Abstract
Background
Hypoendemic malaria transmission in western Kenya highlands is not expected to lead to rapid acquisition of immunity to malaria. However, the asymptomatic subpopulation may play a significant role as an infection reservoir that should be considered in malaria control programs. Determination of spatio-temporal dynamics of asymptomatic subpopulations provides an opportunity to estimate the epidemiological importance of this group to malaria transmission.
Methods
Monthly parasitological surveys were undertaken on a cohort of 246 children for 12 months. Plasmodium falciparum infection prevalence was analyzed by both microscopy and PCR, and infection durations were determined.
Results
Infection prevalence and duration (1–12 months) decreased with age and altitude. Prevalence among age groups 5–9 and 10–14 years was high (34.4% and 34.1%, respectively), but significantly lower in older children (9.1%). Prevalence decreased from (52.4%) at ~1,430 m to 23.3% at 1,580 m.
Conclusions
Prevalence of asymptomatic P. falciparum infections was high, with PCR detecting a significantly higher number of infections than microscopy. Our results are consistent with gradual acquisition of immunity with age upon repeated infection, and also show that malaria transmission risk is highly heterogeneous in the highland area. The results provide strong support for targeted control.
Keywords: Plasmodium falciparum, prevalence, cohort, asymptomatic, highland malaria
INTRODUCTION
Plasmodium falciparum affects about half a billion people annually, with disease manifestations ranging from asymptomatic to severe clinical illness. Africa suffers the largest share (~90%) of malaria-attributable deaths [1]. In the last two decades, malaria epidemics have increased in frequency and intensity in the East African highlands in populations that have little or no previous exposure to plasmodial infections [2]. Whereas the prevalence of symptomatic and asymptomatic infections in the endemic lowlands is well documented, there is a paucity of data from the highlands. Many asymptomatic infections go undetected and untreated while causing little or no clinical manifestation. The extent of asymptomatic parasitemia is inversely related to a population’s susceptibility to clinical disease [3, 4], but more importantly, asymptomatic people (asymptomatics) are major reservoirs of infection [5, 6]. Parasites from asymptomatics are more infectious to mosquitoes than those from their ailing counterparts [7]. Furthermore, field malaria surveys using microscopy may fail to detect very low parasitemias that are common in asymptomatics. The advent of polymerase chain reaction (PCR) [8] has improved the detection of asymptomatic parasitemias, leading to better estimation of their potential for malaria transmission in human populations [6].
In the highlands, small differences in altitude may lead to contrasting suitability and availability of vector breeding habitats and consequently divergent risks of malaria transmission and prevalence [9, 10]. In Tanzania, for example, altitude alone accurately predicted whether a resident had splenomegaly in 73% of households [11]. The success of malaria control in the complex highland eco-epidemiological systems will depend on a systematic understanding of the micro-geographic risk of malaria transmission that would enable identification of high risk spots. The identification, quantification, and spatio-temporal mapping of the asymptomatic subpopulation in the highlands of western Kenya provides an opportunity to estimate the epidemiological importance of this group to malaria transmission [12]. The present study sought to determine the prevalence and dynamics of asymptomatic Plasmodium falciparum infections among children living at different altitudes and distances from major breeding habitats in a highland area with unstable transmission in western Kenya.
MATERIALS AND METHODS
Study area, subjects and protocol
The study was carried out in three neighboring villages: Iguhu, Makhokho and Sigalagala, in Kakamega District in western Kenya. Iguhu is at the valley bottom (~1,430 m), Makhokho lies mid-hill (~1,500 m), while Sigalagala is at the hilltop, ~1,580 m above sea level (figure 1). The distance from the valley bottom to the hilltop is ~4.5 Km. Other details of the study area have previously been reported [13, 14]. Rainfall data for the study area was obtained from Kakamega Weather Station. Monthly parasitological surveys (including data on age, sex, bed net use) were undertaken on 246 school children aged 5–17 years from the three villages from January to December 2006 after obtaining informed assent from the participants and informed consent from parents/guardians. Bed net usage was recorded through questionnaire. The number of children in each cohort was 84 from the valley bottom, 81 from mid-hill and 81 from the hilltop. Table 1 shows the demographic profile of the three cohorts. Inclusion criteria were that primary school children are randomly selected, live in the catchment area, and are willing to participate in the study, regardless of their sex and economic status. Children who intended to relocate during the study period or were unwilling to participate in the study were excluded. The study was approved by Kenya Medical Research Institute Ethical Review Committee and the Institutional Review Board of the University of California, Irvine.
Figure 1.
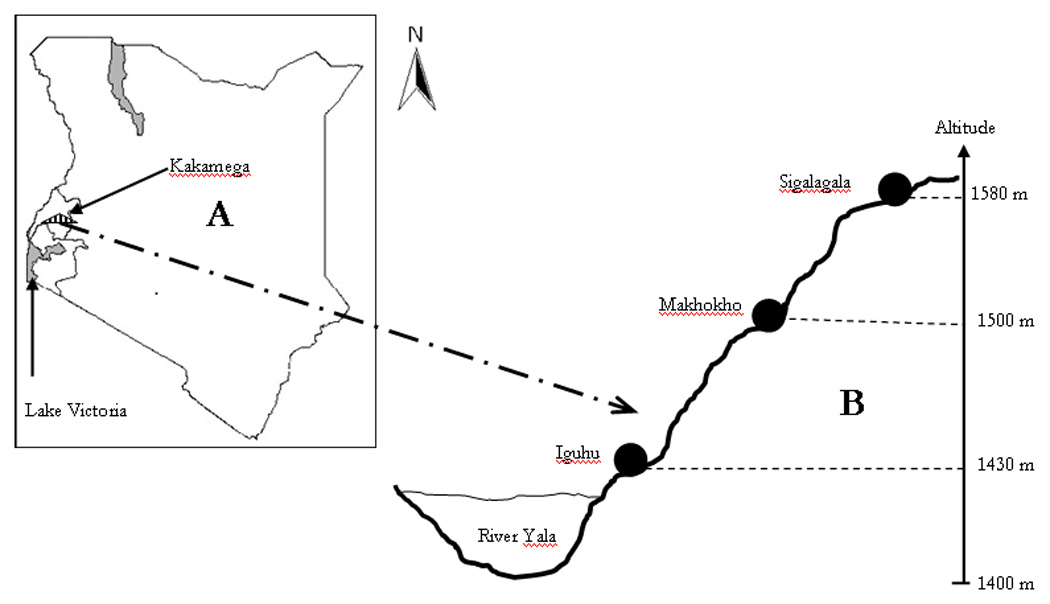
A map of Kenya (A) showing sampling sites (B). Not to scale. River Yala is a major malaria vector breeding site.
Table 1.
Demographic profiles of the cohort study populations. Numbers of children in each category are given, with parentheses containing the corresponding percentages per locality and proportions per category across all sites, respectively. The median age was 10 years.
| Category | Iguhu (n = 84) | Makhokho (n = 81) | Sigalagala (n = 81) | All sites (n = 246) |
|---|---|---|---|---|
| (Valley bottom) | (Mid-hill) | (Hilltop) | ||
| Elevation: 1430 m | Elevation: 1500 m | Elevation: 1580 m | ||
| Elevation: 1430 – 1580 m | ||||
| 5 – 9 years | 31 (37; 0.35) | 40 (49; 0.56) | 17 (21; 0.19) | 88 |
| 10 – 14 years | 52 (62; 0.34) | 41 (51; 0.27) | 60 (74; 0.39) | 153 |
| 15 – 17 years | 1 (1; 0.20) | 0 (0; 0) | 4 (5; 0.80) | 5 |
| Male | 33 (39; 0.35) | 25 (31; 0.26) | 37 (54; 0.39) | 95 |
| Female | 51 (61; 0.34) | 56 (69; 0.37) | 44 (46; 0.29) | 151 |
n = total number of children
Briefly, blood samples were obtained by finger-pricking method. Two to three drops of blood were used to prepare thick and thin smears for microscopy and ~200µl of blood spotted on filter paper (Whatman Corporation, Florham Park, NJ) for PCR. After air-drying, thick smears were dipped twice in acetone and thin smears were fixed in methanol, then stained in 4% Giemsa for 30–45 min. Slides were examined at ×1000 by experienced microscopists. Readings were verified by a second reader, with discrepancies resolved by a third reader. The entire slide was carefully scanned before being declared negative. Parasites were counted against 200 leukocytes, and densities estimated assuming a standard leukocyte count of 8,000/µl, as previously reported [13]. Parasite DNA was extracted from filter papers by the Saponin/Chelex method [15]. While blinded to microscopic results, infections were also diagnosed by nested PCR amplification of the species-specific small-subunit ribosomal RNA (ssrRNA) gene using primers and conditions previously reported [16]. DNA from P. falciparum strain 3D7 (MR4, Manassas, VA, USA) and sterile water (Mediatech, Inc., Herndon, VA, USA) were respectively used as positive and negative controls for PCR. Amplicons were resolved in ethidium bromide-stained 2% agarose and visualized by ultraviolet translumination.
Microscopic results were immediately reported to the study subjects. However, since the guidelines of Kenyan Ministry of Health did not advise treatment of asymptomatics, individuals who had infections were advised to seek anti-malarial treatment at the nearby Iguhu Health Center if they subsequently developed any clinical signs of malaria, such as fever, chills, severe malaise, headache or vomiting.
Definition of P. falciparum infection episodes
A blood sample was considered infected when parasitemia was detected either by microscopy or PCR [17, 18] during the monthly surveys. One or more consecutive positive samples defined a single episode of infection, while a negative intervening sample differentiated between episodes [19]. The duration of a single episode was calculated as the summation of the number of months from the first and last consecutive positive sample [17].
Data analysis
Concordance between microscopy and PCR results was estimated using Cohen’s Kappa (K). Parasite densities and mean number of infections by village and season were compared by ANOVA, while the overall proportions of infections diagnosed by each test method were compared by Fisher’s exact test. Parasite densities for samples positive only by PCR were designated as <40 parasites/µl because microscopic examination would not detect a positive infection below this threshold density [17]. Densities were log-transformed and comparisons done using Tukey's HSD test with repeated measures. Alternating logistic regression [20] was used to the effects of living locality, bed net usage, age and gender on infections. Analysis of the duration episodes was carried out using the Kaplan-Meier survival estimator. Comparisons were based on longest episode an individual experienced during the study period. Duration estimates of episodes for individuals who were positive at the start or end of the study period were censored. Data analyses were performed using SAS 9.2, JMP 5.1 (SAS Institute, Inc., Cary, NC), and GraphPad (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Comparison of microscopic and molecular (PCR) diagnosis of asymptomatic P. falciparum infections
Nineteen subjects (7.7%) were lost to follow-up over the first 11 months, with an additional 170 (69.1%) subjects lost to follow-up during the final month, which coincided with long holidays season. A total of 880 infections were detected by microscopy/PCR among 2,611 blood samples screened across the three villages during the 12 months study period. Microscopy diagnosed only 330 infections, while PCR detected 869 infections, a substantial ~3-fold increase diagnosis (P <0.0001, table 2). Despite repeated DNA extraction and re-amplification, PCR missed 3.3% (11/330) of cases detected by microscopy, while microscopy missed 63.3% (550/869) of cases detected by PCR. Compared to PCR, microscopy had a low (36.7%) sensitivity, but high (99.4%) specificity and positive predictive value (PPV, 96.7%). When microscopy was considered the “gold standard”, PCR showed high (96.7%) sensitivity and negative predictive value (NPV, 99.4%). Overall, there was moderate concordance between microscopy and PCR results (K = 0.427; 95% CI, 0.385 – 0.469). However, in some months, such as October for Makhokho and Sigalagala and December for all sites, infections were detected only by PCR.
Table 2.
Comparison of microscopy and PCR methodologies in the diagnosis of asymptomatic Plasmodium falciparum infections in school children from a highland area of western Kenya.
| Test | Pos | Neg | % Pos | M Pos, PCR Neg | M Neg, PCR Pos | M Neg, PCR Neg | Sensitivity § | Specificity§ | PPV %§ | NPV %§ |
|---|---|---|---|---|---|---|---|---|---|---|
| M | 330 | 2281 | 12.6 | - | - | - | 36.7 (33.5 – 39.9) | 99.4 (99.0 – 99.7) | 96.7 (94.7 – 98.6) | 75.9 (74.1 – 77.6) |
| PCR | 869 | 1742 | 33.3* | 11 | 550 | 1731 | 96.7 (94.7 – 98.6) | 75.9 (74.1 – 77.6) | 36.7 (33.5 – 39.9) | 99.4 (99.0 – 99.7) |
M = Microscopy; PCR = Polymerase chain reaction; Pos = Positive; Neg = Negative; PPV = Positive predictive value; NPV = Negative predictive value.
Significant difference between M and PCR results (Fisher’s two-tailed P <0.0001)
Values under the “M” row were those obtained when PCR results were considered the “true results”, while values under the “PCR” row were those obtained when microscopy results were taken for the “true results”. Corresponding figures in parentheses show the 95% confidence intervals.
Prevalence of asymptomatic P. falciparum infections by age
To assess the overall prevalence of asymptomatic P. falciparum parasitemia by age, data from the three sampling sites were pooled by age-group [19]. Prevalence was similar among ages 5–9 and 10–14 (34.4% and 34.1% by microscopy/PCR). Prevalence, on the other hand, was significantly lower in children above 14 years (9.1% by microscopy/PCR) compared to the younger children.
Number of infected samples per child and parasite densities
The number of infected blood samples per child over the entire study period ranged from 1–12. The altitude/village had a significant effect on the number of infections (F[2, 223] = 19.47, P < 0.0001), with the mean number (± SE) of infected samples per child being twice at Iguhu (5.66 ± 0.41) compared to Makhokho (2.87 ± 0.40) and Sigalagala (2.65 ± 0.33). Post hoc Bonferroni tests showed the mean number of infections to be substantially higher at Iguhu compared to each of the higher altitude villages (P < 0.0001). However, there was no significant difference between Makhokho and Sigalagala (P > 0.05).
Parasite densities declined with age, ranging from <40 to 44,600/µl, <40 to 27,840 and <40 to 360 parasites/µl of blood among children aged 5–9, 10–14 and >14 years, respectively. The geometric mean density for the 5–9 year olds (649 parasites/µl) was 1.6-folds higher than in children aged 10–14 years (400 parasites/µl). Geometric mean density for children >14 years was not calculated, as parasitemia was quantifiable in only one sample which was microscopy positive with 360 parasites/µl. Densities were significantly different among age-groups (F[1, 328] = 8.90, P = 0.003), but not among villages (F[2, 327] = 1.51, P = 0.223). Gametocyte densities were low in all cases, ranging from <40 – 160, ≤40, and <40 – 80 gametocytes/µl blood at the valley bottom, mid-hill and hilltop, respectively.
Prevalence of asymptomatic P. falciparum infections with respect to monthly rainfall patterns
Monthly prevalence showed an over-dispersed distribution of infections in all villages, with no significant correlation between asymptomatic infection prevalence and rainfall intensity (figure 2). Infections were generally prevalent throughout the year. Monthly prevalence by microscopy/PCR ranged from 36.4 – 63.0% at Iguhu, 7.8 – 38.3% at Sigalagala, and 0 – 34.6% at Makhokho (figure 2A). However, gametocyte prevalence was generally low but also decreased with altitude, ranging from 0 – 3.9% at Iguhu, 0 – 2.6% at Makhokho and 0 – 2.5% at Sigalagala, with the highest prevalence at all sites occurring during the long rains season (March – June, figure 2B).
Figure 2.
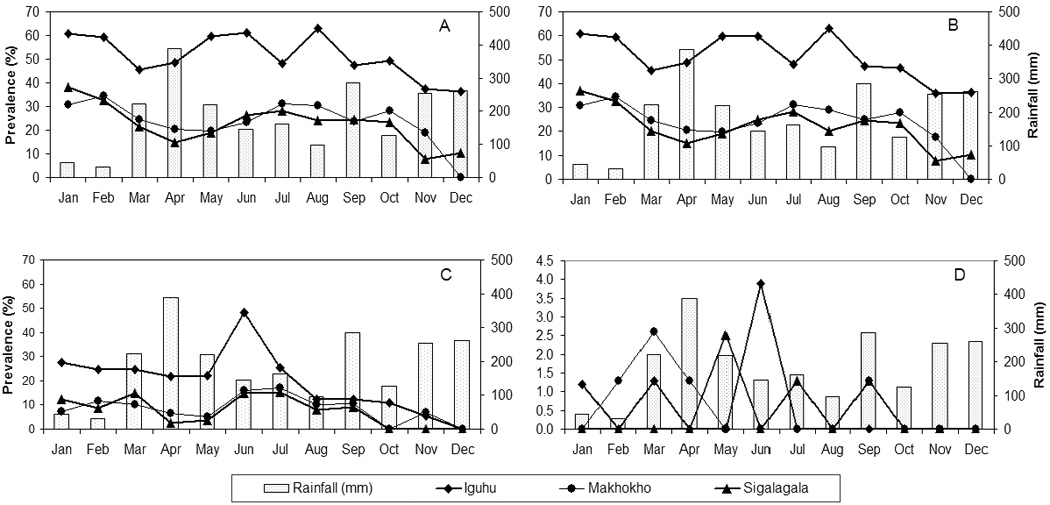
Monthly prevalence of asymptomatic Plasmodium falciparum infections among school children living in three neighboring western Kenya highland villages at different altitudes: (A) assessed by microscopy/PCR; (B) assessed by PCR alone; (C) assessed by microscopy alone; (D) gametocyte prevalence, assessed by microscopy.
Micro-geographic P. falciparum prevalence and infections risk
An inverse relationship was observed between infection prevalence and altitude (table 3). Prevalence was highest at Iguhu (1430 m), followed by Makhokho (1500 m) and Sigalagala (1580 m). Overall prevalence by microscopy/PCR was 52.4%, 25.8% and 23.3% at Iguhu, Makhokho and Sigalagala, respectively.
Table 3.
Prevalence of asymptomatic Plasmodium falciparum infections in three western Kenya highland villages at different altitudes
| Iguhu (n=863) (Valley bottom) Elevation: 1430m |
Makhokho (n=854) (mid-hill) Elevation: 1500m |
Sigalagala (n=894) (Hilltop) Elevation: 1580m |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic method | M | PCR | M/PCR | M | PCR | M/PCR | M | PCR | M/PCR |
| Positives | 181 | 448 | 452 | 78 | 219 | 220 | 71 | 202 | 208 |
| Prevalence (%) | 21.0 | 51.9 | 52.4 | 9.1 | 25.6 | 25.8 | 7.9 | 22.6 | 23.3 |
n = total number of samples tested
M = Microscopy
PCR = Polymerase chain reaction
M/PCR = Microscopy or PCR
Regression analysis (table 4) showed a significantly higher odds ratio (3.3 times) of infection at the valley bottom compared to uphill sites. Insecticide-treated bed net use was generally low (<20% in each village) with no significant difference among localities (F[2, 484] = 2.52, P = 0.08). Nevertheless, bed net users had a 1.4 times less chance of having infections compared to those without bed nets. Children aged 5–9 years had a 6-fold higher chance of having infections compared to older children. Females had significantly less chance of being infected compared to males.
Table 4.
Impact of living locality, bed net use, age and gender on asymptomatic Plasmodium falciparum infections
| Variable | Coefficient* (± Standard error) |
Odds ratio (95% confidence intervals) |
|---|---|---|
| Living at the valley bottom | 1.190 ± 0.090 | 3.292 (2.761 – 3.926) |
| Living at mid-hill | 1.140 (0.908 – 1.430) | |
| Living at hilltop | 0.877 (0.699 – 1.101) | |
| Bed net usage | − 0.328 ± 0.130 | 0.728 (0.567 – 0.935) |
| Age group 1 (5–9 years) | 1.839 ± 0.486 | 5.985 (2.296 – 15.598) |
| Age group 2 (10–14 years) | 1.792 ± 0.483 | 5.842 (2.261 – 15.096) |
| Age group 3 (>14 years) | 0.164 (0.064 – 0.422) | |
| Female | − 0.437 ± 0.089 | 0.646 (0.542 – 0.769) |
| Male | 1.566 (1.313 – 1.867) |
Coefficients of logistic regression and Wald confidence intervals for adjusted odds ratios are shown. Only coefficients with significance at α = 0.05 are reported.
Frequency of recurrent asymptomatic parasitemia and infection episode dynamics
Overall, 334 asymptomatic infection episodes occurred among children from the three villages. The majority (44.0%) of the episodes occurred at Iguhu, which had almost twice as many episodes as Makhokho (24.9%) or Sigalagala (31.1%). A high proportion of children had episodes lasting more than one month (15.0% for 1 month, 38.2% for 2–5 months, 14.2% for 6–12 months, while 32.5% experienced no infection episode at all). The longest episode (12 months) occurred at the valley bottom.
Estimates of the duration of the longest episode of infection in each age group and locality are shown as Kaplan-Meier plots (figure 3). All episodes in children >14 years lasted only one month. A trend of decreasing episode duration with age and elevation is evident. The duration of infection was higher, albeit trivially, among children aged 5–9 compared to those 10–14 years of age (median 5 and 4 months respectively, χ2 = 2.1, df = 1, P = 0.15; figure 3A). Episode durations were significantly different between micro-geographic locales, with children living at the valley bottom having median durations of 6 months compared to 4 months at mid-hill and 3 months at the hilltop (χ2 = 8.6, df = 2, P = 0.01; figure 3B). Significant differences occurred between Iguhu and Makhokho (χ2 = 5.6, df=1, P = 0.02) or Sigalagala (χ2 = 7.4, df = 1, P = 0.01), but not between Makhokho and Sigalagala (χ2 = 0.05, df=1, P = 0.83). Males exhibited marginally longer infection durations than females (median 5 vs. 4 months, χ2 = 3.4, df = 1, P = 0.07).
Figure 3.
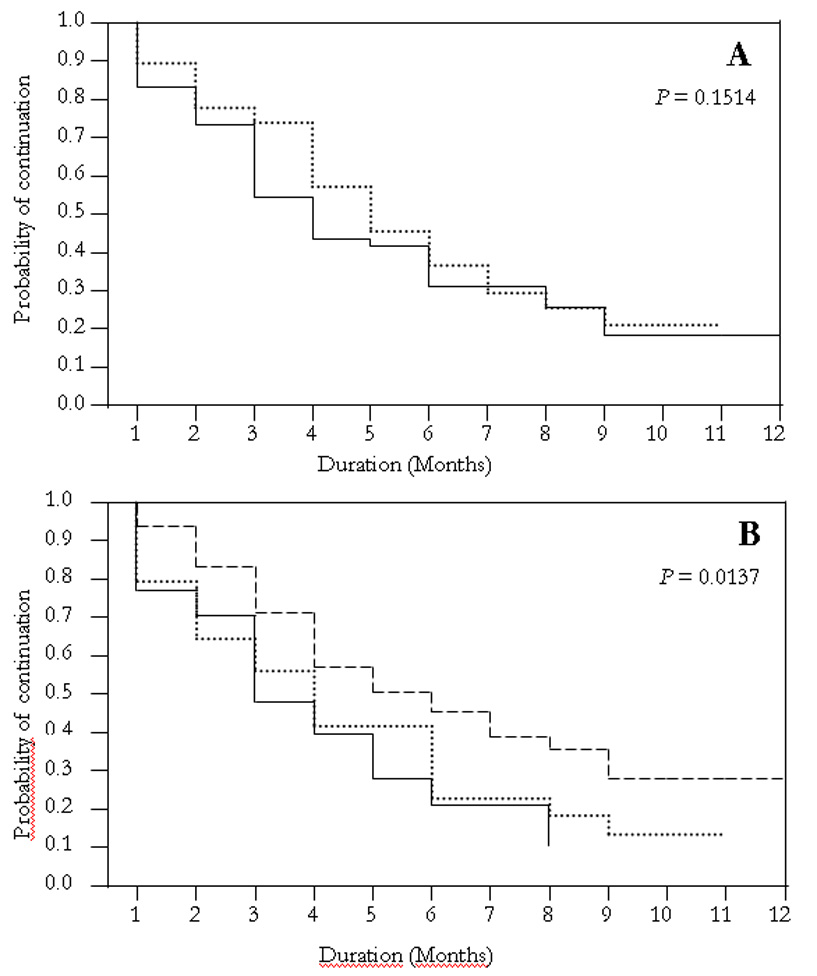
Kaplan-Meir survival plots of duration of episodes of asymptomatic Plasmodium falciparum infection by: (A) age. 5–9 years (dotted line); 10–14 years (solid line). All episodes for ages >14 lasted only one month and were excluded from survival analysis. (B) Altitude/location. Iguhu (~1430 m, broken line), Makhokho (~1500 m, dotted line) and Sigalagala (~1580 m, solid line).
DISCUSSION
The present altitudinal cohort study is one of the few focusing on asymptomatic highland malaria in Africa [5, 21]. The study documents high levels of asymptomatic P. falciparum infections with micro-geographic differences in prevalence within <5 Km radius in an epidemic-prone area in western Kenya. Our findings contrast with studies in Kipsamoite, another highland area in western Kenya, where very low levels of asymptomatic P. falciparum infection (7.9% and 14.5% by microscopy and PCR respectively at peak season) were observed [21]. This suggests that the risk of malaria transmission is highly heterogeneous in the Kenyan highlands, implying that results from one highland area may not be readily extrapolated to other areas.
Estimates of malaria prevalence have primarily been based on microscopy because most cases are detected in healthcare facilities where microscopy remains the standard diagnostic method [22]. In our study, PCR consistently detected a significantly higher number of asymptomatic P. falciparum infections than microscopy. A number of other studies involving asymptomatics have documented similar findings [3, 17, 21, 23], suggesting that many studies and health statistics may have grossly underestimated the true prevalence of malaria infection in various areas. The high specificity (99.4%) and PPV (96.7%) of microscopy compared to PCR attests to the quality of our readings. We cannot absolutely rule out the possibility of increased PCR-sensitivity by detection of dead parasite forms. However, DNA fragments are briskly eliminated from the blood stream, making PCR less likely to detect non-viable parasites [24]. The high prevalence of asymptomatic parasitemia observed here indicates a change to a higher level of malaria endemicity than what would be expected for an area defined as epidemic-prone with unstable, low transmission [25]. New definitions of transmission stability based on PCR are now required. Perhaps the significant drop of parasitemias to sub-microscopic levels may also be indicative of the parasite’s biology and transmission strategies especially through the dry season [18] but this needs further investigation.
The consistently higher levels of infection (36 – 63%) and increased episode durations at the valley bottom (irrespective of transmission season) could be explained by closer proximity to breeding sites, making residents more prone to infection and re-infection [26, 27]. In particular, Iguhu is transected by river Yala, whose environment provides thriving, year-round breeding grounds for malaria vectors [28]. Previous studies found the majority (98%) of malaria vectors at the valley bottom compared to 1% at midhill and the hilltop, with respective entomological inoculation rates (EIRs) of 12.8, 0.05 and 0.04 infective bites per person per year [9], suggesting a significantly higher risk of exposure to infected mosquitoes at the valley bottom compared to uphill sites. The valley bottom is thus a “malaria transmission hotspot” [10]. That highland valleys are usually the springboard of malaria infection has also been demonstrated in Tanzania’s Usambara mountains [11] and in Burundi [26].
A small proportion of children (14.2%) had long periods of persistent infection, lasting a straight 6–12 months, while others (38.2%) had episodes lasting 2–5 months. This is of particular concern, considering that the longer the duration of asymptomatic parasitemia, the larger the contribution to transmission [29]. It is not uncommon for residents of endemic areas to harbor malaria parasites for months without developing clinical symptoms. In Ghana, for example, studies found asymptomatic infections that lasted up to 16 months [17], while in Sudan, infections persisted for over 12 months, with no evidence of re-infection [18]. The observed variation in episode duration, both within and among individuals, with notable reduction in duration with age across all sites is likely due to gradual acquisition of immunity with exposure to infections [3, 30]. The decline in parasite density with increasing age is consistent with studies carried out elsewhere, and is typical of infections in malaria-endemic areas [17, 19, 31]. This is because immune responses suppress parasitemia, or even clinical disease, in proportion to immune levels, which are a function of both age and level of exposure to P. falciparum infections [3, 30].
The observed significantly lower infection rates in females compared to males is consistent with other studies (reviewed in [32]). While the cause of these sex differences merits further investigation, including the possibility that males produce more attractive chemicals for mosquitoes, the production of estrogens by females has been shown to augment [32], whereas testosterone suppresses anti-plasmodial immune responses [33].
All children were apparently healthy at the time of sampling, with only 6.7% of the infections having parasite densities above 2500/µl. Since fever was not measured, we cannot rule out the possibility of its association with some of the higher parasitemias observed. However, studies of asymptomatics in Tanzania have found no significant association between parasite density and the risk subsequent clinical malaria [34]. Still, a follow-up incidence study will be necessary in this highland area.
The present study may have overestimated some episode durations for not differentiating between recrudescent and re-infecting strains. Microsatellite and Msp-2 genotype analysis from a few (33) individuals showed single-genotype persistence for up to 6 months (F. N. Baliraine, unpublished data). Nevertheless, the fact that infections were asymptomatic for months remains of epidemiological significance. Moreover, unlike numerous epidemiological studies that have based solely on microscopy, our analysis complemented microscopy with PCR, which helped detect 63.0% (550/880) infections that would have been missed using microscopy alone.
A number of issues are thus raised. First, while microscopy remains the standard method for diagnosis of clinical malaria [22], our results show the technique to have a substantially low sensitivity in detecting sub-clinical parasitemia. This raises serious doubts about its effectiveness as the sole screening test not only for P. falciparum epidemiological studies especially in places such as our study area, but also in drug efficacy studies and malaria vaccine trials that are very sensitive to minor errors in diagnosis. The high number of false negatives by microscopy and failure of PCR to detect some (albeit few) cases detected by microscopy in the present study supports the view that only the use of both methods can assure high certainty of test results [35]. Nevertheless, while useful for epidemiological purposes, great caution must be exercised using PCR results alone in diagnosing clinical disease, especially in endemic settings where parasitemia is not necessarily associated with clinical malaria [17, 36]. The clinical implications of PCR positives remain an open question that needs further investigation. Although some very low parasitemias may be associated with clinical disease [37], they may reduce the risk of subsequent clinical malaria [3], as well as cause misdiagnosis of conditions such as HIV or bacterial infections that produce cerebral malaria-like symptoms, resulting in poor outcomes [36].
Second, we found that the majority of potential P. faciparum reservoirs are at the valley bottom, where vector breeding sites happen to be concentrated [14, 28]. This allows us to recommend that in the face of limited resources, malaria could be substantially controlled in this highland area if efforts prioritized the valley bottom rather than over-stretch limited budgets to equally spread across all sites. Targeted control has been successfully used against malaria in the highlands of Burundi [25, 26, 38].
Presently, there are renewed calls for malaria eradication [1, 29]. The need to eradicate malaria has been high on the human agenda for centuries, with unrelenting attempts made over the years to achieve this objective [30]. One loophole in the attack strategies however, has been the omission of the human reservoir of the parasites. Since mosquitoes can pick up even submicroscopic parasites [6], targeted therapy even for asymptomatic carriers may be useful as part of an integrated malaria control or eradication program. A combination of mass prophylactic anti-malarial treatment with in-house DDT sprays nearly eliminated malaria in one highland area of Kenya in the late 1940s [2]. Even when asymptomatic, malaria remains a serious problem, as parasites can impair children’s brain development and academic prowess [27, 39], rework the immune system and the body’s response to vaccines, and make the victim more vulnerable to other infections [40]. Our results therefore have implications for Kenya’s malaria control program, which focuses on symptomatic malaria patients [41].
Acknowledgements
We are indebted to the study participants for their time and involvement in the study, and Laith Yakob and Rita Petersen for helpful discussions. We thank two anonymous reviewers whose insightful comments and suggestions helped improve the manuscript. This work is published with the permission of the Director of the Kenya Medical Research Institute.
Financial support: This study was made feasible by grants R01 AI050243, D43 TW001505, and R03 TW007360 from the National Institutes of Health of the USA.
Footnotes
Conflict of Interest: None of the authors has a commercial or other association that might pose a conflict of interest in this work.
References
- 1.Okie S. A new attack on malaria. N Engl J Med. 2008;358:2425–2428. doi: 10.1056/NEJMp0803483. [DOI] [PubMed] [Google Scholar]
- 2.Shanks GD, Hay SI, Omumbo JA, Snow RW. Malaria in Kenya's western highlands. Emerg Infect Dis. 2005;11:1425–1432. doi: 10.3201/eid1109.041131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bereczky S, Liljander A, Rooth I, et al. Multiclonal asymptomatic Plasmodium falciparum infections predict a reduced risk of malaria disease in a Tanzanian population. Microbes Infect. 2007;9:103–110. doi: 10.1016/j.micinf.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Mwangi TW, Fegan G, Williams TN, Kinyanjui SM, Snow RW, Marsh K. Evidence for over-dispersion in the distribution of clinical malaria episodes in children. PLoS ONE. 2008;3:e2196. doi: 10.1371/journal.pone.0002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousema JT, Gouagna LC, Drakeley CJ, et al. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar J. 2004;3:18. doi: 10.1186/1475-2875-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42:777–779. doi: 10.1093/jmedent/42.5.777. [DOI] [PubMed] [Google Scholar]
- 7.Gouagna LC, Okech BA, Kabiru EW, et al. Infectivity of Plasmodium falciparum gametocytes in patients attending rural health centres in western Kenya. East Afr Med J. 2003;80:627–634. doi: 10.4314/eamj.v80i12.8779. [DOI] [PubMed] [Google Scholar]
- 8.Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Githeko AK, Ayisi JM, Odada PK, et al. opography and malaria transmission heterogeneity in western Kenya highlands: prospects for focal vector control. Malar J. 2006;5:107. doi: 10.1186/1475-2875-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC. Malaria hotspot areas in highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar J. 2006;5:78. doi: 10.1186/1475-2875-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balls MJ, Bodker R, Thomas CJ, Kisinza W, Msangeni HA, Lindsay SW. Effect of topography on the risk of malaria infection in the Usambara Mountains, Tanzania. Trans R Soc Trop Med Hyg. 2004;98:400–408. doi: 10.1016/j.trstmh.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Sama W, Killeen G, Smith T. Estimating the duration of Plasmodium falciparum infection from trials of indoor residual spraying. Am J Trop Med Hyg. 2004;70:625–634. [PubMed] [Google Scholar]
- 13.Munyekenye OG, Githeko AK, Zhou G, Mushinzimana E, Minakawa N, Yan G. Plasmodium falciparum spatial analysis, western Kenya highlands. Emerg Infect Dis. 2005;11:1571–1577. doi: 10.3201/eid1110.050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou G, Munga S, Minakawa N, Githeko AK, Yan G. Spatial relationship between malaria vector abundance and environmental factors in western Kenya highlands. Am J Trop Med Hyg. 2007;77:29–35. [PubMed] [Google Scholar]
- 15.Wooden J, Kyes S, Silbley C. PCR and strain identification in Plasmodium falciparum. Parasitology Today. 1993;9:303–305. doi: 10.1016/0169-4758(93)90131-x. [DOI] [PubMed] [Google Scholar]
- 16.Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg. 1999;60:687–692. doi: 10.4269/ajtmh.1999.60.687. [DOI] [PubMed] [Google Scholar]
- 17.Franks S, Koram KA, Wagner GE, et al. Frequent and persistent, asymptomatic Plasmodium falciparum infections in African infants, characterized by multilocus genotyping. J Infect Dis. 2001;183:796–804. doi: 10.1086/318834. [DOI] [PubMed] [Google Scholar]
- 18.Nassir E, Abdel-Muhsin AM, Suliaman S, et al. Impact of genetic complexity on longevity and gametocytogenesis of Plasmodium falciparum during the dry and transmission-free season of eastern Sudan. Int J Parasitol. 2005;35:49–55. doi: 10.1016/j.ijpara.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Bruce MC, Donnelly CA, Packer M, et al. Age- and species-specific duration of infection in asymptomatic malaria infections in Papua New Guinea. Parasitology. 2000;121(Pt 3):247–256. doi: 10.1017/s0031182099006344. [DOI] [PubMed] [Google Scholar]
- 20.Carey V, Zeger SL, Diggle P. Modelling multivariate binary data with alternating logistic regressions. Biometrika. 1993;80:517–526. [Google Scholar]
- 21.John CC, McHugh MM, Moormann AM, Sumba PO, Ofulla AV. Low prevalence of Plasmodium falciparum infection among asymptomatic individuals in a highland area of Kenya. Trans R Soc Trop Med Hyg. 2005;99:780–786. doi: 10.1016/j.trstmh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Ohrt C, Obare P, Nanakorn A, et al. Establishing a malaria diagnostics centre of excellence in Kisumu, Kenya. Malar J. 2007;6:79. doi: 10.1186/1475-2875-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dal-Bianco MP, Koster KB, Kombila UD, et al. High prevalence of asymptomatic Plasmodium falciparum infection in Gabonese adults. Am J Trop Med Hyg. 2007;77:939–942. [PubMed] [Google Scholar]
- 24.Jarra W, Snounou G. Only viable parasites are detected by PCR following clearance of rodent malarial infections by drug treatment or immune responses. Infect Immun. 1998;66:3783–3787. doi: 10.1128/iai.66.8.3783-3787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Protopopoff N, Van Bortel W, Marcotty T, et al. Spatial targeted vector control is able to reduce malaria prevalence in the highlands of Burundi. Am J Trop Med Hyg. 2008;79:12–18. [PubMed] [Google Scholar]
- 26.Protopopoff N, Van Bortel W, Marcotty T, et al. Spatial targeted vector control in the highlands of Burundi and its impact on malaria transmission. Malar J. 2007;6:158. doi: 10.1186/1475-2875-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bin Mohanna MA, Bin Ghouth AS, Rajaa YA. Malaria signs and infection rate among asymptomatic schoolchildren in Hajr Valley, Yemen. East Mediterr Health J. 2007;13:35–40. [PubMed] [Google Scholar]
- 28.Minakawa N, Sonye G, Mogi M, Yan G. Habitat characteristics of Anopheles gambiae s.s. larvae in a Kenyan highland. Med Vet Entomol. 2004;18:301–305. doi: 10.1111/j.0269-283X.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 29.Aguas R, White LJ, Snow RW, Gomes MG. Prospects for malaria eradication in sub-Saharan Africa. PLoS ONE. 2008;3:e1767. doi: 10.1371/journal.pone.0001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Despommier DD, Gwadz RW, Hotez PJ, Knirsch CA. Parasitic Diseases. 5 ed. New York: Apple Trees Productions, LLC; 2005. [Google Scholar]
- 31.Quakyi IA, Leke RG, Befidi-Mengue R, et al. The epidemiology of Plasmodium falciparum malaria in two Cameroonian villages: Simbok and Etoa. Am J Trop Med Hyg. 2000;63:222–230. [PubMed] [Google Scholar]
- 32.Cernetich A, Garver LS, Jedlicka AE, et al. Involvement of gonadal steroids and gamma interferon in sex differences in response to blood-stage malaria infection. Infect Immun. 2006;74:3190–3203. doi: 10.1128/IAI.00008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krucken J, Dkhil MA, Braun JV, et al. Testosterone suppresses protective responses of the liver to blood-stage malaria. Infect Immun. 2005;73:436–443. doi: 10.1128/IAI.73.1.436-443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bereczky S, Montgomery SM, Troye-Blomberg M, Rooth I, Shaw MA, Farnert A. Elevated anti-malarial IgE in asymptomatic individuals is associated with reduced risk for subsequent clinical malaria. Int J Parasitol. 2004;34:935–942. doi: 10.1016/j.ijpara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Rodulfo H, De Donato M, Mora R, Gonzalez L, Contreras CE. Comparison of the diagnosis of malaria by microscopy, immunochromatography and PCR in endemic areas of Venezuela. Braz J Med Biol Res. 2007;40:535–543. doi: 10.1590/s0100-879x2007000400012. [DOI] [PubMed] [Google Scholar]
- 36.Okubadejo NU, Danesi MA. Diagnostic issues in cerebral malaria: a study of 112 adolescents and adults in Lagos, Nigeria. Niger Postgrad Med J. 2004;11:10–14. [PubMed] [Google Scholar]
- 37.Menge DM, Ernst KC, Vulule JM, Zimmerman PA, Guo H, John CC. Microscopy underestimates the frequency of plasmodium falciparum in symptomatic individuals in transmission highland area. Am J Trop Med Hyg. 2008;79:173–177. [PMC free article] [PubMed] [Google Scholar]
- 38.Beier JC. Malaria control in the highlands of Burundi: an important success story. Am J Trop Med Hyg. 2008;79:1–2. [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke SE, Jukes MCH, Njagi KJ, et al. Effect of intermittent preventive treatment malaria on health and education in school children: a cluster-randomised, double-blind, placebo-controlled trial. The Lancet. 2008;372:127–138. doi: 10.1016/S0140-6736(08)61034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 41.Amin AA, Zurovac D, Kangwana BB, et al. The challenges of changing national malaria drug policy to artemisinin-based combinations in Kenya. Malar J. 2007:1–11. doi: 10.1186/1475-2875-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]


