Abstract
Plastid biogenesis and maintenance depend on the coordinated assembly of proteins imported from the cytosol with proteins translated within plastids. Chloroplasts in leaf cells have a greater need for protein import and protein synthesis than plastids in other organs due to the large amount of proteins required for photosynthesis. We previously reported that the Arabidopsis (Arabidopsis thaliana) transcription factor CIA2 specifically up-regulates leaf expression of genes encoding protein translocons Toc33 and Toc75, which are essential for protein import into chloroplasts. Protein import efficiency was therefore reduced in cia2 mutant chloroplasts. To further understand the function of CIA2, gene expression profiles of the wild type and a cia2 mutant were compared by microarray analysis. Interestingly, in addition to genes encoding protein translocon components, other genes down-regulated in cia2 almost exclusively encode chloroplast ribosomal proteins. Isolated cia2 mutant chloroplasts showed reduced translation efficiency and steady-state accumulation of plastid-encoded proteins. When CIA2 was ectopically expressed in roots, expression of both the protein translocon and ribosomal protein genes increased. Further analyses in vivo revealed that CIA2 up-regulated these genes by binding directly to their promoter regions. We propose that CIA2 is an important factor responsible for fulfilling the higher protein demands of leaf chloroplasts by coordinately increasing both protein import and protein translation efficiencies.
Chloroplasts are a major destination of nuclear-encoded proteins in a leaf cell because they are the organelle responsible for photosynthesis and assimilation of nitrogen and sulfur. According to the endosymbiont hypothesis, chloroplasts are derived from a free-living cyanobacterial ancestor. However, >90% of the chloroplast genes have been transferred to the host nucleus during evolution (Martin et al., 2002). A functional chloroplast contains approximately 3,000 proteins (Leister, 2003). Of those, only about 100 proteins are products of the chloroplast genome, translated on 70S ribosomes within chloroplasts. The remaining chloroplast proteins are transcribed in the nucleus, translated on 80S ribosomes in the cytosol, and imported into chloroplasts (Martin and Herrmann, 1998). Transport of nuclear-encoded proteins across the double-membrane envelope is mediated by a translocon complex located at the outer and inner envelope membranes of chloroplasts (Inaba and Schnell, 2008; Jarvis, 2008). These translocon components are named Toc (translocon at the outer envelope membrane of chloroplasts) and Tic (translocon at the inner envelope membrane of chloroplasts) proteins (Schnell et al., 1997).
Many imported proteins must assemble in complexes with chloroplast-synthesized protein partners for their proper function. For example, RbcS (for small subunit of ribulose-1,5-bisP carboxylase) assembles with chloroplast-encoded RbcL (for large subunit of ribulose-1,5-bisP carboxylase) to catalyze the first step of carbon assimilation. Thus, protein import into plastids must be coordinated with protein synthesis within plastids. Since leaf chloroplasts have a higher protein demand than plastids in other organs, both protein import and protein synthesis capacity should be up-regulated in leaf chloroplasts. How this coordinated up-regulation is achieved is not known.
We previously isolated an ethyl methanesulfonate-induced Arabidopsis (Arabidopsis thaliana) mutant, chloroplast import apparatus2 (cia2), which is defective in chloroplast protein import (Sun et al., 2001). CIA2 is a CCT class (for CONSTANS, CONSTANS-like, and TOC1) transcription factor. It is highly expressed in green organs (leaves, flowers, and immature siliques) but not in roots. CIA2 up-regulates the expression of two chloroplast translocon genes, TOC33 and TOC75, specifically in leaves (Sun et al., 2001). In this study, we identified additional CIA2 functions based on microarray analyses of the cia2 mutant. In addition to regulating the expression of translocon genes in leaves, CIA2 also up-regulated expression of genes encoding chloroplast ribosomal proteins. This regulation appears to be direct because CIA2 was enriched in their promoter regions. Therefore, we propose that CIA2 acts as a leaf-specific transcription factor to coordinately up-regulate protein import and protein translation to meet the higher protein demands of leaf chloroplasts.
RESULTS
Identification of Genes Down-Regulated in the cia2 Mutant
To identify CIA2-regulated genes, the Agilent Arabidopsis oligonucleotide microarray was used to compare the transcription profiles of the wild type (Columbia [Col]) and the cia2 mutant. Of the 21,547 gene spots, 19,421 were meaningful with sufficient expression levels in two dye-swap slides. Between the cia2 mutant and the wild type, 40 genes had at least a 1.5 times difference in expression level in both slides. Two genes had a higher transcript level and 38 had a lower transcript level in cia2 (Table I; ratio of cia2 to the wild type higher than 1.5 for up-regulated genes and lower than 0.67 for down-regulated genes). In agreement with our previous data, TOC33 and TOC75 (AT3G46740, referred to as TOC75-III hereafter) were among the 38 genes down-regulated in cia2 (Table I).
Table I.
Genes identified by microarray analysis as consistently up- or down-regulated in the cia2 mutant
| AGI Code | Gene Description | PSLa | Expression Ratio cia2:Wild Type
|
RT-PCRb | ChIPc | |
|---|---|---|---|---|---|---|
| Slide 1 | Slide 2 | |||||
| I. Genes up-regulated in cia2 | ||||||
| Ribosome biosynthesis | ||||||
| AT2G07696 | Ribosomal protein S7 (RPS7) | Nd/Mefg | 1.76 | 1.62 | ||
| Male gametophyte development | ||||||
| AT3G13400 | Multicopper oxidase (SKS13) | EXd/ERefg | 1.62 | 1.52 | ||
| II. Genes down-regulated in cia2 | ||||||
| Protein chloroplast targeting | ||||||
| AT1G02280 | GTP-binding protein (Toc33) | CPde/otherfg | 0.54 | 0.64 | + | + |
| AT3G46740 | Translocon of outer membrane (Toc75) | CPdefg | 0.65 | 0.61 | + | + |
| Ribosome biosynthesis | ||||||
| AT1G32990 | Ribosomal protein L11 (RPL11) | CPdefg | 0.56 | 0.58 | + | + |
| AT1G48350 | Ribosomal protein L18 (RPL18) | CPdefg | 0.63 | 0.47 | + | + |
| AT2G33450 | Ribosomal protein L28 (RPL28) | CPdefg | 0.61 | 0.49 | + | + |
| AT3G17170 | Ribosomal protein S6 (RPS6) | CPdefg | 0.60 | 0.55 | + | + |
| AT3G25920 | Ribosomal protein L15 (RPL15) | CPdefg | 0.54 | 0.64 | + | + |
| AT5G65220 | Ribosomal protein L29 (RPL29) | CPdefg | 0.66 | 0.56 | + | + |
| AT2G38140 | Ribosomal protein S31 (PSRP4) | CPdefg | 0.64 | 0.50 | + | − |
| AT1G35680 | Ribosomal protein L21 (RPL21) | CPdefg | 0.52 | 0.46 | − | |
| AT1G74970 | Ribosomal protein S9 (RPS9) | CPdefg | 0.66 | 0.43 | − | |
| AT1G75350 | Ribosomal protein L31 (RPL31) | CPdefg | 0.58 | 0.54 | − | |
| AT2G43030 | Ribosomal protein L3 (RPL3A) | CPdefg | 0.67 | 0.62 | − | |
| AT3G15190 | Ribosomal protein S20 (RPS20) | CPdefg | 0.57 | 0.48 | − | |
| AT3G44890 | Ribosomal protein L9 (RPL9) | CPdefg | 0.55 | 0.45 | − | |
| AT3G54210 | Ribosomal protein L17 (RPL17) | CPdefg | 0.54 | 0.64 | − | |
| AT4G01310 | Ribosomal protein L5 (RPL5) | CPdefg | 0.52 | 0.52 | − | |
| AT5G47190 | Ribosomal protein L19 (RPL19-2) | CPdefg | 0.66 | 0.56 | − | |
| AT5G51610 | Ribosomal protein L11-like (RPL11-like) | CPdefg | 0.53 | 0.64 | − | |
| RNA binding | ||||||
| AT3G53460 | RNA-binding protein (CP29) | CPdefg | 0.66 | 0.41 | + | + |
| AT3G52150 | RNA-binding protein (CP33) | CPdefg | 0.61 | 0.56 | − | |
| AT4G38160 | Transcription terminator (PDE191) | CPdfg/othere | 0.50 | 0.63 | − | |
| Protein folding | ||||||
| AT2G44650 | Chaperonin 10 (CPN10) | CPdefg | 0.59 | 0.45 | + | + |
| Porphyrin biosynthesis | ||||||
| AT1G03475 | Coproporphyrinogen III oxidase (CPO1) | CPdefg | 0.60 | 0.61 | + | − |
| AT5G08280 | Hydroxymethylbilane synthase (HemC) | CPdefg | 0.66 | 0.59 | − | |
| Removal of superoxide radicals | ||||||
| AT5G23310 | Fe-superoxide dismutase (FSD3) | CPdefg | 0.52 | 0.65 | − | − |
| AT4G25100 | Fe-superoxide dismutase (FSD1) | CPdefg | 0.55 | 0.62 | − | |
| Electron transport | ||||||
| AT1G60600 | Aberrant chloroplast development (ABC4) CPbcde | 0.59 | 0.41 | − | ||
| ATP binding | ||||||
| AT5G63310 | Nucleotide diphosphate kinase2 (NDPK2) CPbde/Nc | 0.60 | 0.64 | − | ||
| Aldo-keto reductase | ||||||
| At1G04420 | Aldo/keto reductase (KAB1) | CPdefg | 0.66 | 0.65 | − | |
| Unknown function | ||||||
| AT5G58250 | Expressed protein | CPdefg | 0.63 | 0.46 | + | + |
| AT5G58260 | Expressed protein | CPdefg | 0.56 | 0.64 | + | + |
| AT3G12930 | Expressed protein | CPdefg | 0.58 | 0.49 | − | |
| AT4G34290 | SWIB BAF60b-containing protein | CPdfg/Ne | 0.67 | 0.40 | − | |
| AT2G01620 | Expressed protein | ERd/otherefg | 0.63 | 0.65 | ||
| AT4G21030 | Zinc finger domain-containing protein | CYd/otherefg | 0.65 | 0.44 | ||
| AT5G05250 | Expressed protein | Nd/otherefg | 0.58 | 0.44 | ||
| AT5G48210 | Expressed protein | ERdfg/othere | 0.64 | 0.63 | ||
PSL, Predicted subcellular location; CP, chloroplast; CY, cytosol; ER, endoplasmic reticulum; EX, extracellular; M, mitochondrion; N, nucleus.
RT-PCR symbol denotation: +, cia2:wild type transcript ratio <67%; −, cia2:wild type transcript ratio between 67% and 100%.
ChIP symbol denotation: +, determined positively; −, determined negatively.
WoLF PSORT, prediction of protein localization (Horton et al., 2007).
Gene Ontology cellular component, predicting cellular component of protein on gene ontology (Ashburner et al., 2000).
Predotar, prediction of organelle targeting sequences (Small et al., 2004).
TargetP, predicting subcellular localization of proteins (Emanuelsson et al., 2000).
To classify the function of genes differentially expressed in cia2, homology searches were performed using BLAST. The resultant homologies listed in Table I are based on the functional classification of the Gene Ontology annotation (Gene Ontology Consortium, 2001). Furthermore, PSORT (Horton et al., 2007), Predotar (Small et al., 2004), and TargetP (Emanuelsson et al., 2000) were also used to predict the subcellular locations of the gene products. We first focused our analyses on genes that were down-regulated in cia2 because they were likely to be targets of CIA2. Among the 38 down-regulated genes, 34 genes were predicted to encode chloroplast-localized proteins. The remaining four genes encode proteins of unknown functions. This suggests that the primary function of CIA2 is related to chloroplast biogenesis. Thus, we decided to focus our analyses on the 34 genes encoding chloroplast-localized proteins. Among these 34 genes, 17 encode plastid-specific ribosomal proteins (PSRPs), which include the 50S large subunits (RPLs), the 30S small subunits (RPSs), and other chloroplast ribosomal-associated proteins. In addition, three genes encode plastid RNA-binding proteins: CP29, CP33 (also named PDE322 for pigment defective 322), and a putative transcription termination factor PDE191 (pigment defective 191). It was elsewhere reported that CP29 and CP33 might participate in translation initiation in chloroplasts (Ohta et al., 1995). Other genes encode proteins participating in various aspects of chloroplast functions, including protein folding, photosynthesis, and other metabolic processes. There were also four genes that encode chloroplast proteins of unknown functions.
Confirmation of the CIA2 Downstream Genes
Reverse transcription (RT)-PCR experiments were used to confirm the microarray analyses. Fifteen genes were confirmed to have an expression level <67% of that in the wild type; their relative expression levels are shown in Figure 1. TOC34 (AT5G05000) and TOC75-IV (AT4G09080) are paralogs of TOC33 and TOC75-III, respectively, but are not down-regulated in cia2 according to the microarray data. Thus, their expression levels were also analyzed as controls. As shown in Figure 1, the transcript levels of TOC34 and TOC75-IV were not significantly altered in cia2. The 15 genes with confirmed reduction of expression in cia2 were further analyzed by chromatin immunoprecipitation (ChIP) assays.
Figure 1.
Relative expression of CIA2 downstream genes. Total RNAs were isolated from the 14-d-old wild type and cia2 mutant, and transcript levels of CIA2 downstream genes were determined by RT-PCR using gene-specific primers (Supplemental Table S3). Transcript levels of each gene were normalized to the transcript level of UBQ10 gene. The change of expression level was calculated by dividing the normalized transcript level of cia2 mutant by that of the wild-type plant. Data are means ± sd of three independent experiments.
Binding of CIA2 to the Promoter Regions of Its Downstream Genes
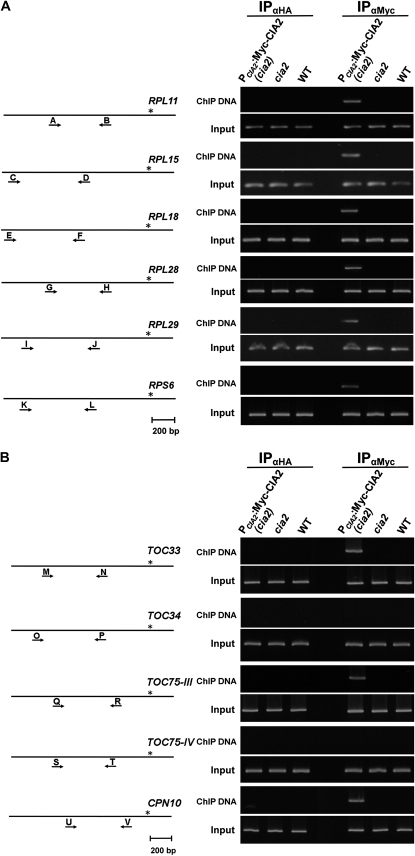
ChIP assays were used to investigate whether CIA2 could specifically bind to the promoter sequences of its downstream genes. Transgenic plants expressing a cMyc-CIA2 fusion protein under the control of the CIA2 promoter in the cia2 mutant background were generated. This line, named PCIA2:Myc-CIA2(cia2), was confirmed to express functional CIA2 by two criteria. First, cMyc-CIA2 rescued the pale-green phenotype of cia2 (Fig. 2). Chlorophyll a, chlorophyll b, and carotenoid levels were reduced by 50% in cia2 and were fully restored in PCIA2:Myc-CIA2(cia2) plants (Fig. 3). Second, the transcript abundance of genes encoding chloroplast ribosomal proteins in the leaves of Col, cia2, and PCIA2:Myc-CIA2(cia2) plants were determined by RT-PCR. As shown in Figure 4, the expression of six PSRP genes was recovered in leaves by the presence of cMyc-CIA2.
Figure 2.
Phenotypic comparison of 21-d-old wild-type (WT), cia2 mutant, PCIA2:Myc-CIA2(cia2), and P35S:CIA2(cia2) transgenic plants.
Figure 3.
Pigment contents in the leaves of wild-type (WT), cia2 mutant, and PCIA2:Myc-CIA2(cia2) transgenic plants. Pigments extracted from 21-d-old leaves were characterized as described in “Materials and Methods.” The relative pigment level was calculated by dividing the amounts in cia2 mutant or PCIA2:Myc-CIA2(cia2) transgenic plants by that in the wild-type plant. Chl a+b, total chlorophylls; Chl a/b, ratio of chlorophyll a to chlorophyll b; x, xanthophylls; c, carotenoids; x+c, total carotenoids; a+b/x+c, ratio of total chlorophylls to total carotenoids. Data are means ± sd of three independent experiments.
Figure 4.
Restoration of ribosomal gene expression by CIA2. Total RNAs were isolated from 14-d-old leaves of wild-type (WT), cia2 mutant, and PCIA2:Myc-CIA2(cia2) transgenic plants. The transcript levels of various genes were determined by RT-PCR using gene-specific primers (Supplemental Table S3). PCR products were fractionated on agarose gel and visualized by SYBR Green I dye. UBQ10, Loading control.
Total genomic DNA was isolated from Col, cia2, and PCIA2:Myc-CIA2(cia2) plants, treated with formaldehyde, immunoprecipitated with anti-cMyc antibody, and analyzed by PCR using target gene-specific primers (Supplemental Table S4). The promoter sequences of six PSRP genes (RPL11, RPL15, RPL18, RPL28, RPL29, and RPS6) could be amplified from DNA precipitated from PCIA2:Myc-CIA2(cia2) DNA but not from DNA precipitated from Col or cia2 (Fig. 5A). The unrelated anti-HA tag antibody was used as a control antibody, and no PCR product was observed in samples precipitated with anti-HA antibody. This result suggests an association between CIA2 and the PSRP gene promoter regions in vivo. Similar results were also obtained for TOC33, TOC75-III, and CPN10 promoters (Fig. 5B). In contrast, no PCR products were detected using TOC34 and TOC75-IV promoter primer sets. ChIP assays were also performed on remaining genes shown in Figure 1. Amplified DNA products were obtained from promoter sequences of CP29 and the two genes encoding chloroplast proteins of unknown function but not from promoter sequences of CPO1, FSD3, and PSRP4 (data not shown).
Figure 5.
Binding of CIA2 to the promoter region of CIA2 downstream genes by ChIP assays. Soluble chromatins isolated from PCIA2:Myc-CIA2(cia2), cia2, and the wild type (WT) were immunoprecipitated using anti-cMyc (αMyc) antiserum or anti-HA (αHA) antiserum as a negative control. These immunoprecipitated DNAs were amplified by PCR for 40 cycles using gene-specific promoter primers for plastid ribosomal proteins (A) and translocon and CPN10 protein (B). Input, PCRs performed using corresponding gene-specific promoter primers and DNA before immunoprecipitation with antiserum. Genomic structure of each target gene is also shown. Lines represent promoter, 5′UTR, and first exon sequences of genes. Asterisk represents the location of start codon. Arrows labeled A to V represent the gene-specific primers and their approximate positions on target genes. All ChIP experiments were repeated at least three times. The primer sequences and the expected lengths of PCR products for ChIP examinations are listed in Supplemental Table S4.
Translational Efficiency of Chloroplast-Encoded Proteins in cia2
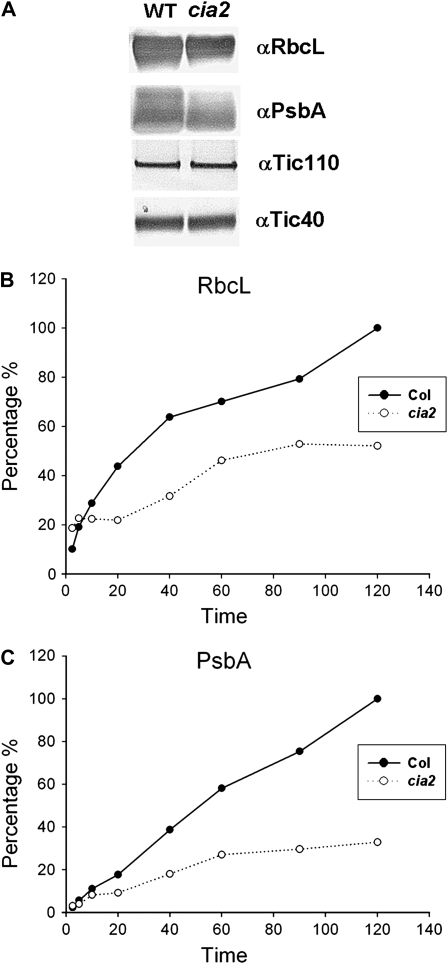
Because the cia2 mutant had reduced transcript levels of genes encoding plastid ribosomal proteins, we next investigated the steady-state level and synthesis rate of chloroplast-encoded proteins in cia2 chloroplasts. For steady-state protein level determination, equal numbers of chloroplasts isolated from Col and cia2 leaves were subjected to immunoblotting using antibodies against RbcL and PsbA (D1 protein of PSII). The amounts of RbcL and PsbA were reduced in cia2 compared to Col (Fig. 6A). For protein synthesis comparison, intact chloroplasts from Col and cia2 were incubated with 35S-Met for various lengths of time, and the labeled proteins were separated by SDS-PAGE. Two labeled chloroplast proteins, RbcL and PsbA, which are the most abundant soluble and membrane proteins, respectively, were quantified by a phosphor imager. The protein synthesis rates for both RbcL and PsbA were lower in cia2 chloroplasts (Fig. 6, B and C). These results suggest that the lower expression of PSRP genes resulted in a reduced rate of protein synthesis in cia2 chloroplasts.
Figure 6.
Steady-state accumulation and translation efficiency of plastid proteins. Intact chloroplasts were isolated from 21-d-old leaves of the wild type (WT) and cia2 mutant. Equal numbers of intact chloroplast were analyzed by SDS-PAGE and immunoblotting using anti-RbcL or anti-PsbA antiserum (A). Tic110 and Tic40 were loading controls in this western blot. For determination of translation rate, equal numbers of intact chloroplasts were incubated with 35S-Met for 2.5, 5, 10, 20, 40, 60, 90, and 120 min. A nine-tenths portion of the sample from each time point was analyzed by SDS-PAGE and the amounts of radioactive RbcL and PsbA quantified on a phosphor imager. The remaining 10% of the sample was separated on another gel and immunoblotted with anti-RbcL or anti-PsbA antiserum to quantify total RbcL and PsbA proteins. After normalization to the total amount of RbcL and PsbA present in each sample, data were plotted using the amount of radioactive RbcL (B) and PsbA (C) at 120 min in the wild type as 100%.
Leaf-Specific Up-Regulation of Ribosomal Protein- and Translocon-Encoding Genes by CIA2
CIA2 is normally only expressed in green tissues but not in roots (Sun et al., 2001). We therefore examined whether the expression of CIA2 downstream genes was modulated by CIA2 in a tissue-specific manner. Compared to the wild type, the transcript levels of RPLs, RPS6, TOC33, and TOC75-III in cia2 were reduced in the leaves but not in the roots (Fig. 7). The expression levels of these genes were recovered in PCIA2:Myc-CIA2(cia2) leaves, while the PCIA2:Myc-CIA2(cia2) transgene had no effect on the expression of these genes in roots. These results indicate that CIA2 is responsible for leaf-specific up-regulation of RPLs, RPS6, TOC33, and TOC75-III. The transcript levels of TOC34 were not significantly altered in the leaves and roots of Col, cia2, and PCIA2:Myc-CIA2(cia2) plants, confirming that TOC34 is not regulated by CIA2.
Figure 7.
Up-regulation of translocon and ribosomal genes by CIA2. Total RNAs were isolated from 10-d-old leaves and roots of wild-type (WT), cia2, PCIA2:Myc-CIA2(cia2), and P35S:CIA2(cia2) plants. The transcript levels of various genes were determined by RT-PCR using gene-specific primers (Supplemental Table S3). PCR products were fractionated on agarose gels and visualized by SYBR Green I dye (A). Relative transcript level of each gene was calculated (B). Signals of transcripts were quantified by image analyzer, normalized to the signals of the UBQ10 transcript, and calibrated based on the transcript amount in the leaves of the wild type. Data are means ± sd of three independent experiments.
Up-Regulation of Genes Encoding Ribosomal Proteins and Translocon Component by Ectopic Expression of CIA2
The above results indicated that loss of CIA2 caused lower expression of genes encoding chloroplast ribosomal proteins and translocon components in leaves. We next asked if CIA2 was sufficient to increase the expression of these genes if ectopically expressed in roots. The plant P35S:CIA2(cia2) was a cia2-based transgenic plant containing the CIA2 coding sequence driven by the constitutive cauliflower mosaic virus 35S promoter. As shown in Figure 7, the P35S:CIA2(cia2) plant indeed had increased expression of RPLs, RPS6, TOC33, and TOC75-III in roots, although the expression levels did not reach those seen in leaves of the wild-type plants. It is possible that additional transcription factors also participate in up-regulating RPLs, RPS6, TOC33, and TOC75-III in leaves.
DISCUSSION
Due to the demands of photosynthesis, leaf chloroplasts need to import more proteins than root plastids do. These photosynthesis-associated proteins are subsequently assembled with partner proteins produced within chloroplasts. Therefore, during chloroplast development, these plastid-encoded partners have to be synthesized at a similar rate as the import rate of nuclear-encoded chloroplast proteins. Our results indicate that CIA2 plays an important role in coordinately increasing both the chloroplast protein import capacity and the translational efficiency by up-regulating the translocon-encoding (TOC33 and TOC75-III) and ribosomal protein-encoding (RPLs and RPSs) genes.
Several CIA2-regulated genes have been previously characterized. An identified Arabidopsis prpl11 mutant (plastid ribosomal protein L11 subunit, the RPL11 mutant) has a pale-green leaf phenotype and reduced growth rate (Pesaresi et al., 2001). The abundance of plastid-encoded proteins, such as RbcL, PsbA, and PsbE (a subunit in PSII), are reduced in prpl11 mutant, indicating that deficiency of RPL11 severely affects translation in chloroplasts (Pesaresi et al., 2001). The ppi1 mutant (plastid protein import 1, the TOC33 mutant) also has pale-green leaves (Jarvis et al., 1998). The import efficiency of four photosynthesis-related proteins (RbcS, chlorophyll a/b-binding protein [Cab], and PORA and PORB [protochlorophyllide oxidoreductases A and B]) into ppi1 mutant chloroplasts is reduced compared to wild-type chloroplasts (Jarvis et al., 1998). Furthermore, the steady-state amount of RbcS has approximately 50% reduction in ppi1 chloroplasts (Kubis et al., 2003), revealing that deficiency of Toc33 reduces the protein import efficiency and accumulation of imported proteins. These results confirm that proper expression of RPL11 and TOC33 is important for chloroplast biogenesis.
Coordinated mechanisms must exist to ensure the simultaneous control of function-related genes from both the nuclear and plastid genomes during the chloroplast development (López-Juez, 2007). Indeed, several studies have shown that reduction of one subunit in one genome resulted in reduced expression of the other subunit in the other genome. For example, the expression of RBCS (nuclear gene) and rbcL (plastid gene) is coordinated to ensure that the small subunits and large subunits of Rubisco accumulate stoichiometrically in chloroplasts. However, RbcL translation declines when RBCS gene is knocked down in tobacco (Nicotiana tabacum; Rodermel et al., 1996). Another example, plastid acetyl-CoA carboxylase, is a tetrameric enzyme catalyzing fatty acid biosynthesis. Arabidopsis acetyl-CoA carboxylase is composed of subunits encoded by three nuclear genes (CAC1, CAC2, and CAC3) and one plastid gene (accD). The subunit mRNAs accumulate at a constant molar stoichiometric ratio, indicating these four genes are coordinately expressed (Ke et al., 2000). In this study, we demonstrate a novel coordinated mechanism that simultaneously increases both imported products of nuclear genes and synthesized products of plastid genes in leaves by CIA2.
Recent large-scale gene expression profiling experiments might provide clues for coordinated expression of nuclear and plastid genomes. Richly et al. (2003) used a nylon array of 3,292 gene sequence tags, including 2,661 nuclear genes encoding chloroplast proteins, to examine the gene expression of chloroplast-targeted proteins under 35 disparate environmental and genetic conditions. They observed three broad classes of gene regulation (up-regulation, down-regulation, and mixed type of gene regulation) and proposed the existence of master switch regulators that coordinate the expression of plastid-specific genes in the nucleus. The response of the same 3,292-gene sequence tag array to a total of 101 different conditions was extensively analyzed by Biehl et al. (2005). There were 1,590 genes differentially expressed that could be assigned to 23 distinct clusters (regulons) of coregulated genes. They found that genes encoding proteins participating in photosynthetic light reactions in regulon 1 (containing genes associated with the photosynthetic apparatus) and chloroplast ribosomal proteins in regulon 2 (containing genes involving in chloroplast gene transcription and translation) were tightly coregulated. However, to ensure photosynthetic function, Cab and RbcS are required to be imported into the chloroplast and assembled therein with their chloroplast-synthesized protein partners. Thus, the import capacity of the chloroplast-targeted proteins must act in harmony with plastid-encoded protein partners during chloroplast biogenesis. Our results strongly support that CIA2 is an up-regulator to comodulate the expression of PSRP and TOC genes in a leaf-specific manner and in consequence balances the protein import capacity and translation efficiency in chloroplasts.
Based on the GENEVESTIGATOR database, many PSRP and TOC genes have increased expression in leaves, presumably to accommodate the higher protein import and synthesis demand of leaf chloroplasts (Zimmermann et al., 2004). Our studies provide insights into the differential up-regulation of RPL11, RPL15, RPL18, RPL28, RPL29, RPS6, TOC33, and TOC75-III, but not the remaining TOC and PSRP genes. Furthermore, ectopic expression of CIA2 in roots only caused a small increase in the expression of its downstream target PSRPs and TOCs. These results indicate that additional transcriptional factors are required to cooperate with CIA2 to increase the expression level of PSRP and TOC genes to the level required for leaf chloroplasts and also to up-regulate the remaining CIA2-independent TOC and PSRP genes.
CPN10 is homologous to GroES of Escherichia coli and also functions as a chloroplast protein folding cochaperonin (Koumoto et al., 2001). Northern blot analysis reveals that CPN10 transcript accumulates in leaves and stems but not in roots (Koumoto et al., 2001). Data from our microarray, RT-PCR, and ChIP analyses indicate that CIA2 directly binds to and regulates the expression of CPN10. It is likely that the increase in protein uptake and protein translation in chloroplasts also requires an increase in protein folding capacity. However, this hypothesis requires further investigation.
CIA2 seems to regulate other photosynthesis-related genes indirectly. Although microarray results (Table I) suggested that seven photosynthesis-related genes showed lower transcript abundance in cia2, they either did not have significant transcript reduction in cia2 in RT-PCR analyses (Fig. 1), or CIA2 enrichment was not observed in their promoter region in ChIP assays. The reduced chlorophyll and carotenoid contents (Fig. 3) and the expression of the photosynthetic genes may be a secondary consequence of reduced protein import and synthesis.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) cia2 mutant was isolated from our previous screening (Sun et al., 2001). Seeds from the wild type (ecotype Col), cia2 mutant, PCIA2:Myc-CIA2(cia2), and P35S:CIA2(cia2) transgenic plants were surface sterilized with 25% (v/v) commercial bleach and grown on 1× Murashige and Skoog (1962) agar medium with Gamborg's vitamins and 2% (w/v) Suc. Plants were grown at 22°C under a 16-h-light/8-h-dark cycle for various numbers of days.
Quantification of Pigment Content
The accumulation of chlorophylls and carotenoid in 21-d-old leaves was measured as described by Lichtenthaler (1987). All measurements were performed in triplicate using three independent leaf samples.
Microarray Analysis
Total RNAs isolated from 2-week-old Col and cia2 plants by TRIzol solution (Invitrogen) were reverse-transcribed to amino allyl-dUTP-labeled cDNAs using SuperScript III reverse transcriptase (BD Biosciences). The cDNA molecules were post-labeled with Cy3 and Cy5 (Amersham) as cDNA probes for use in dual-color microarray hybridization with Agilent Arabidopsis 22 K oligomicroarray slides. A dye-swap experiment was performed with two different RNA populations to eliminate the signal variation caused by the differential labeling efficiency of Cy3 and Cy5 dyes. After hybridization and washing procedures, the fluorescence intensities of each gene in microarray slides were scanned according to the manufacturer's instructions (GenePix 4000B scanner; Molecular Devices), and the original signals are listed in Supplemental Tables S1 and S2. The microarray data were normalized by the LOWESS method, and the expression ratios were analyzed using Genespring GX7.3.1 software (Agilent). The cutoff threshold of fluorescence intensities for both normalized Cy3 and Cy5 was 300. The genes that revealed a consistent expression pattern in cia2 and had at least a 1.5 times difference in expression level in both slides are listed in Table I. Gene annotations were compiled by The Arabidopsis Information Resource.
Quantification of Transcript Level
Amounts of transcripts for various genes were analyzed by RT-PCR. First-strand cDNAs were synthesized using Moloney murine leukemia virus RNase H− reverse transcriptase (Promega) and an oligo(dT)18N primer with total RNAs isolated from 10-d-old or 2-week-old plants. Primers specific for each gene were designed based on the sequences downloaded from The Arabidopsis Information Resource. These gene-specific primers were used to amplify each transcript with 20 to 25 PCR cycles using the first-stranded cDNA as templates. PCR products were fractionated on 1.5% agarose gel, visualized by SYBR Green I dye (Invitrogen), and quantified using a fluorescent image analyzer (Fuji FLA-3000). The relative amount of calculated message was normalized to the level of the UBQ10 transcripts (Sun and Callis, 1997). All PCRs were performed in triplicate using three independent RNA samples. The primer sequences for RT-PCR are described in Supplemental Table S3.
Plasmid Construction and Plant Transformation
The sequences of the cauliflower mosaic virus 35S promoter and NOS terminator from vector pBI121 (Invitrogen) and the coding sequence of CIA2 PCR amplified from Col cDNA were ligated into the binary vector pPZP221 (Hajdukiewicz et al., 1997) to create plasmid pCS146. The cia2 mutant was transformed with pCS146 using the floral dipping method (Clough and Bent, 1998), mediated by Agrobacterium tumefaciens strain GV3101. The transformants were selected on agar plates containing 30 μg/mL G418 and verified by PCR using construct-specific primers. This transgenic line was named P35S:CIA2(cia2).
The promoter and 5′ untranslated region (UTR; 1.5 kb) sequence of CIA2 was PCR-amplified from Col genomic DNA, and cloned into pPZP221 to obtain pCS175. The coding and 3′UTR sequence of CIA2 was PCR-amplified from cDNA, and cloned in-frame into a cMyc epitope-containing vector pCS155 to create plasmid pCS176. The cMyc-CIA2 coding sequence from pCS176 was then ligated downstream to promoter and 5′UTR sequences on pCS175 to obtain pCS181. The pCS181 was transformed into cia2, and the sequential transgenic plants were named PCIA2:Myc-CIA2(cia2). As well as antibiotic selection and PCR confirmation, western blot using antibodies against the cMyc tag (Santa Cruz Biotechnology) was performed to ensure a specific interaction between cMyc antiserum and Myc-CIA2 fusion protein. All the transgenic plants were selected for more than two generations and homozygous transgenic plants (T3) were used for this study.
ChIP
The ChIP procedure was modified from published methods (Gendrel et al., 2002). Two-week-old Col, cia2, and PCIA2:Myc-CIA2(cia2) transgenic plants were cross-linked in 1% formaldehyde. Nuclei were subjected to four cycles of sonication (Misonix XL2000, 10 s each). Soluble chromatin was subjected to ChIP using anti-cMyc antibody-conjugated agarose (Santa Cruz Biotechnology) or anti-HA antibody-conjugated agarose (Santa Cruz Biotechnology) as a negative control. DNA-protein cross-links were reversed by incubating at 65°C overnight. DNA was purified by ethanol precipitation and resuspended in Tris-EDTA (pH 8.0). Immunoprecipitated DNA was amplified by PCR using gene-specific promoter primers. All ChIP experiments were repeated at least three times. The primer sequences for ChIP examinations are described in Supplemental Table S4.
Chloroplast Isolation and Protein Analyses
Isolation of intact chloroplasts from 21-d-old leaves was described previously (Sun et al., 2001). For immunoblot analysis, proteins extracted from chloroplasts were fractionated by SDS-PAGE, blotted onto polyvinylidene difluoride membrane, and hybridized with anti-RbcL or anti-PsbA antibody (Agrisera). The amounts of RbcL and PsbA proteins were quantified using a FLA-3000 image analyzer (Fuji).
In organello pulse labeling experiments were performed as described by Barkan (1998) except that intact chloroplasts were purified on a 40% Percoll gradient after the 2.5- to 120-min 35S-Met incubation. Pellet chloroplasts were resuspended in 15 μL buffer (50 mm HEPES-KOH, pH 7.0, 330 mm sorbitol, 1 mm EDTA, 5% SDS, 0.05% Coomassie Brilliant Blue G, and 12% glycerol), and a 13.5 μL portion of the supernatant (90% of the reaction) was fractionated by SDS-PAGE. The amounts of separated radioactive RbcL and PsbA proteins were then quantified using a Typhoon 9000D phosphor imager (GE Healthcare). The remaining supernatant (10% of the reaction) was used for immunoblot analysis.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers CIA2 (AF359387), RPL11 (AF325023), RPL15 (AK220673), RPL18 (AF336922), RPL28 (AY072373), RPL29 (AK317389), RPS6 (NM112593), Toc33 (AK317629), Toc34 (AK317740), Toc75-III (NM114541), and Toc75-IV (NM116977).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Agilent microarray data control: raw intensity of control channel (Cy3-labeled wild-type cDNA).
Supplemental Table S2. Agilent microarray data control: raw intensity of control channel (Cy3-labeled cia2 cDNA).
Supplemental Table S3. Primers used in the RT-PCR experiments (Figs. 1, 4, and 7).
Supplemental Table S4. Primers used in the ChIP experiments (Fig. 5).
Supplementary Material
Acknowledgments
We thank Shu-Yung Tung for assistance with microarray analyses in the Institute of Molecular Biology, Academia Sinica, Taiwan. We also thank Dr. Hsou-min Li for initial financial support (Academia Sinica Genomic Grant AS92–IMB2 and NSTP-AB Grant 92S501921) and valuable discussions of this work, Drs. Judy Callis and Hsou-min Li for critical review of this manuscript, and Dr. Hsu-Hsing Wu for helpful comments on the microarray analyses.
This work was supported by the National Science Council of Taiwan (grant nos. NSC 92–2321–B–003–001 and 93–2311–B–003–005 to C.-W.S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Chih-Wen Sun (cwsun@ntnu.edu.tw).
The online version of this article contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al (2000) Gene ontology: tool for the unification of biology. Nat Genet 25 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A (1998) Approaches to investigating nuclear genes that function in chloroplast biogenesis in land plants. Methods Enzymol 297 38–57 [Google Scholar]
- Biehl A, Richly E, Noutsos C, Salamini F, Leister D (2005) Analysis of 101 nuclear transcriptomes reveals 23 distinct regulons and their relationship to metabolism, chromosomal gene distribution and co-ordination of nuclear and plastid gene expression. Gene 344 33–41 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, Heijne G (2000) Target P: predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300 1005–1016 [DOI] [PubMed] [Google Scholar]
- Gendrel AV, Lippman Z, Yordan C, Colot V, Martienssen RA (2002) Dependence of heterochromatic histone H3 methylation patterns on the Arabidopsis gene DDM1. Science 297 1871–1873 [DOI] [PubMed] [Google Scholar]
- Gene Ontology Consortium (2001) Creating the gene ontology resource: design and implementation. Genome Res 11 1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz PT, Allison LA, Maliga P (1997) The two RNA polymerases encoded by the nuclear and the plastid compartments transcribe distinct groups of genes in tobacco plastids. EMBO J 16 4041–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35 W585–W587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T, Schnell DJ (2008) Protein trafficking to plastids: one theme, many variations. Biochem J 413 15–28 [DOI] [PubMed] [Google Scholar]
- Jarvis P, Chen LJ, Li H, Peto CA, Fankhauser C, Chory J (1998) An Arabidopsis mutant defective in the plastid general protein import apparatus. Science 282 100–103 [DOI] [PubMed] [Google Scholar]
- Jarvis P (2008) Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol 179 257–285 [DOI] [PubMed] [Google Scholar]
- Koumoto Y, Shimada T, Kondo M, Hara-Nishimura I, Nishimura M (2001) Chloroplasts have a novel CPN10 in addition to CPN20 as co-chaperonins in Arabidopsis thaliana. J Biol Chem 276 29688–29694 [DOI] [PubMed] [Google Scholar]
- Ke J, Wen TN, Nikolau BJ, Wurtele ES (2000) Coordinate regulation of the nuclear and plastidic genes coding for the subunits of the heteromeric acetyl-coenzyme A carboxylase. Plant Physiol 122 1057–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubis S, Baldwin A, Patel R, Razzaq A, Dupree P, Lilley K, Kurth J, Leister D, Jarvis P (2003) The Arabidopsis ppi1 mutant is specifically defective in the expression, chloroplast import, and accumulation of photosynthetic proteins. Plant Cell 15 1859–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister D (2003) Chloroplast research in the genomic age. Trends Genet 19 47–56 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148 350–382 [Google Scholar]
- López-Juez E (2007) Plastid biogenesis, between light and shadows. J Exp Bot 58 11–26 [DOI] [PubMed] [Google Scholar]
- Martin W, Herrmann RG (1998) Gene transfer from organelles to the nucleus: how much, what happens, and why? Plant Physiol 118 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W, Rujan T, Richly E, Hansen A, Cornelsen S, Lins T, Leister D, Stoebe B, Hasegawa M, Penny D (2002) Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc Natl Acad Sci USA 99 12246–12250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15 473–497 [Google Scholar]
- Ohta M, Sugita M, Sugiura M (1995) Three types of nuclear genes encoding chloroplast RNA-binding proteins (cp29, cp31 and cp33) are present in Arabidopsis thaliana: presence of cp31 in chloroplasts and its homologue in nuclei/cytoplasms. Plant Mol Biol 27 529–539 [DOI] [PubMed] [Google Scholar]
- Pesaresi P, Varotto C, Meurer J, Jahns P, Salamini F, Leister D (2001) Knock-out of the plastid ribosomal protein L11 in Arabidopsis: effects on mRNA translation and photosynthesis. Plant J 27 179–189 [DOI] [PubMed] [Google Scholar]
- Richly E, Dietzmann A, Biehl A, Kurth J, Laloi C, Apel K, Salamini F, Leister D (2003) Covariations in the nuclear chloroplast transcriptome reveal a regulatory master-switch. EMBO Rep 4 491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodermel S, Haley J, Jiang CZ, Tsai CH, Bogorad L (1996) A mechanism for intergenomic integration: abundance of ribulose bisphosphate carboxylase small-subunit protein influences the translation of the large-subunit mRNA. Proc Natl Acad Sci USA 93 3881–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell DJ, Blobel G, Keegstra K, Kessler F, Ko K, Soll J (1997) A consensus nomenclature for the protein-impost components of the chloroplast envelope. Trends Cell Biol 7 303–304 [DOI] [PubMed] [Google Scholar]
- Small I, Peeters N, Legeai F, Lurin C (2004) Predotar: a tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 4 1581–1590 [DOI] [PubMed] [Google Scholar]
- Sun CW, Callis J (1997) Independent modulation of Arabidopsis thaliana polyubiquitin mRNAs in different organs and in response to environmental changes. Plant J 11 1017–1027 [DOI] [PubMed] [Google Scholar]
- Sun CW, Chen LJ, Lin LC, Li HM (2001) Leaf-specific upregulation of chloroplast translocon genes by a CCT motif-containing protein, CIA2. Plant Cell 13 2053–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.