Abstract
Low inorganic phosphate (Pi) availability triggers an array of spatiotemporal adaptive responses in Arabidopsis (Arabidopsis thaliana). There are several reports on the effects of Pi deprivation on the root system that have been attributed to different growth conditions and/or inherent genetic variability. Here we show that the gelling agents, largely treated as inert components, significantly affect morphophysiological and molecular responses of the seedlings to deficiencies of Pi and other nutrients. Inductively coupled plasma-mass spectroscopy analysis revealed variable levels of elemental contaminants not only in different types of agar but also in different batches of the same agar. Fluctuating levels of phosphorus (P) in different agar types affected the growth of the seedlings under Pi-deprivation condition. Since P interacts with other elements such as iron, potassium, and sulfur, contaminating effects of these elements in different agars were also evident in the Pi-deficiency-induced morphological and molecular responses. P by itself acted as a contaminant when studying the responses of Arabidopsis to micronutrient (iron and zinc) deficiencies. Together, these results highlighted the likelihood of erroneous interpretations that could be easily drawn from nutrition studies when different agars have been used. As an alternative, we demonstrate the efficacy of a sterile and contamination-free hydroponic system for dissecting morphophysiological and molecular responses of Arabidopsis to different nutrient deficiencies.
Plant development is a dynamic and complex process often subject to biotic and abiotic stresses. On encountering nutrient stress, plants undergo an array of adaptive changes. Phosphorus (P) is an essential plant macronutrient, but low availability of soluble inorganic phosphate (Pi) is common in many natural and agricultural ecosystems (Marschner, 1995; Schachtman and Shin, 2007). Arabidopsis (Arabidopsis thaliana) is used as a model system to answer many of the fundamental questions related to responses of plants to Pi stress. To minimize variation caused by macroenvironmental and microenvironmental conditions, Pi-deficiency responses traditionally have been studied by growing Arabidopsis aseptically in continuously shaken liquid culture or on solid medium in petri plates. Although the use of liquid culture is a convenient technique for generating bulk tissue for assaying biochemical and molecular responses to Pi deprivation (Karthikeyan et al., 2002; Misson et al., 2005), continuous swirling to provide aeration makes the study of root hair development and root system architecture (RSA) all but impossible. Therefore, growth of seedlings on vertically oriented petri plates containing solidified nutrient medium with sufficient (1.0–2.5 mm) or limiting (0–10 μm) Pi would be ideal for morphophysiological and molecular studies (López-Bucio et al., 2002; Jain et al., 2007a).
Agar and phytagel, extracted from red algae and bacteria, respectively, are commonly used gelling agents (http://www.sigmaaldrich.com/sigma/product). Ideally, a gelling agent would be an inert constituent of the plant growth medium. Studies show, however, that the agent itself causes variations in plant growth responses on otherwise identical nutrient media (Nowak and Asiedu, 1992; Scholten and Pierik, 1998a, 1998b; Beruto et al., 1999). Differences in the performance of gelling agents have been attributed to their variable physiochemical characteristics, such as nutrient diffusion rate, elemental and organic impurities, and gel strength (Debergh, 1983; Nairn et al., 1995). While the elemental contaminants in gelling agents used with nutrient-rich media may not significantly affect the growth of Arabidopsis seedlings, they could make a significant difference under nutrient-deficient conditions. It has been shown that a low concentration of Pi (25 μm) in the medium negated the inhibitory effect of Pi deprivation on shoot growth (López-Bucio et al., 2002). Furthermore, the Pi-deficiency response is also influenced by the interaction of Pi with other elements and compounds. For instance, primary roots of Pi-starved seedlings failed to enter the determinant growth phase, a hallmark of the Pi-deficiency response, when the medium was also deprived of iron (Fe; Sánchez-Calderón et al., 2005; Svistoonoff et al., 2007; Ward et al., 2008). Incidents of cross talk between P and other macroelements and microelements (potassium [K] and zinc [Zn]) have also been reported in crop species (Huang et al., 2000; Wang et al., 2002). Therefore, gel medium contaminants other than P could have a significant bearing on overall Pi-deficiency responses.
Inherent genetic variability of Arabidopsis ecotypes is thought to be the likely cause of the large variations noted in morphological responses during Pi-deprivation studies (Chevalier et al., 2003). Some attribute the divergent reports to differences in experimental design (Al-Ghazi et al., 2003; Reymond et al., 2006). For example, photosynthetically active radiation ranging from 30 to 300 μmol m−2 s−1 has been used to grow Arabidopsis (Williamson et al., 2001; López-Bucio et al., 2002; Al-Ghazi et al., 2003; Chevalier et al., 2003; Jain et al., 2007a). Since photosynthates play a pivotal role in the regulation of the Pi-starvation responses (Liu et al., 2005; Jain et al., 2007a; Karthikeyan et al., 2007), variations in photosynthetically active radiation could be a contributory factor to these discrepancies.
To further address disparities in Pi-starvation responses, we investigated the effects of the elemental composition of different batches and types of agar on morphophysiological and molecular responses of Pi-deprived Arabidopsis seedlings. The growth responses of Pi-deprived plants were correlated with P contamination levels of the gelling medium. Also, the effects of Fe contamination in agar were demonstrated under P−Fe− condition on morphological and molecular responses of the seedlings. This study also highlights the fact that P acts as a contaminant in influencing the responses of seedlings to micronutrient (Fe and Zn) deficiencies.
RESULTS
Agar Type Affects Short-Term Pi-Deficiency Responses on Root Traits
Pi deficiency triggers a significant increase in root hair length and density and inhibition of primary root elongation in plants (Gahoonia and Nielsen, 1997; Ma et al., 2001; López-Bucio et al., 2002; Sánchez-Calderón et al., 2005; Jain et al., 2007a). Therefore, we evaluated the effects of short-term Pi deprivation (2 d) on seedlings grown on Pi-deficient medium prepared with four different types of agar: Sigma A-1, Sigma A-2, Sigma E, and Caisson (Fig. 1). There was a significant (P < 0.05) variation in the primary root length of the seedlings grown initially (pretreatment) for 5 d on nutrient-rich medium (one-half-strength Murashige and Skoog [MS] medium + 1.5% [w/v] Suc) prepared with different agars (Fig. 1, A and B). Herein, we refer to nutrient-rich medium as P+. Subsequently, seedlings were transferred to Pi-deprived medium made with the corresponding agar types for 2 d. A similar trend was also observed in root hair emergence in a 5-mm section from the tip of the primary roots (Fig. 1, C and D). Despite a significant reduction in primary root elongation, only a few root hairs were noticed in a 5-mm root tip region of seedlings grown on Caisson agar, which was an unusual response to Pi deprivation. These results clearly demonstrate that different agars impact primary root growth and root hair development under Pi deficiency.
Figure 1.
Pi-deficiency responses on primary root growth and root hair development of Arabidopsis grown on different types of agar. Wild-type (Col-0) seeds were initially grown on one-half-strength MS with different agar types for 5 d and then subsequently transferred to the respective types containing medium deprived of Pi (0 μm) on vertically oriented petri plates for 2 d. A, Mark on the primary root indicates the length achieved before transferring to Pi-deprived medium. C, Development of root hairs in 5-mm sections from the primary root tip. Photographs in A and C are representative of 15 and 10 seedlings each, respectively, grown on different agar types. B and D, Increase in primary root length after transfer to Pi-deprived medium (n = 15; B) and number of root hairs (n = 10; D). Values are means ± se. Different letters on the histograms indicate that the means differ significantly (P < 0.05).
To further determine whether pretreatment on different agar types has any effect on Pi-deficiency-mediated root hair development, seedlings were grown initially for 5 d on P+ medium prepared with Sigma A-2 and Caisson agars and then transferred to Pi-deprived medium (P−) made with Sigma A-2 agar for 2 d. Interestingly, the seedlings grown on Caisson P+/Sigma A-2 P− developed significantly (P < 0.05) fewer (average of 12.75 ± 3.53 [se]) root hairs in a 5-mm section from the tip of the primary roots compared with those grown on Sigma A-2 P+/ Sigma A-2 P− (average of 92.70 ± 10.84 [se]). These data clearly highlight the influence of the agar type used during pretreatment on the subsequent response of the seedling grown on Pi-deprived medium prepared with a different agar type.
Morphophysiological and Molecular Traits Are Influenced by Agar Types during Long-Term Pi-Deficiency Treatment
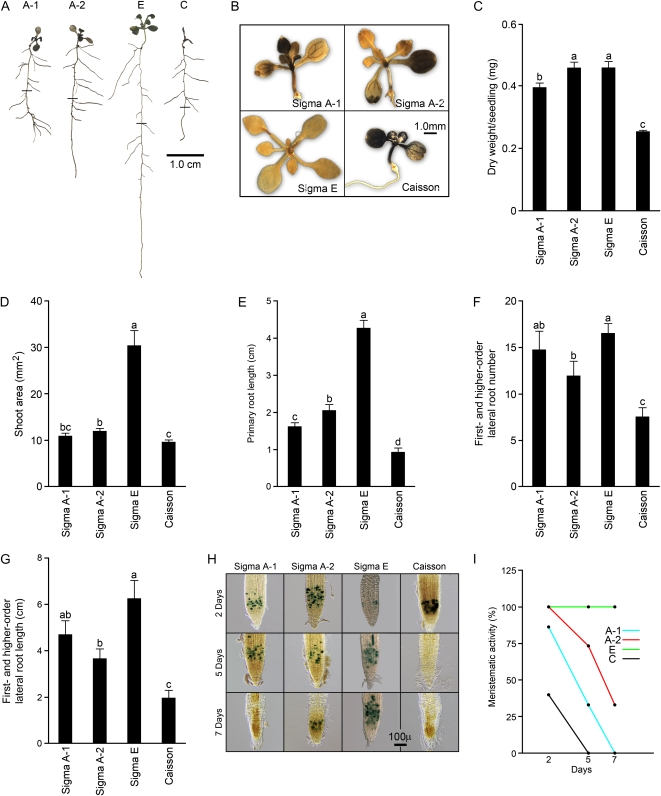
Since some morphophysiological and molecular responses manifest only upon prolonged Pi stress (Misson et al., 2005; Jain et al., 2007b), seedlings grown on different agar types were starved of Pi for 7 d (Fig. 2). The RSA of the seedlings was different on each agar (Fig. 2A). There was significant accumulation of starch in the shoots of the seedlings grown on Sigma A-1, Sigma A-2, and Caisson agars but almost no accumulation on Sigma E (Fig. 2B). Interestingly, significant (P < 0.05) differences between the seedlings grown on Sigma E and Caisson were observed for dry weight, shoot area, primary root length, and number and length of first-order and higher order lateral roots (Fig. 2, C–G). Seedlings raised on Sigma E were relatively robust, whereas those raised on Caisson appeared highly stressed. Although the metrics of the seedlings grown on Sigma A-1 and Sigma A-2 were comparable with respect to shoot area and number and length of first-order and higher order lateral roots (Fig. 2, D, F, and G), the values for dry weight and primary root length were significantly (P < 0.05) higher in the seedlings grown on Sigma A-2 (Fig. 2, C and E). Among the Pi-responsive traits examined, primary root elongation showed the most significant (P < 0.05) differences in seedlings grown on the four different agars (Fig. 2E). Since premature cell differentiation is a specific response to localized Pi deficiency (Nacry et al., 2005; Sánchez-Calderón et al., 2005; Jain et al., 2007a), we examined the meristematic activity of the primary root of transgenic plants expressing the cell cycle marker CycB1;1∷uidA (Ferreira et al., 1994) grown on different agar types (Fig. 2, H and I). After growth intervals of 2, 5, and 7 d, 15 seedlings from different agar types were monitored for GUS activity; the data are presented as percentage expression of CycB1;1∷uidA in the primary root tip (Fig. 2I). After 2 d of Pi starvation, 100% of the seedlings grown on Sigma A-2 and Sigma E, 87% grown on Sigma A-1, and 40% grown on Caisson showed CycB1;1∷uidA expression. By day 5, none of the Caisson seedlings showed expression, indicating a complete loss of meristematic activity. A significant decline in GUS expression was also evident in the seedlings grown on Sigma A-1 and Sigma A-2. Finally, by day 7, 100% of the seedlings grown on Sigma A-1 and 66% grown on Sigma A-2 entered the determinate growth phase, whereas none of the seedlings grown on Sigma E showed any reduction in CycB1;1∷uidA expression. These data thus provided empirical evidence that agar type significantly influences long-term Pi-starvation responses.
Figure 2.
Long-term Pi-deficiency-mediated morphophysiological responses of Arabidopsis grown on different types of agar. Wild-type (Col-0) seeds were grown on different agar types, as described in the legend to Figure 1, on P− medium for 7 d. A, Lateral roots were spread to reveal architectural details. RSA traits are representative of 15 seedlings each for different agar types. Mark on the primary root indicates the length achieved before transferring to P− medium. B, Shoots were stained with iodine solution for detection of starch accumulation. C to G, Dry weight (n = 6 replicates of a pool of 10 seedlings each; C), shoot area (n = 6; D), increase in primary root length after transfer from one-half-strength MS to P− (n = 15; E), and number (F) and length (G) of first-order and higher order lateral roots (n = 15). Values in C to G are means ± se. Different letters on the histograms indicate that the means differ significantly (P < 0.05). H, Histochemical GUS staining of primary root tips of CycB1;1∷uidA seedlings grown for the indicated time intervals on different agar types. GUS-stained seedlings were observed with a compound microscope. Photographs are representative of 12 to 15 seedlings. I, Primary roots of CycB1;1∷uidA seedlings grown on Sigma A-1 (A-1), Sigma A-2 (A-2), Sigma E (E), and Caisson (C) agar types showing meristematic activity (%). [See online article for color version of this figure.]
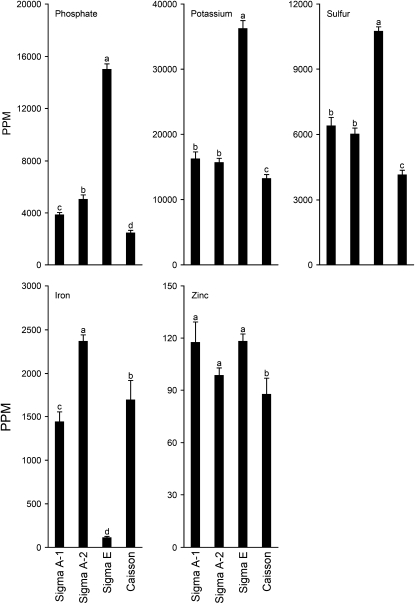
Pi-deficiency-induced reduction in the total Pi content in leaves and roots affects the concentrations of macroelements and microelements such as K, sulfur (S), Fe, and Zn (Misson et al., 2005). Since we observed varied Pi-deficiency responses for the seedlings grown on different agar types, tissue nutrient composition was analyzed by inductively coupled plasma-mass spectroscopy (ICP-MS; Fig. 3). The levels of K and S in seedlings grown on Sigma A-1 and Sigma A-2 and those of Zn grown on Sigma A-1, Sigma A-2, and Sigma E were comparable. However, there were significant (P < 0.05) variations in the levels of P and Fe in Sigma A-1 and Sigma A-2, representing two different lots of the same agar type (A1296). Furthermore, an inverse relation between the levels of P and Fe in seedlings grown on different agar types was apparent. Levels of P, K, S, and Fe in the seedlings grown on Sigma E were significantly (P < 0.05) different from those grown on the other three agars. It was interesting to observe a toxic level of P (>10,000 ppm) content in Pi-deprived seedlings grown on Sigma E. The levels of other macronutrients (calcium [Ca], magnesium [Mg], sodium [Na]) and micronutrients (boron [B], copper [Cu], manganese [Mn], molybdenum [Mo], cobalt [Co]) were different in seedlings grown on different agar types (Supplemental Table S1). These data showed that the agar used in the media significantly impacts the nutrient composition of seedlings under Pi deficiency.
Figure 3.
ICP-MS analysis of the Pi-deprived Arabidopsis grown on different agar types. Wild-type (Col-0) seedlings were grown under Pi-deficient condition, as described in the legend to Figure 2, for 7 d. Data are presented for the ICP-MS analysis of the whole seedling (n = 6 replicates of a pool of 10 seedlings each). Values are means ± se. Different letters on the histograms indicate that the means differ significantly (P < 0.05).
Earlier work has shown that plant Pi status leads to systemic regulation of Pi-starvation-induced (PSI) genes (Burleigh and Harrison, 1999). Further studies have also shown the effects of other mineral nutrients, such as K, Fe, and Zn, on the regulation of PSI genes (Huang et al., 2000; Wang et al., 2002). Therefore, we used real-time PCR to quantify the effects of different agar types on expression of the PSI genes (IPS1, RNS1, PLDZ2, Pht1;4, and At4) in roots (Fig. 4). Expression levels of PSI genes are assigned values based on Sigma A-1 root, which was normalized to 1. Real-time PCR analysis revealed a significant reduction in the relative expression of IPS1, RNS1, PLDZ2, Pht1;4, and At4 in the roots of the seedlings grown on Sigma E, while the relative values for these genes were significantly higher for the roots of seedlings grown on Caisson agar. Although the relative expression values of Pht1;4, At4, and PLDZ2 were comparable for Sigma A-1 and Sigma A-2 seedlings, the values of IPS1 and RNS1 were significantly lower in the latter. Since Pi deficiency leads to an elevated concentration of Fe (Misson et al., 2005), we evaluated the expression of an iron transporter, IRT1. The relative expression of IRT1 increased significantly in the roots of Pi-deficient seedlings grown on Sigma E (25-fold) and Caisson (6-fold) agar media. The variable molecular responses of the Pi-deprived seedlings grown on different agars were consistent with observed morphophysiological traits (Figs. 1–3). The data thus highlight the significant influence of agar type on both locally (RSA traits) and systemically (PSI genes) mediated Pi-deficiency responses in Arabidopsis.
Figure 4.
Effects of agar types on the molecular responses of Pi-deprived Arabidopsis. Real-time PCR analysis of the relative expression levels of PSI genes in the roots of wild-type (Col-0) seedlings grown under Pi-deficient condition, as described in the legend to Figure 2, for 7 d. ACT2 was used as an internal control. Data presented are means of six technical replicates ± se.
Variable Elemental Levels in Different Agars Affect Pi-Starvation Responses
Since seedlings grown on Pi-deficient medium prepared with different lots, different types, and different sources of agar showed different morphophysiological and molecular traits (Figs. 1–4), we measured the levels of macroelements and microelements in them (Fig. 5). The levels of P, K, and S were significantly (P < 0.05) different in the four agar types. The high concentration of P in Sigma E (843 μm) may explain why seedlings grown under Pi-deficient condition on this agar were comparable to P+ plants. Although Sigma A-1 and Sigma A-2 are different lots of the same agar (A1296), the level of P in Sigma A-2 is almost twice that of Sigma A-1.The increased P level in Sigma A-2 was clearly reflected in the altered Pi-deficiency responses of the seedlings (Figs. 1–4). When Sigma A-1 was used as the gelling agent, the classic Pi-deficiency responses in seedlings were observed when total Pi concentration of the medium was kept at or below 15 μm. Although Caisson agar contains three times as much Fe and half the amount of P as Sigma A-1, the unexpected responses of Pi-deprived seedlings grown on Caisson agar suggest that other elements in the gelling medium may interact with P to alter the amount of Pi available to the plant (Figs. 1–4). In addition, large variations in the levels of other macroelements (Ca, Mg, and Na) and microelements (Co, Cu, Mo, B, and Mn) in these agar types were observed (Supplemental Table S1). Together, these data suggested that the fluctuation in levels of P in different agars, in conjunction with other interacting elements, could contribute significantly to variations in the Pi-starvation responses.
Figure 5.
Variable elemental contaminants in different batches and types of agar. Data are presented for the ICP-MS analysis of different agar types (Sigma A-1, Sigma A-2, Sigma E, and Caisson). Values are means ± se (n = 6). Different letters on the histograms indicate that the means differ significantly (P < 0.05). Values in the histograms indicate concentrations (μm) in 1.2% agar.
We investigated whether manipulation of elemental levels in agar by reducing the percentage of agar would impose Pi-starvation responses (Fig. 6). Since Sigma A-1 agar, with a P level of 15 μm, showed normal Pi-starvation responses, we lowered the Sigma A-2 agar concentration from 1.2% to 0.6%, which in turn decreased the level of P from 30 to 15 μm. To eliminate the effect of gel viscosity, 0.6% and 1.2% Sigma E agar were used as controls. Inhibition of growth and loss of meristematic activity in the primary root were monitored. Pi-deprived seedlings grown on 0.6% Sigma A-2 agar showed significant inhibition in primary root growth (Fig. 6, A and C), which was due to the loss of meristematic activity (Fig. 6B). This suggests that higher P in the Sigma A-2 medium is responsible for the attenuated Pi-starvation response. Interestingly, lateral root number and length (data not shown) as well as primary root length of the seedlings grown on 0.6% Sigma E increased significantly (P < 0.05) compared with those grown on 1.2% agar (Fig. 6, A and C). Improved root growth of the Pi-deprived seedlings on lower density (0.6%) Sigma E agar could possibly be due to either increased availability of the nutrients to the roots or attenuated toxic effects of contaminating P. However, meristematic activity was normal at both Sigma E agar concentrations (Fig. 6B). These results demonstrate that nutrient contamination in agar has a significant effect on the Pi-starvation responses. Overall, the data obtained to this point highlight the practical limitations in identifying the best gelling medium for studying plant nutrient responses.
Figure 6.
Low-percentage (0.6%) agar altered Pi-starvation responses of Arabidopsis. Transgenic CycB1;1∷uidA Arabidopsis seedlings were initially grown on one-half-strength MS with 0.6% and 1.2% each of Sigma A-2 and Sigma E and then transferred to the respective types containing P− medium on vertically oriented petri plates for 7 d. A, RSA traits are representative of 15 seedlings each for different treatments. Mark on the primary root indicates the length achieved before transferring to Pi-deprived medium. B, Representative photographs of 12 to 15 histochemical GUS-stained primary root tips of CycB1;1∷uidA seedlings. C, Increase in primary root length after transfer from one-half-strength MS to P− (n = 15). Values are means ± se. Different letters on the histograms indicate that the means differ significantly (P < 0.05). [See online article for color version of this figure.]
Elemental Contamination in the Agar Influences the Responses to P−Fe−
Since different agar types have variable levels of Pi and Fe (Fig. 5), we examined the influence of these nutrients on the responses of seedlings grown under P−Fe− condition (Fig. 7). Consistent with earlier studies, Pi deficiency together with Fe deficiency caused a significant increase in the primary root length of seedlings grown on Sigma A-1, Sigma A-2, and Caisson agars (Fig. 7, A and B). The primary root lengths of seedlings grown on Sigma A agar were significantly (P < 0.05) longer than those grown on Caisson and Sigma E. The variable effects of different agar types were also reflected in the number and length of first-order and higher order lateral roots (Fig. 7, C and D). To further explore the consequences of different agar types on the molecular responses of the P−Fe− seedlings, we used real-time PCR to evaluate the relative expression profiles of Pi (Pht1;4) and Fe (IRT1) high-affinity transporters (Vert et al., 2002; Misson et al., 2005). With the expression values of Pht1;4 and IRT1 in the roots of P−Fe− seedlings grown on Sigma A-1 assigned at 1, the relative expression of these genes in the roots of the seedlings grown on other agar types was compared (Fig. 7, E and F). The relative expression values of Pht1;4 and IRT1 in P−Fe− seedlings grown on Sigma A-1 and Sigma A-2 were comparable. Pht1;4 relative expression values decreased, while IRT1 values increased for seedlings grown on Sigma E; the opposite response was seen in seedlings grown on Caisson agar. These results demonstrate the effect of elemental composition on the morphological and molecular responses of seedlings grown under P−Fe− condition.
Figure 7.
Responses of Arabidopsis to P−Fe− on different agar types. Wild-type (Col-0) seeds, grown on one-half-strength MS with different agar types for 5 d, were transferred to the respective types containing P−Fe− medium on vertically oriented petri plates for 7 d. A, RSA traits are representative of 15 seedlings each for different treatments. Mark on the primary root indicates the length achieved before transferring to P−Fe− medium. B to D, Increase in primary root length after transfer from one-half-strength MS to P−Fe− medium (n = 15; B) and number (C) and length (D) of first-order and higher order lateral roots (n = 10). Values in B to D are means ± se. Different letters on the histograms indicate that the means differ significantly (P < 0.05). E and F, Real-time PCR analysis of the relative expression levels of Pht1;4 (E) and IRT1 (F) in the roots of Pi−Fe− seedlings. ACT2 was used as an internal control. Data presented are means of six technical replicates ± se. [See online article for color version of this figure.]
Elemental Contaminants in the Agar Affect Responses to Micronutrient Deficiencies
In nutrient media, Fe (10−100 μm) and Zn (10 μm) are normally used at low concentrations (Grotz et al., 1998; Thimm et al., 2001; López-Bucio et al., 2002; Hirsch et al., 2006). Among the micronutrients, Zn and Fe are known to interact with P. Therefore, we hypothesized that the presence of high levels of P and other interacting elements in the gelling medium could have an effect on the morphological and molecular responses of seedlings starved for Fe and Zn (Fig. 8).
Figure 8.
Responses of Arabidopsis starved of Fe and Zn on different agar types. Arabidopsis seedlings were grown on one-half-strength MS with different agar types and then transferred to the respective types containing Fe- and Zn-deficient media on vertically oriented petri plates for 7 d. A, RSA traits are representative of 15 seedlings each for different treatments. Mark on the primary root indicates the length achieved before transferring to Zn− and Fe− media. B to D, Increase in primary root length after transfer from one-half-strength MS to Zn− and Fe− media (n = 15; B) and number (C) and length (D) of first-order and higher order lateral roots (n = 5). Values in B to D are means ± se. Different letters on the histograms indicate that the means differ significantly (P < 0.05). E and F, Real-time PCR analysis of the relative expression levels of ZIP3 and ZIP9 in Zn− roots (E) and IRT1 and FER1 in Fe− roots (F). ACT2 was used as an internal control. Data presented are means of six technical replicates ± se. [See online article for color version of this figure.]
Under Fe- and Zn-deficiency conditions, different RSA traits (primary root length, number and length of first-order and higher order lateral roots) did not show significant (P < 0.05) differences between the seedlings grown on Sigma A-1 and Sigma A-2 agars (Fig. 8, A–D). The only notable exception was the longer primary root length of the Zn-deprived seedlings grown on Sigma A-1 compared with those grown on Sigma A-2. On the contrary, these RSA traits were significantly (P < 0.05) lower for the Zn- and Fe-deprived seedlings grown on either Sigma E or Caisson agar. These differential responses of the seedlings to Zn and Fe deficiencies on different agar types, particularly on Sigma E and Caisson, could possibly be attributed to high levels of macroelemental (P and K) and microelemental (Fe) contaminants (Fig. 5). We used real-time PCR to profile the relative expression of Zn- and Fe-responsive genes in the roots of seedlings grown on different agar types (Fig. 8, E and F). About 90- and 200-fold increases in the expression of ZIP3 and ZIP9, respectively, were observed in response to Zn deficiency (A. Jain and R. Meagher, unpublished data). Based on these data, two ZIPs were chosen as candidates for relative gene expression studies. The expression values of ZIPs in the roots of the seedlings grown on Sigma A-1 were assigned as 1. No significant differences were observed in the relative expression of ZIP3 and ZIP9 in the roots of plants grown on Sigma A-1 and Sigma A-2. A marginal (less than 2-fold) increase was observed in the relative expression of ZIP3 in the roots of the seedlings grown on Caisson, but 3- and 12-fold increases in relative expression, respectively, were seen in seedlings grown on Sigma E. Elevated expression of ZIPs in seedlings grown on Sigma E could be due to a lack of Zn and an abundance of P in the gelling medium (Fig. 5). In addition, we evaluated the relative expression of IRT1 and FER1 (Vert et al., 2002; Misson et al., 2005) in Fe-deprived seedlings grown on different agar types (Fig. 8F). The expression values of IRT1 and FER1 in the roots of the seedlings grown on Sigma A-1 were assigned as 1. There was a significant reduction in the relative expression of IRT1 in the roots of the seedlings grown on Sigma A-2 and Sigma E, whereas about 4-fold induction in the expression of this gene was observed in the seedlings grown on Caisson. There was approximately 1.6-fold induction and about 65% suppression of FER1 in the roots of the seedlings raised on Sigma E and Caisson, respectively. These results thus highlight the effects of different agar types on the expression of Zn- and Fe-responsive genes.
Hydroponics Is a Viable Alternative for Studying Nutrient-Deficiency Responses
A practical solution to the problem of agar-introduced contamination would be to raise plants in an agar-free system. To this end, we used a modified aseptic hydroponics system to study Pi-deficiency-mediated effects on RSA and gene expression (Fig. 9). Relative to Pi-replete controls, Pi deficiency induced significant (P < 0.05) reductions in primary root growth and number and length of first-order and higher order lateral roots (Fig. 9, A, B, D, and E). In addition, we used transgenic plants expressing the cell cycle marker CycB1;1∷uidA to investigate the effect of Pi deprivation on primary root meristematic activity (Fig. 9C). There was strong expression of CycB1;1∷uidA in the primary root tips of P+ seedlings but almost none in P− seedlings. Next, we tested the efficacy of the hydroponics system to study other nutrient-starvation (N, K, and Fe) responses (Supplemental Fig. S1). There was a significant (P < 0.05) increase in the primary root length of seedlings starved for Fe and K and a significant (P < 0.05) reduction of this trait during N deficiency compared with seedlings grown under nutrient-rich condition (P+; 6.46 cm ± 0.21 [se]; Fig. 9, A and B; Supplemental Fig. S1, A and B). N-starved seedlings, despite the reduction in primary root growth, showed strong expression of the CycB1;1∷uidA cell cycle marker comparable to that of Fe- and K-deprived seedlings (Supplemental Fig. S1C). These results confirmed an earlier study showing that progressive loss of meristematic cells in the primary root, causing a shift from an indeterminate to a determinate developmental program, is a specific response to Pi deprivation (Sánchez-Calderón et al., 2005). N deficiency also resulted in significant (P < 0.05) reductions in number and length of lateral roots compared with seedlings deprived of Fe and K (Supplemental Fig. S1, D and E). This study thus demonstrated the feasibility of using the hydroponic system to evaluate nutrient-starvation-mediated developmental responses on root growth.
Figure 9.
Hydroponic system for an evaluation of the Pi-starvation responses of Arabidopsis. Transgenic CycB1;1∷uidA Arabidopsis seedlings were initially grown hydroponically on one-half-strength MS for 5 d and then subsequently transferred to P+ (1.25 mm Pi) and P− (0 μm Pi) for 7 d. A, Seedlings were removed from the hydroponic system and spread on agar plates for documenting the RSA traits. B, D, and E, Primary root length (B) and number (D) and length (E) of first-order and higher order lateral roots (n = 10). Values in B, D, and E are means ± se. Different letters on the histograms indicate that the means differ significantly (P < 0.05). C, Histochemical GUS staining of the primary root tip of CycB1;1∷uidA seedlings was documented as described in the legend to Figure 2. F, Real-time PCR analysis of the relative expression levels of genes involved in Pi, Fe, and Zn homeostasis in the roots. ACT2 was used as an internal control. Data presented are means of six technical replicates ± se. [See online article for color version of this figure.]
The nutrient status of plants grown hydroponically under Pi-replete and Pi-deficient conditions was determined by ICP-MS analysis (Supplemental Fig. S2). Pi starvation caused a significant (P < 0.05) drop in the levels of the macronutrients P and K and the micronutrient Mn but increased Fe by 2-fold compared with the P-replete condition. In addition, significant (P < 0.05) reductions in the concentrations of the macronutrients Ca, Mg, and Na and the micronutrients B, Cu, and Mo were also observed in Pi-deprived seedlings (data not shown).
To decipher the molecular responses of hydroponically raised seedlings to Pi deprivation, we used real-time PCR to evaluate the relative expression profiles of genes involved in Pi, Fe, and Zn homeostasis (Fig. 9F). Pi deprivation caused significant inductions of IPS1 (approximately 70-fold), PLDZ2 (approximately 50-fold), and Pht1;4 (approximately 25-fold). Lower levels of induction (approximately 7- to 8-fold) were seen for RNS1 and At4. Expression of IRT1 and ZIP9 was suppressed under Pi deprivation. Suppression of IRT1 was consistent with elevated levels of Fe in Pi-deprived seedlings (Supplemental Fig. S2). Our work also led to the identification of ZIP9, a Zn-responsive gene previously not associated with Pi deficiency. A comparative analysis of the relative expression of PSI genes in the roots of seedlings grown on solid medium and hydroponically is presented in Supplemental Figure S3. Roots from hydroponically raised seedlings had 2- and 2.5-fold higher levels of Pht1;4 and PLDZ2 expression, respectively, than those from seedlings grown on Sigma A-1. Also, there was an approximately 70% suppression of ZIP9 in the roots of hydroponic seedlings compared with those grown on solid medium. Together, these results substantiate the claim that elemental contaminants in solidified media could lead to erroneous conclusions during nutrient studies. Hydroponics could be one of the feasible ways to address this issue.
DISCUSSION
Pi Contamination in the Gelling Agent Contributes to Variable Pi-Deficiency Responses
Development of the Arabidopsis root system is a highly plastic process that can be further modulated by altering Pi levels in the growth medium (Malamy and Ryan, 2001; Williamson et al., 2001; López-Bucio et al., 2002, 2005; Jain et al., 2007a). Seedlings grown on vertically oriented petri plates are well suited for analysis of RSA. However, several studies on Pi-deficiency-induced modulation of different RSA traits have led to conflicting conclusions. For instance, Al-Ghazi et al. (2003) observed no change in total length of the root system under P+ and P– conditions, whereas Williamson et al. (2001) reported Pi-deficiency-induced reduction of this trait. Interestingly, these authors and others (Linkohr et al., 2002) observed significant elongation of lateral roots in Pi-deprived seedlings compared with those grown under Pi-replete condition. On the contrary, temporally regulated highly branched root systems, including secondary, tertiary, and sometimes even quaternary lateral roots, were reported in Pi-deprived seedlings (Sánchez-Calderón et al., 2005; Jain et al., 2007a). There are reports showing both increase (Linkohr et al., 2002) and decrease (Williamson et al., 2001; Al-Ghazi et al., 2003; Chevalier et al., 2003) in lateral root density. Some of these discrepancies have been attributed either to large natural variations in root growth across Arabidopsis accessions (Chevalier et al., 2003) or to the use of different growth conditions such as light intensity, Suc concentration, or duration of Pi starvation (Reymond et al., 2006). Since Pi availability in the growth medium strongly influences RSA (Williamson et al., 2001; Linkohr et al., 2002; López-Bucio et al., 2002; Al Ghazi et al., 2003; Nacry et al., 2005; Jain et al. 2007b), we examined the possibility that the gelling medium is itself a contributor to variations in Pi-starvation responses.
In this study, we show disparities in morphophysiological and molecular responses of Pi-deprived seedlings grown on different agars representing different lots (Sigma A-1 and Sigma A-2) and types (Sigma E) from the same (Sigma) or different (Caisson) companies (Figs. 1–4). Variable levels of P in different agar types could explain at least some of the observed differential responses of Pi-deprived seedlings. For instance, at 1.2%, Sigma A-1 and Sigma A-2 agars contributed about 15 and 30 μm P, respectively, to the nutrient medium (Fig. 5). The higher level of P in Sigma A-2 caused some attenuation of Pi-starvation responses, as revealed by fewer root hairs in a 5-mm region from the tip of the primary root (Fig. 1, C and D), higher dry weight per seedling (Fig. 1C), delayed loss of primary root meristematic activity (Fig. 2, H and I), longer primary roots (Fig. 2, A and E), and relatively lower expression of IPS1 and RNS1 in the roots (Fig. 4). These results suggest that inadvertent alterations in P levels introduced by different agar lots can affect the reproducibility of Pi-starvation responses. Furthermore, a high level of P contamination in the gelling agent (Sigma E; Fig. 5) resulted in a toxic level of P (>10,000 ppm) accumulation in the Pi-deprived seedlings (Fig. 3). Although the physiology of P toxicity is not well understood, symptoms are often associated with nonspecific interactions of high cellular P with Zn and/or Fe, often resulting in the development of symptoms in leaves that resemble micronutrient deficiencies (Marschner, 1995; Lambers et al., 2002). During Pi deficiency, there is an elevated level of Fe and coordinated suppression of the iron transporter IRT1 (Misson et al., 2005). However, Pi-deprived seedlings grown on Sigma E not only showed significantly lower levels of Fe (Fig. 3) but also higher (25-fold) expression of IRT1 compared with those grown on Sigma A-1 (Fig. 4). The seedlings (P+Zn−) grown on Sigma E had significantly higher expression of Zn-deficiency-induced ZIP3 and ZIP9 compared with those grown on other agar types (Fig. 8E). This study thus revealed the influence of P contamination in the gelling medium not only on Pi starvation but also on molecular responses of Arabidopsis to other nutrients.
We further show that by reducing the concentration of Sigma A-2 from 1.2% to 0.6%, effectively halving the level of P, normal Pi-deficiency responses could be restored (Fig. 6). Earlier works have also demonstrated the feasibility of using low-percentage agar to study Pi-starvation responses (0.4% [Narang et al., 2000] and 0.6% [Catarecha et al., 2007]). However, if the level of P contamination is unusually high, as in the case of Sigma E (Fig. 6), this approach may introduce a new set of problems, leading to hyperhydric plants.
Of the several kinds of gelling agents commercially available, agar is most commonly used in nutritional studies (Ticconi et al., 2001; Williamson et al., 2001; López-Bucio et al., 2002; Nacry et al., 2005; Jain et al., 2007a; Ward et al., 2008). Only a few of these studies mention either the type (Linkohr et al., 2002; Reymond et al., 2006; Jain et al., 2007a) or the lot number (Chen et al., 2000; Ticconi et al., 2001; Ward et al., 2008) of the agar used. Although some companies, like Sigma (http://www.sigmaaldrich.com) and DIFCO (http://www.vgdllc.com/Bacto_Agar.htm), provide generic specifications for their gelling agents, none guarantee elemental composition on a lot-to-lot basis. Variability introduced by gelling agents, therefore, could be a contributing factor to some of the conflicting data presented in Pi-starvation-response studies. This raises the question: what is the permissible level of P contamination in the gelling agent that still allows typical morphophysiological and molecular responses of Arabidopsis during Pi starvation? Several studies reported inhibited growth of Arabidopsis raised on Pi-deficient media solidified with gelling agents having P contamination in the range of 1 μm to approximately 25 μm P (Chen et al., 2000; Ticconi et al., 2001; Linkohr et al., 2002; Jain et al., 2007a). Our work also confirmed that P− seedlings grown on Sigma A-1 (15 μm P contamination) showed normal Pi-starvation responses, whereas those grown on Sigma A-2 (30 μm P contamination) revealed attenuation in some of the Pi-starvation responses (Figs. 1–4). Earlier studies and our own observations of seedlings grown on Sigma A-1 and Sigma A-2 suggest that gelling agents with P contamination levels below 25 μm would be ideal for analysis of Arabidopsis Pi-starvation responses. Increase in the number and length of the root hairs at the primary root tip is a typical response to Pi starvation (Gahoonia and Nielsen, 1997; Ma et al., 2001; Jain et al., 2007a). Surprisingly, P− seedlings grown on Caisson agar (7.5 μm P contamination) developed very few root hairs at the primary root tip (Fig. 1, C and D). This unusual response suggests that gelling agent contaminants other than P could also have unexpected effects on the phenotype of Pi-deprived seedlings.
Macroelemental and Microelemental Contaminants in the Gelling Medium Influence Pi-Starvation Responses
Although some earlier studies have acknowledged the potential influence of P-contaminated gelling medium on Pi-starvation responses, the effects of other macroelemental and microelemental contaminants have been largely ignored. We observed significant variations in the levels of macroelements and microelements in different agars (Fig. 5). Since several groups have reported cross talk between Pi and K, S, Fe, and Zn (Huang et al., 2000; Wang et al., 2002; Misson et al., 2005; Svistoonoff et al., 2007; Ward et al., 2008), it was not surprising to observe variable Pi-starvation responses for seedlings grown on two different gelling agents. For instance, both Sigma A-1 and Caisson agars had low levels of P (Fig. 5), but the latter had almost three times more Fe. Perhaps this could be one of the reasons that Pi-deprived seedlings showed significant variations in morphophysiological and molecular responses (Figs. 1–4). More insight as to how P and Fe contaminants in the gelling medium interact to alter Pi-starvation responses was gained by growing seedlings under P−Fe− condition on different agar types (Fig. 7). When compared with P−Fe+ seedlings (Fig. 2), those grown under P−Fe− condition (Fig. 7) showed significantly greater elongation of the primary root on Sigma A-1, Sigma A-2, and Caisson agars; these results were consistent with recent studies highlighting an antagonistic interaction between Fe and Pi influencing primary root growth (Svistoonoff et al., 2007; Ward et al., 2008). P−Fe− seedlings grown on Sigma E agar displayed contrasting morphological and molecular responses compared with those grown on the other types (Fig. 7). Our study thus demonstrates the influence of P and Fe contaminations on both locally and systemically regulated Pi-starvation responses and emphasizes the difficulty in separating the variability induced by the gelling agent from the actual treatment effect. In addition, the contamination of the gelling agent with other interacting elements, such as K and Zn (Fig. 5), could also have a bearing on Pi-deficiency responses. For instance, K deficiency is known to increase the expression of high-affinity Pi transporters (Smith et al., 1999; Wang et al., 2002). Therefore, significantly (P < 0.05) higher levels of K in Sigma E could have contributed to the uncharacteristic phenotypes (Figs. 1 and 2).
P Contamination in the Gelling Medium Affects Responses to Fe and Zn Deficiencies
Fe is a strong Pi chelator (Guerinot and Yi, 1994; Marschner, 1995; Fox and Guerinot, 1998; Schmidt, 1999, Thimm et al., 2001) as well as an essential nutrient that plays a pivotal role in plant metabolism. Studies have shown that Pi deficiency triggers an array of molecular responses linked with Fe transport, accumulation, and homeostasis in Arabidopsis (Misson et al., 2005; Hirsch et al., 2006). Fe-deficient seedlings grown on Sigma A-1 and Sigma A-2 showed comparable RSA and expression of Fe-responsive FER1 (Fig. 8, A–D and F), which could be attributed to the low contamination levels of P and Fe in these two agars (Fig. 5). Fe-deficient seedlings grown on Sigma E and Caisson, however, showed marked variations not only in RSA (Fig. 8, A–D) but also in the expression of IRT1 and FER1 (Fig. 8F). Zn also interacts with P, and high levels of Pi in soils are known to induce Zn deficiency in plants (Webb and Loneragan, 1990; Gianquinto et al., 2000). Our results demonstrate that the high level of P in Sigma E alters the Zn-deficiency responses of the seedlings with regard to RSA (Fig. 8, A–D) and expression of ZIP9 (Fig. 8E). Clearly, a misleading conclusion could be drawn if one ignores the influence of contaminating elements in the gelling agent and their interactions with other elements.
However, some of the responses of the seedlings grown on different agar types under different nutrient deficiencies could not be interpreted merely on the basis of elemental contamination in the gelling medium. For instance, among all four agar types used in this study, Caisson agar has the highest level of Fe contamination (Fig. 5). Therefore, seedlings grown under P+Fe− condition on Caisson agar were expected to show attenuated Fe-starvation responses as compared with those grown on other agar types. On the contrary, P+Fe− seedlings grown on Caisson agar revealed significantly lower values for different root traits (Fig. 8, A–D), relatively higher expression of IRT1, and suppression of FER1 compared with those grown on other agar types (Fig. 8F). These data revealed that P+Fe− seedlings grown on Caisson agar experienced unexpectedly elevated Fe stress as compared with those grown on other agar types. This suggests the likely involvement of other nonelemental contaminants in the gelling agents that could also be exerting influence on the responses of the seedlings.
Hydroponics as a Means to Minimize Elemental Contamination in Nutrient-Deficiency Studies
Conventional nonsterile hydroponics has been used routinely to generate material to examine the biochemical, molecular, and developmental responses of Arabidopsis to Pi deprivation (Gibeaut et al., 1997; Tocquin et al., 2003; Devaiah et al., 2007a, 2007b). A simple sterile hydroponics system could alleviate the problems of elemental contamination imposed by different gelling agents (Fig. 9). Analysis of CycB1;1∷uidA expression in transgenic seedlings grown hydroponically under P+ and P– conditions for 7 d revealed inhibition of mitotic activity in the primary roots of the P– plants (Fig. 9C). Although N-deficient seedlings also showed significantly reduced primary root growth, their meristem tissue remained active (Supplemental Fig. S1, A–C). Our work showed that loss of meristematic activity is a specific response to Pi deprivation, as suggested earlier (Ticconi et al., 2004; Sánchez-Calderón et al., 2005). Previous studies have presented differing views on the effects of Pi deficiency on the density and length of lateral roots of Arabidopsis grown on agars (Williamson et al., 2001; Linkohr et al., 2002; Al-Ghazi et al., 2003; Sánchez-Calderón et al., 2005; Jain et al., 2007a). Our study of hydroponically grown CycB1;1∷uidA seedlings showed that although Pi deprivation causes significant reductions in both the number and length of first-order and higher order lateral roots (Fig. 9, A, D, and E), they remain mitotically active (data not shown). The distinct ontogeny of primary and lateral roots has been suggested as the possible cause for the differential effects of Pi deprivation on their respective meristematic activities (Jain et al., 2007a). Our work demonstrates the utility of a hydroponics system for assessing the developmental responses of Arabidopsis to local Pi availability free from interfering factors in the medium. Significant induction of PSI genes involved in Pi acquisition (Pht1;4), mobilization (IPS1, RNS1, At4), and scavenging (PLDZ2) in hydroponically raised Pi-deprived seedlings (Fig. 9F) further demonstrated the usefulness of hydroponics in the analysis of systemic responses to Pi starvation. Also, hydroponics could be useful in precise analysis of cross talk between nutrient elements in the media.
In conclusion, we provide evidence that elemental contaminants in gelling agents can alter the morphophysiological and molecular responses of Arabidopsis to macronutrient and micronutrient deficiencies. We demonstrate the effectiveness of a hydroponics system as a means to decipher the morphophysiological and molecular responses of Arabidopsis to nutrient deficiencies in a contamination-free environment. However, a hydroponics system is not amenable to some studies, such as nutrient-deficiency-mediated root hair development or split-root experiments, where different parts of the root system are required to be subjected to different nutrient conditions. Alternatively, for these types of studies, one has to resort to ICP-MS-analyzed gelling media.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia (Col-0) and transgenic CycB1;1∷uidA (Colón-Carmona et al., 1999) were used in this study. Seeds were surface sterilized by treating with 70% (v/v) ethanol for 1 min, rinsed once with sterile water, and then dispersed and shaken for 10 min in a solution containing 50% (v/v) commercial bleach and 0.1% (v/v) Tween 20. Traces of bleach were removed by seven sterile water wash/pellet cycles. After 2 d of stratification at 4°C, surface-sterilized seeds were suspended in 0.16% (w/v) agar. Four different types of agar were used in this study: Sigma A-1 (Sigma A1296; lot no. 110K0195), Sigma A-2 (Sigma A1296; lot no. 096K01581), Sigma E (Sigma A6674; lot no. 116K00531), and Caisson (Caisson Agar A038; lot no. 10386). Petri plates (150 × 15 mm) for seed germination were prepared with one-half-strength MS, 1.5% (w/v) Suc, and 1.2% (w/v) agar. Seeds were sown at a density of 20 to 25 per plate. Seeded plates were oriented vertically in racks in a growth room set to these conditions: 16-h/8-h day/night cycle at 22°C with an average photosynthetically active radiation of 60 to 65 μmol m−2 s−1 provided by florescent tubes. Five-day-old seedlings with primary root lengths in the range of 1.5 to 2.5 cm were selected to minimize the effect of intrinsic variability and transferred to the petri plates (five to seven per plate) made with 1.2% (w/v) agar of four types, unless stated otherwise, containing one of the following nutrient media: P+, Fe−, Zn−, P−, and P−Fe−. The tips of the primary roots were marked to facilitate the measurement of increase in primary root length after 7 d. P+ (nutrient-rich) medium was prepared by adding 1.25 mm KH2PO4 to a modified MS medium (López-Bucio et al., 2002), adjusted to pH 5.7, and solidified with 1.2% (w/v) agar. FeSO4/Na2EDTA and ZnSO4·7H2O were removed from the P+ medium to prepare Fe− and Zn− media, respectively. To make P− medium, KH2PO4 in P+ medium was replaced with K2SO4; FeSO4/Na2EDTA was removed from P− medium to prepare P−Fe− medium.
Hydroponic System
Sterile hydroponic system units were made from a standard Magenta (GA-7) box, a mesh screen, and a plastic support (Dr. Owen Hoekenga). A polycarbonate sheet (Laird Plastics) 0.030 inches thick was cut into 4- × 8-cm rectangular pieces. The pieces were notched at the midpoint so that two could fit together into an X-shaped support for a 6- × 6-cm piece of 250-μm polypropylene mesh (catalog no. CMP-0250; Small Parts). Packets of meshes were autoclaved separately from boxes to avoid warping. Enough sterile medium was added to the hydroponic setup so that the liquid level was 2 to 3 mm above the support. Surface-sterilized seeds were sown on a mesh placed on a sterile petri plate; the mesh was then transferred initially to the hydroponic unit containing one-half-strength MS + 1.5% (w/v) Suc for 5 d. To minimize entangling of the roots during RSA and histochemical studies, 16 seeds were sown around the perimeter of the mesh. For bulk tissue collection for ICP-MS and molecular analysis, seeds were sown at higher density (approximately 100 per mesh). Sterile hydroponic units were maintained under controlled growth conditions as described earlier. During this growth period, seeds germinated on the mesh and the hydroponic setup facilitated the penetration of the root system through the mesh into the growth medium. After 5 d, seedlings were washed twice with sterile water and then twice with the nutrient solution to which they were to be subsequently transferred. Seedlings were grown in different nutrient solutions (P+, P−, K−, N−, and Fe−) for 7 d under controlled conditions. P+, P−, and Fe− nutrient solutions were prepared as described earlier. K− medium was made by substituting KNO3 and KH2PO4 for Ca(NO3)2·4H2O and NH4H2PO4, respectively, and omitting KI. N− medium was prepared by replacing NH4NO3 and KNO3 with KCl.
Root Hair Measurements
Seedlings were grown on vertically oriented one-half-strength MS petri plates containing 1.5% (w/v) Suc and 1.2% (w/v) Sigma A-1, Sigma A-2, Sigma E, or Caisson agar. After 5 d, seedlings were transferred to P+ or P− plates made with the corresponding agar. Two days after transfer, images of the root hairs within 5 mm of the root tip were captured using a stereomicroscope (Nikon SMZ-U) equipped with a digital camera. ImageJ (http://rsb.info.nih.gov/ij/) was used to quantify the number and length of the root hairs.
Analysis of Root System Architecture
Seedlings on petri plates were gently spread to reveal architectural details and then scanned in transmissive mode at 600 dpi (UMAX PowerLook 2100 XL) for documentation of RSA traits as described earlier (Jain et al., 2007a). To document the RSA of hydroponically grown seedlings, seedlings along with the mesh were transferred in an inverted position to a pool of water for easier access. Working with a stereomicroscope, the root was separated from the shoot at the hypocotyl and then transferred to an agar plate for documentation.
Quantification of Shoot Area
Leaves were dissected from the shoot, transferred to an agar plate, and scanned in transmissive mode at 600 dpi. ImageJ was used to measure the individual leaf areas.
Determination of Dry Weight
Six replicates of a pool of 10 seedlings (12 d old) were dried to a constant weight at 65°C. Dry weight was computed per seedling.
Staining for Starch Accumulation
Shoots were excised from seedlings, bleached in 95% (v/v) ethanol at room temperature overnight, and rinsed twice with double distilled water. Stain solution was prepared by mixing 1 mL of 300 mm KI with 1 mL of 0.1 n iodine volumetric standard (Aldrich; catalog no. 318981) and diluting to 20 mL with distilled water. Starch was visualized by incubating the shoots in stain solution at room temperature for 5 min, rinsing with water twice, and documenting immediately by photo capture with a stereomicroscope (Nikon SMZ-U).
Histochemical Analysis
For histochemical analysis of GUS activity in the Arabidopsis transgenic line CycB1;1∷uidA, seedlings were incubated for 12 h at 37°C in a reaction mixture of 0.1 m Pi buffer (pH 7) containing 1 mg mL−1 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid, 5 mm K3Fe(CN)6, and 5 mm K4Fe(CN)6·3H2O (Craig, 1992; Malamy and Ryan, 2001). Stained seedlings were cleared with 70% ethanol, and at least 10 to 15 samples for each of the treatments were analyzed for GUS staining by photocapture using differential interference contrast microscopy (Olympus Vanox).
ICP-MS Analysis
ICP-MS analysis was done for the elemental profiling of four different agars (Sigma A-1, Sigma A-2, Sigma E, and Caisson), for P− seedlings grown on these agars, and for seedlings raised hydroponically under P+ and P− conditions. Seedlings were washed thoroughly with distilled water, blot dried, and divided randomly into six replicates of about 50 to 60 mg fresh weight of tissue. Tissues were placed in preweighed Pyrex tubes, dried overnight at 92°C, and reweighed. The dried samples were digested in Pyrex tubes using 0.7 mL of concentrated HNO3 (Mallinckrodt; AR Select grade) at 110°C for 4 h. Each sample was diluted to 6 mL with 18-MΩ water and analyzed on a Perkin-Elmer Elan DRCe ICP-MS apparatus. Indium (EM Science) was used as an internal standard. National Institute of Standards and Technology traceable calibration standards (ULTRA Scientific) were used for the calibration. ICP-MS analysis was performed for macroelements (P, K, S, Ca, Mg, and Na) and microelements (Fe, Zn, Mn, B, Cu, Co, and Mo).
Real-Time PCR
Total RNA was isolated from the roots of seedlings grown on different agar types on petri plates or hydroponically using the RNeasy Plant Mini Kit (Qiagen; http://www.qiagen.com/). The total RNA was treated with RQ1 RNase-free DNase (Promega; http://www.promega.com/), and then 1 μg of the DNase-treated RNA was reverse transcribed using the SuperScript III first-strand synthesis kit (Invitrogen; http://www.invitrogen.com/). Real-time PCR was performed on an Applied Biosystems 7500 real-time PCR system using SYBR Green detection chemistry (Applied Biosystems; http://www.appliedbiosystems.com/) and gene-specific primers. ACT2 was used as an internal control, and the relative expression levels of the genes were computed by the 2−ΔΔCt method of relative quantification (Livak and Schmittgen, 2001). List of primers used for real-time PCR is given in Supplemental Table S2.
Statistical Analysis
Statistical significance of differences between mean values was determined using Student's t test. Different letters on the histograms are used to indicate means that were statistically different at P < 0.05.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. K-, N-, and Fe-deficiency responses of hydroponically grown Arabidopsis.
Supplemental Figure S2. ICP-MS analysis of Arabidopsis grown hydroponically under P+ and P− conditions.
Supplemental Figure S3. Hydroponics is a potent system for studying molecular responses to Pi starvation.
Supplemental Table S1. ICP-MS analysis of different agar types.
Supplemental Table S2. List of primers used for real-time PCR.
Supplementary Material
Acknowledgments
We thank Debra M. Sherman and Chia-Ping Huang (Life Science Microscopy Facility, Purdue University) for their help. We are also grateful to Elizabeth C. Mckinney (University of Georgia) for her help in real-time PCR analysis and to Dr. Owen Hoekenga (Cornell University) for useful tips on designing the hydroponics system.
This work was supported by U.S. Department of Agriculture and McKnight grants to K.G.R.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Kashchandra G. Raghothama (kraghoth@purdue.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Al-Ghazi Y, Muller B, Pinloche S, Tranbarger TJ, Nacry P, Rossignol M, Tardieu F, Doumas P (2003) Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signaling. Plant Cell Environ 26 1053–1066 [Google Scholar]
- Beruto M, Beruto D, Debergh P (1999) Influence of agar on in vitro cultures. I. Physicochemical properties of agar and agar gelled media. In Vitro Cell Dev Biol Plant 35 86–93 [Google Scholar]
- Burleigh SH, Harrison MJ (1999) The down regulation of Mt4-like genes by phosphate fertilization occurs systemically and involves phosphate translocation to the shoots. Plant Physiol 119 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarecha P, Segura MD, Franco-Zorrilla JM, Garcia-Ponce B, Lanza M, Solano R, Paz-Ares J, Leyva A (2007) A mutant of the Arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. Plant Cell 19 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DL, Delatorre CA, Bakker A, Abel S (2000) Conditional identification of phosphate starvation-response mutants in Arabidopsis thaliana. Planta 211 13–22 [DOI] [PubMed] [Google Scholar]
- Chevalier F, Pata M, Nacry P, Doumas P, Rossignol M (2003) Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions. Plant Cell Environ 26 1839–1850 [Google Scholar]
- Colón-Carmona A, You R, Haimovitch-Gal T, Doerner P (1999) Spatiotemporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20 503–508 [DOI] [PubMed] [Google Scholar]
- Craig S (1992) The GUS reporter gene: application to light and transmission electron microscopy. In SR Gallagher, ed, GUS Protocols: Using the GUS Gene as a Reporter of Gene Expression. Academic Press, San Diego, pp 115–124
- Debergh PC (1983) Effects of agar brand and concentration on the tissue culture medium. Physiol Plant 59 270–276 [Google Scholar]
- Devaiah BN, Karthikeyan AS, Raghothama KG (2007. a) WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol 143 1789–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah BN, Nagarajan VK, Raghothama KG (2007. b) Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiol 145 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira PC, Hemerly AS, Engler JD, van Montagu M, Engler G, Inze D (1994) Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell 6 1763–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox TC, Guerinot ML (1998) Molecular biology of cation transport in plants. Annu Rev Plant Physiol Plant Mol Biol 49 669–696 [DOI] [PubMed] [Google Scholar]
- Gahoonia TS, Nielsen NE (1997) Variation in root hairs of barley cultivars doubled soil phosphorus uptake. Euphytica 98 177–182 [Google Scholar]
- Gianquinto G, Abu-Rayyan A, Tola LD, Piccotino D, Pezzarossa B (2000) Interaction effects of phosphorus and zinc on photosynthesis, growth and yield of dwarf bean grown in two environments. Plant Soil 220 219–228 [Google Scholar]
- Gibeaut DM, Hulett J, Cramer GR, Seemann JR (1997) Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol 115 317–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D (1998) Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Natl Acad Sci USA 95 7220–7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML, Yi Y (1994) Iron: nutritious, noxious and not readily available. Plant Physiol 104 815–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J, Marin E, Floriani M, Chiarenza S, Richaud P, Nussaume L, Thibaud MC (2006) Phosphate deficiency promotes modification of iron distribution in Arabidopsis plants. Biochimie 88 1767–1771 [DOI] [PubMed] [Google Scholar]
- Huang C, Barker SJ, Langridge P, Smith FW, Graham RD (2000) Zinc deficiency up-regulates expression of high-affinity phosphate transporter genes in both phosphate-sufficient and -deficient barley roots. Plant Physiol 124 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, Murphy AS, Raghothama KG (2007. a) Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol 144 232–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Vasconcelos MJ, Sahi SV, Raghothama KG (2007. b) Molecular mechanisms of plant adaptation to phosphate deficiency. In J Janick, ed, Plant Breeding Reviews, Vol 29. John Wiley & Sons, New York, pp 359–419
- Karthikeyan AS, Varadarajan DK, Jain A, Held MA, Carpita NC, Raghothama KG (2007) Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta 225 907–918 [DOI] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Mukatira UT, D'Urzo MP, Damsz B, Raghothama KG (2002) Regulated expression of Arabidopsis phosphate transporters. Plant Physiol 130 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Juniper D, Cawthray GR, Veneklass EJ, Martinez-Ferri E (2002) The pattern of carboxylate exudation in Banksia grandis (Proteaceae) is affected by the form of phosphate added to the soil. Plant Soil 238 111–122 [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HMO (2002) Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J 29 751–760 [DOI] [PubMed] [Google Scholar]
- Liu J, Samac DA, Bucciarelli B, Allan DL, Carroll P, Vance CP (2005) Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. Plant J 41 257–268 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129 244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Pérez-Torres A, Rampey RA, Bartel B, Herrera-Estrella L (2005) An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis: identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiol 137 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Bielenberg DG, Brown KM, Lynch JP (2001) Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ 29 459–467 [Google Scholar]
- Malamy JE, Ryan KS (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127 899–909 [PMC free article] [PubMed] [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants. Academic Press, London
- Misson J, Raghothama KG, Jain A, Jouhet J, Block MA, Bligny R, Ortet P, Creff A, Somerville S, Rolland N, et al (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA 102 11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B, Azmi A, Onckelen HV, Rossignol M, Doumas P (2005) A role for auxin redistribution in the response of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol 138 2061–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn BJ, Furneaux RH, Stevenson TT (1995) Identification of an agar constituent responsible for hydric control in micropropagation of radiata pine. Plant Cell Tissue Organ Cult 43 1–11 [Google Scholar]
- Narang RA, Bruene A, Altmann T (2000) Analysis of phosphate acquisition efficiency in different Arabidopsis accessions. Plant Physiol 124 1786–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak J, Asiedu SK (1992) Gelling agent and light effects on in vitro tuberization of potato cultivars. Am Potato J 69 461–470 [Google Scholar]
- Reymond M, Svistoonoff S, Loudet O, Nussaume L, Desnos T (2006) Identification of QTL controlling root growth response to phosphorus starvation in Arabidopsis thaliana. Plant Cell Environ 29 115–125 [DOI] [PubMed] [Google Scholar]
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Cruz-Ramírez A, Nieto-Jacobo F, Dubrovsky JG, Herrera-Estrella L (2005) Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol 46 174–184 [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Shin R (2007) Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol 58 47–69 [DOI] [PubMed] [Google Scholar]
- Schmidt W (1999) Mechanisms and regulation of reduction based iron uptake in plants. New Phytol 141 1–26 [Google Scholar]
- Scholten HJ, Pierik RLM (1998. a) Agar as a gelling agent: chemical and physical analysis. Plant Cell Rep 17 230–235 [DOI] [PubMed] [Google Scholar]
- Scholten HJ, Pierik RLM (1998. b) Agar as a gelling agent: differential biological effects in vitro. Sci Hortic 77 109–116 [Google Scholar]
- Smith FW, Cybinski DH, Rae AL (1999) Regulation of expression of genes encoding phosphate transporters in barley roots. In G Gissel-Nielsen, A Jensen, eds, Plant Nutrition-Molecular Biology and Genetics: Proceedings of the Sixth International Symposium on Genetics and Molecular Biology of Plant Nutrition. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 145–150
- Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T (2007) Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet 39 792–796 [DOI] [PubMed] [Google Scholar]
- Thimm O, Essigmann B, Kloska S, Altman T, Buckhout TJ (2001) Response of Arabidopsis to iron deficiency stress as revealed by microarray analysis. Plant Physiol 127 1030–1043 [PMC free article] [PubMed] [Google Scholar]
- Ticconi CA, Delatorre CA, Abel S (2001) Attenuation of phosphate starvation responses by phosphite in Arabidopsis. Plant Physiol 127 963–972 [PMC free article] [PubMed] [Google Scholar]
- Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S (2004) Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J 37 801–814 [DOI] [PubMed] [Google Scholar]
- Tocquin P, Corbesier L, Havelange A, Pieltain A, Kurtem E, Bernier G, Périlleux C (2003) A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biol 3 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Garvin DF, Kochian LV (2002) Rapid induction of regulatory and transporter genes in response to phosphorus, potassium, and iron deficiencies in tomato: evidence for cross talk and root/rhizosphere mediated signals. Plant Physiol 130 1361–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JT, Lahner B, Yakubova E, David E, Salt DE, Raghothama KG (2008) The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol 147 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb MJ, Loneragan JF (1990) Zinc translocation to wheat roots and its implications for a phosphorus/zinc interaction in wheat plants. J Plant Nutr 13 1499–1512 [Google Scholar]
- Williamson LC, Ribrioux SPCP, Fitter AH, Leyser HMO (2001) Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol 126 875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.