Abstract
Toluene diisocyanate (TDI), a reactive, hazardous irritant, causes respiratory symptoms such as cough, rhinitis, dyspnea, and chest tightness in exposed workers. Although previous animal studies have shown that TDI causes respiratory reflexes that are abolished by desensitization of capsaicin-sensitive sensory nerves, the specific molecular identity of the transducer(s) responsible for sensing this noxious stimulus has, to date, remained elusive. Recent studies have demonstrated that transient receptor potential ankyrin 1 (TRPA1), an ion channel largely restricted to a subset of capsaicin-sensitive sensory nerves, functions as a transducer capable of initiating reflex responses to many reactive chemical stimuli. We therefore hypothesized that TRPA1 is the primary molecular transducer through which TDI causes sensory nerve activation and respiratory reflexes. Consistent with this hypothesis, TDI activated TRPA1, but not the capsaicin-sensitive transient receptor potential vanilloid 1 channel, in heterologous expression systems. TDI also activated a subset of dissociated trigeminal sensory neurons from wild-type but not TRPA1-deficient mice. In vivo, TDI mimicked known TRPA1 agonists by causing a pronounced decrease in breathing rate, indicative of respiratory sensory irritation, and this reflex was abolished in TRPA1-deficient mice. Together, our data suggest that TDI causes sensory nerve activation and airway sensory irritation via the activation of the ion channel, TRPA1.
Keywords: toluene diisocyanate, formaldehyde, sensory irritation, transient receptor potential ankyrin-1
CLINICAL RELEVANCE
Toluene diisocyanate (TDI) causes respiratory symptoms in exposed workers. We demonstrate that TDI activates sensory nerves in vitro and reflexes in vivo in a manner dependent on the expression of the ion channel TRPA1. TRPA1 represents a valid target for treatment of TDI exposures.
Toluene diisocyanate (TDI), a reactive compound extensively used in the manufacture of a variety of polymer-containing products, such as paints and foams, can cause respiratory symptoms, such as cough, rhinitis, dyspnea, and chest tightness in exposed workers (1, 2). Early investigations of TDI led to the hypothesis that the irritant may promote respiratory pathology through two broadly defined mechanisms (3): immunological, driven by TDI-specific antibodies; and pharmacological, driven by TDI's effects on airway structural cells and nerves. Subsequent experiments in animal models demonstrated that capsaicin-sensitive sensory nerves within the respiratory tract are targets of such “pharmacological” effects of TDI, because TDI-induced bronchoconstriction, rhinorrhea, and neurogenic inflammation in these models are inhibited by capsaicin desensitization, the nonspecific inhibition of transient receptor potential (TRP) channels by ruthenium red, or blockade of tachykinin receptors that participate in the effector arm of irritant-induced axon reflex neurotransmission (4–6). Despite this body of functional evidence that capsaicin-sensitive sensory nerves contribute to noxious respiratory sensations evoked by TDI, whether these responses are due to nonspecific tissue damage or to specific molecular substrate(s) remains unknown.
The nonselective cation channel transient receptor potential ankyrin 1 (TRPA1) is expressed predominantly within the plasmamembrane of a subset of capsaicin-sensitive sensory neurons (7, 8). Activation of TRPA1 ion channels (as well as other transducers of external stimuli, such as the capsaicin-sensitive transient receptor potential vanilloid [TRPV1] channel), causes cation influx into the nerve terminal, depolarizing its membrane and initiating action potentials that conduct centrally to produce noxious sensations and subsequent reflexes, such as cough, sneeze, bronchospasm, and mucus secretion (9, 10). TRPA1 is unusual among ion channels in that it can be activated by a variety of structurally unrelated reactive molecules (11, 12). As predicted based on this mode of activation, many irritants, oxidants, and reactive products of inflammation and oxidative stress activate TRPA1 (8, 13–19).
Intriguingly, the functional expression of TRPA1 is necessary for the acute activation of putative nociceptor neurons and pain behaviors evoked by reactive molecules, such as formaldehyde, that were previously thought to evoke noxious sensations through multiple nonspecific mechanisms involving tissue damage and inflammation (15, 16). Of note, TRPA1 is also present in mouse capsaicin-sensitive airway sensory neurons (20), and inhalation of TRPA1 agonist aerosols leads to reflex respiratory rate decreases (20, 21), indicative of sensory irritation. Based on these findings, we hypothesized that the respiratory irritant TDI, which contains reactive electrophilic isocyanate moieties, may evoke respiratory irritation in mice via activation of TRPA1. Indeed, in the current study, we show that TDI activates TRPA1 (but not the capsaicin-sensitive TRPV1) in heterologous expression systems, and functional TRPA1 channels are required for trigeminal neuron activation and sensory irritation evoked by TDI.
MATERIALS AND METHODS
Experimental Animals and Reagents
Male wild-type C57BL/6 mice sufficient with regard to their TRPA1 expression (Trpa1+/+) or their TRPA1-deficient (Trpa1−/−) littermates (19), between 6 and 20 weeks of age, were used in the current study. All procedures were approved by the institutional animal care and use committees of the respective institutions where the experiments were conducted. Toluene-2,4-diisocyanate was purchased from TCI America (Portland, OR), and formalin (10% Lo-odor formalin containing 3.8% formaldehyde wt/vol) was purchased from VWR International (West Chester, PA). All other reagents were purchased from Sigma (St. Louis, MO), unless otherwise noted.
Cell Culture of Human Embryonic Kidney 293 Cells and Mouse Sensory Neurons
Nontransfected (nt) human embryonic kidney (HEK) 293 cells and HEK293 cells stably transfected with either human TRPA1 (TRPA1-HEK) or human TRPV1 (TRPV1-HEK) were cultured according to previously described conditions (18). Briefly, cells were maintained in an incubator (37°C, 5% CO2) in Dulbecco's modified Eagle's medium (containing 110 mg/liter pyruvate) supplemented with 10% FBS and 500 μg/ml G-418 (geneticin) as a selection agent. Cells were removed from their culture flasks by treatment with Accutase (Sigma), then plated onto poly-D-lysine–coated cover slips (BD Biosciences, Bedford, MA) and incubated at 37°C for more than 1 hour before experimentation.
For trigeminal neuron experiments, mice (20–40 g) were killed by CO2 overdose, and the trigeminal ganglia were rapidly dissected and cleared of adhering connective tissue. The medial portions of ganglia (which contains the neurons that innervate the upper airways) were isolated and incubated (50 min, 37°C) in 2 mg/ml collagenase type 1A and 2 mg/ml dispase II in 2 ml of Ca2+-free, Mg2+-free Hank's balanced salt solution. Neurons were dissociated by trituration, washed, resuspended in L-15 medium containing 10% FBS, and then transferred (25 μl) onto circular 25-mm glass coverslips (Bellco Glass Inc., Vineland, NJ) coated with poly-D-lysine (0.1 mg/ml) and laminin (5 μg/ml). Coverslips were used within 24 hours.
Ratiometric Calcium Imaging
HEK293-covered coverslips were loaded with Fura 2 acetyoxymethyl ester (Fura-2 AM; 8 μM) (Molecular Probes, Carlsbad, CA) in Dulbecco's modified Eagle's medium (containing 110 mg/L pyruvate) supplemented with 10% FBS and incubated for 40 minutes at 37°C in 5% CO2. Neuron-covered coverslips were loaded with Fura-2 AM (8 μM) in L-15 media containing 20% FBS and incubated for 40 minutes at 37°C. For imaging, the coverslip was placed in a custom-built chamber (bath volume of 600 μl) and superfused at 4 ml/minute with Locke solution (34°C) for 15 minutes before each experiment by an infusion pump.
Changes in intracellular free calcium concentration (intracellular [Ca2+]free) were measured by digital microscopy (Universal; Carl Zeiss, Inc., Thornwood, NY) equipped with custom-built equipment for ratiometric recording of single cells. The field of cells was monitored by sequential dual excitation—352 and 380 nm—and the analysis of the image ratios used methods described previously to calculate changes in intracellular [Ca2+]free (22). The ratio images were acquired every 6 seconds. Superfused buffer was stopped 30 seconds before each drug application, when 300 μl of buffer was removed from the bath and replaced by 300 μl of 2× test agent solution added between image acquisitions. After treatments, neurons were exposed to KCl (30 s, 75 mM) to confirm voltage sensitivity. At the end of experiments, both neurons and HEK293 cells were exposed to ionomycin (30 s, 1 μM) to obtain a maximal response. For all ratiometric experiments and for each application, TDI was dissolved into Locke solution from pure toluene 2,4 diisocyanate less than 30 seconds before application.
For the analysis of Fura-2 AM–loaded cells, the measurement software converted ratiometric information to intracellular [Ca2+]free using Tsien parameters ([Ca2+] = Kd × ([R − Rmin]/[Rmax − R]) × β) (23), where Kd is the dissociation constant and R is the ratio of the fluorescence at 352 nm and 380 nm, particular to this instrumentation and the HEK cells and dissociated mouse trigeminal neurons. Preliminary calibration studies yielded an Rmin (352:380 ratio under calcium-free conditions) of 0.2 for HEK cells and 0.3 for mouse trigeminal neurons, and an Rmax (352:380 ratio under calcium-saturating conditions) of 18 and 14 for HEK cells and neurons, respectively. The β value (380 in calcium-free conditions/380 in calcium-saturating conditions) was estimated as being 10 and the Kd was estimated as being 224 nM. In the subsequent experimental studies, we did not specifically calibrate the relationship between ratiometric data and absolute calcium concentration for each specific cell, choosing instead to use the parameters provided from the calibration studies and relate all measurements to the peak ionomycin response in each viable cell. This effectively provided the needed cell-to-cell calibration for enumerating individual cellular responses. Only cells that had a robust response to ionomycin were included in analyses. At each time point for each cell, data were presented as the percentage change in intracellular [Ca2+]free, normalized to ionomycin: responsex = 100 × ([Ca2+]x − [Ca2+]bl)/([Ca2+]max − [Ca2+]bl), where [Ca2+]x was the apparent [Ca2+]free of the cell at a given time point, [Ca2+]bl was the cell's mean baseline apparent [Ca2+]free measured over 120 seconds, and [Ca2+]max was the cell's peak apparent [Ca2+]free during ionomycin treatment. For the neuronal experiments, neurons were defined as “responders” to TDI if the mean response was greater than the mean baseline plus twice the SD. Only neurons that responded to KCl were included in analyses.
Nose-Out Whole-Body Plethysmography
For our mouse plethysmography studies, we developed a modification of the Alarie test, wherein decreases in respiratory rate after inhalation of aerosols of test agents are used as an indication of sensory irritation (24).
Mice were briefly anesthetized to effect with isoflurane (≤3% in O2, 1 L/min) using a SurgiVet (Waukesha, WI) Model 100 isoflurane vaporizer, then placed in a BUXCO PLY3351 plethysmography chamber (BUXCO Electronics, Inc., Sharon, CT). Of note, the anesthetic isoflurane was identified as a TRPA1 agonist during the preparation of this manuscript (25). However, isoflurane exposure did not cause any observable genotype-specific confounding effects in our experiments, as baseline respiratory parameters were similar across genotypes (see Results). The respiratory flow signal was obtained through a pneumotachograph connected to a differential pressure transducer (Validyne DP 45–14) and amplifier as part of the PLUGSYS Modular System (HSE-Harvard Apparatus, March-Hugstetten, Germany). Data were analyzed offline with PULMODYN software (HSE-Harvard Apparatus). After a period of 10 to 15 minutes, during which the mice regained consciousness and acclimatized to plethysmography conditions until their respiratory rate reached a steady baseline value, vehicle (1:4 ethyl acetate:olive oil for toluene 2,4 diisocyanate; 50% ethanol in 0.9% PBS for allyl isothiocyanate [AITC]; 0.9% PBS for all other agents; 1–2 μl volume each) was gently pipetted into the mouse's nostril, after which respiratory parameters were recorded. Responses to agonists or their respective vehicles are represented as the maximal effect observed during a 5-minute observation period.
RESULTS
TDI Activates Heterologously Expressed TRPA1
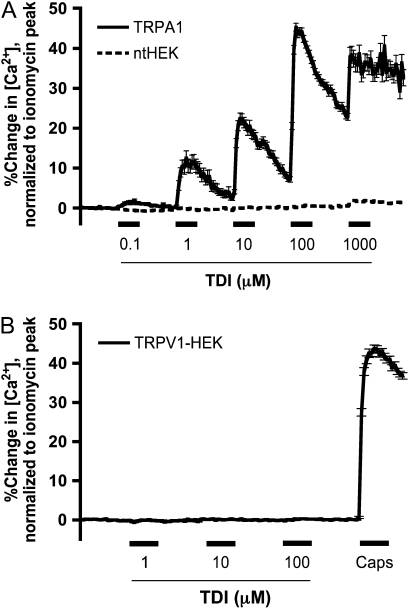
To determine whether TDI can activate TRPA1, we performed ratiometric calcium imaging experiments on HEK293 cells stably transfected with human TRPA1 channels (TRPA1-HEK cells) that we have previously shown to be activated by known TRPA1 agonists, such as AITC and 4-hydroxynonenal (18, 19). TDI (0.1–1,000 μM) caused calcium influx in hTRPA1-HEK cells (n = 284) in a saturating, concentration-dependent manner (Figure 1A), with a maximal effect (45 ± 1.0% of ionomycin) at 100 μM, and an estimated EC50 (the half-maximal effective concentration) of 10 μM. By contrast, TDI (0.1–1000 μM) had no effect in nt-HEK cells (0.8 ± 0.2% of ionomycin; n = 335; Figure 1A), consistent with the hypothesis that these effects of TDI were due to the activation of TRPA1 rather than nonspecific toxicity. Furthermore, TDI (1–100 μM) failed to activate HEK293 cells stably expressing human TRPV1 (TRPV1-HEK; 0.2 ± 0.1% of ionomycin; n = 383; Figure 1B), which were subsequently activated by 300 nM capsaicin (44 ± 1.0% of ionomycin), indicating that the reactive isocyanate does not nonspecifically activate calcium-permeable ion channels.
Figure 1.
Activation of transient receptor potential ankyrin (TRPA1)–human embryonic kidney (HEK) cells by toluene diisocyanate (TDI). (A) Ca2+ responses (mean ± SEM) of TRPA1-HEK cells to TDI (0.1–1,000 μM). Solid line denotes responses of TRPA1-HEK cells (n = 284); dashed line denotes responses of ntHEK cells (n = 335). (B) Ca2+ responses (mean ± SEM) of transient receptor potential vanilloid (TRPV1)-HEK cells (n = 383) to TDI (1–100 μM) and capsaicin (Caps; 300 nM). All agonists (A and B) were applied for 60 seconds (blocked line).
TDI Activation of Mouse Trigeminal Neurons Is Inhibited by Genetic Deletion of TRPA1
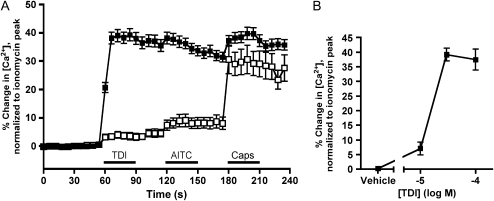
To test the hypothesis that TDI selectively activates TRPA1 on native nociceptive neurons, we performed ratiometric calcium imaging on dissociated trigeminal neurons isolated from wild-type (Trpa1+/+) C57BL/6 mice and TRPA1-deficient (Trpa1−/−) mice (19). In the current study, TDI (30 μM) activated 125 of 181 Trpa1+/+ trigeminal neurons, with a maximum response of 39 (±2.2) % of ionomycin (Figure 2A). This effect of TDI occurred over a narrow concentration range, such that 10 μM evoked a minimal response, whereas 30 μM was a maximally effective concentration (Figure 2B).
Figure 2.
TRPA1 channels are critical for TDI-induced calcium responses in dissociated sensory neurons. (A) Ca2+ responses (mean ± SEM) of trigeminal neurons responding to TDI (30 μM). Responses to AITC (100 μM) and Caps (1 μM) also shown. Data comprise neurons from wild-type mice (Trpa1+/+, solid squares, 125 out of 181 neurons responding) and neurons from Trpa1−/− mice (open squares, 23/237). Blocked line denotes the 30-second application of agonist. All neurons responded to KCl (75 mM) applied immediately before ionomycin. (B) Concentration–response relationship of TDI on Ca2+ responses of trigeminal neurons from wild-type mice (n = 8, 125, and 44 neurons for 10, 30, and 100 μM TDI, respectively).
Sustained neuronal responses to TDI masked further responses to the TRPA1 agonist, AITC (100 μM), although AITC did increase calcium levels of responding neurons from 35 (±2.1) % of ionomycin to 38 (±2.1) % of ionomycin (P < 0.05). Only 3 of 181 neurons that had not already responded robustly to TDI were activated by AITC. In addition, persistent TDI responses largely masked further stimulation by the TRPV1 agonist, capsaicin (1 μM), although, similar to AITC, calcium levels did increase from 31 (±1.7) % of ionomycin to 40 (±2.2) % of ionomycin (P < 0.05) after capsaicin. A total of 14 of 181 neurons responded robustly to capsaicin, but failed to respond to TDI (data not shown).
Because TDI activates heterologously expressed TRPA1, but not TRPV1, we sought to determine whether TDI activation of sensory neurons is dependent upon the presence of TRPA1. Consistent with this hypothesis, the responses of dissociated trigeminal neurons derived from Trpa1−/− mice to TDI (30 μM) were almost abolished, with only 23 of 237 neurons responding with a maximum of 4.0 (±1.2) % of ionomycin (Figure 2A). As expected, these neurons also failed to respond to AITC (100 μM), but were still responsive to TRPA1-independent stimuli, as the TRPV1 agonist, capsaicin (1 μM), activated 108 of 237 Trpa1−/− trigeminal neurons with a maximum response of 31 (±5.0) % of ionomycin.
TRPA1-Deficient Mice Are Protected from TDI-Evoked Respiratory Sensory Irritation
Multiple respiratory irritants, including TRPA1 agonists, such as cinnamaldehyde and sodium hypochlorite, as well as TRPV1 agonists, such as capsaicin, decrease respiratory rate in mice via central reflexes initiated by airway nociceptors (20, 21, 26). Because these irritants are normally administered as aerosols, we sought to initially validate our modified model (see Materials and Methods for details) by administering known TRPA1 agonists.
We first investigated AITC, which is considered a prototypical TRPA1 agonist, as it does not activate cultured sensory neurons in the absence of TRPA1 (19, 27). As summarized in Table 1, respiratory rates (RRs), Vts, and times of braking (T Brs) were similar across genotype at baseline and after vehicle administration. Intranasal instillation of AITC (0.03% and 0.1% vol/vol) produced a dramatic and dose-dependent reduction in RR in Trpa1+/+ mice, which was greatly diminished in Trpa1−/− mice (Figures 3A–3C). This RR reduction was characterized predominantly by an increase in T Br (Table 1), a prototypical sign of respiratory sensory irritation.
TABLE 1.
EFFECTS OF ALLYL ISOTHIOCYANATE ON RESPIRATORY PARAMETERS IN Trpa1+/+ AND Trpa1−/− MICE
| Condition | Genotype | RR (bpm) | VT (μl) | T Br (ms) |
|---|---|---|---|---|
| Basal | Trpa1+/+ | 230 ± 11 | 234 ± 16 | 45 ± 12 |
| Trpa1−/− | 251 ± 19 | 280 ± 13 | 32 ± 6 | |
| Vehicle | Trpa1+/+ | 185 ± 23 | 259 ± 25 | 61 ± 13 |
| Trpa1−/− | 208 ± 25 | 281 ± 29 | 61 ± 16 | |
| 0.03% AITC | Trpa1+/+ | 163 ± 18* | 236 ± 20 | 125 ± 33* |
| Trpa1−/− | 205 ± 22 | 243 ± 17 | 65 ± 10 | |
| 0.1% AITC | Trpa1+/+ | 99 ± 16†‡ | 228 ± 18 | 420 ± 147†‡ |
| Trpa1−/− | 184 ± 21* | 259 ± 18 | 109 ± 21‡ |
Definition of abbreviations: AITC, allyl isothiocyanate; RR, respiratory rate; T Br, time of braking; TDI, toluene diisocyanate; Trpa, transient receptor potential ankyrin.
Values are means (±SEM) (n = 8–10) of respiratory parameters (RR, Vt, T Br) measured after acclimatization period (Basal), delivery of vehicle, or irritant.
P < 0.05, statistically significant effect of treatment compared to baseline (ANOVA followed by Dunnett's multiple comparison test).
P < 0.01, statistically significant differences between Trpa1+/+ and Trpa1−/− (ANOVA followed by Bonferroni post tests).
P < 0.01, statistically significant effect of treatment compared to baseline (ANOVA followed by Dunnett's multiple comparison test).
Figure 3.
Genetic ablation of TRPA1 channels inhibits respiratory sensory irritation produced by AITC. (A) Representative traces of pulmonary airflow waveforms from Trpa1+/+ and Trpa1−/− mice at baseline (Basal), after intranasal instillation of vehicle (VHC), or of the TRPA1 agonist, AITC. Application of AITC in this fashion caused a decrease in respiratory rate (RR) consistent with a sensory irritation response (see text and Table 1 for details). (B) Summary data (mean ± SEM; n = 8–10 each genotype) demonstrating RR in Trpa1+/+ (solid bars) and Trpa1−/− mice (shaded bars) in response to increasing doses of AITC (effect of 0.3% AITC was not determined in Trpa1+/+ mice). *Statistically significant differences (*P < 0.05, **P < 0.01; ANOVA followed by Bonferroni's post test) between genotype for a given dose. (C) The effects (mean ± SEM; n = 8–10) of intranasal dosing of vehicle (VHC) and AITC on time of braking (T Br), a prototypical feature of sensory irritation, in Trpa1+/+ (solid squares) and Trpa1−/− (solid triangles) mice.
Formaldehyde, the active ingredient in formalin solutions, causes pronounced sensory irritation in rodents (28, 29), and evokes symptoms in humans ranging from eye irritation to asthma exacerbations, depending on the extent of exposure and individual susceptibility (30). Recently, two independent groups discovered that formaldehyde is a TRPA1 agonist, and that genetic deletion of TRPA1 dramatically reduced pain behaviors evoked by intraplantar injection of formaldehyde-containing formalin solutions (15, 16). Based on these results, we hypothesized that formalin-evoked respiratory sensory irritation would be reduced in TRPA1-deficient mice. Consistent with this hypothesis, although RRs were similar at baseline (221 ± 11 bpm Trpa1+/+ versus 235 ± 14 bpm Trpa1−/−; n = 6 each) and after vehicle administration (199 ± 21 bpm Trpa1+/+ versus 237 ± 14 bpm Trpa1−/−; n = 6 each), intranasal instillation of 2 μl formalin solution (1%) produced a dramatic reduction in respiratory rate in Trpa1+/+ mice (75 ± 27 bpm; n = 6; as shown in Figure E1 in the online supplement), which was markedly attenuated in Trpa1−/− mice (192 ± 23 bpm; n = 6). This protective effect was also observed after intranasal instillation of 3% formalin in Trpa1−/− mice (185 ± 25 bpm; n = 6).
To further validate our modified model, and to confirm that genetic disruption of TRPA1 did not nonselectively reduce irritant-induced respiratory reflexes, we exposed Trpa1−/− mice to nicotine. Nicotine causes irritation when applied to the nasal mucosa in humans (31) through mechanisms that appear independent of TRPA1 based on studies in animal neurons (32). As predicted, these mice responded robustly to nicotine (100 nmol), which reduced RRs in Trpa1−/− mice from 195 (±30) bpm to 84 (±19) bpm (n = 3).
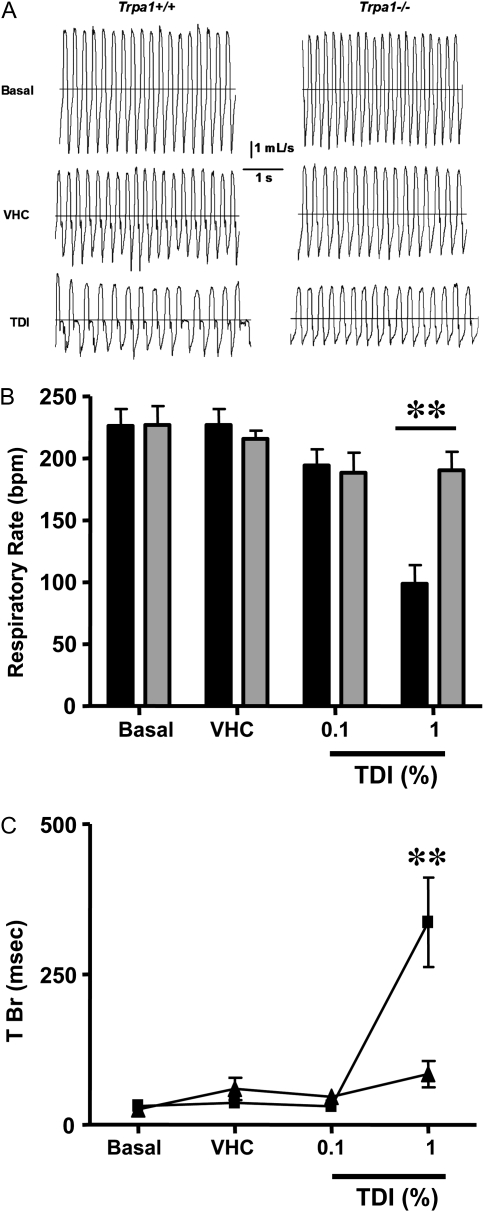
After the validation of our experimental model, we determined whether TRPA1 deletion would also protect mice from TDI-induced respiratory sensory irritation. As in the AITC and formalin studies, respiratory parameters at baseline and after vehicle administration were consistent across genotypes (Table 2). In our model using this intranasal dosing paradigm, 0.1% TDI was without effect; however, instillation of 1% TDI dramatically decreased RR and increased T Br in Trpa1+/+ mice (Table 2; Figure 4). In Trpa1−/− mice, 1% TDI only caused a slight decrease in RR that did not reach statistical significance (Table 2). Trpa1−/− mice were also significantly protected from the 1% TDI-evoked elevation of T Br above its baseline value (Table 2; Figure 4). The residual T Br increase in Trpa1−/− mice was not statistically different from that caused by the TDI vehicle alone (Table 2).
TABLE 2.
EFFECTS OF TOLUENE DIISOCYANATE ON RESPIRATORY PARAMETERS IN Trpa1+/+ AND Trpa1−/− MICE
| Condition | Genotype | RR (bpm) | Vt (μl) | T Br (ms) |
|---|---|---|---|---|
| Basal | Trpa1+/+ | 226 ± 14 | 231 ± 13 | 31 ± 7 |
| Trpa1−/− | 227 ± 15 | 283 ± 28 | 26 ± 8 | |
| Vehicle | Trpa1+/+ | 227 ± 13 | 228 ± 19 | 36 ± 8 |
| Trpa1−/− | 216 ± 7 | 261 ± 29 | 60 ± 19 | |
| 0.1% TDI | Trpa1+/+ | 194 ± 13 | 224 ± 25 | 31 ± 6 |
| Trpa1−/− | 188 ± 16 | 243 ± 22 | 46 ± 8 | |
| 1% TDI | Trpa1+/+ | 99 ± 15*† | 224 ± 25 | 337 ± 74*† |
| Trpa1−/− | 190 ± 15 | 243 ± 22 | 84 ± 22‡ |
Definition of abbreviations: RR, respiratory rate; T Br, time of braking; TDI, toluene diisocyanate; Trpa, transient receptor potential ankyrin.
Values are means (±SEM) (n = 7–10) of respiratory parameters (RR, Vt, T Br) measured after acclimatization period (Basal) and delivery of vehicle or irritant.
P < 0.01, statistically significant differences between Trpa1+/+ and Trpa1−/− (ANOVA followed by Bonferroni post tests).
P < 0.01, statistically significant effect of treatment compared to baseline (ANOVA followed by Dunnett's multiple comparison test).
P < 0.05, statistically significant effect of treatment compared to baseline (ANOVA followed by Dunnett's multiple comparison test).
Figure 4.
Genetic ablation of TRPA1 channels inhibits respiratory sensory irritation produced by TDI. (A) Representative traces of pulmonary airflow waveforms from Trpa1+/+ and Trpa1−/− mice at baseline (Basal) and after intranasal instillation of vehicle (VHC) or 1% TDI. (B) Summary data (mean ± SEM; n = 7–10 for each genotype) demonstrating RR in Trpa1+/+ (solid bars) and Trpa1−/− mice (shaded bars) in response to TDI. **Significant difference in RR between genotypes (P < 0.01; ANOVA followed by Bonferroni post test). (C) The effects of intranasal dosing (mean ± SEM; n = 7–10) of vehicle (VHC) and TDI on T Br, a prototypical feature of sensory irritation, in Trpa1+/+ (solid squares) and Trpa1−/− (solid triangles) mice.
Unlike the standard TRPA1 agonists used in our study, TDI appeared to cause a small amount of airway obstruction in some mice, as suggested by a flattening of the top of the respiratory flow waveforms and slight reduction in their amplitudes (Figure 4A). Based on these observations, we examined the effects of TDI on the expiratory flow at half Vt (EF50), which is considered a measure of airway obstruction (26). Although 1%TDI did produce an apparent decrease in EF50 in Trpa1+/+ mice (−1862 ± 231 μl/s after vehicle versus −1492 ± 318 μl/s; n = 7), this effect did not reach statistical significance (P = 0.08; ANOVA). EF50 values in Trpa1−/− mice were essentially identical to those of vehicle-treated Trpa1+/+ mice, and were unaltered by 1%TDI exposure (−1875 ± 205 μl/s after vehicle versus −1929 ± 290 μl/s after TDI; n = 7).
DISCUSSION
In the current study, we demonstrate that the nonselective cation channel TRPA1 is activated by TDI, and is necessary for TDI-evoked sensory nerve activation and respiratory sensory irritation in mice.
TDI has been known for some time as a hazardous, reactive irritant that can cause heterogeneous respiratory pathologies broadly grouped under the category of occupational lung disease. TDI and similar reactive irritants likely promote such disease by perturbing airway homeostasis through multiple mechanisms. Previous studies in animals have detected a wide variety of effects of TDI exposure, including airway inflammation, elevation of T helper type 1 and/or type 2 cytokines, epithelial hypertrophy, goblet cell metaplasia, hyperreactivity to agonist-induced bronchoconstriction, neurotrophin release, and increased neuropeptide production by airway sensory nerves (4, 33–37). Although each of these phenomena contributes significantly to the chronic airway pathology caused by TDI, exposure evokes defensive airway reflexes that are critically dependent upon capsaicin-sensitive sensory nerves.
Early animal studies revealed that multiple effects of TDI, including airway smooth muscle contraction, sneezing, and rhinorrhea (5, 38), are dependent upon capsaicin-sensitive sensory neurons, although no specific mechanism of activation of these afferent fibers had previously been identified. Given that capsaicin-induced desensitization of airway sensory nerves, the nonselective TRP channel blocker, ruthenium red, and interruption of axon reflex neurotransmission by blockade of tachykinin receptors have all decreased TDI-induced effects in animal studies, one plausible hypothesis to explain how TDI activates airway sensory fibers is through activation of capsaicin-sensitive TRPV1 ion channels. However, we have provided direct evidence against this possibility, as TDI did not activate heterologously expressed TRPV1 ion channels.
Our data suggest an alternative explanation: namely, that TDI initiates respiratory reflexes via activation of TRPA1 in capsaicin-sensitive airway sensory nerve terminals. In support of this explanation, we demonstrate that TDI activates heterologously expressed human TRPA1, that genetic disruption of TRPA1 nearly eliminates activation of mouse trigeminal neurons by TDI, and that TDI-evoked reflex decreases in respiratory rate and reflex increases in T Br are markedly blunted in the absence of TRPA1.
The findings that capsaicin-sensitive sensory neurons and their effector peptide neurotransmitters, but not the capsaicin-sensitive ion channel, TRPV1, are necessary for TDI-evoked neuronal responses in animal models may initially appear contradictory. However, TRPA1 agonists—in a fashion similar to that of TRPV1 agonists, such as capsaicin—can evoke ruthenium red–sensitive, tachykinin-dependent axon reflexes in isolated airways of mice and guinea pigs (19, 32). Furthermore, TRPA1 ion channels are frequently coexpressed with TRPV1 in the cell bodies and terminals of sensory neurons (7), including those that innervate the airways (20). Thus, capsaicin desensitization, which broadly disables TRPV1-containing nerve terminals and their associated molecular transducers of external stimuli, would also render TRPA1 dysfunctional. This type of phenomenon has been observed previously with TRPA1-activating α,β unsaturated aldehydes, such as acrolein and 4-oxononenal (19, 32, 39–41).
In addition to peptidergic axon reflexes, activation of TRPA1 channels on airway sensory nerve fiber terminals produces central reflex decreases in respiratory rate (20, 21). Aerosols of numerous hazardous environmental and industrial irritants, including TDI and formaldehyde, evoke this “sensory irritation” response in rodents (3, 24, 28, 29, 42). As predicted, in our modified version of the Alarie model, in which small volumes (<3 μl) of irritant are directly applied to the nasal mucosa, we observed profound reflex decreases in respiratory rate after delivery of AITC, formaldehyde, or TDI. Despite the widespread tissue damage that these reactive molecules elicit, they provoked only minor respiratory reflexes in the absence of functional TRPA1. Thus, we can conclude that, in our current experiments, the ion channel, TRPA1, is the primary mechanism through which acute exposure to TDI (and formaldehyde) activates mouse airway nociceptors to evoke sensory irritation.
In summary, we have identified TRPA1 as a target of the hazardous irritant, TDI, and demonstrated that TRPA1 is necessary for sensory neuron activation and central respiratory reflexes after acute exposure to TDI. Although the role of TRPA1 after chronic exposure to reactive irritants remains a topic for future exploration, it is worth noting that multiple mediators of inflammation and tissue damage activate or sensitize TRPA1 (8, 13–19, 43, 44), and that the sensory irritation reflex to acrolein and capsaicin (agonists of TRPA1 and TRPV1 channels, respectively) is enhanced in animals experiencing inflammation (26, 40). Considering that TRPA1 activation on airway sensory fiber terminals could evoke noxious respiratory sensations, sensitization of respiratory reflexes, and the local release of proinflammatory neuropeptides that may cause congestion and mucus hypersecretion, our results suggest that TRPA1 merits further study as a potential contributor to the respiratory symptoms evoked by TDI and other reactive hazardous irritants.
Supplementary Material
This work was supported by National Institutes of Health grant R01HL62296 and the Blaustein Pain Research Fund (T.E.T.-C.), and by GlaxoSmithKline Pharmaceuticals (F.K., M.J.C., and M.A.M.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2008-0292OC on December 4, 2008
Conflict of Interest Statement: T.E.T.-C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.K. was a full-time employee of GlaxoSmithKline (GSK) at the time this research was conducted. M.J.C. and M.A.M. are full-time employees of GSK and own shares of stock in GSK.
References
- 1.Brugsch HG, Elkins HB. Toluene di-isocyanate (TDI) toxicity. N Engl J Med 1963;268:353–357. [DOI] [PubMed] [Google Scholar]
- 2.Butcher BT, Salvaggio JE, Weill H, Ziskind MM. Toluene diisocyanate (TDI) pulmonary disease: immunologic and inhalation challenge studies. J Allergy Clin Immunol 1976;58:89–100. [DOI] [PubMed] [Google Scholar]
- 3.Sangha GK, Alarie Y. Sensory irritation by toluene diisocyanate in single and repeated exposures. Toxicol Appl Pharmacol 1979;50:533–547. [DOI] [PubMed] [Google Scholar]
- 4.Kalubi B, Takeda N, Irifune M, Ogino S, Abe Y, Hong SL, Yamano M, Matsunaga T, Tohyama M. Nasal mucosa sensitization with toluene diisocyanate (TDI) increases preprotachykinin A (PPTA) and preproCGRP mRNAs in guinea pig trigeminal ganglion neurons. Brain Res 1992;576:287–296. [DOI] [PubMed] [Google Scholar]
- 5.Mapp CE, Graf PD, Boniotti A, Nadel JA. Toluene diisocyanate contracts guinea pig bronchial smooth muscle by activating capsaicin-sensitive sensory nerves. J Pharmacol Exp Ther 1991;256:1082–1085. [PubMed] [Google Scholar]
- 6.Mapp CE, Boniotti A, Graf PD, Chitano P, Fabbri LM, Nadel JA. Bronchial smooth muscle responses evoked by toluene diisocyanate are inhibited by ruthenium red and by indomethacin. Eur J Pharmacol 1991;200:73–76. [DOI] [PubMed] [Google Scholar]
- 7.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003;112:819–829. [DOI] [PubMed] [Google Scholar]
- 8.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004;427:260–265. [DOI] [PubMed] [Google Scholar]
- 9.Taylor-Clark T, Undem BJ. Transduction mechanisms in airway sensory nerves. J Appl Physiol 2006;101:950–959. [DOI] [PubMed] [Google Scholar]
- 10.Kollarik M, Undem BJ. Sensory transduction in cough-associated nerves. Respir Physiol Neurobiol 2006;152:243–254. [DOI] [PubMed] [Google Scholar]
- 11.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 2006;103:19564–19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007;445:541–545. [DOI] [PubMed] [Google Scholar]
- 13.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004;41:849–857. [DOI] [PubMed] [Google Scholar]
- 14.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A 2007;104:13519–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 2007;104:13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci 2007;27:11412–11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bang S, Kim KY, Yoo S, Kim YG, Hwang SW. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur J Neurosci 2007;26:2516–2523. [DOI] [PubMed] [Google Scholar]
- 18.Taylor-Clark TE, Undem BJ, Macglashan DW Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol 2008;73:274–281. [DOI] [PubMed] [Google Scholar]
- 19.Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol 2008;586:3447–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 2008;586:1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 2008;118:1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacGlashan D Jr. Single-cell analysis of Ca++ changes in human lung mast cells: graded vs. all-or-nothing elevations after IgE-mediated stimulation. J Cell Biol 1989;109:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985;260:3440–3450. [PubMed] [Google Scholar]
- 24.Alarie Y. Sensory irritation of the upper airways by airborne chemicals. Toxicol Appl Pharmacol 1973;24:279–297. [DOI] [PubMed] [Google Scholar]
- 25.Matta J, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP. General anesthetics activate a noiciceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci USA 2008;105:8784–8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun A, Lommatzsch M, Neuhaus-Steinmetz U, Quarcoo D, Glaab T, McGregor GP, Fischer A, Renz H. Brain-derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br J Pharmacol 2004;141:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006;124:1269–1282. [DOI] [PubMed] [Google Scholar]
- 28.Chang JC, Steinhagen WH, Barrow CS. Effect of single or repeated formaldehyde exposure on minute volume of B6C3F1 mice and F-344 rats. Toxicol Appl Pharmacol 1981;61:451–459. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen GD, Hougaard KS, Larsen ST, Hammer M, Wolkoff P, Clausen PA, Wilkins CK, Alarie Y. Acute airway effects of formaldehyde and ozone in BALB/c mice. Hum Exp Toxicol 1999;18:400–409. [DOI] [PubMed] [Google Scholar]
- 30.Leikauf GD. Hazardous air pollutants and asthma. Environ Health Perspect 2002;110:505–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thuerauf N, Kaegler M, Dietz R, Barocka A, Kobal G. Dose-dependent stereoselective activation of the trigeminal sensory system by nicotine in man. Psychopharmacology (Berl) 1999;142:236–243. [DOI] [PubMed] [Google Scholar]
- 32.Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, et al. Cigarette smoke–induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 2008;118:2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheerens H, Buckley TL, Muis TL, Garssen J, Dormans J, Nijkamp FP, Van Loveren H. Long-term topical exposure to toluene diisocyanate in mice leads to antibody production and in vivo airway hyperresponsiveness three hours after intranasal challenge. Am J Respir Crit Care Med 1999;159:1074–1080. [DOI] [PubMed] [Google Scholar]
- 34.Hunter DD, Satterfield BE, Huang J, Fedan JS, Dey RD. Toluene diisocyanate enhances substance P in sensory neurons innervating the nasal mucosa. Am J Respir Crit Care Med 2000;161:543–549. [DOI] [PubMed] [Google Scholar]
- 35.Wilfong ER, Dey RD. Nerve growth factor and substance P regulation in nasal sensory neurons after toluene diisocyanate exposure. Am J Respir Cell Mol Biol 2004;30:793–800. [DOI] [PubMed] [Google Scholar]
- 36.Matheson JM, Johnson VJ, Vallyathan V, Luster MI. Exposure and immunological determinants in a murine model for toluene diisocyanate (TDI) asthma. Toxicol Sci 2005;84:88–98. [DOI] [PubMed] [Google Scholar]
- 37.Johnson VJ, Yucesoy B, Reynolds JS, Fluharty K, Wang W, Richardson D, Luster MI. Inhalation of toluene diisocyanate vapor induces allergic rhinitis in mice. J Immunol 2007;179:1864–1871. [DOI] [PubMed] [Google Scholar]
- 38.Abe Y, Takeda N, Irifune M, Ogino S, Kalubi B, Imamura I, Fukui H, Wada H, Matsunaga T. Effects of capsaicin desensitization on nasal allergy-like symptoms and histamine release in the nose induced by toluene diisocyanate in guinea pigs. Acta Otolaryngol 1992;112:703–709. [DOI] [PubMed] [Google Scholar]
- 39.Lee BP, Morton RF, Lee LY. Acute effects of acrolein on breathing: role of vagal bronchopulmonary afferents. J Appl Physiol 1992;72:1050–1056. [DOI] [PubMed] [Google Scholar]
- 40.Morris JB, Stanek J, Gianutsos G. Sensory nerve–mediated immediate nasal responses to inspired acrolein. J Appl Physiol 1999;87:1877–1886. [DOI] [PubMed] [Google Scholar]
- 41.Symanowicz PT, Gianutsos G, Morris JB. Lack of role for the vanilloid receptor in response to several inspired irritant air pollutants in the C57Bl/6J mouse. Neurosci Lett 2004;362:150–153. [DOI] [PubMed] [Google Scholar]
- 42.Weyel DA, Rodney BS, Alarie Y. Sensory irritation, pulmonary irritation, and acute lethality of a polymeric isocyanate and sensory irritation of 2,6-toleune diisocyanate. Toxicol Appl Pharmacol 1982;64:423–430. [DOI] [PubMed] [Google Scholar]
- 43.Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest 2007;117:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain 2008;131:1241–1251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.