Abstract
Competition for fertile females determines male reproductive success in many species. The priority of access model predicts that male dominance rank determines access to females, but this model has been difficult to test in wild populations, particularly in promiscuous mating systems. Tests of the model have produced variable results, probably because of the differing socioecological circumstances of individual species and populations. We tested the predictions of the priority of access model in the chimpanzees of Gombe National Park, Tanzania. Chimpanzees are an interesting species in which to test the model because of their fission–fusion grouping patterns, promiscuous mating system and alternative male mating strategies. We determined paternity for 34 offspring over a 22-year period and found that the priority of access model was generally predictive of male reproductive success. However, we found that younger males had higher success per male than older males, and low-ranking males sired more offspring than predicted. Low-ranking males sired offspring with younger, less desirable females and by engaging in consortships more often than high-ranking fathers. Although alpha males never sired offspring with related females, inbreeding avoidance of high-ranking male relatives did not completely explain the success of low-ranking males. While our work confirms that male rank typically predicts male chimpanzee reproductive success, other factors are also important; mate choice and alternative male strategies can give low-ranking males access to females more often than would be predicted by the model. Furthermore, the success of younger males suggests that they are more successful in sperm competition.
Keywords: chimpanzee, dominance rank, male reproductive success, Pan troglodytes schweinfurthii, paternity, priority of access
Among mammals, males usually compete over access to fertile females (Trivers 1972; Cunningham & Birkhead 1998). Accordingly, there is a well-documented relationship between male dominance rank and reproductive success in many group-living species (e.g. red deer, Cervus elaphus: Pemberton et al. 1992; northern elephant seals, Mirounga angustirostris: Haley et al. 1994; African wild dogs, Lycaon pictus: Girman et al. 1997). In these species and numerous others, high-ranking males have higher reproductive success than their lower-ranking counterparts.
Many primates likewise show a positive correlation between male rank and reproductive success (e.g. bonobos, Pan paniscus: Gerloff et al. 1999; Hanuman langurs, Semnopithecus entellus: Launhardt et al. 2001; rhesus macaques, Macaca mulatta: Widdig et al. 2004; multimale gorilla, Gorilla gorilla, groups: Bradley et al. 2005). However, the relationship is complex, and there is variation in the strength of the association (Ellis 1995). The relationship between rank and reproductive success was formalized into the ‘priority of access’ model, which predicts how many offspring should be sired by each male based on each male's rank and two demographic factors: the number of male competitors and the number of receptive females present for each conception (Altmann 1962). For example, if five males are in a group with two receptive females, priority of access predicts that the two highest-ranking males will each gain access to one female. Several studies have found support for this model (e.g. Japanese macaques, Macaca fuscata: Soltis et al. 2001; mandrills, Mandrillus sphinx: Setchell et al. 2005; savannah baboon, Papio cynocephalus: Alberts et al. 2006) while another study in grey mouse lemurs, Microcebus murinus, did not (Radespeil et al. 2002). However, lemurs breed synchronously and have a dispersed social system that may make it difficult for males to successfully monitor and defend mates. The influence of breeding synchrony has been observed in other species as well; dominant male domestic cats, Felis catus L., were more successful when females had asynchronous oestrus (Say et al. 2001).
Despite the general correlation between dominance rank and reproductive success, several factors may alter the influence of rank and explain deviations from the priority of access model. Male coalitions, female choice and alternative male mating strategies can alter male access to females (Smuts 1987) and decrease the correlation between rank and reproductive success in primates. Some females may prefer middle- or lower-ranking males, thereby enabling those males to bypass their place in the queue (e.g. rhesus macaques: Chapais 1983; ringtailed lemur, Lemur catta: Pereira & Weiss 1991; Japanese macaques: Soltis et al. 2001; Hayakawa 2007; chimpanzees: Stumpf & Boesch 2005, 2006). Furthermore, low-ranking males can sometimes avoid direct competition with dominant individuals by sneaking copulations, as occurs in Japanese and rhesus macaques (Berard et al. 1994; Soltis et al. 2001). The degree to which these factors are important is probably contingent upon the socioecology of the species or population.
Chimpanzees represent a particularly interesting system in which to investigate the applicability of the priority of access model. Unlike the primate species referenced above, chimpanzees live in a fission–fusion social system in which subgroups, known as parties, are temporary within a permanent community (Nishida 1968; Goodall 1986; Boesch & Boesch-Achermann 2000). Thus, even though males can be ranked in a linear dominance hierarchy (e.g. Boesch & Boesch-Achermann 2000; Mitani & Amsler 2003; Muehlenbein et al. 2004), access to females may be dependent on party composition. For example, lower-ranking males have higher courtship success when higher-ranking males are absent (Matsumoto-Oda 1999). Access to females could also vary across populations given differences in gregariousness and dispersal. Compared to East African chimpanzees, P. t. schweinfurthii (Gombe: Wrangham & Smuts 1980; Williams et al. 2002; Murray et al. 2007), West African female chimpanzees, P. t. verus, are more gregarious and show comparable levels of gregariousness and similar home ranges to males (Bossou, Guinea: Sugiyama 1988; Sakura 1994; Taï National Park, Cote d'Ivoire: Boesch 1996; Boesch & Boesch-Achermann 2000; Lehmann & Boesch 2005). Additionally, while chimpanzee females generally disperse at sexual maturity (Goodall 1986; Nishida et al. 1990; Boesch & Boesch-Achermann 2000), approximately 50% of females in the main study community at Gombe National Park, Tanzania remain in their natal community with related males (Pusey et al. 1997).
Females have conspicuous sexual swellings when they are sexually receptive, and they mate promiscuously (Goodall 1986; Nishida & Hiraiwa-Hasegawa 1987). Hormonal patterns indicate that they ovulate when they are maximally tumescent (Deschner et al. 2003; Emery Thompson 2005). Males can therefore monitor female receptivity and should theoretically concentrate their mating efforts during key periovulatory periods. In fact, males show higher rates of mating during the most fertile days within a cycle, and during conceptive cycles than during nonconceptive cycles (Deschner et al. 2004; Emery Thompson 2005; Emery Thompson & Wrangham 2008). Males also preferentially focus their mating efforts on certain individuals. A recent study from the Kanyawara community in Kibale National Park, Uganda, reported that males preferred older females and suggested that the preference for older females is selected for since they have more maternal experience and their survival may indicate higher genetic quality (Muller et al. 2007).
Male chimpanzees show three different mating strategies: (1) opportunistic, (2) possessiveness or mate guarding and (3) consortship (Tutin 1979). Consortship occurs when a male–female dyad travels alone and mates away from other members of the community. When a male has a successful consortship with a female during the fertile period of a conceptive cycle, he benefits from increased paternity certainty by eliminating competition. However, consortships are also costly since pairs often travel to the edge of the community range where they risk attack by neighbouring chimpanzee communities (Goodall 1986; Gombe Stream Research Centre, unpublished data), and males involved in consortship cannot monitor other females in the group.
Consortships seem to occur more frequently at Gombe (Goodall 1986) than at other study sites (Mahale: Hasegawa & Hiraiwa-Hasegawa 1990; Kibale: Watts 1998; Taï: Boesch & Boesch-Achermann 2000; Budongo: Reynolds 2005). Only 10% of offspring are conceived through consortship at Taï (Boesch & Boesch-Achermann 2000) while 21% of offspring are conceived through consortship at Gombe (Constable et al. 2001). Interestingly, only the alpha male at Taï achieved success through consortship in Boesch & Boesch-Achermann's (2000) study, while low- to middle-ranking fathers achieved success through consortship at Gombe (Constable et al. 2001). Therefore, at Gombe, where females are less gregarious, low-ranking males may have more opportunity to lead females away on consortships.
Besides competing for fertile females through aggression and social dominance, males can also compete via sperm competition. When females copulate with multiple males, the male that produces the most sperm gains an increased chance of fertilizing a receptive female (Parker 1970). Bercovitch & Nürnberg (1996) found that rhesus macaque sires have significantly larger testes than nonsires. Sperm production generally correlates with testes size, and in diverse taxa there is a strong correlation between relative testes size and mating system, with the most polyandrous species having the largest testes (reviewed in Gomendio et al. 1998). Chimpanzees, with their highly promiscuous mating system, have particularly large relative testes size, suggesting that they experience intense sperm competition (Harcourt et al. 1981; Møller 1988; Harcourt et al. 1995). As well as individual differences in testes size, another likely factor influencing a male's success in sperm competition is age. In humans and other primates, various measures of male fertility and physiology decline with age (reviewed in: Kidd et al. 2001; Eskenazi et al. 2003; Bribiescas 2006). The promiscuously mating common lizard, Lacerta vivipara, also shows age-related decline in reproductive success (Richard et al. 2005), and younger bulb mite, Rhizoglyphus robini, males outcompete older males, siring a larger proportion of the females' eggs (Radwan et al. 2005). Thus, as long as young, even low-ranking, males secure copulations during periods of opportunistic mating with fertile females, we might expect them to outcompete older males in sperm competition and achieve fertilization.
Here, we analyse 22 years of data to test whether male chimpanzees at Gombe National Park conform to the priority of access model. Prior work from Taï chimpanzees found that paternity patterns fit well with the model (Boesch et al. 2006). Although we also expected it to hold at Gombe, we predicted that it would not conform as closely since females are less easily monitored in this population than in more socially cohesive groups, such as the Taï population. We further predicted that deviations from the model would result from inbreeding avoidance, whereby females that remained in their natal community would mate with unrelated males that would not necessarily be predicted to have access according to the model parameters. We also expected that low-ranking males would successfully sire offspring with younger, less desirable females and through consortships, and that younger males would be more successful than older males.
Methods
Study Population and Data Collection
Data for this study were from the Kasekela community of Gombe National Park, Tanzania. Study of this community began in 1960, and daily full-day follows on members of the community have been conducted since 1973 (see Goodall 1986, pp. 597–608 for details regarding data collection and interobserver reliability). During follows, female reproductive state (degree of sexual swelling) is noted, and data on group composition, feeding and location are recorded every 15 min. Aggressive, submissive and mating behaviour are recorded throughout the follow. We analysed 22 years of data collected during 1984–2005. During this period, the community contained 7–12 adult males and 12–23 adult females (adult age ≥ 12 years old). We included data from 34 offspring (N = 33 successful pregnancies, including one set of twins) in our analyses.
Male Dominance Rank
We determined dominance rank from the direction of dyadic pant-grunts. Pant-grunts are easily audible and unidirectional submissive vocalizations that function as formal indicators of dominance (Bygott 1979; de Waal 1982). We used MatMan© software (version 1.1, Noldus Information Technology, Wageningen, The Netherlands) and the improved linearity test (de Vries 1995) to calculate annual dominance ranks from pant-grunt data. We included all males that were alive for at least 3 months and turned at least 12 years of age during the year. Twelve years of age is the youngest age at which males have fathered offspring at Gombe (Constable et al. 2001; this study). We found significant linearity (P ≤ 0.05) for 17 of the 21 years in which the infants in this study were conceived using pant-grunts alone and a trend towards linearity (P ≤ 0.1) in 1 year. For the 3 years in which there was no evidence of linearity based on pant-grunt data alone, we repeated the analysis including the outcome of dyadic agonistic interactions that had an unambiguous winner and loser. This resulted in significant linearity for 1 year (P ≤ 0.05) and a trend towards linearity for the other 2 years (P ≤ 0.1).
Paternity Determination and Patterns
We determined paternity for 34 of the 57 offspring born in the Kasekela community between 1984 and 2005. All sampled offspring had survived at least long enough for us to obtain a faecal sample (approximately 2 years), while most unsampled offspring were those that disappeared or died before that age. Paternities for 12 offspring were previously determined (Constable et al. 2001) but were confirmed with the genetic loci used in this study; 22 paternities (for 21 conceptions, including one set of twins) were newly determined (Appendix, Table A1). All but one mother and, on average, 98.6% (range 53.3–100%) of potential fathers from within the community were also sampled (Table A1). We conservatively included all males at least 9 years old at the time of conception as potential fathers. All offspring born from 1992 onwards (N = 25) had 100% sampling of candidate males from within the community. To account for the possibility of extragroup paternity, we included candidate males from the adjacent Mitumba community whenever genotypes were available. Samples could not be collected from some individuals because of the later habituation of this community (see Appendix for details).
We used published genotypes from Constable et al. (2001) when genetic material was not available for deceased individuals. For all others, we isolated DNA from faecal samples, with the exception of one Mitumba male genotyped using tissue collected postmortem. After determining DNA concentrations using quantitative real-time PCR, we genotyped all individuals by amplifying 11 tetranucleotide microsatellite loci using human primers according to the recommendations of Morin et al. (2001). We identified fathers using both simple exclusion and the likelihood-based program CERVUS 2.0 (Marshall et al. 1998). Detailed methods are provided in the Appendix.
We tested for differences in the probability of siring offspring by age after controlling for male rank. For each conception we identified all candidate sires in the community that were alive at the time of conception and turned at least 12 years of age during the conception year. We assigned each candidate a rank for the conception year and categorized them by age, grouping males 12–14, 15–19, 20–24, 25–29, 30–34 and 35–39 years old. The single male over 39 years of age (aged 40) at the time of any conception was excluded from this analysis to prevent statistical bias from having only one male in an age category. We used ANOVA to test the difference in fit between two generalized linear mixed models: the first tested male age category and rank and the second tested just rank, but both controlled for repeated measures on the same male.
Testing the Priority of Access Model
Conception window
We determined the conception swelling for each offspring by backdating from the offspring's date of birth and identifying any swelling within the known range of gestation at Gombe (range 208–235 days; average: 229 days) (Goodall 1986; Wallis 1997). Given that the most likely time of conception is between the third and seventh day before females detumesce (Deschner et al. 2003; Emery Thompson 2005), we used this 5-day window within each swelling as the conception window. Conception could be assigned to a single swelling cycle for all but two pregnancies. In those two cases, we included both cycles in our analyses and averaged our metrics across those cycles. We treated the twins as a single conception since only one male could monopolize the mother according to the priority of access model. Despite being nonidentical twins, both offspring were fathered by the same male (Table 1).
Table 1.
Paternity and demographic parameters of chimpanzees for 33 conceptions between 1984 and 2005
| Offspring | Date of birth | Mother | Age of mother | Father | Age of father | Rank of father | Number of males present | Number of oestrous females present | Father mating strategy |
|---|---|---|---|---|---|---|---|---|---|
| DIA | 12/14/05 | DL | 18 | FE | 12 | 11 | 11 | 6 | Opportunistic |
| BRZ | 11/20/05 | BAH | 16 | KS | 23 | 1 | 11 | 8 | Consortship |
| SHA | 8/25/04 | SR | 12 | WL | 31 | 7 | 10 | 6 | Opportunistic |
| COC | 7/10/04 | CD | 34 | FD | 32 | 3 | 10 | 5 | Opportunistic |
| FAM | 4/18/04 | FN | 22 | SL | 20 | 1 | 10 | 11 | Opportunistic |
| MAM | 2/5/04 | MAK | 12 | GL | 25 | 8 | 10 | 1 | Consortship† |
| GIM | 1/15/04 | GM | 32 | TB | 25 | 2 | 10 | 3 | Opportunistic |
| SAM | 6/17/01 | SA | 27 | FR | 24 | 1 | 10 | 4 | Possessive |
| SDB | 6/9/01 | SW | 40 | FR | 24 | 1 | 10 | 4 | ‡ |
| TOM | 3/7/01 | TA | 11 | KS | 18 | 9 | 10 | 2 | Consortship† |
| TOF | 10/18/00 | TTA | 13 | SL | 16 | 5 | 10 | 1 | ‡ |
| FND* | 5/27/00 | FN | 18 | SL | 16 | 10 | 10 | 5 | Opportunistic |
| ZEL | 11/12/99 | TZ | 19 | KS | 17 | 6 | 10 | 5 | Possessive |
| TZN | 10/1/99 | PI | 38 | FR | 22 | 1 | 10 | 5 | Possessive |
| FLI | 7/20/98 | FF | 39 | KS | 15 | 6 | 12 | 5 | Possessive |
| GLI/GLD | 7/13/98 | GM | 27 | FR | 21 | 2 | 12 | 4 | Opportunistic |
| YAM | 7/22/98 | YD | 11 | WL | 25 | 8 | 12 | 3 | Opportunistic |
| FU (Fudge) | 12/9/96 | FN | 15 | SL | 12 | 10 | 12 | 2 | Consortship |
| FI (Fred) | 9/5/96 | FF | 37 | FR | 19 | 2 | 12 | 2 | Opportunistic |
| SN | 5/24/96 | SA | 21 | AO | 16 | 9 | 12 | 3 | Opportunistic |
| TN (Titan) | 7/10/94 | PI | 32 | FR | 17 | 5 | 10 | 3 | Opportunistic |
| ZS | 12/24/93 | TZ | 14 | FR | 16 | 5 | 10 | 3 | Opportunistic |
| GA (Gaia) | 2/14/93 | GM | 21 | WL | 19 | 1 | 11 | 1 | Possessive |
| FE (Ferdinand) | 8/19/92 | FF | 33 | EV | 39 | 3 | 11 | 2 | Opportunistic |
| SI (Schweini) | 4/15/91 | SW | 30 | WL | 17 | 1 | 11 | 7 | Possessive |
| CN (Conoco) | 1/31/91 | CD | 20 | WL | 17 | 1 | 11 | 7 | Possessive |
| SR | 1/25/91 | SA | 16 | BE | 20 | 2 | 11 | 6 | Opportunistic |
| JK (Jackson) | 9/16/89 | JF | 14 | AL | 21 | 2 | 11 | 2 | Opportunistic |
| FO (Faustino) | 5/8/89 | FF | 30 | WL | 15 | 5 | 9 | 3 | Opportunistic |
| TA (Tanga) | 4/22/89 | PI | 27 | GB | 24 | 1 | 9 | 3 | Possessive |
| GD (Galahad) | 4/5/88 | GM | 16 | AL | 19 | 5 | 7 | 4 | Consortship |
| DL (Dilly)* | 6/17/86 | (DM) | 13 | BE | 16 | 9 | 12 | 1.5 | Consortship† |
| FS | 2/8/85 | FF | 25 | GB | 19 | 1 | 11 | 2 | Possessive |
Paternities from Constable et al. (2001) in italics. Parentheses indicate that the genotype was unavailable for the individual. GLI and GLD were twins and were treated as a single conception.
Numbers of males and oestrous females were averaged across two swelling cycles.
Consortships were included in the less restrictive analysis but removed in the strict analysis of father strategy.
Strategy was not assigned because of the lack of observations.
Demographic parameters
For each conception window, we determined the number of candidate males and the number of simultaneously maximally tumescent females (including the mother) present in the community during the window (Table 1). We calculated the average number of males and simultaneously oestrous females for both cycles when mothers had two cycles of equal probability of conception.
Calculating expected success
We assigned expected success based on the priority of access model following Altmann (1962). The expected paternity for each male was based on his rank and the number of receptive females at the time of conception. For example, if there were four simultaneously swollen females and 10 adult males, the top four males of ranks 1 (alpha) through 4 would each be expected to monopolize one of those females. Those four males would each have a 0.25 chance of monopolizing the conceiving female and siring the offspring, while males ranked 5 and below would have zero likelihood of paternity. These likelihoods were then summed for each rank to give an expected number of offspring to be sired per rank over the entire study period. We then compared the expected proportion of offspring sired to the observed proportion actually sired per rank using the Spearman rank-order correlation coefficient (α level of significance = 0.05), enabling direct comparison to the Taï population (Boesch et al. 2006). Although rank 1 is the lowest numerical rank, it is the highest (alpha) social rank, and was treated as the highest rank in the Spearman correlation.
Examining Sources of Deviation from the Priority of Access Model
Inbreeding avoidance
We modified the priority of access model to test whether close male relatives of the conceiving female in the candidate pool (sons, maternal brothers and maternal uncles) affected male reproductive success. At least one male relative was in the community for 16 of the 33 conceptions. In eight of those cases, the related male had a high enough rank to receive a likelihood of paternity that was greater than zero under the basic model parameters. These related males contributed to seven unique male–female dyadic pairs. Four dyads were maternal siblings, two were mother–son dyads, and one was a niece–uncle dyad. When a related male would have received a likelihood of paternity that was greater than zero under normal model parameters, we instead assigned the related male a likelihood of zero, shifted the greater-than-zero likelihood to the next highest ranking, unrelated male, and tested the modified model as described above.
Mating patterns
We defined the father's mating strategy following Constable et al. (2001). Consortships are difficult to identify conclusively, and so we used both a strict and a less restrictive definition. Our strict definition of consortship required either direct observation of consortship behaviour in the record, or mutual absence of the mother–father pair for at least 3 consecutive days, of which at least one fell in the conception window, the coincidental departure or return of the pair, and no more than two other males absent in the same period. Our less restrictive definition was the same as that described above but without the requirement of the coincidental departure or return of the male–female dyad and without the restriction of no more than two other males also absent. We conducted analyses of fathers' strategies using both strict and less restrictive definitions.
We defined possessive behaviour as when the father disrupted copulations or copulation attempts by other males, showed aggression to other males approaching the female, or otherwise showed persistent attention to the female. We considered fathers to be possessive if they showed these behaviours at least twice when the female was maximally tumescent. We examined all conceptions for evidence of possessiveness, but no instance of possessiveness, according to these criteria, coincided with consortship. If consortship or possessiveness overlapped with opportunistic mating by the father, we assigned the father's strategy to be the most restrictive of the female (i.e. consortship or possessiveness superseded opportunistic mating). If there was no evidence of consortship or possessiveness, then the father was deemed to have mated opportunistically. When we could not differentiate between two cycles for a single conception, we assigned a strategy to each cycle individually and compared them. In both conceptions where this was the case, the strategy was the same for each cycle and therefore was the strategy assigned to the conception. Strategy was not assigned for two conceptions (SDB and TOF) because only one or no chimpanzees were followed or sighted during the mothers' swelling cycles.
To investigate how mate preferences and alternative male mating strategies influenced paternity success, we tested mother's age against father's rank, and father's mating strategy (opportunistic, possessive, consortship) against father's rank, using a linear mixed model in which we controlled for repeated measures on the same father. We assigned the mother's age as her age when conception occurred (Table 1). We used the same model to test mother's age against father's age category (categories as described previously), and father's mating strategy against father's age category.
Results
Paternity Assignment and Distribution
We genotyped 16 of the 23 males (69.6%) that were alive for at least one conception. Despite incomplete sampling of candidate fathers, we successfully determined paternity for all 34 offspring via either simple exclusion or likelihood analysis using CERVUS 2.0 (Marshall et al. 1998). In all but one case (DIA), simple exclusion identified a single male as the only male that could have contributed the complementary set of paternal alleles given the offspring and maternal genotypes. All other candidates had at least one mismatch, and most candidates had two or more mismatches (Table A1). Paternities based on exclusion were also confirmed by CERVUS with at least 80% confidence, and 95% the majority of the time (Table A1). In the case of DIA, the most likely reason neither of the two males could be excluded is because they were maternal brothers and therefore genetically very similar; however, we were still able to assign paternity using CERVUS with 75–95% certainty, depending on the simulation conditions. All 34 offspring were fathered by males within the community, and there was no evidence of extragroup paternity (see Appendix for details, Table A1).
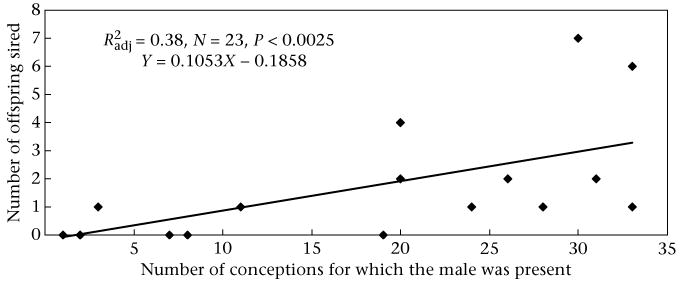
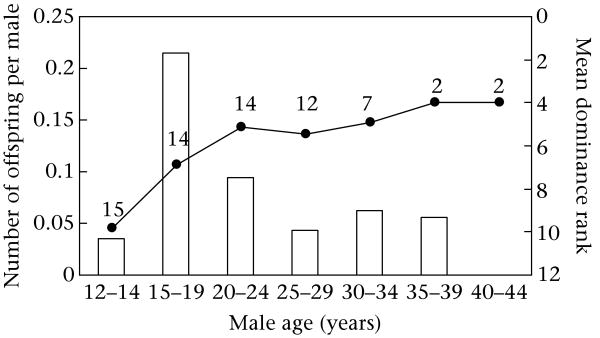
The candidate males sired 0–7 offspring. Most males (56.5%) sired at least one offspring. The mean ± SE number of offspring sired per male was 1.43 ± 0.42 (Table 2). Most of the 10 males that did not produce any offspring were present in the community as adults for the fewest number of conceptions at either the very beginning or the end of the study period, and were often quite young adults. The number of conceptions for which the males were present ranged from 1 to 33, while the percentage of conceptions obtained ranged from 0 to 33.3 (Table 2). There was a positive correlation between the number of conception opportunities and the number of offspring sired ( , N = 23, P< 0.0025; Fig. 1). Paternity distributions differed significantly from expected distributions based simply on the number of males per age category (chi-square test: , P = 0.013). Males aged 15–19 years old were the most successful at gaining paternity (Fig. 2). Per male success dropped considerably after age 19 years and remained low, even though the average male rank continued to rise and remained relatively steady, and the average age of males during their alpha tenures in this study was 22 (N = 6, range 19–26 years). The ANOVA test of the difference in fit between two models, one including both male age (category) and rank, and the other including just male rank, found that including male age as a variable resulted in a significantly better fit (ANOVA: , P = 0.019). Thus, there was a significant difference between age categories in the probability of siring an offspring. Additionally, the probability of siring an offspring remained highest for males that were 15–19 years old, when adjusted for rank.
Table 2.
Total paternity success per candidate male
| Male | Number of offspring sired | Age range during study period | Number of conceptions possible | % Conceptions obtained |
|---|---|---|---|---|
| FR | 7 | 12–29 | 30 | 23.3 |
| WL | 6 | 12–33 | 33 | 18.2 |
| KS | 4 | 12–23 | 20 | 20.0 |
| SL | 4 | 12–22 | 20 | 20.0 |
| GB* | 2 | 20–40 | 31 | 6.5 |
| BE | 2 | 15–33 | 26 | 7.7 |
| AL | 2 | 17–31 | 20 | 10 |
| FD | 1 | 13–34 | 33 | 3.0 |
| GL | 1 | 12–28 | 28 | 3.6 |
| TB | 1 | 12–28 | 28 | 3.6 |
| AO | 1 | 12–26 | 24 | 4.2 |
| EV | 1 | 32–40 | 11 | 9.1 |
| FE | 1 | 12–13 | 3 | 33.3 |
| PF | 0 | 13–26 | 19 | 0 |
| SD† | 0 | 12–14 | 8 | 0 |
| FO | 0 | 12–16 | 7 | 0 |
| ZS | 0 | 12 | 2 | 0 |
| JG† | 0 | 13–15 | 2 | 0 |
| JJ† | 0 | 28–31 | 2 | 0 |
| MM† | 0 | 12–13 | 2 | 0 |
| MU† | 0 | 19–22 | 2 | 0 |
| ST† | 0 | 27–30 | 2 | 0 |
| CT† | 0 | 9–11 | 1 | 0 |
Males (N = 23) are ordered first by number of offspring sired, then by number of conceptions possible for each. Individuals in bold achieved alpha status.
At the end of his alpha tenure, GB sustained bad wounds to his testicles in a fight, after which he was thought to be sterile (he sired no further offspring).
Ungenotyped candidates.
Figure 1.
Number of offspring that each adult male chimpanzee sired as a function of the number of conceptions for which he was at least 12 years of age.
Figure 2.
Observed per male success by age. Number of offspring produced per male for each age category was calculated by dividing the total number of offspring produced by the number of candidate males, including fathers, in that age category during conceptions (open bars). The solid line is the mean dominance rank, and numbers beside the points of the line indicate the total number of males (both fathers and nonfathers) for which rank information was available in each category.
Priority of Access
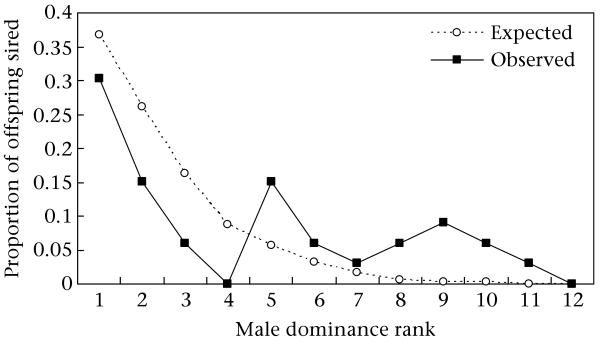
At the time of conception, there were, on average, 10.5 candidate males (range 7–12) and 3.9 simultaneously oestrous females (range 1–11) (Table 1). We found that paternity success decreased according to rank as predicted by the priority of access model, reaching significance in a one-tailed test and a trend towards significance in a two-tailed test (Spearman rank correlation: rS = 0.54, N = 12 rank positions, one-tailed P = 0.034, two-tailed P = 0.068; Fig. 3). The alpha male (rank 1) secured the most offspring, siring 30.3% of the offspring, which was somewhat less than the 36.8% predicted but still 50% more offspring than the next most successful males (ranks 2 and 5). While the highest-ranking males (ranks 1–4) were less successful than predicted, males of lower ranks (5 and below) did as well as or better than predicted, and in some cases, as well as or better than males of higher rank.
Figure 3.
The proportion of offspring expected and observed to be sired by males of different rank. Expected proportions were determined by the priority of access model.
Sources of Deviation
Inbreeding avoidance
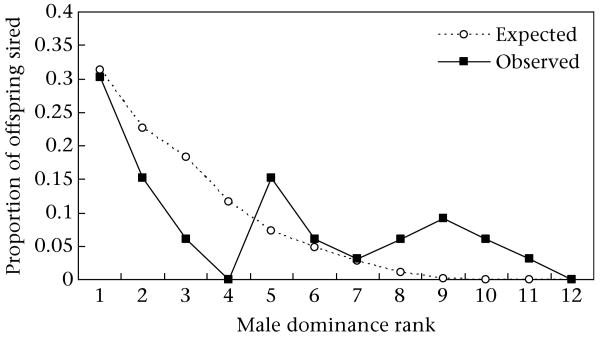
Modification of the priority of access model to account for avoidance of related male candidates did not increase the explanatory power of the model. Shifting likelihoods from a related male to the next highest ranking, unrelated male resulted in a similar rS coefficient (Spearman rank correlation: rS = 0.53, N = 12 rank positions, one-tailed P = 0.039, two-tailed P = 0.078). However, the modification did produce a closer fit between observed and expected proportions of offspring sired by the alpha male (Fig. 4). Alpha males sired offspring solely with unrelated females, or 37% (10 of 27) of the offspring produced by unrelated females.
Figure 4.
The modified proportion of offspring expected and observed to be sired by males of different rank. Expected proportions were determined by the priority of access model with modification to account for related males in the candidate pool (sons, maternal brothers and maternal uncles).
Mating patterns
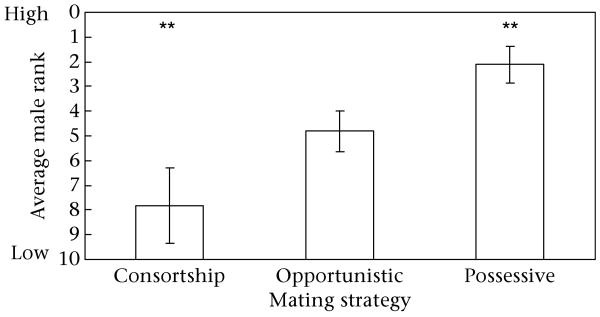
Father's rank was significantly correlated with mother's age, such that lower-ranking fathers sired the offspring of younger females (F1,19 = 10.5, P = 0.004). Father's rank was also significantly correlated with his mating strategy when using the less restrictive definition of consortship (less restrictive: F2,16 = 4.95, P = 0.02; strict: F2,14 = 2.52, P = 0.12). Specifically, the difference between the rank of fathers using the possessive strategy and the rank of fathers using consortship was significant in the less restrictive analysis, with rank being significantly lower for fathers using consortship than that for fathers using the possessive strategy (post hoc group comparison, Tukey–Kramer adjusted: P = 0.02) (Fig. 5). Mother's age and father's strategy did not vary significantly with father's age category. Consortships were associated with 19% of conceptions using the less restrictive definition and 9.7% of conceptions using the strict definition. Fathers were possessive in 29% of conceptions (Table 1).
Figure 5.
Average rank of fathers that achieved success through each mating strategy using the less restrictive definition of consortship. Asterisks denote statistically significant differences in the average male rank of fathers achieving success with different mating strategies. Error bars indicate ±1SE.
Discussion
High dominance rank carries costs such as stress, physiological and metabolic costs, and immunosuppression (Muller & Wrangham 2004; reviewed in Sapolsky 2005). Presumably these costs are offset by higher reproductive success, and evidence for reproductive skew towards high-ranking males is widespread across mammalian taxa (e.g. African wild dogs: Girman et al. 1997; bonobos: Gerloff et al. 1999; yellow-toothed cavy, Galea musteloides: Keil et al. 1999; Hanuman langurs: Launhardt et al. 2001; reindeer, Rangifer tarandus: Røed et al. 2002; rhesus macaques: Widdig et al. 2004; multimale gorilla groups: Bradley et al. 2005). In species where multiple females can be receptive at the same time, the priority of access model predicts the order in which males have mating success. In this study, we found that male chimpanzees tend to conform to the model. However, the model had less explanatory power for the Gombe chimpanzees than was previously reported in the West African subspecies (Boesch et al. 2006). We suggest that differences in female gregariousness partially explain deviations in the model fit and that lower-ranking fathers sire offspring with younger, less desirable females and rely on the consortship strategy to secure matings.
Patterns of Paternity
As in other studies (e.g. baboons: Alberts et al. 2006; chimpanzees: Boesch et al. 2006), alpha males sired more offspring (30.3%) than all other males, but most males sired at least one offspring. Note, however, that two of the unsuccessful males (FO and ZS) were young males, and the seven ungenotyped and unsuccessful males were present for only a few of the earliest conceptions and many were very young adults. Despite the higher success of alpha males, younger males (aged 15–19 years) were more successful per candidate male than were older, often higher-ranking males, and age remained a significant predictor of male success after accounting for rank. This age-related pattern of success is similar to that found in the Taï chimpanzees, although the decline in reproductive success with age is less marked in that population (Boesch et al. 2006). The discordance of the relationship between paternity versus age and rank versus age in this study contrasts with the close fit found for baboons (Alberts et al. 2006). The age-related decline in paternity could be partially explained by male reproductive senescence. Evidence from humans shows that semen volume, count and concentration, as well as sperm motility and morphology, decline gradually as males age, starting even when men are in their 20s (reviewed in Kidd et al. 2001; Eskenazi et al. 2003). Other age-associated changes in mortality, hormone levels and sexual function in nonhuman primates follow patterns similar to those of humans and other mammals (reviewed in Bribiescas 2006). Thus the reproductive benefits of high rank may be countered by a shift in energetic investment more towards survival with increasing age (Bribiescas 2006). This age effect could be compounded in a promiscuous mating system with intense sperm competition such as in chimpanzees (Harcourt et al. 1981; Møller 1988; Harcourt et al. 1995). Because alpha and high-ranking males copulate more frequently than low-ranking males in the periovulatory period (Matsumoto-Oda 1999; Deschner et al. 2004), they may experience sperm depletion due to constraints on sperm production as found in Soay rams, Ovis aries (Preston et al. 2001). Sperm depletion in high-ranking males could enable younger, potentially more potent males to succeed in sperm competition and sire offspring even though they obtain fewer copulations in the conceptive window.
We found no evidence for extragroup paternity (EGP) in our study community despite the fact that extracommunity copulations have been observed (Goodall 1986). Similarly, EGP was not reported in the Budongo population (Uganda), although paternity for 5 of 26 offspring could not be assigned to a genotyped male (Reynolds 2005). In contrast, EGP accounted for 7.1–10.5% of the offspring born into the study communities in Taï (Ivory Coast) (Vigilant et al. 2001; Boesch et al. 2006) and 25% (1 of 4 offspring) at Bossou (Guinea) (Sugiyama et al. 1993); note, however, that the socioecological context at Bossou is unusual (Sugiyama et al. 1993; Sugiyama 1999, 2004). Our results are somewhat surprising since it seems easier for females to seek copulations outside their community in Gombe, given their lower levels of gregariousness in comparison with West African chimpanzees. In addition, Gombe females might gain an advantage from seeking extracommunity copulations since so many females remain in their natal community with close relatives (Pusey et al. 1997). Nevertheless, extracommunity paternity only occurs at low levels at other sites and is probably difficult to achieve given that intercommunity aggression is so severe (Muller 2002; Wilson & Wrangham 2003; Pusey et al. 2008b).
Priority of Access and Sources of Deviation
Patterns of paternity at Gombe generally conformed to the priority of access model (Altmann 1962), with an overall decrease in reproductive success as rank decreased, but the model had less explanatory power at Gombe (rS = 0.54) than at Taï (rS = 0.75; Boesch et al. 2006). In particular, lower-ranking males did better than expected. In addition to a possible advantage gained by young males in sperm competition, it seems likely that the fission–fusion grouping pattern affords low-ranking males more mating opportunities than in spatially cohesive groups such as baboons and macaques. The priority of access model may apply to chimpanzees but on a within-party basis, where lower-ranking males could have access to females when they are higher ranking than the other males in the party (Matsumoto-Oda 1999). Additionally, the fission–fusion system may enable low-ranking males to use alternative mating strategies such as consortship more successfully. This should be true for both chimpanzee subspecies, but more so in Gombe chimpanzees since females at Gombe spend more time alone than do females of West African populations (Wrangham & Smuts 1980; Sugiyama 1988; Sakura 1994; Boesch 1996; Boesch & Boesch-Achermann 2000; Williams et al. 2002; Lehmann & Boesch 2005; Murray et al. 2007).
Given the lower female dispersal rates at Gombe (50%) as compared to nearly complete transfer at other study sites (Gombe: Pusey et al. 1997; Taï: Boesch & Boesch-Achermann 2000; Mahale: Nishida et al. 2003; Kibale: Kahlenberg et al. 2008), we expected that females would mate with lower-ranking males to avoid inbreeding with higher-ranking male relatives. Relatedness is influential in the reproductive success of mandrills, whereby high-ranking males sire significantly fewer offspring with closely related females (Charpentier et al. 2005). Similarly, preference for unrelated mates by female field crickets, Gryllus bimaculatus, decreases the probability of related individuals fertilizing their eggs (Simmons 1991). While copulations do occur between female chimpanzees and their close male relatives, male chimpanzees generally seem uninterested in mating with maternal relatives and females show resistance to their attempts (Tutin 1979; Pusey 1980; Goodall 1986; Pusey 2005). Thus far, there is only a single documented conception of an inbred offspring, between a female and her son, in the wild (Constable et al. 2001). However, when we adjusted the priority of access model to account for inbreeding avoidance of related individuals amongst the pool of male competitors, the general explanatory value of the model did not increase (standard: rS = 0.54; adjusted for relatives: rS = 0.53). Nevertheless, inbreeding avoidance may still be influential because the fit between the observed and the expected proportion of offspring sired improved for alpha males under the modified model. Additionally, no alpha male sired offspring with a female in the six cases where they were close maternal relatives, a pattern similar to white-faced capuchin monkeys, Cebus capucinus, where alpha males sired only a single offspring (of 17) with their daughters (Muniz et al. 2006). Constable et al. (2001) noted that besides resisting mating attempts by male relatives, females might also use consortship as a means to avoid mating with relatives. Small sample size prevented us from testing whether females with relatives in the community participated in consortships more than females without relatives, but this topic warrants further investigation.
Female choice may be influential in male reproductive success in ways not just limited to inbreeding avoidance. Stumpf & Boesch (2005) found that female chimpanzees vary in their individual preferences for and rejection of particular males. This may also account for at least some of the success of low-ranking males in this study, but female choice is difficult to test, and evidence for the effectiveness of female choice in chimpanzees is conflicting. While male mating success in the periovulatory period is negatively correlated with female resistance and positively correlated with female proceptivity (Stumpf & Boesch 2006), male aggression can also coerce fecund females into mating (Muller et al. 2007). Thus, if males can effectively coerce females into mating, then it could negate the influence of female mate choice.
Although inbreeding avoidance did not influence how well the priority of access model fit our population, our results suggest that mate preferences and alternative mating strategies account for at least some of the deviations from the model. At Kibale, Muller et al. (2007) found that males prefer to mate with older females, with higher-ranking males mating more frequently with older females than lower-ranking males. Similarly, we found that higher-ranking males fathered offspring more often with older females. Thus, if higher-ranking males focus their competitive efforts on those females, this could enable low-ranking males to mate and sire offspring with younger, less desirable females.
Consortships occurred more frequently at Gombe than at other study sites (Goodall 1986; Hasegawa & Hiraiwa-Hasegawa 1990; Watts 1998; Boesch & Boesch-Achermann 2000; Reynolds 2005). Although we found no significant effect of fathers' rank on mating strategy when we applied a strict definition of consortship, we found that consortships were used more frequently by low-ranking fathers when defined less restrictively. This finding suggests that lower-ranking males use consortships to ‘steal’ females from high-ranking males. Similar alternative strategies can be successful even in more socially cohesive primates. For example, lower-ranking Japanese and rhesus macaque males succeed by sneaking copulations (Berard et al. 1994; Soltis et al. 2001). The importance of alternative strategies is also evident in nonprimate species. A study of Soay rams, another polygynous and promiscuous species, found that even though the observation of a ram in consort with a ewe made him 18 times more likely to be the sire of the ewe's offspring than other candidate males, young males still sired offspring at higher rates than predicted by their consort time (Coltman et al. 1999). Likewise, while Antarctic fur seal, Arctocephalus gazella, males defending a territory have a reproductive advantage, a large portion of paternity could not be attributed to the territorial male, implying that alternative strategies such as aquatic mating are important for male success (Gemmel et al. 2001).
While the priority of access model is a general predictor of reproductive success in male chimpanzees, it does not take into account recent evidence showing that primate males identify and compete more heavily for females during the females' conceptive cycles and during the periovulatory days of those cycles (longtailed macaques, Macaca fascicularis: Engelhardt et al. 2004; chimpanzees: Deschner et al. 2004; Emery Thompson 2005; Emery Thompson & Wrangham 2008; Hanuman langurs: Ostner et al. 2006; baboons: Gesquiere et al. 2007). The model considers all simultaneously oestrous females, regardless of whether they are fertile, when making predictions for male success. However, if males can reliably distinguish between conceptive and nonconceptive cycles as well as identify the most fertile days within cycles, and higher-ranking males can outcompete others for the conceptive females, then we would expect alpha males to be even more successful than we observed, even when avoiding close female relatives. In this study of 33 conceptions, six conceptions involved temporal overlap with another female's conception window, and, for two of these, paternity of the other female's infant was unknown. Thus, we would predict that the alpha male should have gained all the conceptions with unrelated females that did not have overlapping conception windows and 50% of each overlapping pair of conceiving females, for a total of 24 conceptions (72.7%) in the study. That the alphas did considerably less well, gaining only 30.3% of conceptions, indicates either that alphas did not have complete knowledge of which females were fertile, or that they could not completely monopolize conceptive females. Given the 5-day length of the periovulatory period, it is not surprising that high-ranking males might not be able to completely monopolize a female since mate guarding (possessiveness) is probably costly in terms of the energetic expenditure required to restrict female promiscuity (e.g. Sparkes et al. 1996), as well as in terms of the costs of having to forgo other important activities, such as foraging (e.g. Alberts et al. 1996). Such constraints on the males could be further compounded by evolutionary counter-strategies by females to conceal ovulation and confuse paternity to prevent infanticide of their offspring (reviewed in van Schaik 2000).
Conclusions
Until the advent of noninvasive genetic techniques, it was challenging to test the relationship between male dominance rank and reproductive success in wild populations, particularly in promiscuous mating systems (Hughes 1998; Di Fiore 2003). Any male, regardless of his rank, that copulates with a fertile female has a chance at siring her offspring by means of successful sperm competition. Although rank was initially thought to be unimportant in chimpanzees because males can use alternative strategies (Goodall 1986), our study confirms that male rank generally correlates with reproductive success. However, younger males had the highest success per male, and low-ranking males successfully produced offspring more often than was predicted by the priority of access model. Low-ranking fathers sired offspring with younger, less desirable females and appeared to use the consortship strategy more often than higher-ranking fathers. Thus, even though rank generally serves as a queue for males to have access to reproductive females, and males have some knowledge about female fertility, male age, mate choice and alternative male mating strategies affect patterns of male reproductive success in this species. Future work should further explore the effect of male age, inbreeding avoidance and party composition on male mating frequency and reproductive success.
Acknowledgments
We are grateful to Tanzania National Parks, the Tanzanian Wildlife Research Institute and the Tanzanian Commission for Science and Technology for their permission to work on this project in Gombe National Park. We thank the Jane Goodall Institute for funding long-term research at Gombe and Dr Jane Goodall for granting us permission to work with the long-term data. We are deeply indebted to the entire Gombe Stream Research Center staff, especially the field assistants and faecal sample collectors, for maintaining data collection and data extraction under the direction of Drs D. Anthony Collins, Shadrack Kamenya and Michael Wilson. Dr Jane Raphael and Baraka Gilagiza were instrumental in monitoring faecal sample collection and shipments, with invaluable assistance from the Lincoln Park Zoo (Chicago, IL, U.S.A.). We thank the numerous assistants who have entered long-term dominance data into the database at the Jane Goodall Institute's Center for Primate Studies, particularly Nick Graham and Natasha Tworoski. We also thank Leif Johnson, Julia Molony and Drs Lynn Eberly and Sanford Weisberg for statistical advice. Dr Elizabeth Lonsdorf and three anonymous referees provided insightful comments on the manuscript. The current project was funded primarily by the National Science Foundation (grant no. BCS-0452315) and the National Institutes of Health NIAID (grant no. R01 AI058715), and by grants from Harris Steel Group and the Windibrow Foundation. Emily Wroblewski was supported by the Elmer C. Birney and Florence Rothman Fellowships, and the Dayton and Wilkie Natural History Fund. All research complied with the regulations of the Institutional Animal Care and Use Committee of the University of Minnesota.
Appendix: Genetic and Paternity Analyses
Sampling and DNA Isolation
All faecal samples were collected from individually recognized, habituated chimpanzees no more than several minutes after defecation. An equal volume of faeces was transferred into a vial containing 25 ml of RNAlater (Ambion, Austin, Texas, U.S.A.), frozen in the field (4 °C) as soon as possible until shipment, and then stored at −80 °C in the laboratory. Samples used for analysis ranged from months to years old.
We used the QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA, U.S.A.) to extract DNA from 400 μl to 2 ml of RNAlater preserved faeces. Briefly, faecal samples were incubated in lysis buffer and spun, then the collected supernatant was treated with InhibitEX to remove potential PCR inhibitors. After spinning again, supernatants underwent proteinase K digestion, then were passed through a DNA binding column for DNA purification and final elution in 150–180 μl of elution buffer. Tissue samples were also stored in RNAlater and extracted using the DNeasy Tissue Kit (Qiagen).
Table A1.
Results of CERVUS paternity analysis under three simulation conditions
| Offspring | Mother | Father | Next fewest mismatches | Kasekela candidate males | Kasekela–Mitumba candidate males | All alive Kasekela–Mitumba and 50% unsampled males | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males sampled (total) | Paternity exclusion probability | Confidence | Males sampled (total) | Paternity exclusion probability | Confidence | Males sampled (total) | Paternity exclusion probability | Confidence | ||||
| BRZ | BAH | KS | 4 | 12 (12) | 0.99965 | 95% | 14 (14) | 0.99957 | 95% | 14 (21) | 0.99987 | 95% |
| CN | CD | WL | 3 | 11 (12) | 0.99999 | 95% | 12 (17) | 0.99995 | 95% | 12 (26) | 0.99994 | 95% |
| COC | CD | FD | 2 | 13 (13) | 0.99954 | 95% | 16 (16) | 0.99970 | 95% | 16 (24) | 0.99965 | 95% |
| DIA* | DL | FE | 0 (FO); 1 (FR) | 12 (12) | 0.99230 | 95% | 14 (14) | 0.99354 | 95% | 14 (21) | 0.99682 | 75% |
| DL | (DM) | BE | 1 | 8 (15) | 0.93004 | 80% | 9 (21) | 0.93539 | 80% | 9 (32) | 0.95270 | 80% |
| FAM | FN | SL | 2 | 13 (13) | 0.99950 | 95% | 16 (16) | 0.99952 | 95% | 16 (24) | 0.99900 | 95% |
| FE | FF | EV | 4 | 12 (12) | 0.99993 | 95% | 13 (17) | 0.99993 | 95% | 13 (26) | 0.99992 | 95% |
| FI | FF | FR | 1 | 12 (12) | 0.99970 | 95% | 14 (19) | 0.99973 | 95% | 14 (29) | 0.99977 | 95% |
| FLI | FF | KS | 2 | 13 (13) | 0.99837 | 95% | 15 (18) | 0.99867 | 95% | 15 (27) | 0.99890 | 95% |
| FN | FF | GB | 4 | 5 (11) | 0.99976 | 95% | 5 (16) | 0.99976 | 95% | 5 (24) | 0.99999 | 95% |
| FND | FN | SL | 4 | 13 (13) | 0.99984 | 95% | 16 (18) | 0.99986 | 95% | 14 (27) | 0.99978 | 95% |
| FO | FF | WL | 3 | 11 (12) | 0.99980 | 95% | 12 (17) | 0.99982 | 95% | 12 (26) | 0.99978 | 95% |
| FS | FF | GB | 3 | 7 (12) | 0.99997 | 95% | 7 (17) | 0.99997 | 95% | 7 (26) | 0.99999 | 95% |
| FU | FN | SL | 4 | 12 (12) | 0.99989 | 95% | 14 (18) | 0.99993 | 95% | 14 (27) | 0.99998 | 95% |
| GA | GM | WL | 2 | 13 (13) | 0.99980 | 95% | 14 (18) | 0.99964 | 95% | 14 (27) | 0.99960 | 80% |
| GD | GM | AL | 3 | 10 (11) | 0.99964 | 95% | 11 (17) | 0.99953 | 95% | 11 (26) | 0.99966 | 95% |
| GIM | GM | TB | 3 | 12 (12) | 0.99997 | 95% | 15 (15) | 0.99999 | 95% | 15 (23) | 0.99999 | 95% |
| GLD | GM | FR | 3 | 13 (13) | 0.99999 | 95% | 15 (18) | 0.99999 | 95% | 15 (27) | 0.99999 | 95% |
| GLI | GM | FR | 2 | 13 (13) | 0.99922 | 95% | 15 (18) | 0.99932 | 95% | 15 (27) | 0.99974 | 95% |
| GM* | (ML) | EV | NA | 1 (16) | 0.95759 | 95% | 1 (19) | 0.95760 | 80% | 1 (29) | 0.95759 | 80% |
| JK | JF | AL | 3 | 11 (12) | 0.00059 | 95% | 12 (17) | 0.99967 | 95% | 12 (26) | 0.99933 | 80% |
| MAM | MAK | GL | 3 | 12 (12) | 0.99698† | 95%† | 15 (15) | 0.99803† | 95%† | 15 (23) | 0.99948 | 95% |
| SAM | SA | FR | 2 | 11 (11) | 0.99980 | 95% | 14 (16) | 0.99991 | 95% | 14 (24) | 0.99996 | 95% |
| SDB | SW | FR | 4 | 11 (11) | 0.99997 | 95% | 14 (16) | 0.99997 | 95% | 14 (24) | 0.99997 | 95% |
| SHA | SR | WL | 3 | 13 (13) | 0.99998 | 95% | 16 (16) | 0.99998 | 95% | 16 (24) | 0.99997 | 95% |
| SI | SW | WL | 5 | 11 (12) | 0.99999 | 95% | 12 (17) | 0.99998 | 95% | 12 (26) | 0.99999 | 95% |
| SN | SA | AO | 4 | 12 (12) | 0.99998 | 95% | 14 (19) | 0.99999 | 95% | 14 (29) | 0.99999 | 95% |
| SR | SA | BE | 4 | 11 (12) | 0.99969 | 95% | 12 (17) | 0.99960 | 95% | 12 (26) | 0.99980 | 95% |
| TA | PI | GB | 3 | 11 (12) | 0.99996† | 95%† | 12 (17) | 0.99994† | 95%† | 12 (26) | 0.99992 | 95% |
| TN | PI | FR | 2 (MEL); 3 | 13 (13) | 0.99961† | 95%† | 14 (18) | 0.99962† | 95%† | 14 (27) | 0.99984 | 95% |
| TOF | TTA | SL | 3 | 11 (11) | 0.99989† | 95%† | 14 (16) | 0.99993† | 95%† | 14 (24) | 0.99990 | 95% |
| TOM | TA | KS | 3 | 11 (11) | 0.99973 | 95% | 14 (16) | 0.99972 | 95% | 14 (24) | 0.99982 | 95% |
| TZN | PI | FR | 2 | 13 (13) | 0.99950† | 95%† | 16 (17) | 0.99947† | 95%† | 16 (26) | 0.99996 | 95% |
| YAM | YD | WL | 2 | 13 (13) | 0.99834 | 95% | 15 (18) | 0.99873 | 95% | 15 (27) | 0.99928 | 95% |
| ZEL | TZ | KS | 3 | 13 (13) | 0.99992 | 95% | 16 (17) | 0.99991 | 95% | 16 (26) | 0.99996 | 95% |
| ZS | TZ | FR | 2 | 13 (13) | 0.99987 | 95% | 14 (18) | 0.99992 | 95% | 14 (27) | 0.99984 | 95% |
Offspring in bold were not included in the priority of access analysis because their conceptions preceded 1984; offspring in italics had paternities previously established by Constable et al. (2001); offspring GLD and GLI were twins and were treated as a single paternity in the priority of access analysis. Parentheses around the mother's ID indicate that she was not genotyped. MEL was the only candidate male genotyped at fewer than 10 loci.
Paternity analysis was done slightly differently; see Paternity Analysis in the Appendix for details.
Offspring, candidate male and mother genotypes were compared at fewer than 10 loci because maternal alleles were not in the candidate male allele frequencies.
Quantification of Faecal DNA
We determined DNA extract concentration using quantitative (‘real-time’) PCR before genotyping. Amplification reactions were performed as described in Morin et al. (2001) with minor modifications. BSA (bovine serum albumin, Ambion) was added between 10 μg and 40 μg (current protocol) per reaction. Duplicate sets of DNA standards of known quantity were used to generate a standard curve. Standard DNA amounts were 2000 pg, 1000 pg, 500 pg, 250 pg, 125 pg, 62.5 pg, 31.25 pg and 15.6 pg, and all amplification rounds included a ‘no-template control’. Amplification and quantitative analysis were conducted using an ABI Prism 7700 Sequence Detector and its software, version 1.9 (Applied Biosystems, Foster City, CA, U.S.A.).
Microsatellite Analysis and Genotyping
All individuals for whom DNA samples were available were genotyped using at least 10 of 11 tetranucleotide microsatellite loci amplified by human primers. The 11 loci were a subset of those used by Constable et al. (2001) (D19S431, D9S905, D18S536, D10S676, D4S1627, D2S1333, D4S243, D1S548, D9S922, D11S1366, D2S1326) selected to be on different chromosomes or to avoid linkage by having a maximum of two markers per chromosome that were at least 50 cM apart (genetic maps from Marshfield Center for Medical Genetics, http://research.marshfieldclinic.org/genetics/).
Amplifications were performed in 20 μl reactions with at least 5 μl of faecal DNA extract. Reaction mixes were composed in one of two ways: (1) 1× AmpliTaq Gold PCR Master Mix (Applied Biosystems), 1.25 mM of additional MgCl2, 0.25 mM of additional dNTPs, 0.2 μM of each primer and 25 μg of BSA; (2) (current protocol) 1× AmpliTaq Gold Buffer II, 4.375 mM of MgCl2, 1.5 mM of dNTPs, 0.4 μM of each primer, 50 μg of BSA, 2.5 units of AmpliTaq Gold DNA polymerase, LD (Applied Biosystems). Amplification was performed on ABI Gene Amp PCR System 9700 thermocyclers using an initial denaturation of 95 °C for between 2.5 and 5 min (current protocol), 12 cycles of 95 °C for 30 s, 60 °C (−0.5 °C per cycle) for 30 s, 72 °C for 30 s, followed by 45 cycles of 95 °C for 30 s, 54 °C for 30 s, 72 °C for 30 s, and a single final extension at 72 °C for 10 min.
The forward primer for each microsatellite locus was labelled with a FAM (Invitrogen, Carlsbad, CA, U.S.A.), HEX (Invitrogen) or NED (Applied Biosystems) fluorescent dye for allele sizing. PCR products were pooled into two groups with two of each fluorescent dye type of nonoverlapping product sizes: (1) D19S536 (FAM), D4S243 (FAM), D10S676 (HEX), D9S922 (HEX), D19S431 (NED) and AMEL 212 (NED) (see below); (2) D2S1326 (FAM), D2S1333 (FAM), D4S1627 (HEX), D9S905 (HEX), D1S548 (NED), D11S1366 (NED). A mix of 1.5 μl of pooled sample, 0.5 μl of a ROX (fluorescent dye)-labelled molecular weight ladder (Applied Biosystems), 1 μl of loading dye and 2.5 μl of formaldehyde was denatured at 95 °C for 5 min, then immediately put on ice; 1.5 μl of this mix was then loaded onto a 6% GenePage Plus polyacrylamide gel (Amresco, Solon, OH, U.S.A.) and electrophoresed for 3.2 h at 51 °C and 3000 V on an ABI Prism 377 DNA Sequencer (Applied Biosystems). Sizing of alleles was done using GeneScan software, versions 2.1 and 3.2.1 (Applied Biosystems). Repetition of genotyping for each locus was done, at a minimum, according to the recommendations of Morin et al. (2001) based on the amount of DNA per reaction, but the vast majority of reactions had well over 200 pg.
Sample Identity Verification
The accuracy of the sample identities and genotypes was ensured through several means. First, since mitochondrial DNA is inherited matrilineally, samples from individuals that were maternal relatives were confirmed to be of the same haplotype. Mitochondrial haplotypes were created by amplifying and directly sequencing a 498-base pair region of the hypervariable D loop using primers L15997 (5′-CACCATTAGCACCCAAAGCT-3′) and H16498 (5′-CCTGAAGTAGGAACCAGATG-3′). PCRs were performed in 30 μl (current protocol) or 50 μl reactions containing 1× Expand Long Template PCR Buffer II, 0.58 (30 μl) or 0.35 (50 μl) mM of dNTPs, 1.33 (30 μl), 0.8 or 0.4 (50 μl) μM of each primer, 25 μg of BSA, 2.5 units of Expand Long Template Taq polymerase and 5 μl of DNA extract. Thermocycling initially used the following conditions: initial denaturation of 5 min at 94 °C followed by 55 cycles of 94 °C for 30 s, 55 °C for 1.5 min, 72 °C for 30 s and a final extension of 72 °C for 10 min. The protocol was later modified as follows: initial denaturation of 2 min at 94 °C followed by 55 cycles of 94 °C for 30 s, 55 °C for 45 s, 68 °C for 1 min and a final extension of 68 °C for 10 min. Thirteen different haplotypes have been identified within the Gombe population (Liu et al. 2008). DNA samples were not quantified and genotyped if there was a mismatch between the chimpanzee ID of the sample and the mitochondrial haplotype.
Second, the sex of the sample donor was confirmed whenever possible through amplification of a region of the amelogenin gene using primers AMEL-F212 (5′-ACCTCATCCTGGGCACCCTGG-3′) and AMEL-R212 (5′-AGGCTTGAGGCCAACCATCAG-3′). Because of a known deletion on the X but not the Y chromosome, male chimpanzees amplify two products (212 and 218 base pairs) whereas female chimpanzees have a single amplification product (212 base pairs) (Sullivan et al. 1993). Amplification was conducted under the same conditions as the microsatellite PCRs except each reaction contained 0.25 or 0.5 μM of each primer. Samples were not analysed if there was a mismatch between the sex of the chimpanzee named on the sample and the genetically determined sex.
Additionally, Mendelian inheritance of microsatellite alleles were confirmed by verifying that offspring and their known mothers shared at least one allele at every locus. Finally, the genotypes were confirmed using at least two independent faecal samples whenever more than one was available.
Paternity Analysis
Paternity was first examined through exclusion and confirmed using the likelihood-based program CERVUS 2.0 (Marshall et al. 1998). All genotyped candidate males, mothers and offspring were genotyped at a minimum of 10 of 11 loci with one exception; Mel was only genotyped at five loci (Constable et al. 2001), but it was unlikely that he was a father for the two offspring for which he was a candidate (TN and ZS). He was only 9 years old at the time of their conception, and he had two and three loci, respectively, mismatching with the offspring. We assigned paternity to a male based on exclusion when he was the only male that lacked mismatches with the offspring, given the offspring, mother and male genotypes, and all other male candidates had at least one mismatch. DIA was the only offspring that did not fit the criteria, having two males that lacked mismatches (maternal half-siblings), but CERVUS assigned paternity to one of these two males with high confidence (see below).
We used CERVUS to conduct likelihood-based paternity analysis. Paternity simulation was conducted under the following conditions: 100 000 simulation cycles,1% error rate and confidence levels of 80% and 95%. The proportion of loci typed and candidate males sampled was set to the specific conditions surrounding each conception. Furthermore, we ran simulations under three genetic environments for each conception for the genotype frequencies and proportion of candidate males sampled: (1) within-community candidate males only; (2) all candidate males for both the Mitumba and Kasekela communities (both habituated); and (3) all chimpanzees alive at the time of conception for both the Mitumba and Kasekela communities and an additional 50% unsampled male candidates to account for potential candidates from the non-habituated Kalande community (Constable et al. 2001; Alberts et al. 2006; Boesch et al. 2006). The added 50% unsampled candidates was a conservative estimate and was probably disproportionately high for offspring born after 1999; the southern Kalande community has been in decline since 1999, and currently is believed to have only one adult male (Pusey et al. 2008a). There were two exceptions to these simulation conditions. We used the genotype frequencies of all individuals alive at conception for each of the three candidate sampling conditions for GM because we had only a single candidate male genotype available. We included an advanced simulation parameter to the simulations for DIA whereby we accounted for the four maternal brothers amongst the candidate pool (related 0.25 to each other) because simple exclusion could not differentiate between two of them.
Paternities were assigned for all 36 possible offspring (14 repeated from Constable et al. (2001) and 22 newly assigned). Paternities repeated from Constable et al. (2001) agreed with previous assignments. Paternities based on the exclusion method were in complete agreement with the paternity assignment from CERVUS under all three simulation conditions (Table A1). CERVUS assigned paternity with at least 80% confidence, and with 95% confidence the majority of the time. The single exception was the 75% confidence assigned to the paternity of DIA under the simulation conditions using allele frequencies based on all the individuals alive at the time of conception and 50% unsampled male candidates. To investigate this dramatic drop in confidence from the previous two simulation conditions, we repeated the analysis using the same allele frequencies but removing the unsampled proportion of candidates (therefore leaving complete sampling of Mitumba and Kasekela candidates). Confidence was again 95%, indicating that the unsampled male candidates caused the lowered confidence.
References
- Alberts SC, Altmann J, Wilson ML. Mate guarding constrains foraging activity of male baboons. Animal Behaviour. 1996;51:1269–1277. [Google Scholar]
- Alberts SC, Buchan JC, Altmann J. Sexual selection in wild baboons: from mating opportunities to paternity success. Animal Behaviour. 2006;72:1177–1196. [Google Scholar]
- Altmann SA. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Annals of the New York Academy of Sciences. 1962;102:338–435. doi: 10.1111/j.1749-6632.1962.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Berard JD, Nurnberg P, Epplen JT, Schmidtke J. Alternative reproductive tactics and reproductive success in male rhesus macaques. Behaviour. 1994;129:177–201. [Google Scholar]
- Bercovitch FB, Nürnberg P. Socioendocrine and morphological correlates of paternity in rhesus macaques (Macaca mulatta) Journal of Reproduction and Fertility. 1996;107:59–68. doi: 10.1530/jrf.0.1070059. [DOI] [PubMed] [Google Scholar]
- Boesch C. Social grouping in Taï chimpanzees. In: McGrew WC, Marchant LF, Nishida T, editors. Great Ape Societies. Cambridge: Cambridge University Press; 1996. pp. 101–113. [Google Scholar]
- Boesch C, Boesch-Achermann H. The Chimpanzees of the Taï Forest. Oxford; Oxford University Press; 2000. [Google Scholar]
- Boesch C, Kohou G, Néné H, Vigilant L. Male competition and paternity in wild chimpanzees of the Taï forest. American Journal of Physical Anthropology. 2006;130:103–115. doi: 10.1002/ajpa.20341. [DOI] [PubMed] [Google Scholar]
- Bradley BJ, Robbins MM, Williamson EA, Dieter Steklis H, Gerald Steklis N, Eckhardt N, Boesch C, Vigilant L. Mountain gorilla tug-of-war: silverbacks have limited control over reproduction in multimale groups. Proceedings of the National Academy of Sciences, U S A. 2005;102:9418–9423. doi: 10.1073/pnas.0502019102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bribiescas RG. On the evolution, life history, and proximate mechanisms of human male reproductive senescence. Evolutionary Anthropology. 2006;15:132–141. [Google Scholar]
- Bygott D. Agonistic behavior, dominance, and social structure in wild chimpanzees of the Gombe National Park. In: Hamburg DA, McCown ER, editors. The Great Apes. Menlo Park, California: Benjamin/Cummings; 1979. pp. 405–427. [Google Scholar]
- Chapais B. Reproductive activity in relation to male dominance and the likelihood of ovulation in rhesus monkeys. Behavioral Ecology and Sociobiology. 1983;12:215–228. [Google Scholar]
- Charpentier M, Peignot P, Hossaert-McKey M, Gimenez O, Setchell JM, Wickings EJ. Constraints on control: factors influencing reproductive success in male mandrills (Mandrillus sphinx) Behavioral Ecology. 2005;16:614–623. [Google Scholar]
- Coltman DW, Bancroft DR, Robertson A, Smith JA, Clutton-Brock TH, Pemberton JM. Male reproductive success in a promiscuous mammal: behavioral estimates compared with genetic paternity. Molecular Ecology. 1999;8:1199–1209. doi: 10.1046/j.1365-294x.1999.00683.x. [DOI] [PubMed] [Google Scholar]
- Constable JL, Ashley MV, Goodall J, Pusey AE. Noninvasive paternity assignment in Gombe chimpanzees. Molecular Ecology. 2001;10:1279–1300. doi: 10.1046/j.1365-294x.2001.01262.x. [DOI] [PubMed] [Google Scholar]
- Cunningham EJA, Birkhead TR. Sex roles and sexual selection. Animal Behaviour. 1998;56:1311–1321. doi: 10.1006/anbe.1998.0953. [DOI] [PubMed] [Google Scholar]
- Deschner T, Heistermann M, Hodges K, Boesch C. Timing and probability of ovulation in relation to sex skin swelling in wild West African chimpanzees, Pan troglodytes verus. Animal Behaviour. 2003;66:551–560. [Google Scholar]
- Deschner T, Heistermann M, Hodges K, Boesch C. Female sexual swelling size, timing of ovulation, and male behavior in wild West African chimpanzees. Hormones and Behavior. 2004;46:204–215. doi: 10.1016/j.yhbeh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Di Fiore A. Molecular genetic approaches to the study of primate behavior, social organization, and reproduction. American Journal of Physical Anthropology. 2003;122:62–99. doi: 10.1002/ajpa.10382. [DOI] [PubMed] [Google Scholar]
- Ellis L. Dominance and reproductive success among non-human animals: a cross-species comparison. Ethology and Sociobiology. 1995;16:257–333. [Google Scholar]
- Emery Thompson M. Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): methodological considerations and the role of hormones in sex and conception. American Journal of Primatology. 2005;67:137–158. doi: 10.1002/ajp.20174. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Wrangham RW. Male mating interest varies with female fecundity in Pan troglodytes schweinfurthii of Kanyawara, Kibale National Park. International Journal of Primatology. 2008;29:885–905. [Google Scholar]
- Engelhardt A, Pfeifer JB, Heistermann M, Niemitz C, van Hooff JARAM, Hodges JK. Assessment of female reproductive status by male longtailed macaques, Macaca fascicularis, under natural conditions. Animal Behaviour. 2004;67:915–924. [Google Scholar]
- Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S, Moore D. The association of age and semen quality in healthy men. Human Reproduction. 2003;18:447–454. doi: 10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]
- Gemmel NJ, Burg TM, Boyd IL, Amos W. Low reproductive success in territorial male Antarctic fur seals (Arctocephalus gazelle) suggests the existence of alternative mating strategies. Molecular Ecology. 2001;10:451–460. doi: 10.1046/j.1365-294x.2001.01186.x. [DOI] [PubMed] [Google Scholar]
- Gerloff U, Hartung B, Fruth B, Hohmann G, Tautz D. Intracommunity relationships, dispersal pattern and paternity success in a wild living community of bonobos (Pan paniscus) determined from DNA analysis of faecal samples. Proceedings of the Royal Society of London, Series B. 1999;266:1189–1195. doi: 10.1098/rspb.1999.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Hormones and Behavior. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Girman DJ, Mills MGL, Geffen E, Wayne RK. A molecular genetic analysis of social structure, dispersal, and interpack relationships of the African wild dog (Lycaon pictus) Behavioral Ecology and Sociobiology. 1997;40:187–198. [Google Scholar]
- Gomendio M, Harcourt AH, Roldán ERS. Sperm competition in mammals. In: Birkhead TR, Møller AP, editors. Sperm Competition and Sexual Selection. San Diego: Academic Press; 1998. pp. 667–755. [Google Scholar]
- Goodall J. The Chimpanzees of Gombe. Cambridge, Massachusetts: Belknap Press; 1986. [Google Scholar]
- Haley MP, Deutsch CJ, Le Boeuf Burney J. Size, dominance and copulatory success in male northern elephant seals, Mirounga angustirostris. Animal Behaviour. 1994;48:1249–1260. [Google Scholar]
- Harcourt AH, Harvey PH, Larson SG, Short RV. Testis weight, body weight and breeding system in primates. Nature. 1981;293:55–57. doi: 10.1038/293055a0. [DOI] [PubMed] [Google Scholar]
- Harcourt AH, Purvis A, Liles L. Sperm competition: mating system, not breeding season, affects testes size of primates. Functional Ecology. 1995;9:468–476. [Google Scholar]
- Hasegawa T, Hiraiwa-Hasegawa M. Sperm competition and mating behavior. In: Nishida T, editor. The Chimpanzees of the Mahale Mountains: Sexual and Life History Strategies. Tokyo: University of Tokyo Press; 1990. pp. 115–132. [Google Scholar]
- Hayakawa S. Female defensibility in small troops of Japanese macaques vis-à-vis nontroop males and copulation on the periphery of the troop. International Journal of Primatology. 2007;28:73–96. [Google Scholar]
- Hughes C. Integrating molecular techniques with field methods in studies of social behavior: a revolution results. Ecology. 1998;79:383–399. [Google Scholar]
- Kahlenberg SM, Emery Thompson M, Wrangham RW. Female competition over core areas in Pan troglodytes schweinfurthii, Kibale National Park, Uganda. International Journal of Primatology. 2008;29:931–947. [Google Scholar]
- Keil A, Epplen JT, Sascher N. Reproductive success of males in the promiscuous-mating yellow-toothed cavy (Galea musteloides) Journal of Mammalogy. 1999;80:1257–1263. [Google Scholar]
- Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertility and Sterility. 2001;75:237–248. doi: 10.1016/s0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- Launhardt K, Borries C, Hardt C, Epplen JT, Winkler P. Paternity analysis of alternative male reproductive routes among the langurs (Semnopithecus entellus) of Ramnagar. Animal Behaviour. 2001;61:53–64. doi: 10.1006/anbe.2000.1590. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Boesch C. Bisexually bonded ranging in chimpanzees (Pan troglodytes verus) Behavioral Ecology and Sociobiology. 2005;57:525–535. [Google Scholar]
- Liu W, Worobey M, Li Y, Keele BF, Bibollet-Ruche F, Guo Y, Goepfert PA, Santiago ML, Ndjango JBN, Neel C, Clifford SL, Sanz C, Kamenya S, Wilson ML, Pusey AE, Gross-Camp N, Boesch C, Smith V, Zamma K, Huffman MA, Mitani JC, Watts DP, Peeters M, Switzer WM, Shaw GM, Sharp PM, Hahn BH. Molecular ecology and natural history of simian foamy virus infection in wild-living chimpanzees. PLoS Pathogens. 2008;4:e1000097. doi: 10.1371/journal.ppat.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall TC, Slate J, Kruuk L, Pemberton JM. Statistical confidence for likelihood-based paternity in natural populations. Molecular Ecology. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Oda A. Female choice in the opportunistic mating of wild chimpanzees (Pan troglodytes schweinfurthii) at Mahale. Behavioral Ecology and Sociobiology. 1999;46:258–266. [Google Scholar]
- Mitani JC, Amsler SJ. Social and spatial aspects of male subgrouping in a community of wild chimpanzees. Behaviour. 2003;140:869–884. [Google Scholar]
- Møller AP. Ejaculate quality, testes size and sperm competition in primates. Journal of Human Evolution. 1988;17:479–488. [Google Scholar]
- Morin PA, Chambers KE, Boesch C, Vigilant L. Quantitative polymerase chain reaction analysis of DNA from noninvasive samples for accurate microsatellite genotyping of wild chimpanzees (Pan troglodytes verus) Molecular Ecology. 2001;10:1835–1844. doi: 10.1046/j.0962-1083.2001.01308.x. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Watts DP, Whitten PL. Dominance rank and fecal testosterone levels in adult male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. American Journal of Primatology. 2004;64:71–82. doi: 10.1002/ajp.20062. [DOI] [PubMed] [Google Scholar]
- Muller MN. Agonistic relations among Kanyawara chimpanzees. In: Boesch C, Hohmann G, Marchant LF, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge: Cambridge University Press; 2002. pp. 112–123. [Google Scholar]
- Muller MN, Wrangham RW. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii) Behavioral Ecology and Sociobiology. 2004;55:332–340. doi: 10.1007/s00265-020-02872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Emery Thompson M, Wrangham RW. Male chimpanzees prefer mating with older females. Current Biology. 2007;16:2234–2238. doi: 10.1016/j.cub.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Muniz L, Perry S, Manson JH, Gilkenson H, Gros-Louis J, Vigilant L. Father-daughter inbreeding avoidance in a wild primate population. Current Biology. 2006;16:156–157. doi: 10.1016/j.cub.2006.02.055. [DOI] [PubMed] [Google Scholar]
- Murray CM, Mane SV, Pusey AE. Dominance rank influences female space use in wild chimpanzees, Pan troglodytes: towards an ideal despotic distribution. Animal Behaviour. 2007;74:1795–1804. [Google Scholar]
- Nishida T. The social group of wild chimpanzees in the Mahale Mountains. Primates. 1968;9:167–224. [Google Scholar]
- Nishida T, Hiraiwa-Hasegawa M. Chimpanzees and bonobos: cooperative relationships among males. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 299–305. [Google Scholar]
- Nishida T, Takasaki H, Takahata Y. Demography and reproductive profiles. In: Nishida T, editor. The Chimpanzees of the Mahale Mountains: Sexual and Life History Strategies. Tokyo: University of Tokyo Press; 1990. pp. 63–97. [Google Scholar]
- Nishida T, Corp N, Hamai M, Hasegawa T, Hiraiwa-Hasegawa M, Hosake K, Hunt KD, Itoh N, Kawanaka K, Matsumoto-Oda A, Mitani JC, Nakamura M, Norikoshi K, Sakamaki T, Turner L, Uehara S, Zamma K. Demography, female life history, and reproductive profiles among the chimpanzees of Mahale. American Journal of Primatology. 2003;59:99–121. doi: 10.1002/ajp.10068. [DOI] [PubMed] [Google Scholar]
- Ostner J, Chalise MK, Koenig A, Launhardt K, Nikolei J, Podzuweit D, Borries C. What Hanuman langur males know about female reproductive status. American Journal of Primatology. 2006;68:701–712. doi: 10.1002/ajp.20260. [DOI] [PubMed] [Google Scholar]
- Parker GA. Sperm competition and its evolutionary consequences in insects. Biological Reviews. 1970;45:525–567. [Google Scholar]
- Pemberton JM, Albon SD, Guiness FE, Clutton-Brock TH, Dover GA. Behavioral estimates of male mating success tested by DNA fingerprinting in a polygynous mammal. Behavioral Ecology. 1992;3:66–75. [Google Scholar]
- Pereira ME, Weiss ML. Female mate choice, male migration, and the threat of infanticide in ringtailed lemurs. Behavioral Ecology and Sociobiology. 1991;28:141–152. [Google Scholar]
- Preston BT, Stevenson IR, Pemberton JM, Wilson K. Dominant rams lose out by sperm depletion. Nature. 2001;409:681–682. doi: 10.1038/35055617. [DOI] [PubMed] [Google Scholar]
- Pusey AE. Inbreeding avoidance in chimpanzees. Animal Behaviour. 1980;28:543–552. [Google Scholar]
- Pusey AE. Inbreeding avoidance in primates. In: Wolf AP, Durham WH, editors. Inbreeding, Incest, and the Incest Taboo: the State of Knowledge at the Turn of the Century. Stanford, California: Stanford University Press; 2005. pp. 61–73. [Google Scholar]
- Pusey A, Williams J, Goodall J. The influence of dominance rank on the reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Wilson ML, Collins DA. Human impacts, disease risk, and population dynamics in the chimpanzees of Gombe National Park, Tanzania. American Journal of Primatology. 2008a;70:738–744. doi: 10.1002/ajp.20567. [DOI] [PubMed] [Google Scholar]
- Pusey A, Murray C, Wallauer W, Wilson M, Wroblewski E, Goodall J. Severe aggression among female chimpanzees at Gombe National Park, Tanzania. International Journal of Primatology. 2008b;29:949–973. [Google Scholar]
- Radespeil U, Dal Secco V, Drögemüller C, Braune P, Labes E, Zimmermann E. Sexual selection, multiple mating and paternity in grey mouse lemurs, Microcebus murinus. Animal Behaviour. 2002;63:259–268. [Google Scholar]
- Radwan J, Michalczyk Ł, Prokop Z. Age dependence of male mating ability and sperm competition success in the bulb mite. Animal Behaviour. 2005;69:1101–1105. [Google Scholar]
- Reynolds V. The Chimpanzees of the Budongo Forest: Ecology, Behavior, and Conservation. Oxford: Oxford University Press; 2005. [Google Scholar]
- Richard M, Lecomte J, deFraipont M, Clobert J. Age-specific mating strategies and reproductive senescence. Molecular Ecology. 2005;14:3147–3155. doi: 10.1111/j.1365-294X.2005.02662.x. [DOI] [PubMed] [Google Scholar]
- Røed KH, Holand Ø, Smith ME, Gjøstein H, Kumpula J, Nieminen M. Reproductive success in reindeer males in a herd with varying sex ratio. Molecular Ecology. 2002;11:1239–1243. doi: 10.1046/j.1365-294x.2002.01509.x. [DOI] [PubMed] [Google Scholar]
- Sakura O. Factors affecting party size and composition of chimpanzees (Pantroglodytes verus) Bossou, Guinea. International Journal of Primatology. 1994;15:167–183. [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Say L, Pontier D, Natoli E. Influence of oestrus synchronization on male reproductive success in the domestic cat (Felis catus L) Proceedings of the Royal Society of London, Series B. 2001;268:1049–1053. doi: 10.1098/rspb.2001.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik CP. Infanticide by male primates: the sexual selection hypothesis revisited. In: van Schaik CP, Janson CH, editors. Infanticide by Males and Its Implications. Cambridge: Cambridge University Press; 2000. pp. 27–60. [Google Scholar]
- Setchell JM, Charpentier M, Wickings J. Mate guarding and paternity in mandrills: factors influencing alpha male monopoly. Animal Behaviour. 2005;70:1105–1120. [Google Scholar]
- Simmons LW. Female choice and the relatedness of mates in the field cricket, Gryllus bimaculatus. Animal Behaviour. 1991;41:493–501. [Google Scholar]
- Smuts BB. Sexual competition and mate choice. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 385–399. [Google Scholar]
- Soltis J, Thomsen R, Takenaka O. The interaction of male and female reproductive strategies and paternity in wild Japanese macaques, Macaca fuscata. Animal Behaviour. 2001;62:485–494. [Google Scholar]
- Sparkes TC, Keogh DP, Pary RA. Energetic costs of mate guarding behavior in male stream dwelling isopods. Oecologia. 1996;106:166–171. doi: 10.1007/BF00328595. [DOI] [PubMed] [Google Scholar]
- Stumpf RM, Boesch C. Does promiscuous mating preclude female choice? Female sexual strategies in chimpanzees (Pan troglodytes verus) of the Taï National Park, Côte d'Ivoire. Behavioral Ecology and Sociobiology. 2005;57:511–524. [Google Scholar]
- Stumpf RM, Boesch C. The efficacy of female choice in chimpanzees of the Taï Forest, Côte d'Ivoire. Behavioral Ecology and Sociobiology. 2006;60:749–765. [Google Scholar]
- Sugiyama Y. Grooming interactions among adult chimpanzees at Bossou, Guinea, with special reference to social structure. International Journal of Primatology. 1988;9:393–407. [Google Scholar]
- Sugiyama Y. Socioecological factors of male chimpanzee migration at Bossou, Guinea. Primates. 1999;40:61–68. doi: 10.1007/BF02557702. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y. Demographic parameters and life history of chimpanzees at Bossou, Guinea. American Journal of Physical Anthropology. 2004;124:154–165. doi: 10.1002/ajpa.10345. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Kawamoto S, Takenaka O, Kumazaki K, Miwa N. Paternity discrimination and inter-group relationships of chimpanzees at Bossou. Primates. 1993;34:545–552. [Google Scholar]
- Sullivan KM, Mannucci A, Kimpton CP, Gill P. A rapid and quantitative DNA sex test: fluorescence-based PCR analysis of X-Y homologous gene amelogenin. Biotechniques. 1993;15:636–641. [PubMed] [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man 1871–1971. Chicago: Aldine; 1972. pp. 136–179. [Google Scholar]
- Tutin CEG. Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii) Behavioral Ecology and Sociobiology. 1979;6:29–38. [Google Scholar]
- Vigilant L, Hofreiter M, Siedel H, Boesch C. Paternity and relatedness in wild chimpanzee communities. Proceedings of the National Academy of Sciences, U S A. 2001;98:12890–12895. doi: 10.1073/pnas.231320498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries H. An improved test of linearity in dominance hierarchies containing unknown or tied relationships. Animal Behaviour. 1995;50:1375–1389. [Google Scholar]
- de Waal FBM. Chimpanzee Politics. New York: Harper & Rowe; 1982. [Google Scholar]
- Wallis J. A survey of reproductive parameters in the free-ranging chimpanzees of Gombe National Park. Journal of Reproduction and Fertility. 1997;109:297–307. doi: 10.1530/jrf.0.1090297. [DOI] [PubMed] [Google Scholar]
- Watts DP. Coalitionary mate guarding by male chimpanzees at Ngogo, Kibale National Park, Uganda. Behavioral Ecology and Sociobiology. 1998;44:43–55. [Google Scholar]
- Widdig A, Bercovitch FB, Jürgen Streich W, Sauermann U, Nürnberg P, Krawczak M. A longitudinal analysis of reproductive skew in male rhesus macaques. Proceedings of the Royal Society of London, Series B. 2004;271:819–826. doi: 10.1098/rspb.2003.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Pusey AE, Carlis JV, Farm BP, Goodall J. Female competition and male territorial behaviour influence female chimpanzees' ranging patterns. Animal Behaviour. 2002;63:347–360. [Google Scholar]
- Wilson ML, Wrangham RW. Intergroup relations in chimpanzees. Annual Review of Anthropology. 2003;32:363–392. [Google Scholar]
- Wrangham RW, Smuts BB. Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. Journal of Reproduction and Fertility. 1980;28(Supplement):13–31. [PubMed] [Google Scholar]