Abstract
Potassium (K) is required in large quantities by growing crops, but faced with high fertilizer prices, farmers often neglect K application in favor of nitrogen and phosphorus. As a result, large areas of farmland are now depleted of K. K deficiency affects the metabolite content of crops with negative consequences for nutritional quality, mechanical stability, and pathogen/pest resistance. Known functions of K in solute transport, protein synthesis, and enzyme activation point to a close relationship between K and metabolism, but it is unclear which of these are the most critical ones and should be targeted in biotechnological efforts to improve K usage efficiency. To identify metabolic targets and signaling components of K stress, we adopted a multilevel approach combining transcript profiles with enzyme activities and metabolite profiles of Arabidopsis (Arabidopsis thaliana) plants subjected to low K and K resupply. Roots and shoots were analyzed separately. Our results show that regulation of enzymes at the level of transcripts and proteins is likely to play an important role in plant adaptation to K deficiency by (1) maintaining carbon flux into amino acids and proteins, (2) decreasing negative metabolic charge, and (3) increasing the nitrogen-carbon ratio in amino acids. However, changes in transcripts and enzyme activities do not explain the strong and reversible depletion of pyruvate and accumulation of sugars observed in the roots of low-K plants. We propose that the primary cause of metabolic disorders in low-K plants resides in the direct inhibition of pyruvate kinase activity by low cytoplasmic K in root cells.
Potassium (K) is an essential macronutrient for plants. The high demand of growing crops for K is generally recognized in agriculture, and most farmers in Europe and the United States routinely apply potash fertilizers in the field. However, even in a fertilized field, K deficiency can occur due to unfavorable soil structure (e.g. sandy soils) and depletion zones forming around roots (Kayser and Isselstein, 2005; Moody and Bell, 2006; Andrist-Rangel et al., 2007). In developing countries, the importance of K has sometimes been overlooked, and financial constraints have forced farmers to prioritize applications of nitrogen (N) over K. As a result, a considerable area of farmland has become K deficient (Dobermann et al., 1999; Hoa et al., 2006; Andrist-Rangel et al., 2007). With fertilizer prices on the rise in recent years, nutrient usage efficiency of crops is attracting increasing interest as a trait to be considered for biotechnological improvement (Rengel and Damon, 2008).
Such efforts rely on a good understanding of how inorganic nutrients are used within the plant. Unlike nitrate, phosphate, and sulfate, K is not assimilated into organic matter. Nevertheless, an important role for K in metabolism is evident from the fact that K deficiency affects the contents of primary and secondary metabolites, with important consequences for both mechanical stability and pathogen/pest resistance in crops (for review, see Amtmann et al., 2008). In many respects, K affects plants in an opposite way to nitrate, resulting in a complex interrelationship between these two nutrients and yield. This is well known in the field but is poorly characterized at the level of plant physiology and biochemistry (Koch and Mengel, 1972; Mengel et al., 1976; Gething, 1993).
Molecular research in plant K nutrition over the last two decades has focused on the characterization of K transporters and has provided detailed information on their structure, function, and regulation (Véry and Sentenac, 2003; Amtmann and Blatt, 2009). By contrast, current knowledge of the biochemical and molecular events that form the basis of the interaction between K and primary metabolism is rudimentary. There is no lack of mechanisms by which K could affect primary metabolism. The most obvious ones include transmembrane potentials and pH gradients, long-distance transport, ribosomal function, and changes of enzyme activities (Marschner, 1995). These processes are regularly used to explain metabolic disturbances under K deficiency. However, while all of them depend on K and could potentially affect the biosynthesis, conversion, and allocation of metabolites, very few studies have tried to establish direct causal relationships.
Many enzymes require K as a cofactor (Wyn Jones and Pollard, 1983), and studies in the 1960s and 1970s suggested links between individual K-dependent enzymes (e.g. pyruvate kinase [PK], starch synthase, nitrate reductase [NR], Rubisco) and specific metabolic changes under K deficiency (Sorger et al., 1965; Evans and Sorger, 1966; Nitsos and Evans, 1966; Peoples and Koch, 1979). Due to our increasing understanding of the role of gene expression, protein modification, and protein degradation in the regulation of metabolic pathways, studies of enzyme properties have been largely abandoned without being replaced by research into the latter mechanisms. Meanwhile, publications from the agricultural sector continue to point out the importance of K nutrition for crop metabolism (Pettigrew, 2008).
To achieve real progress in understanding how K deficiency acts on primary metabolism, we have to be able to assess metabolic regulation at different biological levels (e.g. transcripts, proteins, and metabolites) in order to pinpoint candidate targets of K stress and then to manipulate genes and proteins to test their individual roles. Such research is currently best done in the model plant Arabidopsis (Arabidopsis thaliana). Here, we adopted a multilevel approach combining previously established transcript profiles (Armengaud et al., 2004) with enzyme activity and metabolite profiles from Arabidopsis plants grown under control and low-K conditions. The resulting comprehensive data set uncovered changes in individual parameters as well as global tendencies and allowed us to formulate specific hypotheses regarding their causes, which provide strong incentives for future studies in this area.
RESULTS
Metabolite Profiles in Roots and Shoots under Progressing K Deficiency
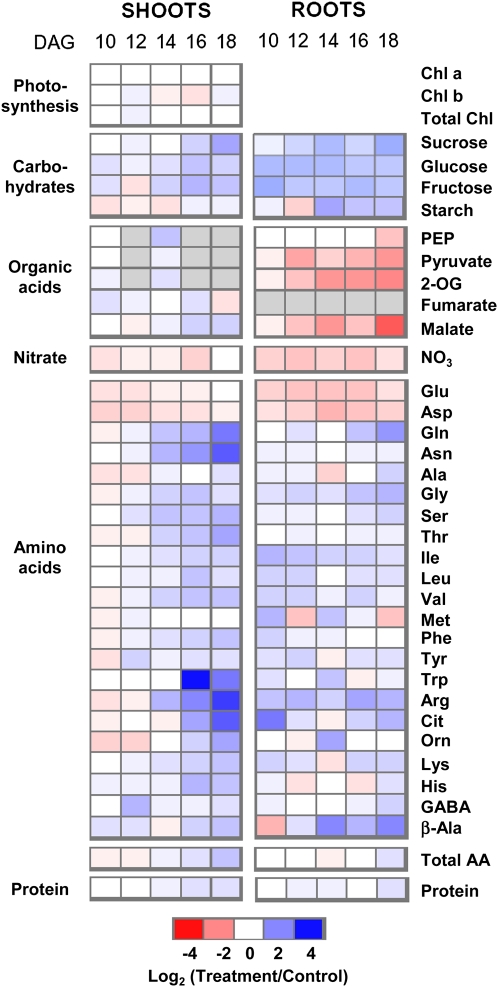
In a first approach to characterize the effects of K on primary metabolism, we monitored metabolite concentrations over a time course of K deficiency (Fig. 1). Concentrations of carbohydrates, organic acids, amino acids, and nitrate as well as chlorophyll and total protein were determined in roots and shoots pooled from approximately 200 Arabidopsis plants grown on nutrient agar in the presence of normal (control) or low-K (for medium composition, see “Materials and Methods”) conditions. Plants were harvested at five time points between 10 and 18 d after germination (DAG). Plants grown in low K were identical in growth and appearance to control plants during the first week of growth. They started to develop visible symptoms of K starvation (e.g. decreased growth rate and arrest of lateral root growth) between 10 and 12 DAG (Armengaud et al., 2004, 2009). Total K concentrations were already considerably lower in low-K plants than in control plants at 10 DAG and decreased further over the next 8 d (from 40% to 20% of the control values in roots, and from 30% to 15% of the control values in shoots; see below for data). As there was very little difference in water content (90.8% ± 0.6% in low-K plants compared with 92.7% ± 0.4% in control plants at 14 DAG; data not shown), the differences in metabolite concentrations based on plant fresh weight reported here are not due to volume effects. For each time point, metabolite concentrations measured in low-K plants were divided by those measured in control plants of similar age and base-2 logarithms calculated to generate the heat map shown in Figure 1. Absolute values are provided in Supplemental Table S1.
Figure 1.
Changes in metabolite concentrations in roots and shoots of Arabidopsis grown on low K. For each time point, approximately 200 plants were pooled. Blue color indicates an increase and red indicates a decrease in metabolite concentration in low-K-grown plants compared with control plants. Different shades of red and blue express the extent of the change according to the color bar provided (log2 ratio of low K to control). White indicates no change; gray indicates not determined. For absolute values, see Supplemental Table S1. AA, Amino acids; Chl, chlorophyll.
The metabolite profiles uncovered a profound effect of K deficiency on the levels of many primary metabolites (but not chlorophyll). The root profile was marked by a decrease in the levels of nitrate, glycolytic intermediates (pyruvate), organic acids (malate, 2-oxoglutarate [2-OG]), and negatively charged amino acids (Glu, Asp) and an increase in the levels of soluble carbohydrates (Suc, Glc, Fru) and many amino acids, notably those with high N-carbon (C) ratio and/or a positive charge (Gln, Gly, Arg). Most of these changes were already evident at 10 DAG. The differences in levels of Fru, Glc, nitrate, Glu, and Asp between low-K and control roots were relatively constant over the assessed period of time, while the deficiency-induced increase in Suc and decrease in organic acids became progressively stronger with time.
In the shoots, metabolite changes under K deficiency occurred generally later than in roots, in particular the increase in carbohydrates, including Suc, reducing sugars, and, to a lesser extent, starch. An increase in N-rich and basic amino acids (Gln, Asn, Arg, His, Lys) was more marked in shoots than in roots, while the decrease in nitrate and negative amino acids was weaker and disappeared with progressive K starvation. The depletion of organic acids observed in roots was absent in shoots.
Reversibility of Metabolite Changes after K Resupply
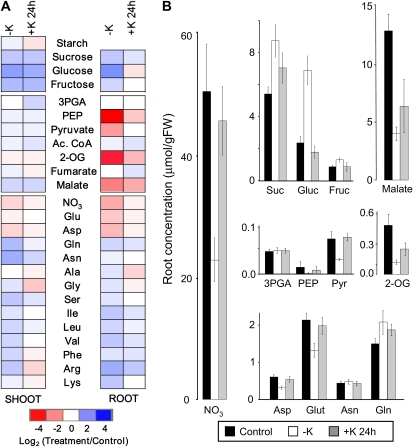
Reversibility of the observed metabolite changes was assessed in an experiment in which plants were grown for 14 d on low K and then resupplied with K for 24 h. We previously reported that within 24 h of resupply, tissue K reaches 60% (roots) and 40% (shoots) of the concentration in control plants (see Fig. 2 in Armengaud et al., 2004). Metabolite levels measured in this experiment are presented as color-coded heat maps in Figure 2A, which displays log ratios between values measured in low-K (or K-resupplied) plants and those measured in control plants at the same time. The latter was chosen as the reference in order to display the extent to which a change in low-K conditions was reversed by K resupply. Absolute levels of selected metabolites measured in the roots are shown in Figure 2B to illustrate pool sizes. Because a single time point was used for this analysis, the experiment was carried out at least three times (each experiment pooling approximately 200 plants). Means and se values of all absolute values and statistical parameters are provided in Supplemental Table S2.
Figure 2.
Reversibility of metabolite changes induced by low K. A, Changes in metabolite concentrations in roots and shoots of 2-week-old Arabidopsis plants grown in low-K (–K) medium for 14 d and subsequently resupplied with K (+K) for 24 h. Changes are in relation to corresponding values measured in plants grown in control medium. Colors and shading are as in Figure 1. B, Absolute concentrations of selected metabolites in the roots of plants grown in control medium (black bars), –K medium (white bars), and –K medium with K added for 24 h (gray bars). Data are means from at least three independently grown and treated plant batches, each of which comprised approximately 100 plants. Raw data and statistics are supplied in Supplemental Table S2. (Note that PEP concentrations were close to the detection limit, so relative changes shown in A should be viewed with caution.) Ac. CoA, Acetyl-CoA; FW, fresh weight; 3PGA, 3-phosphoglycerate; Pyr, pyruvate.
The metabolite changes observed in the K deficiency time course experiment were confirmed in this experiment, namely the accumulation of sugars and nonacidic amino acids and the depletion in nitrate, Glu, and Asp in both roots and shoots as well as the decrease in organic acids in the roots. Most of the changes of metabolites that were induced by K deficiency were reversed within 24 h of K resupply, although the degree of reversibility varied between metabolites and tissues. Thus, soluble carbohydrates reverted partially (Suc) or fully (Fru, Glc) to control levels in roots. Soluble sugars remained high in shoots, whereas starch decreased rapidly to lower levels than in control plants. 2-OG and malate levels were partially restored after 24 h of K resupply in roots. The root-specific decrease of pyruvate was fully reversible. In a separate experiment, we found that the recovery of root pyruvate levels was already complete after 2 h of K resupply (Supplemental Fig. S1). Nitrate, Glu, and Asp levels had almost fully recovered after 24 h of K resupply in both roots and shoots. Amino acids that had significantly accumulated during K starvation reverted either partially (Gln, Ile, Leu, Val, and Arg in roots, Gln, Asn, Ser, and Leu in shoots) or fully (Ser, Lys, Ala, and Gly in roots, Ile, Val, Gly, Phe, Arg, and Lys in shoots) to control levels within 24 h of K resupply; some even dropped below control levels. In summary, most metabolite changes induced by K starvation were readily reversed upon K resupply. Notable exceptions were Suc, Glc, and Fru in the shoots. In roots, malate recovered more slowly than pyruvate, and Gln and Arg showed a relatively slow recovery compared with other amino acids.
Regulation of Metabolic Enzymes by K Deficiency
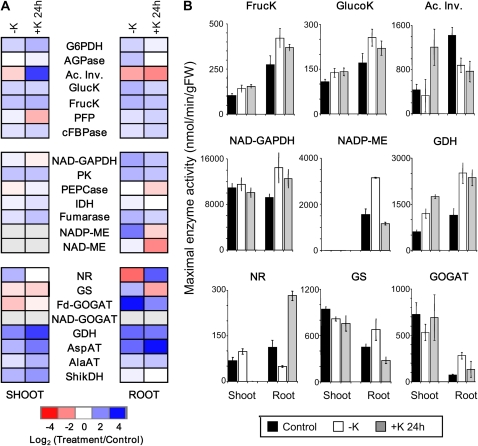
To investigate putative enzymatic targets of K deficiency that could explain the observed changes in metabolite levels, we measured maximal activities of the enzymes that catalyze a set of crucial reactions in primary C and N metabolism. The values reflect the total amounts of active protein producing enzyme activities in the tissues and hence the combined output from transcriptional and posttranscriptional regulation of all contributing isoforms. As before, measurements were carried out in at least three replicates for roots and shoots of plants exposed to control medium at 14 d of K starvation and 24 h of K resupply. Figure 3A shows enzyme activities in low-K and K-resupplied plants relative to control plants (log2 ratios) in a color-coded heat map, with the activities in K-replete control plants being taken as the reference. Again, roots and shoots were analyzed separately. Absolute values are shown in Figure 3B for the most significant responses. Mean activities, se values, and statistical analysis of all measured enzyme activities are provided in Supplemental Table S2.
Figure 3.
Changes in maximal enzyme activities under low K and K resupply. A, Changes in maximal enzyme activities in roots and shoots of 2-week-old Arabidopsis plants grown in low-K (–K) medium for 14 d and subsequently resupplied with K (+K) for 24 h. Changes are in relation to corresponding values measured in plants grown in control medium. Colors and shading are as in Figure 1. B, Absolute activities of selected enzymes (in nmol min−1 g−1 fresh weight [FW]) in the roots and shoots of plants grown in control medium (black bars), –K medium (white bars), and –K medium with K added for 24 h (gray bars). Data are means from three independently grown and treated plant batches, each of which comprised approximately 100 plants. Raw data and statistics are supplied in Supplemental Table S2. Ac. Inv., Acid invertase; AGPase, ADP-Glc pyrophosphorylase invertase; AlaAT, alanine aminotransferase; AspAT, aspartate aminotransferase; cFBPase, cytosolic Fru biphosphatase; FrucK, fructokinase; GlucK, glucokinase; G6PDH, Glc-6-P dehydrogenase; IDH, isocitrate dehydrogenase; PEPCase, PEP carboxylase; PFP, pyrophosphate-dependent phosphofructokinase; ShikDH, shikimate dehydrogenase.
Several enzyme activities related to sugar metabolism were altered in low-K plants. Glucokinase and fructokinase activities were significantly increased in roots and shoots. They reverted partially within 24 h of K resupply in roots but not in shoots. Acid invertase displayed the opposite pattern. Its maximal activity was strongly decreased in roots of K-starved plants but did not revert within 24 h of resupply in this tissue. In shoots, acid invertase activity showed no significant change during K starvation but a strong increase after K resupply.
Changes in the maximal activities of enzymes involved in glycolysis and the metabolism of organic acids were only observed in roots. Here, maximal enzyme activities of NAD-glyceraldehyde-3-phosphate dehydrogenase, PK, NADP-malic enzyme (ME), and fumarase increased in response to K deficiency. The increase of NADP-ME was especially marked. The 24-h K-resupply treatment prompted full reversal of NADP-ME activity but not that of the other enzymes.
Several enzymes involved in N assimilation were strongly affected by K deficiency. Maximal activity of NR (Vmax of phosphorylated NR) was significantly decreased in roots of K-starved plants while showing a small increase in shoot. Within 24 h of K resupply, NR activity in roots was restored to an even higher level than in control plants. Other enzymes catalyzing reactions in ammonium assimilation and amino acid synthesis showed an increase of activity in low K. Thus, several enzymes involved in the (re)assimilation of ammonium exhibited a strong increase in activity in the roots of K-starved plants (i.e. Gln synthetase [GS], ferredoxin-glutamine-2-oxoglutarate aminotransferase [Fd-GOGAT], and Glu dehydrogenase [GDH]). In shoots, GDH activity was also increased, but activities of the former two enzymes decreased. Reversibility of the response induced by K deficiency was observed for Fd-GOGAT (in roots and shoots) and GS (in roots only) but not for GDH after 24 h of K resupply. Asp and Ala aminotransferase activities also increased in low K in the shoot and root and were not reversed by K resupply.
We used our previously published microarray data (Armengaud et al., 2004) to compare K-dependent changes of maximal enzyme activities with changes in the levels of the encoding transcripts (shown in Supplemental Fig. S2 for roots and shoots after 14 d of K starvation and 2 and 6 h of K resupply). Good agreement was observed between changes in transcripts and enzyme activities for GS (GLN1-1 [At5g37600], GLN1-2 [At1g66200]) and GDH (GDH1 [At5g07440], GDH2 [At1g18170]), with transcripts showing up-regulation in K-starved roots and quick down-regulation within 2 h of K resupply. Two transcripts encoding ME (At5g11670, At5g25880) were significantly and reversibly up-regulated in roots of K-starved plants, again mirroring the enzyme activity profile. However, in some cases, significant changes in enzyme activities occurred without a significant change in any of the transcripts in the respective gene families. In other cases, significant changes occurred in individual transcripts (white boxes in Supplemental Fig. S2) but had the opposite direction to the enzymatic activity changes (e.g. At1g47840 encoding glucokinase). Most notably, whereas Nia2 (At1g37130), encoding NR, was significantly up-regulated in K-starved roots and down-regulated after K resupply, root NR activity showed the opposite response. By contrast, regulation of the second NR gene, Nia1 (At1g77760), paralleled the observed increase in NR activity in the shoots. In summary, the observed changes in the maximal activities of root ME, GDH, and GS and shoot NR agree with transcriptional regulation (although this does not exclude other mechanisms). In all other cases, changes in enzyme activities are likely to occur at the level of protein turnover or posttranslational modification.
Cytoplasmic K and pH in Root Cells
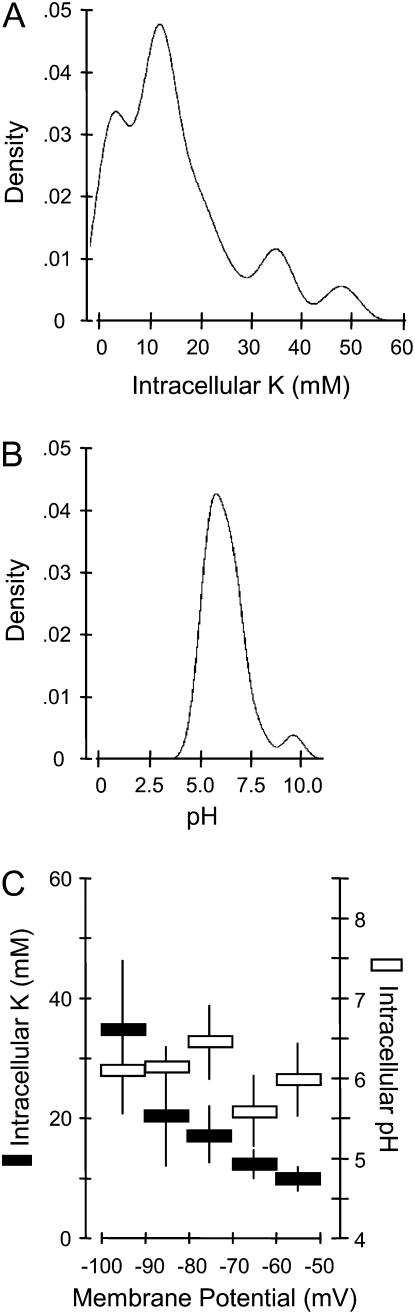
In addition to transcriptional and posttranslational regulation, allosteric and cofactor effects play important roles in determining the actual activities of enzymes in their cellular environment and could make an important contribution to the observed metabolic profiles. K deficiency may lead to changes in the cytoplasmic K concentration. There are also close interactions between cellular K homeostasis and the intracellular pH (Walker et al., 1998). Many enzyme activities are affected by the K concentration and the pH. To investigate a potential role of cytoplasmic K and pH for modulating in vivo enzyme activity, we employed double-barreled ion-selective microelectrodes to measure intracellular K or pH in epidermal and cortical root cells of plants grown for 14 d in low-K medium (Fig. 4).
Figure 4.
Intracellular K concentrations and pH in epidermal root cells of low-K Arabidopsis plants. A, Distribution frequency of intracellular concentrations of K determined in 2-week-old plants by impalement with K-selective microelectrodes (n = 37 plants). B, Distribution frequency of intracellular pH determined by impalement with pH-selective microelectrodes (n = 20 plants). C, K concentration and pH within a particular range of simultaneously measured membrane potentials. Means ± se of measurements in individual plants are shown in black for K (mm) and in white for pH.
Intracellular K concentrations in low-K plants exhibited a peak around 12 mm and included many measurements of only a few millimolar (Fig. 4A). They were thus much lower than K concentrations previously determined in K-sufficient Arabidopsis plants (using the same experimental setup; Shabala et al., 2006). Low-K root cells also displayed only one peak of intracellular pH values (pH 5.8; Fig. 4B). Plotting intracellular K values against simultaneously recorded membrane potentials (Vm) shows that lower K concentrations coincided with more depolarized Vm (Fig. 4C). No correlation was found between intracellular pH and Vm (Fig. 4C).
K Deficiency Induced Changes in Metabolic Charge
Decreasing K concentrations, both at the tissue and at the cellular levels, potentially create an electric charge imbalance. Therefore, it is interesting that the metabolite profiles uncovered a striking relationship between the direction of the concentration change and the electric charge of the metabolite. This was especially so in roots (Fig. 1).
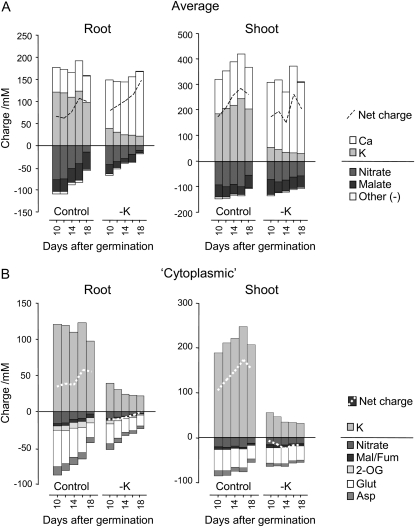
To address this aspect, we added up the positive and negative charges deduced from the concentrations of the main inorganic and organic ions measured in roots and shoots of low-K and control plants (Fig. 5). In control plants, K was the most abundant cation, followed by calcium (Ca), with the concentrations of both ions remaining relatively stable over the assessed time period. Nitrate provided most of the negative charge, together with organic acids (mainly malate in the roots and fumarate in the shoots; see above and Supplemental Data). Compared with these large pools, the contributions to the overall charge of the other metabolites determined here were negligible. In low-K plants, a strong decrease in tissue K concentration was fully compensated by an increase in Ca (while Na levels remained unchanged; Armengaud et al., 2004). The tissue Ca concentration was directly correlated to the external Ca concentration; both increased in the low-K medium (see “Materials and Methods”) but were unaltered during K resupply (Armengaud et al., 2004). Therefore, in low-K roots, the observed decrease in malate and nitrate was “unnecessary” for overall charge balance and led in fact to an increase in net positive charge.
Figure 5.
Electric charges provided by inorganic ions and metabolites. Charge concentration was calculated by multiplying metabolite concentration (as listed in Supplemental Table S1) by the overall charge of the molecule. Plants and growth conditions were as described for Figure 1. A, Charges based on tissue concentrations. These are dominated by the vacuolar lumen. B, Predicted charges in the cytoplasm calculated according to published data for relative cytoplasmic/vacuolar concentrations and volume (see “Materials and Methods”).
However, the measured average tissue ion concentrations shown in Figure 5A will be dominated by vacuolar and apoplastic pools. Changes in organic charges may still be important in the cytoplasm, where free Ca must be maintained at very low concentrations (Kiegle et al., 2000). Based on published data for relative compartmental metabolite concentrations and volumes from spinach (Spinacia oleracea) leaves (Winter et al., 1993), barley (Hordeum vulgare) roots (Zhen et al., 1991), and maize (Zea mays) roots (Radcliffe et al., 2005), we estimated the cytoplasmic concentrations from the average metabolite concentrations shown in Figure 5A (for calculations, see “Materials and Methods”). Although the compartmental distribution of metabolites may vary between different species and change under K deficiency, this exercise provides a useful estimate of metabolite pools that could make important contributions to the cytoplasmic charge balance. Figure 5B shows that Glu, which has an overall low concentration but is preferentially allocated in the cytoplasm, and nitrate, which has an overall high concentration but is preferentially stored in the vacuole, are likely to be the main contributors to negative charge in the cytoplasm. In the same scenario 2-OG, malate (in roots), fumarate (in shoots), and Asp make additional small contributions to cytoplasmic negative charge. Contributions of pyruvate and phosphoenolpyruvate (PEP) to negative charge balance and of His, Arg, and Lys to positive charge balance are negligible, despite an assumed high cytoplasmic/vacuolar distribution. Based on the measured intracellular K concentrations, we assumed that K is present at similar concentrations in the vacuole and cytoplasm. When the estimated charge balance is inspected, there is an excess of positive charge in the cytoplasm of K-replete plants, indicating that the K concentration in the cytoplasm may actually be lower than in the vacuole. In K-starved plants, the summed positive and negative charges are similar, indicating that more K is located in the cytoplasm. Irrespective of these uncertainties, the analysis shows that the decreases of nitrate, organic acids, and acidic amino acids in low K result in a decrease of the total negative charge, which is in the same order of magnitude as the decrease of K.
K Deficiency Induced Changes in the C-N Ratio of Amino Acids
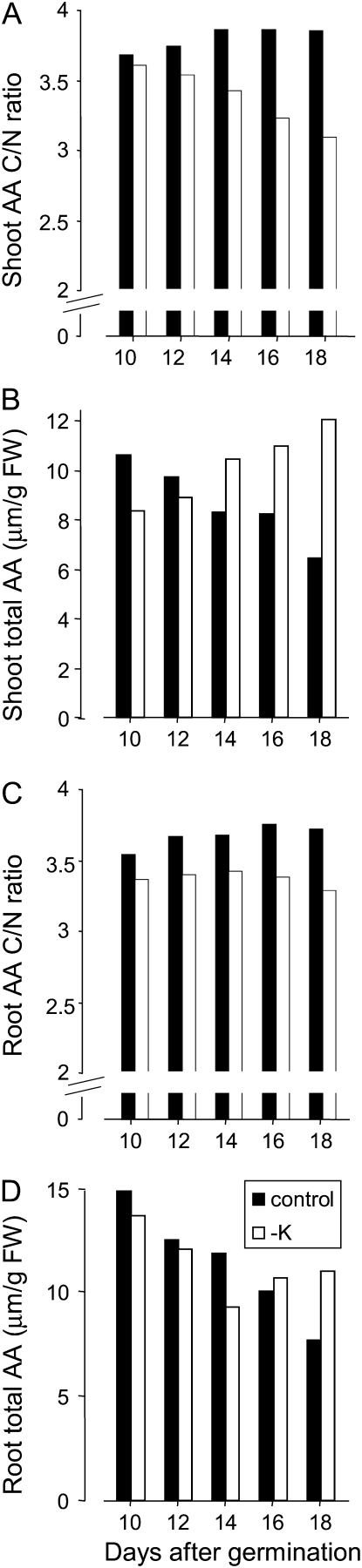
Another trend uncovered by the metabolite profiles was an increase in N-rich amino acids under K deficiency. As in the case of charges, the relative pool sizes of individual metabolites have to be considered to assess the net outcome of this observation. To do so, we multiplied the number of C and N atoms in each amino acid with the concentration of the amino acid and added the respective products to obtain the C-N ratio of the total amino acid pool. As shown in Figure 6, there was a slight increase in amino acid C-N ratio in shoots (Fig. 6A) and roots (Fig. 6C) of control plants over the assessed period of time. In low-K plants, the C-N ratio was lower than in control plants and, particularly in shoots, steadily decreased with time. As a result, amino acid C-N ratios at 18 DAG were markedly lower in low-K plants compared with control plants (3.3 compared with 3.7 in roots and 3.1 compared with 3.9 in shoots; note that the time course experiment was only carried out once but that different time points provide an internal control). These data have to be interpreted in relation to total amino acid concentrations, which over the assessed time period decreased in roots and shoots of control plants and in roots of low-K plants but increased in shoots of low-K plants (Fig. 6, B and D). Hence, in K-deficient plants, prioritization of N-rich amino acids is likely to occur both at the level of biosynthesis and at the level of root-shoot allocation.
Figure 6.
Effect of K deficiency on the C-N ratio of amino acids. C-N ratios of all measured amino acids (A and C) and total amino acid concentrations (B and D) in shoots (A and B) and roots (C and D) of plants growing in control (black bars) and low-K (white bars) medium over the indicated course of time are shown. Plants and growth conditions are as described for Figure 1. C and N concentrations were calculated by multiplying the number of C and N atoms in each amino acid (AA) by the concentration of the respective amino acid (as listed in Supplemental Table S1). FW, Fresh weight.
DISCUSSION
Effect of K on Metabolite Concentrations
In this study, we measured metabolite concentrations and enzyme activities in the root and shoot of Arabidopsis plants during the progressive development of a K deficiency (10–18 DAG) and after short-term K resupply (24 h). Metabolite profiles of low-K Arabidopsis plants were characterized by a strong increase in the concentrations of soluble sugars (Suc, Fru, Glc) and a slight net increase in total protein content and the overall amino acid level. Several basic or neutral amino acids accumulated during K deficiency, while acidic amino acids (Glu, Asp) decreased. In addition to these changes, which occurred in both roots and shoots (albeit to a different degree and with different dynamics), a strong decrease of pyruvate and organic acids was recorded in the roots. The metabolite analysis carried out here was more comprehensive (with respect to type of metabolite, tissue, and timing) than previous studies, but some of the metabolite changes reported here have been observed before in K-deficient crops. For example, increased concentrations of soluble sugars were found in leaves of K-deficient bean (Phaseolus vulgaris; Cakmak et al., 1994a), cotton (Gossypium hirsutum; Bednarz and Oosterhuis, 1999; Pettigrew, 1999), soybean (Glycine max; Huber, 1984), and wheat (Triticum aestivum; Ward, 1960) and in roots of alfalfa (Medicago sativa; Li et al., 1997) and sugar beet (Beta vulgaris; Farley and Draycott, 1975). Amino acid accumulation under K deficiency was reported for tobacco (Nicotiana tabacum; Koch and Mengel, 1974), rice (Oryza sativa; Mengel et al., 1976), and barley (Helal and Mengel, 1979). There is little information on concentrations of individual amino acids in K-deficient crops, but our results agree with those from rice seedlings (Yamashita and Fujiwara, 1967), which showed higher Gln and lower Glu and Asp concentrations in K-deficient plants. Therefore, it appears that Arabidopsis is a good model system for characterizing the effects of K deficiency on primary metabolism in plants.
Possible Causes for K-Dependent Changes in Metabolite Profile
Most metabolite changes occurring during K deficiency were at least partially reversed within 24 h of K resupply. During this period of time, there was no change in plant appearance, indicating that the observed metabolite profiles were not linked to irreversible deficiency symptoms such as leaf senescence and chlorosis but were indeed directly related to the external K supply. However, they were not always related to tissue K content. While concentrations of sugars and nitrate reverted faster in roots than in shoots (Fig. 2) and thus mirrored tissue K concentrations (see Fig. 2 in Armengaud et al., 2004), many amino acids (e.g. Gln, Arg, Phe, Lys) reverted faster in shoots than in roots. This could be due to different pool sizes and different turnover rates in the two tissues but could also imply the existence of a root-shoot signal other than K itself. Candidates for such a signal are nitrate and Glu as well as hormones and other growth-related signaling compounds.
Carbohydrates accumulate in the shoot and root in low K. Sugar accumulation in leaves of K-deficient bean plants has been explained by a requirement for K in long-distance transport (Cakmak et al., 1994a, 1994b). However, an impairment of phloem transport should lead to decreased sugar levels in the roots, whereas the opposite was observed here. The fact that sugars accumulated considerably earlier in roots than in shoots (Fig. 1) also argues against this explanation and suggests instead that sugar accumulation in the leaves is caused by impaired sugar usage in the roots, which is followed by a buildup of sugar levels in the shoots.
K acts as a counter-ion for the transport of nitrate into and in the xylem (Blevins et al., 1978; Rufty et al., 1981). One would expect that inhibition of xylem loading under K deficiency would lead at least temporarily to a buildup of nitrate in the roots. Instead, we observed a decrease of nitrate concentration in the roots. This was even stronger than the decrease in the shoots. Our combined data on root and shoot metabolites, therefore, provide no evidence that defective long-distance transport causes the changes in nitrate or (see above) sugar concentrations under K deficiency. In contrast to what has been suggested before (Marschner, 1995), there was also no indication for a link between the metabolite profile and a K deficiency-induced decrease in photosynthetic rate (which should lead to a decrease rather than a buildup of sugars) or protein synthesis (which should lead to a decrease in protein concentration rather than the observed increase).
One conspicuous feature of the K-dependent metabolite profiles was the reversible depletion of pyruvate, 2-OG, and malate in roots of low-K plants. This decrease of organic acids could contribute to maintaining charge balance, especially in conjunction with the selective decrease of acidic amino acids (Fig. 5B). Considering the parallel (but opposite) changes in the concentrations of reducing sugars and amino acids, the most likely interpretation of this observation is that (1) glycolysis is inhibited and (2) biosynthesis of amino acids is maintained at a net cost of organic acid carbon pools. This scenario has partial support from a 14C-feeding experiment carried out by Yamada et al. (2002) with sunflower (Helianthus annuus) and other crops, which revealed a decrease of carbon flux into the tricarboxylic acid (TCA) cycle and amino acids under K deficiency. The obvious follow-on question is what causes this decrease in glycolytic carbon flux and increased utilization of organic acids.
Reprogramming of Metabolism under K Stress?
One possibility is that the observed metabolite changes are evidence of an active stress response that “reprograms” metabolism (e.g. in preparation for a switch from vegetative growth to reproductive investment; Kolar and Senkova, 2008). This would imply that changes in metabolite concentrations are caused by transcriptional and/or posttranscriptional regulation of specific enzymes, which represent end points of signaling pathways related to growth and development. To investigate this possibility, we complemented previously created transcript profiles (Armengaud et al., 2004) with an analysis of maximal enzyme activities as a direct measure of the amount of active protein. Several enzyme activities indeed showed significant changes in response to K deficiency (Fig. 3; Supplemental Table S2). Only a few of these (root ME, GDH, and GS and shoot NR) were accompanied by parallel changes in the encoding transcripts, suggesting an important role of posttranscriptional processes as well as transcription. In most cases, the changes in enzyme activities did not readily explain the measured changes in metabolite concentrations. For example, the observed down-regulation of acid invertase and up-regulation of glucokinase and fructokinase (Fig. 3) in the roots would be expected to decrease rather than increase Glc and Fru concentrations. Similarly, the measured increase in activities of root ME and PK was accompanied by a decrease, rather than an increase, of pyruvate in roots. The observed decrease in root NR activity did not preserve the nitrate pool. Rather than causing the observed changes in metabolites, the measured changes in enzyme activities may reflect the necessity to adjust fluxes through particular pathways to a primary metabolic disturbance.
In this context, the strong up-regulation of ME activity in roots is particularly interesting, as it might indicate the stimulation of an anaplerotic pathway to use organic acids as a substrate to maintain carbon flux through the TCA cycle, despite an inhibition of pyruvate delivery from glycolysis. In this scenario, NADP-ME converts some of the malate to pyruvate, which is then converted to acetyl-CoA and recombined with oxaloacetate derived from the remainder of the malate. An analogous situation has been proposed for phosphate-starved plants, where an anaplerotic PEP carboxylase/MDH/ME sequence has been suggested to bypass PK when the supply of ADP is low (Plaxton, 1996; Plaxton and Podesta, 2006). Similarly, increasing activity of the GS/GOGAT/GDH cycle in K-starved roots could be a means to maintain N flux into amino acids and proteins in the face of decreased glycolytic carbon flux. Alternatively, regulation of NADP-ME and GDH may be part of an adaptive response aiming to reduce levels of organic and anionic amino acids for charge balance in low-K conditions (Fig. 5B). Indeed, it was shown recently that levels of malate and fumarate can be altered in Arabidopsis by overexpression of NADP-ME (Tronconi et al., 2008) and that overexpression of GDH lowers Asp levels in tobacco (Purnell et al., 2005).
We conclude that our data do not provide evidence that the K deficiency-induced changes in metabolite concentrations are the result of transcriptional and posttranscriptional regulation, at least as far as glycolysis and nitrate reduction are concerned. However, some active reprogramming of metabolism seems to occur downstream of glycolysis, in particular in the TCA and GS/GOGAT/GDH cycles, and could partly explain why organic acid levels are decreased in low-K plants while total amino acid and protein concentrations are not.
Allosteric and Direct Effects of K
Lacking an explanation at the level of maximal enzyme activities for the observed imbalance between hexose and pyruvate in low-K roots, we have to consider allosteric regulators and cofactors, which could modulate these activities in vivo. For example, Glu and Asp are known to be allosteric regulators of PK (Smith et al., 2000). Since they exert opposite effects, the net outcome of the observed parallel change in both amino acids for PK activity is likely to be small. It should be noted, however, that inhibition of PK by Glu is pH dependent (Smith et al., 2000) and could thus be released by cytoplasmic alkalinization.
Most importantly, K itself is an essential cofactor of PK (Kachmar and Boyer, 1953). The binding site for K in PK has been identified and is well conserved in most eukaryotic PK isoforms, including all Arabidopsis isoforms apart from one that is exclusively expressed in pollen (Schmid et al., 2005; Oria-Hernandez et al., 2006). Rabbit muscle PK shows a 10,000-fold increase of in vitro activity when K is present (Ramirez-Silva et al., 2001; Oria-Hernandez et al., 2005, 2006). The response is better described with the Hill model than with Michaelis-Menten kinetics (Ramirez-Silva and Oria-Hernandez, 2003). This in vitro enzymology suggests that PK is a cooperative enzyme that will respond with great sensitivity to relatively small changes in cytoplasmic K, when these are in the range of K0.5 (the concentration at which half of the maximal activity is reached). The observed increase in sugar and concomitant decrease in pyruvate and other organic acid levels are consistent with the hypothesis that PK activity is directly inhibited in low-K root cells, as is the quick reestablishment of pyruvate levels following the resupply of K (Supplemental Fig. S1).
Although an involvement of PK in K stress physiology has often been discussed in the past (Evans and Sorger, 1966; Memon et al., 1985; Marschner, 1995; Ruiz et al., 1999), it is generally discarded on the basis of the discrepancy between high cytoplasmic K concentrations (around 100 mm; Walker et al., 1996) and the low Km of PK for K (between 0.2 and 4 mm; Baysdorfer and Bassham, 1984; Memon et al., 1985; Smith et al., 2000; Turner and Plaxton, 2000). However, under prolonged K deficiency, these two values may not be as far apart as previously assumed. Using intracellular K-selective microelectrodes, we measured intracellular K concentrations (5–15 mm; Fig. 4) in root cells of Arabidopsis plants grown for 2 weeks on low K. Several features of the measured data strongly suggest that these concentrations reflect low cytoplasmic K concentrations. First, the measured K concentrations in our treatments lie in the range where vacuolar stores are depleted in barley, resulting in a steep decrease in cytoplasmic K (Walker et al., 1996). Second, unlike others (Maathuis and Sanders, 1993, 1994; Nieves-Cordones et al., 2008), we could not separate two populations of intracellular K concentrations. This indicates that the cytoplasmic and vacuolar K concentrations are very similar in our material. Third, in contrast to a hyperpolarization of the membrane potential observed in other studies for low-K root cells (Maathuis and Sanders, 1993; Nieves-Cordones et al., 2008), we measured a depolarization, which increased with lower intracellular K values. A depolarization is in accordance with low cytoplasmic K concentrations causing a positive shift of the K equilibrium potential (Nernst equation) and possibly also directly inhibiting the proton pump (Churchill et al., 1983). One notable difference between the plants sampled here and those used in other studies is that they were not allowed to grow in a K-rich medium prior to exposure to low K. We conclude that cytoplasmic K in root cells of living plants can adopt much lower values than hitherto assumed. Another important point to consider is that most studies dealing with the kinetic properties of PK are performed in vitro. Such conditions may be very different from those in situ, particularly in terms of aqueous environment. Effects of K on the water structure surrounding proteins are at the heart of its mode of action (Page and Di Cera, 2006); for example, it was found that partial replacement of water with dimethyl sulfoxide lowers the affinity of PK for K (Ramirez-Silva and Oria-Hernandez, 2003).
We conclude that inhibition of glycolysis as a result of direct inhibition of PK by low cytoplasmic K in root cells of K-starved plants is a compelling hypothesis that merits further investigation. In this context, it is interesting that many prokaryotic PKs lack a K-binding site and are K independent (Oria-Hernandez et al., 2006). Overexpression of a K-independent bacterial PK in Arabidopsis could be one way to test our hypothesis.
As in the case of K, intracellular pH values of low-K plants did not readily separate into cytoplasmic and vacuolar populations, suggesting alkalinization of the vacuole and acidification of the cytoplasm, which is in accordance with a previous study in barley (Walker et al., 1996). A decrease in pH is unlikely to affect PK activity directly, as this enzyme has a broad pH optimum (Smith et al., 2000). By contrast, a small decrease in pH below 7.5 inhibits PEP carboxylase and activates ME (Davies, 1986; Lepiniec et al., 1994; Drincovich et al., 2001). The latter would occur on top of the observed up-regulation at the level of transcripts and maximum activity (Fig. 3).
Metabolic Signals
N metabolism in the roots of K-starved plants showed interesting features. On the one hand, we measured down-regulation of NRT2 transporters (Armengaud et al., 2004), decreased maximal activity of NR, and preferential assimilation of N into N-rich amino acids. All of these are typical responses to C starvation (Rolland et al., 2002). On the other hand, we measured up-regulation of the GS/GOGAT/GDH cycle, which suggests that reactions involved in ammonium (re)assimilation receive a signal of C abundance (Morcuende et al., 1998). The observation that N assimilation pathways in low-K plants receive conflicting signals with respect to C availability is interesting because (1) it reflects the fact that low-K plants show C accumulation in hexoses but C depletion in organic acids and (2) it suggests that regulatory signals for different N-assimilatory enzymes originate either upstream or downstream of glycolysis. Hexokinase has been postulated to be the main sugar sensor mediating cross talk between C and N metabolism (Sheen et al., 1999; Moore et al., 2003), but our results indicate that some signals for nitrate uptake and reduction as well as aminotransferases are created farther down in the glycolytic pathway and override or modulate hexose signaling. In this context, it is interesting that the signal for sugar derepression of NRT2.1 in the dark is Glc-6-P (Lejay et al., 2003, 2008).
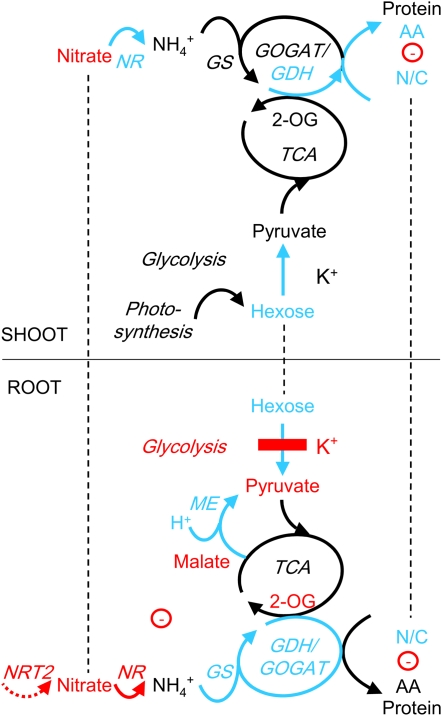
CONCLUSION
In this study, we have investigated the effects of external K supply on primary metabolism in young Arabidopsis plants by measuring metabolite concentrations, enzyme activities, and intracellular K and pH. The combined data suggest the scenario shown in Figure 7, in which the primary causes of K deficiency-induced changes in metabolism reside in the direct inhibition of PK by low cytoplasmic K and the increase of NADP-ME activity due to low cytoplasmic pH. The inhibition of glycolysis explains the observed buildup in root sugar levels and the decrease in pyruvate concentration. Many other changes in metabolites and enzyme activities in the roots can be interpreted as consequences of this event. Thus, up-regulation of enzymes related to hexose metabolism could reflect feedback regulation by accumulating Fru and Glc while preferential synthesis (and root-shoot allocation) of N-rich amino acids, as well as down-regulation of root nitrate transporters and NR, could be a response to diminished production of carbon skeletons by the TCA cycle. Up-regulation of ME and the GS/GOGAT/GDH cycle in the roots can be interpreted as an adaptive response to maintain carbon flux through the TCA cycle and into amino acids and proteins in the face of inhibited glycolysis. Metabolite changes in the shoots (sugar accumulation and nitrate depletion) are likely to be knockon effects of the same changes in the roots.
Figure 7.
Scheme summarizing the effects of low K on primary metabolism in root and shoot cells of Arabidopsis. Biochemical and transport pathways are indicated with solid and dashed arrows, respectively. Increases in metabolite concentrations and enzyme activities under K deficiency are shown in blue, and decreases are shown in red. Putative direct inhibition of PK by low K is indicated with the red bar. Dashed lines indicate the exchange of metabolites between roots and shoots. Negative electric charge is given as a circled minus. AA, Amino acids; N/C, N-C ratio of the total amino acid pool; NRT2, nitrate transporter 2.
Our study provides an essential knowledge platform to test the integration of metabolism with plant K status. Important novel information was obtained from the separate analyses of root and shoot tissues. Taking into account that K, pH, metabolites, enzymes, and pathways are differentially localized in different cells and organelles, this is clearly only the very first step toward understanding the effects of K on metabolism. In the future, we should seek to achieve a much higher resolution of the changes in K, pH, and metabolites both temporally and spatially. Thus, metabolic fluxes should be measured in roots, and imaging techniques should be explored to analyze the dynamics of K and metabolite changes at the single, subcellular level (Lalonde et al., 2005; Looger et al., 2005). Such research can be expected to make an important contribution to understanding the complex interaction between mineral nutrition and nutritional quality of crops.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) seeds were surface sterilized (2.5% sodium hypochlorite and 0.1% Tween 20) for 5 min, rinsed five times with sterile water, and placed in darkness at 4°C for 3 to 4 d to synchronize germination. Seeds were then sown in 120- × 120-mm square petri dishes (approximately 15–20 seeds per plate) containing 70 mL of nutrient medium with 3% Suc and 1% agar type A (Sigma) added. The control nutrient medium contained 1.25 mm KNO3, 0.5 mm Ca(NO3)2, 0.5 mm MgSO4, 42.5 μm FeNaEDTA, 0.625 mm KH2PO4, 2 mm NaCl, and micronutrients (Maathuis et al., 2003) at pH 5.6. In the “K-free” medium, KNO3 was replaced by Ca(NO3)2, KH2PO4 was replaced by NaH2PO4, and NaCl was lowered to 1.375 mm. Final ion concentrations in the two media, control (K-free), were 1.875 (0) mm K, 0.5 (1) mm Ca2+, 1.25 (1) mm NO3−, and 2 (1.375) mm Cl− (all other ions were unchanged). K contamination from the agar and other chemicals was measured at 80 μm. Petri dishes were sealed with Parafilm and placed vertically under the light source (16 h per day at 100 μE) at a constant temperature of 22°C. Resupply experiments were carried out with 2-week-old seedlings and consisted of replacing the condensed solution at the bottom of the petri dishes with 5 mL of liquid K-free medium supplemented with 10 mm KCl (+K treatment). For procedures and tissue ion concentrations of plants subjected to the different treatments, see Armengaud et al. (2004).
Metabolite Analysis
Chlorophylls, Glc, Fru, Suc, starch, total soluble protein, total amino acid, malate, fumarate, and nitrate contents were measured using spectrophotometric analyses of soluble and residual fractions of an ethanol-water extract as described by Cross et al. (2006). Malate and fumarate contents were determined as described by Nunes-Nesi et al. (2007). Amino acids were measured in the same extract by HPLC after derivatization of the primary amino group with o-phthalic acid dialdehyde according to Geigenberger et al. (1996). 3-Phosphoglycerate, PEP, pyruvate, 2-OG, and isocitrate were measured using perchloric extracts according to Stitt et al. (1989) and Bergmeyer (1989). All presented measurements are averages of at least three independent experiments.
Enzymatic Assays
Aliquots of 20 mg of frozen tissue powder were extracted with 0.5 to 1 mL of the extraction buffer consisting of 20% (v/v) glycerol, 0.25% (w/v) bovine serum albumin, 1% (v/v) Triton X-100, 50 mm HEPES/KOH, pH 7.5, 10 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 1 mm benzamidine, 1 mm aminocapronic acid, 1 mm phenylmethylsulfonyl fluoride, 10 mm leupeptin, and 0.25 mm dithiothreitol. Crude extracts were diluted to generate appropriate conditions for the measurement of all of the different enzymes as described by Gibon et al. (2004). NAD-ME was determined as described by Jenner et al. (2001) with slight modifications. Briefly, the reaction was stopped by 0.5 m HCl containing 0.1 m HEPES/KOH, pH 9, buffer and neutralized after 5 min of incubation at room temperature with 0.5 m NaOH before adding the determination mix. NADP-ME was determined using the same protocol, except that NAD was replaced by NADP in the assay mix. All presented measurements are averages of three to five independent experiments.
Intracellular K and pH Measurements
Ion-selective microelectrodes were constructed from silanized borosilicate glass capillaries and prepared using ionophore sensor cocktails for K and pH as described previously (Walker et al., 1995, 1996; Miller et al., 2001). The open barrel measuring the membrane potential was filled with 200 mm NaCl for both ion-selective electrodes. The ion-selective barrel was filled with K-free growth medium (omitting Suc) for the K-selective electrode or with pH 4 calibration solution for the pH-selective electrode. Electrode calibration was performed before and after measurements with K calibration solutions containing 1, 10, 100, and 200 mm KCl or pH calibration solutions (using 1 mm BisTris/MES buffers) at pH 4, 6, 7, and 8.5. Calibration curves were fitted with the Nicholson-Eisenman equation. VISER software, developed by I.R. Jennings (University of York), was used to analyze the data. A whole plant grown for 14 DAG in K-free medium was removed from the petri dish, and its primary root was placed in a plexiglass chamber filled with K-free growth medium. Epidermal and cortical cells were impaled at 20 to 30 mm from the root tip. Membrane potential recorded with the open pipette was subtracted from the voltage determined by the ion-selective electrode, and the difference was compared with the calibration curve. After the measurements, electrodes were recalibrated, and only those measurements were considered for analysis that showed good agreement in precalibration and postcalibration.
Calculation of Metabolic Charge
For the calculation of average metabolic charges (Fig. 5A), we multiplied for each molecule its determined concentration with its electric charge (−1 for pyruvate, PEP, Glu, Asp, and nitrate, −2 for 2-OG, malate, and fumarate, +1 for K, His, Arg, and Lys, +2 for Ca, and 0 for all others). To estimate cytoplasmic charges (Fig. 5B), we calculated a cytoplasmic-vacuolar concentration ratio (cytoplasmic factor [Fcyt]) for each compound from previously published data and combined it with the relative volumes of cytoplasm (Volcyt) and vacuole (Volvac). Cytoplasmic charge concentrations (Concyt) were then calculated from the determined average charge concentrations (Conave) with the following equation:
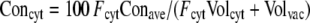 |
For the graphs shown in Figure 5B, values for Fcyt were taken from a compilation by Winter et al. (1993): 68 (for 2-OG), 38 (for Glu), 26 (for Asp), and 0.12 (for malate). These factors were also applied to pyruvate and PEP (68), His, Arg, and Lys (26), and fumarate (0.12). For nitrate, we used an Fcyt of 0.2, which is the average of previously determined values (Zhen et al., 1991; Winter et al., 1993; Radcliffe et al., 2005). Fcyt for K was taken as 1, assuming that vacuolar K has reached its lowest value and cytoplasmic K has decreased (Walker et al., 1996), and Fcyt for Ca was taken as 0.00001 (Walker et al., 1996; Kiegle et al., 2000). We further used a cytoplasmic proportion of 7% as an average value for different cell types (Volcyt was 0.07 and Volvac was 0.93).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Full recovery of root pyruvate levels after 2 h of K resupply.
Supplemental Figure S2. Changes in transcript levels of metabolic enzymes in response to K deficiency and resupply (data from Armengaud et al., 2004).
Supplemental Table S1. Absolute metabolite concentrations for the K-deficiency time course experiment shown in Figure 1.
Supplemental Table S2. Absolute metabolite concentrations and enzyme activities for the K-deficiency and -resupply experiment (Figs. 2 and 3), including means, se values, and P values.
Supplementary Material
Acknowledgments
We thank Melanie Hoehne, Manuela Guenther, Hendrick Tschoep, Marie-Caroline Durand (all Max Planck Institute of Plant Molecular Physiology), Naomi Donald (University of Glasgow), and Susan J. Smith (Rothamsted Research) for excellent technical assistance with the wide range of measurements presented in this study.
This work was supported by the Biotechnology and Biological Sciences Research Council (Wain travel fellowship to P.A. and grant no. BB/D006775 to A.A.) and the German Ministry of Education and Research within the German Plant Genome Initiative GABI-GNADE and GABI-GENOPLANTE (to M.S.). Rothamsted Research is grant aided by the Biotechnology and Biological Sciences Research Council.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Anna Amtmann (a.amtmann@bio.gla.ac.uk).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Amtmann A, Blatt MR (2009) Regulation of macronutrient transport. New Phytol 181 35–52 [DOI] [PubMed] [Google Scholar]
- Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plant 133 682–691 [DOI] [PubMed] [Google Scholar]
- Andrist-Rangel Y, Edwards AC, Hillier S, Oborn I (2007) Long-term K dynamics in organic and conventional mixed cropping systems as related to management and soil properties. Agric Ecosyst Environ 122 413–426 [Google Scholar]
- Armengaud P, Breitling R, Amtmann A (2004) The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol 136 2556–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengaud P, Zambaux K, Hills A, Sulpice R, Pattison RJ, Blatt MR, Amtmann A (2009) EZ-Rhizo: integrated software for fast and accurate measurement of root system architecture. Plant J 57 945–956 [DOI] [PubMed] [Google Scholar]
- Baysdorfer C, Bassham JA (1984) Spinach pyruvate kinase isoforms: partial purification and regulatory properties. Plant Physiol 74 374–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarz CW, Oosterhuis DM (1999) Physiological changes associated with potassium deficiency in cotton. J Plant Nutr 22 303–313 [Google Scholar]
- Bergmeyer HU (1989) Methods in Enzymatic Analysis, Vol 7. Verlag Chemie, Weinheim, Germany
- Blevins DG, Barnett NM, Frost WB (1978) Role of potassium and malate in nitrate uptake and translocation by wheat seedlings. Plant Physiol 62 784–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I, Hengeler C, Marschner H (1994. a) Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J Exp Bot 45 1245–1250 [Google Scholar]
- Cakmak I, Hengeler C, Marschner H (1994. b) Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J Exp Bot 45 1251–1257 [Google Scholar]
- Churchill KA, Holaway B, Sze H (1983) Separation of 2 types of electrogenic H+-pumping ATPases from oat roots. Plant Physiol 73 921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross JM, von Korff M, Altmann T, Bartzetko L, Sulpice R, Gibon Y, Palacios N, Stitt M (2006) Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiol 142 1574–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DD (1986) The fine control of cytosolic pH. Physiol Plant 67 702–706 [Google Scholar]
- Dobermann A, Cassman KG, Mamaril CP, Shesby JE (1999) Management of phosphorus, potassium, and sulfur in intensive irrigated lowland rice. Field Crops Res 56 113–118 [Google Scholar]
- Drincovich MF, Casati P, Andreo CS (2001) NADP-malic enzyme from plants: a ubiquitous enzyme involved in different metabolic pathways. FEBS Lett 490 1–6 [DOI] [PubMed] [Google Scholar]
- Evans HJ, Sorger GJ (1966) Role of mineral elements with emphasis on the univalent cations. Annu Rev Plant Physiol 17 47–76 [Google Scholar]
- Farley RF, Draycott PA (1975) Growth and yield of sugar beet in relation to potassium and sodium supply. J Sci Food Agric 26 385–392 [Google Scholar]
- Geigenberger P, Lerchi J, Stitt M, Sonnewald U (1996) Phloem-specific expression of pyrophosphatase inhibits long distance transport of carbohydrates and amino acids in tobacco plants. Plant Cell Environ 19 43–55 [Google Scholar]
- Gething PA (1993) The Potassium-Nitrogen Partnership. International Potash Institute, Basel
- Gibon Y, Blaesing OE, Hannemann J, Carillo P, Hohne M, Hendriks JH, Palacios N, Cross J, Selbig J, Stitt M (2004) A Robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helal H, Mengel K (1979) Nitrogen metabolism of young barley plants as affected by NaCl-salinity and potassium. Plant Soil 51 457–462 [Google Scholar]
- Hoa NM, Janssen BH, Oenema O, Dobermann A (2006) Comparison of partial and complete soil K budgets under intensive rice cropping in the Mekong Delta, Vietnam. Agric Ecosyst Environ 116 121–131 [Google Scholar]
- Huber SC (1984) Biochemical basis for effects of K-deficiency on assimilate export rate and accumulation of soluble sugars in soybean leaves. Plant Physiol 76 424–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner HL, Winning BM, Millar AH, Tomlinson KL, Leaver CJ, Hill SA (2001) NAD malic enzyme and the control of carbohydrate metabolism in potato tubers. Plant Physiol 126 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachmar JF, Boyer PD (1953) Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J Biol Chem 200 669–682 [PubMed] [Google Scholar]
- Kayser M, Isselstein J (2005) Potassium cycling and losses in grassland systems: a review. Grass Forage Sci 60 213–224 [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR (2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23 267–278 [DOI] [PubMed] [Google Scholar]
- Koch K, Mengel K (1972) Effect of a varied potassium nutrition on the uptake and incorporation of labelled nitrate by young tobacco plants (Nicotiana tabacum L.). J Sci Food Agric 23 1107–1112 [Google Scholar]
- Koch K, Mengel K (1974) The influence of the level of potassium supply to young tobacco plants (Nicotiana tabacum L.) on short-term uptake and utilisation of nitrate nitrogen (15N). J Sci Food Agric 25 465–471 [Google Scholar]
- Kolar J, Senkova J (2008) Reduction of mineral nutrient availability accelerates flowering of Arabidopsis thaliana. J Plant Physiol 165 1601–1609 [DOI] [PubMed] [Google Scholar]
- Lalonde S, Ehrhardt DW, Frommer WB (2005) Shining light on signaling and metabolic networks by genetically encoded biosensors. Curr Opin Plant Biol 8 574–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejay L, Gansel X, Cerezo M, Tillard P, Muller C, Krapp A, von Wiren N, Daniel-Vedele F, Gojon A (2003) Regulation of root ion transporters by photosynthesis: functional importance and relation with hexokinase. Plant Cell 15 2218–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejay L, Wirth J, Pervent M, Cross JM, Tillard P, Gojon A (2008) Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis. Plant Physiol 146 2036–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepiniec L, Vidal J, Chollet R, Gadal P, Cretin C (1994) Phosphoenolpyruvate carboxylase: structure, regulation and evolution. Plant Sci 99 111–124 [Google Scholar]
- Li R, Volenec JJ, Joern BC, Cunningham SM (1997) Potassium and nitrogen effects on carbohydrate and protein metabolism in alfalfa roots. J Plant Nutr 20 511–529 [Google Scholar]
- Looger LL, Lalonde S, Frommer WB (2005) Genetically encoded FRET sensors for visualizing metabolites with subcellular resolution in living cells. Plant Physiol 138 555–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJ, Filatov V, Herzyk P, Krijger GC, Axelsen KB, Chen S, Green BJ, Li Y, Madagan KL, Sánchez-Fernández R, et al (2003) Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. Plant J 35 675–692 [DOI] [PubMed] [Google Scholar]
- Maathuis FJ, Sanders D (1994) Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc Natl Acad Sci USA 91 9272–9276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D (1993) Energization of potassium uptake in Arabidopsis thaliana. Planta 191 302–307 [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants, Ed 2. Academic Press, London
- Memon AR, Siddiqi MY, Glass ADM (1985) Efficiency of K+ utilization by barley varieties: activation of pyruvate kinase. J Exp Bot 36 79–90 [Google Scholar]
- Mengel K, Viro M, Hehl G (1976) Effect of potassium on uptake and incorporation of ammonium-nitrogen of rice plants. Plant Soil 44 547–558 [Google Scholar]
- Miller AJ, Cookson SJ, Smith SJ, Wells DM (2001) The use of microelectrodes to investigate compartmentation and the transport of metabolized inorganic ions in plants. J Exp Bot 52 541–549 [PubMed] [Google Scholar]
- Moody PW, Bell MJ (2006) Availability of soil potassium and diagnostic soil tests. Aust J Soil Res 44 265–275 [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300 332–336 [DOI] [PubMed] [Google Scholar]
- Morcuende R, Krapp A, Hurry V, Stitt M (1998) Sucrose-feeding leads to increased rates of nitrate assimilation, increased rates of alpha-oxoglutarate synthesis, and increased synthesis of a wide spectrum of amino acids in tobacco leaves. Planta 206 394–409 [Google Scholar]
- Nieves-Cordones M, Miller AJ, Alemán F, Martínez V, Rubio F (2008) A putative role for the plasma membrane potential in the control of the expression of the gene encoding the tomato high-affinity potassium transporter HAK5. Plant Mol Biol 68 521–532 [DOI] [PubMed] [Google Scholar]
- Nitsos RE, Evans HJ (1966) Effects of univalent cations on the inductive formation of nitrate reductase. Plant Physiol 41 1499–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Nesi A, Carrari F, Gibon Y, Sulpice R, Lytovchenko A, Fisahn J, Graham J, Ratcliffe RG, Sweetlove LJ, Fernie AR (2007) Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J 50 1093–1106 [DOI] [PubMed] [Google Scholar]
- Oria-Hernandez J, Cabrera N, Perez-Montfort R, Ramirez-Silva L (2005) Pyruvate kinase revisited: the activating effect of K+. J Biol Chem 280 37924–37929 [DOI] [PubMed] [Google Scholar]
- Oria-Hernandez J, Riveros-Rosas H, Ramirez-Silva L (2006) Dichotomic phylogenetic tree of pyruvate kinase family: K+-dependent and independent enzymes. J Biol Chem 281 30717–30724 [DOI] [PubMed] [Google Scholar]
- Page MJ, Di Cera E (2006) Role of Na+ and K+ in enzyme function. Physiol Rev 86 1049–1092 [DOI] [PubMed] [Google Scholar]
- Peoples TR, Koch DW (1979) Role of potassium in carbon dioxide assimilation in Medicago sativa L. Plant Physiol 63 878–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew WT (1999) Potassium deficiency increases specific leaf weights and leaf glucose levels in field-grown cotton. Agron J 91 962–968 [Google Scholar]
- Pettigrew WT (2008) Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol Plant 133 670–681 [DOI] [PubMed] [Google Scholar]
- Plaxton WC (1996) The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol 47 185–214 [DOI] [PubMed] [Google Scholar]
- Plaxton WC, Podesta FE (2006) The functional organization and control of plant respiration. Crit Rev Plant Sci 25 159–198 [Google Scholar]
- Purnell MP, Skopelitis DS, Roubelakis-Angelakis KA, Botella JR (2005) Modulation of higher-plant NAD(H)-dependent glutamate dehydrogenase activity in transgenic tobacco via alteration of beta subunit levels. Planta 222 167–180 [DOI] [PubMed] [Google Scholar]
- Radcliffe SA, Miller AJ, Ratcliffe RG (2005) Microelectrode and Cs-133 nuclear magnetic resonance evidence for variable cytosolic and cytoplasmic nitrate pools in maize root tips. Plant Cell Environ 28 1379–1387 [Google Scholar]
- Ramirez-Silva L, Ferreira ST, Nowak T, Tuena de Gomez-Puyou M, Gomez-Puyou A (2001) Dimethylsulfoxide promotes K+-independent activity of pyruvate kinase and the acquisition of the active catalytic conformation. Eur J Biochem 268 3267–3274 [DOI] [PubMed] [Google Scholar]
- Ramirez-Silva L, Oria-Hernandez J (2003) Selectivity of pyruvate kinase for Na+ and K+ in water/dimethylsulfoxide mixtures. Eur J Biochem 270 2377–2385 [DOI] [PubMed] [Google Scholar]
- Rengel Z, Damon PM (2008) Crops and genotypes differ in efficiency of potassium uptake and use. Physiol Plant 133 624–636 [DOI] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell (Suppl) 14 S185–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty TW, Jackson WA, Raper CD (1981) Nitrate reduction in roots as affected by the presence of potassium and by flux of nitrate through the roots. Plant Physiol 68 605–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JM, Moreno DA, Romero L (1999) Pyruvate kinase activity as an indicator of the level of K+, Mg2+, and Ca2+ in leaves and fruits of the cucumber: the role of potassium fertilization. J Agric Food Chem 47 845–849 [DOI] [PubMed] [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37 501–506 [DOI] [PubMed] [Google Scholar]
- Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA (2006) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol 141 1653–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J, Zhou L, Jang JC (1999) Sugars as signaling molecules. Curr Opin Plant Biol 2 410–418 [DOI] [PubMed] [Google Scholar]
- Smith CR, Knowles VL, Plaxton WC (2000) Purification and characterization of cytosolic pyruvate kinase from Brassica napus (rapeseed) suspension cell cultures: implications for the integration of glycolysis with nitrogen assimilation. Eur J Biochem 267 4477–4485 [DOI] [PubMed] [Google Scholar]
- Sorger GJ, Ford RE, Evans HJ (1965) Effects of univalent cations on the immunoelectrophoretic behavior of pyruvic kinase. Proc Natl Acad Sci USA 54 1614–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Lilley RM, Gerhardt R, Heldt HW (1989) Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol 174 518–552 [Google Scholar]
- Tronconi MA, Fahnenstich H, Weehler MCG, Andreo CS, Flugge UI, Drincovich MF, Maurino VG (2008) Arabidopsis NAD-malic enzyme functions as a homodimer and heterodimer and has a major impact on nocturnal metabolism. Plant Physiol 146 1540–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner WL, Plaxton WC (2000) Purification and characterization of cytosolic pyruvate kinase from banana fruit. Biochem J 352 875–882 [PMC free article] [PubMed] [Google Scholar]
- Véry AA, Sentenac H (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54 575–603 [DOI] [PubMed] [Google Scholar]
- Walker DJ, Black CR, Miller AJ (1998) The role of cytosolic potassium and pH in the growth of barley roots. Plant Physiol 118 957–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DJ, Leigh RA, Miller AJ (1996) Potassium homeostasis in vacuolate plant cells. Proc Natl Acad Sci USA 93 10510–10514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DJ, Smith SJ, Miller AJ (1995) Simultaneous measurement of intracellular pH and K+ or NO3− in barley root-cells using triple-barreled, ion-selective microelectrodes. Plant Physiol 108 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward GM (1960) Potassium in plant metabolism. III. Some carbohydrate changes in the wheat seedling associated with varying rates of potassium supply. Can J Plant Sci 40 729–735 [Google Scholar]
- Winter H, Robinson DG, Heldt HW (1993) Subcellular volumes and metabolite concentrations in barley leaves. Planta 191 180–190 [Google Scholar]
- Wyn Jones RJ, Pollard A (1983) Proteins, enzymes and inorganic ions. In A Lauchli, A Pirson, eds, Encyclopedia of Plant Physiology. Springer, Berlin, pp 528–562
- Yamada S, Osaki M, Shinano T, Yamada M, Ito M, Permana AT (2002) Effect of potassium nutrition on current photosynthesized carbon distribution to carbon and nitrogen compounds among rice, soybean and sunflower. J Plant Nutr 25 1957–1973 [Google Scholar]
- Yamashita T, Fujiwara A (1967) Metabolism of acetate-1-14C in excised leaves from potassium deficient rice seedlings. Plant Cell Physiol 8 557–565 [Google Scholar]
- Zhen RG, Koyro HW, Leigh RA, Tomos AD, Miller AJ (1991) Compartmental nitrate concentrations in barley root cells measured with nitrate-selective microelectrodes and by single-cell sap sampling. Planta 185 356–361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.