Abstract
Reversible protein phosphorylation is a key regulatory mechanism governing polar auxin transport. We characterized the auxin transport and gravitropic phenotypes of the pinoid-9 (pid-9) mutant of Arabidopsis (Arabidopsis thaliana) and tested the hypothesis that phosphorylation mediated by PID kinase and dephosphorylation regulated by the ROOTS CURL IN NAPHTHYLPHTHALAMIC ACID1 (RCN1) protein might antagonistically regulate root auxin transport and gravity response. Basipetal indole-3-acetic acid transport and gravitropism are reduced in pid-9 seedlings, while acropetal transport and lateral root development are unchanged. Treatment of wild-type seedlings with the protein kinase inhibitor staurosporine phenocopies the reduced auxin transport and gravity response of pid-9, while pid-9 is resistant to inhibition by staurosporine. Staurosporine and the phosphatase inhibitor, cantharidin, delay the asymmetric expression of DR5∷revGFP (green fluorescent protein) at the root tip after gravistimulation. Gravity response defects of rcn1 and pid-9 are partially rescued by treatment with staurosporine and cantharidin, respectively. The pid-9 rcn1 double mutant has a more rapid gravitropic response than rcn1. These data are consistent with a reciprocal regulation of gravitropism by RCN1 and PID. Furthermore, the effect of staurosporine is lost in pinformed2 (pin2). Our data suggest that reduced PID kinase function inhibits gravitropism and basipetal indole-3-acetic acid transport. However, in contrast to PID overexpression studies, we observed wild-type asymmetric membrane distribution of the PIN2 protein in both pid-9 and wild-type root tips, although PIN2 accumulates in endomembrane structures in pid-9 roots. Similarly, staurosporine-treated plants expressing a PIN2∷GFP fusion exhibit endomembrane accumulation of PIN2∷GFP, but no changes in membrane asymmetries were detected. Our data suggest that PID plays a limited role in root development; loss of PID activity alters auxin transport and gravitropism without causing an obvious change in cellular polarity.
A variety of important growth and developmental processes, including gravity response, embryo and vascular development, and the branching of roots and shoots, are controlled by the directional and regulated transport of auxin in higher plants. Reversible protein phosphorylation is an important regulatory strategy that may modulate auxin transport and dependent processes such as root gravitropism, perhaps through action of the PINOID (PID) kinase (for review, see DeLong et al., 2002; Galvan-Ampudia and Offringa, 2007). PID is an AGC family Ser/Thr kinase (Christensen et al., 2000) and belongs to an AGC kinase clade containing WAG1, WAG2, AGC3-4, and D6PK/AGC1-1 (Santner and Watson, 2006; Galvan-Ampudia and Offringa, 2007; Zourelidou et al., 2009). PID activity has been demonstrated in vitro and in vivo (Christensen et al., 2000; Michniewicz et al., 2007), and several pid mutant alleles exhibit altered auxin transport in the inflorescence and a floral development defect resembling that of auxin transport mutants (Bennett et al., 1995). Overexpression of the PID gene results in profound alterations in root development and responses to auxin transport inhibitors, reduced gravitropism and auxin accumulation at the root tip (Christensen et al., 2000; Benjamins et al., 2001; Michniewicz et al., 2007), as well as enhanced indole-3-acetic acid (IAA) efflux in tobacco (Nicotiana tabacum) cell cultures (Lee and Cho, 2006) and altered PINFORMED1 (PIN1), PIN2, and PIN4 localization patterns (Friml et al., 2004; Michniewicz et al., 2007), consistent with PID being a positive regulator of IAA efflux. However, the effects of pid loss-of-function mutations on auxin transport activities and gravitropic responses in roots have not yet been reported (Robert and Offringa, 2008).
In contrast, auxin transport and gravitropism defects of a mutant with reduced protein phosphatase activity have been characterized in detail. The roots curl in naphthylphthalamic acid1 (rcn1) mutation, which ablates the function of a protein phosphatase 2A regulatory subunit, causes reduced PP2A activity in vivo and in vitro (Deruère et al., 1999). Roots and hypocotyls of rcn1 seedlings have elevated basipetal auxin transport (Deruère et al., 1999; Rashotte et al., 2001; Muday et al., 2006), and rcn1 roots exhibit a significant delay in gravitropism, consistent with altered auxin transport (Rashotte et al., 2001; Shin et al., 2005). These data indicate that PP2A is a negative regulator of basipetal transport and suggest that if PID-dependent phosphorylation regulates root auxin transport and gravitropism, then it may act in opposition to PP2A-dependent dephosphorylation.
In roots, auxin transport is complex, with distinct sets of influx and efflux carriers that define tissue-specific and opposing directional polarities (for review, see Leyser, 2006). IAA moves acropetally, from the shoot toward the root apex, through the central cylinder (Tsurumi and Ohwaki, 1978), and basipetally, from the root apex toward the base, through the outer layer of cells (for review, see Muday and DeLong, 2001). When plants are reoriented relative to the gravity vector, auxin becomes asymmetrically distributed across the root tip, as a result of a process termed lateral auxin transport (for review, see Muday and Rahman, 2008). Several carriers that mediate root basipetal IAA transport have been clearly defined and include the influx carrier AUXIN-INSENSITIVE1 (AUX1; Marchant et al., 1999; Swarup et al., 2004; Yang et al., 2006) and efflux carriers of two classes, PIN2 (Chen et al., 1998; Müller et al., 1998; Rashotte et al., 2000) and ATP-BINDING CASSETTE TYPE B TRANSPORTER4/MULTIDRUG-RESISTANT4/P-GLYCOPROTEIN4 (ABCB4/MDR4/PGP4; Geisler et al., 2005; Terasaka et al., 2005; Lewis et al., 2007). Lateral transport at the root tip may be mediated by PIN3, an efflux carrier with a gravity-dependent localization pattern (Friml et al., 2002; Harrison and Masson, 2007).
Gravitropic curvature of Arabidopsis (Arabidopsis thaliana) roots requires changes in IAA transport at the root tip (for review, see Muday and Rahman, 2008). Auxin transport inhibitors (Rashotte et al., 2000) and mutations in genes encoding basipetal transporters, including aux1 (Bennett et al., 1996), pin2/agr1 (Chen et al., 1998; Müller et al., 1998), and abcb4/mdr4/pgp4 (Lin and Wang, 2005; Lewis et al., 2007), alter gravitropism. Auxin-inducible reporters exhibit asymmetric expression across the root tip prior to differential growth, and this asymmetry is abolished by treatment with auxin transport inhibitors that prevent gravitropic curvature (Rashotte et al., 2001; Ottenschläger et al., 2003). Additionally, the pin3 mutant exhibits slightly reduced rates of gravitropic curvature (Harrison and Masson, 2007), and PIN3 is expressed in the columella cells, which are the site of gravity perception (Blancaflor et al., 1998; Friml et al., 2002). The PIN3 protein relocates to membranes on the lower side of columella cells after gravitropic reorientation, consistent with a role in facilitating asymmetric IAA transport at the root tip (Friml et al., 2002; Harrison and Masson, 2007).
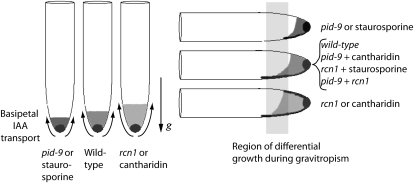
The available data suggest a model in which PID and RCN1 antagonistically regulate basipetal transport and gravitropic response in root tips (Fig. 1). In this model, the regions with the highest IAA concentrations in the epidermal and cortical cell layers are indicated by shading, and the arrows indicate the direction and relative amounts of basipetal auxin transport. Our previous work suggests that elevated basipetal IAA transport in rcn1 roots impairs gravitropic response, presumably due to the inability of roots either to form or to perceive a lateral auxin gradient in the context of a stronger polar IAA transport stream (Rashotte et al., 2001). Enhanced basipetal transport may increase the initial auxin concentration along the upper side of the root, impeding the establishment or perception of a gradient in rcn1 and cantharidin-treated wild-type roots (Fig. 1, right). Based on the published pid inflorescence transport data (Bennett et al., 1995), we hypothesize that pid seedling roots and staurosporine-treated wild-type roots have reduced basipetal auxin transport (Fig. 1, left). Upon reorientation of roots relative to the gravity vector, the reduced basipetal IAA transport in pid may lead to slower establishment of an auxin gradient across the root. This model then predicts that cantharidin treatment of pid-9 or staurosporine treatment of rcn1 seedlings would enhance or restore gravitropism in these mutants. Similarly, a double mutant might be expected to exhibit a corrected gravitropic response relative to the single mutants.
Figure 1.
Auxin transport defects in pid-9 and rcn1 mutants alter auxin redistribution after reorientation relative to the gravity vector. This model predicts that differences in basipetal auxin transport activities of wild-type, pid-9, and rcn1 roots will affect the formation of lateral auxin gradients. The shaded area in each root represents the region of highest IAA concentration in epidermal and cortical cells, with darker shading in the central columella cells, believed to be the auxin maxima. The direction and amount of basipetal IAA transport are indicated by arrows. The region of differential growth during gravitropic bending is indicated by the shaded rectangle. If auxin transport is reduced (as shown in the pid-9 mutant or in staurosporine-treated seedlings), this would lead to a slower formation of an auxin gradient in root tips. The rcn1 mutation (or treatment with cantharidin) has already been shown to lead to increased basipetal transport and a reduced rate of gravitropic bending, consistent with altered formation or perception of an auxin gradient. The antagonistic effects of kinase and phosphatase inhibition are predicted to lead to normal gravity responses in the pid-9 rcn1 double mutant as well as in pid-9 and rcn1 single mutants treated with the “reciprocal” inhibitor.
The experiments described here were designed to test this model by examining gravitropism and root basipetal IAA transport in pid and staurosporine-treated seedlings. We investigated the regulation of gravity response by PID kinase and RCN1-dependent PP2A activities and observed antagonistic interactions between the rcn1 and pid-9 loss-of-function phenotypes that are consistent with reciprocal kinase/phosphatase regulation. We found that loss of kinase activity in the pid mutant and in staurosporine-treated wild-type plants inhibits basipetal auxin transport and the dependent physiological process of root gravitropism. Our results suggest that staurosporine acts to regulate these processes through inhibition of PID kinase and that PID effects are PIN2 dependent. In both wild-type and pid-9 roots, we observed polar membrane distribution of the PIN2 protein; unlike wild-type roots, though, pid-9 roots exhibited modest accumulation of PIN2 in endomembrane structures. Similarly, we detected asymmetric distribution and endomembrane accumulation of PIN2∷GFP in staurosporine-treated roots. Our data suggest that PID plays a limited role in root development; loss of PID activity alters PIN2 trafficking, auxin transport, and gravitropism without causing an obvious loss of cellular polarity. Together, these experiments provide insight into phosphorylation-mediated control of the gravity response and auxin transport in Arabidopsis roots.
RESULTS
Genetic and Chemical Reductions in Kinase Activity Reduce Root Gravitropism
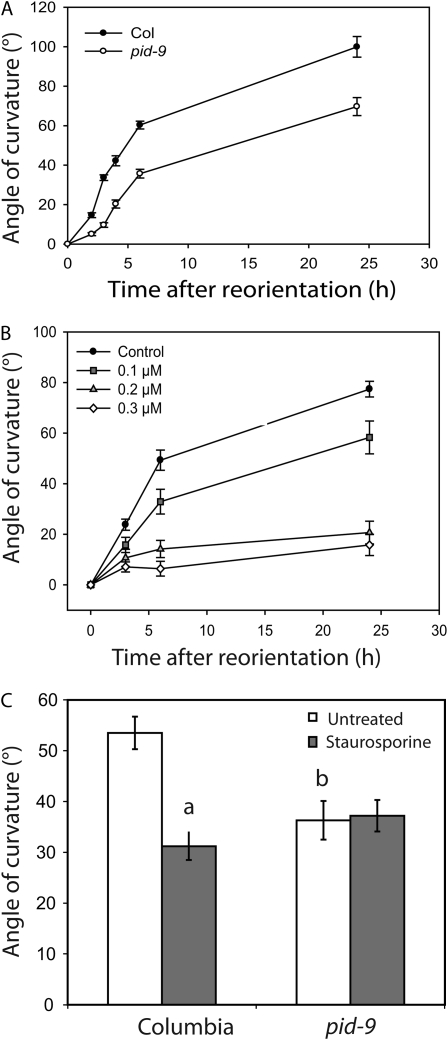
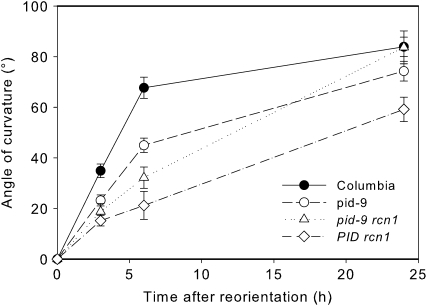
Gravitropic response is reduced in roots of transgenic plants overexpressing PID (Benjamins et al., 2001), but the gravitropic phenotype of pid loss-of-function mutants has not been reported previously (Robert and Offringa, 2008). We measured the gravity response in light-grown pid-9 seedlings (a strong loss-of-function pid allele; Christensen et al., 2000) after reorientation by 90° in the dark. For these experiments, pid homozygotes were selected from a segregating family using the tricot phenotype exhibited by approximately 5% of pid mutant seedlings, but with extremely low frequency (less than one in 1,000) in wild-type seedlings (Bennett et al., 1995; Zhou et al., 2004). Gravity response was quantified by measuring the angle of curvature at various times after reorientation, and the results of four pooled experiments are shown in Figure 2A. The pid-9 mutant exhibits significant decreases in gravitropic bending at all time points (P < 0.005). The greatest difference between pid-9 and ecotype Columbia (Col) was observed at early time points after gravity stimulation, with a 3.4-fold reduction in curvature 3 h after reorientation. Overall elongation of pid-9 roots is normal (data not shown).
Figure 2.
Gravity response in pid-9 and staurosporine-treated roots. Seedlings were reoriented by 90° relative to the gravity vector, and the angle of curvature of the root was plotted as a function of time after reorientation or at 6 h after reorientation. A, For the wild type (Col) and pid-9, each value represents the average ± se for 37 to 49 seedlings from four separate experiments. Gravity response in pid-9 exhibits statistically significant delays at all time points as judged by Student's t test (P < 0.005). B, For staurosporine treatment, each value represents the average ± se for at least 19 seedlings from three separate experiments. The effect of staurosporine on gravity response was significant at all doses and time points as judged by Student's t test (P < 0.05 at 0.1 μm at all time points and P < 0.005 at 0.2 and 0.3 μm at all time points). C, Gravity response was measured at 6 h after reorientation in 17 to 20 seedlings with or without 0.1 μm staurosporine. Data are from two separate experiments. Statistical comparisons were performed by Student's t test (P < 0.005). a, Statistical differences between untreated and 0.1 μm staurosporine treatment; b, significant differences between genotypes.
We also used staurosporine, a broad-spectrum protein kinase inhibitor, to ask if this compound produces a phenocopy of the pid gravitropic defect in wild-type roots. Staurosporine treatment causes reductions in gravity response beginning 3 h after gravity stimulation (Fig. 2B). Wild-type seedlings treated with 0.1 μm staurosporine exhibit significantly reduced curvature relative to untreated controls (P < 0.05 at all time points) and show gravitropic bending kinetics similar to those of pid-9 mutant seedlings; at this dose, staurosporine had only a small effect on root elongation (Supplemental Fig. S1). Higher doses of staurosporine have a much more profound effect on the gravity response, particularly at later time points, with 0.2 and 0.3 μm staurosporine having significant effects at all times points (P < 0.005).
If the pid mutation and kinase inhibition by staurosporine are affecting the same pathway, then pid-9 seedlings might be insensitive to the effect of staurosporine on root gravitropism. We measured the curvature of wild-type (Col) and pid-9 seedlings 6 h after reorientation in the presence and absence of 0.1 μm staurosporine (Fig. 2C). While staurosporine treatment significantly reduced curvature in wild-type seedlings (P < 0.005), there were no significant changes in curvature in pid-9 with staurosporine treatment (P > 0.05). These results are consistent with PID kinase being a primary target through which low-dose staurosporine treatment inhibits gravitropism.
Staurosporine and Cantharidin Alter DR5∷revGFP Expression
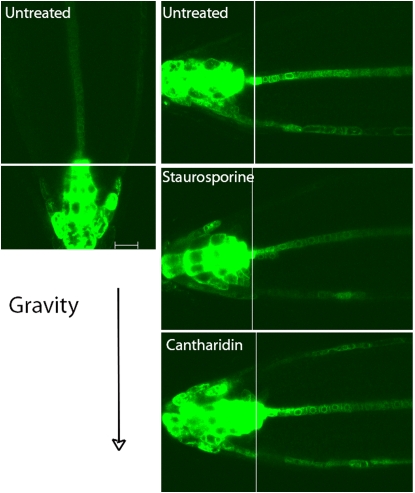
We also examined the effect of staurosporine on the asymmetric expression of the auxin-responsive reporter, DR5∷revGFP, after reorientation of seedlings relative to the gravity vector. DR5∷revGFP is expressed in the columella in vertically growing seedlings, with limited expression in the epidermis and lateral root cap beyond the root tip (Fig. 3). Consistent with previous reports (Ottenschläger et al., 2003; Paciorek et al., 2005), asymmetric DR5rev∷GFP gene expression is observed after reorientation of the seedlings relative to the gravity vector. The asymmetry in GFP expression in untreated roots is evident from the reduced expression along the upper side of the root tip and in the enhanced expression along the lower side of the lateral root cap and epidermal tissues in all samples examined at 3 or 4 h after reorientation. This asymmetric DR5∷revGFP pattern extends 100 to 200 μm from the root tip, into the root distal elongation zone, where asymmetric growth drives gravitropic bending.
Figure 3.
Staurosporine and cantharidin alter asymmetric DR5rev∷GFP expression across reoriented roots. Roots of seedlings carrying the DR5rev∷GFP transgene were grown for 18 h on inhibitor-free medium or medium containing 0.2 μm staurosporine or 10 μm cantharidin. Confocal images were captured 4 h after a 90° reorientation of the seedlings. These images were captured at high magnification with a 63× objective, so it was not possible to see the entire root tip expression domain of the DR5rev∷GFP pattern in a single frame. Individual optical sections were captured in two positions under identical conditions, and the junctions between these two images are marked by thin white lines. These panels contain representative images from 12 to 17 seedlings for each treatment. Bar = 20 μm.
In contrast, staurosporine treatment (performed under conditions identical to those described above) delays the initiation of bending and minimizes the asymmetry in DR5∷revGFP expression. Strong asymmetries in DR5∷revGFP expression, as defined above, were observed in less than 25% of roots tested (n = 17). Other roots exhibited expression on the upper side and lower flanks of the root, or no detectable expression farther from the root tip. A representative image of the most common DR5∷revGFP expression pattern after staurosporine treatment, observed in more than 50% of seedlings, is shown in Figure 3. In this image, the reduction in expression on the upper side of the root tip is not observed, some expression is evident in the upper epidermis, and there is uneven/discontinuous expression along the lower flank of the epidermis, with expression extending a shorter distance than in normal untreated seedlings.
As cantharidin had previously been shown to phenocopy the delayed gravitropic response of rcn1 (Rashotte et al., 2001; Shin et al., 2005), we also examined its effect on the asymmetric expression of DR5rev∷GFP. Cantharidin treatment led to a third expression pattern. In 87% of the cantharidin-treated roots (n = 15), there was DR5∷revGFP expression evident in epidermal cells on both the upper and lower flanks. This pattern is consistent with enhanced basipetal IAA transport in cantharidin-treated roots (Rashotte et al., 2001), which may interfere with the formation of auxin gradients across roots reoriented relative to the gravity vector. Similar gravity stimulation experiments were previously performed with a DR5-GUS reporter in rcn1 or cantharidin-treated wild-type roots, and development of a DR5-GUS expression asymmetry was significantly delayed (Muday and DeLong, 2001; Rashotte et al., 2001).
Impaired Kinase and Phosphatase Activities Reciprocally Affect Gravitropic Response
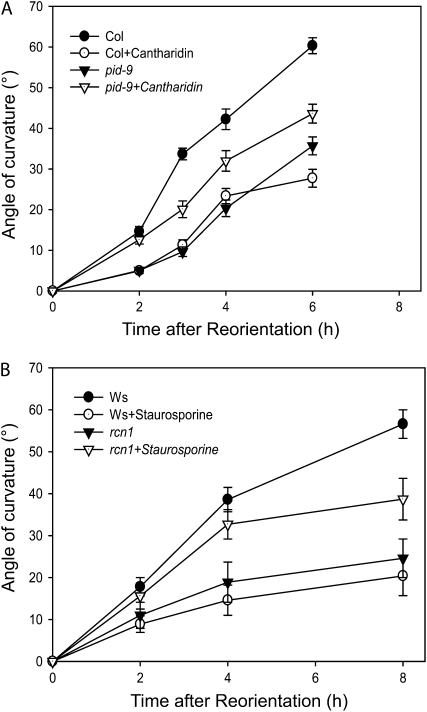
One question raised by these experiments is whether PID and a protein phosphatase activity reciprocally regulate gravitropism. Reduction in protein phosphatase activity due to cantharidin treatment or the rcn1 mutation results in decreased gravitropic bending (Rashotte et al., 2001). We asked whether protein phosphatase inhibition might rescue the gravity defect caused by loss of PID function, measuring the gravity response of cantharidin-treated wild-type and pid-9 mutant seedlings (Fig. 4A). Cantharidin at 10 μm reduced root gravitropism in wild-type roots with statistically significant effects at all time points (P < 0.001), consistent with previous reports (Rashotte et al., 2001; Shin et al., 2005), while growth was not significantly altered by this dose (data not shown). Cantharidin treatment of pid-9 plants significantly increased the root gravitropic response relative to the untreated pid-9 seedlings at all time points (P < 0.05; Fig. 4A).
Figure 4.
Inhibitor treatments suppress gravity response defects of pid and rcn1 mutant seedlings. Five-day-old seedlings were gravity stimulated, and the average angles of root curvature were plotted as a function of time after reorientation. A, Col and pid-9 roots were compared with or without 10 μm cantharidin treatment. Both the pid-9 mutation and cantharidin treatment statistically reduce gravity response at all time points as compared with untreated Col (P < 0.001). Cantharidin treatment of pid-9 enhances gravity response relative to untreated pid-9 at all time points (P < 0.05). B, Ws and rcn1 were compared with or without 0.1 μm staurosporine treatment. Both the rcn1 mutation and staurosporine treatment of Ws statistically reduce gravity response at 4 and 8 h after reorientation with staurosporine (P < 0.001) as compared with untreated Ws. Staurosporine treatment of rcn1 enhances gravity response relative to untreated rcn1 (P < 0.05) at 4 and 8 h after reorientation. For both experiments, each value represents the average ± se for at least 23 to 51 seedlings from five separate experiments.
We also asked whether kinase inhibition could rescue the gravitropic defect of rcn1 roots. Wild-type (ecotype Wassilewskija [Ws]) and rcn1 seedlings were treated with 0.1 μm staurosporine, with identical dose and treatment time as described above, prior to gravity stimulation (as described above for Col and pid-9 seedlings), and root gravitropic curvature and growth were measured after gravity stimulation. As reported previously, rcn1 roots were much slower than wild-type roots in gravitropic bending, with significant reductions in bending at 4 and 8 h after reorientation (P < 0.001; Fig. 4B; Rashotte et al., 2001). Treatment of wild-type Ws seedlings with 0.1 μm staurosporine resulted in inhibition of gravitropic curvature similar to that described above for the wild-type Col, with significant effects at 4 and 8 h (P < 0.001). Interestingly, treatment of rcn1 roots with 0.1 μm staurosporine significantly increased the gravity response relative to untreated rcn1 and relative to staurosporine-treated wild-type plants at 4 and 8 h after gravity stimulation (P < 0.05; Fig. 4B). The effect of staurosporine treatment on rcn1 is most profound at 2 and 4 h after gravity stimulation, when the treatment restores gravitropic bending to levels that are not statistically different from those of the untreated control seedlings (P > 0.05).
To confirm the results of the inhibitor experiments described above, we took a genetic approach and studied the root gravity response in pid-9 rcn1 double mutant seedlings. The inhibitor experiments described above suggested that gravity response defects of the parental single mutants might be suppressed in pid-9 rcn1 double mutant plants. The rcn1 mutation was isolated in the Ws genetic background (Garbers et al., 1996), while pid-9 is in the Col background. To control for these different genetic backgrounds, the gravity response of the rcn1 pid-9 double mutant was compared with that of an rcn1 PID sibling family. Seven-day-old seedlings were rotated 90° in the dark, and angles of curvature were measured at several time points (Fig. 5). Like the parental rcn1 mutant, the rcn1 PID line exhibited a significantly reduced gravity response (Supplemental Table S1). At all time points, the pid-9 rcn1 seedlings exhibited a greater gravity response than rcn1 PID seedlings, with these differences being statistically significant at all time points (P < 0.05). The gravity response in rcn1 pid-9 seedlings is slower than that in pid-9 until 24 h, when it surpasses the curvature of the pid-9 mutant. At this last time point, the curvatures of these two genotypes (pid-9 and the rcn1 pid-9 double mutant) become equivalent to that of the wild type (P > 0.05). Collectively, these results suggest that protein kinase and protein phosphatase activities antagonistically regulate the root gravitropic response.
Figure 5.
Suppression of the rcn1 gravitropism defect by pid-9. Seven-day-old seedlings were gravity stimulated, and the average angles of root curvature were plotted as a function of time after reorientation. Each value represents the average ± se for at least 17 seedlings from three separate experiments. Statistical differences between treatments are indicated in Supplemental Table S1.
Kinase-Regulated Root Basipetal Auxin Transport Is PID-PIN2 Dependent
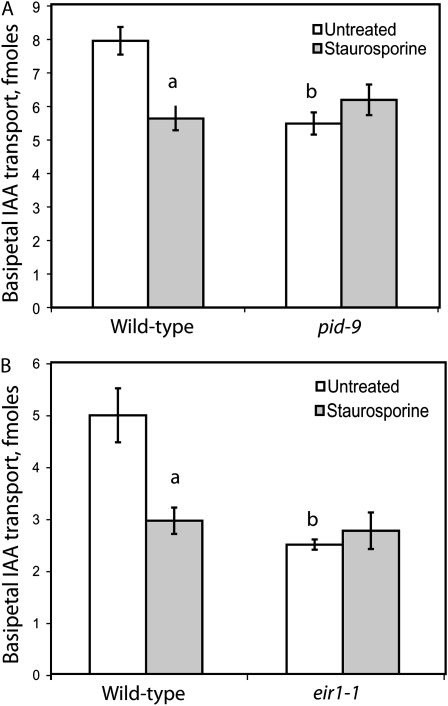
Basipetal auxin transport was measured in roots of pid-9 and staurosporine-treated wild-type seedlings to determine if transport, like gravitropism, is altered by reduced kinase activity. Basipetal transport assays were performed by application of an agar line of [3H]IAA directly to the root tips of intact living plants. After 5 h, the amount of [3H]IAA transported basipetally was measured, and the results from multiple pooled experiments are shown in Figure 6A. Both pid-9 plants and seedlings treated with 0.2 μm staurosporine exhibited significant reductions in basipetal IAA transport compared with untreated Col seedlings, as judged by Student's t test (P < 0.0001). The magnitude of the reduction in transport by staurosporine and the pid-9 mutation was similar (31% and 29%, respectively). Similar effects were observed with seedlings treated with 0.1 and 0.3 μm staurosporine (data not shown). Naphthylphthalamic acid (NPA) inhibition of transport in both pid-9 and staurosporine-treated roots was equivalent to that observed in wild-type controls in the absence of staurosporine (data not shown). Staurosporine treatment of pid-9 seedlings at the same range of concentrations failed to inhibit basipetal IAA transport. In conjunction with the observed staurosporine insensitivity of the pid-9 gravitropic response (Fig. 2C), this result suggests that the PID kinase is a target of staurosporine inhibition in basipetal transport and gravitropism.
Figure 6.
Loss of PID or PIN2 function abrogates staurosporine sensitivity of basipetal auxin transport. A, Basipetal IAA transport was measured in 5-d-old seedlings of Col and pid-9. The average and se are reported for 37 seedlings in four separate experiments. B, Basipetal IAA transport was measured in 5-d-old Col and eir1-1 seedlings using a modified transport assay protocol (see “Materials and Methods”). The average and se are reported for 10 seedlings. Statistical comparisons were performed by Student's t test (P < 0.005). a, Statistical differences between untreated and 0.2 μm staurosporine treatment; b, significant differences between genotypes. In both pid-9 and eir1-1 seedlings, staurosporine treatment did not cause a statistically significant change in basipetal transport (P > 0.05).
As PIN2 is one of the IAA efflux carriers that mediate basipetal auxin transport (Chen et al., 1998; Müller et al., 1998), we asked whether staurosporine altered IAA transport in a pin2 mutant. Transport was measured in 5-d-old Col or eir1-1 (a pin2 allele) seedlings that had been treated for 18 h with 0.2 μm staurosporine and in untreated controls (Fig. 6B). The magnitude of the reduction in basipetal IAA transport in eir1-1 (>50%) is consistent with two previous reports that indicate only partial reduction in transport in this mutant (Rashotte et al., 2001; Shin et al., 2005). The magnitude of reduction in transport is greater in eir1-1/pin2 than in pid-9, consistent with the role of PIN2 as a transporter rather than a regulator of transport. Strikingly, staurosporine has no effect on basipetal transport in the eir1-1 mutant. These results suggest that PIN2 may be a target for the staurosporine-sensitive kinase that regulates basipetal transport. What is perhaps surprising is that pin2 alleles still exhibit significant transport, even though they are agravitropic. It has been shown that PIN1 expression is increased in a pin2 background in tissues that mediate basipetal IAA transport (Vieten et al., 2005), which may compensate for the role of PIN2 in this polar transport. Additionally, other proteins contribute to basipetal IAA transport in the roots, including AUX1 and ABCB4/MDR4 (Rashotte et al., 2001; Lewis et al., 2007), suggesting redundant mechanisms to control basipetal IAA. Nonetheless, the effect of staurosporine is completely lost in the pin2 mutant, consistent with this being a primary target for phosphorylation.
PID Action Is Not Required for Root Acropetal Transport and Lateral Root Formation
Root acropetal IAA transport was measured in pid-9 mutant seedlings to determine whether this polarity of IAA transport is sensitive to reduced kinase activity. Acropetal transport assays were performed by applying an agar cylinder of [3H]IAA to the root-shoot junction using methods that have been optimized for measurement of auxin transport in Arabidopsis (Lewis and Muday, 2009). The amount of acropetal [3H]IAA was measured in a 5-mm segment including the root tip. As Col and pid-9 seedlings had similar root lengths, the distance IAA traveled was matched between these genotypes. Acropetal IAA transport activities were similar in pid-9 and Col (Table I). We also asked whether reduced kinase activity affected lateral root development and elongation, processes tied to acropetal IAA transport (Reed et al., 1998; Bhalerao et al., 2002; Lewis et al., 2007). The total length of roots and number of emerged lateral roots were measured in Col and pid-9 on day 10 after planting. To control for differences in primary root lengths, lateral root density was calculated by dividing the number of lateral roots by the length of the roots (Table I). No differences in lateral root densities or in the number of elongated lateral roots or root lengths were observed in the pid-9 mutant. Thus, loss of PID function alters neither acropetal transport nor lateral root formation but appears to have a specific effect on root basipetal IAA transport and dependent physiological processes. These data suggest that the loss of PIN1 polarity observed in pid embryos (Friml et al., 2004) has no significant impact on root developmental processes, in contrast to the profound effects of PIN1 polarity shift on embryogenesis and shoot organogenesis.
Table I.
Acropetal IAA transport and lateral root development are not altered in pid-9
| Variable | Col | pid-9 |
|---|---|---|
| Acropetal IAA transport (fmol)a | 2.38 ± 0.24 | 2.45 ± 0.38 |
| Lateral root density (roots cm−1)b | 1.42 ± 0.1 | 1.54 ± 0.13 |
Each value represents the average ± se for 18 seedlings from a representative experiment in which seedlings were used at 5 d after planting.
Total root lengths were measured and emerged lateral roots were counted in seedlings at 10 d after planting, and lateral root density was calculated. Each value represents the average ± se for at least 17 seedlings from a representative experiment. Transport and lateral root density did not differ between Col and pid-9 as judged by Student's t test (P > 0.05).
RCN1 and PID Are Expressed in Cells That Mediate Basipetal IAA Transport and Root Gravitropism
We compared the expression patterns of PID and RCN1 with those of genes encoding proteins that are involved in basipetal and acropetal IAA transport in Arabidopsis roots (Supplemental Fig. S2A). The tissue-specific expression patterns of these genes link them to specific transport polarities, with genes expressed at the highest levels in the epidermis, cortex, and stele being tied to acropetal IAA transport, while expression in epidermis and lateral root cap is consistent with proteins mediating basipetal IAA transport. For this comparison, we used the previously published microarray data set in which gene expression was characterized in Arabidopsis root protoplasts isolated from distinct cell populations expressing tissue-specific GFP reporter lines (Birnbaum et al., 2003). For clarity, we show only the data for stage 2, the transition zone between the root tip and the elongation zone, which comprises the region from 0.3 to 0.45 mm from the root tip. (The results for other stages are nearly identical [data not shown].) PID gene expression is highest in the epidermal and lateral root cap cells, consistent with its specific effect in regulation of basipetal IAA transport, mediated by AUX1, PIN2, and ABCB4/MDR4/PGP4, which all localize to these same tissues. Additionally, the PID-like WAG1 and WAG2 kinase genes exhibit a similar expression pattern in this data set (data not shown). This pattern is consistent with a PID∷YFP expression pattern reported previously (Michniewicz et al., 2007). In contrast, PIN1 expression is higher in the stele, endodermis, and cortex, tissues implicated in acropetal IAA transport, and substantially lower in those tissues showing strong PID expression. ABCB19/PGP19/MDR1 also is more highly expressed in tissues involved in acropetal transport, although the difference is less striking. In contrast, RCN1 expression is roughly equivalent in all tissues of the root.
We also examined the localization of RCN1∷YFP (yellow fluorescent protein) and PIN2∷GFP protein fusions in root tips to determine whether RCN1 protein accumulates in appropriate positions to directly regulate the activity of basipetal transport proteins. This RCN1∷YFP fusion was previously shown to complement the rcn1 mutation, restoring normal root tip organization and hypocotyl elongation, and exhibits robust accumulation in all cells of the root tip (Blakeslee et al., 2008). As reported previously for native PIN2 and PIN2∷GFP, we observed PIN2 localization at the plasma membranes at the upper (basal) ends of cells in the epidermis and lateral root cap of the root tip, consistent with its role mediating basipetal transport from the tip toward the base of the root (Supplemental Fig. S2B; Chen et al., 1998; Müller et al., 1998; Paciorek et al., 2005). RCN1∷YFP was detected in the cytoplasm and closely associated with the plasma membrane in the root tip, basal elongation, and central elongation zones. Membrane association of native RCN1 protein in roots also has been documented by cell fractionation (Blakeslee et al., 2008). These data show that PIN2-GFP, which marks the cells that mediate basipetal IAA transport, and RCN1∷YFP are expressed in a common group of cells and both exhibit membrane-associated populations in live cells. These results suggest that RCN1 is properly localized to regulate proteins involved in basipetal IAA transport and root gravitropism.
The pid-9 Mutation and Staurosporine Treatment Cause Endomembrane PIN2 Accumulation without a PIN2 Polarity Shift
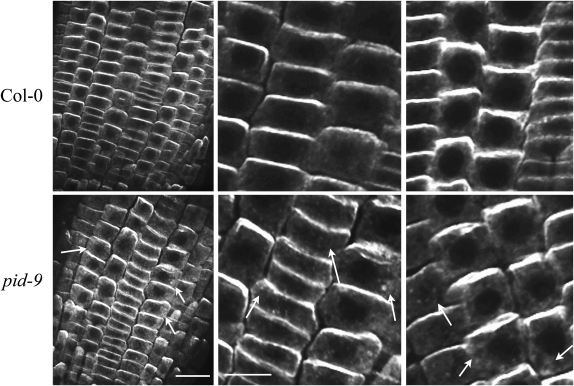
To determine if kinase activity modulates IAA transport by altering the abundance or localization of auxin transport proteins, we examined the localization of PIN2 protein by immunofluorescence in wild-type and pid-9 seedling roots (Fig. 7). The anti-PIN2 antibody is highly specific, as it produces no plasma membrane staining in eir1-1/pin2 mutant roots (Supplemental Fig. S3). In wild-type roots, PIN2 is detected in epidermal and cortical cell files with localization in epidermal and lateral root cap cells on the basal membrane (the side away from the root tip), consistent with the role of PIN2 in directing basipetal IAA transport polarity. The pid-9 mutation does not alter the asymmetric localization of PIN2 protein in any of the 12 roots examined but leads to accumulation of PIN2 protein in endomembrane structures in 77% of the roots examined (Fig. 7). This result suggests that, in contrast to PID overexpression, which reduces/eliminates PIN2 asymmetries (Friml et al., 2004), loss of PID function may alter PIN2 trafficking but does not lead to loss of polarity as measured by PIN2 localization.
Figure 7.
PIN2 protein accumulates in endomembrane structures in the pid-9 mutant but exhibits its normal asymmetric membrane localization. PIN2 localization was examined in epidermal tissues of fixed root sections isolated from wild-type Col and pid-9 seedlings using immunofluorescence labeling. Arrows indicate cells with PIN2 protein visible in endomembrane structures. For each genotype, more than 12 different seedlings were analyzed and two representative images are shown. Bars = 20 μm in low-magnification images and 10 μm in high-magnification images.
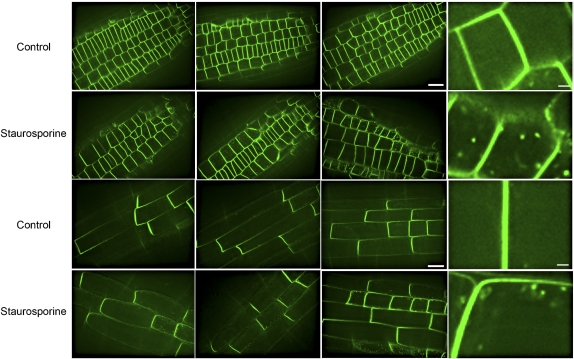
We also examined the effect of staurosporine treatment on PIN2∷GFP localization. To mirror the conditions that alter auxin transport and gravitropism, 5-d-old seedlings were transferred to agar plates containing 0.2 μm staurosporine for 18 h, and the effect on PIN2∷GFP localization was determined in epidermal cells in the root tip and in the transition zone (Fig. 8). When roots are treated with staurosporine, PIN2∷GFP is still membrane associated and has the same directional polarity as in untreated roots (100% of more than 50 roots examined). After staurosporine treatment, 12 out of 17 roots tested (71%) exhibited PIN2∷GFP in internal structures similar to those observed for the native PIN2 protein in pid-9 roots. Multiple representative images from this analysis are shown in Figure 8. We examined the PIN2∷GFP pattern in untreated seedlings on three different confocal instruments and found GFP fluorescence in endomembranes in less than 10% of the more than 50 untreated roots that were imaged under identical confocal settings to those used in staurosporine treatments. These fluorescent structures are not due to autofluorescence, as staurosporine treatment of nontransformed plants yielded no detectable fluorescence at equivalent confocal settings (data not shown). In contrast, staurosporine treatment of seedlings expressing PIN1∷GFP did not alter the PIN1∷GFP localization pattern (n > 30; Supplemental Fig. S4). These results are consistent with the effect of the pid-9 mutation on gravitropism and auxin transport and with the lack of a pid-9 effect on acropetal IAA transport and lateral root development, two processes tied to PIN1 (Benkova et al., 2003).
Figure 8.
Staurosporine does not alter PIN2∷GFP polar localization but does increase PIN2∷GFP accumulation in endomembrane structures. PIN2-GFP seedlings were transferred for 18 h to inhibitor-free medium or medium containing 0.2 μm staurosporine, and individual confocal sections were captured from the outer layer of epidermal cells either close to the root tip (top two rows) or in the transition zone cells that are beginning to elongate (bottom two rows). The images were captured using identical confocal settings and are representative of 20 roots. Bars = 20 μm except on the higher zooms, in which bars = 3 μm.
DISCUSSION
Here, we present a detailed analysis of the role of PID kinase in regulating gravitropism and auxin transport in Arabidopsis roots. Inflorescences of pid mutants have reduced auxin transport (Bennett et al., 1995), but auxin transport in pid roots had not been previously examined. Together, our data indicate that the PID kinase regulates basipetal IAA transport and gravitropism in Arabidopsis roots and support the hypothesis that PIN2 is a kinase target that regulates root gravitropism and auxin transport. We also provide evidence for a reciprocal regulation of the gravity response by PID kinase and the RCN1-PP2A complex. Staurosporine treatment and the pid-9 mutation cause accumulation of PIN2∷GFP and PIN2 protein in endomembrane structures, respectively, consistent with a kinase-dependent mechanism controlling the movement of auxin transport proteins to the plasma membrane. Previous reports had shown that PIN1 is mislocalized to a different plasma membrane face in pid shoots and in roots of PID-overexpressing transgenics (Friml et al., 2004; Michniewicz et al., 2007), but the pattern of protein localization has not been reported previously for pid roots. Despite the PIN polarity shifts observed during pid embryogenesis, our data indicate that pid loss of function has a relatively limited impact on root developmental processes and does not significantly perturb the asymmetric localization of PIN1 and PIN2 proteins in seedling roots. The clear PID-dependent regulation of PIN polarity observed in embryos and shoots may be attenuated in roots, such that basipetal auxin transport is reduced and acropetal transport is unaffected.
The pid-9 mutation and low-dose staurosporine treatment reduce basipetal IAA transport and delay root gravitropism and the initiation of asymmetric DR5-revGFP expression across gravity-stimulated roots. Strikingly, auxin transport and gravity response in pid-9 roots are unaffected by staurosporine treatment at the dose that phenocopies the pid-9 mutation. This result is consistent with the hypothesis that PID kinase is a primary target of staurosporine inhibition in basipetal IAA transport and root gravitropism. It is possible that additional kinases are affected by staurosporine treatment, especially at higher doses. PID is part of a clade of AGC kinases that are expressed in roots and have effects on root growth (e.g. WAG1 and WAG2; Santner and Watson, 2006). However, the insensitivity of gravitropism to 0.1 μm staurosporine in the pid-9 mutant strongly argues that activity of PID kinase is crucial for the inhibitory effect of staurosporine at this dose. Furthermore, PID kinase appears to have a specific regulatory effect on subsets of IAA transport proteins, since acropetal transport and lateral root development are normal in pid seedlings. Consistent with these findings, a PID∷YFP marker is expressed in the epidermal and cortical cell layers (Michniewicz et al., 2007), in which PIN2 also is expressed (Müller et al., 1998) and in which basipetal IAA transport occurs. PID∷YFP is not expressed in the stele and endodermis, tissues that mediate acropetal IAA transport and express auxin transport proteins appropriate for this transport polarity, including PIN1 (Friml et al., 2004). Two other AGC3 kinase genes, WAG1 and WAG2, have expression domains similar to that of PID, and the WAG gene products have been shown to alter root waving but not root gravitropism (Santner and Watson, 2006). It is possible that overlapping functions of these kinases will be revealed only at higher staurosporine doses. Because of partial overlap between the regulatory A subunits of PP2A, low-dose cantharidin treatment mimics rcn1 phenotypes, while higher doses of cantharidin mimic rcn1 pp2aa2 and rcn1 pp2aa3 double mutants (Zhou et al., 2004; Shin et al., 2005). Altered root branching when PID is constitutively overexpressed may change the expression domain of PID, leading to changes in phosphorylation of carriers in cells beyond those in which PID is normally expressed (Christensen et al., 2000; Benjamins et al., 2001). In contrast, RCN1∷YFP is expressed in all tissues of the root, consistent with its role in regulating both acropetal and basipetal IAA transport polarities (Rashotte et al., 2001).
Second, we tested the hypothesis that gravitropism is reciprocally regulated by PID kinase and RCN1-dependent PP2A activities. Support for this model includes the ability of cantharidin treatment to reduce root gravitropism in the wild type and to enhance gravitropism in pid-9. Similarly, staurosporine reduces root gravitropism in the wild type but enhances curvature in rcn1 roots. Furthermore, pid-9 partially suppresses the gravitropism defect of rcn1 seedlings. This finding parallels the report of Michniewicz et al. (2007), which indicates that the meristem collapse and severe elongation defect of an rcn1 pp2aa3 double mutant is partially reversed by a pid mutation. Taken together, these results support the model of reciprocal regulation by a coordinated kinase/phosphatase pair that functions in Arabidopsis roots.
Previous experiments have shown that inhibition of PP2A activity enhanced basipetal auxin transport, consistent with the model of reciprocal regulation (Rashotte et al., 2001). Paradoxically, both enhanced transport in rcn1 and cantharidin-treated seedlings and reduced transport in pid-9 and staurosporine-treated seedlings are accompanied by reduced gravitropic bending. The DR5-revGFP expression patterns we observed (Fig. 3) suggest that formation of auxin asymmetries in gravity-stimulated roots is delayed via different mechanisms in roots with reduced kinase or phosphatase activity, consistent with our model (Fig. 1). Reduced kinase activity reduces basipetal transport and limits auxin movement to the lower side. In contrast, reduced PP2A/phosphatase activity leads to enhanced auxin transport into epidermal cells: after reorientation relative to the gravity vector, the auxin depletion required for asymmetric growth and reduced DR5-revGFP expression occurs only slowly. Consistent with this model, the auxin transport inhibitor NPA was able to reverse the inhibitory effects of the rcn1 mutation, cantharidin treatment (Rashotte et al., 2001), or PID overexpression (Benjamins et al., 2001) on gravity response in roots.
What are the targets of kinase regulation in gravitropism and auxin transport? Here, we have shown that staurosporine does not further reduce auxin transport in a pin2 mutant, consistent with PIN2 being a major target of staurosporine action. Although pin2 mutants are completely agravitropic, pid-9 mutant seedlings do respond to gravity, exhibiting a delayed response that is statistically significant at all time points. In fact, the gravitropism defect of pid-9 is of greater magnitude than that of some other gravitropic mutants, such as pin3 (Harrison and Masson, 2007). These results suggest that PID kinase promotes and enhances root gravitropism but is not absolutely required, while pin2 is essential for the response. Similarly, in PID overexpression lines, root tip collapse has been reported in the wild type but not in a pin2 mutant allele (Friml et al., 2004). A recent report shows that a His-tagged PID protein can phosphorylate a peptide derived from the hydrophilic (intracellular) loop of PIN2 in vitro, providing biochemical evidence that supports direct action of PID kinase on PIN2 (Michniewicz et al., 2007).
The reciprocal action of PID and RCN1 in the regulation of gravitropism suggests that they might act on the same target. However, we and others had previously asked whether RCN1 enhanced auxin transport by acting through PIN2 and did not find evidence of PIN2 as a target using genetic approaches (Rashotte et al., 2001; Shin et al., 2005; Michniewicz et al., 2007). Basipetal IAA transport measurements of cantharidin-treated pin2 and aux1 mutant alleles and an rcn1 eir1-1(pin2) double mutant suggested that phosphatase inhibition increases transport by a mechanism that is not dependent on either PIN2 or AUX1 (Rashotte et al., 2001). Additionally, no differences in the localization of the PIN2/AGR1/EIR1 protein were reported in rcn1 root tips (Shin et al., 2005), although an rcn1 pp2aa3 double mutant exhibits several root developmental defects and altered PIN2 accumulation in cortical but not epidermal cells (Michniewicz et al., 2007). Overexpression of RCN1 did not increase the dephosphorylation of a PIN2-derived peptide, but phosphorylation of the PIN2 peptide in protein extracts from rcn1 pp2aa2 and rcn1 pp2aa3 double mutants was increased relative to that observed in extracts from wild-type or pid plants (Michniewicz et al., 2007). While these results suggest that severely PP2A-deficient seedlings may dephosphorylate PIN2 less efficiently than wild-type seedlings, they do not account for the PIN2-independent increase in basipetal transport and the apparently normal localization of PIN2 observed in the rcn1 single mutant (Rashotte et al., 2001; Shin et al., 2005). These conflicting results might be the result of compensatory changes in the expression of other PIN genes in the pin2 mutant (Vieten et al., 2005). In pin2 roots, the PIN1 expression domain expands outward from the central cylinder, into cortical and epidermal cells that normally express PIN2 but not PIN1 (Vieten et al., 2005). RCN1 regulates both acropetal and basipetal IAA transport polarities (Rashotte et al., 2001), which are believed to be mediated by PIN1 and PIN2, respectively, suggesting that PP2A may regulate the function of both PIN1 and PIN2 proteins in roots. Our data showing loss of staurosporine sensitivity in the pin2 mutant suggest that compensatory basipetal transport functions of PIN1 in pin2 mutants would be PID kinase independent. Additionally, a recent report showed that rcn1 pgp1 pgp19 seedlings exhibit agravitropic root growth that is more severe than the agravitropic phenotype of either rcn1 or the pgp1 pgp19 double mutant (Mravec et al., 2008), consistent with the ABCB transporters ABCB1/PGP1 and ABCB19/PGP19 being targets of RCN1 dephosphorylation.
Finally, we examined the effect of altered kinase activity on the localization and accumulation of the native PIN2 protein as well as PIN2∷GFP and PIN1∷GFP fusion proteins. Staurosporine treatment of PIN2∷GFP consistently led to altered accumulation of this fusion protein in endomembrane compartments, with the pid-9 mutant leading to similar alterations in accumulation of PIN2 protein as detected by immunofluorescence. Neither staurosporine treatment nor the pid-9 mutation altered the polar localization of PIN2 or PIN2∷GFP, respectively. Staurosporine had no effect on the localization of PIN1∷GFP and did not cause any endomembrane accumulation of this reporter. In conjunction with the normal acropetal auxin transport and root development observed in pid-9 mutant seedlings (Table I) and the lack of overlap in PID and PIN1 expression patterns (Michniewicz et al., 2007), these data suggest that PIN1 in roots is not a major target of PID action, although PID in shoots appears to regulate PIN1-dependent auxin transport and inflorescence morphology (Bennett et al., 1995).
Together, these results indicate that PID kinase regulates basipetal IAA transport and root gravitropism in Arabidopsis roots. The ability of staurosporine to produce a phenocopy of the pid-9 mutation in wild-type roots and the absence of additional phenotypes in staurosporine-treated pid-9 seedlings support this conclusion. PIN2 is likely to be a phosphorylation target for PID-mediated control of root gravitropism. We also provide evidence for reciprocal regulation of the gravity response by PID kinase and the RCN1-PP2A complex, although the target for PP2A-mediated dephosphorylation remains unclear. Staurosporine and the pid-9 mutation lead to accumulation of PIN2 and PIN2∷GFP in endomembrane structures, consistent with a phosphorylation mechanism controlling the membrane targeting of auxin transport proteins, but without a loss or reversal of PIN2 membrane asymmetry.
MATERIALS AND METHODS
Chemicals
Staurosporine was purchased from Calbiochem or Sigma. NPA was purchased from Chemical Services. Absolute ethanol was purchased from McCormick Distilling. [3H]IAA (26 and 25 Ci mmol−1) was purchased from Amersham or American Radiolabeled Chemicals. Glufosinate ammonium was purchased from Crescent Chemicals. ScintiVerse scintillation fluid and Triton X-100 were purchased from Fisher Scientific. All other chemicals were purchased from Sigma.
Seed Germination and Plant Growth
Wild-type Arabidopsis (Arabidopsis thaliana ecotypes Ws and Col) seeds were obtained from the Arabidopsis Biological Resource Center (Ohio State University). The pid-9 seeds were generously provided by Sioux Christensen. Transgenic PIN1∷GFP seeds were generously provided by Markus Heisler (ecotype Landsberg erecta) and Jiri Friml (ecotype Col; Benkova et al., 2003; Heisler et al., 2005). PIN2∷GFP-transformed eir1-1 seeds were provided by Jiri Friml (Xu and Scheres, 2005).
Seeds were surface sterilized with 20% bleach and 0.01% Triton X-100 for 5 min and with 95% ethanol for 1 to 5 min. Seeds were then washed five times in sterile distilled water and were sown on 9-cm petri plates containing sterile control medium (0.8% agar [Sigma type M, plant tissue culture], 1× Murashige and Skoog salts, pH 5.8 to 6.0, 1.5% Suc, 1 μg mL−1 thiamine, 1 μg mL−1 pyridoxine HCl, and 0.5 μg mL−1 nicotinic acid). Seeds were usually stratified for 2 d at 4°C in the dark. Plates were then placed vertically in racks in continuous fluorescent light (100 μmol m−2 s−1) at room temperature (22°C). Plates were not wrapped with Parafilm or surgical tape, in order to make sure that ethylene levels were not elevated.
Selection of pid-9 Mutants
The pid-9 mutant is sterile and therefore must be propagated as a heterozygote. Approximately 5% of homozygous pid-9 mutants will develop as tricots rather than the normal dicots, while very few wild-type seedlings exhibit this phenotype. Selecting tricots served as an effective screen for homozygous pid-9 seedlings. The pid-9 mutant was produced by T-DNA insertion mutagenesis (Christensen et al., 2000) and carries a BASTA resistance determinant in the T-DNA. For propagation, pid-9/+ heterozygotes (as well as homozygous mutants) were selected by growth on agar plates containing 10 mg L−1 glufosinate ammonium, which is the active ingredient in BASTA.
Analysis of Gravitropism
Root gravity response was measured using 5- and 7-d-old seedlings transferred to either control agar or agar supplemented with staurosporine and cantharidin at the indicated concentrations. Staurosporine and cantharidin were dissolved in dimethyl sulfoxide (DMSO) and were added to 50°C molten agar at 0.1 to 0.3 and 10 μm concentrations, respectively, and poured into petri dishes. The final DMSO concentration was 0.015% or less in agar medium. For each experiment, these compounds were added to agar immediately before use to minimize their breakdown. After 12 to 24 h of vertical growth, plates were reoriented by 90°. Pictures of plants were taken at specific time points after reorientation using a digital camera. The angle of root tip curvature and root elongation after reorientation were measured by Adobe Photoshop (version 10.0).
Auxin Transport Assays
Root basipetal transport was measured in two ways as described previously (Rashotte et al., 2000; Lewis and Muday, 2009). Seeds were germinated on medium and then 10 5-d-old seedlings were transferred to medium with or without 0.2 μm staurosporine. After 12 to 18 h, root tips were aligned. A 1-mm-thick agar cylinder (1% agar in 5 mm MES, pH 5.5) containing 100 nm [3H]IAA was applied to the root tips. Plates were then placed vertically in the dark for 5 h. A 1- to 2-mm section of the root tip was then excised and discarded. Radioactivity transported into the adjacent 5-mm section of root tip was quantified by scintillation counting using a Beckman LS 6500 scintillation counter for 2 min. A second assay was performed for measurement of auxin transport in the eir1-1/pin2 mutant. For this assay, agar containing 500 nm [3H]IAA was dispensed into small droplets, which were applied to individual seedlings. After a 2-h incubation assay, roots were excised and counted as described above.
Root acropetal auxin transport was measured as described previously (Reed et al., 1998; Lewis and Muday, 2009). Seedlings were transferred to fresh agar medium, and root-shoot junctions were aligned. A 1-mm-thick agar cylinder containing [3H]IAA was stretched along multiple seedlings in a position just below the root-shoot junction. The plates were then oriented vertically but with the shoot facing down in the dark for 18 h. For experiments comparing transport in Col and pid-9, a 5-mm section of the root tip was then excised, as these roots are equivalent in length.
To rule out the possibility that [3H]IAA may be transferred to the root tip by diffusion through the agar and not by active transport, an additional control for the acropetal transport assay was performed. For this experiment, seedlings were cut approximately 3 mm below the site of [3H]IAA application immediately after the IAA was applied. The radioactivity reaching the root tip in these seedlings was at background levels, indicating that [3H]IAA does not diffuse through the agar to reach the root tip.
Lateral Root Analyses
Six-day-old Col and pid-9 seedlings were transferred to agar plates. On day 10 after planting, the length of the primary root was measured and the number of lateral roots that had clearly emerged was counted with a dissecting microscope. The density of lateral roots was estimated by dividing the number of lateral roots by the length of the primary root in centimeters.
DR5∷revGFP
Five-day-old DR5∷revGFP seedlings were transferred to either control agar plates or plates with 0.2 μm staurosporine or 10 μm cantharidin. Following an 18-h incubation, the plates were reoriented 90° relative to gravity. Four hours after reorientation, DR5∷rev GFP was visualized using a Zeiss LSM510 fluorescence laser scanning confocal microscope with fluorescein isothiocyanate for frame filter (500–550 band pass) and an excitation of 488 nm using an argon laser and a 63× water objective. A pinhole setting of 2.5 airy units (AU) and consistent gain were used for all images. Images of the root tip and a second image overlapping with the first were captured. The composites of these two images are shown.
PIN2 Antibody Localization Studies
To localize PIN proteins, we used the protocol from Rahman et al. (2007) with minor modifications. Briefly, roots were fixed in PIPES buffer containing paraformaldehyde and calcium, permeabilized in 1% (v/v) Triton and cold methanol successively, and rehydrated in phosphate-buffered saline. Seedlings were incubated in 10% (v/v) DMSO and 3% (v/v) Nonidet P-40 in PME (50 mm PIPES, pH 7, 2 mm MgSO4, and 5 mm EGTA) for 1 h. After incubation, seedlings were rinsed with PME (3 × 5 min) and blocked in 1% (w/v) bovine serum albumin, 0.01% sodium azide in phosphate-buffered saline, and after 1 h, the blocking solution was replaced carefully with primary antibody. The primary antibody was anti-PIN2 (1:100 dilution; a gift of P. Masson, University of Wisconsin). The secondary antibody was Cy-3 goat anti-rabbit IgG (1:200; Jackson Immunoresearch; http://www.jacksonimmuno.com/). All imaging was done on a spinning-disc confocal microscope (Olympus BX-61 equipped with Bx-DSU; Olympus; http://www.olympus.com/) equipped with a 60× oil-immersion objective, with images captured at identical pinhole, gain, and laser intensity settings.
PIN1∷GFP, PIN2∷GFP, and RCN1-YFP Localization and Intensity
The GFP fluorescence from a PIN1∷GFP transgenic line was visualized using a Zeiss LSM510 fluorescence laser scanning confocal microscope using the argon laser for excitation at 488 nm. Four- to 6-d-old seedlings were transferred to plates containing 0.3 μm staurosporine or DMSO control for 12 to 18 h. Seedlings were then mounted on slides with 1× Murashige and Skoog salts and were examined immediately. A 63× water-immersion lens with a zoom of 0.7 to 2.3 was used to visualize PIN1∷GFP with a fluorescein isothiocyanate narrow band filter (505–530 of 500–550 band pass). A pinhole setting of 1 AU was used for PIN1∷GFP. Gain was kept constant for each experiment, with a range of 800 to 1,250 throughout all experiments.
PIN2-GFP seedlings were treated by transfer to fresh agar medium with or without addition of 0.2 μm staurosporine for 18 h before visualization. Roots were imaged on a spinning-disc confocal microscope (Olympus BX-61 equipped with Bx-DSU; Olympus; http://www.olympus.com/) equipped with a 60× oil-immersion objective.
RCN1-YFP fluorescence was visualized using a Zeiss LSM510 fluorescence laser scanning confocal microscope using an excitation wavelength of 514 nm. Pinhole was set at 1.5 to 2.5 AU, and images were taken using a 63× water immersion lens with a gain setting of 900 to 1,000.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: MDR1/ABCB19 (At3G28860), PIN1 (At1G73590), ABCB4/MDR4 (At2G47000), PIN2 (At5G57090), AUX1 (AtG2G38120), PIN3 (At1G70940), PID (At2G34650), and RCN1 (At1G25490).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effects of staurosporine on root gravity response and growth.
Supplemental Figure S2. Expression of genes encoding auxin transport proteins and their regulators.
Supplemental Figure S3. PIN2 antibody does not show membrane-localized signal in the eir1-1/pin2 mutant.
Supplemental Figure S4. PIN1∷GFP localization is unaffected by staurosporine treatment.
Supplemental Table S1. Statistical analysis of root gravitropic curvature in Col, Ws, pid-9, rcn1, rcn1 pid-9, and rcn1 PID.
Supplementary Material
Acknowledgments
We appreciate the generosity of Sioux Christensen, Jiri Friml, Marcus Heisler, and Jian Xu in sharing seeds, and Patrick Masson for sharing PIN2 antibody. Thoughtful comments on the manuscript by Daniel Lewis, Cassie Mattox, Josh Blakeslee, Kyle Skottke, Sangeeta Negi, and Heather Fairfield are gratefully acknowledged. We appreciate the microscopy assistance of Anita McCauley.
This work was supported by the National Aeronautics and Space Agency and the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service (grant nos. NAG2–1507 and 2006–35304–17311 to G.K.M.) and by the National Science Foundation (grant no. IOB0446039 to A.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Gloria K. Muday (muday@wfu.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128 4057–4067 [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602 [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273 948–950 [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR (1995) Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J 8 505–520 [Google Scholar]
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29 325–332 [DOI] [PubMed] [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN (2003) A gene expression map of the Arabidopsis root. Science 302 1956–1960 [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, Zhou HW, Heath JT, Skottke KR, Barrios JA, Liu SY, DeLong A (2008) Specificity of RCN1-mediated protein phosphatase 2A regulation in meristem organization and stress response in roots. Plant Physiol 146 539–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S (1998) Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol 116 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RJ, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH (1998) The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA 95 15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D (2000) Regulation of auxin response by the protein kinase PINOID. Cell 100 469–478 [DOI] [PubMed] [Google Scholar]
- DeLong A, Mockaitis K, Christensen S (2002) Protein phosphorylation in the delivery of and response to auxin signals. Plant Mol Biol 49 285–303 [PubMed] [Google Scholar]
- Deruère J, Jackson K, Garbers C, Söll D, DeLong A (1999) The RCN1-encoded A subunit of protein phosphatase 2A increases phosphatase activity in vivo. Plant J 20 389–399 [DOI] [PubMed] [Google Scholar]
- Friml J, Wiœniewska J, Benková E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415 806–809 [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, et al (2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306 862–865 [DOI] [PubMed] [Google Scholar]
- Galvan-Ampudia CS, Offringa R (2007) Plant evolution: AGC kinases tell the auxin tale. Trends Plant Sci 12 541–547 [DOI] [PubMed] [Google Scholar]
- Garbers C, DeLong A, Deruère J, Bernasconi P, Soll D (1996) A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J 15 2115–2124 [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, et al (2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44 179–194 [DOI] [PubMed] [Google Scholar]
- Harrison BR, Masson PH (2007) ARL2, ARG1 and PIN3 define a gravity signal transduction pathway in root statocytes. Plant J 53 380–392 [DOI] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15 1899–1911 [DOI] [PubMed] [Google Scholar]
- Lee SH, Cho HT (2006) PINOID positively regulates auxin efflux in Arabidopsis root hair cells and tobacco cells. Plant Cell 18 1604–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Miller ND, Splitt BL, Wu G, Spalding EP (2007) Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis multidrug resistance-like ABC transporter genes. Plant Cell 19 1838–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Muday GK (2009) Measurement of auxin transport in Arabidopsis thaliana. Nat Protocols 4 437–451 [DOI] [PubMed] [Google Scholar]
- Leyser O (2006) Dynamic integration of auxin transport and signalling. Curr Biol 16 R424–R433 [DOI] [PubMed] [Google Scholar]
- Lin R, Wang H (2005) Two homologous ATP-binding cassette transporter proteins, AtMDR1 and AtPGP1, regulate Arabidopsis photomorphogenesis and root development by mediating polar auxin transport. Plant Physiol 138 949–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M, Zago M, Abas L, Wijers D, Schweighofer A, Meskiene I, Heisler M, Ohno C, Zhang J, Huang F, et al (2007) Antagonistic regulation of PIN phosphorylation by PPSA and PINOID directs auxin flux. Cell 130 1044–1056 [DOI] [PubMed] [Google Scholar]
- Mravec J, Kubes M, Bielach A, Gaykova V, Petrasek J, Skupa P, Chand S, Benkova E, Zazimalova E, Friml J (2008) Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development 135 3345–3354 [DOI] [PubMed] [Google Scholar]
- Muday GK, Brady SR, Argueso C, Deruere J, Kieber JJ, DeLong A (2006) RCN1-regulated phosphatase activity and EIN2 modulate hypocotyl gravitropism by a mechanism that does not require ethylene signaling. Plant Physiol 141 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Sci 6 535–542 [DOI] [PubMed] [Google Scholar]
- Muday GK, Rahman A (2008) Auxin transport and the integration of gravitropic growth. In S Gilroy, P Masson, eds, Plant Tropisms. Blackwell Publishing, Oxford, pp 47–78
- Müller A, Guan C, Galweiler L, Tanzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17 6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jurgens G, Geldner N, et al (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435 1251–1256 [DOI] [PubMed] [Google Scholar]
- Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI (2007) Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J 50 514–528 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert HS, Offringa R (2008) Regulation of auxin transport polarity by AGC kinases. Curr Opin Plant Biol 11 495–502 [DOI] [PubMed] [Google Scholar]
- Santner AA, Watson JC (2006) The WAG1 and WAG2 protein kinases negatively regulate root waving in Arabidopsis. Plant J 45 752–764 [DOI] [PubMed] [Google Scholar]
- Shin H, Guo Z, Blancaflor EB, Masson PH, Chen R (2005) Complex regulation of Arabidopsis AGR1/PIN2-mediated root gravitropic response and basipetal auxin transport by cantharidin-sensitive protein phosphatases. Plant J 42 188–200 [DOI] [PubMed] [Google Scholar]
- Swarup R, Kargul J, Marchant A, Zadik D, Rahman A, Mills R, Yemm A, May S, Williams L, Millner P, et al (2004) Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16 3069–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka K, Blakeslee JJ, Titapiwatanakun B, Peer WA, Bandyopadhyay A, Makam SN, Lee OR, Richards EL, Murphy AS, Sato F, et al (2005) PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 17 2922–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi S, Ohwaki Y (1978) Transport of 14C-labeled indoleacetic acid in Vicia root segments. Plant Cell Physiol 19 1195–1206 [Google Scholar]
- Vieten A, Vanneste S, Wisniewska J, Benkova E, Benjamins R, Beeckman T, Luschnig C, Friml J (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132 4521–4531 [DOI] [PubMed] [Google Scholar]
- Xu J, Scheres B (2005) Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E (2006) High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol 16 1123–1127 [DOI] [PubMed] [Google Scholar]
- Zhou HW, Nussbaumer C, Chao Y, DeLong A (2004) Disparate roles for the regulatory A subunit isoforms in Arabidopsis protein phosphatase 2A. Plant Cell 16 709–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zourelidou M, Muller I, Willige BC, Nill C, Jikumaru Y, Li H, Schwechheimer C (2009) The polarly localized D6 PROTEIN KINASE is required for efficient auxin transport in Arabidopsis thaliana. Development 136 627–636 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.