Abstract
Oxytocin (Oxt) is a nonapeptide hormone best known for its role in lactation and parturition. Since 1906 when its uterine-contracting properties were described until 50 years later when its sequence was elucidated, research focused on its peripheral roles in reproduction. Only over the past several decades have researchers focused on what functions Oxt might have in the brain, the subject of this review.
Immunohistochemical studies revealed that magnocellular neurons of the hypothalamic paraventricular and supraoptic nuclei are the neurons of origin for the Oxt released from the posterior pituitary. Smaller cells in various parts of the brain, as well as release from magnocellular dendrites, provide the Oxt responsible for modulating various behaviors at its only identified receptor.
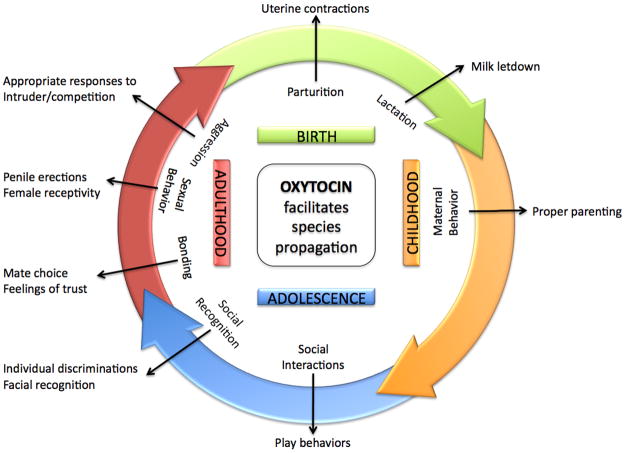
Although Oxt is implicated in a variety of “non-social” behaviors, such as learning, anxiety, feeding and pain perception, it is Oxt’s roles in various social behaviors that have come to the fore recently. Oxt is important for social memory and attachment, sexual and maternal behavior, and aggression. Recent work implicates Oxt in human bonding and trust as well. Human disorders characterized by aberrant social interactions, such as autism and schizophrenia, may also involve Oxt expression. Many, if not most, of Oxt’s functions, from social interactions (affiliation, aggression) and sexual behavior to eventual parturition, lactation and maternal behavior, may be viewed as specifically facilitating
1. Introduction
Oxytocin (Oxt) is a nonapeptide hormone best known for its role in lactation and parturition. The word “oxytocin” was coined from the Greek words (ω k ν ξ, τ o k ox ξ) meaning “quick birth” after its uterine-contracting properties were discovered by Dale (Dale, 1906). Shortly thereafter, the milk ejection property of Oxt was described (Ott and Scott, 1910; Schafer and Mackenzie, 1911). The nine amino acid sequence of Oxt was elucidated in 1953 (du Vigneaud et al., 1953b; Tuppy, 1953) and synthesized soon after (du Vigneaud et al., 1953a; du Vigneaud et al., 1954). Prior to the determination of the structure of the preprohormone from the cloned gene for Oxt (Ivell and Richter, 1984), oxytocin was shown to be cleaved from a precursor containing a neurophysin polypeptide during axonal transport to the posterior pituitary (Brownstein et al., 1980). Its sole known receptor (Oxtr) was cloned in 1992 (Kimura et al., 1992). These landmark studies have paved the way for a large body of work, covering not only on the roles of Oxt in the periphery, but as we will review, in the central nervous system control of behavior.
1.1 Structure and evolution of oxytocin
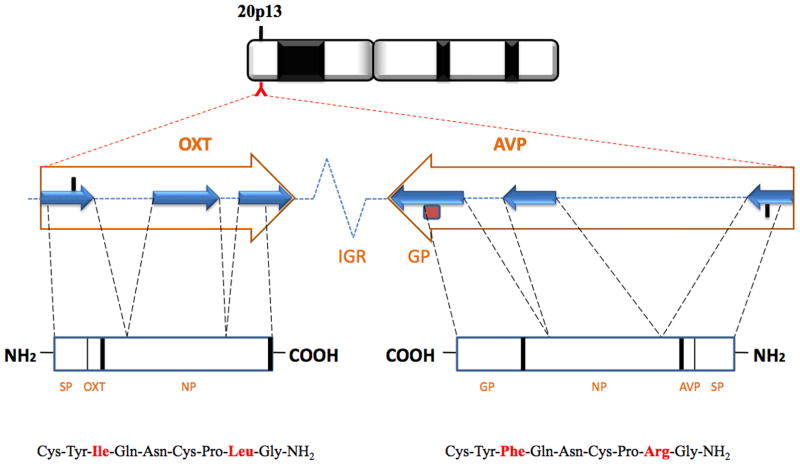
Oxt is composed of nine amino acids (Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-GlyNH2) with a sulfur bridge between the two cysteines (Fig. 1). The structure of Oxt is very similar to another nonapeptide, vasopressin (Avp), which differs from Oxt by two amino acids. Oxt and Avp are neuropeptides that are evolutionarily well conserved across phyla (Acher et al., 1995; Caldwell and Young, 2006). As a result of gene duplication, the Oxt gene is located on the same chromosome (chromosome 2 in mice and 20 in humans) as Avp but is oriented in opposite transcriptional direction in mammals. Both Oxt and Avp contain three exons and two introns and are highly homologous. The two genes are separated by an intergenic region (IGR) that varies in length across species (e.g., 11 kb in rat and human, and 3.6 kb in mouse). The IGR harbors regulatory DNA sequences within conserved portions for both Oxt and Avp (Gainer et al., 2001; Young and Gainer, 2003, 2009). The preprohormone consists, in order, of the signal peptide, the nanopeptide (Oxt), and the neurophysin (Fig. 1).
Fig. 1.
Schematic diagram of the oxytocin and vasopressin genes (large arrows), preprohormones (boxes), and neuropeptides (bottom). Their human chromosomal location is shown at the top. Both genes are composed of three exons shown as small blue arrows and separated by two introns (shown as dotted lines between the exons). The genes are located on the same chromosome but are transcribed in opposite directions and separated by an intergenic region (IGR). The IGR length varies across species. The preprohormones each contain a signal peptide (SP), a neuropeptide (Avp or Oxt), and a neurophysin (NP). In the case of Avp, a glycopeptide (GP) as well. Protein processing signals are represented by thick lines. Cysteine residues form a disulfide bond to create a cyclic six amino acid ring for both neuropeptides. Seven out of the nine amino acids are identical in the neuropeptides and two are different (red). Adapted and modified from (Caldwell et al., 2008).
1.2 Pharmacology of the oxytocin receptor
Oxytocin is currently known to have only one receptor (Oxtr), unlike Avp which has at least three different subtypes (Caldwell et al., 2008). Oxtr belongs to the rhodopsin-type (class I) G protein-coupled receptor (GPCR) family and is coupled to phospholipase C through Gαq11 (Gimpl and Fahrenholz, 2001;Young and Gainer, 2003). Much work has gone into creating agonists and antagonists (both peptides and small molecules) with specificity for the Oxtr and little if any activity at the Avp receptors (Manning et al., 2008). Two well known Oxt antagonists are Atosiban (deamino-[D-Tyr2-(O-ethyl)-Thr4-Orn8]vasotocin) (Akerlund et al., 1985), and OVTA (Elands et al., 1988). Atosiban is clinically used to delay premature delivery (Zingg and Laporte, 2003). However, both antagonists have an affinity for the vasopressin receptor (Avpr) 1a (Akerlund et al., 1999; Manning et al., 1995). Non-peptide Oxtr antagonists such as SSR126768 (Serradeil-Le Gal et al., 2004) and GSK2211149A (McCafferty et al., 2007) have higher specificity and may eventually find clinical use. Synthetic Oxt (known as Pitocin) is used to induce labor and to help milk production (Hayes and Weinstein, 2008). Non-peptide agonists are also under development (Manning et al., 2008). As central Oxt is involved in many behaviors, the use of agonists and antagonists for peripheral indications will need close scrutiny to assess unintended behavioral effects due to passage of the agents through the blood brain barrier. Excellent reviews are available (Gimpl, 2008; Manning et al., 2008).
1.3 Distribution of oxytocin and its receptor in the brain
Oxytocin is primarily synthesized in magnocellular neurons of the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus. The bulk of the peptide is transported to the posterior pituitary where it is released to regulate parturition and lactation. However, some of the Oxt is transported into the dendrites where regulation of its release is critical for controlling the firing patterns of the Oxt neurons (Rossoni et al., 2008). Lesser amounts of Oxt are generated by smaller, parvocellular neurons of the PVN and, depending on species, the bed nucleus of the stria terminalis (BNST), medial preoptic area, and lateral amygdala for release within the brain (Young and Gainer, 2003, 2009).
The distribution of Oxtr expression within the central nervous system (CNS) of numerous species has been examined using in situ hybridization histochemistry (Ostrowski, 1998; Yoshimura et al., 1993), a transgenic mouse model (Gould and Zingg, 2003), and receptor autoradiography. A number of species, including rat (De Kloet et al., 1985a; Freund-Mercier et al., 1987; Kremarik et al., 1993; van Leeuwen et al., 1985; Veinante and Freund-Mercier, 1997), mouse (Insel et al., 1991), vole (Insel and Shapiro, 1992), and human (Loup et al., 1991; Loup et al., 1989), have been studied by receptor autoradiography, a technique that indicates where the receptor is transported after synthesis. Sexual and species differences exist in the distributions of the Oxtr, even within the same genus, and these differences are believed to explain certain variations in behavior (see Section 2). In general, however, Oxtr is widely distributed throughout the brain. In rodents, it is often especially prominent in the olfactory bulb (OB) and tubercle, neocortex, endopiriform cortex, hippocampal formation (especially subiculum), central and lateral amygdala, BNST, nucleus accumbens (NAcc), and ventromedial hypothalamus (VMH) (Insel et al., 1991; Veinante and Freund-Mercier, 1997). In humans, expression is prominent in the basal nucleus of Meynert, the nucleus of the vertical limb of the diagonal band of Broca, the ventral part of the lateral septal nucleus, the preoptic/anterior hypothalamic area, the posterior hypothalamic area, the substantia nigra pars compacta, and the substantia gelatinosae of the caudal spinal trigeminal nucleus and of the dorsal horn of the upper spinal cord, as well as in the medio-dorsal region of the nucleus of the solitary tract (Loup et al., 1991; Loup et al., 1989).
It should be emphasized, as Leng and colleagues discuss (Leng et al., 2008b), that there is not always a match between Oxt immunoreactive terminals and receptor concentrations. Their work shows that dendritic release from magnocellular PVN and SON neurons can influence behavior (Ludwig and Leng, 2006). They suggest, for example, that the lordotic response (see below), shown to rely on VMH Oxtr, may reflect action of Oxt that diffused to the VMH from the PVN and SON after dendritic release (Sabatier et al., 2007).
1.4 Sex differences in expression of oxytocin and its receptor
Oxt and Oxtr expression is usually higher in females (Carter, 2007; Zingg and Laporte, 2003). The central roles of Oxt on behaviors and physiology are strongly dependent on steroid hormones (discussed below) and gender, and Oxt and Oxtr distributions between brains of different sexes have been reported (Carter, 2007; Insel et al., 1991; Tribollet et al., 1997; Tribollet et al., 1992). For example, the numbers of Oxt-immunostained cells and the amounts of Oxt found in females far exceed the numbers and amounts found in males along with greater numbers of oxytocin-immunostained axons (Haussler et al., 1990). In female but not male, rats Oxt binding is increased by high levels of maternal stimulation during infancy, suggesting that epigenetic influence can alter the Oxtr expression in a sex-specific manner (Francis et al., 2002). Sexually dimorphic expression of Oxt binding is observed in some brain regions where Oxt is known to have behavioral effects, such as the ventromedial hypothalamic nucleus (VMH), while other areas such as the central nucleus of the amygdala, do not show sexual dimorphism (Uhl-Bronner et al., 2005).
1.5 Control of oxytocin and oxytocin receptor by steroid hormones
Estrogen receptor (ER) β is present in magnocellular neurons of the PVN and SON (Forsling et al., 2003; Hrabovszky et al., 2004). The ERβ mRNA expression there is negatively regulated by basal glucocorticoid secretion and by hyperosmotic stimulation (Somponpun et al., 2004). The rat and human Oxt gene promoters contain estrogen-response elements (ERE) and are stimulated by estrogen (E) and thyroid hormones (Mohr and Schmitz, 1991; Richard and Zingg, 1990). A recent in vivo study suggests that E action on the Oxt gene is more likely to involve a DNA-independent mechanism than direct regulation by ERs (Stedronsky et al., 2002). Interestingly, the poly(A) tail, which is important for mRNA stability, is increased by osmotic stimulation (Carter and Murphy, 1989) and blocked by gonadectomy (Crowley and Amico, 1993).
It is well known that E stimulates expression of Oxtr in the uterus (see (Richter et al., 2004) and references therein) and greatly increases the expression in the kidney (Breton et al., 1996; Ostrowski et al., 1995). Oxtr expression increases in the myoepithelial cells of the breast during pregnancy and lactation (Soloff, 1982). In rats, Oxtr binding and mRNA levels in the brain are increased with E and testosterone treatment and decreased by castration (Breton and Zingg, 1997; Larcher et al., 1995; Stevenson et al., 1994; Tribollet et al., 1990). However, this effect may depend on the species studied (Insel et al., 1993). Oxtr expression also increases in a number of brain areas just prior to parturition (Meddle et al., 2007), accompanied by a concomitant increase in gonadal hormones, particularly E (Rosenblatt et al., 1988).
The VMH is an important nucleus for the regulation of sex behavior and the role of Oxtr within the VMH has been the focus of intense study. Within the VMH of males and females, Oxtr is particularly sensitive to gonadal steroids (Bale and Dorsa, 1995; Bale et al., 1995; Coirini et al., 1989; De Kloet et al., 1986; De Kloet et al., 1985b; Johnson et al., 1991; Quinones-Jenab et al., 1997).
There are complete palindromic EREs in the promoters of the Oxtr genes of mice and rats (Bale and Dorsa, 1997; Kubota et al., 1996), as well as half-palindromic EREs in those of mice, rats and humans (Inoue et al., 1994; Kubota et al., 1996; Rozen et al., 1995). It is likely that E can act on the half palindromic EREs, albeit with lower affinity (Sanchez et al., 2002). A recent study suggests that a membrane bound receptor for E may also regulate expression within the PVN and SON (Sakamoto et al., 2007). The Oxtr gene has several other response elements in its promoter, including an interleukin response element, a cAMP response element, and AP-1, AP-2, AP-3, and AP-4 sites (Bale and Dorsa, 1998; Gimpl and Fahrenholz, 2001; Rozen et al., 1995). E-induced Oxtr binding in the brain is abolished in ER-α knockout (KO) mice, whereas the basal Oxtr expression in the brain of the KO mice is similar to controls (Young et al., 1998).
1.6 Gene inactivation in mice
A large number of studies have utilized KO mouse models. Oxt KO mice were first introduced in 1996 by two groups (Nishimori et al., 1996; Young et al., 1996b). Oxt KO mice display normal parturition even though female KO do not show milk ejection (Nishimori et al., 1996; Young et al., 1996b). These two groups later independently generated Oxtr KO mice (Lee et al., 2008; Takayanagi et al., 2005) with one line showing late-onset obesity (Takayanagi et al., 2008). The other line is a conditional KO that allows temporal and spatial Oxtr elimination (Lee et al., 2008). Detailed descriptions of the behavioral deficits in these Oxt and Oxtr KOs are presented in Section 2.
Other eliminated genes have significant effects on Oxt expression or effectiveness. For example, absence of the basic helix-loop-helix-PAS Sim1 (Michaud et al., 1998) or the POU protein Brn-2 (Nakai et al., 1995; Schonemann et al., 1995) genes lead to elimination of magnocellular neurons of the PVN and SON. As absence of Sim1 also leads to lack of Brn-2, it would seem that Sim1 functions upstream of Brn-2 (Michaud et al., 1998). Knockout mice lacking CD38, a protein that aids in release of Oxt show decreased release of Oxt accompanied by defects in maternal and social behaviors (Jin et al., 2007).
2. Behavior
Oxt is involved in the regulation of a wide variety of behaviors; many times, these behaviors are intertwined (e.g., more social species tend to have monogamous relationships and to be biparental). To better elucidate the role Oxt has in each of these behaviors, we have separated this review into two broad categories: social behaviors and non-social behaviors. Within each category we will separately discuss various behaviors affected by Oxt, with a concerted effort to draw out the similarities between them. An additional section has been added at the end discussing the role Oxt has in various behaviors that do not readily fit under either of the two aforementioned categories. Also, it should be noted that while Oxt is often discussed in concert with Avp, the main focus of this review is Oxt, so mention of the role of Avp on these same behaviors will be limited. For recent Avp reviews, see ((Caldwell et al., 2008); (Raggenbass, 2008); (Lim and Young, 2006); and (Landgraf, 2005)).
2.1 Social Behavior
The ability to recognize a conspecific is imperative in determining the proper response to that individual, and formation of a ‘social memory’ of individuals is vital for display of appropriate behaviors within a social group. For inter-sex interactions, the appropriate behaviors are often affiliative in nature, which allows for reproduction, pair-bonding, and parental behaviors. For same-sex interactions, particularly male-male interactions, the appropriate behaviors are often aggressive in nature and center around competition for mates and other resources. Across species, Oxt is important in regulating the formation of social memories, as well as displays of affiliative and aggressive behaviors. The next three sections will delve into the particular roles that Oxt has in regulating social behaviors (social recognition; affiliation; aggression); these sections are summarized in Table 1. For recent reviews of Oxt effects on social behaviors, see (Neumann, 2008; Neumann and Landgraf, 2008).
Table.
Summary of the behavioral effects of Oxt and the Oxtr
| Behavioral Classes | Behaviors | Effects of Oxt in rodents | Effects of Oxt in humans |
|---|---|---|---|
| Social Behaviors | |||
| Social memory | Social recognition |
|
|
| Affiliation | Sexual behavior |
|
|
| Paternal behavior |
|
no known effect | |
|
|||
| Maternal behavior |
|
no known effect | |
| Aggression | Female aggression |
|
no known effect |
| Male aggression |
|
|
|
| Non-Social Behavior | |||
| Learning and Memory | Non-spatial memory |
|
|
| Spatial memory |
|
no known effect | |
| Anxiety & Depression | Anxiety |
|
|
| Depression |
|
|
|
2.1.1. Social memory and social recognition
Recognition of individuals is important for everyday life. Without the ability to determine friend from foe, it is difficult to display the appropriate behaviors (either affiliative or aggressive, respectively). The formation of a social memory of individuals is therefore vital, and in rodents relies primarily on volatile and pheremonal olfactory cues. This differs from primates, which rely primarily on both visual and auditory cues. In rodents, Oxt influences social memories by affecting the processing of these olfactory cues (for a recent review, see Sanchez-Andrade and Kendrick, 2008).
Social memory is commonly examined in rodents through three paradigms: habituation-dishabituation, social recognition, and social discrimination. In the first, a subject animal is exposed to the same “stimulus” animal over repeated trials, and decreases investigation through habituation. Following habituation, a novel animal is presented, which typically results in an increase in investigation time, or dishabituation (Winslow and Camacho, 1995). In the social recognition paradigm, a subject animal is exposed to a stimulus animal and after a predetermined period of time is either re-exposed to the same stimulus animal or to a novel stimulus animal. Typically, the subject spends a greater amount of time investigating the novel animal (Dantzer et al., 1987). The third paradigm, social discrimination, is similar to social recognition, except that on the re-exposure trial both the same and novel stimulus animals are presented simultaneously, forcing the subject animal to choose between the two (Engelmann et al., 1995). For detailed protocols, see (Winslow, 2003).
Choleris and coworkers have suggested a gene micronet involving the four genes coding ERα, ERβ, Oxt and Oxtr as the regulatory basis of social recognition in the brain (Choleris et al., 2004). The interactions between the E and Oxt systems are quite intricate. For example, neonatal manipulations of Oxtr stimulation lead to changes in brain ER and Oxt levels in both juveniles and adults (Cushing et al., 2003; Kramer et al., 2007). Even this proposed micronet can only be part of the basis of social recognition, as it is known that other substances (e.g., vasopressin (Bielsky et al., 2004; Wersinger et al., 2002)) are necessary.
2.1.1.1. Social memory/recognition in males
In males, a great body of evidence indicates that both neuropeptides Avp and Oxt influence social memory. The role of Avp in social memory/recognition has been described previously (Caldwell et al., 2008); this review will focus on the role of Oxt in social memory/recognition. Early work by Dluzen and colleagues indicates that Oxt likely influences social recognition responses in males by affecting the ability to process odors. Infusion of Oxt into the OB facilitates social recognition at both a 30-min and 120-min delay compared to vehicle-treated animals, but infusion of the Oxt antagonist desGly-NH2,d(CH2)5[Try(Me)2,Thr4,Orn8]vasotocin (AOVT) into the same region of the OB fails to block social recognition at either time delay (Dluzen et al., 1998a). Retrodialysis of Oxt into the OB increases norepinephrine (NE) release (Dluzen et al., 2000). Infusion of 6-hydroxydopamine, which destroys NE terminals, directly into the OB results in a complete lack of social recognition, even with co-infusion of Oxt (Dluzen et al., 1998b). Specifically, the actions of NE on the α-adrenoceptors in the OB are necessary, as infusion of clonidine (an alpha-adrenoceptor agonist) preserves social recognition, while phentolamine (an alpha-adrenoceptor antagonist) prevents social recognition, even in the presence of Oxt (Dluzen et al., 2000).
Oxt also facilitates social recognition when administered in other regions. Infusion into the lateral ventricles significantly enhances social recognition 120 minutes later at doses of 1fg – 10 ng/rat when injected after the first encounter (Benelli et al., 1995), indicating a role for Oxt in the acquisition phase of social memory (see (Ferguson et al., 2002) for review). Similarly, infusion of the Oxt antagonist d(CH2)5[Tyr(Me)2-Orn8]vasotocin (CPOVT) 5 minutes before Oxt injection abolishes the memory-enhancing effect (Benelli et al., 1995). Social recognition is facilitated by Oxt injection into the medial preoptic area of the hypothalamus (mPOA) with a wide range of doses (0.3–1000pg), but not when injected into the septum (Popik and van Ree, 1991). Interestingly, Avp facilitates social recognition when injected into the septum (Engelmann and Landgraf, 1994), but not the mPOA (Popik and van Ree, 1991); see (Caldwell et al., 2008) for review), indicating that these two neuropeptides influence social recognition in different brain regions. Finally, subcutaneous administration of Oxt and related peptides containing the C-terminal glycinamide (i.e., Oxt-(1–9), Oxt-(7–9), and Oxt-(8–9)) have been shown to facilitate social recognition at low doses (Popik et al., 1996). Access to the CNS by this route of administration is problematical, as is the site of action.
The development of Oxt and Oxt receptor KO mice has led to the further characterization of Oxt’s role in social recognition responses. Male Oxt KO mice fail to develop social memory on both the habituation-dishabituation test (Ferguson et al., 2000; Lee et al., 2008) and the social recognition test (Ferguson et al., 2001). Oxt in the medial amygdala is necessary to facilitate social recognition, as demonstrated by c-fos activation in the medial amygdala of wildtype (WT) but not Oxt KO mice during the initial exposure (Ferguson et al., 2001). Interestingly, two independently derived lines of Oxt KO mice fail to show any deficits in general sociability (as measured by the social approach task; (Crawley et al., 2007), indicating that Oxt is primarily involved in the memory component of social recognition (see Section 2.2.1 for the role Oxt plays in learning and memory).
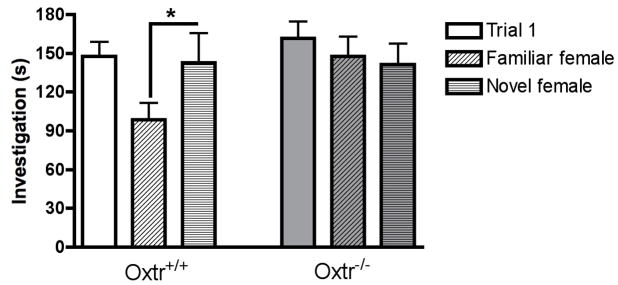
Recently, a similar impairment in social recognition in two lines of Oxtr KO mice was described. Specifically, unlike WT controls, Oxtr KO mice continue to investigate a ‘familiar’ female as if she were ‘novel’ (Takayanagi et al., 2005). Furthermore, we generated a line of conditional Oxtr KO mice to reduce Oxtr expression in parts of the forebrain (OxtrFB/FB). Compared to WT littermate controls, OxtrFB/FB mice also have a social recognition impairment but in a different manner, with decreased investigation of both ‘familiar’ and ‘novel’ females on the second trial, but at intermediate levels (Figure 3) (Lee et al., 2008). As OxtrFB/FB mice can distinguish between familiar and novel stimulus females on the habituation-dishabituation task, it is unlikely that the decreased investigation is due to a loss of interest in the social recognition tasks. Why the Oxtr KO and OxtrFB/FB mice should differ in social recognition performance is unclear, and is currently being investigated.
Fig. 3.
Social recognition by Oxtr WT and KO males, as examined with the two-trial social recognition task. Investigation times of stimulus females during Trial 1 were equal across genotypes, indicating no motivational or olfactory differences. During Trial 2, Oxtr WT males display remember the previous stimulus females, represented by significantly less time investigating those female from Trial 1 (“familiar”) as compared to new females (“novel”); *p < 0.05 by t-test between familiar and novel. In contrast, Oxtr KO males spend equal times investigating the “familiar” and “novel” females, indicating a reduced ability to remember the familiar female and impaired social recognition. Adapted from (Lee et al., 2008).
Generation of KO mice has allowed for investigation into the importance of proteins that regulate Oxt secretion. Oxt release is controlled, in part, by intracellular calcium (Ca2+) stores (reviewed in Ludwig and Leng, 2006). CD38 is a transmembrane glycoprotein that, through formation of cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate, mobilizes Ca2+ and affects Oxt secretion. CD38 KO mice show normal synthesis and storage of Oxt in axon terminals, but abnormally low plasma and high hypothalamic Oxt concentrations, indicating abnormal release of Oxt in these mice, from both axon terminals and soma and/or dendrites (Jin et al., 2007). Interestingly, these mice show deficits in social recognition similar to those of the Oxt and Oxtr KOs described above.
2.1.1.2. Social memory/recognition in females
Social recognition differs between males and females. Females investigate stimulus animals less than males (Bluthe and Dantzer, 1990), but seem to retain the social memory for longer durations than males (Bluthe and Dantzer, 1990; Engelmann et al., 1998). Intracerebroventricular administration of the oxytocin antagonist AOVT abolishes social recognition in females, whereas the Avpr1a receptor antagonist d(CH2)5Tyr(Me)2AVP does not (Engelmann et al., 1998).
Similar to male social recognition, the medial amygdala modulates female social recognition. Antisense oligonucleotides specific for the Oxtr administered i.c.v. into the medial amygdala several days prior to testing significantly reduce social recognition in females, indicating that Oxtr expression in that region is necessary for proper social recognition (Choleris et al., 2007). Additionally, social recognition is mediated by neuropeptide - steroid hormone interactions. In males, testosterone regulates the effects of neuropeptides, particularly Avp (Bluthe et al., 1990), on social recognition; in females, E appears to regulate Oxt effects on social recognition. Specifically, ERα, ERβ, and Oxt KO mice all have very similar deficits in social recognition on the habituation-dishabituation test, with all three KO lines failing to habituate to a ‘familiar’ animal or dishabituate with a ‘novel’ animal (Choleris et al., 2003). Similarly, in a social discrimination task, the three lines show either complete impairment (Oxt and ER-α KO mice) or partial impairment (ERβ KO mice), indicating that all three genes are necessary to some degree for social recognition in females (Choleris et al., 2006). Lastly, Oxt facilitates long-term potentiation in mitral cells of the accessory OB (Fang et al., 2008). Oxt release in the OB modulates social recognition, with vaginocervical stimulation during proestrus/estrus (high E levels) significantly increasing release of Oxt in the OB, thereby enhancing social recognition 5 hours after first exposure (Larrazolo-Lopez et al., 2008).
Another olfactory-based social memory in females is the Bruce effect, where housing a female with an unfamiliar male blocks pregnancy (Bruce, 1959) due to the chemosensory signals present in the new male’s urine (Brennan, 2003; Dominic, 1966). Oxt KO, heterozygote (HET), and WT females display pregnancy block in response to an unfamiliar male, as expected; only Oxt KO females block pregnancy when a familiar male (their previous mate) is encountered, indicating a social memory deficit (Wersinger et al., 2008). Interestingly, continuously paired Oxt KO females do not pregnancy block, likely because with constant exposure, they do not have the opportunity to ‘forget’ their mates. Avpr1b plays a role in the Bruce effect as well, but in a different manner, as Avpr1b KO females do not exhibit pregnancy block with an unfamiliar male (Wersinger et al., 2008).
2.1.1.3. Human studies
Recently, researchers have begun to examine Oxt effects on human social recognition, primarily by testing recognition of faces. Generally, Oxt seems to enhance memory for faces; whether or not the emotion displayed on the faces is relevant remains unclear. Oxt administered intranasally to males and females after viewing male faces (with happy, angry, or neutral expressions) significantly improves recognition memory 30 minutes and 24 hours later for neutral and angry faces only (Savaskan et al., 2008). Similarly, intranasal Oxt increases memory for angry faces in both males and females without influencing response time, accuracy, or gaze time (Guastella et al., 2009), indicating that Oxt may influence face recognition at very early stages of perceptual processing. In contrast, an earlier study by the same group reports that Oxt (administered to males only) increases memory for previously seen faces with happy expressions only, not angry or neutral (Guastella et al., 2008c). Why this discrepancy exists remains unclear, but may be due to presentation of different stimulus faces (male: (Savaskan et al., 2008); male and female: (Guastella et al., 2008c); drawings: (Guastella et al., 2009)). Perhaps significantly, the former study gave Oxt after the acquisition phase while it was given prior to the acquisition phase in the latter two. In a more recent study, intranasal Oxt was given to male subjects 40 minutes prior to presentation of faces. Twenty-four hours later, subjects treated with Oxt had better memory for faces seen the previous day, with no influence on memory for previously seen non-social stimuli (Rimmele et al., 2009). This is a further indication that Oxt is specifically involved in memory of social stimuli.
As discussed above (Sections 2.1.1.1 and 2.1.1.2), Oxt in the medial amygdala underlies social recognition in rodents. Similarly, the human amygdala seems important for facial recognition in humans. Lower blood oxygenation level dependent functional magnetic resonance imaging (fMRI) activity is seen in the right amygdala after intranasal Oxt, as compared with placebo, when viewing emotional (happy, fearful, or angry expressions) faces, regardless of type of emotion displayed (Domes et al., 2007a). Similarly, male subjects have reduced amygdala activation when shown angry or fearful expressions following Oxt administration (Kirsch et al., 2005). The same reduction is not seen when presented with non-social stimuli, such as fearful or threatening scenes. Furthermore, functional amygdala connections to upper brainstem regions (such as the periaqueductal gray) are significantly reduced with intranasal Oxt (Kirsch et al., 2005), indicating that Oxt can affect a circuit for social recognition and response. However, it should be remembered that although intranasal application of large proteins may reach the olfactory bulbs (Balin et al., 1986) and small peptides the CSF (Born et al., 2002), no studies have shown that the intranasal Oxt is reaching the CNS areas involved in facial recognition.
2.1.1.4. Conclusion
Administration of Oxt agonists and antagonists, as well as studies using Oxt and Oxtr KO mice, indicate a positive relationship between Oxt and social memory in both males and females. Data from clinical studies reveal similar results, with Oxt promoting face recognition in humans. In both non-humans and humans, the amygdala seems important for recognition of social stimuli. Further investigation of social memory using techniques such as conditional knockouts (Lee et al., 2008) and site-specific lentivirus injections will allow more detailed analyses of the role of Oxt and the Oxtr in social memory. Earlier reviews have also been written detailing Oxt and Avp effects on social memory and recognition (Ferguson et al., 2002);(Winslow and Insel, 2004); and (Bielsky and Young, 2004).
2.1.2. Affiliation
Affiliation, or social bonding between individuals, is one of the most highly motivated social behaviors, as forming social bonds can counteract the stress and anxiety provoked by social isolation in rodents (Grippo et al., 2007). Affiliative behaviors are highly species-specific (for review see (Carter, 1998). In rodents, the most readily quantifiable affiliative behaviors are sexual behavior, pair bonding, and parental care. The regulation of these behaviors by Oxt will be discussed in this section (for review of Avp regulation of affiliative behaviors see (Caldwell et al., 2008)).
Approximately 3% of all mammals display monogamous sexual relationships (Kleiman, 1977). Monogamy consists of a set of distinguishing characteristics including (1) sharing of nest and territory by a breeding pair during breeding and non-breeding seasons; (2) aggressive displays by both sexes towards unfamiliar conspecifics; (3) bi-parental care; (4) socially regulated reproduction; and (5) incest avoidance via suppression of puberty in the natal group (Carter et al., 1995; Insel and Fernald, 2004). Pair bond formation has been largely studied in voles, as both monogamous (prairie and pine voles) and non-monogamous (montane and meadow voles) species exist, with corresponding differences in Oxtr binding across monogamous and non-monogamous species (Winslow et al., 1993b). Comparisons between these species allows for quantification of the development of social bonds, such as pair bonding (see (Young et al., 2008) for review).
In the laboratory, pair bonding is measured with the partner preference task, in which animals are given a choice between spending time with a familiar, mated partner and a novel “stranger” animal. Partner preference is operationally defined as an animal spending twice as much time with the familiar partner compared to the novel ‘stranger’. Monogamous male voles readily make this distinction, and spend less than 20% of their time alone, compared with polygynous voles, which remain alone about 90% of the time (Carter et al., 1995).
Oxt facilitates pair bonding in monogamous female voles, likely due to its release during mating (see Section 2.1.3; but mating is not always required for partner preference: see (Williams et al., 1992). Partner preference in females seems to be exclusively under Oxt control, as infusion of Avp or Avp antagonist fails to affect partner preference in female prairie voles (Insel and Hulihan, 1995; Insel et al., 1998), and pretreatment with an Oxtr antagonist prevents partner preference (Cho et al., 1999; Cushing and Carter, 2000). Furthermore, i.c.v. or s.c. Oxt infusion (over 24 hrs) into female prairie voles facilitates formation of pair bonds, while treatment with an Oxtr antagonist prior to mating prevents pair bonding (Williams et al., 1994). The ability of Oxt-treated females to develop a partner preference is dose dependent, with high doses (above 2 mg/kg) decreasing partner preference (Bales et al., 2007b). Oxt may interact with E to facilitate pair bonding, as administration of the endocrine disrupting chemical methoxychlor (MEX) to female pine voles significantly reduces Oxt binding in the cingulate cortex and time spent engaged in social activity (Engell et al., 2006).
Oxt’s role in male partner preference is less clear. Acute Oxt treatment (just prior to testing) has been shown to facilitate partner preference in males (Cho et al., 1999). Furthermore, developmental exposure to Oxt (1 injection administered on postnatal day 1 (PND 1)) does result in formation of partner preference in male prairie voles as adults (Bales and Carter, 2003a). However, other studies indicate no role for Oxt in male partner preference (Winslow et al., 1993a); Cushing & Carter, 2000), or an interactive regulation of pair bonding by both Oxt and Avp (Cho et al., 1999; Liu et al., 2001). Recent data indicates that the latter is likely, as Oxt seems to alter expression of Avpr1a (Bales et al., 2007). Cross-communication between the two systems could regulate pair bonding in males.
The sexually dimorphic effects of Oxt on pair bonding may be due to differences in Oxt and Avp pathways and receptor distributions throughout the vole brain. The female brain is generally more susceptible to effects of Oxt. Administration of Oxt on PND1 alters aggression in female prairie voles, but not males (Bales and Carter, 2003b). Treatment with Oxt and the Oxtr antagonist CPOVT on PND1 results in increased Oxt immunoreactive (Oxt-ir) neurons in females, but not males (Yamamoto et al., 2004). Recently, the same Oxt antagonist was shown to increase c-fos activity in the central amygdala of females when mated, which could affect later social behavior (Kramer et al., 2006).
Interestingly, distribution of OT neurons is not gender-specific in either monogamous or non-monogamous voles (Wang et al., 1996). The prairie vole does have fewer Oxt-ir neurons in the mPOA and BNST when compared to the montane vole, however (Insel et al., 1995). Furthermore, distribution patterns and quantity of Oxtr differ between monogamous and polygamous voles (Insel and Shapiro, 1992). Generally, monogamous prairie voles have greater Oxtr in the frontal cortex, NAcc, BNST, amygdala, and thalamus, while Oxtr in the polygamous montane vole is concentrated in lateral septum and VMH (Insel and Shapiro, 1992). While both Oxtr and Avpr1a in the ventral forebrain seem important in regulating pair bonding and partner preference (Insel and Shapiro, 1992; Insel et al., 1994), the two receptors have different expression patterns in prairie voles, with Oxtr specifically relegated to the shell and core of the NAcc in prairie voles (Lim et al., 2004), where Oxt-dopamine interactions aid in partner preference formation in female prairie voles (Liu and Wang, 2003). In contrast, non-monogamous voles, rats and mice have very low levels of Oxtr binding in the NAcc (Olazabal and Young, 2006b). Administration of adeno-associated viral vector encoding the vole Oxtr gene into the NAcc of monogamous prairie voles results in accelerated formation of partner preference in comparison to untreated prairie voles (Ross et al., 2009). However, the same virus administered into the NAcc of polygamous meadow voles does not facilitate partner preference, indicating that some other mechanism likely underlies partner preference in polygamous species (Ross et al., 2009). The Oxtr genes of monogamous and non-monogamous species have highly homologous coding and near promoter regions, so that differences in more distant regulatory elements or levels of their cognate binding proteins may explain the expression differences (Young et al., 1996a).
Due to the ability to perform comparative studies across different vole species, Microtus voles are the most heavily studied in terms of pair bonding and partner preference (Young et al., 2008). Despite the difficulties in studying ‘partner preference’ in other mammalian species, Oxt has been shown to affect sociability. Intracranial and subcutaneous Oxt injections increase the amount of social contact in male rats (Witt et al., 1992), female mongolian gerbils (Razzoli et al., 2003), and male squirrel monkeys (Winslow and Insel, 1991). Oxt increases flank marking (Harmon et al., 2002b) and decreases aggression (Harmon et al., 2002a) in female Syrian hamsters. The social and non-social species of tuco-tuco (genus Ctenomys), a South American rodent, differ highly in Oxtr binding throughout the brain, although not in the same manner as microtine rodents (Beery et al., 2008). Bonnet macaques, which have a more affiliative social structure than the closely related pigtail macaques, have higher levels of Oxt in CSF than do pigtails (Rosenblum et al., 2002).
2.1.2.3. Parental Behavior
2.1.2.3.1. Maternal Care
During pregnancy and when nursing pups, both Oxt and the Oxtr are altered in the female rat brain, particularly the ventral septum (Landgraf et al., 1991), SON (Caldwell et al., 1987; Landgraf et al., 1992; Mezey and Kiss, 1991), PVN (Caldwell et al., 1987), and, perhaps, within the dorsal hippocampus (Landgraf et al., 1992). Similar increases in Oxt expression are seen in the PVN and SON of postpartum female prairie and montane voles (Wang et al., 2000), in the PVN, SON, and lateral hypothalamic area on postpartum day 1 in rabbits (Caba et al., 1996), and in the OB of primiparous and multiparous ewes at parturition (Levy et al., 1995).
Oxtr expression is also significantly increased at parturition throughout the brain (particularly in the SON, mPOA, BNST, OB, and amygdala), with Oxtr expression returning to the levels seen in virgin rats by 12 hours postpartum (Meddle et al., 2007). Similarly, female prairie and montane voles have significantly greater Oxtr binding in the VMH than virgin females or males, although the expression patterns differ between the two species (Wang et al., 2000). Interestingly, specific Oxtr binding sites have not been found in the OB of postpartum ewes (Levy et al., 1992), despite an increase in Oxt levels in the same region (Levy et al., 1995).
A likely reason for increased expression of Oxt and the Oxtr is to facilitate the onset and maintenance of maternal behavior, which is strongly regulated by Oxt (see (Leng et al., 2008a) for a recent review). Injections of Oxt i.c.v. to gonadally-intact females significantly increases the display of “full maternal behavior” (display all of the following: grouping pups, licking pups, crouching over pups, nest building, and pup retrieval), but only with high endogenous E levels (Pedersen and Prange, 1979). In OVX females, Oxt is only able to induce maternal behavior in females primed with estradiol benzoate (EB) (Pedersen and Prange, 1979). Furthermore, Oxt effects on maternal behavior are dose-dependent, with higher doses eliciting higher maternal behavior responses in EB-primed rats (Pedersen et al., 1982). Oxt may specifically alter grooming and posturing over pups, as i.c.v. infusion of the selective Oxt antagonist OVTA significantly increases self-grooming and frequency of lying prone on pups instead of facilitating nursing by remaining elevated and upright (Pedersen and Boccia, 2003). Oxt seems to interact with the dopamine and serotonin systems to control certain aspects of maternal behavior, including grooming (Johns et al., 2005). Oxt induces full maternal behavior more readily than Avp, and maintains full maternal behavior for up to 6 hours after administration (Pedersen et al., 1982). Similarly, in OVX females primed with E and progesterone (P), anti-Oxt antiserum i.c.v. significantly reduces maternal behavior after 2, 6, and 25 hours compared to animals receiving anti-vasopressin antiserum or normal rabbit serum (Pedersen et al., 1985). A recent review detailing the role of E and ERs on maternal carefully describes the interactions between E and Oxt in facilitating maternal behavior (Cameron et al., 2008).
In many species, including rats, natural variations in maternal behavior are seen. Some dams show high levels of pup licking and grooming and arched-back nursing (High LG-ABN), while others show low levels of these behaviors (Low LG-ABN); High LG-ABN mothers have higher levels of Oxtr than Low LG-ABN mothers in a number of regions known to underlie maternal behavior (i.e., the BNST, mPOA, and lateral septum) and maternal aggression (i.e., the central nucleus of the amygdala; Champagne et al., 2001; Francis et al., 2000). I.c.v. injections of the Oxtr antagonist OVTA effectively turns High LG-ABN mothers into Low LG-ABN mothers (Champagne et al., 2001), further indicating that activation of Oxtr in the brain is required for proper display of maternal behavior. E is again shown to play a role as s.c. administration significantly increases Oxtr binding in the mPOA and lateral septum of High, but not Low, LG-ABN mothers (Champagne et al., 2001). Individual variations in maternal behavior are transmitted across generations, with female offspring (biological or cross-fostered) of High LG-ABN mothers growing up to be High LG-ABN mothers themselves (Francis et al., 1999). Interestingly, Oxtr binding in the central nucleus of the amygdala and BNST is higher in female offspring of High LG-ABN mothers (Francis et al., 2002).
Rodent species differ in expression of alloparental behavior (caring for non-related pups). In a highly social rodent species, the naked mole rat, only the queen gives birth and nurses pups, but many individuals participate in caring for pups. Interestingly, high levels of Oxt immunoreactive fibers are found in the NAcc and mPOA (Rosen et al., 2008), areas that are implicated in maternal care. Juvenile and adult prairie voles readily express ‘spontaneous’ maternal behavior (Olazabal and Young, 2005), juvenile rats require pup exposure (Mayer and Rosenblatt, 1979), and juvenile mice failing to show any spontaneous maternal behavior (Gandelman, 1973). The Oxtr may underlie species differences in spontaneous maternal behavior, as Oxtr density is highest in the NAcc of juvenile prairie voles, intermediate in juvenile rats, and lowest in juvenile mice and meadow voles (Olazabal and Young, 2006b). The exact opposite pattern of Oxtr expression is seen in the lateral septum, with Oxtr binding lowest in juvenile prairie voles and rats (Olazabal and Young, 2006b). A follow-up study with sexually-naïve adult female prairie voles shows that maternal behavior display and Oxtr binding in the NAcc are positively correlated; injection of the Oxtr antagonist OVTA into the NAcc completely abolishes maternal behavior (Olazabal and Young, 2006a). However, elevation of Oxtr through adeno-associated virus delivery in female prairie voles does not increase further alloparental behavior (Ross, et al., 2009).
The recent development of Oxt and Oxtr KO mice permits further examination of the role of Oxt and the Oxtr in maternal behaviors. Two separate lines of Oxt KO mice have been developed; in both lines, females fail to successfully eject milk but display maternal behavior identical to WT mice (Nishimori et al., 1996; Young et al., 1996b). However, a recent detailed study of maternal behavior by Oxt KO and WT nulliparous females towards foster pups indicates that fewer Oxt KO females retrieve pups; those that do retrieve pups retrieve fewer pups, and Oxt KO females groom themselves and pups less than WT females (Pedersen et al., 2006). Similar deficits are seen in both Oxtr KO virgin postpartum females (Takayanagi et al., 2005).
2.1.2.3.2 Paternal Care
Much less is known about the role of Oxt in paternal behavior, likely due to the small number of species in which males care for young. However, certain species of rodents (prairie voles and California mice, Peromyscus californicus) are biparental, providing a model with which to examine a possible role for Oxt in paternal care. In male California mice, plasma Oxt levels are significantly higher in expectant fathers from days 1–15 of pregnancy, compared to virgin males or non-expectant fathers (Gubernick et al., 1995), indicating that Oxt may prepare monogamous males for display of paternal behavior. However, postpartum Oxt levels do not differ between paternal and non-paternal males, and Oxt levels are elevated in males removed from their partner and pups (Gubernick et al., 1995).
I.c.v. Oxt does not increase alloparental behavior in reproductively-naïve male prairie voles (Bales et al., 2004). Furthermore, only combined treatment with Oxt and Avp antagonists reduces alloparental behavior, not treatment with either antagonist alone (Bales et al., 2004), suggesting that either the Oxt or Avp system may be sufficient for alloparental behavior. However, administration of the Oxt antagonist CPOVT on postnatal day 1 results in significantly reduced alloparental care by males when 21 days old (Bales et al., 2004). Neonatal exposure to Oxt or an Oxt antagonist may affect later paternal care, but there is little evidence that Oxt during adulthood is responsible for paternal care in the same manner as has been shown for maternal care. Instead, Oxt may indirectly promote paternal care by promoting release of prolactin (Liu and Ben-Jonathan, 1994) that is more directly implicated in paternal care in rodents (Wynne-Edwards and Timonin, 2007) and primates (Ziegler, 2000).
Interestingly, changes in Oxt binding are seen in non-monogamous male voles as well. Sexually and parentally experienced male meadow voles have greater Oxt binding compared to inexperienced males in the accessory olfactory nucleus, BNST, lateral septum, and lateral amygdala (Parker et al., 2001). Whether these changes in Oxt underlie paternal behavior has yet to be assessed, as non-monogamous species such as the meadow vole do not readily display alloparental behavior.
2.1.2.4. Conclusion
Oxt is greatly implicated in the formation of affiliative bonds for both partners and pups, although in the latter case there is greater evidence that Oxt primarily acts in females. Generally, Oxt release due to vagino-cervical stimulation during mating aids in sexual receptivity, the formation of affiliative bonds, and later display of maternal behavior. For detailed reviews on Oxt and social bonding, see (Carter, 2003; Carter et al., 1995; Insel, 1997; Insel and Fernald, 2004; Insel et al., 1998; Lim and Young, 2006). For a recent detailed review on the contribution of Avp and both Avpr receptor subtypes to social bonding see Caldwell et al. (Caldwell et al., 2008). As discussed next, Oxt is also important for male and female sexual behavior.
2.1.3. Sexual behavior
One of the earliest discovered functions of Oxt was the facilitation of smooth muscle contractions in the uterus during labor (Sheldrick and Flint, 1985). Since then, Oxt has been found to regulate maternal behavior (see Section 2.1.2.3.1) and to aid in forming social bonds as adults (see Section 2.1.2). Additionally, Oxt plays a key role in regulating sexual behaviors in both male and female rodents. In male rodents, Oxt is implicated in erectile functioning, copulatory activity, and ejaculation (reviewed in Witt, 1995). In female rodents, Oxt activity is most studied in voles, rats, and rabbits. In female rats, in particular, regulation of copulatory behavior occurs through interactions between E and Oxt (reviewed in Witt, 1995). The following sections discuss how Oxt influences behaviors relating to sexual activities in both male and female rodents, as well as humans.
2.1.3.1. Sex behavior in males
Acute administration of Oxt enhances male sexual behavior, while intravenous Oxt injections accelerate time to ejaculation and number of ejaculations in rabbits (Melin and Kihlstroem, 1963) and the number of intromissions prior to ejaculation in rats, although only at low doses (Stoneham et al., 1985). Similarly, both i.c.v. and i.p. Oxt injections accelerate time to ejaculation and decrease time between mating attempts in rats (Arletti et al., 1985). Oxt facilitates erections in an inverted U-shaped manner, with high doses inhibiting erection frequency (Argiolas et al., 1987) as well as decreasing mounting bouts and increasing intromission latencies in male rats (Stoneham et al., 1985).
One hypothesis is that Oxt, at high levels, contributes to feelings of sexual satiety and therefore inhibits male sexual behavior. During mating bouts with a receptive female, Oxt is released within the PVN of male rats and is accompanied by reduced anxiety-like behavior up to 30 minutes after mating (Waldherr and Neumann, 2007). The release of Oxt during mating could contribute to sexual satiety. Indeed, acute i.c.v. Oxt can inhibit sexual behavior in male prairie voles (Mahalati et al., 1991). Unlike acute administration, chronic i.c.v. Oxt infusion has no long-term effects on number of mounts, intromissions, or ejaculations in male rats (Witt et al., 1992), perhaps due to decreased Oxt receptor density throughout the brain (Insel et al., 1992). However, chronic Oxt infusion does increase interaction time with the female without increasing sexual behavior (Witt et al., 1992), further implicating Oxt in sexual satiety (for review see (Carter, 1992) and general male social behavior (see Section 2.1.1.1).
Oxt does not act alone to bring about penile erections. Oxt is unable to induce erections without testosterone, as castration eliminates erections even with administration of Oxt and apomorphine; erections can later be re-established with co-administration of testosterone (Melis et al., 1994). Additionally, Oxt interacts with the dopamine and serotonin systems. The dopamine agonist apomorphine injected s.c. induces penile erections in a manner similar to that of Oxt injections into the lateral ventricles (Melis et al., 1989). More recently, Melis and coworkers (Melis et al., 2007) found that: (1) injections of both the Oxt receptor antagonist CPOVT and the dopamine receptor antagonist haloperidol into the shell of the NAcc or the PVN abolishes Oxt-induced penile erections; (2) injections of Oxt into the ventral tegmental area (VTA) increases extracellular dopamine and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) in the NAcc, which occurs concomitant with penile erection; and (3) Oxt-containing axons from the PVN to the VTA closely contact dopaminergic neurons in the shell of the NAcc, providing evidence that both dopamine and Oxt influence sexual behavior. Furthermore, i.c.v. injections of CPOVT dose-dependently inhibit the sexual response (erection) normally occurring in response to the dopamine D3 receptor agonist 7-OH-DPAT (Clement et al., 2008).
Serotonin depletion is an underlying factor in premature ejaculation in rats (Olivier et al., 2006) and humans (reviewed in (Giuliano and Clement, 2006). Pharmacological studies indicate that treatment with selective serotonin reuptake inhibitors induce serotonin and Oxt release, which may help to maintain erections and delay ejaculation (reviewed in (de Jong et al., 2007).
Potential interactions between Oxt and nitric oxide (NO) systems in mediating penile erections have been the subject of investigation. Administration of NO synthesis inhibitors (NG-nitro-L-arginine methyl ester and NG-monomethyl-L-arginine), and the oxytocin antagonist CPOVT into the PVN all prevent Oxt-induced erections (Argiolas & Melis, 1995). However, subsequent studies indicate that the same antagonist only prevents erections when injected into the lateral ventricles (Melis et al., 1999). Injections of Oxt into the VTA stimulate dopaminergic neurons that aid in production of NO and ultimately induce penile erection (Succu et al., 2008). However, not all studies agree that the Oxt-NO interaction is necessary for penile erection. Although Oxt i.c.v. increases both the number of erections and the concentration of NO2- and NO3- in the PVN (Melis et al., 1997), administration of oxytocin antagonist CPOVT into the lateral ventricles reduces non-contact erections without modifying NO2- and NO3- concentrations (Melis et al., 2000).
Finally, Oxt KO males produce normal litters when mated (Nishimori et al., 1996; Young et al., 1996b), indicating apparent normal sexual behavior, and the KOs are still potent sexual triggers to hormone-primed females (Agmo et al., 2008). Therefore, other hormones and mechanisms are more critically involved in sexual behavior in males. For review, see (Argiolas and Melis, 2004, 2005; Carter, 1992).
2.1.3.2 Sex behavior in females
Oxt acts in females to coordinate the onset of sexual maturity through interactions with gonadatropin-releasing hormone (GnRH): treatment with the Oxt antagonist desGly-NH2-d(CH2)2[D-Tyr2,Thr4]-vasotocin for 6 days significantly decreases GnRH pulse frequency, as well as age of vaginal opening and first estrus (Parent et al., 2008). Upon entering sexual maturity, female sexual behavior is examined in rodents primarily via examining the lordosis response. Lordosis is a reflexive posture displayed by receptive females in response to male mounting, and is primarily under control of E (see (Kow and Pfaff, 1998) for review).
Oxt also induces female sexual behavior, primarily by actions in the mPOA of the hypothalamus and the VMH (both regions underlie lordosis display; see Kow & Pfaff, 1998). Oxt immunoreactivity is enhanced by E (Caldwell et al., 1989a), and E increases affinity for Oxtr in the mPOA (Caldwell et al., 1994b). Similarly, Oxt release in the VMH due to mounting by males occurs only in females pretreated with E and P (Caldwell et al., 1989b). In females pretreated with E and P, Oxt injection into the mPOA significantly increases sexual receptivity as measured by the lordosis quotient (LQ: number of lordosis postures/100 mounts), while Oxt injection into the VMH increases lordosis duration only (Schulze and Gorzalka, 1991). Oxt injection into the mesencephalic central gray, or ventral tegmental area (other regions implicated in lordosis behavior) is not shown to alter LQ (Caldwell et al., 1989b).
Oxt facilitates sexual receptivity as measured by an increase in lordosis behavior, but only when the females have been pretreated with either E alone (Caldwell et al., 1986b), P alone (Gorzalka and Lester, 1987), or E and P (Arletti et al., 1985; Caldwell et al., 1989a). In intact, non-ovariectomized females, Oxt significantly increases lordosis quotient (LQ) and duration during estrus, when P levels are highest, while the Oxt antagonist CPOVT decreases LQ and duration (Benelli et al., 1994). Similarly, administration of the Oxt antagonist OVTA prior to treatment with P significantly decreases lordosis posturing, and increases duration of fighting with males (Caldwell et al., 1994a), but only when the Oxt antagonist is injected into the mPOA.
Early work indicates that Oxt may primarily affect facilitation of sexual behavior by P. The selective Oxtr antagonist OVTA reduces female sexual behavior in females primed with E and P, but not in females primed with E alone (Witt and Insel, 1991). In contrast, a later study indicates that E (conjugated to bovine serum albumin at position 6) and Oxt infusion to mPOA and medial basal hypothalamus significantly increases sexual receptivity (LQ), whereas E and P with Oxt does not (Caldwell and Moe, 1999). However, the Oxtr antagonist used by Witt and Insel is also an Avpr1a receptor antagonist (see (Pedersen and Boccia, 2006) for a recent study investigating Avp and Oxt interactions in controlling female sexual behavior). Treatment with the more selective Oxtr antagonist AOVT to ovariectomized (OVX) females primed with E significantly decreases LQ, and increases male-directed antagonistic behavior prior to P injection (Pedersen and Boccia, 2002). This Oxt antagonist does not decrease female sexual behavior at 4 and 6 hours after P injection, but does decrease lordosis 8–12 hours after P (Pedersen and Boccia, 2002). It is likely, therefore, that shortly after P injection, Oxtr activation facilitates the onset of female sexual behavior, and contributes to maintaining sexual behavior for up to 8 hours.
Prolactin (see Section 1.5) is released in the presence of Oxt (Egli et al., 2004; Samson et al., 1986) and with vagino-cervical stimulation (Erskine and Kornberg, 1992). Prolactin is released with mating in a twice-daily surge termed pseudopregnancy; females infused with the Oxtr antagonist OVTA into the VMH show only a 22% induction of pseudopregnancy, compared with 100% in females infused with control or an Avpr1a antagonist (Northrop and Erskine, 2008).
Oxt regulates female sexual behavior in other rodent species as well. In a manner similar to rats, Oxt infused into the VMH and mPOA induces sexual receptivity (increased duration of lordosis) in female Syrian hamsters (Whitman and Albers, 1995), while the Oxt antagonist OVTA reduces sexual receptivity. As in rats, female hamsters require pretreatment with some combination of E and P for Oxt to exert an effect (Whitman and Albers, 1995).
Unlike rats, prairie voles do not have a spontaneous estrus cycle. Female voles require social interactions with unfamiliar males for sexual behavior to be displayed (Carter et al., 1987). Accordingly, simple injections of Oxt (i.c.v. or i.p.) do not facilitate sexual receptivity in female prairie voles pretreated with E (Witt et al., 1990), and treatment with an Oxt antagonist does not inhibit sexual behavior (Witt et al., 1991). However, in sexually-naïve females (no exposure to males after weaning), daily Oxt injection (s.c.) for 5 days increases the likelihood of mating (compared to saline treated females), and treatment of Oxt with E increases sexual receptivity greater than E alone (Cushing and Carter, 1999). Therefore, prior exposure to Oxt can mimic the effects of social contact on female sexual behavior. Treatment with the Oxt antagonist CPOVT increases the likelihood of carrying a litter to term when the father is removed (a manipulation that ordinarily leaves a 50:50 chance of producing a litter; (Cushing et al., 2005).
2.1.3.3. Human Studies
In men, Oxtr can be found in the corpus cavernosum and epididymis of the penis; binding to the Oxtr in this region may affect contractility (Vignozzi et al., 2004) and subsequent ejaculation (Filippi et al., 2003). Plasma Oxt levels increase during sexual arousal, and orgasm significantly raises levels in men (Carmichael et al., 1987). In men, intranasal inhalation of Oxt significantly increases plasma Oxt and epinephrine levels for at least one hour, and increases self-perception of arousal during masturbation (Burri et al., 2008). Additionally, a recent case study indicates that intranasal Oxt administered during coitus may treat anorgasmia in men in cases where medical conditions, drug abuse, and psychological issues have been ruled out (Ishak et al., 2008).
In women, the primary medical use of Oxt treatment is to bring about labor, as it quickly advances uterine contractions (Carter, 2003). Oxt has also been used to facilitate breast-feeding, as it aids in milk let-down, but its efficacy is uncertain (Anderson and Valdes, 2007). One case study reports that intranasal inhalation of Oxt to stimulate breast-feeding increases vaginal lubrication and feelings of arousal (Anderson-Hunt and Dennerstein, 1994). Furthermore, plasma Oxt levels significantly correlate with higher levels of arousal and lubrication as measured by the Female Sexual Function Index (Salonia et al., 2005). Plasma Oxt levels increase in women during sexual arousal and are elevated further by orgasm (Blaicher et al., 1999; Carmichael et al., 1987).
2.1.4. Aggression
Aggression is part of the complex repertoire of social behaviors that function to increase the likelihood of survival and reproduction. Aggressive behavior occurs in situations of competition (e.g., for food, mates or space) to establish hierarchy in a social group or in defense of altricial young. The type of situation that will elicit aggressive behavior, as well as the behavior display, depends on the species and sex of the animal studied (Miczek et al., 2007). In laboratory settings, rodents are often used to model aggressive interactions (Blanchard and Blanchard, 2003; Blanchard et al., 1975; Malick, 1975; Miczek et al., 2001). In this section, we will describe the role that Oxt plays in male and female aggressive interactions.
2.1.4.1. Animal Models of Aggression in Males
Generally, aggression in male rodents is believed to be heavily under the control of Avp (see (Caldwell et al., 2008) for review). The role of Oxt in controlling aggressive behavior in males is ambiguous and likely depends upon the species used, the test animals’ sexual status and the age at which Oxt levels are manipulated. In prairie voles, ventricular delivery of Oxt reduces sexual behavior but has no effect on aggression (Mahalati et al., 1991). Oxt has been shown to affect aggressive behavior in prairie voles after, but not prior to, mating (Winslow et al., 1993b). This effect is not seen in montane voles, a non-monogamous vole species.
Interestingly, male Wistar rats introduced as an intruder to the cage of a singly housed male rat have between two- and five-fold increases in Oxt levels in the SON and anterior ventrolateral portion of the hypothalamus (Engelmann et al., 1999). This suggests that Oxt’s role in aggressive encounters may have more to do with the stress response to this type of social interaction than aggression per se. This mirrors the link between maternal aggression and an anxious phenotype reported in female rats (Bosch et al., 2005). However, inducing subordination in males through use of a chronic subordinate housing condition increases anxiety but does not result in a change in hypothalamic Oxt mRNA levels, and slightly decreases hypothalamic Avp mRNA only at 20 days of chronic subordinate housing (Reber and Neumann, 2008).
In male squirrel monkeys pair-housed for a sufficient length of time in which to form a stable dominant-subordinate relationship, Oxt significantly increases sexual and aggressive behaviors in the dominant, but not subordinate male, during interaction with a female (Winslow and Insel, 1991). Similarly, the increase in aggression was blocked following concomitant administration of Oxt and the Oxt antagonist OVTA (Winslow and Insel, 1991).
A clear picture has yet to emerge from studies using transgenic mice. One line with inactivation of the Oxt gene is mildly less aggressive than WT or HET controls, and shows no difference in anxiety behavior in an open field (DeVries et al., 1997). In a different line of Oxt KO mice, other researchers have reported increased aggressive behavior in the resident-intruder paradigm and decreased anxiety in the elevated plus maze (EPM) (Winslow et al., 2000). These effects were noted only in KO mice born to obligate mice (KO-KO matings); KO mice, and their WT controls, were cross-fostered to WT mothers. Non-obligates (KO’s produced from HET-HET matings) show no reduction in anxiety and a small increase in aggression only on the third aggressive encounter (Winslow et al., 2000). This suggests that the effects on aggression and anxiety are due to the lack of Oxt in the prenatal environment, or an interaction of genotype and the stress of cross-fostering. Elevated levels of aggression are also reported in Oxtr knockouts generated from non-obligates, consistent with the idea that a lack of prenatal activation of the Oxt system results in increased adult aggression (Takayanagi et al., 2005).
2.1.4.2. Animal Models of Aggression in Females
Female mammals are most aggressive during the postpartum period. In rodents, the mother will attack an unfamiliar male introduced to the cage for several days after giving birth. This type of aggression, dubbed maternal aggression, is a complex behavior that is influenced by a variety of factors that have been extensively reviewed elsewhere (Lonstein and Gammie, 2002).
There are several brain areas that appear to be critical to the mediation of maternal aggression, including the PVN. The expression of the immediate early gene c-Fos is elevated in aggressive, but not non-aggressive, lactating female rats following exposure to intruders, and the immediate early gene EGR-1 is elevated by aggressive experience above levels associated with lactation in lactating female rats (Gammie and Nelson, 2001; Hasen and Gammie, 2006).
The results from PVN lesions are less clear since not all findings report effects in the same direction. Most do suggest a role for the PVN in maternal aggression, however. Electrolytic lesions of the PVN have been shown to decrease maternal aggression in rats (Consiglio and Lucion, 1996). Ibotenic acid lesions directed at the parvocellular portion of the PVN increase maternal aggression, an effect also obtained by blockade of Oxt synthesis by injection of Oxt antisense mRNA into the same region (Giovenardi et al., 1998). Fiber sparing kainic acid lesions of the PVN fail to reduce aggressive behavior, however (Olazabal and Ferreira, 1997). Both studies used female Wistar rats, lesioned on the second day postpartum (selectively targeting the parvocellular region of the PVN) and tested for maternal aggression within five days of giving birth. Thus, it is difficult to reconcile these two findings. The preponderance of evidence does point to PVN involvement in maternal aggression.
The amygdala is another region with demonstrable involvement in maternal aggression. Aggressive encounters in female rats have been shown to elevate c-Fos and EGR-1 levels in several amygdalar nuclei (Gammie and Nelson, 2000; Hasen and Gammie, 2006). Administration of bicuculline, a GABA antagonist, into the amygdala decreases aggression in lactating rats (Hansen and Ferreira, 1986). Infusion of the dual Oxtr/Avpr1a antagonist d(CH2)5[Try(Me)2-Thr4-Tyr-NH29]-vasotocin in the central nucleus of the amygdala (CeA) increases the number of attacks postpartum female rats made against intruders (Lubin et al., 2003). Oxt infused into the CeA and BNST decreases the frequency of biting and frontal attacks (Consiglio et al., 2005). Oxt has the opposite effect in golden hamsters, however. Administration of Oxt into the CeA increases aggression against a male intruder in postpartum females (Ferris et al., 1992). It is unclear whether this difference is species specific or somehow related to dosage.
Oxt may exert its influence on maternal aggression through its role in modulating anxiety. Oxtr are found in the PVN and CeA, two areas that are part of the circuitry mediating anxiety responses. Administration of Oxt in these areas has been linked to increases in maternal aggression (Ferris et al., 1992; Harmon et al., 2002a; Lubin et al., 2003); but see (Consiglio et al., 2005). Moreover, increased levels of aggression and Oxt release are found in lactating rats bred for high levels of anxiety but not in a less aggressive, low anxiety strain (Bosch et al., 2005). This increase in aggressive behavior is blocked in the high anxiety rats by administration of the Oxtr antagonist AOVT but has no effect on the low anxiety group (Bosch et al., 2005). Aggression levels in the low anxiety group are increased by delivery of Oxt to the PVN, however. Thus Oxt may influence anxiety and aggression together in a manner dependent upon circulating levels of Oxt.
2.1.3.3. Oxt and Aggression in Humans
Little is known about the role of Oxt in human aggression. Higher levels of autoantibodies reactive for Oxt are found in males with conduct disorder than in controls (Fetissov et al., 2006). Oxt administration has been shown to reduce amygdalar activity in response to fear-inducing visual stimuli (Kirsch et al., 2005), and anxiety levels appear to be linked to aggression in several animal models (Bosch et al., 2005; Bosch et al., 2007; Winslow et al., 2000). In humans, Oxt may act to decrease anxiety by increasing recognition (Savaskan et al., 2008) and feelings of affiliation (Kosfeld et al., 2005) (see Sections 2.1.1. and 2.1.2).
2.2. Non-social Behavior
In addition to its effects on social behaviors, oxytocin also impacts non-social behaviors such as non-social memory, anxiety, depression, and stress. The following sections will describe the role that Oxt is believed to play in these behaviors in both non-humans (primarily rodents) and humans via clinical studies. The results are summarized in Table 1.
2.2.1. Learning and Memory
2.2.1.1. Rodent studies
Memory processes are highly influenced by neuropeptides. Generally, Avp seems to enhance both non-spatial and spatial memory, likely through connections between the hippocampus and septum (see (Caldwell et al., 2008) for review). In contrast, Oxt seems to attenuate memory processes. Pioneering work by De Wied and colleagues (Bohus et al., 1978; De Wied, 1971; Kovacs et al., 1978) consistently demonstrated that passive avoidance behavior is either unaffected (Bohus et al., 1978; De Wied, 1971), or impaired by administration of Oxt (Kovacs et al., 1978), even when administered at doses equivalent to effective doses of Avp. Specifically, Oxt decreases “step-down latency” (latency to jump off of a platform onto a floor with which the animals had been trained to associate a shock; (Kovacs et al., 1978) and passive-avoidance behavior (latency to enter a dark chamber in which a shock had previously been given (Kovacs et al., 1979). Region-specific effects of Oxt on passive-avoidance behavior are discussed below. For a complete review of this early work see (Kovacs and Telegdy, 1982). Recently, de Oliveria and colleagues also showed that i.p. Oxt administered prior to testing impairs inhibitory avoidance measuring “step-down latency”, without causing increases in anxiety alone (tested on EPM), and with an accompanying decrease in corticosterone levels (de Oliveira et al., 2007). Stress hormones and effects on the hypothalamic-pituitary-adrenal (HPA) axis may, therefore, mediate the amnesic effects of Oxt.
Later research by De Wied and colleagues indicates that Oxt impairs passive avoidance behavior when administered s.c. both after the conditioning trial (post-learning) and one hour prior to the retention trial (pre-retention; (De Wied et al., 1987). Interestingly, fragments of Oxt containing just the C-terminal [Oxt (4–8), Oxt (4–9), Oxt (5–8), Oxt (5–9)] inhibit passive avoidance behavior as well as or better than the ‘parent’ molecule [Oxt (1–9)] (De Wied et al., 1987). Similarly, Oxt (4–9) significantly decreases conditioned freezing behavior (Stoehr et al., 1992). Oxt effects on passive avoidance memory are bimodal, with low doses of Oxt and its C-terminal peptides facilitating, and higher doses inhibiting, passive avoidance behavior (Gaffori and De Wied, 1988). It is not clear how peripheral administration of these peptides could gain entrance to the brain to influence behavior.
The site of injection can also affect Oxt-induced changes in memory. Passive avoidance behavior is impaired with post-learning injections of Oxt in either the dentate gyrus or dorsal raphe nucleus; however, memory is facilitated with Oxt injection into the dorsal septal nucleus (Kovacs et al., 1979). Furthermore, Oxt antiserum injected into dorsal hippocampus, dorsal raphe nucleus, or lateral habenula does not impair passive avoidance (Greidanus and Baars, 1993), contradicting the idea that Oxt is generally an amnesic peptide (reviewed in (Engelmann et al., 1996).
Whether Oxt influences spatial memory is unclear. Oxtr are highly expressed in the hippocampus of mice (Insel et al., 1991). Hippocampal slices treated with Oxt in vitro maintain long-term potentiation (LTP) significantly longer than untreated slices, and have higher levels of phosphorylated CREB (Tomizawa et al., 2003). Additionally, i.c.v. Oxt into virgin females significantly improves reference memory on the radial arm maze (i.e., fewer entries into arms that never contained food) on retention testing 3 days after acquisition. As anxiety-like and locomotor behaviors were not altered in the open field, it is believed that Oxt acts directly on the hippocampus to affect memory, and not indirectly through amygdala-based effects on anxiety (Tomizawa et al., 2003). Furthermore, Oxt seems to underlie parity-induced enhancements to spatial memory, as multiparous females treated with the Oxtr antagonist CPOVT had significantly lower long-term LTP and phosphorylated CREB, as well as poorer reference memory on the radial arm maze (Monks et al., 2003; Tomizawa et al., 2003).
In other brain regions, Oxt also appears to inhibit spatial memory. Injections of Oxt into the nucleus basalis of Meynert (NBM), a region that provides primary cholinergic pathways to the cortex and is involved attention and memory (Wenk, 1997), significantly increases latency to escape onto the hidden platform in the Morris water maze (MWM) (Wu and Yu, 2004). Furthermore, injection of the Oxt antagonist Atosiban into the NBM blocks the Oxt induced impairment, indicating that action at the Oxtr in the NBM is responsible for inhibition of spatial memory (Wu and Yu, 2004). Interestingly, mice lacking the Oxt gene throughout the brain and body do not show deficits on the MWM or Y-maze, indicating that Oxt is not necessary for spatial memory (Ferguson et al., 2000). However, Engelmann and colleagues have recently shown that exposure to the MWM for at least 3 days can significantly increase intra-SON Oxt release (Engelmann et al., 2006). The stress of the MWM could underlie the increased Oxt release, indicating that the MWM is not an optimal test of Oxt effects on spatial memory.
2.2.1.2. Human studies
Similar to rodent studies, the available data in humans indicate that Oxt is generally amnesic in both men and women. Infusion of Oxt into women for therapeutic abortions significantly decreases memory, accuracy, and decisiveness when administered for both four and eight hours (Ferrier et al., 1980). Specifically, word recall ability is decreased (increased number of errors) and picture matching is impaired, i.e., increased number of errors and changes in picture selection (Ferrier et al., 1980). A follow-up study indicates that the treatment itself is not amnesic, as six women undergoing therapeutic abortion without Oxt infusion (treatment otherwise identical to (Ferrier et al., 1980) maintain memory abilities on word recall and picture matching (Kennett et al., 1982). Similarly, in men treated with intranasal Oxt, word recall is significantly impaired in comparison to both placebo controls and subjects administered Lys8-vasopressin (Fehm-Wolfsdorf et al., 1984). In particular, the ability to recall the most recent words presented on a list (i.e., the ‘recency effect’) is impaired. Recently, Heinrichs et al. (Heinrichs et al., 2004) found that Oxt impairs cued recall, as well as generation of words with reproduction-related meaning, but does not impair generation of “neutral” words. The authors conclude that when greater processing is needed, Oxt selectively impairs implicit word memory based upon the meaning of the words (Heinrichs et al., 2004).
Healthy males given Oxt show deficits in learning processes (Bruins et al., 1992). Initial word storage (correctly remembered words after first presentation) and rate of storage (number of trials to recall words at least once) are significantly impaired, with no differences between groups treated with Oxt, Avp, or placebo in measures of attention or arousal (Bruins et al., 1992). Other studies examining Oxt and Avp effects on physiological arousal (heart rate, blood pressure, etc.) also indicate no change in these measures with Oxt administration (reviewed in (Fehm-Wolfsdorf and Born, 1991). These amnesic effects of Oxt are consistent with direct actions of Oxt on memory processes, but access to the brain remains problematical.
2.2.2 Stress, Anxiety and Depression
All creatures engage in allostatic defense in response to stressors, be they physiological or psychogenic. Myriad neural systems are engaged in this complicated process, and a complete description is beyond the scope of this review. Oxt can modulate the physiological and behavioral responses to stress by direct and indirect modulation of the HPA axis at the level of the hypothalamus, amygdala, BNST and septum. As discussed in this section, Oxt appears to have a dampening effect on stress responses. The circuits involved and the mechanisms by which they achieve their coordinated effect still remain to be elucidated.
2.2.2.1. Hypothalamic-pituitary-adrenal Axis and Oxytocin
Oxt plays a role in HPA activity, both in terms of basal function and stress induced activation (Neumann et al., 2000). Two excellent reviews detail Oxt’s effects on HPA functioning (Engelmann et al., 2004; Neumann, 2002), but we will highlight the salient points here. In general, plasma concentrations of Oxt are increased following physiological and psychological stresses, including forced swim, restraint, cold stress, shaker stress, hyperosmolarity and social stress (Engelmann et al., 1999; Gibbs, 1984; Hashiguchi et al., 1997; Jezova et al., 1995; Lang et al., 1983; Neumann et al., 2000).
Oxt was originally thought to have an agonistic effect on adrenocorticotropin hormone (ACTH) release and a facilitative effect on corticotropin-releasing factor (CRF)-mediated ACTH release at the level of the pituitary: Oxt administered to superfused hemipituitaries or pituitary cells in vitro causes the release of ACTH (Link et al., 1992) and potentiates ACTH release in response to CRF (Antoni et al., 1983; Gibbs et al., 1984; Link et al., 1992). This potentiation of ACTH release is due to Oxt’s action at the Avpr1b receptor in the pituitary (Schlosser et al., 1994). I.c.v. Oxt administration decreases plasma ACTH levels in anesthetized rats, possibly by affecting catecholamine levels (Gibbs, 1986). Infusion of the Oxt antagonist AOVT into either the lateral ventricles or directly into the PVN increases basal levels of ACTH, as well as stress induced (e.g., after forced swim or EPM) secretion of ACTH and corticosterone (Neumann et al., 2000).
2.2.2.2. Oxytocin and Anxiety
Oxytocin is thought to work as an anxiolytic as it decreases release of stress hormones in both humans (reviewed in Legros, 2001) and rats (Stachowiak et al., 1995). As stated above (section 2.1.3.1), endogenous release of Oxt in males during mating can reduce anxiety-like behaviors (Waldherr and Neumann, 2007). In male rats, Oxt administration reduces anxiety-like behaviors in a number of behavioral tests (e.g., EPM); in mice, this effect is blocked in some tests by the Oxt antagonist WAY-162720 (Ring et al., 2006). Bilateral Oxt infusions to the PVN produce anxiolytic effects in both the EPM and the light-dark box, likely through activation of the extracellular signal-regulated kinase 1/2 cascade (Blume et al., 2008). However, as mentioned above (section 2.1.4.1), inducing subordination in males through use of a chronic subordinate housing condition increases anxiety but does not result in a change in hypothalamic Oxt mRNA levels (Reber and Neumann, 2008).
Oxt has anxiolytic effects in females as well. Ovariectomized female rats show a dose dependent decrease in plasma corticosterone levels following an auditory stressor after chronic i.c.v. injection of Oxt (Windle et al., 1997). An anxiolytic effect of Oxt is also found when female rats are exposed to a novel environment (Windle et al., 1997). Oxt also has an anxiolytic effect on EPM behavior in female ovariectomized mice, but only with combined E + Oxt treatment (McCarthy et al., 1996). Oxt seems to mediate postpartum reductions in anxiety, as delivery of the Oxtr antagonist desGly-NH2,d(CH2)5[D-Tyr2,Thr4]OVT in the ventrocaudal periaqueductal gray reduces the percentage of time lactating dams spend in open arms of the EPM, but does not affect virgin females’ performance (Figueira et al., 2008).
The anxiolytic properties of Oxt may be mediated, at least in part, via action at the Oxtr in the amygdala. Oxt infusion into the amygdala, but not the VMH, has an anxiolytic effect on ovariectomized female rats in an open field (Bale et al., 2001). The amygdala is well known for its role in the acquisition, modulation and storage of emotional memory (Davis and Whalen, 2001; LeDoux, 2007; Pare et al., 2004; Schulkin et al., 2003). Projections from the septum and the amygdala may modulate the HPA axis via connections to Oxt neurons in the PVN and SON (Oldfield et al., 1985). Within the extended amygdala there are many regions with binding sites for Oxt (Veinante and Freund-Mercier, 1997). This includes a population of neurons with Oxtr in the lateral portion of the CeA that have inhibitory connections to neurons that excite brainstem areas associated with species-specific defensive responses (Huber et al., 2005). Whether this population of neurons plays a functional role in learned fear behavior has yet to be established, but it is clear that Oxt neurons within the CeA modulate some behaviors such as maternal aggression (Bosch et al., 2005; Consiglio et al., 2005; Ferris et al., 1992; Lubin et al., 2003). A recent study (Yoshida, et al., 2009) in mice indicates that many serotonergic raphe neurons express Oxtr and that infusion of Oxt into the median raphe results in serotonin release. Further, i.c.v. Oxt reduces anxiety that is prevented by i.p. administration of a serotonin 5-HT2A/2C receptor antagonist (Yoshida, et al., 2009).
In terms of trait anxiety, results are mixed. Wistar rats bred for high or low levels of anxiety behavior have similar plasma levels of ACTH, corticosterone and Oxt following an EPM stressor (Landgraf et al., 1999). This suggests that Oxt does not play a role in the anxiety displayed by the high anxiety group. However, transgenic female mice lacking endogenous Oxt do show an anxious behavioral. Virgin female Oxt KO mice tested on the EPM make fewer open arm entries than WT, an effect that is reversed by i.c.v. Oxt administration (Mantella et al., 2003). Corticosterone levels are also higher in KO females following acute and chronic shaker stress, though basal levels remain unaffected (Amico et al., 2004a). In a follow-up study, a similar stressor (insertion of rectal probe to record body temperature) also results in higher levels of plasma corticosterone in female Oxt KO mice compared with WT mice (Amico et al., 2008). Interestingly, a physical stressor (insulin-induced hypoglycemia) does not cause female Oxt KO mice to release greater amounts of corticosterone (Amico et al., 2008), implicating the central Oxt pathways in modulating different types of stressors.
In one line of Oxt KO mice, male offspring of KO-KO parents show reduced anxiety on the EPM, though this effect may be due to lack of exposure to Oxt in utero (Winslow et al., 2000); see Section 2.1.4. for more discussion). Others have reported no effect on anxiety-like behavior in Oxtr KO mice or in a partial forebrain-specific Oxtr knockout line (Lee et al., 2008). Future work using temporal and spatial KO of Oxtr populations in the amygdala, septum and striatum through the use of lentiviral gene delivery, for example, will be useful in clarifying the role of Oxt in anxiety.
2.2.2.3. Human Studies
In humans, intranasal Oxt generally facilitates correct classification of emotional faces into either positive or negative categories (Di Simplicio et al., 2008). Oxt may exert effects on emotionality by acting within the amygdala. Oxt appears to reduce amygdalar activation in response to vague or threatening stimuli and may increase feelings of trust and affiliation by reducing social- or novelty-induced amygdalar activation (Baumgartner et al., 2008; Meyer-Lindenberg, 2008). Intranasal Oxt administration reduces amygdalar activation in response to fearful or threatening scenes (Kirsch et al., 2005). Oxt also modulates amygdalar responses to socially relevant stimuli such as faces (Domes et al., 2007b; Kirsch et al., 2005). Pre-stress intranasal administration of Oxt blunts the social stress of speaking in front of an audience, a situation that increases reported feelings of stress as well as cortisol levels (Heinrichs et al., 2003). The anxiolytic effect of Oxt is enhanced by concomitant social support from a friend prior to the stressor (Heinrichs et al., 2003). Lower plasma Oxt levels have been linked to higher levels of psychological distress and less parental attachment (Gordon et al., 2008). Oxt has also been shown to modulate learning about socially relevant stimuli. Subjects trained using the pairing of a mild shock with images of people directing their gaze towards or away from the observer do not show an increase in reaction time or amygdalar responding above control levels to those images paired with shock (Petrovic et al., 2008). The suppression of amygdalar activity by Oxt is even greater for images with direct gaze than indirect gaze (Petrovic et al., 2008). This suggests that Oxt within the amygdala plays a role in processing negative information about negative stimuli, but Oxt is particularly important for more socially relevant (gaze directed) stimuli.
There is also strong evidence that oxytocin is involved in the regulation of affect in humans. Lower levels of plasma Oxt have been reported in humans with major depression (Scantamburlo et al., 2007) while increased post-mortem levels of Oxt mRNA in the PVN are found in patients with melancholic depression (Meynen et al., 2007). Oxt levels are also negatively correlated with self-reported psychological distress, including depressive symptoms (Gordon et al., 2008). In one study of depressed patients, those with the lowest Oxt plasma levels also were those least likely to find social interactions rewarding (Bell et al., 2006). Increases in the number of Oxt-ir neurons in the PVN of people with major depression or bipolar disorder have also been reported (Purba et al., 1996).
Increased Oxt levels postpartum have been associated with elevated mood and decreased anxiety. Administration of the Oxt antagonist AOVT increases ACTH levels in lactating rats (Neumann et al., 2001). In humans, breast-feeding similarly decreases cortisol levels while increasing Oxt levels and is associated with a decrease in negative feelings (Mezzacappa and Katlin, 2002). Compared with non-lactating women, postpartum mothers (2 days after birth) who have received Oxt during labor, just after birth, or were not medicated, have significantly lower scores on the anxiety and aggression scales, and higher on the socialization scale, of the Karolinska Scales of Personality (Jonas et al., 2008). Scores remained similar at 2 and 6 months. Women who received epidural analgesia without Oxt did not differ from the non-lactating women at 2 days postpartum but did at 2 and 6 months, suggesting that both exogenous and endogenous Oxt maintain lower anxiety levels and promote sociability in women through the early postpartum period. A recent study of depressed women reveals increased pulsatile variability and total Oxt release during an affiliation-focused image task (Cyranowski et al., 2008).
2.2.2.5. Conclusion
Both physiological and psychological stressors increase Oxt plasma concentrations and central release in a few key brain regions. This increase dampens the release of HPA axis-mediated stress hormones and creates an anxiolytic behavioral profile. Oxt imbalances have been associated with both anxiety and depression, though direct evidence for Oxt’s role as a therapeutic agent is still awaited. Given the presence of Oxt receptors in the hypothalamus and extended amygdala and its link to HPA function and mood, there is potential for therapeutic use of Oxt in mood disorders.
2.3. Other Behavioral Effects
2.3.1. Oxytocin and feeding behavior
Oxt regulates intake of food and various other solutions, such as NaCl and sucrose. Generally, Oxt suppresses food intake, while at the same time facilitating the onset of sexual behavior. Appetitive control may occur, in part, in the VMH through hormonal like actions after Oxt release from PVN and SON magnocellular dendrites and possibly by yet-to-be-discovered synaptic contact from PVN parvocellular fibers (reviewed in (Leng et al., 2008b; Sabatier et al., 2007). Oxt parvocellular fibers project to the nucleus of the solitary tract as well where regulation of feeding may occur (Blevins, et al., 2003). Oxytocin is also co-expressed with the satiety factor nesfatin-1 in about a quarter of PVN Oxt neurons and 35% of SON Oxt neurons (Kohno et al., 2008). Centrally-administered Oxt and Oxt agonists i.c.v. strongly inhibit feeding (Olson et al., 1991), as does i.p. Oxt, albeit in a dose-dependent manner, with the highest doses most strongly inhibiting food intake (Arletti et al., 1989, 1990). Oxt antagonists prevent this inhibition (Arletti et al., 1989, 1990; Olson et al., 1991). Furthermore, Oxt dose-dependently inhibits water intake in freely-drinking animals, as well as induces thirst (Arletti et al., 1990). During pregnancy, activity of magnocellular Oxt neurons is reduced and Oxt release is inhibited, which may contribute to hyperphagia during pregnancy (Douglas et al., 2007).
Oxt is implicated (along with neuropeptide Y and orexin) in ingestion of sugar, as during a one hour scheduled intake of palatable sucrose pellets, Oxt gene expression is significantly up-regulated in the hypothalamus (Olszewski et al., 2009). However, Oxt KO mice maintain appetite after dehydration, whereas WT littermates do not (Rinaman et al., 2005). Furthermore, compared to WT mice, Oxt KO mice increase ingestion of a sodium (NaCl) solution (Amico et al., 2003), sucrose and saccharine solutions (Amico et al., 2005; Billings et al., 2006), and both sweet and non-sweet carbohydrate solutions (Sclafani et al., 2007), with only an initial (but not sustained) preference for a palatable fat-containing solution (Miedlar et al., 2007). Oxt KO mice show slightly increased weight gain over WT, as well as impaired thermoregulation when exposed to cold (Kasahara et al., 2007). Oxt mRNA and peptide are depleted by approximately 80% in Sim-1 haploinsufficient mice, which have abnormal makeup of the PVN (including PVN neuropeptides) and are severely obese (Kublaoui et al., 2008; Michaud et al., 2001). Treatment with Oxt inhibits food intake in Sim-1 haploinsufficient mice (Kublaoui et al., 2008).
Some evidence indicates that the Oxtr is also involved in feeding behavior. Oxtr WT and KO mice do not differ in overall daily food intake (Leng et al., 2008b; Takayanagi et al., 2008), but late-onset obesity (beginning after 12 weeks old) is observed in Oxtr KO males, and is likely due to heavier fat tissue (Takayanagi et al., 2008). In contrast, no weight differences are found between KO and WT in a different line of Oxtr mice, and partial forebrain-specific Oxtr KO male and female mice (Oxtr FB/FB) weigh significantly less than WT littermates (Lee et al., 2008).
2.3.2. Oxytocin and pain perception
Pain perception is generally measured in rodents through the tail flick test, in which heat is applied to the tail and latency to remove the tail from the heat source is measured. The hot plate test is also used and the latency for the animal to lick his paw, vocalize or jump is measured. Oxt lowers the pain threshold in rats, after either i.c.v. or intra-spinal administration (Yang et al., 2007a, b). Specifically, Oxt increases latency to remove the tail from heat, while the Oxt antagonist CPOVT prevents the antinociceptive properties of Oxt (Arletti et al., 1993; Lundeberg et al., 1994; Uvnas-Moberg et al., 1992). Oxt KO mice have significantly reduced antinociception following stress compared to WT mice, and the Oxt antagonist CPOVT given to WT mice attenuates antinociception (Robinson et al., 2002). Recent research indicates that central Oxt (i.c.v. or intra-spinal) combined with acupuncture treatment significantly increases the pain threshold in compared to non-treated controls (Yang et al., 2007b). Oxt seems to mediate antinociception by connections from Oxt neurons in the PVN to the dorsal horn of the spinal cord (Robinson et al., 2002), specifically by acting upon a subpopulation of lamina II glutamatergic interneurons in the dorsal horn. This generally elevates inhibition at the level of the spinal cord (Breton et al., 2008). Furthermore, pain stimulation decreases Oxt concentration throughout the brain, particularly in hypothalamic regions, although notably not in the PVN (Yang et al., 2007a, b).
Interestingly, a recent study reports that Oxt may underlie ethnic differences in pain perception. African American and non-Hispanic white women given three types of pain-testing procedures display differences in overall tolerance to pain that are correlated with the amount of basal Oxt (Grewen et al., 2008). Oxt levels are also correlated with other measures of pain perception and tolerance, such as norepinephrine and beta-endorphin levels (Grewen et al., 2008).
2.3.3. Oxytocin and grooming
Oxt administered i.c.v. dose-dependently enhances grooming activity in both male and female rats, as does s.c. Oxt, albeit not as strongly (Drago et al., 1986b). Other studies confirm the relationship between Oxt and increased grooming (Caldwell et al., 1986a; Drago et al., 1986a), and find that select brain regions seem to control Oxt-mediated grooming behavior, including the NAcc (Drago et al., 1986a), various hypothalamic nuclei (the so-called “hypothalamic grooming area”, which includes the VMN and dorsal hypothalamic area; (Kaltwasser and Andres, 1989; Roeling et al., 1993), and most notably the ventral tegmentum (Kaltwasser and Andres, 1989; Kaltwasser and Crawley, 1987; Stivers et al., 1988). Furthermore, i.c.v. Oxt significantly increases grooming in Oxt KO mice to greater levels than in WT mice (Amico et al., 2004b). Oxt elicits its effects on grooming by action at the Oxtr, as the Oxt antagonist Atosiban abolishes Oxt, but not Avp, effects on grooming (Amico et al., 2004b). Oxt also seems to underlie grooming of offspring, as administration of an Oxt antagonist increases self-grooming in lactating dams, redirecting grooming behavior from pup-focused to self-focused (Pedersen and Boccia, 2003).
Hypergrooming is one component of animal models of obsessive-compulsive disorder (OCD) (Berridge et al., 2005). As Oxt elevates grooming, and has been shown to be elevated in CSF levels of patients with OCD (Leckman et al., 1994b), some have proposed that Oxt may mediate some forms of OCD (Leckman et al., 1994a). However, not all studies have found elevated Oxt in OCD patients (Altemus et al., 1999), and whether intranasal Oxt can improve OCD symptoms remains unclear (Ansseau et al., 1987; den Boer and Westenberg, 1992). Recently, an Oxt-induced hypergrooming model of OCD was developed by Oxt administration to the CeA (Marroni et al., 2007). Oxt in the CeA significantly increases all components of grooming, likely through connections from the CeA to the hypothalamic grooming area (Marroni et al., 2007).
3. Implications for human behavior
3.1. Love, bonding, and Trust
While many studies indicate Oxt as a facilitator to pair bonding and parental care in animal studies (see Sections 2.1.2.), data from human studies is inconclusive. Generally, it is believed that Oxt facilitates social interactions and feelings of attachment in humans. Heterosexual couples receiving intranasal Oxt prior to a videotaped “conflict discussion” display significantly increased positive communication behaviors (such as eye contact, self-disclosure, and positive body language) than couples treated with placebo (Ditzen et al., 2008). Oxt further strengthens the anxiolytic effect of social support (presence of a friend) during the Trier Social Stress Test (public speaking and arithmetic in front of an audience), as measured by decreased corticosterone in men (Heinrichs et al., 2003). In women, Oxt is positively correlated with higher self-reported feelings of attachment (tendency to express and share emotions) on the Temperament and Character Inventory (Tops et al., 2007). Women viewing pictures of loved ones have high brain activity in dopaminergic pathways associated with reward, which also contain high levels of Oxt and Avp receptors (Bartels and Zeki, 2004), as do people self-describing as being “intensely in love” (Fisher et al., 2005; Fisher et al., 2006). However, no study has conclusively shown that being in a relationship, or “in love,” is associated with high levels of Oxt (reviewed in (Campbell, 2008). Interestingly, dog owners who receive a “long” duration of gaze from their dogs, and report a high degree of attachment to their dogs, have higher urinary Oxt (but not Avp) levels after interacting with the dogs than owners receiving “short” duration of gaze and who report a lower degree of attachment (Nagasawa et al., 2008). This study indicates that Oxt may be involved in other forms of bonding, such as owner-pet, although little other evidence exists to support this idea.
Greater evidence implicates Oxt in human maternal care. Oxt levels are very stable throughout all trimesters of pregnancy, and are positively associated with maternal-fetal attachment as measured by the maternal-fetal attachment scale (self-reported feelings regarding the mothers’ views of their fetus; (Levine et al., 2007). Oxt levels in the first trimester and early postpartum period have been positively correlated with certain maternal bonding behaviors, such as gaze with infant, vocalizations to infant, and affectionate touch (Feldman et al., 2007). Viewing images of their children activates dopaminergic pathways in the mothers’ brains associated with reward that also contain high levels of Oxt and Avp receptors (Bartels and Zeki, 2004). Mothers with variants of the serotonin transporter and the Oxtr genes (the 5-HTT SLC6A4 and OXTR rs53576 polymorphisms, respectively) show lower levels of sensitive responsiveness to their toddlers (rated by observers on the aid given by the mothers to their children on cognitively difficult tasks (Bakermans-Kranenburg and van Ijzendoorn, 2008), implicating systems involved in production and bonding of Oxt in maternal responsiveness. The effect of the rs53576 polymorphism on Oxtr pharmacology is not known.
As adults, plasma Oxt levels are positively correlated with self-report of the affiliative bond to the subjects’ parents, and negatively correlated to reports of depression and anxiety (Gordon et al., 2008). In men with early parental separation (long-term separation from at least one parent due to divorce or death prior to age 13), Oxt does not decrease salivary cortisol levels as in control males, indicating a possible abnormal HPA response to Oxt in these subjects (Meinlschmidt and Heim, 2007). Additionally, it is unclear what effects, if any, exposure to Oxt during breast-feeding could possibly have on development of the offspring (reviewed in (Carter, 2003). It is possible that that Oxt may promote parental care and subsequent feelings of attachment in both the parent and the offspring; however, more study is needed.
Recent work has focused on one route in which Oxt promotes sociability: by increasing feelings of trust. Zak and colleagues have implemented a money transfer game, in which Subject 1 (investor) sends some quantity of money (through a computer) to Subject 2 (trustee), who is told that Subject 1 sent the trustee money. Both subjects are aware that the amount sent will be tripled, and that Subject 2 will be prompted to send an amount back to Subject 1. Trustworthiness is measured by the amounts sent from investor to trustee, and back to investor (Zak, 2008). Plasma Oxt levels are higher in Subject 2 when Subject 1 chooses how much to give (an intentional signal of trust) than when the amount Subject 1 sends is randomly assigned (Zak et al., 2004, 2005), although it should be remembered that peripheral peptide levels do not necessarily reflect central levels (Bartz and Hollander, 2006).
When given intranasal Oxt 50 minutes prior to testing, the average amount of money given by Subject 1 is substantially higher than with placebo treatment (Kosfeld et al., 2005). Interestingly, when subjects play the same game but send money to a “project”, not a “trustee”, Oxt does not increase the amount of money sent, so overall risk-taking behavior is not increased by intranasal Oxt (Kosfeld et al., 2005). Intranasal Oxt maintains trusting behavior, even when subjects learn that their trust has been breached (i.e., “trustee” fails to return money 50% of the time; (Baumgartner et al., 2008). Furthermore, Oxt decreases activity in the amygdala, caudate nucleus, and midbrain regions compared to placebo, indicating that these regions modulate Oxt effects on trust (Baumgartner et al., 2008). Similar to the trust game, intranasal Oxt increases generosity (Subject 1 gives money to Subject 2 while taking into consideration the amount Subject 2 finds acceptable), but not overall altruism (Subject 1 gives to Subject 2 with no feedback from Subject 2; (Zak et al., 2007). Therefore, Oxt seems to particularly affect the ability to understand others’ emotions, i.e., affects empathy.
Intranasal Oxt increases the amount of time spent gazing at the eye region of human faces (Guastella et al., 2008a) as well as the likelihood of recalling a happy face (Guastella et al., 2008b). Intranasal Oxt also improves the ability to infer the mental state of others from social cues in the eye region (Domes et al., 2007b). Similarly, intranasal Oxt attenuates feelings of negativity towards faces conditioned with negative affective ratings, particularly in faces with direct gaze (Petrovic et al., 2008). Oxt may thus increase feelings of trust and empathy by increasing eye gaze and subsequent understanding of social cues. Overall, this line of research indicates that Oxt enhances feelings of generosity, trust, and may aid in detection and understanding of others’ feelings (empathy).
3.2. Autism
Autism is a neuropsychological disorder characterized by abnormal social relationships, including impaired interaction and communication, as well as repetitive and stereotyped behaviors. Autism spectrum disorders (ASD; includes autism) are labeled as pervasive developmental disorders, and can include other medical disorders, such as retardation and seizures, and psychological problems, such as heightened anxiety (Fombonne, 2005). Recently, much effort has gone into determining the underlying causes of autism and related disorders. The positive relationship between Oxt and formation of social bonds in animal studies (reviewed in (Hammock and Young, 2006) has led many to believe that Oxt abnormalities may play a part in autism. Indeed, several studies indicate that single nucleotide polymorphisms (SNPs) in the Oxt (Yrigollen et al., 2008) and Oxtr genes (Jacob et al., 2007; Lerer et al., 2008; Wu et al., 2005) are linked with ASD. Intravenous infusion of Oxt into adults with autism and Asperger’s disorder significantly reduces both number and severity of repetitive behaviors (such as repeating, self-injury, touching; (Hollander et al., 2003), and increases ability to comprehend and remember the affective component of spoken words (happy, indifferent, angry, or sad; (Hollander et al., 2007).
However promising the link between Oxt and autism may be, it is important to remember that many gene systems, in many combinations, contribute to any observable phenotype, and that many systems are currently being explored in relation to autism (Abu-Elneel et al., 2008; McCauley et al., 2005; Morrow et al., 2008; Yan et al., 2008). For a recent review of the possible link beween Oxt and autism, as well as other neuropsychiatric disorders, see (Marazziti and Dell’osso, 2008).
3.3. Oxytocin and schizophrenia
Prepulse inhibition (PPI) of the startle reflex is a form of sensorimotor gating displayed across a variety of species in which the reflexive reaction to a sudden, intense sensory stimulus is reduced by a preceding, weaker sensory stimulus. This gating process is an attentional mechanism that filters potentially distracting stimuli so that attention can be focused on relevant information. Deficits in sensorimotor gating are a feature of many psychiatric and neurological disorders including schizophrenia (Braff et al., 2001; Braff et al., 1992; Grillon et al., 1992; Light and Braff, 1999). Using animal models, PPI has been disrupted in a manner similar to that seen in schizophrenics by the administration of psychotomimetic drugs (Davis et al., 1990; Mansbach et al., 1989; Mansbach and Geyer, 1989; Mansbach et al., 1988), particularly those that affect the dopamine and glutamate/NMDA receptors (Martinez et al., 2000).
Oxt levels may be elevated in patients with psychiatric disorders such as schizophrenia (Beckmann et al., 1985) and OCD (Leckman et al., 1994b), although not all studies find such a difference (Glovinsky et al., 1994). Also, a recent study reports lower levels of Oxt in hyponatremic schizophrenics who display altered HPA activity (Goldman et al., 2008). However, use of antipsychotics such as amperozide (serotonin antagonist) and clozapine (dopamine and partial serotonin agonist) significantly increases plasma Oxt levels (Uvnas-Moberg et al., 1992), indicating that Oxt may act as a natural antipsychotic. Indeed, Oxt restores PPI that is disrupted by dizocilpine (non-competitive NMDA antagonist) and amphetamine (indirect dopamine agonist(Feifel and Reza, 1999). Furthermore, Oxt KO mice exhibit greater PPI deficits with treatment of phencyclidine (PCP, an NMDA antagonist) than do WT mice (Caldwell et al., 2009), indicating that Oxt in particular affects the glutamatergic component of PPI, and likely underlies disruptions in sensory gating observed in schizophrenic patients (Swerdlow et al., 2006).
In addition to disrupting PPI, chronic PCP (14 days) causes social deficits and decreased Oxt binding in the hypothalamus, but increases Oxt binding in the CeA; bilateral Oxt administered to the CeA reverses the social deficits from PCP (Lee et al., 2005). Interestingly, levels of plasma Oxt in schizophrenics positively predicts their ability to identify facial emotions (Goldman et al., 2008), further implicating Oxt in the social aspects of schizophrenia.
4. Concluding Remarks
In the current review, we have detailed the role of Oxt in a variety of behaviors, including recognition of previously met conspecifics, affiliation, sexual behavior, and reduction of anxiety. In light of the prominent role in parturition and essential role in lactation, we are drawn to the view that Oxt serves the continued propagation of a species. As indicated in Figure 5, the cycle of life has numerous points at which Oxt intercedes. In ancient species such as earthworms, leeches, and gastropods, progenitors of Oxt were likely involved in reproduction (Fujino et al., 1999; Oumi et al., 1996; Satake et al., 1999; Van Kesteren et al., 1995). Amazingly, through eons of evolution, Oxt’s repertoire has expanded to maintain a central role in more complicated aspects of reproductive behavior. For these reasons, we call Oxt the great facilitator of life.
Fig. 5.
A simple cycle of life illustrates numerous points at which Oxt may affect behaviors and physiology to facilitate the propagation of the species.
Fig. 2.
Disruption of the Oxtr in mouse forebrain by Camk2a-driven Cre recombinase expression. Oxtr levels were examined by receptor binding in coronal sections from adult WT (A B, C), OxtrFB/FB (D, E, F), and Oxtr−/− (G, H, I) mice. Most areas in the forebrain of OxtrFB/FB mice show decreased levels of binding, with the notable exception of the medial amygdala (MA). Oxtr−/− mice show only background levels. Exposure was for 3 weeks to X-ray film (C). Abbreviations: Am, amygdala; AON, anterior olfactory nucleus; CP, caudate-putamen; Ctx, cerebral cortex; Hi, hippocampal formation; LS, lateral septum; MA, medial amygdala; OB, olfactory bulb; PC, piriform cortex; VP, ventral pallidum. Scale bar equals 0.5 cm. From (Lee et al., 2008).
Fig. 4.
The effects of treatment with saline, 10 mg/kg of amphetamine, 10 mg/kg of apomorphine, or 6 mg/kg of phencyclidine on the prepulse inhibition of the startle reflex (PPI) percentage in Oxt WT (A) and KO (B) mice. Data were analyzed using a repeated measures analysis of variance. There were main effects of drug treatment, but not genotype. Compared to saline, treatment with amphetamine, apomorphine, and phencyclidine all had an effect on PPI percentage and there was a prepulse intensity-dependent increase in PPI percentage across all groups. There was an interaction between drug and genotype. Specifically, in Oxt KO mice, treatment with phencyclidine resulted in impaired PPI compared to saline treatment in Oxt WT mice. From (Caldwell et al., 2009).
Acknowledgments
This work was supported by the NIMH Intramural Research Program (Z01-MH-002498-20).
Abbreviations
- ACTH
adrenocorticotropin hormone
- AOVT
desGly-NH2,d(CH2)5[Try(Me)2,Thr4,Orn8]vasotocin
- Avp
vasopressin
- Avpr
vasopressin receptor
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- CPOVT
d(CH2)5[Tyr(Me)2-Orn8]vasotocin
- CRF
corticotropin-releasing factor
- CSF
cerebrospinal fluid
- E
estrogen
- EB
estradiol benzoate
- EPM
elevated plus maze
- ERE
estrogen-response element
- FB
forebrain
- GnRH
gonadatropin-releasing hormone
- HET
heterozygous
- HPA
hypothalamic-pituitary-adrenal
- i.c.v
intracerebroventricular
- IGR
intergenic region
- i.p
intrperitoneal
- ir
immunoreactive
- KO
knockout
- LQ
lordosis quotient
- mPOA
medial preoptic area of the hypothalamus
- MWM
Morris water maze
- NAcc
nucleus accumbens
- NBM
nucleus basalis of Meynert
- NE
norepinephrine
- OB
olfactory bulb
- OCD
obsessive compulsive disorder
- OVTA
d(CH2)5[Try(Me)2,Thr4,Orn8,Tyr9-NH2]-vasotocin
- OVX
ovariectomized
- Oxt
oxytocin
- Oxtr
oxytocin receptor
- P
progesterone
- PCP
phencyclidine
- PPI
prepulse inhibition
- PVN
paraventricular nucleus of the hypothalamus
- s.c
subcutaneous
- SON
supraoptic nucleus
- VMH
ventromedial hypothalamus
- VTA
ventral tegmental area
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Elneel K, Liu T, Gazzaniga FS, Nishimura Y, Wall DP, Geschwind DH, Lao K, Kosik KS. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics. 2008;9:153–161. doi: 10.1007/s10048-008-0133-5. [DOI] [PubMed] [Google Scholar]
- Acher R, Chauvet J, Chauvet MT. Man and the chimaera. Selective versus neutral oxytocin evolution. Adv Exp Med Biol. 1995;395:615–627. [PubMed] [Google Scholar]
- Agmo A, Choleris E, Kavaliers M, Pfaff DW, Ogawa S. Social and sexual incentive properties of estrogen receptor alpha, estrogen receptor beta, or oxytocin knockout mice. Genes, brain, and behavior. 2008;7:70–77. doi: 10.1111/j.1601-183X.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- Akerlund M, Bossmar T, Brouard R, Kostrzewska A, Laudanski T, Lemancewicz A, Serradeil-Le Gal C, Steinwall M. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects on isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol. 1999;106:1047–1053. doi: 10.1111/j.1471-0528.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- Akerlund M, Carlsson AM, Melin P, Trojnar J. The effect on the human uterus of two newly developed competitive inhibitors of oxytocin and vasopressin. Acta Obstet Gynecol Scand. 1985;64:499–504. doi: 10.3109/00016348509156728. [DOI] [PubMed] [Google Scholar]
- Altemus M, Jacobson KR, Debellis M, Kling M, Pigott T, Murphy DL, Gold PW. Normal CSF oxytocin and NPY levels in OCD. Biol Psychiatry. 1999;45:931–933. doi: 10.1016/s0006-3223(98)00263-7. [DOI] [PubMed] [Google Scholar]
- Amico JA, Cai HM, Vollmer RR. Corticosterone release in oxytocin gene deletion mice following exposure to psychogenic versus non-psychogenic stress. Neurosci Lett. 2008;442:262–266. doi: 10.1016/j.neulet.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico JA, Mantella RC, Vollmer RR. Consumption of solutions containing sodium chloride is enhanced in female oxytocin-deficient mice. Behav Neurosci. 2003;117:32–37. doi: 10.1037//0735-7044.117.1.32. [DOI] [PubMed] [Google Scholar]
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol. 2004a;16:319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1798–1806. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- Amico JA, Vollmer RR, Karam JR, Lee PR, Li X, Koenig JI, McCarthy MM. Centrally administered oxytocin elicits exaggerated grooming in oxytocin null mice. Pharmacol Biochem Behav. 2004b;78:333–339. doi: 10.1016/j.pbb.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Anderson PO, Valdes V. A critical review of pharmaceutical galactagogues. Breastfeed Med. 2007;2:229–242. doi: 10.1089/bfm.2007.0013. [DOI] [PubMed] [Google Scholar]
- Anderson-Hunt M, Dennerstein L. Increased female sexual response after oxytocin. BMJ. 1994;309:929. doi: 10.1136/bmj.309.6959.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansseau M, Legros JJ, Mormont C, Cerfontaine JL, Papart P, Geenen V, Adam F, Franck G. Intranasal oxytocin in obsessive-compulsive disorder. Psychoneuroendocrinology. 1987;12:231–236. doi: 10.1016/0306-4530(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Antoni FA, Holmes MC, Jones MT. Oxytocin as well as vasopressin potentiate ovine CRF in vitro. Peptides. 1983;4:411–415. doi: 10.1016/0196-9781(83)90041-4. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. The role of oxytocin and the paraventricular nucleus in the sexual behaviour of male mammals. Physiol Behav. 2004;83:309–317. doi: 10.1016/j.physbeh.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. Central control of penile erection: role of the paraventricular nucleus of the hypothalamus. Prog Neurobiol. 2005;76:1–21. doi: 10.1016/j.pneurobio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR, Vargiu L, Gessa GL. d(CH2)5Tyr(Me)-[Orn8]vasotocin, a potent oxytocin antagonist, antagonizes penile erection and yawning induced by oxytocin and apomorphine, but not by ACTH-(1–24) Eur J Pharmacol. 1987;134:221–224. doi: 10.1016/0014-2999(87)90168-3. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bazzani C, Castelli M, Bertolini A. Oxytocin improves male copulatory performance in rats. Horm Behav. 1985;19:14–20. doi: 10.1016/0018-506x(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10:89–93. doi: 10.1016/0196-9781(89)90082-x. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav. 1990;48:825–830. doi: 10.1016/0031-9384(90)90234-u. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A. Influence of oxytocin on nociception and morphine antinociception. Neuropeptides. 1993;24:125–129. doi: 10.1016/0143-4179(93)90075-l. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM. Regulation of oxytocin receptor messenger ribonucleic acid in the ventromedial hypothalamus by testosterone and its metabolites. Endocrinology. 1995;136:5135–5138. doi: 10.1210/endo.136.11.7588251. [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM. Cloning, novel promoter sequence, and estrogen regulation of a rat oxytocin receptor gene. Endocrinology. 1997;138:1151–1158. doi: 10.1210/endo.138.3.4998. [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM. NGF, cyclic AMP, and phorbol esters regulate oxytocin receptor gene transcription in SK-N-SH and MCF7 cells. Brain Res Mol Brain Res. 1998;53:130–137. doi: 10.1016/s0169-328x(97)00287-8. [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM, Johnston CA. Oxytocin receptor mRNA expression in the ventromedial hypothalamus during the estrous cycle. J Neurosci. 1995;15:5058–5064. doi: 10.1523/JNEUROSCI.15-07-05058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2003a;117:854–859. doi: 10.1037/0735-7044.117.4.854. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003b;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kim AJ, Lewis-Reese AD, Sue Carter C. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007;144:38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm Behav. 2007b;52:274–279. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balin BJ, Broadwell RD, Salcman M, el-Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J Comp Neurol. 1986;251:260–280. doi: 10.1002/cne.902510209. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Beckmann H, Lang RE, Gattaz WF. Vasopressin--oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology. 1985;10:187–191. doi: 10.1016/0306-4530(85)90056-3. [DOI] [PubMed] [Google Scholar]
- Beery AK, Lacey EA, Francis DD. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis) J Comp Neurol. 2008;507:1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Nicholson H, Mulder RT, Luty SE, Joyce PR. Plasma oxytocin levels in depression and their correlation with the temperament dimension of reward dependence. Journal of psychopharmacology (Oxford, England) 2006;20:656–660. doi: 10.1177/0269881106060512. [DOI] [PubMed] [Google Scholar]
- Benelli A, Bertolini A, Poggioli R, Menozzi B, Basaglia R, Arletti R. Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides. 1995;28:251–255. doi: 10.1016/0143-4179(95)90029-2. [DOI] [PubMed] [Google Scholar]
- Benelli A, Poggioli R, Luppi P, Ruini L, Bertolini A, Arletti R. Oxytocin enhances, and oxytocin antagonism decreases, sexual receptivity in intact female rats. Neuropeptides. 1994;27:245–250. doi: 10.1016/0143-4179(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW, Houchard KR, Zhuang X. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: a model of obsessive compulsive disorder and Tourette’s. BMC Biol. 2005;3:4. doi: 10.1186/1741-7007-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Billings LB, Spero JA, Vollmer RR, Amico JA. Oxytocin null mice ingest enhanced amounts of sweet solutions during light and dark cycles and during repeated shaker stress. Behav Brain Res. 2006;171:134–141. doi: 10.1016/j.bbr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Blaicher W, Gruber D, Bieglmayer C, Blaicher AM, Knogler W, Huber JC. The role of oxytocin in relation to female sexual arousal. Gynecol Obstet Invest. 1999;47:125–126. doi: 10.1159/000010075. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. What can animal aggression research tell us about human aggression? Horm Behav. 2003;44:171–177. doi: 10.1016/s0018-506x(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Fukunaga K, Blanchard DC, Kelley MJ. Conspecific aggression in the laboratory rat. J Comp Physiol Psychol. 1975;89:1204–1209. doi: 10.1037/h0077177. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 2003;993:30–41. doi: 10.1016/j.brainres.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R. Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Res. 1990;535:301–304. doi: 10.1016/0006-8993(90)91613-l. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Schoenen J, Dantzer R. Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res. 1990;519:150–157. doi: 10.1016/0006-8993(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Bohus B, Urban I, van Wimersma Greidanus TB, De Wied D. Opposite effects of oxytocin and vasopressin on avoidance behaviour and hippocampal theta rhythm in the rat. Neuropharmacology. 1978;17:239–247. doi: 10.1016/0028-3908(78)90107-7. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Sartori SB, Singewald N, Neumann ID. Extracellular amino acid levels in the paraventricular nucleus and the central amygdala in high- and low-anxiety dams rats during maternal aggression: regulation by oxytocin. Stress. 2007;10:261–270. doi: 10.1080/10253890701223197. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, Swerdlow NR. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr Res. 2001;49:171–178. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Brennan PAPP. Towards an understanding of the pregnancy-blocking urinary chemosignals of mice. Biochem Soc Trans. 2003;31:152–155. doi: 10.1042/bst0310152. [DOI] [PubMed] [Google Scholar]
- Breton C, Neculcea J, Zingg HH. Renal oxytocin receptor messenger ribonucleic acid: characterization and regulation during pregnancy and in response to ovarian steroid treatment. Endocrinology. 1996;137:2711–2717. doi: 10.1210/endo.137.7.8770890. [DOI] [PubMed] [Google Scholar]
- Breton C, Zingg HH. Expression and region-specific regulation of the oxytocin receptor gene in rat brain. Endocrinology. 1997;138:1857–1862. doi: 10.1210/endo.138.5.5127. [DOI] [PubMed] [Google Scholar]
- Breton JD, Veinante P, Uhl-Bronner S, Vergnano AM, Freund-Mercier MJ, Schlichter R, Poisbeau P. Oxytocin-induced antinociception in the spinal cord is mediated by a subpopulation of glutamatergic neurons in lamina I–II which amplify GABAergic inhibition. Mol Pain. 2008;4:19. doi: 10.1186/1744-8069-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Bruins J, Hijman R, Van Ree JM. Effect of a single dose of des-glycinamide-[Arg8]vasopressin or oxytocin on cognitive processes in young healthy subjects. Peptides. 1992;13:461–468. doi: 10.1016/0196-9781(92)90075-e. [DOI] [PubMed] [Google Scholar]
- Burri A, Heinrichs M, Schedlowski M, Kruger TH. The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology. 2008;33:591–600. doi: 10.1016/j.psyneuen.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Caba M, Silver R, Gonzalez-Mariscal G, Jimenez A, Beyer C. Oxytocin and vasopressin immunoreactivity in rabbit hypothalamus during estrus, late pregnancy, and postpartum. Brain Res. 1996;720:7–16. doi: 10.1016/0006-8993(96)00036-4. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Stephens SL, Young WS., 3rd Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol Psychiatry. 2009;14:190–196. doi: 10.1038/sj.mp.4002150. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Young WS., 3rd . Oxytocin and Vasopressin: Genetics and Behavioral Impolications. In: Lim R, editor. Neuroactive Proteins and Peptides. Springer; New York: 2006. pp. 573–607. [Google Scholar]
- Caldwell JD, Brooks PJ, Jirikowski GF, Barakat AS, Lund PK, Pedersen CA. Estrogen Alters Oxytocin Messenger-Rna Levels in the Preoptic Area. J Neuroendocrinol. 1989a;1:273–278. doi: 10.1111/j.1365-2826.1989.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Greer ER, Johnson MF, Prange AJ, Jr, Pedersen CA. Oxytocin and vasopressin immunoreactivity in hypothalamic and extrahypothalamic sites in late pregnant and postpartum rats. Neuroendocrinology. 1987;46:39–47. doi: 10.1159/000124794. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Hruby VJ, Hill P, Prange AJ, Jr, Pedersen CA. Is oxytocin-induced grooming mediated by uterine-like receptors? Neuropeptides. 1986a;8:77–86. doi: 10.1016/0143-4179(86)90068-5. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Jirikowski GF, Greer ER, Pedersen CA. Medial preoptic area oxytocin and female sexual receptivity. Behav Neurosci. 1989b;103:655–662. doi: 10.1037//0735-7044.103.3.655. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Johns JM, Faggin BM, Senger MA, Pedersen CA. Infusion of an oxytocin antagonist into the medial preoptic area prior to progesterone inhibits sexual receptivity and increases rejection in female rats. Horm Behav. 1994a;28:288–302. doi: 10.1006/hbeh.1994.1024. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Moe BD. Conjugated estradiol increases female sexual receptivity in response to oxytocin infused into the medial preoptic area and medial basal hypothalamus. Horm Behav. 1999;35:38–46. doi: 10.1006/hbeh.1998.1494. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Prange AJ, Jr, Pedersen CA. Oxytocin facilitates the sexual receptivity of estrogen-treated female rats. Neuropeptides. 1986b;7:175–189. doi: 10.1016/0143-4179(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Walker CH, Pedersen CA, Barakat AS, Mason GA. Estrogen increases affinity of oxytocin receptors in the medial preoptic area-anterior hypothalamus. Peptides. 1994b;15:1079–1084. doi: 10.1016/0196-9781(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA, Meaney MJ. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. J Neuroendocrinol. 2008;20:795–801. doi: 10.1111/j.1365-2826.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biol Psychol. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carter CS. Oxytocin and sexual behavior. Neurosci Biobehav Rev. 1992;16:131–144. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Carter DA, Murphy D. Independent regulation of neuropeptide mRNA level and poly(A) tail length. J Biol Chem. 1989;264:6601–6603. [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001a;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Kavaliers M, Pfaff DW. Functional genomics of social recognition. J Neuroendocrinol. 2004;16:383–389. doi: 10.1111/j.0953-8194.2004.01178.x. [DOI] [PubMed] [Google Scholar]
- Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci U S A. 2007;104:4670–4675. doi: 10.1073/pnas.0700670104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes, brain, and behavior. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Clement P, Peeters M, Bernabe J, Denys P, Alexandre L, Giuliano F. Brain oxytocin receptors mediate ejaculation elicited by 7-hydroxy-2-(di-N-propylamino) tetralin (7-OH-DPAT) in anaesthetized rats. Br J Pharmacol. 2008;154:1150–1159. doi: 10.1038/bjp.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coirini H, Johnson AE, McEwen BS. Estradiol modulation of oxytocin binding in the ventromedial hypothalamic nucleus of male and female rats. Neuroendocrinology. 1989;50:193–198. doi: 10.1159/000125221. [DOI] [PubMed] [Google Scholar]
- Consiglio AR, Borsoi A, Pereira GA, Lucion AB. Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav. 2005;85:354–362. doi: 10.1016/j.physbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Consiglio AR, Lucion AB. Lesion of hypothalamic paraventricular nucleus and maternal aggressive behavior in female rats. Physiol Behav. 1996;59:591–596. doi: 10.1016/0031-9384(95)02117-5. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Crowley RS, Amico JA. Gonadal steroid modulation of oxytocin and vasopressin gene expression in the hypothalamus of the osmotically stimulated rat. Endocrinology. 1993;133:2711–2718. doi: 10.1210/endo.133.6.8243294. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Carter CS. Prior exposure To oxytocin mimics the effects Of social contact and facilitates sexual behaviour In females. J Neuroendocrinol. 1999;11:765–769. doi: 10.1046/j.1365-2826.1999.00382.x. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Levine K, Cushing NL. Neonatal manipulation of oxytocin influences female reproductive behavior and success. Horm Behav. 2005;47:22–28. doi: 10.1016/j.yhbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Yamamoto Y, Hoffman GE, Carter CS. Central expression of c-Fos in neonatal male and female prairie voles in response to treatment with oxytocin. Brain Res Dev Brain Res. 2003;143:129–136. doi: 10.1016/s0165-3806(03)00105-6. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale HH. On some physiological actions of ergot. The Journal of physiology. 1906;34:163–206. doi: 10.1113/jphysiol.1906.sp001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- Davis M, Mansbach RS, Swerdlow NR, Campeau S, Braff DL, Geyer MA. Apomorphine disrupts the inhibition of acoustic startle induced by weak prepulses in rats. Psychopharmacology (Berl) 1990;102:1–4. doi: 10.1007/BF02245735. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Veening JG, Olivier B, Waldinger MD. Oxytocin involvement in SSRI-induced delayed ejaculation: a review of animal studies. J Sex Med. 2007;4:14–28. doi: 10.1111/j.1743-6109.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Rotteveel F, Voorhuis TA, Terlou M. Topography of binding sites for neurohypophyseal hormones in rat brain. Eur J Pharmacol. 1985a;110:113–119. doi: 10.1016/0014-2999(85)90036-6. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Voorhuis DA, Boschma Y, Elands J. Estradiol modulates density of putative ‘oxytocin receptors’ in discrete rat brain regions. Neuroendocrinology. 1986;44:415–421. doi: 10.1159/000124680. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Voorhuis TA, Elands J. Estradiol induces oxytocin binding sites in rat hypothalamic ventromedial nucleus. Eur J Pharmacol. 1985b;118:185–186. doi: 10.1016/0014-2999(85)90679-x. [DOI] [PubMed] [Google Scholar]
- de Oliveira LF, Camboim C, Diehl F, Consiglio AR, Quillfeldt JA. Glucocorticoid-mediated effects of systemic oxytocin upon memory retrieval. Neurobiol Learn Mem. 2007;87:67–71. doi: 10.1016/j.nlm.2006.05.006. [DOI] [PubMed] [Google Scholar]
- De Wied D. Long term effect of vasopressin on the maintenance of a conditioned avoidance response in rats. Nature. 1971;232:58–60. doi: 10.1038/232058a0. [DOI] [PubMed] [Google Scholar]
- De Wied D, Gaffori O, Burbach JP, Kovacs GL, van Ree JM. Structure activity relationship studies with C-terminal fragments of vasopressin and oxytocin on avoidance behaviors of rats. J Pharmacol Exp Ther. 1987;241:268–274. [PubMed] [Google Scholar]
- den Boer JA, Westenberg HG. Oxytocin in obsessive compulsive disorder. Peptides. 1992;13:1083–1085. doi: 10.1016/0196-9781(92)90010-z. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Young WS, 3rd, Nelson RJ. Reduced aggressive behaviour in mice with targeted disruption of the oxytocin gene. J Neuroendocrinol. 1997;9:363–368. doi: 10.1046/j.1365-2826.1997.t01-1-00589.x. [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Massey-Chase R, Cowen P, Harmer C. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. Journal of psychopharmacology (Oxford, England) 2008 doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal Oxytocin Increases Positive Communication and Reduces Cortisol Levels During Couple Conflict. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, Ebner K, Landgraf R. Oxytocin induces preservation of social recognition in male rats by activating alpha-adrenoceptors of the olfactory bulb. Eur J Neurosci. 2000;12:760–766. doi: 10.1046/j.1460-9568.2000.00952.x. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, Landgraf R. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998a;19:999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Landgraf R. Olfactory bulb norepinephrine depletion abolishes vasopressin and oxytocin preservation of social recognition responses in rats. Neurosci Lett. 1998b;254:161–164. doi: 10.1016/s0304-3940(98)00691-0. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007a;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007b;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Dominic CJ. Block to pseudopregnancy in mice caused by exposure to male urine. Experientia. 1966;22:534–535. doi: 10.1007/BF01898676. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Johnstone LE, Leng G. Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol Behav. 2007;91:352–365. doi: 10.1016/j.physbeh.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Drago F, Caldwell JD, Pedersen CA, Continella G, Scapagnini U, Prange AJ., Jr Dopamine neurotransmission in the nucleus accumbens may be involved in oxytocin-enhanced grooming behavior of the rat. Pharmacol Biochem Behav. 1986a;24:1185–1188. doi: 10.1016/0091-3057(86)90168-1. [DOI] [PubMed] [Google Scholar]
- Drago F, Pedersen CA, Caldwell JD, Prange AJ., Jr Oxytocin potently enhances novelty-induced grooming behavior in the rat. Brain Res. 1986b;368:287–295. doi: 10.1016/0006-8993(86)90573-1. [DOI] [PubMed] [Google Scholar]
- du Vigneaud V, Ressler C, Swan CJM, Roberts CW, Katsoyannis PG, Gordon S. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J Am Chem Soc. 1953a;75:4879–4880. [Google Scholar]
- du Vigneaud V, Ressler C, Swan JM, Roberts CW, Katsoyannis PG. The synthesis of oxytocin. J Am Chem Soc. 1954;76:3115–3121. [Google Scholar]
- du Vigneaud V, Ressler C, Trippett S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem. 1953b;205:949–957. [PubMed] [Google Scholar]
- Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology. 2004;145:3386–3394. doi: 10.1210/en.2003-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elands J, Barberis C, Jard S, Tribollet E, Dreifuss JJ, Bankowski K, Manning M, Sawyer WH. 125I-labelled d(CH2)5[Tyr(Me)2, Thr4, Tyr-NH2(9)]OVT: a selective oxytocin receptor ligand. Eur J Pharmacol. 1988;147:197–207. doi: 10.1016/0014-2999(88)90778-9. [DOI] [PubMed] [Google Scholar]
- Engell MD, Godwin J, Young LJ, Vandenbergh JG. Perinatal exposure to endocrine disrupting compounds alters behavior and brain in the female pine vole. Neurotoxicol Teratol. 2006;28:103–110. doi: 10.1016/j.ntt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J Neuroendocrinol. 1999;11:867–872. doi: 10.1046/j.1365-2826.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Wotjak CT. Effects of Morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Horm Behav. 2006;50:496–501. doi: 10.1016/j.yhbeh.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Wotjak CT, Landgraf R. Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav Brain Res. 1998;90:89–94. doi: 10.1016/s0166-4328(97)00084-3. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav. 1994;55:145–149. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci Biobehav Rev. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Kornberg E. Acute Luteinizing-Hormone and Prolactin Responses to Paced Mating Stimulation in the Estrous Female Rat. J Neuroendocrinol. 1992;4:173–179. doi: 10.1111/j.1365-2826.1992.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Fang LY, Quan RD, Kaba H. Oxytocin facilitates the induction of long-term potentiation in the accessory olfactory bulb. Neurosci Lett. 2008;438:133–137. doi: 10.1016/j.neulet.2007.12.070. [DOI] [PubMed] [Google Scholar]
- Fehm-Wolfsdorf G, Born J. Behavioral effects of neurohypophyseal peptides in healthy volunteers: 10 years of research. Peptides. 1991;12:1399–1406. doi: 10.1016/0196-9781(91)90226-f. [DOI] [PubMed] [Google Scholar]
- Fehm-Wolfsdorf G, Born J, Voigt KH, Fehm HL. Human memory and neurohypophyseal hormones: opposite effects of vasopressin and oxytocin. Psychoneuroendocrinology. 1984;9:285–292. doi: 10.1016/0306-4530(84)90007-6. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza T. Oxytocin modulates psychotomimetic-induced deficits in sensorimotor gating. Psychopharmacology (Berl) 1999;141:93–98. doi: 10.1007/s002130050811. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Ferrier BM, Kennett DJ, Devlin MC. Influence of oxytocin on human memory processes. Life Sci. 1980;27:2311–2317. doi: 10.1016/0024-3205(80)90499-3. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Foote KB, Meltser HM, Plenby MG, Smith KL, Insel TR. Oxytocin in the amygdala facilitates maternal aggression. Ann N Y Acad Sci. 1992;652:456–457. doi: 10.1111/j.1749-6632.1992.tb34382.x. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Hallman J, Nilsson I, Lefvert AK, Oreland L, Hokfelt T. Aggressive behavior linked to corticotropin-reactive autoantibodies. Biol Psychiatry. 2006;60:799–802. doi: 10.1016/j.biopsych.2006.03.081. [DOI] [PubMed] [Google Scholar]
- Figueira RJ, Peabody MF, Lonstein JS. Oxytocin receptor activity in the ventrocaudal periaqueductal gray modulates anxiety-related behavior in postpartum rats. Behav Neurosci. 2008;122:618–628. doi: 10.1037/0735-7044.122.3.618. [DOI] [PubMed] [Google Scholar]
- Filippi S, Vignozzi L, Vannelli GB, Ledda F, Forti G, Maggi M. Role of oxytocin in the ejaculatory process. J Endocrinol Invest. 2003;26:82–86. [PubMed] [Google Scholar]
- Fisher H, Aron A, Brown LL. Romantic love: an fMRI study of a neural mechanism for mate choice. J Comp Neurol. 2005;493:58–62. doi: 10.1002/cne.20772. [DOI] [PubMed] [Google Scholar]
- Fisher HE, Aron A, Brown LL. Romantic love: a mammalian brain system for mate choice. Philos Trans R Soc Lond B Biol Sci. 2006;361:2173–2186. doi: 10.1098/rstb.2006.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry 66 Suppl. 2005;10:3–8. [PubMed] [Google Scholar]
- Forsling ML, Kallo I, Hartley DE, Heinze L, Ladek R, Coen CW, File SE. Oestrogen receptor-beta and neurohypophysial hormones: functional interaction and neuroanatomical localisation. Pharmacol Biochem Behav. 2003;76:535–542. doi: 10.1016/j.pbb.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Stoeckel ME, Palacios JM, Pazos A, Reichhart JM, Porte A, Richard P. Pharmacological characteristics and anatomical distribution of [3H]oxytocin-binding sites in the Wistar rat brain studied by autoradiography. Neuroscience. 1987;20:599–614. doi: 10.1016/0306-4522(87)90113-8. [DOI] [PubMed] [Google Scholar]
- Fujino Y, Nagahama T, Oumi T, Ukena K, Morishita F, Furukawa Y, Matsushima O, Ando M, Takahama H, Satake H, Minakata H, Nomoto K. Possible functions of oxytocin/vasopressin-superfamily peptides in annelids with special reference to reproduction and osmoregulation. J Exp Zool. 1999;284:401–406. doi: 10.1002/(sici)1097-010x(19990901)284:4<401::aid-jez6>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]
- Gaffori OJ, De Wied D. Bimodal effect of oxytocin on avoidance behavior may be caused by the presence of two peptide sequences with opposite action in the same molecule. Eur J Pharmacol. 1988;147:157–162. doi: 10.1016/0014-2999(88)90774-1. [DOI] [PubMed] [Google Scholar]
- Gainer H, Fields RL, House SB. Vasopressin gene expression: experimental models and strategies. Exp Neurol. 2001;171:190–199. doi: 10.1006/exnr.2001.7769. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. Maternal and mating-induced aggression is associated with elevated citrulline immunoreactivity in the paraventricular nucleus in prairie voles. J Comp Neurol. 2000;418:182–192. [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 2001;898:232–241. doi: 10.1016/s0006-8993(01)02189-8. [DOI] [PubMed] [Google Scholar]
- Gandelman R. The ontogeny of maternal responsiveness in female Rockland-Swiss albino mice. Horm Behav. 1973;4:257–268. doi: 10.1016/0018-506x(73)90010-x. [DOI] [PubMed] [Google Scholar]
- Gibbs DM. Dissociation of oxytocin, vasopressin and corticotropin secretion during different types of stress. Life Sci. 1984;35:487–491. doi: 10.1016/0024-3205(84)90241-8. [DOI] [PubMed] [Google Scholar]
- Gibbs DM. Oxytocin inhibits ACTH and peripheral catecholamine secretion in the urethane-anesthetized rat. Regul Pept. 1986;14:125–132. doi: 10.1016/0167-0115(86)90213-2. [DOI] [PubMed] [Google Scholar]
- Gibbs DM, Vale W, Rivier J, Yen SS. Oxytocin potentiates the ACTH-releasing activity of CRF(41) but not vasopressin. Life Sci. 1984;34:2245–2249. doi: 10.1016/0024-3205(84)90212-1. [DOI] [PubMed] [Google Scholar]
- Gimpl G. Oxytocin receptor ligands: a survey of the patent literature. Expert Opinion on Therapeutic Patents. 2008;18:1239–1251. [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: effects of ibotenic acid lesion and oxytocin antisense. Physiol Behav. 1998;63:351–359. doi: 10.1016/s0031-9384(97)00434-4. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Clement P. Serotonin and premature ejaculation: from physiology to patient management. Eur Urol. 2006;50:454–466. doi: 10.1016/j.eururo.2006.05.055. [DOI] [PubMed] [Google Scholar]
- Glovinsky D, Kalogeras KT, Kirch DG, Suddath R, Wyatt RJ. Cerebrospinal fluid oxytocin concentration in schizophrenic patients does not differ from control subjects and is not changed by neuroleptic medication. Schizophr Res. 1994;11:273–276. doi: 10.1016/0920-9964(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Goldman M, Marlow-O’Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr Res. 2008;98:247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: associations with bonding and psychological distress. Psychophysiology. 2008;45:349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Lester GL. Oxytocin-induced facilitation of lordosis behaviour in rats is progesterone-dependent. Neuropeptides. 1987;10:55–65. doi: 10.1016/0143-4179(87)90089-8. [DOI] [PubMed] [Google Scholar]
- Gould BR, Zingg HH. Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor-LacZ reporter mouse. Neuroscience. 2003;122:155–167. doi: 10.1016/s0306-4522(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Greidanus TBV, Baars A. The Role of Endogenous Vasopressin and Oxytocin in Restricted Brain-Areas on Passive-Avoidance Behavior. Neuroscience Research Communications. 1993;13:133–142. [Google Scholar]
- Grewen KM, Light KC, Mechlin B, Girdler SS. Ethnicity is associated with alterations in oxytocin relationships to pain sensitivity in women. Ethn Health. 2008;13:219–241. doi: 10.1080/13557850701837310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Charney DS, Krystal J, Braff D. Startle gating deficits occur across prepulse intensities in schizophrenic patients. Biol Psychiatry. 1992;32:939–943. doi: 10.1016/0006-3223(92)90183-z. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Carson DS, Dadds MR, Mitchell PB, Cox RE. Does oxytocin influence the early detection of angry and happy faces? Psychoneuroendocrinology. 2009;34:220–225. doi: 10.1016/j.psyneuen.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008a;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry. 2008b;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, Winslow JT, Jensen P, Jeanotte L, Bowen J. Oxytocin changes in males over the reproductive cycle in the monogamous, biparental California mouse, Peromyscus californicus. Horm Behav. 1995;29:59–73. doi: 10.1006/hbeh.1995.1005. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Ferreira A. Food intake, aggression, and fear behavior in the mother rat: control by neural systems concerned with milk ejection and maternal behavior. Behav Neurosci. 1986;100:64–70. doi: 10.1037//0735-7044.100.1.64. [DOI] [PubMed] [Google Scholar]
- Harmon AC, Huhman KL, Moore TO, Albers HE. Oxytocin inhibits aggression in female Syrian hamsters. J Neuroendocrinol. 2002a;14:963–969. doi: 10.1046/j.1365-2826.2002.00863.x. [DOI] [PubMed] [Google Scholar]
- Harmon AC, Moore TO, Huhman KL, Albers HE. Social experience and social context alter the behavioral response to centrally administered oxytocin in female Syrian hamsters. Neuroscience. 2002b;109:767–772. doi: 10.1016/s0306-4522(01)00523-1. [DOI] [PubMed] [Google Scholar]
- Hasen NS, Gammie SC. Maternal aggression: new insights from Egr-1. Brain Res. 2006;1108:147–156. doi: 10.1016/j.brainres.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Hashiguchi H, Ye SH, Morris M, Alexander N. Single and repeated environmental stress: effect on plasma oxytocin, corticosterone, catecholamines, and behavior. Physiol Behav. 1997;61:731–736. doi: 10.1016/s0031-9384(96)00527-6. [DOI] [PubMed] [Google Scholar]
- Haussler HU, Jirikowski GF, Caldwell JD. Sex differences among oxytocin-immunoreactive neuronal systems in the mouse hypothalamus. J Chem Neuroanat. 1990;3:271–276. [PubMed] [Google Scholar]
- Hayes EJ, Weinstein L. Improving patient safety and uniformity of care by a standardized regimen for the use of oxytocin. Am J Obstet Gynecol. 2008;198:622 e621–627. doi: 10.1016/j.ajog.2008.01.039. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Wippich W, Ehlert U, Hellhammer DH. Selective amnesic effects of oxytocin on human memory. Physiol Behav. 2004;83:31–38. doi: 10.1016/j.physbeh.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Steinhauser A, Merchenthaler I, Coen CW, Petersen SL, Liposits Z. Estrogen receptor-beta in oxytocin and vasopressin neurons of the rat and human hypothalamus: Immunocytochemical and in situ hybridization studies. J Comp Neurol. 2004;473:315–333. doi: 10.1002/cne.20127. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kimura T, Azuma C, Inazawa J, Takemura M, Kikuchi T, Kubota Y, Ogita K, Saji F. Structural organization of the human oxytocin receptor gene. J Biol Chem. 1994;269:32451–32456. [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43:623–630. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Winslow JT, Wang Z, Young LJ. Oxytocin, vasopressin, and the neuroendocrine basis of pair bond formation. Adv Exp Med Biol. 1998;449:215–224. doi: 10.1007/978-1-4615-4871-3_28. [DOI] [PubMed] [Google Scholar]
- Insel TR, Winslow JT, Wang ZX, Young L, Hulihan TJ. Oxytocin and the molecular basis of monogamy. Adv Exp Med Biol. 1995;395:227–234. [PubMed] [Google Scholar]
- Insel TR, Winslow JT, Witt DM. Homologous regulation of brain oxytocin receptors. Endocrinology. 1992;130:2602–2608. doi: 10.1210/endo.130.5.1315251. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young L, Witt DM, Crews D. Gonadal steroids have paradoxical effects on brain oxytocin receptors. J Neuroendocrinol. 1993;5:619–628. doi: 10.1111/j.1365-2826.1993.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Ishak WW, Berman DS, Peters A. Male anorgasmia treated with oxytocin. J Sex Med. 2008;5:1022–1024. doi: 10.1111/j.1743-6109.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- Ivell R, Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984;81:2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. Ann N Y Acad Sci. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Johns JM, Joyner PW, McMurray MS, Elliott DL, Hofler VE, Middleton CL, Knupp K, Greenhill KW, Lomas LM, Walker CH. The effects of dopaminergic/serotonergic reuptake inhibition on maternal behavior, maternal aggression, and oxytocin in the rat. Pharmacol Biochem Behav. 2005;81:769–785. doi: 10.1016/j.pbb.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AE, Coirini H, Insel TR, McEwen BS. The regulation of oxytocin receptor binding in the ventromedial hypothalamic nucleus by testosterone and its metabolites. Endocrinology. 1991;128:891–896. doi: 10.1210/endo-128-2-891. [DOI] [PubMed] [Google Scholar]
- Jonas W, Nissen E, Ransjo-Arvidson AB, Matthiesen AS, Uvnas-Moberg K. Influence of oxytocin or epidural analgesia on personality profile in breastfeeding women: a comparative study. Arch Womens Ment Health. 2008;11:335–345. doi: 10.1007/s00737-008-0027-4. [DOI] [PubMed] [Google Scholar]
- Kaltwasser MT, Andres U. The interaction of central oxytocin and cholecystokinin regulates grooming behavior in the rat. Brain Res. 1989;496:380–384. doi: 10.1016/0006-8993(89)91093-7. [DOI] [PubMed] [Google Scholar]
- Kaltwasser MT, Crawley JN. Oxytocin and cholecystokinin induce grooming behavior in the ventral tegmentum of the rat. Brain Res. 1987;426:1–7. doi: 10.1016/0006-8993(87)90418-5. [DOI] [PubMed] [Google Scholar]
- Kasahara Y, Takayanagi Y, Kawada T, Itoi K, Nishimori K. Impaired thermoregulatory ability of oxytocin-deficient mice during cold-exposure. Biosci Biotechnol Biochem. 2007;71:3122–3126. doi: 10.1271/bbb.70498. [DOI] [PubMed] [Google Scholar]
- Kennett DJ, Devlin MC, Ferrier BM. Influence of oxytocin on human memory processes: validation by a control study. Life Sci. 1982;31:273–275. doi: 10.1016/0024-3205(82)90589-6. [DOI] [PubMed] [Google Scholar]
- Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356:526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology. 2008;149:1295–1301. doi: 10.1210/en.2007-1276. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Bohus B, Versteeg DH, De Kloet ER, De Wied D. Effect of oxytocin and vasopressin on memory consolidation: sites of action and catecholaminergic correlates after local microinjection into limbic-midbrain structures. Brain Res. 1979;175:303–314. doi: 10.1016/0006-8993(79)91009-6. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Telegdy G. Role of oxytocin in memory and amnesia. Pharmacol Ther. 1982;18:375–395. doi: 10.1016/0163-7258(82)90038-9. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Vecsei L, Telegdy G. Opposite action of oxytocin to vasopressin in passive avoidance behavior in rats. Physiol Behav. 1978;20:801–802. doi: 10.1016/0031-9384(78)90309-8. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav Brain Res. 1998;92:169–180. doi: 10.1016/s0166-4328(97)00189-7. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Choe C, Carter CS, Cushing BS. Developmental effects of oxytocin on neural activation and neuropeptide release in response to social stimuli. Horm Behav. 2006;49:206–214. doi: 10.1016/j.yhbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Yoshida S, Papademetriou E, Cushing BS. The organizational effects of oxytocin on the central expression of estrogen receptor alpha and oxytocin in adulthood. BMC neuroscience. 2007;8:71. doi: 10.1186/1471-2202-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremarik P, Freund-Mercier MJ, Stoeckel ME. Histoautoradiographic detection of oxytocin- and vasopressin-binding sites in the telencephalon of the rat. J Comp Neurol. 1993;333:343–359. doi: 10.1002/cne.903330304. [DOI] [PubMed] [Google Scholar]
- Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Kimura T, Hashimoto K, Tokugawa Y, Nobunaga K, Azuma C, Saji F, Murata Y. Structure and expression of the mouse oxytocin receptor gene. Mol Cell Endocrinol. 1996;124:25–32. doi: 10.1016/s0303-7207(96)03923-8. [DOI] [PubMed] [Google Scholar]
- Landgraf R. Neuropeptides in anxiety modulation. Handbook of experimental pharmacology. 2005:335–369. doi: 10.1007/3-540-28082-0_12. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann I, Pittman QJ. Septal and hippocampal release of vasopressin and oxytocin during late pregnancy and parturition in the rat. Neuroendocrinology. 1991;54:378–383. doi: 10.1159/000125917. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann I, Russell JA, Pittman QJ. Push-pull perfusion and microdialysis studies of central oxytocin and vasopressin release in freely moving rats during pregnancy, parturition, and lactation. Ann N Y Acad Sci. 1992;652:326–339. doi: 10.1111/j.1749-6632.1992.tb34364.x. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wigger A, Holsboer F, Neumann ID. Hyper-reactive hypothalamo-pituitary-adrenocortical axis in rats bred for high anxiety-related behaviour. J Neuroendocrinol. 1999;11:405–407. doi: 10.1046/j.1365-2826.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- Lang RE, Heil JW, Ganten D, Hermann K, Unger T, Rascher W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology. 1983;37:314–316. doi: 10.1159/000123566. [DOI] [PubMed] [Google Scholar]
- Larcher A, Neculcea J, Breton C, Arslan A, Rozen F, Russo C, Zingg HH. Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology. 1995;136:5350–5356. doi: 10.1210/endo.136.12.7588281. [DOI] [PubMed] [Google Scholar]
- Larrazolo-Lopez A, Kendrick KM, Aburto-Arciniega M, Arriaga-Avila V, Morimoto S, Frias M, Guevara-Guzman R. Vaginocervical stimulation enhances social recognition memory in rats via oxytocin release in the olfactory bulb. Neuroscience. 2008;152:585–593. doi: 10.1016/j.neuroscience.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL, Anderson GM, Riddle MA, McDougle CJ, Barr LC, et al. The role of central oxytocin in obsessive compulsive disorder and related normal behavior. Psychoneuroendocrinology. 1994a;19:723–749. doi: 10.1016/0306-4530(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL, Anderson GM, Riddle MA, McSwiggan-Hardin M, McDougle CJ, et al. Elevated cerebrospinal fluid levels of oxytocin in obsessive-compulsive disorder. Comparison with Tourette’s syndrome and healthy controls. Arch Gen Psychiatry. 1994b;51:782–792. doi: 10.1001/archpsyc.1994.03950100030003. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology. 2005;30:1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- Legros JJ. Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying-yang neurohormones? Psychoneuroendocrinology. 2001;26:649–655. doi: 10.1016/s0306-4530(01)00018-x. [DOI] [PubMed] [Google Scholar]
- Leng G, Meddle SL, Douglas AJ. Oxytocin and the maternal brain. Curr Opin Pharmacol. 2008a;8:731–734. doi: 10.1016/j.coph.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Leng G, Onaka T, Caquineau C, Sabatier N, Tobin VA, Takayanagi Y. Oxytocin and appetite. Prog Brain Res. 2008b;170:137–151. doi: 10.1016/S0079-6123(08)00413-5. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Levy F, Dreifuss JJ, Dubois-Dauphin M, Berti M, Barberis C, Tribollet E. Autoradiographic detection of vasopressin binding sites, but not of oxytocin binding sites, in the sheep olfactory bulb. Brain Res. 1992;595:154–158. doi: 10.1016/0006-8993(92)91467-s. [DOI] [PubMed] [Google Scholar]
- Levy F, Kendrick KM, Goode JA, Guevara-Guzman R, Keverne EB. Oxytocin and vasopressin release in the olfactory bulb of parturient ewes: changes with maternal experience and effects on acetylcholine, gamma-aminobutyric acid, glutamate and noradrenaline release. Brain Res. 1995;669:197–206. doi: 10.1016/0006-8993(94)01236-b. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Human and animal studies of schizophrenia-related gating deficits. Current psychiatry reports. 1999;1:31–40. doi: 10.1007/s11920-999-0008-y. [DOI] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Link H, Dayanithi G, Fohr KJ, Gratzl M. Oxytocin at physiological concentrations evokes adrenocorticotropin (ACTH) release from corticotrophs by increasing intracellular free calcium mobilized mainly from intracellular stores. Oxytocin displays synergistic or additive effects on ACTH-releasing factor or arginine vasopressin-induced ACTH secretion, respectively. Endocrinology. 1992;130:2183–2191. doi: 10.1210/endo.130.4.1312449. [DOI] [PubMed] [Google Scholar]
- Liu JW, Ben-Jonathan N. Prolactin-releasing activity of neurohypophysial hormones: structure-function relationship. Endocrinology. 1994;134:114–118. doi: 10.1210/endo.134.1.8275925. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss JJ. Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain Res. 1989;500:223–230. doi: 10.1016/0006-8993(89)90317-x. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav Neurosci. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature reviews. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lundeberg T, Uvnas-Moberg K, Agren G, Bruzelius G. Anti-nociceptive effects of oxytocin in rats and mice. Neurosci Lett. 1994;170:153–157. doi: 10.1016/0304-3940(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Mahalati K, Okanoya K, Witt DM, Carter CS. Oxytocin inhibits male sexual behavior in prairie voles. Pharmacol Biochem Behav. 1991;39:219–222. doi: 10.1016/0091-3057(91)90426-3. [DOI] [PubMed] [Google Scholar]
- Malick JB. Effects of age and food deprivation on the development of muricidal behavior in rats. Physiol Behav. 1975;14:171–175. doi: 10.1016/0031-9384(75)90162-6. [DOI] [PubMed] [Google Scholar]
- Manning M, Cheng LL, Klis WA, Stoev S, Przybylski J, Bankowski K, Sawyer WH, Barberis C, Chan WY. Advances in the design of selective antagonists, potential tocolytics, and radioiodinated ligands for oxytocin receptors. Adv Exp Med Biol. 1995;395:559–583. [PubMed] [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V(1a), V(1b), V(2) and OT receptors: research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Braff DL, Geyer MA. Prepulse inhibition of the acoustic startle response is disrupted by N-ethyl-3,4-methylenedioxyamphetamine (MDEA) in the rat. Eur J Pharmacol. 1989;167:49–55. doi: 10.1016/0014-2999(89)90746-2. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA. Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neuropsychopharmacology. 1989;2:299–308. doi: 10.1016/0893-133x(89)90035-3. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berl) 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144:2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Dell’osso MC. The role of oxytocin in neuropsychiatric disorders. Curr Med Chem. 2008;15:698–704. doi: 10.2174/092986708783885291. [DOI] [PubMed] [Google Scholar]
- Marroni SS, Nakano FN, Gati CD, Oliveira JA, Antunes-Rodrigues J, Garcia-Cairasco N. Neuroanatomical and cellular substrates of hypergrooming induced by microinjection of oxytocin in central nucleus of amygdala, an experimental model of compulsive behavior. Mol Psychiatry. 2007;12:1103–1117. doi: 10.1038/sj.mp.4002015. [DOI] [PubMed] [Google Scholar]
- Martinez ZA, Halim ND, Oostwegel JL, Geyer MA, Swerdlow NR. Ontogeny of phencyclidine and apomorphine-induced startle gating deficits in rats. Pharmacol Biochem Behav. 2000;65:449–457. doi: 10.1016/s0091-3057(99)00217-8. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Ontogeny of maternal behavior in the laboratory rat: early origins in 18- to 27-day-old young. Dev Psychobiol. 1979;12:407–424. doi: 10.1002/dev.420120502. [DOI] [PubMed] [Google Scholar]
- McCafferty GP, Pullen MA, Wu C, Edwards RM, Allen MJ, Woollard PM, Borthwick AD, Liddle J, Hickey DM, Brooks DP, Westfall TD. Use of a novel and highly selective oxytocin receptor antagonist to characterize uterine contractions in the rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R299–305. doi: 10.1152/ajpregu.00057.2007. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Li C, Jiang L, Olson LM, Crockett G, Gainer K, Folstein SE, Haines JL, Sutcliffe JS. Genome-wide and Ordered-Subset linkage analyses provide support for autism loci on 17q and 19p with evidence of phenotypic and interlocus genetic correlates. BMC Med Genet. 2005;6:1. doi: 10.1186/1471-2350-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148:5095–5104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol Psychiatry. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Melin P, Kihlstroem JE. Influence of Oxytocin on Sexual Behavior in Male Rabbits. Endocrinology. 1963;73:433–435. doi: 10.1210/endo-73-4-433. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A, Gessa GL. Evidence that apomorphine induces penile erection and yawning by releasing oxytocin in the central nervous system. Eur J Pharmacol. 1989;164:565–570. doi: 10.1016/0014-2999(89)90265-3. [DOI] [PubMed] [Google Scholar]
- Melis MR, Mauri A, Argiolas A. Apomorphine-and oxytocin-induced penile erection and yawning in intact and castrated male rats: effect of sexual steroids. Neuroendocrinology. 1994;59:349–354. doi: 10.1159/000126677. [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, Boi A, Ferri GL, Argiolas A. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur J Neurosci. 2007;26:1026–1035. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- Melis MR, Spano MS, Succu S, Argiolas A. The oxytocin antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin reduces non-contact penile erections in male rats. Neurosci Lett. 1999;265:171–174. doi: 10.1016/s0304-3940(99)00236-0. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Iannucci U, Argiolas A. Oxytocin increases nitric oxide production in the paraventricular nucleus of the hypothalamus of male rats: correlation with penile erection and yawning. Regul Pept. 1997;69:105–111. doi: 10.1016/s0167-0115(97)00002-5. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Spano MS, Argiolas A. Effect of excitatory amino acid, dopamine, and oxytocin receptor antagonists on noncontact penile erections and paraventricular nitric oxide production in male rats. Behav Neurosci. 2000;114:849–857. doi: 10.1037//0735-7044.114.4.849. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. Impact of prosocial neuropeptides on human brain function. Prog Brain Res. 2008;170:463–470. doi: 10.1016/S0079-6123(08)00436-6. [DOI] [PubMed] [Google Scholar]
- Meynen G, Unmehopa UA, Hofman MA, Swaab DF, Hoogendijk WJ. Hypothalamic oxytocin mRNA expression and melancholic depression. Mol Psychiatry. 2007;12:118–119. doi: 10.1038/sj.mp.4001911. [DOI] [PubMed] [Google Scholar]
- Mezey E, Kiss JZ. Coexpression of vasopressin and oxytocin in hypothalamic supraoptic neurons of lactating rats. Endocrinology. 1991;129:1814–1820. doi: 10.1210/endo-129-4-1814. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Katlin ES. Breast-feeding is associated with reduced perceived stress and negative mood in mothers. Health Psychol. 2002;21:187–193. [PubMed] [Google Scholar]
- Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, Levy E, Mitchell GA, Himms-Hagen J, Fan CM. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet. 2001;10:1465–1473. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998;12:3264–3275. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, de Almeida RM, Kravitz EA, Rissman EF, de Boer SF, Raine A. Neurobiology of escalated aggression and violence. J Neurosci. 2007;27:11803–11806. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Mohr E, Schmitz E. Functional characterization of estrogen and glucocorticoid responsive elements in the rat oxytocin gene. Brain Res Mol Brain Res. 1991;9:293–298. doi: 10.1016/0169-328x(91)90075-9. [DOI] [PubMed] [Google Scholar]
- Monks DA, Lonstein JS, Breedlove SM. Got milk? Oxytocin triggers hippocampal plasticity. Nat Neurosci. 2003;6:327–328. doi: 10.1038/nn0403-327. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa M, Kikusui T, Onaka T, Ohta M. Dog’s gaze at its owner increases owner’s urinary oxytocin during social interaction. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Nakai S, Kawano H, Yudate T, Nishi M, Kuno J, Nagata A, Jishage K, Hamada H, Fujii H, Kawamura K, et al. The POU domain transcription factor Brn-2 is required for the determination of specific neuronal lineages in the hypothalamus of the mouse. Genes Dev. 1995;9:3109–3121. doi: 10.1101/gad.9.24.3109. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Advances in vasopressin and oxytocin--from genes to behaviour to disease. Preface Prog Brain Res. 2008;170:xi–xiii. doi: 10.1016/S0079-6123(08)00448-2. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Toschi N, Ohl F, Torner L, Kromer SA. Maternal defence as an emotional stressor in female rats: correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. Eur J Neurosci. 2001;13:1016–1024. doi: 10.1046/j.0953-816x.2001.01460.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A. 1996;93:11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop LE, Erskine MS. Selective oxytocin receptor activation in the ventrolateral portion of the ventromedial hypothalamus is required for mating-induced pseudopregnancy in the female rat. Endocrinology. 2008;149:836–842. doi: 10.1210/en.2007-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazabal DE, Ferreira A. Maternal behavior in rats with kainic acid-induced lesions of the hypothalamic paraventricular nucleus. Physiol Behav. 1997;61:779–784. doi: 10.1016/s0031-9384(96)00567-7. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Variability in “spontaneous” maternal behavior is associated with anxiety-like behavior and affiliation in naive juvenile and adult female prairie voles (Microtus ochrogaster) Dev Psychobiol. 2005;47:166–178. doi: 10.1002/dev.20077. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006a;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006b;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Hou-Yu A, Silverman AJ. A combined electron microscopic HRP and immunocytochemical study of the limbic projections to rat hypothalamic nuclei containing vasopressin and oxytocin neurons. J Comp Neurol. 1985;231:221–231. doi: 10.1002/cne.902310209. [DOI] [PubMed] [Google Scholar]
- Olivier B, Chan JS, Pattij T, de Jong TR, Oosting RS, Veening JG, Waldinger MD. Psychopharmacology of male rat sexual behavior: modeling human sexual dysfunctions? Int J Impot Res. 2006;18(Suppl 1):S14–23. doi: 10.1038/sj.ijir.3901330. [DOI] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991;12:113–118. doi: 10.1016/0196-9781(91)90176-p. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Shaw TJ, Grace MK, Hoglund CE, Fredriksson R, Schioth HB, Levine AS. Complexity of neural mechanisms underlying overconsumption of sugar in scheduled feeding: Involvement of opioids, orexin, oxytocin and NPY. Peptides. 2009;30:226–233. doi: 10.1016/j.peptides.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski NL. Oxytocin receptor mRNA expression in rat brain: implications for behavioral integration and reproductive success. Psychoneuroendocrinology. 1998;23:989–1004. doi: 10.1016/s0306-4530(98)00070-5. [DOI] [PubMed] [Google Scholar]
- Ostrowski NL, Young WS, 3rd, Lolait SJ. Estrogen increases renal oxytocin receptor gene expression. Endocrinology. 1995;136:1801–1804. doi: 10.1210/endo.136.4.7895693. [DOI] [PubMed] [Google Scholar]
- Ott I, Scott JC. The Action of Infundibulum upon Mammary Secretion. Proc Soc Exp Biol. 1910;8:48–49. [Google Scholar]
- Oumi T, Ukena K, Matsushima O, Ikeda T, Fujita T, Minakata H, Nomoto K. Annetocin, an annelid oxytocin-related peptide, induces egg-laying behavior in the earthworm, Eisenia foetida. J Exp Zool. 1996;276:151–156. doi: 10.1002/(SICI)1097-010X(19961001)276:2<151::AID-JEZ8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parent AS, Rasier G, Matagne V, Lomniczi A, Lebrethon MC, Gerard A, Ojeda SR, Bourguignon JP. Oxytocin facilitates female sexual maturation through a glia-to-neuron signaling pathway. Endocrinology. 2008;149:1358–1365. doi: 10.1210/en.2007-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Kinney LF, Phillips KM, Lee TM. Paternal behavior is associated with central neurohormone receptor binding patterns in meadow voles (Microtus pennsylvanicus) Behav Neurosci. 2001;115:1341–1348. doi: 10.1037//0735-7044.115.6.1341. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin maintains as well as initiates female sexual behavior: effects of a highly selective oxytocin antagonist. Horm Behav. 2002;41:170–177. doi: 10.1006/hbeh.2001.1736. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin antagonism alters rat dams’ oral grooming and upright posturing over pups. Physiol Behav. 2003;80:233–241. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Vasopressin interactions with oxytocin in the control of female sexual behavior. Neuroscience. 2006;139:843–851. doi: 10.1016/j.neuroscience.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Johnson MF, Fort SA, Prange AJ., Jr Oxytocin antiserum delays onset of ovarian steroid-induced maternal behavior. Neuropeptides. 1985;6:175–182. doi: 10.1016/0143-4179(85)90108-8. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes, brain, and behavior. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol. 1991;1:555–560. doi: 10.1016/0924-977x(91)90010-r. [DOI] [PubMed] [Google Scholar]
- Popik P, Vetulani J, Van Ree JM. Facilitation and attenuation of social recognition in rats by different oxytocin-related peptides. Eur J Pharmacol. 1996;308:113–116. doi: 10.1016/0014-2999(96)00215-4. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S, Ogawa S, Adan RA, Burbach JP, Pfaff DW. Effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the uterus, pituitary, and forebrain of the female rat. Neuroendocrinology. 1997;65:9–17. doi: 10.1159/000127160. [DOI] [PubMed] [Google Scholar]
- Raggenbass M. Overview of cellular electrophysiological actions of vasopressin. Eur J Pharmacol. 2008;583:243–254. doi: 10.1016/j.ejphar.2007.11.074. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Cushing BS, Carter CS, Valsecchi P. Hormonal regulation of agonistic and affiliative behavior in female mongolian gerbils (Meriones unguiculatus) Horm Behav. 2003;43:549–553. doi: 10.1016/s0018-506x(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Reber SO, Neumann ID. Defensive behavioral strategies and enhanced state anxiety during chronic subordinate colony housing are accompanied by reduced hypothalamic vasopressin, but not oxytocin, expression. Ann N Y Acad Sci. 2008;1148:184–195. doi: 10.1196/annals.1410.003. [DOI] [PubMed] [Google Scholar]
- Richard S, Zingg HH. The human oxytocin gene promoter is regulated by estrogens. J Biol Chem. 1990;265:6098–6103. [PubMed] [Google Scholar]
- Richter ON, Kubler K, Schmolling J, Kupka M, Reinsberg J, Ulrich U, van der Ven H, Wardelmann E, van der Ven K. Oxytocin receptor gene expression of estrogen-stimulated human myometrium in extracorporeally perfused non-pregnant uteri. Mol Hum Reprod. 2004;10:339–346. doi: 10.1093/molehr/gah039. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Vollmer RR, Karam J, Phillips D, Li X, Amico JA. Dehydration anorexia is attenuated in oxytocin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1791–1799. doi: 10.1152/ajpregu.00860.2004. [DOI] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Robinson DA, Wei F, Wang GD, Li P, Kim SJ, Vogt SK, Muglia LJ, Zhuo M. Oxytocin mediates stress-induced analgesia in adult mice. The Journal of physiology. 2002;540:593–606. doi: 10.1113/jphysiol.2001.013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeling TA, Veening JG, Peters JP, Vermelis ME, Nieuwenhuys R. Efferent connections of the hypothalamic “grooming area” in the rat. Neuroscience. 1993;56:199–225. doi: 10.1016/0306-4522(93)90574-y. [DOI] [PubMed] [Google Scholar]
- Rosen GJ, de Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience. 2008;155:809–817. doi: 10.1016/j.neuroscience.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD, Giordano AL. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Smith EL, Altemus M, Scharf BA, Owens MJ, Nemeroff CB, Gorman JM, Coplan JD. Differing concentrations of corticotropin-releasing factor and oxytocin in the cerebrospinal fluid of bonnet and pigtail macaques. Psychoneuroendocrinology. 2002;27:651–660. doi: 10.1016/s0306-4530(01)00056-7. [DOI] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F. Emergent synchronous bursting of oxytocin neuronal network. PLoS Comput Biol. 2008;4:e1000123. doi: 10.1371/journal.pcbi.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen F, Russo C, Banville D, Zingg HH. Structure, characterization, and expression of the rat oxytocin receptor gene. Proc Natl Acad Sci U S A. 1995;92:200–204. doi: 10.1073/pnas.92.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Rowe I, Leng G. Central release of oxytocin and the ventromedial hypothalamus. Biochem Soc Trans. 2007;35:1247–1251. doi: 10.1042/BST0351247. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Morris JF, Prossnitz ER, Kawata M. Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology. 2007;148:5842–5850. doi: 10.1210/en.2007-0436. [DOI] [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, Fabbri F, Zanni G, Rigatti P, Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47:164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Samson WK, Lumpkin MD, McCann SM. Evidence for a physiological role for oxytocin in the control of prolactin secretion. Endocrinology. 1986;119:554–560. doi: 10.1210/endo-119-2-554. [DOI] [PubMed] [Google Scholar]
- Sanchez R, Nguyen D, Rocha W, White JH, Mader S. Diversity in the mechanisms of gene regulation by estrogen receptors. Bioessays. 2002;24:244–254. doi: 10.1002/bies.10066. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andrade G, Kendrick KM. The main olfactory system and social learning in mammals. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Satake H, Takuwa K, Minakata H, Matsushima O. Evidence for conservation of the vasopressin/oxytocin superfamily in Annelida. J Biol Chem. 1999;274:5605–5611. doi: 10.1074/jbc.274.9.5605. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Schafer EA, Mackenzie K. The action of animal extracts on milk secretion. Proceedings of the Royal Society of London Series B-Containing Papers of a Biological Character. 1911;84:16–22. [Google Scholar]
- Schlosser SF, Almeida OF, Patchev VK, Yassouridis A, Elands J. Oxytocin-stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology. 1994;135:2058–2063. doi: 10.1210/endo.135.5.7956927. [DOI] [PubMed] [Google Scholar]
- Schonemann MD, Ryan AK, McEvilly RJ, O’Connell SM, Arias CA, Kalla KA, Li P, Sawchenko PE, Rosenfeld MG. Development and survival of the endocrine hypothalamus and posterior pituitary gland requires the neuronal POU domain factor Brn-2. Genes Dev. 1995;9:3122–3135. doi: 10.1101/gad.9.24.3122. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Thompson BL, Rosen JB. Demythologizing the emotions: adaptation, cognition, and visceral representations of emotion in the nervous system. Brain Cogn. 2003;52:15–23. doi: 10.1016/s0278-2626(03)00004-6. [DOI] [PubMed] [Google Scholar]
- Schulze HG, Gorzalka BB. Oxytocin effects on lordosis frequency and lordosis duration following infusion into the medial pre-optic area and ventromedial hypothalamus of female rats. Neuropeptides. 1991;18:99–106. doi: 10.1016/0143-4179(91)90008-7. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Rinaman L, Vollmer RR, Amico JA. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1828–1833. doi: 10.1152/ajpregu.00826.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Valette G, Foulon L, Germain G, Advenier C, Naline E, Bardou M, Martinolle JP, Pouzet B, Raufaste D, Garcia C, Double-Cazanave E, Pauly M, Pascal M, Barbier A, Scatton B, Maffrand JP, Le Fur G. SSR126768A (4-chloro-3-[(3R)-(+)-5-chloro-1-(2,4-dimethoxybenzyl)-3-methyl-2-oxo-2,3-dihydro-1H-indol-3-yl]-N-ethyl-N-(3-pyridylmethyl)-benzamide, hydrochloride): a new selective and orally active oxytocin receptor antagonist for the prevention of preterm labor. J Pharmacol Exp Ther. 2004;309:414–424. doi: 10.1124/jpet.103.061200. [DOI] [PubMed] [Google Scholar]
- Sheldrick EL, Flint AP. Endocrine control of uterine oxytocin receptors in the ewe. J Endocrinol. 1985;106:249–258. doi: 10.1677/joe.0.1060249. [DOI] [PubMed] [Google Scholar]
- Soloff MS. Oxytocin receptors and mammary myoepithelial cells. J Dairy Sci. 1982;65:326–337. doi: 10.3168/jds.S0022-0302(82)82194-2. [DOI] [PubMed] [Google Scholar]
- Somponpun SJ, Holmes MC, Seckl JR, Russell JA. Modulation of oestrogen receptor-beta mRNA expression in rat paraventricular and supraoptic nucleus neurones following adrenal steroid manipulation and hyperosmotic stimulation. J Neuroendocrinol. 2004;16:472–482. doi: 10.1111/j.1365-2826.2004.01190.x. [DOI] [PubMed] [Google Scholar]
- Stachowiak A, Macchi C, Nussdorfer GG, Malendowicz LK. Effects of oxytocin on the function and morphology of the rat adrenal cortex: in vitro and in vivo investigations. Res Exp Med (Berl) 1995;195:265–274. doi: 10.1007/BF02576797. [DOI] [PubMed] [Google Scholar]
- Stedronsky K, Telgmann R, Tillmann G, Walther N, Ivell R. The affinity and activity of the multiple hormone response element in the proximal promoter of the human oxytocin gene. J Neuroendocrinol. 2002;14:472–485. doi: 10.1046/j.1365-2826.2002.00799.x. [DOI] [PubMed] [Google Scholar]
- Stevenson KR, Riley PR, Stewart HJ, Flint AP, Wathes DC. Localization of oxytocin receptor mRNA in the ovine uterus during the oestrous cycle and early pregnancy. J Mol Endocrinol. 1994;12:93–105. doi: 10.1677/jme.0.0120093. [DOI] [PubMed] [Google Scholar]
- Stivers JA, Kaltwasser MT, Hill PS, Hruby VJ, Crawley JN. Ventral tegmental oxytocin induces grooming. Peptides 9 Suppl. 1988;1:223–231. doi: 10.1016/0196-9781(88)90248-3. [DOI] [PubMed] [Google Scholar]
- Stoehr JD, Cramer CP, North WG. Oxytocin and vasopressin hexapeptide fragments have opposing influences on conditioned freezing behavior. Psychoneuroendocrinology. 1992;17:267–271. doi: 10.1016/0306-4530(92)90067-h. [DOI] [PubMed] [Google Scholar]
- Stoneham MD, Everitt BJ, Hansen S, Lightman SL, Todd K. Oxytocin and sexual behaviour in the male rat and rabbit. J Endocrinol. 1985;107:97–106. doi: 10.1677/joe.0.1070097. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Cocco C, Melis T, Boi A, Ferri GL, Argiolas A, Melis MR. Oxytocin induces penile erection when injected into the ventral tegmental area of male rats: role of nitric oxide and cyclic GMP. Eur J Neurosci. 2008;28:813–821. doi: 10.1111/j.1460-9568.2008.06385.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6:384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- Tops M, van Peer JM, Korf J, Wijers AA, Tucker DM. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology. 2007;44:444–449. doi: 10.1111/j.1469-8986.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Audigier S, Dubois-Dauphin M, Dreifuss JJ. Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats. An autoradiographical study. Brain Res. 1990;511:129–140. doi: 10.1016/0006-8993(90)90232-z. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Arsenijevic Y. Distribution of vasopressin and oxytocin receptors in the rat spinal cord: sex-related differences and effect of castration in pudendal motor nuclei. Neuroscience. 1997;78:499–509. doi: 10.1016/s0306-4522(96)00591-x. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Barberis C, Jard S. Oxytocin receptors in the central nervous system. Distribution, development, and species differences. Ann N Y Acad Sci. 1992;652:29–38. doi: 10.1111/j.1749-6632.1992.tb34343.x. [DOI] [PubMed] [Google Scholar]
- Tuppy H. The amino-acid sequence in oxytocin. Biochim Biophys Acta. 1953;11:449–450. doi: 10.1016/0006-3002(53)90071-7. [DOI] [PubMed] [Google Scholar]
- Uhl-Bronner S, Waltisperger E, Martinez-Lorenzana G, Condes Lara M, Freund-Mercier MJ. Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat. Neuroscience. 2005;135:147–154. doi: 10.1016/j.neuroscience.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Alster P, Svensson TH. Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology (Berl) 1992;109:473–476. doi: 10.1007/BF02247726. [DOI] [PubMed] [Google Scholar]
- Van Kesteren RE, Smit AB, De Lange RP, Kits KS, Van Golen FA, Van Der Schors RC, De With ND, Burke JF, Geraerts WP. Structural and functional evolution of the vasopressin/oxytocin superfamily: vasopressin-related conopressin is the only member present in Lymnaea, and is involved in the control of sexual behavior. J Neurosci. 1995;15:5989–5998. doi: 10.1523/JNEUROSCI.15-09-05989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen FW, van Heerikhuize J, van der Meulen G, Wolters P. Light microscopic autoradiographic localization of [3H]oxytocin binding sites in the rat brain, pituitary and mammary gland. Brain Res. 1985;359:320–325. doi: 10.1016/0006-8993(85)91443-x. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- Vignozzi L, Filippi S, Luconi M, Morelli A, Mancina R, Marini M, Vannelli GB, Granchi S, Orlando C, Gelmini S, Ledda F, Forti G, Maggi M. Oxytocin receptor is expressed in the penis and mediates an estrogen-dependent smooth muscle contractility. Endocrinology. 2004;145:1823–1834. doi: 10.1210/en.2003-0962. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci U S A. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu Y, Young LJ, Insel TR. Hypothalamic vasopressin gene expression increases in both males and females postpartum in a biparental rodent. J Neuroendocrinol. 2000;12:111–120. doi: 10.1046/j.1365-2826.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wenk GL. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol Learn Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Temple JL, Caldwell HK, Young WS., 3rd Inactivation of the oxytocin and the vasopressin (Avp) 1b receptor genes, but not the Avp 1a receptor gene, differentially impairs the Bruce effect in laboratory mice (Mus musculus) Endocrinology. 2008;149:116–121. doi: 10.1210/en.2007-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman DC, Albers HE. Role of oxytocin in the hypothalamic regulation of sexual receptivity in hamsters. Brain Res. 1995;680:73–79. doi: 10.1016/0006-8993(95)00233-g. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Winslow JT. Mouse social recognition and preference. Curr Protoc Neurosci. 2003 doi: 10.1002/0471142301.ns0816s22. Chapter 8, Unit 8 16. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Camacho F. Cholinergic modulation of a decrement in social investigation following repeated contacts between mice. Psychopharmacology (Berl) 1995;121:164–172. doi: 10.1007/BF02245626. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993a;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J Neurosci. 1991;11:2032–2038. doi: 10.1523/JNEUROSCI.11-07-02032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Neuroendocrine basis of social recognition. Curr Opin Neurobiol. 2004;14:248–253. doi: 10.1016/j.conb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Shapiro L, Carter CS, Insel TR. Oxytocin and complex social behavior: species comparisons. Psychopharmacol Bull. 1993b;29:409–414. [PubMed] [Google Scholar]
- Witt DM. Oxytocin and rodent sociosexual responses: from behavior to gene expression. Neurosci Biobehav Rev. 1995;19:315–324. doi: 10.1016/0149-7634(95)00006-z. [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter CS, Insel TR. Oxytocin Receptor-Binding in Female Prairie Voles - Endogenous and Exogenous Estradiol Stimulation. J Neuroendocrinol. 1991;3:155–161. doi: 10.1111/j.1365-2826.1991.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter CS, Walton DM. Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster) Pharmacol Biochem Behav. 1990;37:63–69. doi: 10.1016/0091-3057(90)90042-g. [DOI] [PubMed] [Google Scholar]
- Witt DM, Insel TR. A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinology. 1991;128:3269–3276. doi: 10.1210/endo-128-6-3269. [DOI] [PubMed] [Google Scholar]
- Witt DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol Biochem Behav. 1992;43:855–861. doi: 10.1016/0091-3057(92)90418-f. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Wu W, Yu LC. Roles of oxytocin in spatial learning and memory in the nucleus basalis of Meynert in rats. Regul Pept. 2004;120:119–125. doi: 10.1016/j.regpep.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Wynne-Edwards KE, Timonin ME. Paternal care in rodents: weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience. 2004;125:947–955. doi: 10.1016/j.neuroscience.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Yan J, Feng J, Schroer R, Li W, Skinner C, Schwartz CE, Cook EH, Jr, Sommer SS. Analysis of the neuroligin 4Y gene in patients with autism. Psychiatr Genet. 2008;18:204–207. doi: 10.1097/YPG.0b013e3282fb7fe6. [DOI] [PubMed] [Google Scholar]
- Yang J, Yang Y, Chen JM, Liu WY, Wang CH, Lin BC. Central oxytocin enhances antinociception in the rat. Peptides. 2007a;28:1113–1119. doi: 10.1016/j.peptides.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Yang J, Yang Y, Chen JM, Liu WY, Wang CH, Lin BC. Effect of oxytocin on acupuncture analgesia in the rat. Neuropeptides. 2007b;41:285–292. doi: 10.1016/j.npep.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotoninergic neurons in mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura R, Kiyama H, Kimura T, Araki T, Maeno H, Tanizawa O, Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993;133:1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z. The neurobiology of social attachment: A comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol C Toxicol Pharmacol. 2008;148:401–410. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Huot B, Nilsen R, Wang Z, Insel TR. Species differences in central oxytocin receptor gene expression: comparative analysis of promoter sequences. J Neuroendocrinol. 1996a;8:777–783. doi: 10.1046/j.1365-2826.1996.05188.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Donaldson R, Rissman EF. Estrogen receptor alpha is essential for induction of oxytocin receptor by estrogen. Neuroreport. 1998;9:933–936. doi: 10.1097/00001756-199803300-00031. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Gainer H. Transgenesis and the study of expression, cellular targeting and function of oxytocin, vasopressin and their receptors. Neuroendocrinology. 2003;78:185–203. doi: 10.1159/000073702. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Gainer H. Vasopressin/Oxytocin and Receptors. In: Squire L, editor. Encyclopedia of Neuroscience. Academic Press; Oxford: 2009. pp. 51–59. [Google Scholar]
- Young WS, 3rd, Shepard E, Amico J, Hennighausen L, Wagner KU, LaMarca ME, McKinney C, Ginns EI. Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. J Neuroendocrinol. 1996b;8:847–853. doi: 10.1046/j.1365-2826.1996.05266.x. [DOI] [PubMed] [Google Scholar]
- Yrigollen CM, Han SS, Kochetkova A, Babitz T, Chang JT, Volkmar FR, Leckman JF, Grigorenko EL. Genes controlling affiliative behavior as candidate genes for autism. Biol Psychiatry. 2008;63:911–916. doi: 10.1016/j.biopsych.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ. The neurobiology of trust. Sci Am. 2008;298:88–92. 95. doi: 10.1038/scientificamerican0608-88. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. The neurobiology of trust. Ann N Y Acad Sci. 2004;1032:224–227. doi: 10.1196/annals.1314.025. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS ONE. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE. Hormones associated with non-maternal infant care: a review of mammalian and avian studies. Folia Primatol (Basel) 2000;71:6–21. doi: 10.1159/000021726. [DOI] [PubMed] [Google Scholar]
- Zingg HH, Laporte SA. The oxytocin receptor. Trends in endocrinology and metabolism: TEM. 2003;14:222–227. doi: 10.1016/s1043-2760(03)00080-8. [DOI] [PubMed] [Google Scholar]