neurotransmitter-induced airway muscle contraction.
DISCUSSION
Increasing evidence indicates that Ca2+ sparks, due to the opening of RyRs, play important roles in numerous physiological and pathological responses in many types of cells, including SMCs. We and other investigators have shown that PKC stimulation can result in a significant reduction in the activity of RyR-mediated Ca2+ sparks in the cerebral arterial and airway SMCs (10–12). Furthermore, our most recent study reveals that PKCɛ is a major PKC isoform in the regulation of Ca2+ sparks in airway SMCs (12). In a natural extension of these exciting results, here we have found that the inhibition of PKCɛ with a specific peptide inhibitor or gene deletion significantly increases, while the activation of PKCɛ with PMA reduces, the frequency of Ca2+ sparks in the presence of xestospongin-C to effectively eliminate functional IP3Rs in airway SMCs (Figures 1 and 2). In addition, specific PKCɛ inhibition also decreases, whereas PKCɛ activation increases Ca2+ spark amplitude in the absence of functional IP3Rs. We have also observed that, in PKCɛ−/− cells, PKCɛ inhibition with a peptide inhibitor and PKCɛ activation with PMA fails to affect either Ca2+ spark frequency or amplitude (Figures 1 and 2). These results not only indicate that the PKCɛ peptide inhibitor and PMA specifically target PKCɛ, but also suggest that PKCɛ may serve as a major PKC isoform in specifically regulating RyR-mediated Ca2+ sparks in airway SMCs.
A number of reports have shown that IP3Rs can crosstalk to RyRs to regulate the activity of Ca2+ sparks in airway and other types of SMCs through a local Ca2+-induced Ca2+ release mechanism (12, 21, 22). In the present study, we have revealed that PKCɛ is able to modulate Ca2+ sparks in the absence of functional IP3Rs. In addition, our data also indicate that both specific inhibition and activation of PKCɛ neither alter the resting level of [Ca2+]i nor caffeine-induced maximal Ca2+ release (SR Ca2+ load). These results, together with previous findings that PKC may phosphorylate RyRs and subsequently increase [3H] ryanodine binding in cardiac microsomes (8), suggest that PKCɛ is likely to directly interact with RyRs modulating the activity of Ca2+ sparks in SMCs.
RyR1, a predominant subtype of RyRs in skeletal muscle cells, is also expressed in SMCs. Inhibition of RyR1 gene expression with antisense oligonucleotides significantly suppresses membrane depolarization–induced Ca2+ sparks in cultured portal vein myocytes (23). Additionally, RyR1 gene deletion abolishes depolarization-induced Ca2+ sparks in embryonic bladder SMCs (24). These results suggest an important role of RyR1 in the generation of spontaneous Ca2+ sparks in SMCs. Consistent with this view, in the present study, we have observed that the activity of spontaneous Ca2+ sparks is significantly reduced in embryonic RyR1−/−, embryonic RyR+/−, and adult RyR+/− airway SMCs. Furthermore, our data indicate that, in embryonic RyR1−/− airway myocytes, specific PKCɛ inhibition neither increases the frequency of Ca2+ sparks nor decreases the amplitude of Ca2+ sparks (Figure 3). Similarly, PKCɛ activation fails to produce an effect on either Ca2+ spark frequency or amplitude in RyR1−/− SMCs. Moreover, PKCɛ inhibition or activation is unable to affect Ca2+ spark frequency and amplitude in cells from adult and embryonic RyR1+/− mice (Figure 4). It is well known that Ca2+ sparks are generated due to the simultaneous opening of a cluster of multiple RyRs. Because of this underlying mechanism, a reduction in RyR1 expression by heterozygous gene deletion results in the inability of the rest of the channels to form the functional cluster necessary to generate Ca2+ sparks, wherein PKCɛ fails to regulate RyR1 and the associated Ca2+ sparks in RyR1+/− cells. Collectively, PKCɛ may specifically interact with RyR1 to regulate Ca2+ sparks in SMCs. It has been reported that native RyR1 in skeletal myocytes generates Ca2+ sparks (25). Apparently, PKCɛ may also regulate Ca2+ sparks in skeletal muscle cells, contributing to physiological and pathological responses in this type of cells.
FKBP12.6 is an endogenous molecule that can physically bind to RyR2 and biologically control its activity in airway and vascular SMCs (17, 26). Gene deletion of this molecule significantly increases the frequency of Ca2+ sparks in bladder myocytes (27), implying a significant role of RyR2 in the generation of Ca2+ sparks in SMCs. A previous study has shown that PKA can cause phosphorylation of RyR2, leading to its disassociation with FKBP12.6, which increases the open probability of these Ca2+ release channels in cardiac cells (20). This inspired us to investigate whether an analogous functioning process could operate in the role of PKCɛ in the regulation of Ca2+ sparks in airway SMCs. Our data reveal that FKBP12.6 gene deletion does not block PKCɛ inhibition–induced increase in the frequency of Ca2+ sparks and decrease in the amplitude of Ca2+ sparks (Figure 5). The effect of PKCɛ activation on Ca2+ spark frequency and amplitude are unaltered in FKBP12.6−/− cells as well. In agreement with the effect of FKBP12.6 gene deletion, RyR2 heterozygous gene deletion to reduce its activity does not block the effect of PKCɛ peptide inhibitor on Ca2+ sparks, either. These results further suggest that RyR2 is unlikely to be involved in PKCɛ-mediated regulation of Ca2+ sparks in airway myocytes.
The role of native RyR3 in the generation of Ca2+ sparks in SMCs is unclear. A previous study has shown that RyR3 gene deletion does not alter the activity of Ca2+ sparks in bladder SMCs (27). Similarly, antisense oligonucleotides–mediated inhibition of RyR3 gene also has no effect on depolarization-triggered Ca2+ sparks in cultured portal vein myocytes. In contrast, a report has shown that Ca2+ spark activity is increased in RyR3−/− cerebral arterial SMCs (28). These diverse findings seem to reflect the distinct expression and organization of this Ca2+ release channel in different types of cells, although further experiments are needed to verify this view. Nevertheless, we have shown that the effect of PKCɛ inhibition on the frequency and amplitude of Ca2+ sparks is not blocked in cells from RyR3−/− mice (Figure 6). Furthermore, the effect of PKCɛ activation on Ca2+ spark frequency and amplitude are not prevented in RyR3−/− cells, either. These data reinforce the specific role of RyR1 in the PKCɛ-induced regulation of Ca2+ sparks in SMCs.
It is well established that Ca2+ sparks can produce hyperpolarizing STOCs to inhibit Ca2+ influx and then cause relaxation in cerebral arterial SMCs (2). In contrast, these local Ca2+ signals may generate depolarizing STICs, which promote Ca2+ influx and contraction in pulmonary vascular and airway myocytes (3, 4). In this article, we present data showing that PKCɛ inhibition to increase Ca2+ spark activity can promote airway muscle contraction induced by stimulation of muscarinic receptors with methacholine, whereas PKCɛ activation to decrease Ca2+ spark activity produces an opposite effect. The regulatory effect of PKCɛ inhibition and activation on agonist-induced muscle contraction is abolished in PKCɛ−/− mouse tracheal rings (Figure 7). We have also found that PKCɛ inhibition enhances, whereas PKCɛ activation reduces, the integrated local Ca2+ signals, which is in line with their effect on airway muscle contraction. These findings provide experimental proof that PKCɛ may play an important role in physiological responses by regulating the activity of RyR-mediated Ca2+ sparks in SMCs. We have recently reported that the firing of spontaneous Ca2+ sparks is finely controlled by the basal activity of phospholipase C through a positive IP3–IP3R pathway and a negative diacylglycerol–PKCɛ pathway in airway SMCs (12). Moreover, phospholipase C has long been recognized as a key signaling player in contractile responses after stimulation of neurotransmitter, hormone, and growth factor receptors by generating IP3 and diacylglycerol in virtually every type of SMC. All these findings further support the physiological significance of PKCɛ in the regulation of RyR-mediated local Ca2+ release and attendant contraction in airway myocytes.
In conclusion, we have, for the first time, provided clear evidence showing that specific PKCɛ inhibition significantly increases, whereas PKCɛ activation decreases, the frequency of Ca2+ sparks in airway SMCs in the absence of functional IP3Rs. PKCɛ inhibition and activation also results in an increase and a decrease, respectively, in Ca2+ spark amplitude. However, neither PKCɛ inhibition nor activation alters the resting level of [Ca2+]i and SR Ca2+ load. The regulatory effects of PKCɛ on Ca2+ sparks are completely abolished in RyR1−/− and RyR1+/−, but not in FKBP12.6−/−, RyR2+/−, or RyR3−/− airway SMCs. PKCɛ inhibition enhances the integrated local Ca2+ signals and promotes agonist-induced airway muscle contraction, whereas PKCɛ activation produces the opposite effects. Thus, PKCɛ regulates Ca2+ sparks by interacting with RyR1 in airway SMCs. This specific mode of regulation of Ca2+ sparks has a significant physiological impact on contractile responses in airway SMCs and also potentially in other types of cells.
Acknowledgments
The authors thank Krista Wadsworth and Jodi Heim for technical assistance.
This work was supported by National Institutes of Health grant R01HL071000 (Y.-X.W.), American Heart Association Established Investigator Award 0340160N (Y.-X.W.), and Scientist Development grants 0730242N (Q.-H.L.) and 0630236N (Y.-M.Z.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0323OC on November 14, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 1993;262:740–744. [DOI] [PubMed] [Google Scholar]
- 2.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 1995;270:633–637. [DOI] [PubMed] [Google Scholar]
- 3.Kotlikoff MI, Wang YX. Calcium release and calcium-activated chloride channels in airway smooth muscle cells. Am J Respir Crit Care Med 1998;158:S109–S114. [DOI] [PubMed] [Google Scholar]
- 4.Remillard CV, Zhang WM, Shimoda LA, Sham JS. Physiological properties and functions of Ca2+ sparks in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2002;283:L433–L444. [DOI] [PubMed] [Google Scholar]
- 5.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res 2006;99:398–406. [DOI] [PubMed] [Google Scholar]
- 6.Kohlhaas M, Zhang T, Seidler T, Zibrova D, Dybkova N, Steen A, Wagner S, Chen L, Brown JH, Bers DM, et al. Increased sarcoplasmic reticulum calcium leak but unaltered contractility by acute CaMKII overexpression in isolated rabbit cardiac myocytes. Circ Res 2006;98:235–244. [DOI] [PubMed] [Google Scholar]
- 7.Yang D, Zhu WZ, Xiao B, Brochet DX, Chen SR, Lakatta EG, Xiao RP, Cheng H. Ca2+/calmodulin kinase II–dependent phosphorylation of ryanodine receptors suppresses Ca2+ sparks and Ca2+ waves in cardiac myocytes. Circ Res 2007;100:399–407. [DOI] [PubMed] [Google Scholar]
- 8.Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem 1991;109:163–170. [DOI] [PubMed] [Google Scholar]
- 9.Mironov SL, Hermann A. Ethanol actions on the mechanisms of Ca2+ mobilization in rat hippocampal cells are mediated by protein kinase C. Brain Res 1996;714:27–37. [DOI] [PubMed] [Google Scholar]
- 10.Bonev AD, Jaggar JH, Rubart M, Nelson MT. Activators of protein kinase C decrease Ca2+ spark frequency in smooth muscle cells from cerebral arteries. Am J Physiol 1997;273:C2090–C2095. [DOI] [PubMed] [Google Scholar]
- 11.Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 2000;279:C1528–C1539. [DOI] [PubMed] [Google Scholar]
- 12.Liu QH, Zheng YM, Wang YX. Two distinct signaling pathways for regulation of spontaneous local Ca2+ release by phospholipase C in airway smooth muscle cells. Pflugers Arch 2007;453:531–541. [DOI] [PubMed] [Google Scholar]
- 13.Takeshima H, Iino M, Takekura H, Nishi M, Kuno J, Minowa O, Takano H, Noda T. Excitation–contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature 1994;369:556–559. [DOI] [PubMed] [Google Scholar]
- 14.Xin HB, Senbonmatsu T, Cheng DS, Wang YX, Copello JA, Ji GJ, Collier ML, Deng KY, Jeyakumar LH, Magnuson MA, et al. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature 2002;416:334–337. [DOI] [PubMed] [Google Scholar]
- 15.Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J 1998;17:3309–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeshima H, Ikemoto T, Nishi M, Nishiyama N, Shimuta M, Sugitani Y, Kuno J, Saito I, Saito H, Endo M, et al. Generation and characterization of mutant mice lacking ryanodine receptor type 3. J Biol Chem 1996;271:19649–19652. [DOI] [PubMed] [Google Scholar]
- 17.Wang YX, Zheng YM, Mei QB, Wang QS, Collier ML, Fleischer S, Xin HB, Kotlikoff MI. FKBP12.6 and cADPR regulation of Ca2+ release in smooth muscle cells. Am J Physiol Cell Physiol 2004;286:C538–C546. [DOI] [PubMed] [Google Scholar]
- 18.Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol 2005;125:427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rathore R, Zheng YM, Li XQ, Wang QS, Liu QH, Ginnan R, Singer HA, Ho YS, Wang YX. Mitochondrial ROS-PKCɛ signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun 2006;351:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 2000;101:365–376. [DOI] [PubMed] [Google Scholar]
- 21.Gordienko DV, Bolton TB. Crosstalk between ryanodine receptors and IP(3) receptors as a factor shaping spontaneous Ca2+-release events in rabbit portal vein myocytes. J Physiol 2002;542:743–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boittin FX, Coussin F, Macrez N, Mironneau C, Mironneau J. Inositol 1,4,5-trisphosphate– and ryanodine-sensitive Ca2+ release channel-dependent Ca2+ signalling in rat portal vein myocytes. Cell Calcium 1998;23:303–311. [DOI] [PubMed] [Google Scholar]
- 23.Coussin F, Macrez N, Morel JL, Mironneau J. Requirement of ryanodine receptor subtypes 1 and 2 for Ca2+-induced Ca2+ release in vascular myocytes. J Biol Chem 2000;275:9596–9603. [DOI] [PubMed] [Google Scholar]
- 24.Fritz N, Morel JL, Jeyakumar LH, Fleischer S, Allen PD, Mironneau J, Macrez N. RyR1-specific requirement for depolarization-induced Ca2+ sparks in urinary bladder smooth muscle. J Cell Sci 2007;120:3784–3791. [DOI] [PubMed] [Google Scholar]
- 25.Schneider MF, Ward CW. Initiation and termination of calcium sparks in skeletal muscle. Front Biosci 2002;7:d1212–d1222. [DOI] [PubMed] [Google Scholar]
- 26.Zheng YM, Mei QB, Wang QS, Abdullaev I, Lai FA, Xin HB, Kotlikoff MI, Wang YX. Role of FKBP12.6 in hypoxia- and norepinephrine-induced Ca2+ release and contraction in pulmonary artery myocytes. Cell Calcium 2004;35:345–355. [DOI] [PubMed] [Google Scholar]
- 27.Ji G, Feldman ME, Greene KS, Sorrentino V, Xin HB, Kotlikoff MI. RYR2 proteins contribute to the formation of Ca2+ sparks in smooth muscle. J Gen Physiol 2004;123:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohn M, Jessner W, Furstenau M, Wellner M, Sorrentino V, Haller H, Luft FC, Gollasch M. Regulation of calcium sparks and spontaneous transient outward currents by RyR3 in arterial vascular smooth muscle cells. Circ Res 2001;89:1051–1057. [DOI] [PubMed] [Google Scholar]