Abstract
Previous studies have shown that leukotriene B4 (LTB4), a proinflammatory lipid mediator, is linked to the development of airway hyperresponsiveness through the accumulation of IL-13–producing CD8+ T cells, which express a high affinity receptor for LTB4, BLT1 (Miyahara et al., Am J Respir Crit Care Med 2005;172:161–167; J Immunol 2005;174:4979–4984). By using leukotriene A4 hydrolase–deficient (LTA4H−/−) mice, which fail to synthesize LTB4, we determined the role of this lipid mediator in allergen-induced airway responses. Two approaches were used. In the first, LTA4H−/− mice and wild-type (LTA4H+/+) mice were systemically sensitized and challenged via the airways to ovalbumin. In the second, mice were passively sensitized with anti-ovalbumin IgE and exposed to ovalbumin via the airways. Mast cells were generated from bone marrow of LTA4H+/+ mice or LTA4H−/− mice. After active sensitization and challenge, LTA4H−/− mice showed significantly lower airway hyperresponsiveness compared with LTA4H+/+ mice, and eosinophil numbers and IL-13 levels in the bronchoalveoloar lavage of LTA4H−/− mice were also significantly lower. LTA4H−/− mice also showed decreased airway reactivity after passive sensitization and challenge. After LTA4H+/+ mast cell transfer, LTA4H−/− mice showed increased airway reactivity after passive sensitization and challenge, but not after systemic sensitization and challenge. These data confirm the important role for LTB4 in the development of altered airway responses and suggest that LTB4 secretion from mast cells is critical to eliciting increased airway reactivity after passive sensitization with allergen-specific IgE.
Keywords: rodent, T cells, cytokines, lipid mediators, lung
CLINICAL RELEVANCE
Lipid mediators play a major role in the pathophysiology of allergen-induced lung disease. Leukotriene B4 (LTB4) is emerging as a major chemoattractant for subsets of T cells in the lung which contribute to the pathophysiology of asthma. Mice genetically incapable of synthesizing LTB4 have limited capacity to develop allergen-induced altered airway dysfunction and inflammation. Mast cells are a major source of this lipid mediator and can reconstitute responses in LTB4-deficient mice.
Allergic asthma is a complex syndrome characterized by airway obstruction, airway inflammation, and airway hyperresponsiveness (AHR) (1, 2). It is well established that acquired immunity plays an important role in the development of these responses; central to the pathogenesis of airway disease are antigen-specific memory T cell responses and antigen-specific B cell responses (2, 3).
Leukotriene B4 (LTB4) is a potent lipid inflammatory mediator derived from membrane phospholipids by the sequential actions of cytosolic phospholipase A2, 5-lipoxygenase, and leukotriene A4 (LTA4) hydrolase (4, 5). LTB4 is a chemoattractant for leukocytes, including neutrophils, macrophages, monocytes, and eosinophils (6–10). LTB4 activates leukocytes through a G protein–coupled high-affinity surface receptor, BLT1 (11, 12). Recently, BLT1 has been shown to be expressed on CD8+ effector memory T cells, and it has also been shown that the LTB4-BLT1 pathway is important for effector T cell accumulation at sites of inflammation (13, 14). LTB4 levels are increased in patients with asthma compared with healthy subjects (15, 16), and in animal models, we and others have shown that the LTB4-BLT1 pathway appears critical to the development of allergen-induced AHR and inflammation (17–21).
In the present study, we investigated the role of LTB4 production in the development of allergen-induced AHR and airway inflammation using LTA4 hydrolase-deficient (LTA4H−/−) mice, which lack the capacity to synthesize LTB4. To define the role of LTA4H, we used two models of allergen-induced AHR: an IgE mast cell–independent and an IgE mast cell–dependent model. LTA4H−/− mice showed significantly reduced allergen-induced AHR in both models.
MATERIALS AND METHODS
Animals
Female mice with a targeted disruption of the LTA4H (LTA4H−/− mice, 129Seve genetic background) were kindly provided by Dr. B. H. Koller (University of North Carolina, Chapel Hill, NC) (22), and LTA4H+/+ mice 8 to 12 weeks of age (on the same genetic background, 129Seve) were used in all experiments. The mice were bred and housed under specific pathogen–free conditions and maintained on an ovalbumin (OVA)-free diet in the Biological Resources Center at National Jewish Health. All experimental animals used in this study were under a protocol approved by the Institutional Animal Care and Use Committee of National Jewish Health.
Sensitization and Airway Challenge
LTA4H−/− and LTA4H+/+ mice were assigned to the following groups: (1): nonsensitized—airway challenge with nebulized OVA alone (PBS/OVA group); and (2) intraperitoneal sensitization with OVA and OVA airway challenge (OVA/OVA). Mice were sensitized by intraperitoneal injection with 20 μg of OVA (Grade V; Sigma, St. Louis, MO) emulsified in 2 mg of alum (Imuject Alum; Pierce, Rockford, IL) in a total volume of 100 μl on Days 1 and 14. Mice were subsequently challenged via the airways by inhalation of aerosols of OVA (1% in saline) for 20 minutes on Days 28, 29, and 30. OVA aerosols were produced by an ultrasonic nebulizer (particle size 1–5 μm; OMRON, Kyoto, Japan). On Day 32, airway function was measured as described below, followed by collection of samples for further analyses.
Assessment of Airway Function
Airway responsiveness was assessed as a change in lung function after provocation with aerosolized methacholine using a method previously described in detail (23). Methacholine aerosol was administered for 10 seconds (160 breaths/min, 0.5 ml tidal volume) in increasing concentrations. Maximum values of lung resistance (RL) were recorded and expressed as percent change from baseline after saline aerosol. There were no significant differences in baseline values among the different groups.
Bronchoalveolar Lavage
Immediately after measurements of AHR, lungs were lavaged and bronchoalveolar lavage (BAL) fluid recovered. Total leukocyte numbers were obtained (Beckman Coulter, Fullerton, CA). Cytospin slides were stained with Leukostat (Fisher Diagnostics, Pittsburgh, PA) and differentiated by standard hematologic procedures.
Histologic Studies
After lavage, the lungs were fixed in 10% formalin and processed into paraffin blocks. Tissue sections, 5 μm thick, were stained with hematoxylin and eosin and periodic acid-Schiff (PAS) for identification of mucus-containing cells. The number of mucus-containing cells per millimeter of basement membrane was determined using IPLAB2 software (Signal Analytics, Vienna, VA) for the Macintosh computer, counting six to eight different fields per animal as previously described (24).
Cells containing eosinophilic major basic protein (MBP) were identified by immunohistochemical staining as previously described using rabbit-anti mouse MBP (kindly provided by Dr. J. J. Lee, Mayo Clinic, Scottsdale, AZ) (24). Numbers of peribronchial eosinophils in the tissues were evaluated as previously described (24).
Immunofluorescence Staining for Detecting LTA4H-Positive BMMC in LTA4H−/− Mice
The lungs of LTA4H−/− mice with or without LTA4H+/+ BMMC transfer were inflated and embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA). Frozen lung sections were cut to 15-μm thickness and fixed with cold acetone and methanol. After blocking with 5% normal donkey serum, LTA4H was detected using goat anti-human LTA4H polyclonal Ab (Santa Cruz Biotechnology, Santa Cruz, CA) as a primary Ab and rhodamine red–conjugated donkey anti-goat IgG polyclonal Ab (Jackson ImmunoResearch, West Grove, PA) as a secondary Ab. Nuclei were stained (blue) using Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). The immunofluorescent images were obtained with a Leica microscope camera system and Slidebook 4.2 digital microscopy software (Intelligent Imaging Innovations, Denver, CO). LTA4H-positive cells in the peribronchial regions (within 0.1 mm in depth under the subepithelial basement membrane) or alveolar regions were counted using a photo processing software (ImageJ, developed at the National Institute of Mental Health, Bethesda, MD, and available at http://rsb.info.nih.gov/ij/index.html). Data are expressed as the number of LTA4H-positive cells per millimeter square of peribronchial or alveolar regions.
Cell Preparation and Culture
Lung mononuclear cells (MNC) were isolated as previously described (25) using collagenase digestion. MNC (4 × 105) were cultured for 24 hours in 96-well round-bottom plates in the presence or absence of OVA (10 μg/ml) as previously described (26).
Measurement of Cytokines and LTB4
Cytokine levels in the BAL fluid and cell culture supernatants were measured by ELISA using IL-4, IL-5, IFN-γ (BD Biosciences/Pharmingen, San Diego, CA), and IL-13 (R&D Systems, Minneapolis, MN) kits. LTB4 levels in the BAL fluid were also measured by ELISA (R&D Systems). ELISAs were performed according to the manufacturer's directions. The limits of detection were 4 pg/ml for IL-4, IL-5, and IFN-γ, 5 pg/ml for IL-13, and 5.6 pg/ml for LTB4.
Flow Cytometry
After purification, cells were incubated with APC-conjugated anti-CD3, PE-conjugated anti-CD4, FITC-conjugated anti-CD8 antibodies (BD Pharmingen), and then analyzed by flow cytometry (FACScalibur; Becton Dickinson Immunocytometry Systems, San Jose, CA) as previously described (17). The number of cytokine-producing CD3+, CD4+, or CD8+ T cells per lung was calculated from the percent of cytokine-producing cells and the number of CD3+, CD4+, or CD8+ T cells isolated from the lung.
Experimental Protocols for Passive Sensitization
Experimental groups consisted of four mice per group and each experiment was performed at least twice. Mice were injected intravenously on 2 consecutive days with 2 μg of OVA-specific IgE, derived from an anti-OVA–secreting hybridoma cell line (25), in a volume of 200 μl and then exposed to OVA (Grade V; Sigma-Aldrich) via the airways as described previously (25). Briefly, a solution of 1% OVA in 0.9% saline was delivered by ultrasonic nebulization for 20 minutes on Days 3 and 4 after the second OVA-IgE injection. AHR was assessed 48 hours after the last nebulization.
Assessment of Airway Smooth Muscle Responsiveness
Forty-eight hours after the last airway challenge, AHR was assessed by measuring airway smooth muscle responsiveness to increasing frequency of electric field stimulation (EFS), as described previously (27). Frequencies resulting in 50% of the maximal contraction (ES50) were calculated for each individual animal and were compared between the groups.
Transfer of Bone Marrow–Derived Mast Cells
Mast cells were derived from bone marrow as previously described (28). Bone marrow was obtained from LTA4H+/+ and LTA4H−/− mice and cultured in Iscove Modified Dulbecco's Medium (Life Technologies, Grand Island, NY) supplemented with 5% FBS (Summit Biotechnology, Fort Collins, CO), 50 μM 2-ME (Life Technologies), 2 mM glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin, 0.5 μg/ml amphotericin B, IL-3 obtained from medium conditioned by X63-AG8-653 myeloma cells transfected with a vector expressing IL-3 (29), and c-kit ligand from medium conditioned by CHO cells (provided by S. Webb, National Jewish Health, Denver, CO). After 4 weeks of culture, more than 98% of nonadherent cells contained granules that stained positively with toluidine blue, and over 98% expressed the high-affinity IgE receptor (FcɛRI) and c-kit on their surface as determined by FACS analysis using anti-FcɛRI mAb and anti–c-kit mAb.
LTA4H−/− recipients received 0.5 to 10 × 106 bone marrow–derived mast cells (BMMC) intravenously 8 weeks before initiating the systemic sensitization with OVA plus alum or passive sensitization with OVA-IgE. Assessment of airway responses was performed 48 hours after the last OVA challenge. This protocol for mast cell transfer has been shown to result in mast cell accumulation in the airways and to reconstitute development of lung allergic responses in mast cell–deficient mice after a mast cell–dependent protocol for the development of lung allergic responses (30).
In some experiments, after transfer of mast cells, passive sensitization, and challenge, the LTB4H−/− recipients received an LTB4 receptor antagonist (CP105, 696, provided by Pfizer Pharmaceuticals, Groton, CT) (31, 32) or vehicle by gavage at 50 mg/kg (suspended in 100 μl of hydroxypropylmethylcellulose [Abbott Laboratories, Lake Bluff, IL]), 2 days and 1 day before initiating the OVA challenges and 1 hour before each OVA challenge.
Transfer of T Cells or MNC
Spleens of LTA4H+/+ or LTA4H−/− mice, which were sensitized twice (Days 1 and 14) with OVA plus alum, were removed 14 days after the last sensitization (Day 28). MNC were first isolated by Histopaque gradient centrifugation (Sigma-Aldrich). T cells from MNC were isolated by Mouse T Cell Recovery Column Kit (Cedarlane, Burlington, ON, Canada) (purity > 95%) and 1 × 107 T cells were transferred into LTA4H−/− mice on Day 28, 2 hours before OVA challenge.
To assess the degree of purification, cells were incubated with APC-conjugated anti-CD3, FITC-conjugated anti-CD4, and PE-conjugated antibodies (anti-CD8, anti-CD11c, anti-mouse pan-NK cells, anti-B220, anti-γδ TCR, or anti-αβ TCR) (BD Pharmingen), and then analyzed by flow cytometry (FACScalibur).
Recipient LTA4H−/− mice were sensitized twice with OVA plus alum on Days 1 and 14. OVA-primed LTA4H+/+ T cells (1 × 107) or MNC (1 × 107) were administered intravenously via the tail vein into OVA-sensitized LTA4H−/− mice, 14 days after the last sensitization (Day 28). After transfer, the mice were exposed to three allergen challenges via the airways on Days 28, 29, and 30. Assays were performed on Day 32.
In some experiments, 2 × 107 MNC were injected intravenously 4 weeks before the first sensitization (Day −28) into recipient LTA4H−/− mice. Recipients were then sensitized twice with OVA plus alum on Days 1 and 14, followed by OVA challenge on Days 28, 29, and 30; airway responses were assessed on Day 32.
Transfer of Alveolar Macrophages
Lungs of naive LTA4H+/+ or LTA4H−/− mice were lavaged and BAL fluid was recovered. More than 95% of the cells in BAL fluid were judged to be alveolar macrophages. Under anesthesia, 1 × 106 alveolar macrophages in 40 μl of PBS were instilled into OVA-sensitized LTA4H−/− or passively sensitized LTA4H−/− mice through the trachea, under fiberoptic illumination. Twenty-four hours after macrophage transfer, mice were exposed to OVA, then airway responses were assessed.
Statistical Analysis
All results were expressed as the means ± SEM. ANOVA was used to determine the levels of difference between all groups. Pairs of groups of samples distributed parametrically were compared by unpaired two-tailed Student's t test, and those samples distributed nonparametrically were compared by Mann-Whitney U test. Significance was assumed at P values of < 0.05.
RESULTS
LTA4H−/− Mice Develop Significantly Lower Allergic Airway Responses Compared with LTA4H+/+ Mice after Systemic Sensitization and Challenge
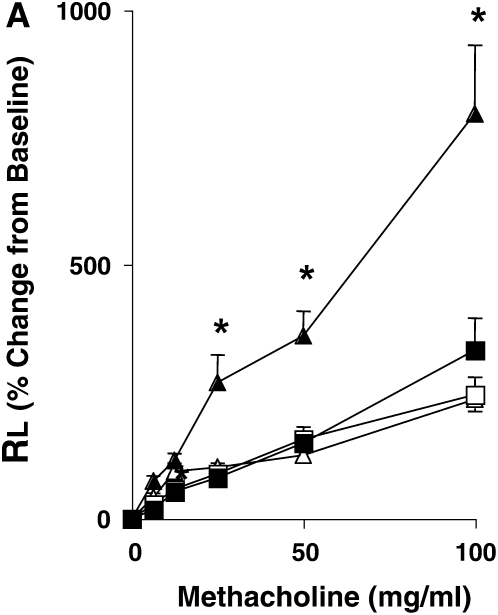
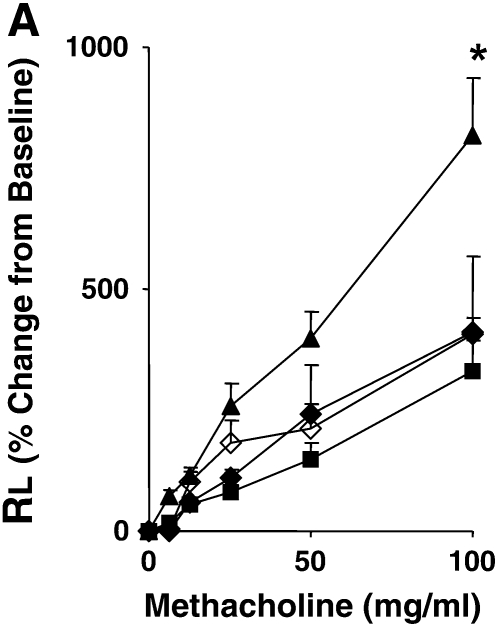
Systemic (intraperitoneal) sensitization to allergen with alum leads to the development of AHR independent of mast cells and IgE and airway inflammation as documented by responses in mast cell– and B cell–deficient mice and in mice lacking FcɛRI (23, 33). OVA sensitization and airway challenge led to the development of increased AHR in LTA4H+/+ mice, illustrated by significant increases in RL, as compared with mice challenged (nonsensitized) alone with OVA (Figure 1A). In contrast, OVA-sensitized and -challenged LTA4H−/− mice developed significantly lower increases in RL compared with sensitized and challenged LTA4H+/+ mice (Figure 1A).
Figure 1.
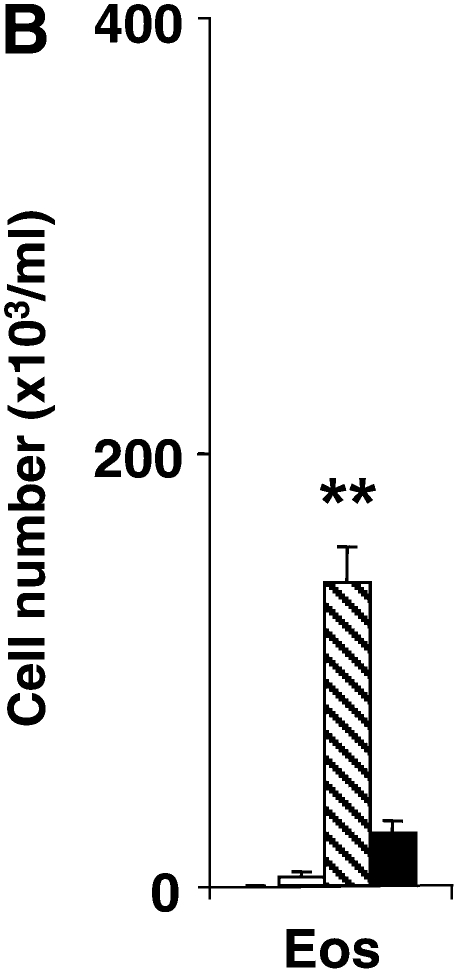
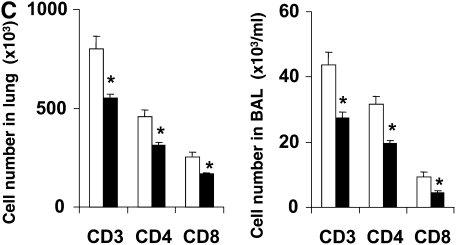
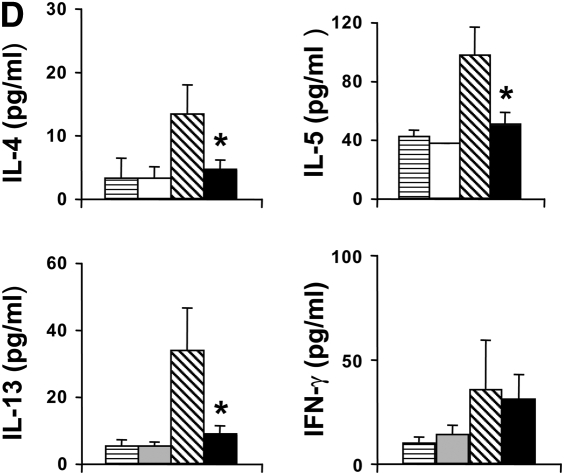
Development of reduced allergic airway responses in sensitized and challenged LTA4H−/− mice (ovalbumin [OVA]/OVA). (A) Lung resistance (RL) was determined in response to increasing concentrations of inhaled methacholine as described in Materials and Methods (n = 12 in each group). Open triangles, LTA4H+/+ PBS/OVA; open squares, LTA4H−/− PBS/OVA; solid triangles, LTA4H+/+ OVA/OVA; solid squares, LTA4H−/− OVA/OVA. (B) Eosinophil numbers in bronchoalveolar lavage (BAL) fluid. Horizontally striped bar, LTA4H+/+ PBS/OVA; open bar, LTA4H−/− PBS/OVA; hatched bar, LTA4H+/+ OVA/OVA; solid bar, LTA4H−/− OVA/OVA. (C) Numbers of CD3+, CD4+, and CD8+ T cells in the lung and BAL fluid after sensitization and challenge. Numbers of cells in the lung and BAL were determined as described in Materials and Methods. Open bars, LTA4H+/+ OVA/OVA; solid bars, LTA4H−/− OVA/OVA. (D) Cytokine levels in BAL fluid. IL-4, IL-5, IL-13, and IFN-γ levels in BAL fluid were measured in supernatants by enzyme-linked immunosorbent assay (ELISA). Horizontally striped bars, LTA4H+/+ PBS/OVA; open bars, LTA4H−/− PBS/OVA; hatched bars, LTA4H+/+ OVA/OVA; solid bars, LTA4H−/− OVA/OVA. (E) IL-4, IL-5, and IL-13 production in lung cells from LTA4H−/− mice after sensitization and challenge. Cytokine levels in culture supernatants of cultured lung mononuclear cells (MNC), in the presence or absence of OVA (100 μg/ml), were determined by ELISA. Open bars, LTA4H+/+ OVA/OVA; solid bars, LTA4H−/− OVA/OVA.*P < 0.05 and **P < 0.01 between LTA4H−/− mice (LTA4H−/− OVA/OVA) and LTA4H-sufficient mice (LTA4H+/+ OVA/OVA).
In parallel to the assessment of lung function, the inflammatory cell composition in the BAL fluid was examined (Figure 1B). After sensitization and allergen challenge, the proportion of eosinophils reached up to 60% of total BAL cells in LTA4H+/+ mice but was significantly lower (∼ 15% of total cells) in the LTA4H−/− mice (P < 0.01). There were no statistically significant differences between the two groups in the numbers of other inflammatory cells (macrophages, neutrophils) recovered in the BAL fluid.
To determine if the accumulation of T cells in the airways of sensitized and challenged mice was affected by a deficiency of LTB4, BAL cells and lung cells were isolated and numbers of CD3+, CD4+, and CD8+ T cells were determined. The numbers of CD3+, CD4+, and CD8+ T cells in the lungs of sensitized and challenged LTA4H−/− mice were significantly lower than in LTA4H+/+ mice (Figure 1C). The numbers of CD3+, CD4+, and CD8+ T cells in the BAL fluid of LTA4H−/− mice were also lower compared with the BAL of LTA4H+/+ mice (Figure 1C). These data suggested that accumulation of CD4+ and CD8+ T cells into the airways of sensitized mice after challenge was reduced in the absence of LTB4 production.
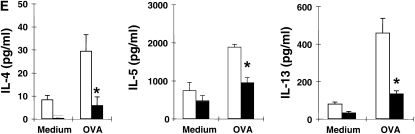
Concentrations of IL-4, IL-5, IL-13, and IFN-γ in the BAL fluid were determined by ELISA. OVA sensitization and challenge resulted in significant increases in IL-4, IL-5, and IL-13 levels in LTA4H+/+ mice (Figure 1D). However, after sensitization and challenge, the levels of IL-4, IL-5, and IL-13 were significantly lower in the BAL fluid recovered from LTA4H−/− mice as compared with LTA4H+/+ mice; IFN-γ levels were similar in both strains.
In Vitro Cytokine Production from Lung Cells Is Lower in LTA4H−/− Mice
To determine if the differences observed between the two strains of mice in their levels of Th2-type cytokines in BAL fluid were due to differences in antigen-specific T cell responsiveness, lung cells were isolated from sensitized and challenged mice, and were restimulated in culture with OVA. Levels of IL-4, IL-5, IL-13, and IFN-γ were measured in the culture supernatants 24 hours later. Consistent with the in vivo observations, after culture in vitro with OVA, LTA4H−/− lung cells secreted significantly lower amounts of IL-4, IL-5, and IL-13 than did the cells from LTA4H+/+ mice (Figure 1E). IFN-γ levels were similar (data not shown). These data suggested that not only accumulation of T cells but also activation of Th2-type cells in the airways were impaired in the absence of LTB4 production.
Development of Lung Allergic Responses after Passive Sensitization Is Dependent on LTB4 Production
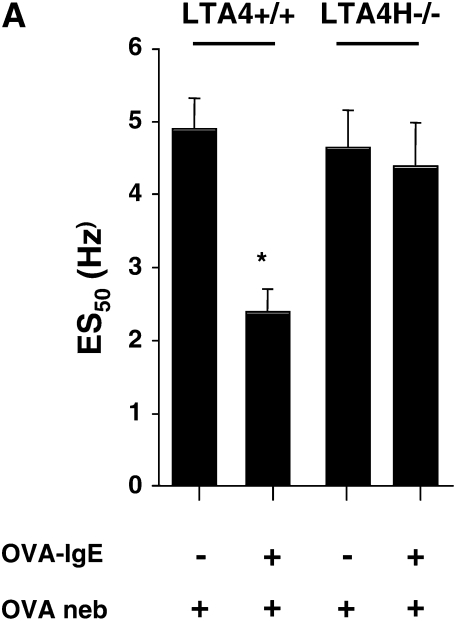
In contrast to systemic sensitization, passive sensitization with allergen-specific IgE followed by limited airway challenge is dependent on mast cells, IgE, and FcɛRI (21). LTA4H−/− and LTA4H+/+ mice were passively sensitized with OVA-specific IgE and then challenged with nebulized OVA on 2 consecutive days. Passively sensitized and challenged LTA4H+/+ mice showed an increased response to EFS (defined as a decrease in ES50) when compared with nonsensitized but allergen-challenged animals (Figure 2A), or sensitized but not challenged mice (data not shown). In contrast, LTA4H−/− mice passively sensitized with OVA-IgE and challenged with OVA demonstrated EFS responsiveness that was similar to that in nonsensitized control mice (Figure 2A). These data extend our previous findings (21) and identify the critical role for LTB4 in the development of altered airway reactivity in this passive sensitization and allergen challenge model.
Figure 2.
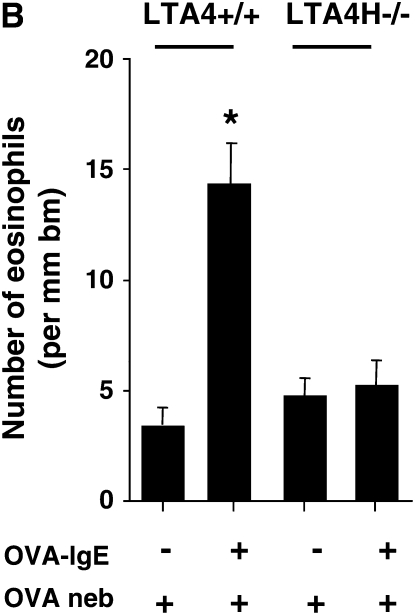
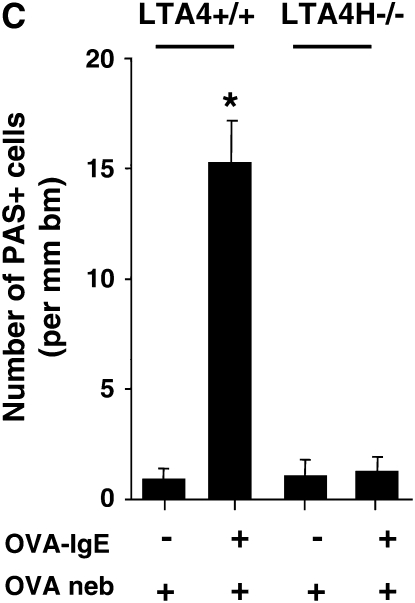
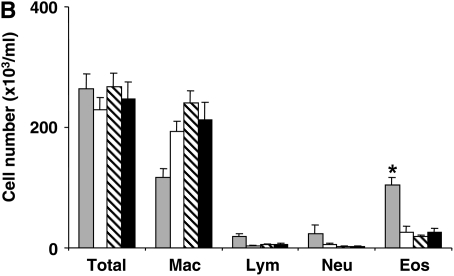
Airway responses in LTA4H−/− are reduced after passive sensitization and challenge. (A) Airway reactivity determined by electrical field stimulation of tracheal smooth muscle preparations. LTA4H−/− mice (n = 8 in each group) and similar numbers of the controls (LTA4H+/+) were passively sensitized with OVA-IgE, and exposed to nebulized OVA. Some mice received OVA challenge without passive sensitization. (B) Numbers of eosinophils in lung tissue. Numbers of eosinophils in the lung tissue were quantified in MBP-stained sections as described in Materials and Methods. (C) Numbers of PAS+ cells. Goblet cell numbers were quantified in PAS-stained sections as described in Materials and Methods. *P < 0.05 compared with other groups.
Following this protocol, few inflammatory cells are detectable in the BAL fluid (25). Nonetheless, eosinophils can be detected in lung tissue. Lung tissue inflammation and, in particular, eosinophil accumulation were assessed using immunohistochemistry with anti-MBP. After passive sensitization and challenge, LTA4H+/+ mice demonstrated an increase in numbers of peribronchial eosinophils compared with the nonsensitized but allergen-challenged control mice (Figure 2B). In OVA-challenged LTA4H−/− mice, no such increase in eosinophil tissue inflammation was observed (Figure 2B). Associated with increases in airway reactivity were increases in goblet cell metaplasia and mucus hyperproduction. To assess goblet cell metaplasia, tissue sections were stained with PAS and numbers of positive cells quantitated. After passive sensitization and airway challenge, LTA4H+/+ mice demonstrated increased numbers of PAS+ cells, whereas passively sensitized and challenged LTA4H−/− mice and the nonsensitized but allergen-challenged animals showed few PAS+ cells (Figure 2C).
Reconstitution of Airway Responsiveness in LTA4H−/− Mice by Mast Cells
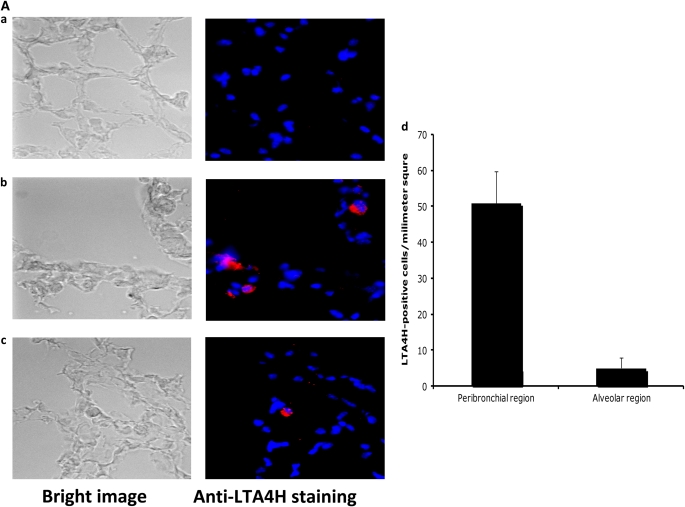
Eight weeks before passive sensitization, in vitro differentiated BMMC (1 × 107) were injected into LTA4H−/− recipients. These LTA4H−/− mice were then passively sensitized with OVA-IgE followed by OVA challenge. We first confirmed the transfer of BMMC by staining LTA4H-positive cells in the lungs of LTA4H−/− recipient mice. Staining intensity of LTA4H-positive BMMC generated from LTA4H+/+ mice was not homogeneous from cell to cell, and only a proportion of the BMMC from LTA4H+/+ mice, but none of BMMC from LTA4H−/− mice, were detected as staining red with the anti-LTA4H Ab (data not shown). Since not all of the LTA4H+/+ BMMC could be detected in this way with the anti-LTA4H Ab, it is difficult to discriminate between (negative, nonstaining) resident LTA4H−/− BMMC and transferred LTA4H+/+ BMMC in the LTA4H−/− recipient mice. However, many LTA4H-positive BMMC were detected in the peribronchial regions and some in the alveolar regions of LTA4H−/− recipient mice 8 weeks after transfer of LTA4H+/+ BMMC (Figure 3A).
Figure 3.
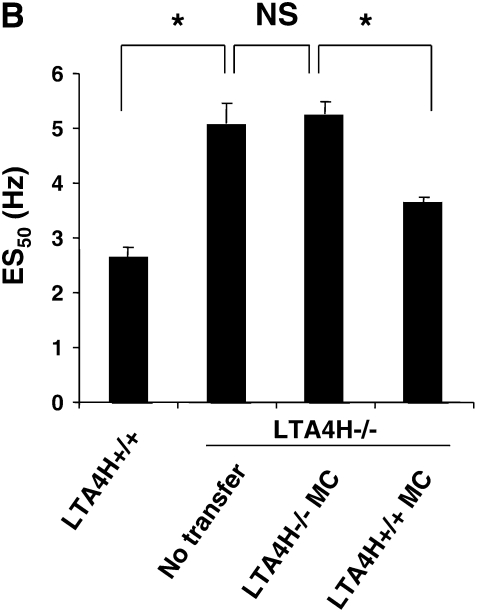
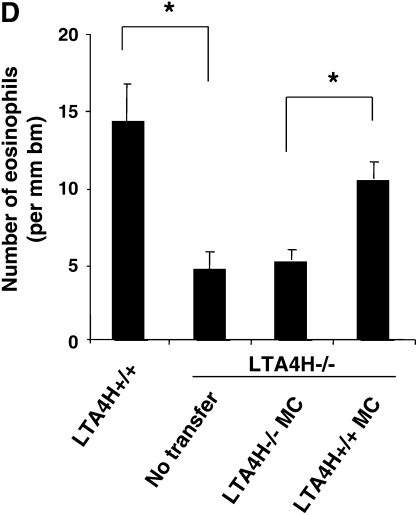
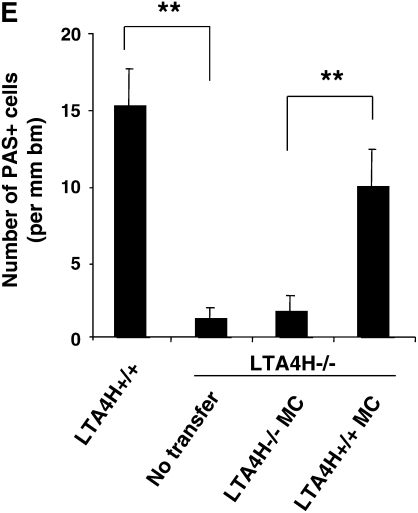
Reconstitution of LTA4H−/− mice with LTA4H+/+ mast cells after passive sensitization and challenge restores lung allergic responses. Bone marrow–derived mast cells (BMMC) were generated in culture and transferred into LTA4H−/− mice as described in Materials and Methods. Eight weeks later, LTA4H−/− mice received passive sensitization with OVA-IgE followed by OVA challenge. Three groups of LTA4H−/− mice were studied: no reconstitution (n = 8), reconstitution with BMMC from LTA4H−/− mice (LTA4H−/− MC, n = 8), and BMMC generated from LTA4H+/+ mice (LTA4H+/+ MC, n = 8). (A) Localization of LTA4H-positive BMMC in LTA4H−/− recipient mice. Anti-LTA4H staining (red for LTA4H, blue for nuclei) of lungs of naive LTA4H−/− mice (a), peribronchial region (b), and alveolar region (c) of lungs of LTA4H−/− recipients of LTA4H+/+ BMMC transfer. Bright field images (left panel) of anti-LTA4H Ab-stained sections were obtained at the same time. Original magnification: ×400. (d) LTA4H-positive BMMC numbers in peribronchial regions (within the limits of 0.1 mm in depth under subepithelial basement membrane) or alveolar regions were quantified as described in Materials and Methods. Data are expressed as the number of LTA4H-positive cells per millimeter square of peribronchial region or alveolar region.
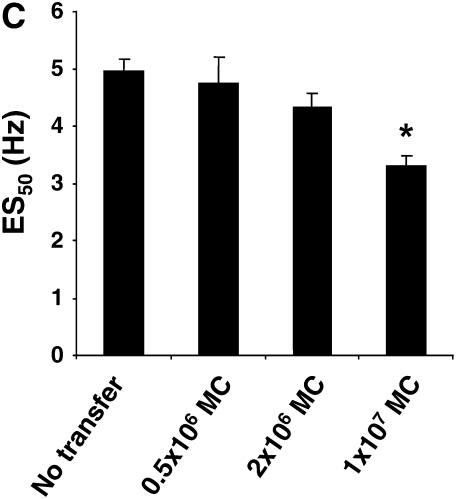
Figure 3B shows the results on airway reactivity after transfer of LTA4H+/+ mast cells. Transfer of LTA4H+/+ mast cells into the LTA4H−/− mice restored the development of increased airway reactivity, to levels that were comparable to those seen in passively sensitized and challenged LTA4H+/+ mice. In contrast, transfer of LTA4H−/− mast cells into LTA4H−/− recipients failed to restore airway reactivity. When fewer mast cells were transferred (2 × 106), the development of airway reactivity in LTA4H−/− recipients was enhanced to a lower degree, without full restoration of airway reactivity (Figure 3B).
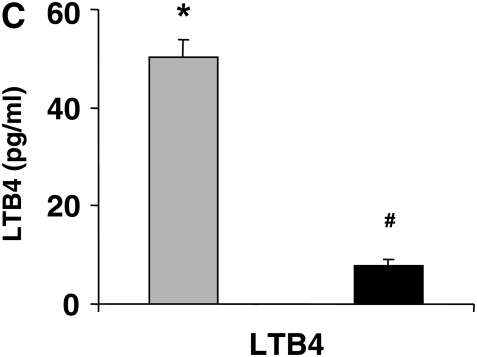
Figure 3C shows the numbers of eosinophils in the lung tissue of recipients of mast cells. The numbers of eosinophils in LTA4H−/− mice that received LTA4H+/+ mast cells were significantly higher than those in the mice that received LTA4H−/− mast cells. Transfer of LTA4H+/+ mast cells into the LTA4H−/− mice also restored the numbers of PAS+ cells in the lung tissue, to levels similar to those seen in passively sensitized and challenged LTA4H+/+ mice (Figure 3D).
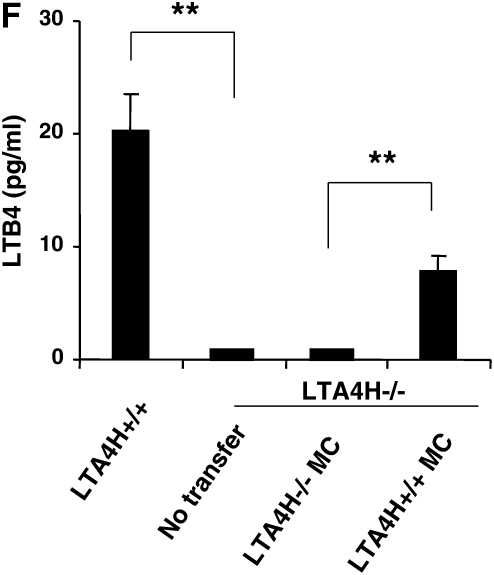
Analysis of LTB4 levels in the BAL fluid of LTA4H−/− recipient mice showed that after transfer of LTA4H+/+ mast cells, a significant increase in LTB4 levels in the BAL fluid could be detected compared with mice that received LTA4H−/− mast cells (Figure 3E).
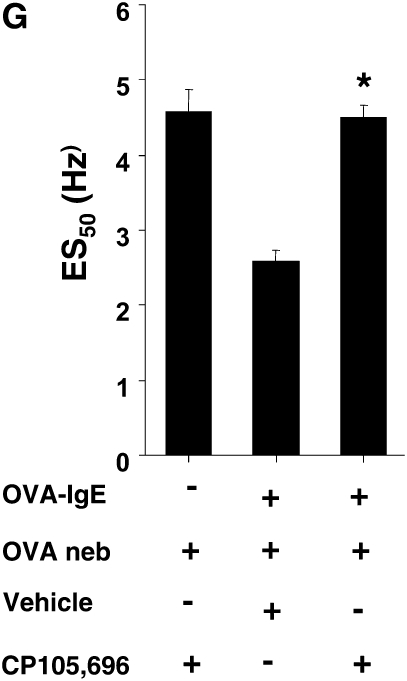
To determine whether the effect of LTA4H+/+ mast cell transfer into LTA4H−/− recipients was in fact dependent on the LTB4-BLT1 pathway, we treated passively sensitized LTA4H−/− recipients with an LTB4 receptor antagonist beginning 2 days and 1 day before each OVA challenge. Treatment with the LTB4 receptor antagonist completely abolished the effect of LTA4H+/+ mast cell transfer on the development of increased airway reactivity (Figure 3F).
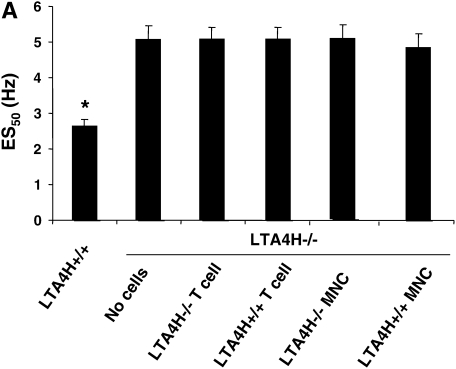
Transfer of LTA4H+/+ T Cells, MNC, or Alveolar Macrophages Fails to Restore Responses in Passively Sensitized LTA4H−/− Mice
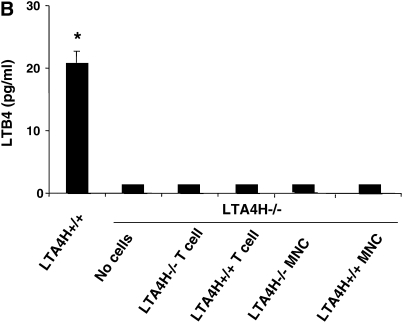
We next determined if we could bypass the need for mast cell–derived LTB4 by transferring allergen-primed T cells to the passively sensitized LTA4H−/− mice. Although T cells are not a source of LTB4, transfer of allergen-primed T cells can reconstitute lung allergic responses under certain conditions (26). We transferred LTA4H+/+ T cells into LTA4H−/− mice to determine if sensitized T cells could restore AHR, bypassing the need for endogenous (recipient) production of LTB4. Figure 4A illustrates the results of airway reactivity after LTA4H+/+ T cell transfer into LTA4H−/− mice; transfer of LTA4H+/+ T cells did not restore airway reactivity in recipient LTA4H−/− mice. A similar lack of effect was seen after transfer of LTA4H+/+ MNC into the passively sensitized LTA4H−/− mice before challenge (Figure 4A). After transfer of LTA4H+/+ T cells, LTB4 levels in the BAL fluid of LTA4H−/− recipient mice did not increase and were similar to the levels in recipients of LTA4H−/− T cells (Figure 4B). Transfer of LTA4H+/+ MNC similarly did not increase LTB4 levels in the BAL fluid of recipient LTA4H−/− mice (Figure 4B).
Figure 4.
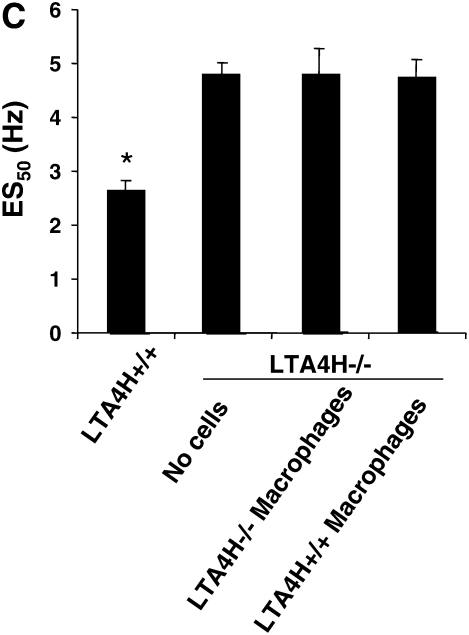
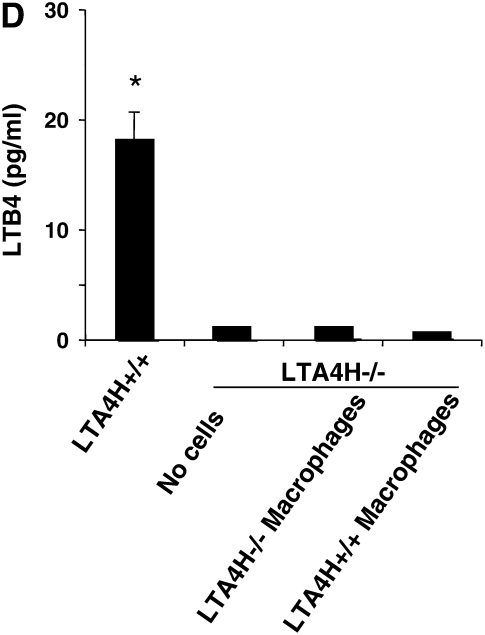
Failure to reconstitute LTA4H−/− mice with antigen-primed LTA4H+/+ T cells, MNCs, or alveolar macrophages after passive sensitization and challenge. (A) LTA4H−/− mice (recipient mice) received 1 × 107 LTA4H+/+ or LTA4H−/− T cells, or MNC cells intravenously, 2 hours before the first airway challenge with aerosolized OVA. Airway reactivity was assessed by electrical field stimulation of tracheal smooth muscle preparations as described in Materials and Methods. LTA4H+/+ and LTA4H−/− mice receiving no cells are also shown. (B) LTB4 levels in BAL fluid after transfer of T cells or MNC. (C) Passively sensitized LTA4H−/− mice (recipient mice) received 1 × 106 LTA4H+/+ or LTA4H−/− alveolar macrophages intratracheally 24 hours before the first airway challenge with aerosolized OVA as described in Materials and Methods. Airway reactivity was assessed by electrical field stimulation of tracheal smooth muscle preparations. (D) LTB4 levels in BAL fluid after transfer of macrophages. n = 8 in each group. *P < 0.05 comparing passively sensitized and challenged LTA4H+/+ mice to all other groups.
To determine if alveolar macrophages as a source of LTB4 were capable of restoring airway responses in the passively sensitized LTA4H−/−, we transferred LTA4H+/+ macrophages into LTA4H−/− mice. The alveolar macrophages were transferred intratracheally 24 hours before the first of two OVA challenges, and airway responsiveness was monitored 48 hours after the last OVA challenge. Transfer of LTA4H+/+ macrophages, did not reconstitute AHR in the LTA4H−/− mice (Figure 4C). Analysis of LTB4 levels in the BAL fluid of LTA4H−/− recipient mice showed that transfer of LTA4H+/+ macrophages did not increase LTB4 levels in the BAL fluid (Figure 4D).
Failure of LTA4H+/+ Cells to Restore Responsiveness in Sensitized and Challenged LTA4H−/− Recipients
In contrast to the passive sensitization, mast cell–dependent model, systemic sensitization (together with adjuvant) appears to bypass an essential role for mast cells but nonetheless remains dependent on LTB4-BLT1 interactions (17). As sensitized and challenged LTA4H−/− mice failed to develop the full array of lung allergic responses, we sought to determine if transfer of mast cells, T cells, MNC, or macrophages from LTA4H+/+ donors could restore responsiveness. Eight weeks after mast cell transfer, LTA4H−/− mice were actively sensitized with OVA (plus alum) on Days 1 and 14, then challenged with OVA from Days 26 to 28. On Day 30, airway responses were monitored. Figure 5 shows the results after LTA4H+/+ mast cell transfer. Transfer of the LTA4H+/+ mast cells into LTA4H−/− mice did not enhance AHR (Figure 5A) or affect the numbers of eosinophils in the BAL fluid after sensitization and challenge (Figure 5B). To determine if the failure of mast cells to reconstitute the responses was due to a failure of LTB4 release by the transferred cells, we quantitated LTB4 levels in the BAL fluid 48 hours after the last challenge in sensitized mice. As shown in Figure 5C, recipient LTA4H−/− mice showed a significant increase in LTB4 levels in the BAL fluid and were comparable to the levels achieved in the passively sensitized LTA4H−/− recipients of LTA4H mast cells (Figure 3D) that were fully reconstituted.
Figure 5.
Failure to reconstitute sensitized and challenged LTA4H−/− mice with LTA4H+/+ mast cells. BMMC were generated in culture and transferred into LTA4H−/− mice as described in Materials and Methods. Eight weeks later, LTA4H−/− mice were sensitized and challenged with OVA. Recipient mice were composed of two groups: in one group, mice received BMMC generated from LTA4H+/+ mice (LTA4H−/− recipients of LTA4H+/+ MC). In the other group, mice received BMMC from LTA4H−/− mice (LTA4H−/− recipients of LTA4H−/− MC). Sensitized and challenged LTA4H+/+ and LTA4H−/− mice, receiving no cells, are also shown. (A) Airway resistance to inhaled methacholine. Solid triangles, LTA4H+/+; solid squares, LTA4H−/−; open diamonds, LTA4−/− recipients of LTA4H−/− MC; solid diamonds, LTA4−/− recipients of LTA4H+/+ MC. (B) BAL eosinophil numbers. Shaded bars, LTA4H+/+; open bars, LTA4H−/−; hatched bars, LTA4−/− recipients of LTA4H−/− MC; solid bars, LTA4−/− recipients of LTA4H+/+ MC. (C) LTB4 levels in BAL fluid. Shaded bars, LTA4H+/+; open bars, LTA4H−/−; hatched bars, LTA4−/− recipients of LTA4H−/− MC; solid bars, LTA4−/− recipients of LTA4H+/+ MC.*P < 0.05 comparing sensitized and challenged LTA4H+/+ mice (LTA4H+/+) to all other groups, #P < 0.05 comparing LTA4H−/− recipients of LTA4H+/+ MC and LTA4H−/− recipients of LTA4H−/−MC.
Studies were also performed to determine whether transfer of T cells, MNC, or macrophages were capable of restoring responsiveness in the sensitized and challenged LTA4H−/− recipients. None of these cell transfers altered airway reactivity or BAL cell composition, and analysis of LTB4 levels and Th2 cytokine levels in the BAL fluid of LTA4H−/− recipient mice showed that transfer of LTA4H+/+ T cells, MNC, or macrophages did not increase LTB4 or Th2 cytokine levels in the BAL fluid (data not shown). Even transferring as many as 2 × 107 MNC up to 8 weeks before sensitization failed to restore any lung allergic responses (data not shown).
DISCUSSION
To investigate the mechanisms underlying development of allergen-induced airway responses, a number of animal models have been used. Many studies have used systemic sensitization to a soluble antigen such as OVA together with an adjuvant (alum) to induce a robust increase in AHR and eosinophilic airway inflammation. This protocol has been shown to serve as an IgE mast cell–independent model of allergen-induced airway responses, since mast cell– and B cell–deficient mice, which lack IgE production, have been shown to develop airway responses comparable to those of their normal littermates (34–36). To supplement this approach, we developed a passive sensitization model using OVA-specific IgE, and demonstrated that this model is IgE- and mast cell–dependent (5, 21). In both of these models, BLT1, the high-affinity receptor for LTB4, appears to play a pivotal role. Using BLT1-deficient mice or a specific BLT1 antagonist, we showed that BLT1 is essential to the development of lung allergic responses in the IgE mast cell–independent model (17) as well as in the IgE mast cell–dependent model of allergic airway responses (21). Given this apparent role of BLT1, in the present study we determined whether LTB4 is truly required for the development of allergen-induced airway responses. To this end, we investigated the capacity of LTA4H−/− mice, which completely lack LTB4 production but maintain production of other lipid mediators (22), to develop AHR in both models. Previous studies demonstrated that in the absence of 5-lipoxygenase (in knockout mice or drug-treated mice), lung allergic responses were diminished (37, 38). Our data suggest that LTB4 is essential to both the IgE mast cell–independent and –dependent models, further establishing the importance of the LTB4-BLT1 pathway in development of these lung allergic responses.
LTB4 is a potent chemoattractant produced by leukocytes including neutrophils, macrophages, and mast cells, and functions in the recruitment of leukocytes to sites of inflammation (39). In addition, LTB4 induces migration of human blood T cells and affects T cell proliferation (39). The ability of LTB4 to affect such a wide range of cell types indicates that LTB4 may function broadly in the regulation of innate and adaptive immune responses. LTB4 is essential in host defense against bacterial infection (40), and is linked to many inflammatory disorders, including arthritis (41–43), atherogenesis (44), and aortic aneurysm formation (45). In animal models and in patients with asthma, the levels of LTB4 have been reported to be elevated in the airways and to correlate with asthma severity (15, 16, 38, 46). However, the source of LTB4 evoking allergic airway inflammation has not been investigated directly. In the present study, we investigated which cell type produced LTB4 after different allergen exposures, and which was essential to development of allergic airway responses. These results showed that LTB4 release from mast cells was critical to the development of IgE-mediated, mast cell–dependent lung allergic responses but was insufficient in the mast cell–independent model.
Two populations of memory CD8+ T cells, termed central and effector memory CD8+ T cells, have been described (47–49). These two populations can be distinguished by their distinct localization to lymph nodes and peripheral sites of inflammation such as the lung and liver, respectively. Consistent with the well-described functions of adhesion molecules and chemokines in orchestrating the migration of immune cells, the homing of central memory CD8+ T cell to lymph nodes is attributed to their expression of CD62L and CCR7. The mechanism by which effector CD8+ T cells are recruited to peripheral tissues has also been studied, and BLT1 expression on effector memory CD8+ T cells has been shown to be crucial to the recruitment of these cells to inflamed tissues (13, 14). We previously demonstrated that the IgE mast cell–dependent passive sensitization model is also dependent on CD8+ T cells, and BLT1+/+ effector CD8 T cells in particular (21). Effector CD8+ T cells can secrete large amounts of IL-13 after stimulation, and accumulate in the airways after passive sensitization with OVA-specific IgE and OVA challenge. BLT1+/+ but not BLT1−/− effector CD8+ T cells are recruited to the airways and are capable of eliciting AHR, at least in part by secreting IL-13 (21). Effector CD8+ T cells, which express BLT1, showed chemotactic responses to LTB4 secreted from activated mast cells in vitro (50).
Mast cells bearing the high-affinity IgE receptor FcɛRI reside in peripheral tissues and are thus well-positioned to respond quickly to environmental stimuli. Activation of mast cells through FcɛRI stimulates rapid degranulation and production of lipid-derived mediators such as leukotrienes and prostaglandins (51). At later time points after activation, mast cells secrete a wide range of cytokines and chemokines that function in the recruitment and activation of effector cells, including eosinophils, neutrophils, and lymphocytes. Recently, it has been shown that LTB4 released from activated mature mast cells has an important autocrine role in regulating the release of mast cell progenitors from the bone marrow and/or their recruitment into tissues before maturation (52).
In the present study, we showed that in vivo LTB4 release from mast cells alone can induce allergen-induced airway responses after passive sensitization and challenge. Following a mast cell transfer protocol that has been effective in other models (30), we showed that the transfer of LTA4H+/+ mast cells could reconstitute the responses in passively sensitized, LTA4H−/− recipients. Transfer of LTA4H+/+ mast cells resulted in increased BAL LTB4 levels and restoration of AHR, lung eosinophilia, Th2 cytokine production, and goblet cell metaplasia. Tracking these transferred mast cell by LTA4H staining showed the accumulation of these cells throughout the lungs. To confirm that the transferred cells were indeed reconstituting the responses through LTB4–BLT1 interactions, administration of an effective BLT1 antagonist (30) completely prevented the reconstitution of the AHR response.
In this passive sensitization model, transfer of alveolar macrophages, primed spleen T cells, or spleen MNC were not capable of reconstituting these lung allergic responses. Most likely, these cells were either not a source of LTB4 (MNC) after adoptive transfer, or the macrophages, a potential source of LTB4, were not activated in an appropriate manner (i.e., there was no source of IgG), or were activated but did not accumulate at the appropriate site. The failure of primed T cells to reconstitute the responses in LTA4H−/− hosts provides further support for the need for LTB4 in the directed accumulation of critical T effector cells in the development of lung allergic responses (17, 18). Together, these data confirm the role of mast cells and their synthesis and release of LTB4 after activation through FcɛRI by allergen-specific IgE and allergen cross-linking, consequently triggering the accumulation of effector memory CD8+ T cells and mediating IgE–mast cell–LTB4–BLT1–CD8+ airway reactivity.
Surprisingly, transfer of LTA4H+/+ BMMC was not sufficient to restore AHR in the systemically sensitized and challenged LTA4H−/− mice, despite similar increases in LTB4 levels in the BAL fluid of recipient mice. Although this model of OVA-systemic sensitization together with alum followed by OVA challenge has been demonstrated to be mast cell– and IgE-independent (23, 33) but LTB4-BLT1–dependent (17), the LTB4 release from transferred mast cells appeared insufficient to induce AHR under this protocol. Perhaps not surprising given the importance of LTB4 in attracting effector T cells to the lung, LTA4H+/+ T cells or MNC transferred under these experimental conditions also did not restore airway responses in the LTA4H−/− mice in the absence of a source of LTB4. As many as 2 × 107 MNC transferred up to 8 weeks before sensitization failed to restore any of the responses. Although macrophages are believed to be a major source of LTB4, under the experimental conditions used, increases in BAL LTB4 levels were not detected and transfer of macrophages as performed here in different ways was not able to restore AHR. Studies are underway to determine other potential sources of LTB4 after systemic sensitization and challenge and why the macrophage transfers as performed here were insufficient. LTA4H has been shown to be expressed ubiquitously, not only in mast cells and other leukocytes, but also in bronchial epithelial cells in both humans and rodents (53, 54). Thus, production of LTB4 from several kinds of cells may orchestrate allergic inflammation after systemic sensitization and challenge.
In summary, we have identified a critical requirement for LTB4 synthesis and release in the development of allergen-induced AHR and airway inflammation under different experimental conditions, and have shown that LTB4 release from mast cells is sufficient to induce IgE mast cell–mediated airway reactivity. Manipulating LTB4 production in mast cells or other cell types will provide a novel approach for controlling IgE-mediated AHR and eosinophilic airway disease, with the potential to improve the treatment of asthma.
Acknowledgments
The authors thank L. N. Cunningham and D. Nabighian (National Jewish Health) for their assistance.
The project described was supported by NIH grants HL-36577 and HL-61005, and by EPA grant R825702. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH. N.M. was supported in part by a grant from the Ministry of Education, Science and Culture of Japan.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0095OC on November 21, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Busse WW, Lemanske RF Jr. Asthma. N Engl J Med 2001;344:350–362. [DOI] [PubMed] [Google Scholar]
- 2.De Sanctis GT, Itoh A, Green FH, Qin S, Kimura T, Grobholz JK, Martin TR, Maki T, Drazen JM. T-lymphocytes regulate genetically determined airway hyperresponsiveness in mice. Nat Med 1997;3:460–462. [DOI] [PubMed] [Google Scholar]
- 3.Oshiba A, Hamelmann E, Haczku A, Takeda K, Conrad DH, Kikutani H, Gelfand EW. Modulation of antigen-induced B and T cell responses by antigen-specific IgE antibodies. J Immunol 1997;159:4056–4063. [PubMed] [Google Scholar]
- 4.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 1987;237:1171–1176. [DOI] [PubMed] [Google Scholar]
- 5.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway: biochemistry and relation to pathobiology in human diseases. N Engl J Med 1990;323:645–655. [DOI] [PubMed] [Google Scholar]
- 6.Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 1980;286:264–265. [DOI] [PubMed] [Google Scholar]
- 7.Serhan CN, Prescott SM. The scent of a phagocyte: advances on leukotriene B4 receptors. J Exp Med 2000;192:F5–F8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang WW, Garcia-Zepeda EA, Sauty A, Oettgen HC, Rothenberg ME, Luster AD. Molecular and biological characterization of the murine leukotriene B4 receptor expressed on eosinophils. J Exp Med 1998;188:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tager AM, Luster AD. BLT1 and BLT2: The leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids 2003;69:123–134. [DOI] [PubMed] [Google Scholar]
- 10.Miyahara N, Miyahara S, Takeda K, Gelfand EW. Role of the LTB4/BLT1 pathway in allergen-induced airway hyperresponsiveness and inflammation. Allergol Int 2006;55:91–97. [DOI] [PubMed] [Google Scholar]
- 11.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 1997;387:620–624. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Yokomizo T, Izumi T, Shimizu T. Cell-specific transcriptional regulation of human leukotriene B4 receptor gene. J Exp Med 2000;192:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodarzi K, Goodarzi M, Tager AM, Luster AD, von Andrian UH. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol 2003;4:965–973. [DOI] [PubMed] [Google Scholar]
- 14.Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol 2003;4:982–990. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel SE, Trudeau JB, Kaminsky DA, Cohn J, Martin RJ, Westcott JY. Effect of 5-lipoxygenase inhibition on bronchoconstriction and airway inflammation in nocturnal asthma. Am J Respir Crit Care Med 1995;152:897–899. [DOI] [PubMed] [Google Scholar]
- 16.Csoma Z, Kharitonov SA, Balint B, Bush A, Wilson NM, Barnes PJ. Increased leukotrienes in exhaled breath condensate in childhood asthma. Am J Respir Crit Care Med 2002;166:1345–1349. [DOI] [PubMed] [Google Scholar]
- 17.Miyahara N, Takeda K, Miyahara S, Matsubara S, Koya T, Matsubara S, Joetham A, Krishnan E, Dakhama A, Haribabu B, et al. Requirement for the leukotriene B4 receptor-1 in allergen-induced airway hyperresponsiveness. Am J Respir Crit Care Med 2005;172:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyahara N, Takeda K, Miyahara S, Taube C, Joetham A, Koya T, Matsubara S, Dakhama A, Tager AM, Luster AD, et al. Leukotriene B4 receptor-1 is essential for allergen-mediated recruitment of CD8+ T cells and airway hyperresponsiveness. J Immunol 2005;174:4979–4984. [DOI] [PubMed] [Google Scholar]
- 19.Terawaki K, Yokomizo T, Nagase T, Toda A, Taniguchi M, Hashizume K, Yagi T, Shimizu T. Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J Immunol 2005;175:4217–4225. [DOI] [PubMed] [Google Scholar]
- 20.Medoff BD, Tager AM, Jackobek R, Means TK, Wang L, Luster AD. Antibody-antigen interaction in the airway drives early granulocyte recruitment through BLT1. Am J Physiol Lung Cell Mol Physiol 2006;290:L170–L178. [DOI] [PubMed] [Google Scholar]
- 21.Taube C, Miyahara N, Ott V, Swanson B, Takeda K, Loader L, Shultz LD, Tager AM, Luster AD, Dakhama A, et al. The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness. J Immunol 2006;176:3157–3164. [DOI] [PubMed] [Google Scholar]
- 22.Byrum RS, Goulet JL, Snouwaert JN, Griffiths RJ, Koller BH. Determination of the contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase- and leukotriene A4 hydrolase-deficient mice. J Immunol 1999;163:6810–6819. [PubMed] [Google Scholar]
- 23.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med 1997;186:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomkinson A, Cieslewicz G, Duez C, Larson KA, Lee JJ, Gelfand EW. Temporal association between airway hyperresponsiveness and airway eosinophilia in ovalbumin-sensitized mice. Am J Respir Crit Care Med 2001;163:721–730. [DOI] [PubMed] [Google Scholar]
- 25.Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, Gelfand EW. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice. J Clin Invest 1996;97:1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyahara N, Takeda K, Kodama T, Joetham A, Taube C, Park JW, Miyahara S, Balhorn A, Dakhama A, Gelfand EW. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol 2004;172:2549–2558. [DOI] [PubMed] [Google Scholar]
- 27.Larsen GL, Renz H, Loader JE, Bradley KL, Gelfand EW. Passive transfer of increased responsiveness with peribronchial lymph nodes. J Clin Invest 1992;89:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishizuka T, Chayama K, Takeda K, Hamelmann E, Terada N, Keller GM, Johnson GL, Gelfand EW. Mitogen-activated protein kinase activation through Fc epsilon receptor I and stem cell factor receptor is differentially regulated by phosphatidylinositol 3-kinase and calcineurin in mouse bone marrow-derived mast cells. J Immunol 1999;162:2087–2094. [PubMed] [Google Scholar]
- 29.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol 1988;18:97–104. [DOI] [PubMed] [Google Scholar]
- 30.Taube C, Wei X, Joetham A, Takeda K, Loader J, Miyahara N, Kodama T, Swasey CH, Shultz LD, Donaldson DD, et al. Mast cells, Fc epsilon RI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. J Immunol 2004;172:6398–6406. [DOI] [PubMed] [Google Scholar]
- 31.Koch K, Melvin LS Jr, Reiter LA, Biggers MS, Showell HJ, Griffiths RJ, Pettipher ER, Cheng JB, Milici AJ, Breslow R, et al. (+)-1-(3S,4R)-[3-(4-phenylbenzyl)-4-hydroxychroman-7-yl]cyclopentane carboxylic acid, a highly potent, selective leukotriene B4 antagonist with oral activity in the murine collagen-induced arthritis model. J Med Chem 1994;37:3197–3199. [DOI] [PubMed] [Google Scholar]
- 32.Showell HJ, Breslow R, Conklyn MJ, Hingorani GP, Koch K. Characterization of the pharmacological profile of the potent LTB4 antagonist CP-105,696 on murine LTB4 receptors in vitro. Br J Pharmacol 1996;117:1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamelmann E, Wahn U, Gelfand EW. Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness–a murine model. Allergy 1999;54:297–305. [DOI] [PubMed] [Google Scholar]
- 34.Korsgren M, Erjefält JS, Korsgren O, Sundler F, Persson CG. Allergic eosinophil-rich inflammation develops in lungs and airways of B cell-deficient mice. J Exp Med 1997;185:885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamelmann E, Takeda K, Schwarze J, Vella AT, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness requires interleukin-5 but not immunoglobulin E or B lymphocytes. Am J Respir Cell Mol Biol 1999;21:480–489. [DOI] [PubMed] [Google Scholar]
- 36.MacLean JA, Sauty A, Luster AD, Drazen JM, De Sanctis GT. Antigen-induced airway hyperresponsiveness, pulmonary eosinophilia, and chemokine expression in B cell-deficient mice. Am J Respir Cell Mol Biol 1999;20:379–387. [DOI] [PubMed] [Google Scholar]
- 37.Irvin CG, Tu YP, Sheller JR, Funk CD. 5-Lipoxygenase products are necessary for ovalbumin-induced airway responsiveness in mice. Am J Physiol 1997;272:L1053. [DOI] [PubMed] [Google Scholar]
- 38.Henderson WR Jr, Lewis DB, Albert RK, Zhang Y, Lamm WJ, Chiang GK, Jones F, Eriksen P, Tien YT, Jonas M, et al. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J Exp Med 1996;184:1483–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokomizo T, Izumi T, Shimizu T. Leukotriene B4: metabolism and signal transduction. Arch Biochem Biophys 2001;385:231–241. [DOI] [PubMed] [Google Scholar]
- 40.Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol 1996;157:5221–5224. [PubMed] [Google Scholar]
- 41.Griffiths RJ, Smith MA, Roach ML, Stock JL, Stam EJ, Milici AJ, Scampoli DN, Eskra JD, Byrum RS, Koller BH, et al. Collagen-induced arthritis is reduced in 5-lipoxygenase-activating protein-deficient mice. J Exp Med 1997;185:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med 2006;203:829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao WH, Del Prete A, Bock CB, Haribabu B. Targeted disruption of leukotriene B4 receptors BLT1 and BLT2: a critical role for BLT1 in collagen-induced arthritis in mice. J Immunol 2006;176:6254–6261. [DOI] [PubMed] [Google Scholar]
- 44.Heller EA, Liu E, Tager AM, Sinha S, Roberts JD, Koehn SL, Libby P, Aikawa ER, Chen JQ, Huang P, et al. Inhibition of atherogenesis in BLT1-deficient mice reveals a role for LTB4 and BLT1 in smooth muscle cell recruitment. Circulation 2005;112:578–586. [DOI] [PubMed] [Google Scholar]
- 45.Ahluwalia N, Lin AY, Tager AM, Pruitt IE, Anderson TJ, Kristo F, Shen D, Cruz AR, Aikawa M, Luster AD, et al. Inhibited aortic aneurysm formation in BLT1-deficient mice. J Immunol 2007;179:691–697. [DOI] [PubMed] [Google Scholar]
- 46.Turner CR, Breslow R, Conklyn MJ, Andresen CJ, Patterson DK, Lopez-Anaya A, Owens B, Lee P, Watson JW, Showell HJ. In vitro and in vivo effects of leukotriene B4 antagonism in a primate model of asthma. J Clin Invest 1996;97:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–712. [DOI] [PubMed] [Google Scholar]
- 48.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 2001;291:2413–2417. [DOI] [PubMed] [Google Scholar]
- 49.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8+ T cells. J Exp Med 2001;194:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ott VL, Cambier JC, Kappler J, Marrack P, Swanson BJ. Mast cell-dependent migration of effector CD8+ T cells through production of leukotriene B4. Nat Immunol 2003;4:974–981. [DOI] [PubMed] [Google Scholar]
- 51.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 1997;77:1033–1079. [DOI] [PubMed] [Google Scholar]
- 52.Weller CL, Collington SJ, Brown JK, Miller HR, Al-Kashi A, Clark P, Jose PJ, Hartnell A, Williams TJ. Leukotriene B4, an activation product of mast cells, is a chemoattractant for their progenitors. J Exp Med 2005;201:1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minami M, Mutoh H, Ohishi N, Honda Z, Bito H, Shimizu T. Amino-acid sequence and tissue distribution of guinea-pig leukotriene A4 hydrolase. Gene 1995;161:249–251. [DOI] [PubMed] [Google Scholar]
- 54.Qiu H, Gabrielsen A, Agardh HE, Wan M, Wetterholm A, Wong CH, Hedin U, Swedenborg J, Hansson GK, Samuelsson B, et al. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc Natl Acad Sci USA 2006;103:8161–8166. [DOI] [PMC free article] [PubMed] [Google Scholar]