Abstract
In open places, plants are exposed to higher fluence rates of photosynthetically active radiation and to higher red to far-red ratios than under the shade of neighbor plants. High fluence rates are known to increase stomata density. Here we show that high, compared to low, red to far-red ratios also increase stomata density in Arabidopsis (Arabidopsis thaliana). High red to far-red ratios increase the proportion of phytochrome B (phyB) in its active form and the phyB mutant exhibited a constitutively low stomata density. phyB increased the stomata index (the ratio between stomata and epidermal cells number) and the level of anphistomy (by increasing stomata density more intensively in the adaxial than in the abaxial face). phyB promoted the expression of FAMA and TOO MANY MOUTHS genes involved in the regulation of stomata development in young leaves. Increased stomata density resulted in increased transpiration per unit leaf area. However, phyB promoted photosynthesis rates only at high fluence rates of photosynthetically active radiation. In accordance to these observations, phyB reduced long-term water-use efficiency estimated by the analysis of isotopic discrimination against 13CO2. We propose a model where active phyB promotes stomata differentiation in open places, allowing plants to take advantage of the higher irradiances at the expense of a reduction of water-use efficiency, which is compensated by a reduced leaf area.
Photosynthesis, transpiration, and transpiration efficiency, the ratio of carbon fixation to water loss, are key physiological traits considered by plant breeders when selecting productive and water-use efficient plants (Rebetzke et al., 2002; Richards, 2006; Passioura, 2007). Opening of the stomata allows the uptake of CO2 necessary for photosynthesis but it simultaneously increases the loss of water and the potential deterioration of the water status. Plants are finely tuned to efficiently face this dilemma. Under low levels of photosynthetically active radiation (PAR), stomata open just enough to prevent the limitation of photosynthesis by CO2 influx and the photochemical phase of photosynthesis is the limiting step. If PAR increases, allowing higher rates of photochemical reactions, which leads to more ATP and NADPH, stomatal conductance also increases to allow sufficient CO2 to use these products in the Calvin cycle (Donahue et al., 1997; Yu et al., 2004). If instead of following this response coordinated to photosynthetic rates, stomata opened maximally in response to low PAR, more CO2 than needed would be allowed to reach the chloroplast at the expense of unnecessary water loss.
Canopy shade light is characterized not only by reduced PAR levels but also by a reduced proportion of red light (R) compared to far-red light (FR) caused by the selective absorption of visible light by photosynthetic pigments and the reflection and transmission of FR (Holmes and Smith, 1977a). This low R/FR ratio compared to unfiltered sunlight is perceived by phytochromes (Smith, 1982; Ballaré et al., 1987; Pigliucci and Schmitt, 1999), mainly phytochrome B (phyB; Yanovsky et al., 1995). In Arabidopsis (Arabidopsis thaliana), the high R/FR signals perceived by phyB decrease the length of the stem and petioles, cause a more prostrate position of the leaves, and promote branching and delay flowering, among other responses (Reed et al., 1993; Franklin and Whitelam, 2005).
Transgenic plants of potato (Solanum tuberosum) expressing the PHYB gene of Arabidopsis show higher stomatal conductance, transpiration rates, and photosynthesis rates per unit leaf area than the wild type (Thiele et al., 1999; Boccalandro et al., 2003; Schittenhelm et al., 2004). Stomata density is unaffected, indicating that phyB enhances the aperture of the stomatal pore in these transgenic plants. Stomatal conductance is higher in Fuchsia magellanica plants exposed to R than to FR pulses at the end of the photoperiod (Aphalo et al., 1991). However, there are no general effects of R/FR treatments on the aperture of the stomatal pore. The stomata of Commelina communis (Roth-Bejerano, 1981) and of the orchid of the genus Paphiopedilum (Talbott et al., 2002) open in response to R and this effect is reversed by FR, indicating a control by phytochrome. Nevertheless, this FR reversal of the effect of R is absent in wild-type Arabidopsis (Talbott et al., 2003). In Phaseolus vulgaris, FR accelerates stomatal movements during dark to light (opening) and light to dark (closing) transitions and this effect is R reversible, but phytochrome status has no effects under constant conditions of light or darkness (Holmes and Klein, 1985). In the latter species, prolonged FR added to a white-light background promotes stomatal conductance but this effect cannot be ascribed to phytochrome (Holmes et al., 1986).
In addition to this rapid adjustment of the CO2 and water vapor fluxes to daily fluctuations in light levels via the regulation of the stomatal pore aperture, plants acclimate to the prevailing PAR conditions by changing stomatal density (number of stomata per unit area) and stomatal index (the ratio between the number of stomata in a given area and the total number of stomata and other epidermal cells in that same area). Stomatal density and stomatal index are higher in plants grown in full sunlight at high levels of PAR than in plants grown in shade (Willmer and Fricker, 1996; Lake et al., 2001; Thomas et al., 2004; Casson and Gray, 2008). Mature leaves sense the environment (light intensity and CO2) and produce a systemic signal that regulates stomatal density and index in young leaves (Coupe et al., 2006). A change in CO2 concentrations or PAR levels affects photosynthesis and therefore it was suggested that a metabolic compound associated to this process (i.e. a sugar) may regulate stomatal development (Coupe et al., 2006). However, there is no correlation between photosynthetic rate and stomatal index in poplar (Populus spp.; Miyazawa et al., 2006) and transgenic anti-small subunit of Rubisco tobacco (Nicotiana tabacum) plants, show reduced photosynthesis and normal responses of stomatal density and stomatal index to PAR, suggesting that other photoreceptors could be involved in this regulation (Baroli et al., 2008).
Here we demonstrate that high, compared to low, R/FR ratios perceived by phyB increase stomata density, stomata index, and amphistomy in the leaves of Arabidopsis. This behavior results in an enhanced photosynthetic rate at high PAR at the expense of reduced water-use efficiency.
RESULTS
Light Signals Perceived by phyB Increase Transpiration Rate
Plants of Arabidopsis of the wild-type Landsberg erecta (Ler) and of the phyB-4 and phyB-5 mutants were grown under white-light photoperiods (12 h) terminated with or without a pulse of FR (+FR). The classical end-of-day pulse of FR (Downs et al., 1957) provides a simulation of canopy shade light that is more rudimentary than FR given simultaneously with white light throughout the photoperiod but it avoids potential effects linked to differential excitation of PSI and PSII by FR, which are known to be important in the acclimation of photosynthesis-related traits (Dietzel and Pfannschmidt, 2008; Wagner et al., 2008).
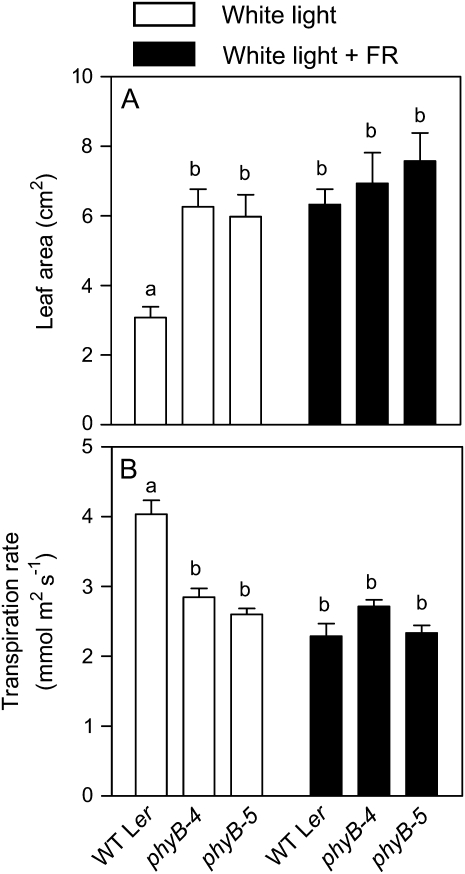
Transpiration per plant depends on leaf area per plant and transpiration per unit leaf area. The +FR treatment and the phyB mutation increased the leaf area per plant (Fig. 1A) and reduced the rate of transpiration per unit leaf area (Fig. 1B), compared to wild-type plants grown under the high R/FR ratio control conditions. A highly significant interaction occurred between light conditions and genotype because under the +FR conditions, the phyB mutation had no effects compared to the wild type, and in the phyB mutant, the +FR treatment had no effect compared to the high R/FR ratio control. These observations indicate a control of leaf area and transpiration per unit leaf area by the +FR treatment perceived by phyB, without any obvious participation of other phytochromes in the response to R/FR ratio.
Figure 1.
Phytochrome controls leaf transpiration rate. Leaf area per plant (A) and transpiration per unit leaf area (B) in seedlings of the wild type and of the phyB mutants grown under white light with or without exposure to FR at the end of the photoperiod. Data are means and se of at least 21 plant replicates. Factorial ANOVA indicates significant interaction (P < 0.0001) between the effects of the phyB mutations and the +FR treatment because the phyB mutation had effects under white light but not under white light + FR (A and B). Different letters denote significant differences (P < 0.05) among means according to Bonferroni post tests.
phyB Increases Stomata Density
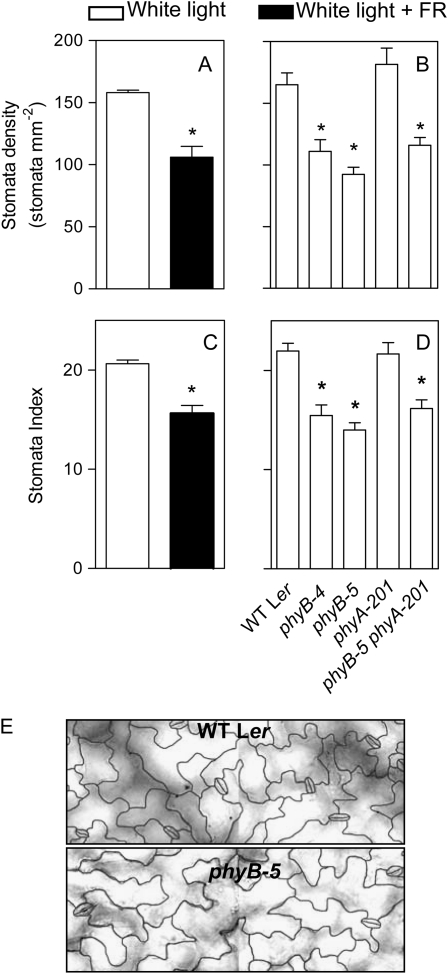
To investigate the basis for the differences in the rate of transpiration per unit leaf area we recorded the number of stomata per unit leaf area. The +FR treatment (Fig. 2A) and the phyB mutation (Fig. 2B) reduced stomata density compared to wild-type plants grown under the high R/FR ratio control conditions. Light and genotype effects on stomata density correlate positively with the effects on the rate of transpiration per unit leaf area (compare with Fig. 2, A and B, and Fig. 1B). The phyA-201 mutant presented a wild-type-like phenotype and the phyB-5 phyA-201 double mutant behaved as the phyB single mutants (Fig. 2B), indicating no significant role of phyA.
Figure 2.
Phytochrome controls stomata density (A and B) and stomata index (C and D). Plants of the wild type were grown under white light and white light + FR (A and C) and plants of the phyA, phyB, and phyA phyB mutants (B and D) were grown with their respective wild type under white light. Data are means and se of at least 12 plant replicates. Asterisk (*) denotes significant differences (P < 0.05) between the indicated condition or genotype and its control according to ANOVA followed by Bonferroni post tests. E, Representative sections of the epidermis are shown for a phyB mutant and its wild type (WT Ler). Stomata density, index, and images correspond to the adaxial epidermis of fully expanded leaves.
phyB Increases Stomata Index
Differences in stomata density can result either from a general effect on cell density per unit leaf area (larger cells would reduce density and account for the enhanced area in response to +FR or the phyB mutation) or from a specific reduction in stomata differentiation. We calculated the stomatal index, i.e. the ratio between the number of stomata and the number of epidermal cells in a given area. The +FR treatment (Fig. 2C) and the phyB mutation (Fig. 2D) reduced stomatal index, revealing the existence of a specific control by phyB of the proportion of epidermal cells that differentiate into stomata. The phyA-201 mutant presented a wild-type-like phenotype and the phyB-5 phyA-201 double mutant was similar to the phyB single mutants (Fig. 2), indicating no significant role of phyA. The phyA and phyB mutant backgrounds caused small but statistically significant increments or reductions, respectively, of nonguard epidermal cell density (cells per mm2, mean ± se, wild-type Ler: 764 ± 19; phyB-5: 710 ± 26; phyB-4: 772 ± 25; phyA-201: 849 ± 29; phyB-5 phyA-201: 772 ± 21, P < 0.05 for phyA versus PHYA and phyB-5 versus PHYB). The phyB mutation caused no obvious stomata aberrant distribution phenotype (e.g. contiguous stomata).
phyB Enhances the Amphistomy Level
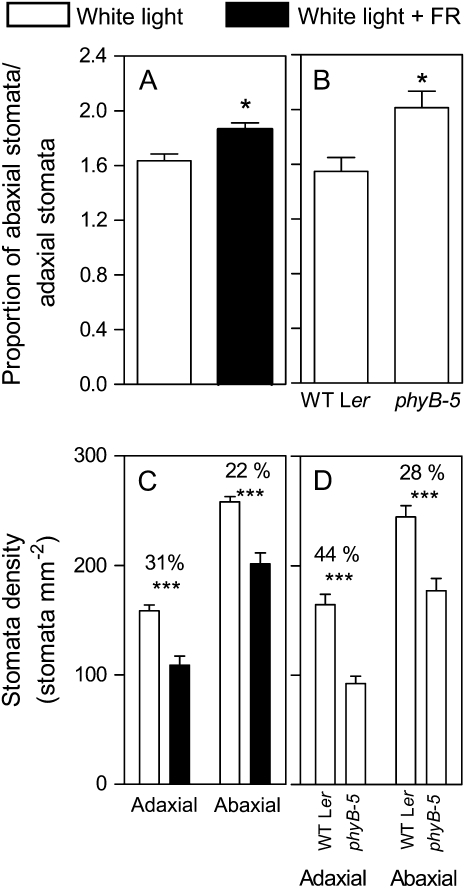
Arabidopsis has amphistomatous leaves, with higher stomata density in the abaxial respect to the adaxial leaf surface (Hetherington and Woodward, 2003). Depending on the species, PAR levels increases not only stomata density but also the level of amphistomy, a trait that affects acclimation to sunny environments (Mott et al., 1982; Mott and Michaelson, 1991). The +FR treatment and the phyB mutation reduced stomata density more in the adaxial than in the abaxial face of the leaf and therefore increased the abaxial/adaxial ratio (Fig. 3).
Figure 3.
Phytochrome increases the level of amphistomy (A and B). The abaxial and abaxial stomatal densities and the percent decrease caused by +FR or by the phyB mutation are also shown (C and D). Plants of the wild type were grown under white light and white light + FR (A and C) and plants of the phyB mutant were grown with its wild type under white light (B and D). Data are means and se of at least 12 plant replicates. Asterisk (*) denotes significant differences (P < 0.05) according to ANOVA. *** denotes significant differences (P < 0.001) according to Bonferroni post tests.
phyB Reduces Water-Use Efficiency
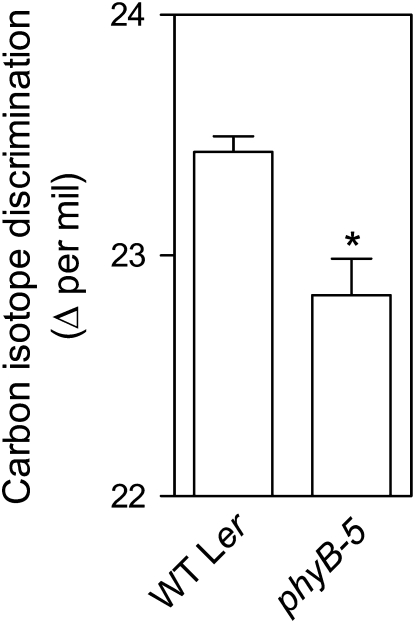
Transpiration efficiency (the ratio between fixed CO2 and water vapor lost by transpiration) was estimated through the analysis of isotopic discrimination against 13CO2 with respect to 12CO2 (Δ). This parameter is a reliable and sensitive marker negatively correlated with water-use efficiency or photosynthesis per unit transpiration (Farquhar and Richards, 1984; Masle et al., 2005). The phyB mutation decreased isotope discrimination (Δ; Fig. 4), indicating that phyB decreases water use efficiency.
Figure 4.
Phytochrome reduces water-use efficiency. Plants of the phyB mutant and its wild type were grown under white light. Data are means and se of three plant replicates (pool of three plants each one). Asterisk (*) denotes significant differences (P < 0.05) according to ANOVA.
Overexpression of PHYB Increases Stomatal Density and Stomatal Index and Reduces Water-Use Efficiency
Transgenic plants overexpressing PHYB (PHYB OX) had a phenotype opposite in many respects to that of the phyB mutants (Supplemental Fig. S1). Compared to the wild type, the transgenic overexpressors showed reduced leaf area per plant, increased transpiration rate per unit leaf area, increased stomata density, increased stomatal index, increased amphistomatous character, and increased isotope discrimination (Δ). The proportion between abaxial and adaxial stomata decreased because stomata density increased more in the adaxial (147%) than in the abaxial face (76%). In addition, PHYB overexpression increased total nonguard epidermal cell density (wild-type Nossen [No], 468 ± 30; PHYB OX, 645 ± 46, P < 0.05). PHYB overexpression caused no obvious aberrant stomata distribution phenotype (e.g. contiguous stomata).
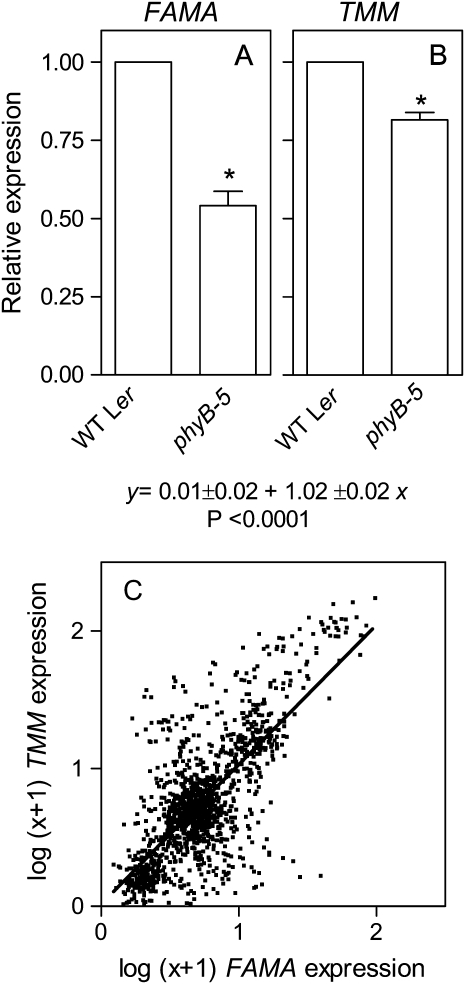
phyB Increases the Expression of FAMA and TOO MANY MOUTHS
FAMA causes the guard mother cell to divide into the guard cells that define the stomatal pore (MacAlister et al., 2007; Casson and Gray, 2008). TOO MANY MOUTHS (TMM) negatively regulates the development of supernumerary stomata and enhances spacing among stomata (Geisler et al., 2000; Nadeau and Sack, 2002). We investigated the level of expression of these genes in developing leaves of wild-type and phyB mutant plants. Other genes involved in stomatal development were also investigated but the expression levels did not allow a clear resolution. The phyB mutation reduced both FAMA and TMM expression compared to the wild type (Fig. 5, A and B). At first glance it was surprising to see that both genes with contrasting consequences on stomata density were affected in the same direction in the phyB mutant, which shows reduced stomata density. To explore this issue in further detail we analyzed the correlation of expression between FAMA and TMM across 633 conditions representing different tissues, developmental stages, and differentially treated plants (www.arabidopsis.org). Publicly available data for each of the two genes were normalized to the median of each experiment and ln (x + 1) transformed as described (Buchovsky et al., 2008). This analysis demonstrates a highly significant positive correlation between the expression of FAMA and TMM beyond the action of phyB (Fig. 5C).
Figure 5.
Phytochrome promotes the expression of FAMA and TMM in the leaves and the positive correlation in the expression of these two genes extends beyond phyB action. A and B, Plants of the phyB mutant and its wild type were grown under white light. Data are means and se of three biological replicates. Asterisk (*) denotes significant differences (P < 0.05) according to ANOVA. C, Positive correlation between the expression of FAMA and TMM. The figure includes 633 data points corresponding to different developmental contexts and biotic or abiotic treatments (1–3 biological replicates per point) taken from 46 experiments (1,388 microarrays; www.arabidopsis.org). Data were normalized to the median of each experiment and transformed as ln (x + 1) (Buchovsky et al., 2008). The line shows least square linear fit of the 633 points and the significance is indicated.
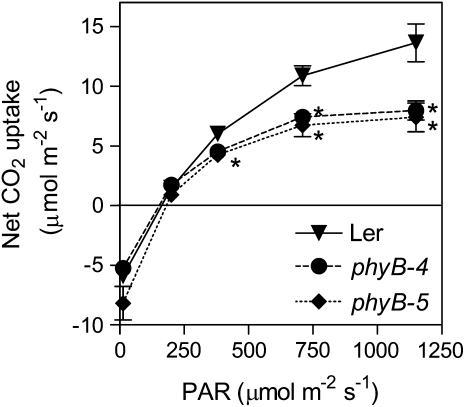
phyB Promotes Photosynthesis at High PAR
phyB increased stomata density and the level of amphistomy. Both traits potentially favor CO2 diffusion from the atmosphere to the chloroplasts. We investigated whether phyB affects CO2 uptake in wild-type and phyB mutant leaves exposed to a range of PAR (Fig. 6). Net CO2 uptake was unaffected by the phyB mutation at or below the PAR that the plants had experienced during the growth period (250 μmol m−2 s−1). However, the phyB mutants presented lower photosynthetic rates than the wild type under higher PAR (380 μmol −2 s−1 or more; Fig. 6).
Figure 6.
Phytochrome promotes photosynthesis in Arabidopsis. Plants of the wild type and of the phyB mutants were grown under white light and then exposed to the indicated PAR during measurements. Data are means and se of six plant replicates. Asterisk (*) denotes significant differences (P < 0.05) according to ANOVA and Bonferroni post tests.
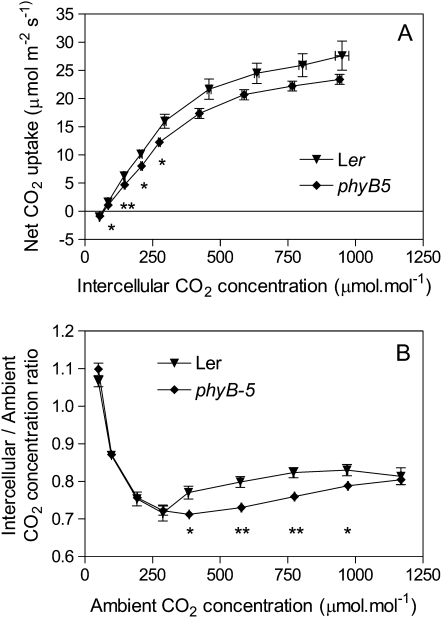
To investigate whether the differences in CO2 uptake were caused by stomatal limitations to CO2 diffusion, consistent with the lower stomata density, we obtained curves of net CO2 uptake against the intercellular CO2 concentration (Fig. 7A). The wild type showed higher CO2 conductance than the phyB-5 mutant (mean ± se in mol m−2 s−1 for 400 μmol mol−1 of CO2 in the reference infrared gas analyzer; n = 5; wild type = 0.36 ± 0.02; phyB-5 = 0.21 ± 0.02; P < 0.0005). The lower ratios between intercellular and ambient CO2 concentrations in the phyB mutant compared to the wild type indicate stomatal limitations to CO2 diffusion (Fig. 7B). The residual differences in the curves of net CO2 uptake against the intercellular CO2 concentration (Fig. 7A) suggest that additional nonstomatal effects of phyB on photosynthesis could occur.
Figure 7.
Analysis of stomatic and nonstomatic limitations to photosynthesis. A, Curves of leaf net CO2 uptake against intercellular CO2 concentrations were obtained with a LI-COR 6400 system for the wild type and the phyB-5 mutant. B, Ratio between intercellular and ambient CO2 concentrations plotted against ambient CO2 concentration (i.e. the concentration in the sample infrared gas analyzer of the LI-COR 6400 system). Data are means and se of five plant replicates. Asterisk (*) denotes significant differences (P < 0.05) according to t tests. The tests were done between wild type and the phyB having the closest intercellular (A) or ambient (B) CO2 concentrations (in A, this overestimates the significance of the difference for the samples around 280 μmol mol−1).
DISCUSSION
In Arabidopsis, the density of stomata and the stomata index increase in response to PAR and in response to low CO2 (Willmer and Fricker, 1996; Lake et al., 2001). Low R/FR ratios are typical of canopy shade light and establish low proportions of active phytochrome (Holmes and Smith, 1977a, 1977b). Here we show that light treatments (+FR) that reduce the proportion of phytochrome in its active form also reduce stomata density and stomata index of Arabidopsis leaves (Fig. 2, A and C). The phyB mutants having reduced or null levels of phyB, the main photoreceptor of R/FR ratios, showed low stomata density and stomata index under high R/FR ratios (Fig. 2, B, D, and E). The level of amphistomy (i.e. the presence of stomata on both leaf blade surfaces) can increase with PAR (Mott et al., 1982). The +FR treatment and the phyB mutation reduced stomata density more in the adaxial than in the abaxial face and therefore reduced the level of amphistomy (Fig. 3). This picture is completed by the observation that lines overexpressing the PHYB gene increased stomata density, stomata index, and the level of amphistomy compared to the wild type (Supplemental Fig. S1). Therefore, active phyB increases stomata density, stomata index, and amphistomy in Arabidopsis.
Low irradiance or low R/FR ratio do not change stomata density in the leaves of C. communis (Assmann, 1992), indicating that not all the species follow the pattern of response to shade light signals exhibited by Arabidopsis. However, given the contribution of Arabidopsis as a model experimental system to study the regulation of transpiration and drought tolerance (Nilson and Assmann, 2007), it is important to uncover the environmental signals and the receptors controlling stomatal density and the physiological consequences of this regulation in Arabidopsis.
Stomata index is defined relatively early during the development of the leaves (Larkin et al., 1997; Serna et al., 2002) and therefore, the adjustment to the future conditions that the leaf is more likely to face would be beneficial. In closing canopies, the reduction in R/FR ratio anticipates actual shading among neighbors due to selective FR reflection on green leaves (Ballaré et al., 1987). The perception of low R/FR signals by phyB would provide a mechanism to adjust stomata density of developing leaves to the likely occurrence of shade, before mutual shading (i.e. reduced irradiance) actually takes place.
We are largely ignorant of the genes that relate environmental signals to the control of stomata density (Wang et al., 2007). One exception is the HIGH CARBON DIOXIDE gene, which encodes a putative 3-keto acyl coenzyme A synthase involved in the synthesis of very-long-chain fatty acids, involved in the response to carbon dioxide (Gray et al., 2000). Here we place phyB between light quality signals and genes involved in stomata differentiation. The expression of FAMA, a basic helix-loop-helix (bHLH) transcription factor that promotes stomata differentiation (MacAlister et al., 2007; Casson and Gray, 2008), is reduced in developing leaves of the phyB mutant compared to the wild type (Fig. 5A). SPEECHLESS (SPCH) is a bHLH transcription factor that positively regulates the asymmetric divisions that form stomata and the expression of FAMA (MacAlister et al., 2007; Casson and Gray, 2008). The expression of SPCH in 8-d-old seedlings with very young primordia is reduced by exposure to 1 h or 4 d to low R/FR ratio (Carabelli et al., 2007). Based on these observations we propose a model where phyB-mediated promotion of stomata differentiation involves positive regulation of bHLH transcription factors that play a key role in stomata development. Interestingly, the expression of TMM, a putative cell-surface Leu-rich repeat-containing receptor-like protein that negatively regulates stomata differentiation (Geisler et al., 2000; Nadeau and Sack, 2002) was also lower in the phyB mutant than in the wild type (Fig. 5B). The analysis of the expression levels of FAMA and TMM across different developmental, abiotic, and biotic conditions revealed that, despite their opposite effects on stomata density, the positive expression correlation between these genes is not limited to the comparison between the wild type and the phyB mutant (Fig. 5C). TMM is not only a repressor of stomatal development, it also seems to provide competence to enter into the stomatal pathway (Nadeau and Sack, 2002). It is likely that phyB positively regulates stomata formation by increasing the number of cells from which the stomatal lineage may develop. The fact that TMM also represses stomatal development, playing an opposite role to phyB, suggests that its expression might be positively controlled by a feedback mechanism during stomata differentiation. These findings suggest that the expression of TMM might be positively controlled by a feedback mechanism during stomata differentiation. There are several examples where an environmental cue initiates downstream signaling and sets into motion mechanisms that negatively regulate that signaling (Casal et al., 2004). The complex interaction among TMM and three ERECTA family of Leu-rich repeat-containing receptor kinases revealed by the mutant phenotypes is not fully understood (Nadeau, 2008) and these sort of feedback regulations could be part of the complexity. The ERECTA gene product is a putative partner of TMM in the regulation of stomata development (Casson and Gray, 2008). We observed changes in stomata density and index in response to +FR or to the phyB mutation in the Landsberg erecta background, indicating that phyB-mediated responses of these traits can occur in the absence of a functional ERECTA gene.
Changes in stomata density do not necessarily translate into changes in water vapor and carbon dioxide fluxes. In transgenic Arabidopsis plants overexpressing the STOMATAL DENSITY AND DISTRIBUTION1 (SDD1) gene and in the sdd1 mutant stomata density is significantly decreased or increased, respectively, compared to the wild type (Bussis et al., 2006). However, carbon dioxide assimilation rate and stomatal conductance of overexpressers and sdd1 mutant were unchanged compared with wild type because changes in stomatal density were compensated for by opposite changes in stomatal aperture (Bussis et al., 2006). Conversely, the control of stomatal density by phyB correlated with changes in the rate of transpiration when the wild type is compared to either the phyB mutant (Fig. 1B) or the PHYB OX (Supplemental Fig. S1). This resulted in a negative regulation of water-use efficiency by phyB (Fig. 4; Supplemental Fig. S1) consistent with the positive regulation by ERECTA, which reduces stomata density (Masle et al., 2005).
The activity of phyB is higher in open places, where the radiation load is stronger and atmospheric water demand is more intense. Therefore, the increased stomatal density caused by phyB, which results in reduced water-use efficiency, cannot easily be associated with a strategy aimed to conserve water. Along the same line of arguments, increased amphistomy is a feature often linked to adequate water availability (Mott et al., 1982; Lynn and Waldren, 2002). Low R/FR ratios and the phyB mutation increased leaf area per plant (Fig. 1A). Increased leaf area in response to +FR has been observed in other species such us Cucumis sativus (López Juez et al., 1990), Taraxacum officinale (Cogliatti and Sánchez, 1987), Terminalia ivorensis (Kwesiga and Grace, 1986), and Petunia axilaris (Casal et al., 1987), and for some leaf positions in Arabidopsis (Robson et al., 1993). However, the opposite pattern has also been found (Kasperbauer, 1971; Holmes and Smith, 1977b; Frankland and Letendre, 1978; Robson et al., 1993; Devlin et al., 1999), suggesting that the actual effect may depend on the species and growing conditions. The negative regulation of leaf area by phyB observed here would help to reduce water loss per plant in open places. However, the opposite regulation of leaf area and transpiration per unit leaf area by phyB largely compensate each other, indicating that the main function of these changes is not the adjustment of water use. Rather, phyB perception of the high R/FR ratios of open places allows Arabidopsis plants to increase photosynthesis in response to the higher PAR levels that the plant may experience in those conditions (Fig. 6). The increased stomata density and amphistomy mediated by phyB would be an adaptation to the high PAR of open places by favoring the diffusion of carbon dioxide (Fig. 7B) and by facilitating leaf cooling due to the concomitant increase in transpiration rate reducing the stress imposed by high radiation loads (Mott et al., 1982; Parkhurst and Mott, 1990; Mott and Michaelson, 1991). Excitation energy that is not used for photochemistry and not dissipated as fluorescence or heat can be transferred to molecular oxygen, creating highly damaging reactive oxygen species (Golan et al., 2006, and refs. therein). Therefore, the higher rate of photosynthesis at high PAR caused by phyB could also reduce the diversion of excitation energy to the generation of reactive species of oxygen in open places.
MATERIALS AND METHODS
Plant Material
The accessions Ler, Columbia, and No of Arabidopsis (Arabidopsis thaliana) were used as wild type in this study. phyA-201 (formerly fre-1; Nagatani et al., 1993), phyB-5 (formerly hy3), phyB-4 (Koornneef et al., 1980; Reed et al., 1993), and the double mutant phyA-201 phyB-5 are in the Ler background; the transgenic line expressing 35S:PHYB:GFP (PHYB:GFP; Más et al., 2000) is in the Columbia background and the overexpressor line 35S:PHYB (PHYB OX) is in the No background (Wagner et al., 1991).
Seeds were sown on 0.8% agar water and 4-d-old seedlings were transplanted to 230 cm3 pots containing equal amounts of perlite (Perlome; Perfiltra), peat moss (Cuidad Floral), and vermiculite (Intersum; Aislater) and watered as needed with a solution containing 1 g per L of Hakaphos R (COMPO).
Light Conditions
Plants were grown under white-light photoperiods of 12 h at 250 μmol m−2 s−1 of PAR (LI-188B sensor; LI-COR) provided by a combination of mercury and sodium lamps and temperature was 23°C ± 1°C. The R/FR ratio (Skye meter SKR 100, remote probe SKR110; Skye Instruments) was 4.1. During 1 h after the end of white-light photoperiod, FR (10 μmol m−2 s−1, R/FR ratio = 0.04) was provided from one side of the plants by incandescent lamps in combination with a water filter (10 cm width), a red filter (no. 026; Lee Filters), and two blue acrylics filters (1 mm thick; Paolini 2031).
Stomata Density, Stomata Index, and Amphistomy
Fully expanded leaves of the first pair were collected from 25-d-old plants. The number of stomata and epidermal cells were counted in clarified leaves or in imprints performed with transparent nail varnish, under the microscope (40×) in six portions of the adaxial surface of the leaf blade, at both sides of the midrib (two determinations in the distal, medium, and proximal zone). In some experiments, epidermal cell counting was performed in the adaxial and abaxial sides of the second pair of leaves to investigate the amphistomy level (Fig. 3) or the effect of +FR on stomata density and index (Fig. 2). Representative photographs were taken using Nomarsky optics with a Leica DC 300F camera attached to a Leica DMIRB inverted microscope. To improve their visualization, cell walls were draw on the image, using the brush tool of the Photoshop v.7.0.
Carbon Isotope Discrimination
Analysis of carbon isotope composition was performed on 35-d-old rosette leaves (vegetative stage). Three plants per genotype were pooled for each independent biological replicate. Carbon isotope composition (δ) was measured at the Stable Isotope Ratio Facility for Environmental Research (University of Utah) following the standard protocol to determinate stable isotopes (http://ecophys.biology.utah.edu/sirfer.html). The δ values were then converted to carbon isotopic discrimination values (Δ). Δ was calculated according to Masle et al. (2005) using the equation Δ = (δa − δp)/1 + δp, where δa and δp are the δ of the source air and the plant, respectively. δ of the source air (δa) was assumed to be −8 per mil.
Gene Expression
Total RNA was isolated from 200 mg of developing leaves (less than 5 mm in length), the top part of the shoot and shoot apex of 25-d-old plants by using RNeasy kit (Qiagen). Complementary DNA was synthesized from 1 μg of total RNA using 0.1 μg oligo(dT) primer and reverse transcriptase (SuperScript III; Invitrogen). Reverse transcription-PCR was run for 40 cycles. Real-time reverse transcription-PCR analysis was carried out with a 7500 real-time PCR system with TaqMan fast universal PCR master mix (Applied Biosystems). The expression of TMM1 (At1g80080) and FAMA (At3g24140) was normalized to the expression of ACT8. The primers sequences were as follows: TMM1Fw, 5′-AACAGTCTTCGGGTCCTTCAC-3′; TMM1Rv, 5′-GCTTTCTCCTCATCCTCCACA-3′; FAMA1Fw, 5′-GACCATAACCAAACCCAACA-3′; FAMA1Rv, 5′-GCTCTCTTCCTCTTGCTCTTCA-3′; ACT8Fw, 5′-AGTGGTCGTACAACCGGTATTGT-3′; and ACT8Rv, 5′-GAGGATAGCATGTGGAACTGAGAA-3′.
Correlation of Expression of FAMA and TMM
The correlation between the expression of FAMA and TMM was analyzing using 633 data points corresponding to different developmental contexts and biotic or abiotic treatments (1–3 biological replicates per point) taken from 46 experiments (1,388 microarrays, www.arabidopsis.org). Data were normalized to the median of each experiment and transformed as ln (x + 1) as it was performed by Buchovsky et al. (2008).
Whole-Plant Transpiration Rate
Whole-plant transpiration rate was measured as described (Masle et al., 2005). Briefly, the night before transpiration was assayed, the pots containing one 35-d-old plant (vegetative stage) were watered at field capacity and the surface of the soil was wrapped with 0.025-mm thick transparent film (Rolopac) to avoid evaporation. The next day, pots were weighted immediately prior to the beginning of the photoperiod and 12 h later. Relative humidity during the photoperiod was 70% ± 4%. The rosette leaves were harvested immediately and photographed to determinate leaf area of each plant using the Photoshop v.7.0. Transpiration per plant per hour (mg of water h−1) was calculated as the difference between the initial and the final weight of each pot at the end of the photoperiod, divided by the number of hours of the photoperiod (12 h). The transpiration rate (mg of water cm−2 h−1) was calculated dividing the former parameter by the total leaf area of each plant. Units were converted to mmol of water m−2 s−1.
Leaf Photosynthesis
Leaf CO2 exchange responses to PAR were obtained by using a closed infrared gas analysis system (LI-COR 6200; LI-COR). CO2 exchange at 0, 200, 380, 710, and 1,150 μmol m−2 s−1 PAR was measured in fully expanded leaves of phyB-4, phyB-5, and wild-type Ler 30-d-old plants, using a 0.25-L chamber attached to a regulated portable R power (QB1205LI-670; Quantum Devices). Actual leaf temperature during measurements was between 27°C and 29°C and CO2 concentration was 390 μmol mol−1.
A portable gas-exchange system (LI-COR 6400; LI-COR) was used to obtain curves of leaf CO2 exchange against intercellular CO2 concentration in fully expanded leaves of phyB-5 and wild-type Ler 30-d-old plants. The area included in the 6-cm2 chamber was recorded for each leaf. Measurements of leaf CO2 exchange started at 400 μmol mol−1 CO2 in the reference chamber, decreased stepwise to 50 μmol mol−1, returned to 400 μmol mol−1, and increased stepwise to 1,200 μmol mol−1 CO2. Photosynthetic photon flux density was 1,200 μmol m−2 s−1 provided by the red and blue diodes of the gas-exchange system (6400-02B LED light source; LI-COR). Actual leaf temperature during measurements was between 27°C and 29°C. The flow rate of air was set at 300 μmol s−1. Ambient and reference CO2 concentrations are the concentration in the sample and reference infrared gas analyzers, respectively.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. phyB overexpression increases transpiration per area, stomata density, stomata index, and amphistomy level and reduces leaf area per plant and transpiration efficiency.
Note Added in Proof
While this article was under review, Casson et al. (Casson SA, Franklin KA, Gray JE, Grierson CS, Whitelam GC, Hetherington AM [2009] Phytochrome B and PIF4 regulate stomatal development in response to light quantity. Curr Biol 19: 229–234) demonstrated a role of phyB in the promotion of stomata density by irradiance. The latter is consistent with and complements the function of phyB in the response to light quality reported here.
Supplementary Material
Acknowledgments
We thank Juan Pablo Sáez and Carina Verónica González (both from IFEVA) for their help with stomata determinations, Amy Austin (IFEVA) for helping in the processing and analysis of the samples for isotope discrimination, Paloma Más (Instituto de Biología Molecular de Barcelona) for her kind provision of seeds of the 35S:PHYB:GFP line, and Antonio Hall (IFEVA) and Pedro Aphalo (Helsinki University) for their helpful comments. We are grateful to Instrumentalia S.A. for their kind lending of the LI-COR 6400.
This work was supported by grants from the University of Buenos Aires (grant no. G044 to J.J.C.), Agencia Nacional de Promoción Científica y Tecnológica (grant no. PICT 1026 to M.J.Y. and grant nos. PICT 32924 and 00492 to H.E.B.), International Centre for Genetic Engineering and Téchnology (grant no. CRP/ARG07–02 to J.J.C.), and Consejo Nacional de Investigaciones Científicas y Técnicas (grant no. PIP5958 to J.J.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jorge J. Casal (casal@ifeva.edu.ar).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aphalo PJ, Gibson D, Di Benedetto AH (1991) Responses of growth, photosynthesis, and leaf conductance to white light irradiance and end-of-day red and far-red pulses in Fuchsia magellanica Lam. New Phytol 117 461–471 [DOI] [PubMed] [Google Scholar]
- Assmann SM (1992) Effects of light quantity and quality during development on the morphology and stomatal physiology of Commelina communis. Oecologia 92 188–195 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Sánchez RA, Scopel AL, Casal JJ, Ghersa CM (1987) Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ 10 551–557 [Google Scholar]
- Baroli I, Dean Price G, Badger M, von Caemmerer S (2008) The contribution of photosynthesis to the red light response of stomatal conductance. Plant Physiol 146 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccalandro HE, Ploschuk EL, Yanovsky MJ, Sanchez RA, Gatz C, Casal JJ (2003) Increased phytochrome B alleviates density effects on tuber yield of field potato crops. Plant Physiol 133 1539–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchovsky AS, Strasser B, Cerdán PD, Casal JJ (2008) Suppression of pleiotropic effects of functional CRYPTOCHROME genes by TERMINAL FLOWER 1. Genetics 180 1467–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussis D, Von Groll U, Fisahn J, Altmann T (2006) Stomatal aperture can compensate altered stomatal density in Arabidopsis thaliana at growth light conditions. Funct Plant Biol 33 1037–1043 [DOI] [PubMed] [Google Scholar]
- Carabelli M, Possenti M, Sessa G, Ciolfi A, Sassi M, Morelli G, Ruberti I (2007) Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes Dev 21 1863–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Aphalo PJ, Sánchez RA (1987) Phytochrome effects on leaf growth and chlorophyll content in Petunia axillaris. Plant Cell Environ 10 509–514 [Google Scholar]
- Casal JJ, Fankhauser C, Coupland G, Blázquez MA (2004) Signalling for developmental plasticity. Trends Plant Sci 9 309–314 [DOI] [PubMed] [Google Scholar]
- Casson S, Gray JE (2008) Influence of environmental factors on stomatal development. New Phytol 178 9–23 [DOI] [PubMed] [Google Scholar]
- Cogliatti DH, Sánchez RA (1987) Influencia del fitocromo sobre el crecimiento foliar en Taraxacum officinale. Phyton 42 191–199 [Google Scholar]
- Coupe SA, Palmer BG, Lake JA, Overy SA, Oxborough K, Woodward FI, Gray JE, Quick WP (2006) Systemic signalling of environmental cues in Arabidopsis leaves. J Exp Bot 57 329–341 [DOI] [PubMed] [Google Scholar]
- Devlin PF, Robson PRH, Patel SR, Goosey L, Sharrock RA, Whitelam GC (1999) Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol 119 909–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel L, Pfannschmidt T (2008) Photosynthetic acclimation to light gradients in plant stands comes out of shade. Plant Signal Behav 3 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RA, Poulson ME, Edwards GE (1997) A method for measuring whole plant photosynthesis in Arabidopsis thaliana. Photosynth Res 52 263–269 [Google Scholar]
- Downs RJ, Hendricks SB, Borthwick HA (1957) Photoreversible control of elongation of pinto beans and other plants under normal conditions of growth. Bot Gaz 118 199–208 [Google Scholar]
- Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11 539–552 [Google Scholar]
- Frankland B, Letendre RJ (1978) Phytochrome and effects of shading on growth of woodland plants. Photochem Photobiol 27 223–230 [Google Scholar]
- Franklin KA, Whitelam G (2005) Phytochromes and shade-avoidance responses in plants. Ann Bot (Lond) 96 169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Nadeau J, Sack FD (2000) Oriented asymmetric divisions that generate the stomatal spacing pattern in Arabidopsis are disrupted by the too many mouths mutation. Plant Cell 12 2075–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan T, Müller-Mouleì P, Niyogi KK (2006) Photoprotection mutants of Arabidopsis thaliana acclimate to high light by increasing photosynthesis and specific antioxidants. Plant Cell Environ 29 879–887 [DOI] [PubMed] [Google Scholar]
- Gray J, Holroyd G, van der Lee F, Bahrami A, Sijmons P, Woodward F, Schuch W, Hetherington A (2000) The HIC signalling pathway links CO2 perception to stomatal development. Nature 408 713–716 [DOI] [PubMed] [Google Scholar]
- Hetherington A, Woodward F (2003) The role of stomata in sensing and driving environmental change. Nature 424 901–908 [DOI] [PubMed] [Google Scholar]
- Holmes MG, Klein WH (1985) Evidences for phytochrome involvement in light-mediated stomatal movement in Phaseolus vulgaris L. Planta 166 348–353 [DOI] [PubMed] [Google Scholar]
- Holmes MG, Sager JC, Klein WH (1986) Sensitivity to far-red radiation in stomata of Phaseolus vulgaris L.: rhythmic effects on conductance and photosynthesis. Planta 168 516–522 [DOI] [PubMed] [Google Scholar]
- Holmes MG, Smith H (1977. a) The function of phytochrome in the natural environment. I. Characterization of daylight for studies in photomorphogenesis and photoperiodism. Photochem Photobiol 25 533–538 [Google Scholar]
- Holmes M, Smith H (1977. b) The function of phytochrome in the natural environment. III. Measurement and calculation of phytochrome photoequilibria. Photochem Photobiol 25 547–550 [Google Scholar]
- Kasperbauer MJ (1971) Spectral distribution of light in a tobacco canopy and effects of end-of-day light quality on growth and development. Plant Physiol 47 775–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Rolf E, Spruit CJP (1980) Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol 100 147–160 [Google Scholar]
- Kwesiga F, Grace J (1986) The role of red/far-red ratio in the response of tropical tree seedlings to shade. Ann Bot (Lond) 57 283–290 [Google Scholar]
- Lake JA, Quick WP, Beerling DJ, Woodward FI (2001) Plant development: signals from mature to new leaves. Nature 411 154. [DOI] [PubMed] [Google Scholar]
- Larkin J, Marks M, Nadeau J, Sack F (1997) Epidermal cell fate and patterning in leaves. Plant Cell 9 1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Juez E, Buurmeijer WF, Heeringa GH, Kendrick RE, Wesselius JC (1990) Response of light-grown wild type and long hypocotyl mutant cucumber plants to end-of-day far-red light. Photochem Photobiol 52 143–149 [Google Scholar]
- Lynn D, Waldren S (2002) Physiological variation in populations of Ranunculus repens L. (creeping buttercup) from the temporary limestone lakes (turloughs) in the west of Ireland. Ann Bot (Lond) 89 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister CA, Ohashi-Ito K, Bergmann DC (2007) Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 445 537–540 [DOI] [PubMed] [Google Scholar]
- Más P, Devlin P, Panda S, Kay S (2000) Functional interaction of phytochrome B and cryptochrome 2. Nature 408 207–211 [DOI] [PubMed] [Google Scholar]
- Masle J, Gilmore SR, Farquhar GD (2005) The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 436 866–870 [DOI] [PubMed] [Google Scholar]
- Miyazawa SI, Livingston NJ, Turpin DH (2006) Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpa x P. deltoides). J Exp Bot 57 373–380 [DOI] [PubMed] [Google Scholar]
- Mott K, Gibson A, O'Leary J (1982) The adaptative significance of amphistomatic leaves. Plant Cell Environ 5 455–460 [Google Scholar]
- Mott KA, Michaelson O (1991) Amphistomy as an adaptation to high light intensity in Ambrosia cordifolia (Compositae). Am J Bot 78 76–79 [Google Scholar]
- Nadeau JA (2008) Stomatal development: new signals and fate determinants. Curr Opin Plant Biol 12 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD (2002) Control of stomatal distribution on the Arabidopsis leaf surface. Science 296 1697–1700 [DOI] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J (1993) Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol 102 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson S, Assmann SM (2007) The control of transpiration: insights from Arabidopsis. Plant Physiol 143 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst DF, Mott KA (1990) Intercellular diffusion limits to CO2 uptake in leaves. Plant Physiol 94 1024–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura J (2007) The drought environment: physical, biological and agricultural perspectives. J Exp Bot 58 113–117 [DOI] [PubMed] [Google Scholar]
- Pigliucci M, Schmitt J (1999) Genes affecting phenotypic plasticity in Arabidopsis: pleiotropic effects and reproductive fitness of photomorphogenic mutants. J Evol Biol 12 551–562 [Google Scholar]
- Rebetzke GJ, Condon AG, Richards RA, Farquhar GD (2002) Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. J Exp Bot 42 739–745 [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RA (2006) Physiological traits used in the breeding of new cultivars for water-scarce environments. Agric Water Manage 80 197–211 [Google Scholar]
- Robson PRH, Whitelam GC, Smith H (1993) Selected components of the shade-avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome B. Plant Physiol 102 1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Bejerano C (1981) Involvement of phytochrome in stomatal movement: effect of blue and red light. Physiol Plant 52 201–206 [Google Scholar]
- Schittenhelm S, Menge-Hartmann U, Oldenburg E (2004) Photosynthesis, carbohydrate metabolism, and yield of phytochrome-B-overexpressing potatoes under different light regimes. Crop Sci 44 131–143 [Google Scholar]
- Serna L, Torres-Contreras J, Fenoll C (2002) Clonal analysis of stomatal development and patterning in Arabidopsis leaves. Dev Biol 241 24–33 [DOI] [PubMed] [Google Scholar]
- Smith H (1982) Light quality, photoperception and plant strategy. Annu Rev Plant Physiol 33 481–518 [Google Scholar]
- Talbott LD, Shmayevich IJ, Chung Y, Hammad JW, Zeiger E (2003) Blue light and phytochrome-mediated stomatal opening in the npq1 and phot1 phot2 mutants of Arabidopsis. Plant Physiol 133 1522–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zhu J, Han SWEZ (2002) Phytochrome and blue light-mediated stomatal opening in the orchid, Paphiopedilum. Plant Cell Physiol 43 639–646 [DOI] [PubMed] [Google Scholar]
- Thiele A, Herold M, Lenk I, Quail PH, Gatz C (1999) Heterologous expression of Arabidopsis phytochrome B in transgenic potato influences photosynthetic performance and tuber development. Plant Physiol 120 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Woodward FI, Quick WP (2004) Systemic signalling in tobacco. New Phytol 161 193–198 [Google Scholar]
- Wagner D, Tepperman JM, Quail PH (1991) Overexpression of phytochrome B induces a short hypocotyl phenotype in transgenic Arabidopsis. Plant Cell 3 1275–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Dietzel L, Brautigam K, Pfannschmidt T (2008) The long-term response to fluctuating light quality is an important and distinct light acclimation mechanism that supports survival of Arabidopsis thaliana under low light conditions. Planta 228 573–587 [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen X, Xiang CB (2007) Stomatal density and bio-water saving. J Integr Plant Biol 49 1435–1444 [Google Scholar]
- Willmer C, Fricker M (1996) Stomata, Ed 2. Chapman and Hall, London
- Yanovsky MJ, Casal JJ, Whitelam GC (1995) Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ 18 788–794 [Google Scholar]
- Yu Q, Zhang Y, Liu Y, Shi P (2004) Simulation of the stomatal conductance of winter wheat in response to light, temperature and CO2 changes. J Exp Bot 93 435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.