Abstract
Bacterial pneumonia remains a serious disease. Pattern recognition receptors play an integral role in neutrophil accumulation during pneumonia. Although myeloid differentiation protein (MD)-2 has been recognized as a key molecule for LPS signaling, the role of MD-2 in neutrophil accumulation in the lung during bacterial infection has not been explored. Here, we investigate the role of MD-2 in Escherichia coli LPS–induced lung inflammation and E. coli–induced pneumonia. LPS-induced CD14-independent neutrophil accumulation was abolished in CD14/MD-2−/− mice. MD-2−/− mice challenged with LPS displayed attenuated neutrophil influx, NF-κB activation, cytokine/chemokine expression, and lung histopathology. MD-2−/− mice transplanted with MD-2+/+ bone marrow demonstrated decreased neutrophil influx and cytokine/chemokine expression in the lungs when challenged by LPS. MD-2−/− mice infected with E. coli demonstrated reduced neutrophil influx and cytokine/chemokine expression in the lungs, whereas heat-killed E. coli did not induce either neutrophil accumulation or cytokine/chemokine expression in MD-2−/− mice infected with E. coli. Furthermore, MD-2−/− mice displayed increased bacterial burden in the lungs and enhanced bacterial dissemination. Toll-like receptor (TLR)-5−/− mice infected with E. coli exhibited attenuated neutrophil accumulation, whereas MD-2/TLR5−/− mice inoculated with E. coli showed further attenuated neutrophil influx and impaired bacterial clearance. Taken together, these new findings demonstrate: (1) the important role of MD-2 in the CD14-independent LPS-mediated cascade of neutrophil influx; (2) the relative importance of bone marrow– and non–bone marrow cell–derived MD-2 in LPS-induced inflammation; and (3) the essential role of MD-2–dependent and MD-2–independent (TLR5) signaling in E. coli–induced neutrophil accumulation and pulmonary host defense.
Keywords: neutrophil, host defense, mouse model
CLINICAL RELEVANCE
This study will improve our understanding of the pathogenesis of bacterial pneumonia and help design improved treatment strategies.
Pneumonia caused by gram-negative pathogens is associated with a high mortality rate (1–3), and is manifested by microvascular leakage, up-regulation of proinflammatory cytokines/chemokines, enhanced up-regulation of cell surface adhesion molecules, and extensive migration of neutrophils into the alveolar spaces, with consequent damage of lung parenchyma (4–7). Neutrophil recruitment is a pathological hallmark of bacterial pulmonary diseases (8). Although neutrophil migration to the lung is a critical event in pulmonary defense (8), excessive neutrophil influx may lead to diffuse parenchymal damage of the lung. However, a better understanding of the interaction between bacteria and lung cells is a prerequisite for the design of therapeutic strategies to attenuate excessive neutrophil recruitment to the lung.
Cell surface pattern recognition receptors, such as Toll-like receptors (TLRs), expressed in bone marrow and non–bone marrow (resident) cells, play a vital role in host defense in the lung via recognition of pathogen-associated molecular patterns (9, 10). TLR2, TLR4, and TLR5 are known to recognize bacterial products, such as peptidoglycan, LPS, and flagellin (9). LPS recognition is mediated by a complex of proteins, including TLR4, LPS-binding protein (LBP), myeloid differentiation protein (MD)-2, and CD14 (11–14). Because CD14, LBP, and MD-2 have no intrinsic signaling capabilities (11–14), TLR4 is required to induce downstream signaling cascades (14). MD-2 is a 25-kD glycoprotein that binds to the extracellular domain of TLR4 and is therefore tethered on TLR4-expressing cells (15). Soluble and membrane-bound forms of MD-2 have been reported (15). It has been demonstrated that TLR4 does not induce a complete response in the absence of MD-2 (15). The pathways underlying TLR4-induced signaling are identical to that of IL-1 receptor activation (16). The binding of LPS to TLR4 induces both MyD88-dependent and -independent signaling cascades, resulting in, respectively, expression of cytokine/chemokine genes and expression of IFN-inducible genes and, ultimately, modulating the innate immune response in the lung (17–22).
The roles of TLR4, LBP, and CD14 in lung inflammation elicited by bacteria and/or their products have been under intense investigation (23–25), although the role of MD-2 has not been examined in detail. More importantly, the role of MD-2 in antibacterial defense in the lungs remains to be elucidated. Furthermore, the role of MD-2–independent cascades in response to bacterial pathogens has not been delineated. In this investigation, we sought to determine the contribution that MD-2 makes to the pulmonary immune response and host defense against a gram-negative pathogen, Escherichia coli, using MD-2 gene–disrupted mice in which both soluble and membrane-bound forms of MD-2 are deficient. We also used the E. coli virulence factor, LPS, to demonstrate the role of MD-2 in CD-14–dependent and/or –independent cascades of LPS-induced lung inflammation. Finally, we also studied the MD-2–independent cascades in lung host response against this gram-negative pathogen.
MATERIALS AND METHODS
Experimental Animals
CD14 and MD-2 gene–deficient mice were backcrossed eight times with C57Bl/6 before use (25, 26), and, therefore, C57Bl/6 mice were used as littermate control animals. TLR5 (27) and MD-2/TLR5 gene–deficient mice on a C57Bl/6 background were used. Pathogen-free 8- to 10-week-old female gene-deficient and control (C57Bl/6) mice, ranging from 21 to 25 g in weight, were used in all experiments. Animals were kept under specific pathogen-free conditions and maintained on a 12-hour:12-hour light:dark cycle with ad libitum food and water. All experiments were performed in accordance with protocols approved by the National Jewish Center and Louisiana State University.
Materials
Ultrapure E. coli LPS (O111:B4) purchased from Sigma-Aldrich (St. Louis, MO) was dissolved in pyrogen-free isotonic saline to yield a concentration of 300 μg/ml (low dose) or 3,000 μg/ml (high dose). Flagellin from Salmonella typhimurium was purchased from InvivoGen. The manufacturer reported that this purified flagellin activates TLR5, but not TLR4 or TLR2. LPS-free glass- and plasticware were used in experiments.
LPS or Flagellin Challenge
Mice were exposed to 300 μg/ml or 3,000 μg/ml of LPS by aerosolization using a bang nebulizer (CH Technologies Inc., Westwood, NJ) for 20 minutes in a plexiglass chamber, as described previously (25, 28–32). Control animals were treated similarly with nebulized vehicle control (isotonic saline). At 2, 8, and 24 hours after inhalation, the whole lungs were harvested, because LPS-induced inflammatory parameters in the airspace and lung parenchyma were remarkable at these time points, as described in our previous studies (25, 28–32).
In another set of experiments, 1 μg of LPS-free recombinant flagellin from S. typhimurium was used in mice.
Bone Marrow Transplantation
Donor and recipient mice (6 wk old) were used to generate chimeras, as described previously (33). Recipient mice were γ irradiated from a cesium source in two 525-rad doses 3 hours apart. Bone marrow cells (8 × 106/mouse) were injected into the tail vein of the irradiated recipients. Transplanted mice were maintained on 0.2% neomycin sulfate for the first 2 weeks. The reconstituted mice were used 2 months after the transplantation. In parallel experiments, we used green fluorescent protein–expressing donor cells. Sample blood was collected from these recipients between 6 and 8 weeks after transplantation, and hematological parameters (red and white blood cell and differential counts) were assessed. We found that greater than 90% of blood leukocytes were derived from donor mice at the time the mice were used for experiments (8 wk after transplantation; data not shown). Irradiated mice that were not transplanted with donor cells died between Days 20 and 22 after transplantation (data not shown).
Isolation of Primary Murine Cells
Isolation of alveolar epithelial type II (AEII) cells (34), bone marrow–derived neutrophils (30), and alveolar macrophages was performed as previously described (30). (1) A total of 2.5 × 106 AEII cells were cultured on a collagen/matrigel system. The apical and basolateral surfaces were stimulated with 30 ng/ml LPS for 18 hours at 37°C. (2) A total of 5 × 106 mature neutrophils were stimulated with 30 ng of LPS and incubated for 4 hours at 37°C. (3) Alveolar macrophages (AMs) were grown at a density of 2 × 106 cells/ml/well on a six-well plate for 3 days using RPMI 1,640 media containing 10% FBS and were stimulated with 30 ng of LPS for 18 hours at 37°C.
At the end of incubation, the cells were centrifuged, and supernatant was collected for CXCL5 protein determination by ELISA.
Induction of Pneumonia
To determine the role of MD-2 and TLR5 in pulmonary inflammation and antibacterial defense against a gram-negative bacterium, we used viable E. coli (American Type Culture Collection 25,922) (28, 32) and heat-killed E. coli (boiled for 30 min). A frozen 1 ml (107 CFU/ml) aliquot of the bacterium was grown for 12 hours at 37°C in 50 ml tryptic Soy broth (Becton Dickinson, Sparks, MD) on a shaker at 200 rpm, pelleted by centrifugation at 1,200 × g for 2 minutes, and washed twice in sterile isotonic saline. The bacteria were then resuspended in sterile saline at a concentration of 106 CFU/50 μl/mouse. The MD-2−/−, TLR5−/−, MD-2/TLR5−/−, and their littermate control mice (C57Bl/6) were anesthetized with avertin (250 mg/kg), and a midventral incision was made. After isolation of surrounding muscles, the trachea was exposed and each mouse was inoculated with 106 CFU of E. coli suspension in 50 μl saline (pH 7.4). A 50-μl aliquot of serially diluted suspension of initial inoculum was plated onto a tryptic soy agar (TSA) plate and a MacConkey plate to confirm the inoculum.
Bronchoalveolar Lavage Fluid Collection
At the designated time points after E. coli infection or LPS challenge, bronchoalveolar lavage fluid (BALF) was collected by canulating the trachea after animals were killed with 100 mg/kg pentobarbital, as described in our earlier reports (25, 28–32). A total of 3.0 ml BALF was obtained from each mouse, and 0.5 ml of BALF was centrifuged and placed on glass cytospin slides, which were then stained by Diff-Quick reagents (Fisher, Chicago, IL) to enumerate leukocyte subtypes based on their cellular and nuclear morphology. Undiluted BALF (2 ml) was centrifuged, passed via a 0.22-μm filter, and used immediately or kept at −20°C for the determination of cytokine/chemokine protein concentrations.
Cytokine/Chemokine Determination
Cytokine/chemokine protein concentrations in BALF were measured using a specific sandwich ELISA, as reported in our previous publications (25, 28–32). The minimum cytokine/chemokine detection limit is 2 pg/ml of protein (25, 28–32).
Lung Harvesting
At the indicated time points, the whole (nonlavaged) lungs were harvested from mice and immediately snap frozen, followed by storage at −70°C. The lungs were processed for the determination of NF-κB translocation and myeloperoxidase (MPO) activity. In another set of experiments, the entire (unlavaged) lungs were harvested and sectioned for histology.
NF-κB Activation
An ELISA-based NF-κB assay (Active Motif, Carlsbad, CA) was used to detect the translocation of the p65 subunit of NF-κB into the nucleus of lung cells, as described in our earlier reports (25, 28) and according to the manufacturer's recommendations. Briefly, unlavaged lungs were weighed and diced. They were then homogenized in lysis buffer containing 10 mM DTT and a cocktail of protease inhibitors. Solubilized proteins were then separated from cell debris by centrifugation (10 min at 10,000 × g at 4°C). Then, nuclear and cytosolic fraction was separated by centrifugation. The concentration of protein in the nuclear fraction was measured by Bradford assay. A total of 20 μg of nuclear extract was added to the NF-κB–specific oligonucleotide–coated 96-well plate and incubated for 1 hour at room temperature. After three washings, a primary antibody specific for p65 was added, followed by incubation for 1 hour at room temperature. After washing three times to remove excess primary antibody, an anti–horseradish peroxidase conjugate was added to the plate, and the color development was measured at an optical density of 450 nm in triplicate, according to the manufacturer's recommendations. According to the manufacturer, the oligonucleotides are highly specific for NF-κB and, therefore, do not display cross-reactivity with other transcription factors.
MPO Determination
Lung MPO activity was measured as described in our previous reports (25, 28). Harvested whole lungs were weighed, kept frozen at −70°C, and then homogenized for 30 seconds. The resulting homogenates were centrifuged, and the pellet was resuspended in 50 mM potassium phosphate buffer at pH 6.0 (supplemented with 0.5% hexadecyltrimethylammonium bromide). Lung homogenates were then incubated at 60°C for 2 hours and assayed for MPO activity in a hydrogen peroxide/O-dianisidine buffer at 460 nm at 0 and 90 seconds. The MPO activity in the lungs was calculated between these time points. Lungs were used for MPO activity within 2 weeks of collection.
Actin Cytoskeleton Assembly Determination
To determine whether neutrophils from MD-2−/− mice display equal or defective actin assembly relative to MD-2+/+ mice, we measured actin polymerization in response to keratinocyte-derived chemokine (KC), macrophage inflammatory protein (MIP)-2, TNF-α, or LPS. Bone marrow–derived polymorphonuclear cells were isolated using a Ficoll gradient, as described in our previous reports (29, 32). Isolated polymorphonuclear cells (106/ml) were assayed for actin polymerization in response to E. coli LPS (1 μg/ml; 1 h), TNF-α (1 μg/ml, 1 h), KC (5 ng/ml; 15 min), or MIP-2 (5 ng/ml; 15 min). After the incubation at 37°C, cells were labeled with nitrobenzoxadiazole-phallacidin, and actin assembly was assessed by flow cytometry, as previously described in our studies (29, 32). The data were normalized to unstimulated controls, giving a relative fluorescence index of 1.
Histopathological Analysis
At 24 hours after LPS or bacterial challenge, lungs were inflated via the trachea with Streck tissue fixative (Streck Laboratories, Omaha, NE), as previously described (25, 28). For paraffin sections, the lungs were fixed with Streck tissue fixative overnight at room temperature. The lungs were then embedded in paraffin, and 5-μm serial sections were made. These sections were mounted on super frost Matsunami adhesive silane–coated slides and stained with hematoxylin and eosin. Nonquantitative histology of lung tissue was performed by a pathologist who was blinded to treatment groups.
Bacterial Enumeration
Mice were killed at 6 and 24 hours after E. coli infection and their whole lungs were excised and homogenized in 10 ml of sterile saline, as described previously (28, 32). Several 2- and 10-fold dilutions of the homogenates were plated on TSA and MacConkey plates, and colonies were counted after 12- to 18-hour incubation at 37°C. Spleens from the same animals were excised and homogenized in 1 ml of sterile saline, as reported previously (28, 32). Several 2- and 10-fold dilutions of the homogenates were plated on TSA and MacConkey plates, and colonies were enumerated at 18 hours after incubation.
Survival Studies
MD-2−/−, TLR5−/−, MD-2/TLR5−/−, and C57Bl/6 (control) mice were inoculated intratracheally with E. coli (108 CFU in 50 μl 0.9% saline/mouse), and their survival was monitored as described previously (28).
Statistical Analysis
Results are expressed as mean values (±SEM). To assess the significance of the difference between groups, a Student's t test was used for data between two groups and one-way ANOVA was used for comparisons of data involving more than two groups. Survival data were compared by Wilcoxon rank sign test. All calculations were determined using Kaleidagraph version 6.0 (Synergy Software, Reading, PA). Differences were considered statistically significant at a P value less than 0.05.
RESULTS
Effect of MD-2 on the Lung Inflammatory Response to E. coli LPS
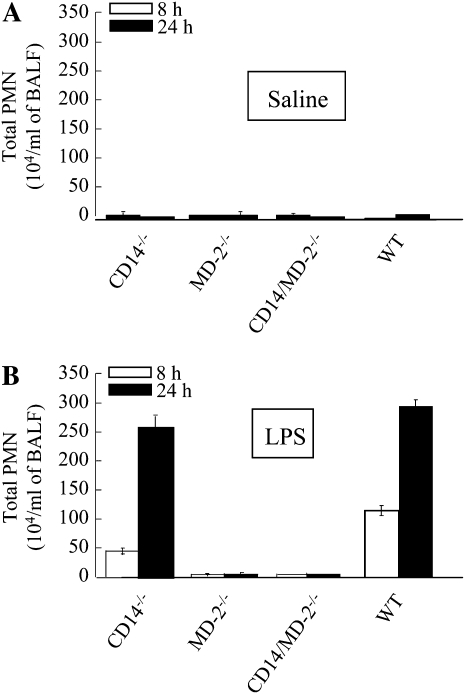
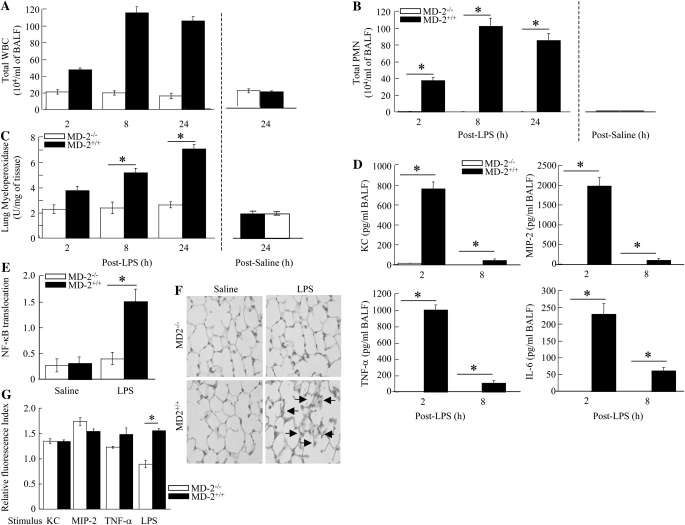
Our laboratory has recently obtained evidence of CD14-dependent (at low LPS dose [300 μg/ml]) and -independent (at high LPS dose [3,000 μg/ml]) cascades in LPS-induced lung inflammation (25). We also found that LPS-induced lung inflammation at both doses is entirely TLR4 dependent (25). However, it is not known whether these CD14 cascades are MD-2 dependent or independent. To determine the role of MD-2 in CD14 cascades, we investigated lung inflammation in response to LPS. Although significant neutrophil accumulation was detected in the lungs of CD14−/− mice in response to high-dose LPS (3,000 μg/ml), no neutrophil migration was observed in the lungs of MD-2−/− or CD14/MD-2−/− mice (Figure 1). These observations suggest that MD-2 is downstream of CD14, and is absolutely required for both CD14-dependent and -independent neutrophil recruitment. We further investigated the role of MD-2 in neutrophil influx, cytokine/chemokine expression, NF-κB activation, and lung histopathology after 300 μg/ml (low-dose) LPS exposure. No neutrophil accumulation was induced in the lungs of CD14−/− (25) or MD2−/− mice after challenge with low-dose LPS (Figures 2A–2C). We also found that there was decreased cytokine/chemokine expression (Figure 2D), NF-κB translocation (Figure 2E), and lung histological changes (Figure 2F) in MD2−/− mice after LPS challenge. These results suggest that MD-2 is a critical mediator of LPS-induced lung inflammation.
Figure 1.
Neutrophil accumulation in the lung after saline or LPS exposure. Animals were treated with aerosolized saline (control) (A) or 3,000 μg of LPS (B) for 20 minutes, and bronchoalveolar lavage fluid (BALF) was collected at 8 or 24 hours after challenge. BALF neutrophil counts were determined. A total of three to five mice were used in each group at each time point. Data are presented as mean (±SEM).
Figure 2.
Innate immune responses in the lung after LPS challenge. Animals were treated with aerosolized LPS (300 μg) or saline (control) for 20 minutes, and BALF and lungs were collected at various time points after LPS challenge. BAL total white blood cell (WBC) (A) and neutrophil (B) counts, myeloperoxidase activity in the lung homogenates (C), BALF cytokine/chemokine levels (D), lung NF-κB activation at 2 hours (E), lung histology at 24 hours (arrows indicate infiltrating inflammatory cells and edema; original magnification: ×400) (F), and actin filament assembly in bone marrow–derived neutrophils (G) were determined in MD-2−/− and MD-2+/+ mice. A total of five to seven animals were examined in each group at each time point. *Significant differences between MD-2−/− and MD-2+/+ mice (P < 0.05). Histopathology shown is representative of three lungs in each group. Data are presented as mean (±SEM).
Neutrophil trafficking to the airspaces from the circulation is also dependent on cytokine/chemokine-induced assembly of the actin cytoskeleton in neutrophils (35–38). To determine whether the absence of neutrophil accumulation in the lungs of MD-2−/− mice is because of improper neutrophil cytoskeleton remodeling in response to proinflammatory mediators, such as KC, MIP-2, and TNF-α, we assessed actin assembly in isolated neutrophils from MD-2−/− and MD-2+/+ mice in response to these mediators. We found that KC, MIP-2, and TNF-α cause similar degrees of actin polymerization in neutrophils obtained from both MD2−/− and MD-2+/+ mice (Figure 2G). These findings suggest that impaired actin assembly is not a mechanism for reduced neutrophil recruitment into the lungs after LPS challenge.
Role of Bone Marrow– and Non–Bone Marrow Cell–Derived MD-2 in LPS-Induced Lung Inflammation
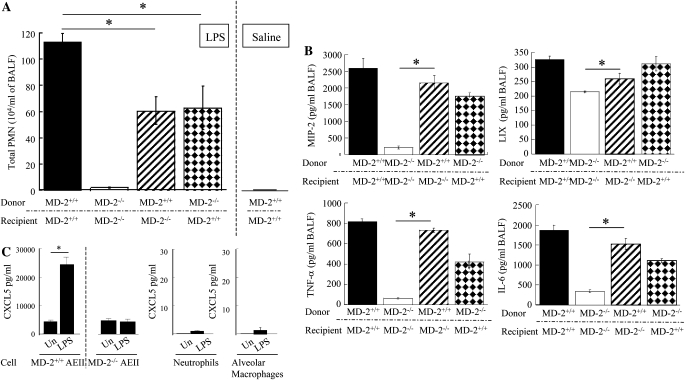
We next investigated the role of bone marrow cell–associated/derived MD-2 versus non–bone marrow (resident) cell–associated MD-2 in LPS-mediated lung inflammation using bone marrow chimeras. Neutrophil influx in response to LPS challenge was attenuated in irradiated MD-2+/+ mice reconstituted with MD-2−/− bone marrow (wild type [WT]→knockout [KO]; Figure 3A). LPS-induced neutrophil influx was also reduced in irradiated MD-2−/− mice reconstituted with MD-2+/+ bone marrow (KO→WT; Figure 3A). As anticipated, no neutrophil accumulation was observed in MD-2+/+ mice after saline challenge (Figure 3A). In addition, MIP-2, TNF-α, and IL-6, but not CXCL5/LPS-induced CXC chemokine (LIX), expression was enhanced in MD-2−/− mice transplanted with MD-2+/+ bone marrow as compared with MD−/− mice (Figure 3B). In fact, our results, using isolated cells, demonstrate that resident murine AEII cells, but not bone marrow–derived cells (macrophages and neutrophils), generate CXCL5 in response to LPS stimulation in an MD-2–dependant manner (Figure 3C). Thus, LPS-induced neutrophil influx in the lungs is dependent on MD-2 in both bone marrow and non–bone marrow cells, and MIP-2, TNF-α, and IL-6 originate from bone marrow cells, whereas CXCL5 originates from resident cells.
Figure 3.
Role of MD-2 originating from bone marrow and non–bone marrow cells in LPS-induced inflammation (A and B). Bone marrow chimeras were prepared by lethal irradiation of MD-2−/− and MD-2+/+ mice and reconstituted with bone marrow cells via tail vein injection. BALF neutrophil counts (A) and cytokine/chemokine expression (B) in BALF were obtained at 8 h after LPS (300 μg aerosolized for 20 min) challenge.*Significant differences between groups (P < 0.05); a total of four to seven animals were used. (C) LIX expression by alveolar epithelial type II (AEII) cells after LPS exposure. Murine primary AEII cells were stimulated with LPS (30 ng/ml) for 18 hours at 37°C, and the protein levels were measured. These data are an average (±SEM) of four or five wells from two animals.
Effect of MD-2 on Neutrophil Trafficking in the Lung in E. coli Pneumonia
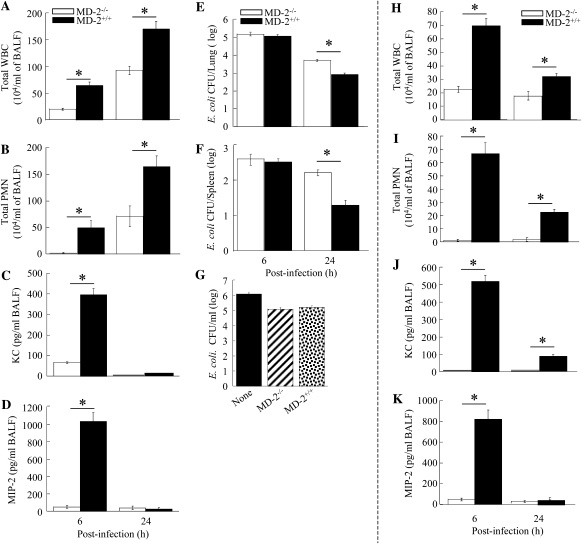
Because the role of MD-2 in the host response to bacterial pathogens has not been characterized, we next determined the role of MD-2 in E. coli–induced host response in the lung. We performed BALF and lung homogenate studies at 6 and 24 hours after intratracheal administration of 106 CFU E. coli/mouse. In MD-2−/− mice, neutrophil influx into the airspaces was abolished at 6 hours as compared with their control littermates (MD-2+/+), and was attenuated at 24 hours (Figures 4A–4B). Saline challenge did not induce neutrophil accumulation in the lungs in either MD2−/− or MD2+/+ mice (data not shown). These data support the conclusion that, although MD-2 is an important mediator for E. coli–induced neutrophil recruitment into the airspace and lung parenchyma, MD-2–independent cascade(s) also plays an essential role in neutrophil accumulation in E. coli pneumonia, particularly at a late time point (24 h).
Figure 4.
(A–D) Effect of viable Escherichia coli (106 CFU/mouse) on total WBC (A), neutrophil accumulation (B), and KC and macrophage inflammatory protein (MIP)-2 expression in the airspaces (C and D) in MD-2−/− and MD-2+/+ mice at 6 and 24 hours after infection (n = 4–7 mice in each group; *Significant differences between MD-2−/− and MD-2+/+ [P < 0.05]). (E–G) Bacterial clearance of E. coli after intratracheal inoculation. The MD-2−/− and MD-2+/+ mice were administered E. coli intratracheally on Day 0 at a concentration of 106 CFU/mouse, and bacterial burden in the lungs (E) and spleens (F) were determined at 6 and 24 hours after infection (n = 4–6 mice in each group; *Significant differences between MD-2−/− and MD-2+/+ mice [P < 0.05]). (G) Neutrophil killing assay. A total of 106 CFU/ml of E. coli was cultured alone or cocultured with 106 bone marrow–derived polymorphonuclear cells from MD-2−/− or MD-2+/+ mice. Bacteria were then quantified after 2 hours of incubation (n = 3/group). (H–K) Effect of heat-killed E. coli (106 CFU/mouse) on neutrophil accumulation, and KC and MIP-2 expression in the lungs in MD-2−/− and MD-2+/+ mice at 6 and 24 h after infection (n = 4–6 mice in each group at 6 and 24 h; *Significant differences between MD-2−/− and MD-2+/+ [P < 0.05]). Data are presented as mean (±SEM).
Effect of MD-2 on KC and MIP-2 Expression in E. coli Pneumonia
Because it is well documented that the presence of high concentrations of cytokines/chemokines in the lungs can contribute to neutrophil recruitment, we measured levels of KC and MIP-2 in the BALF at 6 and 24 hours after E. coli challenge. Concentrations of both these cytokines/chemokines were decreased in MD2−/− mice to varying degrees after E. coli inoculation (Figures 4C–4D). These observations demonstrate that MD-2–mediated signaling is essential for the expression of KC and MIP-2 in the lung in E. coli pneumonia. This finding could serve as an explanation for the reduced neutrophil influx observed in the lungs of MD-2−/− mice after E. coli challenge.
Effect of MD-2 Signaling on Pulmonary Host Defense against E. coli
Having established that MD-2 is important for the immune response to E. coli infection in the lungs, we determined the importance of MD-2 signaling in antibacterial defense. Both MD-2−/− and MD-2+/+ mice were infected intratracheally with E. coli and killed at 6 and 24 hours after infection to determine the bacterial load in their lungs (the primary site of infection) and spleens (to evaluate bacterial dissemination). Relative to MD-2+/+ mice, MD-2−/− mice had a higher bacterial load in the lungs (Figure 4E) and enhanced bacterial dissemination at 24 hours (Figure 4F). Higher bacterial load found in the lungs could be due to either: (1) attenuated neutrophil recruitment into the lungs of MD2−/− mice after E. coli challenge; and/or (2) impairment of the bactericidal capacity of MD-2−/− neutrophils. To investigate the latter possibility, we performed a killing assay of E. coli by isolated neutrophils. E. coli was quantitatively cultured in the presence of MD-2−/− and MD-2+/+ neutrophils in vitro, and the CFUs were enumerated at the end of a 2-hour incubation. There were no significant differences in E. coli CFUs between neutrophils observed from MD-2−/− and MD-2+/+ mice (Figure 4G). These findings suggest that MD-2 is required for host defense, and that the critical mechanism involves neutrophil recruitment rather than bactericidal capacity.
To demonstrate whether a heat-labile agent (protein) is responsible for MD-2–independent neutrophil responses in the lungs after E. coli infection, we used heat-killed E. coli. Both neutrophil influx and KC/MIP-2 expression in response to heat-killed E. coli was totally dependent on MD-2 (Figures 4H–4K). These data suggest that a heat-labile component of E. coli is responsible for MD-2–independent signaling cascade.
Role of MD-2–Independent Cascade in Neutrophil Accumulation and Antibacterial Defense
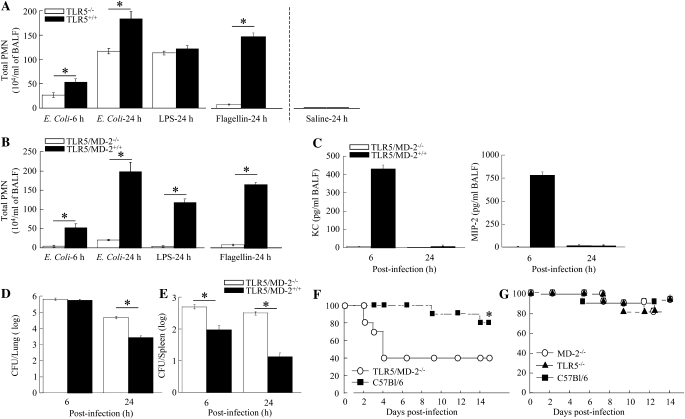
Having established that both MD-2–dependent and –independent cascades are required for neutrophil influx into the lung after E. coli infection, we next investigated the role of MD-2–independent signaling cascades in antibacterial defense. Because E. coli is a flagellated bacterium, we investigated the importance of E. coli flagellin protein recognition in the lungs. To determine the role of E. coli flagellin in the induction of the MD-2–independent innate response, we used TLR5−/− and MD-2/TLR5−/− mice. TLR5−/− mice displayed attenuated neutrophil accumulation in the lungs after E. coli inoculation (Figure 5A). These findings demonstrate that the MD-2–independent innate immune response to E. coli infection is important for neutrophil accumulation, and that this response is regulated via TLR5. When MD-2/TLR5−/− animals were infected with WT E. coli, we observed attenuated neutrophil influx in the lungs at 6 and 24 hours (Figure 5B). Furthermore, no KC or MIP-2 expression was detected in BALF obtained from MD-2/TLR5−/− animals (Figure 5C). Finally, MD-2/TLR5−/− mice demonstrated a heavier bacterial burden in the lungs at 24 hours, similar to MD-2−/− mice (Figure 5D). Unlike MD-2−/− mice, greater E. coli dissemination was observed in MD-2/TLR5−/− mice at 6 and 24 hours (Figure 5E). These data together demonstrate that both MD-2–dependent and –independent cascades are important for pulmonary neutrophil recruitment against E. coli, and that these responses limit bacterial growth in the lungs and bacterial dissemination. To further demonstrate whether reduced neutrophil accumulation in the lungs resulted in attenuated protective defense, we inoculated MD-2−/−, TLR5−/−, or MD-2−/−/TLR5 mice with E. coli. We observed impaired survival in MD-2/TLR5 double-KO mice (Figure 5F), but not in TLR5−/− or MD-2−/− mice (Figure 5G).
Figure 5.
(A and B) Effect of viable E. coli (106 CFU/mouse) on neutrophil accumulation in the lungs in Toll-like receptor (TLR)-5−/− and MD-2−/−/TLR5 mice (n = 4–5 mice in each group at 6 and 24 h; *significant differences between knockout [KO] mice and their control littermates [P < 0.05]). (C) KC and MIP-2 expression in the lungs in MD-2−/−/TLR5 and wild-type (WT) mice at 6 and 24 hours after infection (n = 4–5). *Significant differences between MD-2−/−/TLR5−/− and their littermate controls (P < 0.05). (D and E) Bacterial clearance of E. coli after intratracheal inoculation. The MD-2/TLR5−/− and MD-2+/+/TLR5−/− mice were administered E. coli at a concentration of 106 CFU/ml on Day 0, and bacterial burden in the lungs (D) and bacterial dissemination (E) were determined. *Significant differences between MD-2/TLR5−/−−/− and WT mice. (F) Survival in MD-2−/−/TLR5 mice and control animals (C57Bl/6) after intratracheal E. coli infection. Mice were inoculated intratracheally with a dose of E. coli (108 CFU/mouse) on Day 0, and for survival (n = 16 mice from 2 separate experiments in each group; *significant differences between KO and WT mice [P < 0.05] determined by Wilcoxon rank sign test between groups). (G) Survival in MD-2−/−, TLR5−/− mice, and control animals (C57Bl/6) after intratracheal E. coli (108 CFU/mouse) infection (n = 18 mice from 2 separate experiments in each group; *P < 0.05 determined by Wilcoxon rank sign test between groups). Data are presented as mean (±SEM).
DISCUSSION
Pulmonary diseases caused by gram-negative pathogens are a leading cause of morbidity and mortality among healthy and immunocompromised people worldwide (1–3). Although antibiotics reduce the mortality rates of pneumonia, the efficacy of antibiotic therapy has recently been limited by: (1) increasing numbers of immunocompromised patients; (2) a growing population of elderly individuals; and (3) the emergence of antibiotic-resistant bacterial pathogens. Therefore, modulation of the host immune response may be a better therapeutic strategy, particularly among patients with impaired immune systems or those infected with antibiotic-resistant bacteria. However, an understanding of the role of the innate immune responses in the lung microenvironment is a prerequisite to designing early interventions that minimize the severe parenchymal damage associated with infections.
Because the alveolar microenvironment is continuously exposed to microbes and their products, it has developed a complex defense mechanism to maintain sterility, including neutrophil recruitment from the bloodstream, primarily in response to bacterial invasion (4–8). Although the migration of neutrophils into lung is a critical event in host defense during bacterial pneumonia (8), excessive neutrophil influx may contribute to tissue damage. Therefore, attenuating excessive neutrophil influx is of great interest. From a therapeutic point of view, due to the identified and unidentified complex mechanisms associated with neutrophil accumulation in the lung in response to LPS, blocking an individual adhesion molecule may not be a viable approach to minimizing LPS-mediated neutrophil migration. However, blocking the initial signaling steps could possibly attenuate the excessive neutrophil accumulation and, thereby, minimize damage to lung parenchyma. Although some recent studies have focused on the role of pattern recognition receptor, such as TLRs and CD-14 in lung infection models (12–14, 25), none has examined the role of MD-2. The aim of this investigation was to define the role of MD-2 in lung inflammation and host defense.
Neutrophil sequestration within capillaries and migration into the lung during infection is a multistep sequence that involves neutrophil stiffening, retention in capillaries, adhesion, and eventual migration into the alveolar spaces (35, 36). Reports have shown that cytokine/chemokine expression and impaired neutrophil cytoskeletal assembly are key events in the migration of neutrophils into the lung (37–39). Our findings from this investigation suggest that LPS-induced MD-2 signaling leads to activation of NF-κB and subsequent expression of chemokines/cytokines, and that these events contribute to neutrophil influx into the lungs after LPS exposure. This conclusion is further supported by the fact that MD-2−/− neutrophils were not defective in actin assembly in response to chemokines/cytokines.
LPS is implicated as a major virulence factor in the induction of lung inflammation. We have reported that low-dose (300 μg/ml) LPS–induced neutrophil influx was dependent on CD-14, whereas high-dose (3,000 μg/ml) LPS–induced influx was not dependent on CD14 (25). Our observations demonstrate that LPS-mediated neutrophil accumulation is completely dependent on MD-2, regardless of the LPS concentration, which is surprising given the fact that MD-2 does not have an intracellular signaling domain. Although these findings are similar to what we have reported regarding the role of TLR4 in LPS-induced lung inflammation (25), this is the first investigation to demonstrate the important role of MD-2 in LPS-induced lung inflammation.
It has been reported that bone marrow and non–bone marrow (resident) cells in the lung produce proinflammatory mediators. It is well documented that hematopoietic cells produce neutrophil chemoattractants, such as KC and MIP-2, whereas the resident AEII cells produce the neutrophil chemoattractant, LIX. Our results are the first to demonstrate that MD-2 in both cell types is important for neutrophil-mediated inflammation in the lungs. The conclusions obtained from this investigation are consistent with several prior studies of the role of hematopoietic and nonhematopoietic cells. For example, a recent investigation has shown that MyD88 derived from hematopoietic cells is more important for LPS-induced bronchial constriction and expression of TNF-α and IL-12p40 (40), despite the fact that both hematopoietic and resident cell–derived MyD88 are important for LPS-induced neutrophil influx (41–44).
We also demonstrate that neutrophil influx into the lungs in response to live E. coli challenge was diminished, but not abolished, in MD-2−/− mice, suggesting the presence of an MD-2–independent cascade(s), probably via another virulence factor of E. coli. Due to the involvement of several cell types and multiple signaling cascades simultaneously activated by E. coli, it is difficult to dissect out cascades other than MD-2. Despite these limitations, we demonstrate that TLR5 is the other mediator triggering downstream events that lead to inflammation via binding to E. coli flagellin. In this regard, recent investigations have demonstrated similar findings in which neutrophil influx in response to Pseudomonas aeruginosa (45) and Legionella pneumophila (46) in the lungs involves TLR5.
In conclusion, the present study has clearly established that MD-2 plays a critical role in inducing lung inflammation in response to LPS. In addition, MD-2 derived from both hematopoietic and resident lung cells is important for LPS-induced lung inflammation. We also found that MD-2 plays an important role in antibacterial host defense, including the limitation of bacterial growth in the lung and dissemination, and survival. Our data further demonstrate that the MD-2–independent cascade in response to E. coli involves TLR5. Because MD-2 lacks an intracellular signaling domain, based on previous reports (15), we speculate that conformational changes upon ligand binding leads to downstream signaling cascades, resulting in cytokine/chemokine production and subsequent neutrophil influx.
Recognition of these cascades, the cell types involved, and the responsible bacterial virulence factors will provide fundamental information about the immune mechanisms by which gram-negative pathogens and/or their products induce lung inflammation. These findings suggest that MD-2 may be a useful therapeutic target in the development of novel strategies to counter infection or attenuate excessive lung inflammation in pathological settings caused or complicated by gram-negative pathogens. Extending upon our observations, we propose that functional gene polymorphisms in human MD-2 may have important consequences in host responses against gram-negative pathogens.
Acknowledgments
The authors thank Kensuke Miyake at the University of Tokyo for providing MD-2−/−, Shizuo Akira for providing TLR5−/−, and Mason Freeman for providing CD14−/− strains of mice. The authors are indebted to the Jeyaseelan laboratory members (Ann Craig, Yanru Zhang, Gayathriy Balamayooran, and Jonathan Mai) and the Worthen laboratory members (Junjie Mei and Kenneth Malcolm) for their helpful discussions. They also thank Jay Westcott at ELISA Tech (Denver, CO) for providing cytokine/chemokine assay kits.
This work was supported by American Lung Association research grant RG-22442-N (S.J.), Flight Attendant Medical Research Institute Scientist Award YCSA-062466 (S.J.), and National Institutes of Health grants R01 HL-091958 (S.J.), R01 HL-068876 (G.S.W.), and P20 RR-020159 (to LSU).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0152OC on November 6, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Mizgerd JP. Lung infection: a public health priority. PLoS Med 2006;3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fein AM. Pneumonia in the elderly: overview of diagnostic and therapeutic approaches. Clin Infect Dis 1999;28:726–729. [DOI] [PubMed] [Google Scholar]
- 3.Lynch JP, Martinez FJ. Community-acquired pneumonia. Curr Opin Pulm Med 1998;4:162–172. [PubMed] [Google Scholar]
- 4.Mizgerd JP, Skerrett SJ. Animal models of human pneumonia. Am J Physiol Lung Cell Mol Physiol 2008;294:L387–L398. [DOI] [PubMed] [Google Scholar]
- 5.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med 2008;358:716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strieter RM, Kunkel SL. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Investig Med 1994;42:640–651. [PubMed] [Google Scholar]
- 7.Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc 2005;2:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol 2002;14:123–132. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev 2000;173:39–51. [DOI] [PubMed] [Google Scholar]
- 11.Fenton MJ, Golenbock DT. LPS-binding proteins and receptors. J Leukoc Biol 1998;64:25–32. [DOI] [PubMed] [Google Scholar]
- 12.da Silva Correia J, Ulevitch J. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J Biol Chem 2002;277:1845–1854. [DOI] [PubMed] [Google Scholar]
- 13.Pugin J, Schurer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA 1993;90:2744–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990;249:1431–1433. [DOI] [PubMed] [Google Scholar]
- 15.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, et al. Crystal structure of the TLR4–MD-2 complex with bound endotoxin antagonist eritoran. Cell 2007;130:906–917. [DOI] [PubMed] [Google Scholar]
- 16.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 1998;2:253–258. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)–deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 1999;162:3749–3752. [PubMed] [Google Scholar]
- 18.Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol 2004;40:861–868. [DOI] [PubMed] [Google Scholar]
- 19.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1– and IL-18–mediated function. Immunity 1998;9:143–150. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 2003;301:640–643. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho K, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4–mediated MyD88-independent signaling pathway. Nat Immunol 2003;4:1144–1150. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi T, Fujita T, et al. Essential role for TIRAP in activation of the signaling cascade shared by TLR2 and TLR4. Nature 2002;420:324–329. [DOI] [PubMed] [Google Scholar]
- 23.Fan MH, Klein RD, Steinstraesser L, Merry AC, Nemzek JA, Remick DG, Wang SC, Su GL. An essential role for lipopolysaccharide-binding protein in pulmonary innate immune responses. Shock 2002;18:248–254. [DOI] [PubMed] [Google Scholar]
- 24.Branger J, Florquin S, Knapp S, Leemans JC, Pater JM, Speelman P, Golenbock DT, van der Poll T. LPS-binding protein–deficient mice have an impaired defense against gram-negative but not gram-positive pneumonia. Int Immunol 2004;16:1605–1611. [DOI] [PubMed] [Google Scholar]
- 25.Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infect Immun 2005;73:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol 2002;3:667–672. [DOI] [PubMed] [Google Scholar]
- 27.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol 2006;7:868–874. [DOI] [PubMed] [Google Scholar]
- 28.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Toll–IL-1 receptor domain-containing adaptor protein is critical for early lung immune responses against Escherichia coli lipopolysaccharide and viable Escherichia coli. J Immunol 2005;175:7484–7495. [DOI] [PubMed] [Google Scholar]
- 29.Jeyaseelan S, Young SK, Yamamoto M, Arndt PG, Akira S, Kolls JK, Worthen GS. Toll–IL-1 receptor domain–containing adaptor protein is a critical mediator of antibacterial defense in the lung against Klebsiella pneumoniae but not Pseudomonas aeruginosa. J Immunol 2006;177:538–547. [DOI] [PubMed] [Google Scholar]
- 30.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol 2005;32:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun 2004;72:7247–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeyaseelan S, Young SK, Fessler MB, Liu Y, Malcolm KC, Yamamoto M, Akira S, Worthen GS. Toll/IL-1 receptor domain–containing adaptor inducing IFN-beta (TRIF)–mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol 2007;178:3153–3160. [DOI] [PubMed] [Google Scholar]
- 33.Andonegui G, Bonder CS, Green F, Mullaly SC, Zbytnuik L, Raharjo E, Kubes P. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Invest 2003;111:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 2002;283:L256–L264. [DOI] [PubMed] [Google Scholar]
- 35.Doerschuk CM. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation 2001;8:71–88. [PubMed] [Google Scholar]
- 36.Wang Q, Doerschuk CM, Mizgerd JP. Neutrophils in innate immunity. Semin Respir Crit Care Med 2004;25:33–41. [DOI] [PubMed] [Google Scholar]
- 37.Bazan-Socha S, Bukiej A, Marcinkiewicz C, Musial J. Integrins in pulmonary inflammatory diseases. Curr Pharm Des 2005;11:893–901. [DOI] [PubMed] [Google Scholar]
- 38.Worthen GS, Schwab B, Elson EL, Downey GP. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science 1989;245:183–186. [DOI] [PubMed] [Google Scholar]
- 39.Andonegui G, Goyert SM, Kubes P. Lipopolysaccharide-induced leukocyte-endothelial cell interactions: a role for CD14 versus Toll-like receptor 4 within microvessels. J Immunol 2002;169:2111–2119. [DOI] [PubMed] [Google Scholar]
- 40.Noulin N, Quesniaux VFJ, Schnyder-Candrian S, Schnyder B, Maillet I, Robert T, Vargaftig BB, Ryffel B, Couillin I. Both hematopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. J Immunol 2005;175:6861–6869. [DOI] [PubMed] [Google Scholar]
- 41.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol 2004;287:L143–L152. [DOI] [PubMed] [Google Scholar]
- 42.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol 2008;38:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J Immunol 2007;178:1896–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hajjar AM, Harowicz H, Liggitt HD, Fink J, Wilson CB, Skerrett SJ. An essential role for non–bone marrow–derived cells in control of Pseudomonas aeruginosa pneumonia. Am J Respir Cell Mol Biol 2005;33:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2007;292:L312–L322. [DOI] [PubMed] [Google Scholar]
- 46.Hawn TR, Berrington WR, Smith IA, Uematsu S, Akira S, Aderem A, Smith KD, Skerrett SJ. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J Immunol 2007;179:6981–6987. [DOI] [PubMed] [Google Scholar]