Abstract
The strawberry (Fragaria × ananassa ‘Chandler’) fruit undergoes a fast softening during ripening. Polygalacturonase (PG) activity is low during this process, but two ripening-related PG genes, FaPG1 and FaPG2, have been cloned. Both genes were up-regulated during fruit ripening and were also negatively regulated by auxin. To further assess the role of FaPG1 on strawberry softening, transgenic plants containing an antisense sequence of this gene under the control of the 35S promoter (APG lines) were obtained. Sixteen out of 30 independent transgenic lines showed fruit yields similar to those of the control. Several quality parameters were measured in ripe fruits from these 16 lines. Fruit weight was slightly reduced in four lines, and most of them showed an increase in soluble solid content. Half of these lines yielded fruits significantly firmer than did the control. Four APG lines were selected, their ripened fruits being on average 163% firmer than the control. The postharvest softening of APG fruits was also diminished. Ripened fruits from the four selected lines showed a 90% to 95% decrease in FaPG1 transcript abundance, whereas the level of FaPG2 was not significantly altered. Total PG activity was reduced in three of these lines when compared with control fruits. Cell wall extracts from APG fruits showed a reduction in pectin solubilization and an increase in pectins covalently bound to the cell wall. A comparative transcriptomic analysis of gene expression between the ripened receptacle of the control and those of the APG fruits (comprising 1,250 receptacle expressed sequence tags) did not show any statistically significant change. These results indicate that FaPG1 plays a central role in strawberry softening.
Cultivated strawberry (Fragaria × ananassa) is one of the most economically important small fruits. World production and consumer demand for strawberry have been increasing since the 1970s. Today, it represents the third most valuable crop in the noncitrus fruit category, behind grapes and apples, within the United States (Sjulin, 2003). Strawberry is a soft fruit characterized by a great softening during its ripening stage (Manning, 1993). The rapid loss of firm texture determines the short postharvest life of this fruit and the enormous cost of spoiling associated with strawberry bruising and subsequent pathogen infection. Several factors contribute to the overall fruit texture (e.g. turgor pressure, cell shape, mechanical properties of the cell walls, and the strength and extension of adhesion areas between neighboring cells; Harker et al., 1997). However, it is generally believed that the cell wall disassembly and the reduction of cell adhesion, resulting from dissolution of the middle lamella, are the main factors that cause fruit softening (Brummell and Harpster, 2001; Brummell, 2006). During strawberry ripening, the middle lamella of the cortical parenchyma cells is extensively degraded (Perkins-Veazie, 1995), and the cells appear separated by a considerable intercellular space and a little cell-to-cell contact area (Redgwell et al., 1997b). At the molecular level, the largest changes in the cell wall occur in the pectin fraction. The proportion of soluble pectins increases from 30% in undeveloped fruits to 65% in ripe fruits (Huber, 1984). However, this solubilization is not accompanied by pectin depolymerization (Huber, 1984). By contrast, the Mr of hemicellulose is slightly reduced during strawberry ripening (Huber, 1984; Rosli et al., 2004). Several cell wall-degrading enzymes have been involved in strawberry softening. Cellulase activity increased 6-fold during fruit development, and the largest increase was associated with overripe fruits (Abeles and Takeda, 1990). Two cellulase genes, cel1 and cel2, whose expression is correlated with strawberry ripening, have been cloned (Harpster et al., 1998; Llop-Tous et al., 1999; Trainotti et al., 1999). However, the down-regulation of these genes by antisense technology did not modify fruit texture, even when both genes were simultaneously silenced (Woolley et al., 2001; Palomer et al., 2006). As to pectin-degrading enzymes, polygalacturonase (PG) appears to play a minor role in strawberry softening. PG activity is low in ripe strawberry fruit (Perkins-Veazie, 1995), which would be in accordance with the absence of pectin depolymerization during ripening. By contrast, pectin methyl esterase increases as the strawberry ripens (Barnes and Patchett, 1976), but the degree of esterification of strawberry polyuronides is low and does not change during development (Wade, 1964). More recently, an increased expression of a fruit-specific esterase gene, FaPE1, has been reported (Castillejo et al., 2004). In general, classical studies assumed a minor role of pectin processing in strawberry softening, but recent evidence, such as the increased firmness of ripe fruits with pectate lyase down-regulated (Jiménez-Bermúdez et al., 2002), challenges this assumption. This enzyme cleaves deesterified polyuronides by a β-elimination reaction, in contrast to the hydrolytic mechanism of PG. Its silencing in ripe strawberry fruits reduced cell wall swelling, pectin solubilization, and depolymerization of the pectin fraction covalently linked to the cell wall (Santiago-Doménech et al., 2008).
PG is the best characterized cell wall hydrolytic enzyme in fruits and the first to be analyzed using transgenic methods. In tomato (Solanum lycopersicum), transgenic fruits expressing an antisense PG sequence resulted in 99% suppression of PG mRNA. This silencing did not reduce pectin solubilization but suppressed pectin depolymerization (Smith et al., 1990). Despite these changes, fruit softening was not altered, but important horticultural traits, such as storage life of overripe fruits, postharvest pathogen susceptibility (Kramer et al., 1992), and viscosity of processed tomato paste (Brummell and Labavitch, 1997), were improved. Altogether, these studies led to the hypothesis that PG activity alone is not sufficient to affect texture, but it contributes significantly to tissue deterioration in the later stages of ripening (Hadfield and Bennett, 1998; Giovannoni, 2001). As far as we know, the role of PG in fruit softening has not been analyzed by transgenic technology in species other than tomato. In the case of strawberry, two ripening-related genes codifying putative functional PGs have been described. Salentijn et al. (2003) analyzed gene expression by cDNA microarrays on two cultivars differing in fruit firmness and detected an up-regulation of a clone, B4 (FaPG2, AY280662), putatively encoding a PG, on the soft cultivar. A different PG gene, spG (FaPG1, AF380299), was cloned by Redondo-Nevado et al. (2001). The expression pattern of this gene indicated that it could be involved in the release of pectic oligosaccharins, which could be elicitors of the ripening process, rather than the bulk hydrolysis of pectins within the cell wall, since its expression was only observed at the onset of ripening (Redondo-Nevado et al., 2001). This hypothesis pointed at FaPG1 as a key gene in the regulation of strawberry softening. The aim of this work was to assess the role of FaPG1 on strawberry softening, underexpressing the gene by antisense transformation. In contrast to the above-mentioned results in tomato, we have observed that inhibition of FaPG1 improves significantly the firmness of ripe fruits and the postharvest behavior of strawberries without affecting other fruit characteristics or fruit yield.
RESULTS
Expression Pattern of FaPG1 and FaPG2 Genes during Fruit Development
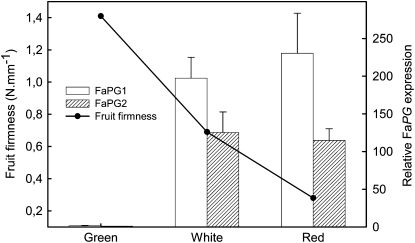
As an initial step to assess the role of PG on strawberry fruit softening, the expression of both FaPG1 and FaPG2 genes was quantified by quantitative real-time PCR (QRT-PCR) at different fruit developmental stages (Fig. 1). FaPG1 gene expression increased notably from green to white stage, and contrary to previous results obtained by Redondo-Nevado et al. (2001), the expression of the FaPG1 gene was maintained at a high level also in the ripened fruit. Also, the FaPG2 gene showed an expression pattern similar to that of the FaPG1 gene, although in this case the maximum gene expression level was achieved at the white stage. Fruit receptacle firmness declined linearly from the green to the ripened stage (Fig. 1).
Figure 1.
Expression of FaPG1 and FaPG2 genes, in relative units, and evolution of fruit firmness during fruit development. Bars represent means ± sd of three independent RNA quantifications.
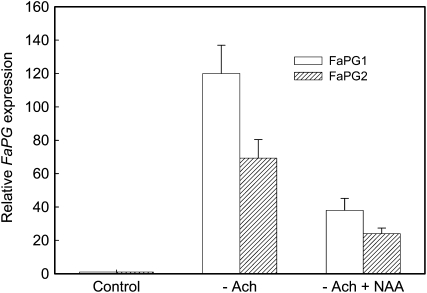
In strawberry fruit, a gradual decline in the supply of auxin from achenes in the later stages of growth has been implicated as the basis of ripening (Nitsch, 1950; Perkins-Veazie, 1995). Thus, removing the achenes in developing fruits accelerates ripening and induces the expression of ripening-related genes, whereas the application of a synthetic auxin to deachened fruits prevents this process (Manning, 1994). To investigate if FaPG1 and FaPG2 genes are under the control of auxin, midsized green fruits were carefully deachened and kept “in planta” and gene expression was studied after 5 d. Additionally, deachened fruits were treated with a lanolin paste containing naphthalene acetic acid (NAA), a synthetic auxin. The expression of both PG genes increased substantially after 5 d in deachened fruits and could be largely reversed by the auxin treatment (Fig. 2). These results show that the expression of FaPG1 and FaPG2 genes is negatively regulated by auxin.
Figure 2.
Effect of auxin on FaPG gene expression, expressed as relative units. –Ach, Deachened fruits; –Ach + NAA, deachened fruits treated with lanolin containing 1% NAA. Bars represent means ± sd of three independent RNA quantifications.
Obtaining FaPG1 Antisense Transgenic Plants
Three independent transformation experiments were performed with more than 200 Agrobacterium tumefaciens-inoculated explants per experiment. After 20 weeks of selection in 25 mg L−1 kanamycin, 80 kanamycin-resistant shoots were recovered, which represents an average transformation rate of 12.6%. After micropropagation, 30 independent anti-FaPG1 transgenic lines (APG lines) were acclimated, transferred to the greenhouse, and propagated by runners for agronomic and biochemical analysis of the daughter plants. The presence of the transgenes in these putative transgenic lines was confirmed by PCR amplification of a 220-bp fragment belonging to the nptII gene (data not shown).
Phenotypic Analysis of Transgenic Plants
Transgenic APG plants showed a vegetative growth pattern similar to that of the control. Dwarfism and leaf chlorosis, the most common variants as a result of in vitro regeneration in strawberry (Nehra et al., 1992), were not observed on either mother or daughter plants. In relation to fruit yield, during the first year of analysis, almost 50% of transgenic lines showed a fruit yield similar to that of control plants, in terms of grams of fruit per plant. The rest of the lines yielded a low number of fruits, and three of them did not set fruits during the first year of analysis. A similar percentage of low fruit-producing plants has been obtained in previous work (Jiménez-Bermúdez et al., 2002; Palomer et al., 2006), and this effect could be mainly attributed to somaclonal variations induced by the in vitro regeneration phase.
To estimate fruit quality, well-shaped fruits with weights higher than 5 g were harvested at the stage of full ripening, and the weight, color, soluble solids, and firmness were recorded. Only 16 transgenic lines produced enough fruits to be analyzed. Results are summarized in Table I. As it is generally found in micropropagated strawberry plants (López-Aranda et al., 1994), mean fruit weight was slightly lower in all the transgenic lines, although the differences were only statistically significant in four lines (APG21, -27, -32, and -35). Color did not differ between the control and the transgenic fruits. By contrast, soluble solids increased significantly in most of the APG lines. As to firmness, eight of the 16 transgenic lines analyzed showed a fruit firmness higher than the control fruits, the differences being statistically significant. The increment in firmness ranged from 144% of APG2 to 111% of APG53 fruits, with an average increment in firmness of 129.8%.
Table I.
Characteristics of ripened fruits in control and transgenic APG plants
Data represent means ± sd of a minimum of 10 fruits per line, evaluated during the first year of analysis. Lines that are significantly different from the control by Mann-Whitney U test at P = 0.05 are indicated with asterisks.
| Genotype | Weight | Color | Soluble Solids | Firmness |
|---|---|---|---|---|
| g | °brix | N | ||
| Control | 12.3 ± 4.8 | 5.9 ± 0.3 | 8.2 ± 0.9 | 3.4 ± 0.5 |
| APG1 | 11.2 ± 4.7 | 5.8 ± 0.4 | 8.6 ± 1.4 | 3.6 ± 0.9 |
| APG2 | 10.2 ± 3.2 | 5.7 ± 0.5 | 10 ± 1.7* | 4.9 ± 1.0* |
| APG5 | 10.9 ± 4.0 | 5.7 ± 0.4 | 9.6 ± 2.1* | 4.5 ± 0.9* |
| APG10 | 11.1 ± 2.0 | 5.3 ± 0.4 | 8.2 ± 1.2 | 4.7 ± 0.6* |
| APG12 | 11.0 ± 3.9 | 5.1 ± 0.5 | 10.3 ± 2.2* | 3.3 ± 0.3 |
| APG14 | 10.5 ± 4.0 | 5.5 ± 0.5 | 8.6 ± 1.9 | 3.4 ± 0.5 |
| APG21 | 9.0 ± 2.9* | 5.6 ± 0.5 | 9.4 ± 2.1* | 3.6 ± 0.6 |
| APG25 | 11.8 ± 5.3 | 5.6 ± 0.5 | 9.6 ± 1.7* | 3.7 ± 0.6 |
| APG27 | 7.8 ± 3.1* | 5.7 ± 0.5 | 9.5 ± 1.3* | 3.3 ± 0.7 |
| APG28 | 11.1 ± 4.1 | 5.7 ± 0.4 | 9.3 ± 1.3* | 3.5 ± 0.8 |
| APG29 | 12.2 ± 5.5 | 5.7 ± 0.4 | 9.4 ± 1.9* | 4.5 ± 0.7* |
| APG32 | 9.9 ± 3.8* | 5.7 ± 0.4 | 8.4 ± 0.9 | 3.5 ± 0.7 |
| APG35 | 9.3 ± 4.1* | 5.8 ± 0.4 | 9.4 ± 2.1 | 4.3 ± 1.2* |
| APG44 | 9.6 ± 1.6 | 5.7 ± 0.5 | 9.6 ± 0.8* | 4.1 ± 0.9* |
| APG53 | 11.6 ± 5.0 | 5.5 ± 0.5 | 9.8 ± 1.8* | 3.8 ± 0.7* |
| APG62 | 10.1 ± 3.0 | 5.8 ± 0.4 | 9.0 ± 1.6 | 4.5 ± 1.1* |
Based on optimal fruit yield and increase in fruit firmness, lines APG2, -5, -29, and -62 were selected for further studies. These lines have been vegetatively propagated by runners yearly, since cultivated strawberry is not propagated by seed. The firmness of the ripe fruit was evaluated on the daughter plants during 3 consecutive years. The four selected APG lines showed higher fruit firmness than did the control in the 3 years of analysis, the average increment in firmness being 162.7% ± 3.6%. Thus, the transgenic phenotype was stably maintained during vegetative propagation. On the other hand, no significant differences in fruit weight or soluble solids between the control and the transgenic lines were observed during the 3 years, except for APG2, which recurrently showed higher contents of soluble solids than control fruits.
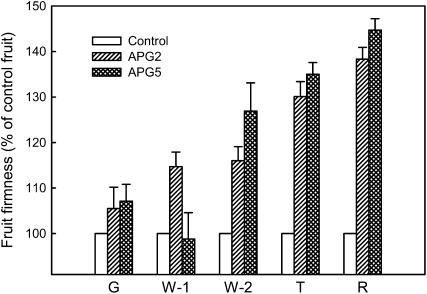
In the case of APG2 and APG5, firmness was measured during different stages of fruit development, and the results, expressed as a percentage of control fruits, are shown in Figure 3. No significant differences in firmness between control and transgenics were detected at the green stage. The same was observed at the white 1 stage, when achenes were still immature in the APG5 line; the APG2 fruits, though, showed incremental firmness when compared with the control. After these early phases of fruit development, fruit firmness increased steeply in both transgenic lines, reaching the maximum difference between control and transgenic fruits at the red stage. These results are in accordance with the expression pattern of the FaPG1 gene shown in Figure 1.
Figure 3.
Fruit firmness at different stages of maturation in two transgenic APG lines. Firmness was evaluated during the second year of analysis and is expressed as the percentage of control fruit firmness at each developmental stage. G, Green fruit; W-1, white fruit with green achenes; W-2, white fruit with brown achenes; T, turning fruit; R, full red fruit. Bars represent means ± se of a minimum of 20 fruits per line and stage.
Postharvest Quality of APG Fruits
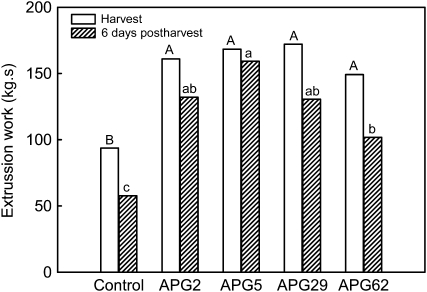
To analyze the behavior of transgenic APG fruits during postharvest, ripened fruits from the four APG selected lines were stored for 4 d at 5°C. Firmness was evaluated after an additional 2 d at 25°C. In the puncture test, the bioyield point was measured in individual fruits both before and after the postharvest period. At harvest, the bioyield point of control fruit was 1.4 ± 0.4 N. In the case of APG fruits, no differences in this parameter were found among the four lines analyzed, the average mean value being 2.1 ± 0.6 N, 151.4% higher than that obtained from control fruits. After the postharvest treatment, the bioyield point decreased in both control and transgenic fruits, but the differences between both genotypes were maintained (1.1 ± 0.9 versus 1.6 ± 1.3 N in control and APG fruits, respectively). However, estimating fruit firmness by this puncture test after 6 d of postharvest was difficult because a high percentage of fruits did not show a clear bioyield point due to excessive softening. Thus, firmness was also evaluated by an Ottawa cell test, which is based on the measurement of the force needed to extrude a pool of five to six fruits through a holed plate. Transgenic fruits showed significantly higher values of extrusion work than the control fruits both at harvest and after 6 d of postharvest (Fig. 4). Interestingly, the decrement in firmness after the postharvest treatment was reduced in APG fruits, 19.8% in transgenic versus 38.2% in control fruits.
Figure 4.
Firmness of control and transgenic ripe APG fruits at harvest and after storage for 4 d at 5°C plus 2 d at 25°C. Firmness was measured with a TAXT-Plus texturometer using an Ottawa cell. Bars with different letters indicate statistically significant differences by T2-Tamhane in the case of fruits at harvest or by lsd in postharvest fruits, both at P = 0.05. Uppercase letters represent mean separation at harvest, and lowercase letters represent mean separation at postharvest.
Molecular Analysis of Selected APG Lines
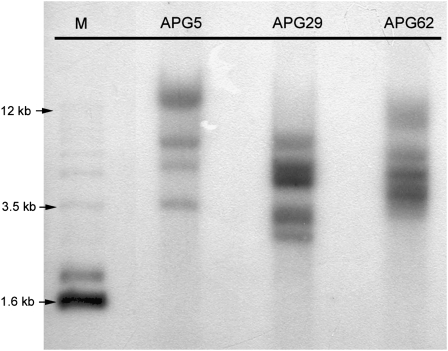
The stable integration of the antisense FaPG1 sequence in the selected lines was confirmed by Southern-blot analysis using a 2.3-kb probe, which included part of the 35S promoter and the nptII gene (Fig. 5). All of the transgenic lines showed between four and five hybridization signals.
Figure 5.
Southern-blot analysis of DNA isolated from selected APG lines. Filter was hybridized with a 2.3-kb probe, including part of the 35S promoter and the nptII gene. M, Molecular mass marker.
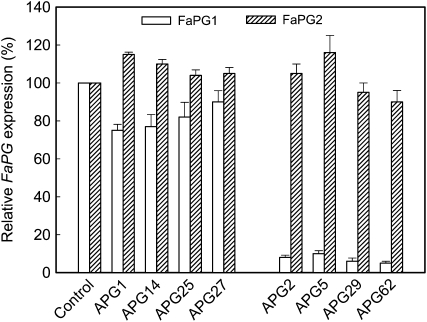
To quantify FaPG gene expression levels, mRNA was extracted from ripe fruits of the four selected APG lines and analyzed by QRT-PCR. Four transgenic APG lines, which showed similar fruit firmness as the control (APG1, -14, -25, and -27), were also included in this analysis. A significant down-regulation of the FaPG1 gene was observed in the four selected APG lines, the silencing ranging from 90% in APG5 to 95% in APG62 (Fig. 6). However, FaPG2 gene expression was not down-regulated in the same transgenic fruits (Fig. 6), and even more, APG5 showed a slight increase in FaPG2 mRNA levels when compared with the control. As to APG lines that did not show an improved, firmer phenotype, FaPG1 down-regulation was quite low in these lines, with silencing ranging from 25% in APG1 to 10% in APG27 (Fig. 6). FaPG2 expression level in these lines was also unaffected. These results clearly indicate that fruit firmness was negatively correlated with FaPG1 expression. Furthermore, the strong silencing of FaPG1 did not significantly affect the expression of FaPG2 in transgenic fruits.
Figure 6.
Expression of FaPG genes in ripened fruits from transgenic APG lines showing the “firmer fruit” phenotype (APG2, -5, -29, and -62) and those not showing the phenotype (APG1, -14, -25, and -27). The level of gene expression was evaluated by QRT-PCR; it is expressed as the percentage of gene expression in control nontransformed fruits. Bars represent means ± sd of three independent quantifications.
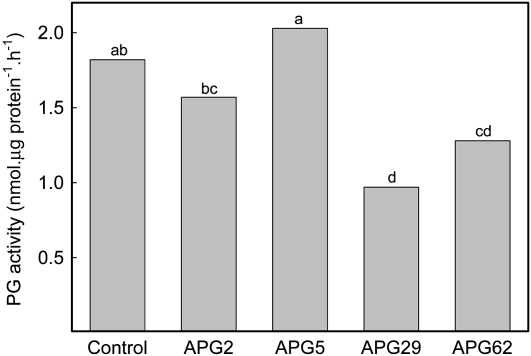
PG activity was also measured in protein extracts from ripe fruits of the four selected APG lines, following the protocol described by Villarreal et al. (2008). Total PG activity was reduced in APG2, -29, and -62 when compared with control fruits, with decrements ranging from 13.5% in APG2 to 47% in APG29 (Fig. 7). By contrast, total PG activity in extracts from APG5, which showed the lowest FaPG1 silencing, was similar to that in control fruits (Fig. 7).
Figure 7.
Total PG activity in ripened fruits from control and selected APG lines. Data correspond to three independent extractions per line. Bars with different letters indicate statistically significant differences by lsd at P = 0.05.
Cell Wall Analysis
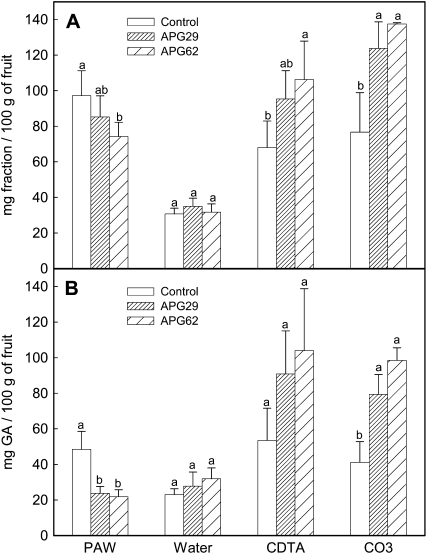
Cell wall from ripe fruits of lines APG29 and APG62 was extracted according to Redgwell et al. (1992) using PAW (phenol:acetic acid:water, 2:1:1, w/v/v) for enzyme inactivation. The yield of cell wall material (CWM) obtained per fresh weight of fruit was significantly higher in both transgenic lines, 0.90 ± 0.06 versus 1.08 ± 0.1 g per 100 g of fruit, in the control and transgenic fruits, respectively. By contrast, the amount of the soluble PAW fraction, which contains in vivo solubilized pectins, was lower in transgenic fruits than in the control (Fig. 8A). Pectin fractions from CWM were sequentially extracted with water, cyclohexane-trans-1,2-diaminetetraacetic acid (CDTA), and sodium carbonate. The results obtained are also shown in Figure 8A. No differences in the water fraction were observed between control and transgenic CWM. However, the amounts of the ionically bound (CDTA) and covalently bound (carbonate) fractions were both significantly higher in the transgenic fruits. Uronic acid was measured in all of these fractions, and the results are shown in Figure 8B. Transgenic PAW fractions contained less uronic acid than the control, but the CDTA and, especially, the carbonate fractions were enriched in pectins in the case of transgenic fruits. As previously observed, no differences in the content of uronic acid in the water fraction were detected between the control and the transgenic fruits. It is noteworthy that both APG lines showed the same cell wall modification pattern. Altogether, these results indicate a lower pectin solubilization in transgenic APG fruits.
Figure 8.
Cell wall analysis of ripe fruits from control and transgenic APG lines APG29 and APG62. A, Yield of the different fractions obtained after sequential extraction of the CWM. B, Uronic acid content, expressed as mg of GalUA per 100 g of fruit, in the different cell wall fractions. Bars represent means ± sd of five independent fractionations. Within each fraction, bars with different letters indicate statistically significant differences by lsd at P = 0.05.
Transcriptomic Analysis of Ripened APG Transgenic Fruits
To determine if oligogalacturonic acids (OGAs) potentially released by FaPG1 could play a physiological role in the regulation of gene expression patterns related to the receptacle ripening process, we have done a transcriptomic comparative study between red, ripened fruit receptacles from transgenic line APG29 versus control fruit receptacles. We used a microarray platform containing 1,250 ESTs corresponding to genes expressed in the strawberry fruit receptacle. In this study, no statistically significant differences between the transcriptome of control and transgenic fruits were found (Supplemental Table S1). This result does not support the direct involvement of FaPG1 in the production of OGAs, which acted as developmental signals during the strawberry fruit-ripening process. However, we should be cautious with this conclusion, since important genes may have been missed with the array format used.
DISCUSSION
The presence of PG activity during strawberry fruit development has been an issue of discussion. Early works did not detect PG activity in strawberry fruit (Huber, 1984; Abeles and Takeda, 1990). Nogata et al. (1993) found a low level of endo- and exo-PG, which decreased consistently during the development from a small green to an overripe fruit. This activity consisted of three isoenzymes, one with endo- and exo-PG activity and the rest with exo-type PG. Contrary to these research findings, Villarreal et al. (2008) recently found that PG activity increases in strawberry fruit during development and that total PG activity correlates positively with fruit softening. Similarly, Lefever et al. (2004) found that softness in strawberry varieties correlated positively with PG activity in ripening fruits. At the molecular level, three ripening-related PG genes have been cloned. The B4 or FaPG2 PG gene shows homology with Arabidopsis and microbial PGs (Salentijn et al., 2003). Based on northern-blot analysis, the spG or FaPG1 gene was initially thought to be expressed mainly in the white fruit at the onset of ripening (Redondo-Nevado et al., 2001). However, in this work, a more detailed study by QRT-PCR analysis has shown that FaPG1 expression was maintained at high levels in ripe fruits. A similar result was obtained by Villarreal et al. (2008) in different strawberry cultivars, and interestingly, the highest FaPG1 expression level occurred in the softest cultivar. Besides these two genes, a third putative PG gene, T-PG, has been isolated by Villarreal et al. (2008). This cDNA is identical to FaPG1, but it showed a deletion of 85 bp that caused a frameshift and produced an inactive protein. In this work, FaPG1 gene silencing resulted in a significant increment in fruit firmness at the ripening stage, without affecting other fruit characteristics such as color, weight, or soluble solids. Furthermore, in the four anti-PG lines selected, this phenotype has been stably maintained through vegetative propagation for several years. Additionally, fruit softening several days after harvest was reduced in the transgenic APG fruits. These results indicate that the product of the FaPG1 gene plays a crucial role in establishing strawberry fruit texture. Experiments are in progress to obtain transgenic lines overexpressing FaPG1 to firmly confirm this hypothesis.
The importance of pectin processing in strawberry fruit ripening has previously been experimentally supported. In a previous work, we obtained transgenic strawberry plants expressing an antisense sequence of a fruit-specific pectate lyase gene (Jiménez-Bermúdez et al., 2002). Ripened fruits from these plants, which showed a high silencing of the gene, were also significantly firmer than the control fruits. However, the firmness increment, as a result of FaPG1 inhibition, had been on average 25% higher than that achieved by the pectate lyase silencing. On the other hand, most of the antipectate lyase plants showed a decrease in yield due to a reduction in both fruit set and fruit weight. This result could be related to a putative role of pectate lyase activity on pollen tube emergence and/or pollen tube growth down the style. In contrast, the expression of the antisense FaPG1 did not significantly affect fruit yield, and almost 50% of the lines showed yields similar to those of control nontransformed plants. The yield reduction in the rest of the APG lines could be ascribed to the in vitro tissue culture phase, since a decrease in fruit size and yield is generally observed when micropropagated strawberry plants are grown directly for fruit production (López-Aranda et al., 1994). High levels of exo-PG activity have been measured in the pollen of many species (Hadfield and Bennett, 1998). As in pectate lyase, the role of PG in pollen tubes would be to degrade the walls on the stylar cells to allow penetration of the pollen tube or, alternatively, to act on the tube cell wall to facilitate growth. Pollen PGs are included in clade C and are likely to express exo-PGs (Hadfield et al., 1998). Although Villarreal et al. (2008) included the FaPG1 gene within this clade, northern-blot analysis showed the absence of FaPG1 expression in the reproductive tissue (Redondo-Nevado et al., 2001; Villarreal et al., 2008); therefore, the pollination process is unlikely to be affected by FaPG1 down-regulation.
The constitutive expression of the antisense FaPG1 in the four selected transgenic lines reduced the levels of FaPG1 mRNA by 90% to 95% when compared with control fruits. As expected, the expression of the other PG gene, FaPG2, was not significantly modified, as both genes share a low homology. Total PG activity was measured in ripened fruits. In the control, PG activity was similar to that reported by Villarreal et al. (2008) in strawberry soft cultivar Toyonaka, about 50-fold lower than PG activity found in ripe tomato fruits (Carrington et al., 1993). In transgenic APG fruits, total PG activity was only reduced in the three most FaPG1-silenced selected lines, but even in these lines, the decrements in PG activity were low. The functional analysis of cell wall activities by single gene silencing is usually constrained by the complementary action of different isoenzymes within the same protein family (Vicente et al., 2007). Apparently, this complementary action could not be ascribed to FaPG2, since the expression of this gene in the APG lines was unaffected, with the exception of APG5 fruits, which showed a slight increment in FaPG2 mRNA level. The low decrement in PG activity as a result of a strong FaPG1 silencing could indicate that most PG activity in strawberry fruits is due to the expression of FaPG2 or other undiscovered PG genes. Interestingly, the presence of the firmer phenotype in the transgenic lines, despite the low decrement in PG activity, could indicate that both FaPG1 and FaPG2 PGs do not act synergistically and could have different functions in the ripening process. Additionally, cell wall targets for both PG enzymes may be different.
At the cell wall level, the main features of strawberry softening are the reduction of cell wall content, a moderate increase in soluble pectins without pectin depolymerization, and a slight hemicellulose depolymerization (Knee et al., 1977; Huber, 1984; Redgwell et al., 1997b; Koh and Melton, 2002; Rosli et al., 2004). Apparently, the loss of neutral sugars, especially Gal, does not correlate with softening (Redgwell et al., 1997a). The different methodologies used to isolate the cell wall and carbohydrate fractions make difficult the achievement of a clear picture of the changes taking place during strawberry softening. In our study, we have employed the protocol described by Redgwell et al. (1992), based on the use of PAW to inactivate endogenous cell wall enzymes. Studies performed on strawberry fruits at different developmental stages with a similar cell wall extraction methodology showed an increase in the amount of the phenol fraction as well as an enriched proportion of uronic acid on this fraction as the fruits ripen (Redgwell et al., 1997b; Koh and Melton, 2002). PAW carbohydrates represent, jointly with the water fraction, those solubilized by in vivo processes but remaining in the apoplast (Redgwell et al., 1992; Carrington et al., 1993). Contrary to PAW, the CDTA and CO3Na2 fractions, which contain, respectively, pectins ionically and covalently linked to the cell wall (Brummell, 2006), declined during ripening (Koh and Melton, 2002). Rosli et al. (2004) also found a decrease in the amount of hydrochloric acid-soluble pectins, which would be equivalent to the CO3Na2 fraction, during strawberry ripening. Presumably, pectin solubilization occurs at the expense of the sodium-carbonate soluble pectins (Brummell, 2006). Several hypotheses have been proposed to explain this solubilization. Huber (1984) pointed out a role for the synthesis of a modified, more freely soluble form of pectins during ripening. Koh and Melton (2002) postulated that solubilization could be associated with the disentanglement of pectins in the cell walls due to the gradual degradation of arabinan side chains or with the cleavage of linkages between pectins and hemicelluloses. However, Rosli et al. (2004) suggested that pectin solubilization in Toyonaka could be related to a slight depolymerization of covalently linked pectins. The analysis of cell wall from antipectate lyase fruits also supports a role for pectin depolymerization in strawberry softening (Santiago-Doménech et al., 2008). Our results confirm that FaPG1 contributes significantly to pectin solubilization and fruit softening, despite the low PG activity detected in ripened strawberry fruit. In the transgenic APG fruits, the yield of CWM on a fresh weight basis was significantly higher than that in control fruits. Conversely, the soluble PAW fraction, as well as the uronic acid present within this fraction, was significantly reduced as a result of FaPG1 silencing. Contrary to soluble polymers, the amounts of CDTA and CO3Na2 fractions were higher and contained more uronic acids in transgenic APG lines than in control fruits. These results clearly indicate that pectin solubilization is diminished in fruits with low FaPG1 expression, and this is correlated with a firmer fruit phenotype. Along this line, postharvest manipulations that slightly reduce softening, such as heat or calcium treatment, increase ionically or covalently bound pectins (Lara et al., 2004; Vicente et al., 2005).
Historically, PG has been implicated as the primary agent of polyuronide degradation, and hence fruit softening, in several commodities that showed a peak of PG activity during ripening (e.g. tomato, avocado [Persea americana], and peach [Prunus persica]; Hadfield and Bennett, 1998). However, functional analyses of PG genes by transgenic experiments in tomato have deemphasized the role of this enzyme in fruit softening. The down-regulation of PG mRNA accumulation by constitutive expression of an antisense PG gene in tomato (Carrington et al., 1993) induced cell wall changes in a way similar to that described for APG strawberry fruits. However, these modifications have a very modest effect on tomato fruit softening (Smith et al., 1990; Kramer et al., 1992). Additionally, the up-regulation of a PG gene in the nonsoftening tomato mutant rin failed to induce softening, although polyuronide solubilization was restored to similar levels found in control fruits (Giovannoni et al., 1989). Consequently, the most accepted opinion is that PG-mediated pectin disassembly is neither necessary nor sufficient for fruit softening, especially at the initial stages of the process, and therefore, efforts to modulate fruit softening should be focused on other cell wall enzymes (Hadfield and Bennett, 1998; Brummell and Harpster, 2001; Vicente et al., 2007). However, the results obtained in this work show that the role of PG on fruit ripening merits a reevaluation and further testing in different fruit models. Through the antisense silencing of FaPG1, we have demonstrated that this gene plays a crucial role in strawberry pectin solubilization and softening, in spite of the low PG activity present in this fruit. The contrasting results between tomato and strawberry could be due to the different botanical origins of these fruits. Botanically, a strawberry is an aggregate fruit originating from the development of a flower receptacle, whereas a tomato fruit develops directly from the ovary. It is likely that the softening process occurs in different ways in both tissues. Understanding the mechanism by which PG induces this process in strawberry needs further investigation. As a first hypothesis, FaPG1 could be involved in the release of pectin oligosaccharins that will induce fruit ripening. In the strawberry-ripening process, pectin breakdown fragments of [1→4]-α-linked oligogalacturonides can, potentially, be released through the action of PG or other pectinolytic enzymes. These OGAs have been demonstrated to modify gene expression and to play a role in eliciting different cellular responses as a plant-pathogen interaction in strawberry fruits (Osorio et al., 2008). However, in our case, the absence of significant transcriptomic changes in the microarray studies does not support this explanation. As an alternative, FaPG1 product could act within specific homogalacturonan domains, releasing more freely soluble pectins. Along this line, detailed studies of cell wall changes induced by silencing of a pectate lyase gene showed a decreased depolymerization of soluble and carbonate pectins (Santiago-Doménech et al., 2008).
In conclusion, the experiments described here provide evidence that the silencing of the FaPG1 PG gene in strawberry significantly reduces the softening of ripened fruits at harvest and after several days of storage. This increase in firmness in transgenic fruits was mainly related to a decrease in pectin solubilization and an enhancement of the amount of pectins covalently bound to the cell wall. This work suggests that the modification of pectin metabolism through PG gene inhibition can be a useful approach to improve the texture of a soft fruit such as strawberry with a high economic impact.
MATERIALS AND METHODS
Plant Material and Agrobacterium-Mediated Transformation
The clone FaPG1-cDNA containing a 0.6-kb DNA fragment from the PG strawberry (Fragaria × ananassa) gene FaPG1 (spG, AF380299; Redondo-Nevado et al., 2001) was digested with EcoRI and cloned in the antisense orientation in the pGUSINT plasmid (Vancanneyt et al., 1990) flanked by the cauliflower mosaic virus 35S promoter and polyadenylation signal. The pGUSINT binary plasmid was previously digested with SmaI and KpnI to release the GUS gene. The presence of the FaPG1 gene insert in the antisense orientation was confirmed by restriction analysis. The binary plasmid containing the antisense FaPG1 fragment was introduced into Agrobacterium tumefaciens strain LBA4404 by electroporation. For plant transformation, leaf discs of in vitro strawberry (cv Chandler) plants, micropropagated in a N30K medium supplemented with 2.2 μm kinetin, were transfected according to Barceló et al. (1998). Shoots were selected in 25 mg L−1 kanamycin and maintained in this selection medium until acclimatization, approximately 20 to 30 weeks after leaf inoculation. Kanamycin-resistant plants were acclimated, transferred to a screenhouse, and propagated vegetatively by runners to obtain enough copies for further analysis.
Phenotypic Analysis of Transgenic Plants
Fruit yield and quality of 30 independent antisense PG lines (APG lines) as well as a control line, obtained by conventional runner propagation, were evaluated during the first year of analysis. Ripe fruits were harvested from March to June, and the yield was estimated in terms of grams of fruit per plant. The quality of the ripe fruits was evaluated using only well-shaped fruits of uniform size and coloration and weight higher than 5 g. Color was estimated using the Centre Technique Interprofessionel des Fruits et Legumes code. This code comprises eight categories, increasing the red color from 1 (light orange-red) to 8 (dark wine-red). Soluble solids were measured using a refractometer (Atago N1), and firmness was measured using a hand penetrometer (Effegi) with a cylindrical needle of 9.62 mm2 area. Eight plants per line and a minimum of 10 ripe fruits per line were evaluated.
After the first analysis, four transgenic lines, which showed good yield and fruit firmness at ripeness higher than those of the control, were selected for further studies. These plants were propagated by runner yearly and analyzed for 3 consecutive years, evaluating the weight, color, soluble solids, and firmness of ripe fruits, as previously mentioned. Thirty plants per line and 50 to 100 ripe fruits per line were analyzed each year. Fruit firmness at different developmental stages was also evaluated during the second year of analysis. In this experiment, fruits were harvested at the green, white 1 (white receptacle with green achenes), white 2 (white receptacle with brown achenes), turning (lower than 25% surface red), and full ripe stages; firmness was measured with a hand penetrometer using different needles (0.78 mm2 surface needle for green fruits, 3.16 mm2 for white and pink fruits, and 9.62 mm2 for mature fruits).
To analyze the postharvest quality of transgenic fruits, control and APG ripe fruits were stored for 4 d at 4°C and evaluated after an additional 2 d at 25°C. Fruit firmness was measured with a TAXT-Plus texturometer (Stable Microsystem) using a puncture test with a 4-mm-diameter needle and an Ottawa cell, which measures the force needed to extrude a group of fruits, approximately 60 g, through a holed plate. In the last test, the curve force-strain was recorded and the area of the curve, namely the extrusion work, was used as an estimator of fruit firmness.
Auxin Treatment
The hormone treatment was performed as described previously (Medina-Escobar et al., 1997). Briefly, achenes from midsized green fruits were removed carefully with a scalpel blade. One set of fruits was treated with a lanolin paste containing 1 mm NAA in 1% (v/v) dimethyl sulfoxide. The other set of deachened fruits was treated with the same paste without the synthetic auxin NAA. Fruits were harvested after 5 d and immediately frozen in liquid nitrogen.
DNA Extraction
Strawberry genomic DNA was extracted from young leaves kept in distilled water in the dark at 4°C for 2 d. After that, 2 g of leaves was carefully ground under liquid nitrogen into a fine powder and gently resuspended in 25 mL of a warm (65°C) buffer solution (50 mm Tris-HCl, 100 mm NaCl, 50 mm EDTA, 1% 2-mercaptoethanol, 4% SDS, and 6% polyvinylpolypyrrolidone [PVPP]). The 2-mercaptoethanol, SDS, and PVPP were added just before use. The mixture was incubated at 65°C for 1 h, and then 8 mL of 3 m potassium acetate (pH 4.8) was added. Afterward, the mixture was incubated on ice and centrifuged at 10,000 rpm for 10 min. The supernatant mixture was gently filtered through a double layer of Miracloth, 2 volumes of ice-cold ethanol was added, and DNA was recovered with a microcapillary pipette. The sample was washed two to three times in ice-cold ethanol, dried at room temperature, and resuspended in water. To increase DNA quality, the samples were purified by a column (Kit G-Spin IIp; Intron Biotechnology) and quantified again before Southern-blot analysis.
Southern-Blot Analysis
Genomic DNA (15 μg) was digested with the restriction enzyme HindIII, fractionated on a 1% agarose gel, and then alkaline transferred to Hybond-N+ membranes (GE Healthcare). DNA was fixed by UV light using the Stratalinker (Stratagene), and the blot was prehybridized at 65°C for 1 h in 15 mL of hybridization solution (0.25 m NaH2PO4, pH 7, 7% SDS, and 0.1 mm EDTA-Na2). Denatured probes were added to the same hybridization solution, and hybridization was carried out at 65°C for 14 to 16 h. Filter was washed (twice) for 15 min in 100 mL of 0.2× SSC and 0.1% SDS and then exposed to x-ray film at −70°C for 3 d.
A 2.3-kb PCR-amplified fragment was used as a probe, using 35S and nptII primers. This fragment includes part of the 35S promoter and the nptII gene. The probe was labeled to a specific activity of approximately 108 cpm μg−1 using a commercial random priming kit (GE Healthcare).
RNA Isolation
Total RNA from a pool of several strawberry fruits at different stages of fruit growth and ripening or of red-ripe transgenic fruits was isolated according to Asif et al. (2000). Achenes were removed from fruit samples, and only receptacle RNA was purified. RNA samples were also treated with DNaseI, until absence of genomic contamination was observed by absence of amplicons corresponding to genes analyzed using RNA as template in a standard PCR.
Analysis of Gene Expression by QRT-PCR
Quantitative gene expression analyses corresponding to FaPG1 and FaPG2 (B4, AY280662) during strawberry fruit growth and ripening were performed by QRT-PCR. For this purpose, the iCycler (Bio-Rad) system was used. First-strand cDNA was obtained using 2 μg of total RNA and the iScript (Bio-Rad) according to the manufacturer's instructions. The PCR consisted of 25 μL of a mixture containing 1× PCR buffer, 1.5 mm MgCl2, 0.2 mm each deoxyribonucleotide triphosphate, 0.2 μm of each sequence-specific primer, 3 μL of SYBR Green I (diluted 1:15,000), 3 μL of transcribed cDNA, and 0.5 units of Taq polymerase (Biotools). Primer sequences for quantitative amplification of FaPG1 and FaPG2 genes were 5′-CGACAGAGTGAAAAATTCCTTAG-3′ and 5′-AGGACTGGGTTAGCAAAATTATTC-3′ for FaPG1 and 5′-GTGAATTCTGGTGGCAACAGTTCC-3′ and 5′-CCTGACGGCTGATTCGGTAATGAT-3′ for FaPG2. In QRT-PCR analysis, quantification is based on threshold cycle (Ct) values. The Ct is a measurement taken during the exponential phase of amplification, when limiting reagents and small differences in starting reagent amount have not yet influenced PCR efficiency. Ct is defined as the cycle at which fluorescence is first detectable above background and is inversely proportional to the log of the initial copy number. In this system, each 10-fold difference in initial copy number produced a 3.2 cycle difference in Ct. Each reaction was done at least in triplicate, and the corresponding Ct values were normalized using the Ct value corresponding to an interspacer 26S-18S strawberry RNA gene (housekeeping gene). The primers corresponding to this interspacer 26S-18S gene were 5′-ACCGTTGATTCGCACAATTGGTCATCG-3′ and 5′-TACTGCGGGTCGGCAATCGGACG-3′. The efficiency of each particular QRT-PCR was also calculated. All of these values were then used to determinate the fold changes of gene expression in the different PG transgenic plants analyzed according to the following expressions:
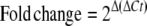 |
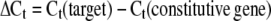 |
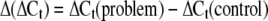 |
Microarray Generation
The microarray platform used contains 1,250 ESTs corresponding to unigenes expressed in receptacles from red-ripened strawberry fruits. To print the slides, the ESTs were amplified by PCR and purified with the PCR 96 Cleanup Kit (Millipore). The concentration of each EST was adjusted to 200 to 400 ng μL−1 with milliQ water and diluted in dimethyl sulfoxide (50%, v/v). The ESTs were organized on 384 plates and arrayed on UltraGAPS Coated Slides (Corning) using a MicroGridII Pro Arrayer (BioRobotics) spotter equipped with a head of 16 pins in a 4 × 4 format. DNA elements were deposited in duplicate with a spot diameter of 150 to 200 μm. The Universal ScoreCard (Amersham Biosciences) spikes were also included in the array. After printing, DNA was dry at room temperature and exposed to darkness for 24 h; then, it was rehydrated with 80°C vapor water for 5 s. To dry the slides, they were immediately deposited on a thermal block at 90°C during 5 s. Finally, the EST fragments were cross-linked to the slides by UV treatment (Stratalinker, Stratagene).
Microarray-Labeled cDNA Preparation and Hybridization
Total RNA from control and APG29 fruits was extracted as described previously. Afterward, the RNA was purified using the RNAeasy Purification Kit (Qiagen) and then 7.5 μg of purified total RNA was amplified using the Message Amp II aRNA Amplification Kit (Ambion). The amplified RNA (aRNA) was used to obtain fluorescently labeled cDNA using the SuperScript Plus Indirect cDNA Labeling System (Invitrogen) with some modifications. Briefly, cDNA was generated by retrotranscription of 2 μg of aRNA where RNA corresponding to spike controls was added. After 3 h of incubation at 46°C, the aRNA was degraded by adding 0.33 m NaOH and 0.16 m EDTA for 15 min at 65°C. Then, the mixture was neutralized by the addition of 0.33 m ClH. The cDNA obtained was purified using the MiniElute PCR Purification Kit (Qiagen). The purified cDNA was vacuum dried to 3 μL. Then, the phluorophores Alexa Fluor 555 and 647 were coupled according to the manufacturer's instructions. Finally, the labeled cDNA was purified with the MiniElute PCR Purification Kit (Qiagen). The control and test-labeled cDNAs were mixed in an equal amount (1 μg each) and dissolved in 1× hybridization buffer (Amersham Biosciences) containing 25% deionized formamide. Volume was completed to 250 μL with milliQ water, and prior to hybridization, the probe was denaturized by heating for 2 min at 95°C.
The slides were hybridized in a Lucidea APS Automated Slide Processor (Amersham). Prehybridization was carried out for 30 min at 42°C in 5× SSC and 0.1% SDS containing 25% formamide. After hybridization during 16 h at 42°C, the slides were cleaned, at room temperature, using a series of washing buffers: first, 1× SSC and 0.2% SDS; then, 0.1× SSC and 0.1% SDS; and finally, 0.1× SSC. Finally, they were washed with isopropanol at 37°C, dried, and immediately scanned.
Scanning and Data Analysis
The arrays were scanned using a GenePix Microarray Scanner 4000B (Axon Instruments) with 10-μm resolution. The signal intensity ratios of the two channels were corrected by modification of the gain of photomultiplicator in each wavelength until the ratio of the control spikes (ratio spikes) was 1.0. Bioinformatic image analysis and normalization were performed using the GenePix Pro 4.1 (Axon Instruments) program. Each spot was defined by manual positioning of a grid of circles over the image, and bad or absent spots were flagged manually. Visually flagged and low-quality spots were filtered from subsequent analysis. The average signal intensities of the replica spots were determined after subtraction of the local background intensities. The expression ratios of data were normalized by adjusting to 1.0 the ratio of medians corresponding to the normalization features (ratio spikes). Four replica experiments (two biological replicas and two methodological replicas for each biological one) were performed for each sample. To identify differentially expressed genes, the normalized data for each gene were converted as log2 of ratios and standardized as z-scores, and the P value for each z-score was determined. We used a significant level of confidence (P < 0.05) to consider genes that were statistically differentially expressed.
PG Activity
PG activity was measured in ripe fruits according to Villarreal et al. (2008). Frozen fruits (10 g) were homogenized using an Ultra-Turrax (Janke & Kunkel) with 30 mL of cold extraction buffer, 0.05 m Na-acetate buffer, pH 6, containing 1% (w/v) PVPP. Homogenates were centrifuged at 20,000g for 30 min at 4°C, and the pellets were extracted with 30 mL of extraction buffer containing 1 m NaCl. Then, extracts were stirred for 12 h and centrifuged at 20,000g for 30 min at 4°C, and supernatants were dialyzed overnight against extraction buffer. The dialysates were used as the crude extract.
PG activity was assayed as described by Nogata et al. (1993). Briefly, reaction mixtures containing 50 mm NaOAc, pH 5.5, 0.2% polygalacturonic acid (washed with 80% ethanol prior to use), and an appropriate amount of extract in a total volume of 0.6 mL were incubated at 37°C for 17 h. Then, solutions were analyzed for reducing groups by the cyanoacetamide method, using GalUA as a standard (Gross, 1982). Controls in which the enzyme extracts were heat inactivated or omitted were also included. Three independent PG extractions per line were performed, and each extract was assayed in triplicate. The protein content of extracts was estimated by the Bradford assay.
Cell Wall Analysis
Cell walls were extracted from frozen ripe fruits following the method of Redgwell et al. (1992) with some modifications. Fruits were pulverized with a mortar and pestle under liquid N2. Approximately 20 g of frozen powder was mixed with 40 mL of PAW and homogenized with an Ultra-Turrax. Homogenates were centrifuged at 4,000g for 15 min at 4°C, and the pellet was washed twice with water. The PAW and water supernatants were mixed, filtered through Miracloth, and dialyzed against distilled water for 4 d at 4°C (Mr cutoff of 7,000). The dialysate was centrifuged at 23,000g for 15 min at 4°C, and the supernatant was concentrated on a rotary evaporator, lyophilized, and weighed to obtain the PAW-soluble fraction. The pellet obtained after PAW extraction was resuspended in 20 mL of 90% aqueous dimethyl sulfoxide to eliminate the starch. After incubation overnight on an orbital shaker, the suspension was centrifuged at 4,000g for 15 min at 4°C. The supernatant was discarded, and the pellet was washed twice with distilled water. After washing, the pellet, largely consisting of CWM, was lyophilized and weighed.
Fractions of 150 mg of CWM were sequentially extracted with deionized water, 0.05 m CDTA in 0.05 m acetate buffer, pH 6, and 0.1 m Na2CO3 containing 0.02 m NaBH4. Each extraction was performed as follows. CWM was incubated for 24 h in 40 mL of each solvent on an orbital shaker. After incubation, the extract was centrifuged at 4,000g for 20 min, and the residue was incubated for another 24 h under the same conditions. Both supernatants were combined, filtered through FV/C glass fiber filters, and exhaustively dialyzed (Mr cutoff of 7,000) against water during 4 d at 4°C. After dialysis, the extract was centrifuged at 23,000g for 20 min, and the supernatant was concentrated on a rotary evaporator to approximately 3 to 5 mL, lyophilized, and weighed. The residue insoluble in each solvent was washed twice with water and extracted with the next reagent following the same procedure. Five independent fractionations were performed.
The uronic acid content in the different fractions was estimated using the Blumenkrantz and Asboe-Hansen (1973) assay as modified by Van den Hoogen et al. (1998).
Statistical Analysis
Data were analyzed by ANOVA using SPSS software, version 14 (SPSS). The Levene test for homogeneity of variances was performed prior to ANOVA, and mean separation was done by lsd at P = 0.05. In the case of nonhomogeneous variances, the nonparametric Mann-Whitney U test and the T2-Tamhane test were used for pair and multiple mean comparisons, respectively, both at P = 0.05.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AF380299 (FaPG1) and AY280662 (FaPG2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Comparative transcriptomic study between ripened receptacles from control and transgenic APG29 fruits.
Supplementary Material
Acknowledgments
We thank Dr. Jose M. López-Aranda (Instituto de Investigación y Formación Agraria y Pesquera [IFAPA], Centro de Churriana) for his support and advice on growing the plants. We thank Mari C. Molina (IFAPA, Centro de Churriana) for her work in plant growth and maintenance.
This work was supported by the Ministerio de Educación y Ciencia of Spain and Feder European Union Funds (grant nos. AGL2005–08128, AGL2008–02356, and MEC–BIO2007–67509–C02–02) and by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria of Spain (grant no. RTA04–079). J.A.G.-G. and S.P. were supported by Consejería de Agricultura y Pesca (Junta de Andalucía) and Ministerio de Educación y Ciencia predoctoral fellowships, respectively.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: José A. Mercado (mercado@uma.es).
The online version of this article contains Web-only data.
References
- Abeles FB, Takeda F (1990) Cellulase activity and ethylene in ripening strawberry and apple fruits. Sci Hortic (Amsterdam) 42 269–275 [Google Scholar]
- Asif MH, Dhawan P, Nath P (2000) A simple procedure for the isolation of high quality RNA from ripening banana fruit. Plant Mol Biol Rep 18 109–115 [Google Scholar]
- Barceló M, El-Mansouri I, Mercado JA, Quesada MA, Pliego-Alfaro F (1998) Regeneration and transformation via Agrobacterium tumefaciens of the strawberry cultivar Chandler. Plant Cell Tissue Organ Cult 54 29–36 [Google Scholar]
- Barnes MF, Patchett BJ (1976) Cell wall degrading enzymes and the softening of senescent strawberry fruit. J Food Sci 41 1392–1395 [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 41 1392–1395 [DOI] [PubMed] [Google Scholar]
- Brummell DA (2006) Cell wall disassembly in ripening fruit. Funct Plant Biol 33 103–119 [DOI] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47 311–340 [PubMed] [Google Scholar]
- Brummell DA, Labavitch JM (1997) Effect of antisense suppression of endopolygalacturonase activity on polyuronide molecular weight in ripening tomato fruit and in fruit homogenates. Plant Physiol 115 717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington CM, Greve LC, Labavitch JM (1993) Cell wall metabolism in ripening fruit. VI. Effect of the antisense polygalacturonase gene on cell wall changes accompanying ripening in transgenic tomatoes. Plant Physiol 103 429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillejo C, de la Fuente JI, Iannetta P, Botella MA, Valpuesta V (2004) Pectin esterase gene family in strawberry fruit: study of FaPE1, a ripening-specific isoform. J Exp Bot 398 909–918 [DOI] [PubMed] [Google Scholar]
- Giovannoni J (2001) Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol 52 725–749 [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ, DellaPenna D, Bennett AB, Fischer RL (1989) Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell 1 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross KC (1982) A rapid and sensitive spectrophotometric method for assaying polygalacturonase using 2-cyanoacetamide. HortScience 17 933–934 [Google Scholar]
- Hadfield KA, Bennett AB (1998) Polygalacturonase: many genes in search of a function. Plant Physiol 117 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield KA, Rose JKC, Yaver DS, Berka RM, Bennett AB (1998) Polygalacturonase gene expression in ripe melon fruit supports a role for polygalacturonase in ripening-associated pectin disassembly. Plant Physiol 117 363–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker FR, Redgwell RJ, Hallett IC, Murray SH (1997) Texture of fresh fruit. Hortic Rev (Am Soc Hortic Sci) 20 121–224 [Google Scholar]
- Harpster MH, Brummell DA, Dunsmuir P (1998) Expression analysis of a ripening-specific, auxin-repressed endo-1,4-β-glucanase gene in strawberry. Plant Physiol 118 1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber DJ (1984) Strawberry fruit softening: the potential roles of polyuronides and hemicelluloses. J Food Sci 49 1310–1315 [Google Scholar]
- Jiménez-Bermúdez S, Redondo-Nevado J, Muñoz-Blanco J, Caballero JL, López-Aranda JM, Valpuesta V, Pliego-Alfaro F, Quesada MA, Mercado JA (2002) Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol 128 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knee M, Sargent JA, Osborne DJ (1977) Cell wall metabolism in developing strawberry fruits. J Exp Bot 28 377–396 [Google Scholar]
- Koh TH, Melton LD (2002) Ripening-related changes in cell wall polysaccharides of strawberry cortical and pith tissues. Postharvest Biol Technol 26 23–33 [Google Scholar]
- Kramer M, Sanders R, Bolkan H, Waters C, Sheehy RE, Hiatt WR (1992) Postharvest evaluation of transgenic tomatoes with reduced levels of polygalacturonase: processing, firmness and disease resistance. Postharvest Biol Technol 1 241–255 [Google Scholar]
- Lara I, García P, Vendrell M (2004) Modifications in cell wall composition after cold storage of calcium-treated strawberry (Fragaria × ananassa Duch.) fruit. Postharvest Biol Technol 34 331–339 [Google Scholar]
- Lefever G, Vieuille M, Delage N, D'Harlingue A, de Monteclerc J, Bompeix G (2004) Characterization of cell wall enzyme activities, pectin composition, and technological criteria of strawberry cultivars (Fragaria × ananassa Duch). J Food Sci 69 221–226 [Google Scholar]
- Llop-Tous I, Domínguez-Puigjaner E, Palomer X, Vendrell M (1999) Characterization of two divergent endo-β-1,4-glucanase cDNA clones highly expressed in the nonclimateric strawberry fruit. Plant Physiol 119 1415–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Aranda JM, Pliego-Alfaro F, López-Navidad I, Barceló-Muñoz M (1994) Micropropagation of strawberry (Fragaria × ananassa Duch.): effect of mineral salts, benzyladenine levels and number of subcultures on the in vitro and field behaviour of the obtained microplants and the fruiting capacity of their progeny. J Hortic Sci 69 625–637 [Google Scholar]
- Manning K (1993) Soft fruit. In GB Seymour, JE Taylor, GA Tucker, eds, Biochemistry of Fruit Ripening. Chapman and Hall, Cambridge, UK, pp 347–377
- Manning K (1994) Changes in gene expression during strawberry fruit ripening and their regulation by auxin. Planta 194 62–68 [Google Scholar]
- Medina-Escobar N, Cárdenas J, Moyano E, Caballero JL, Muñoz-Blanco J (1997) Cloning, molecular characterization and expression pattern of a strawberry ripening-specific cDNA with sequence homology to pectate lyase from higher plants. Plant Mol Biol 34 867–877 [DOI] [PubMed] [Google Scholar]
- Nehra NS, Kartha KK, Stushnoff C, Giles KL (1992) The influence of plant growth regulator concentrations and callus age on somaclonal variation in callus culture regenerants of strawberry. Plant Cell Tissue Organ Cult 29 257–268 [Google Scholar]
- Nitsch JP (1950) Growth and morphogenesis of the strawberry as related to auxin. Am J Bot 37 211–215 [Google Scholar]
- Nogata Y, Ohta H, Voragen AGJ (1993) Polygalacturonase in strawberry fruit. Phytochemistry 34 617–620 [Google Scholar]
- Osorio S, Castillejo C, Quesada MA, Medina-Escobar N, Brownsey GJ, Suau R, Heredia A, Botella MA, Valpuesta V (2008) Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca). Plant J 54 43–55 [DOI] [PubMed] [Google Scholar]
- Palomer X, Llop-Tous I, Vendrell M, Krens FA, Schaart JG, Boone MJ, van der Valk E, Salentijn EMJ (2006) Antisense down-regulation of strawberry endo-β-(1,4)-glucanase genes does not prevent fruit softening during ripening. Plant Sci 171 640–646 [Google Scholar]
- Perkins-Veazie P (1995) Growth and ripening of strawberry fruit. Hortic Rev (Am Soc Hortic Sci) 17 267–297 [Google Scholar]
- Redgwell RJ, Fischer M, Kendall E, MacRae EA, Perry J, Harker R (1997. a) Galactose loss and fruit ripening: high-molecular-weight arabinogalactans in the pectic polysaccharides of fruit cell walls. Planta 203 174–181 [Google Scholar]
- Redgwell RJ, MacRae EA, Hallett I, Fischer M, Perry J, Harker R (1997. b) In vivo and in vitro swelling of cell walls during fruit ripening. Planta 203 162–173 [Google Scholar]
- Redgwell RJ, Melton LD, Brasch DJ (1992) Cell-wall dissolution in ripening kiwifruit (Actinidia deliciosa): solubilization of the pectic polymers. Plant Physiol 98 71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo-Nevado J, Moyano E, Medina-Escobar N, Caballero JL, Muñoz-Blanco J (2001) A fruit-specific and developmentally regulated endopolygalacturonase gene from strawberry (Fragaria × ananassa cv. Chandler). J Exp Bot 52 1941–1945 [DOI] [PubMed] [Google Scholar]
- Rosli HG, Civello PM, Martinez GA (2004) Changes in cell wall composition of three Fragaria x ananassa cultivars with different softening rate during ripening. Plant Physiol Biochem 42 823–831 [DOI] [PubMed] [Google Scholar]
- Salentijn EMJ, Aharoni A, Schaart JG, Boone MJ, Krens FA (2003) Differential gene expression analysis of strawberry cultivars that differ in fruit-firmness. Physiol Plant 118 571–578 [Google Scholar]
- Santiago-Doménech N, Jiménez-Bermúdez S, Matas AJ, Rose JKC, Muñoz-Blanco J, Mercado JA, Quesada MA (2008) Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. J Exp Bot 59 2769–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjulin TM (2003) The North American small fruit industry 1903–2003. II. Contributions of public and private research in the past 25 years and a view to the future. HortScience 38 960–967 [Google Scholar]
- Smith CJS, Watson CF, Morris PC, Bird CR, Seymour GB, Gray JE, Arnold C, Tucker GA, Schuch W, Harding S, et al (1990) Inheritance and effect on ripening of antisense polygalacturonase genes in transgenic tomatoes. Plant Mol Biol 14 369–379 [DOI] [PubMed] [Google Scholar]
- Trainotti L, Spolaore S, Pavanello A, Baldan B, Casadoro G (1999) A novel E-type endo-β-1,4-glucanase with a putative cellulose-binding domain is highly expressed in ripening strawberry fruits. Plant Mol Biol 40 323–332 [DOI] [PubMed] [Google Scholar]
- Vancanneyt G, Schmidt R, O'Connor-Sanchez A, Willmitzer L, Rocha-Sosa M (1990) Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220 245–250 [DOI] [PubMed] [Google Scholar]
- Van den Hoogen BM, van Weeren PR, Lopes-Cardozo M, van Golde LMG, Barneveld A, van de Lest CHA (1998) A microtiter plate assay for the determination of uronic acids. Anal Biochem 257 107–111 [DOI] [PubMed] [Google Scholar]
- Vicente AR, Costa ML, Martínez GA, Chaves AR, Civello PM (2005) Effect of heat treatments on cell wall degradation and softening in strawberry fruit. Postharvest Biol Technol 38 213–222 [Google Scholar]
- Vicente AR, Saladié M, Rose JKC, Labavitch JM (2007) The linkage between cell wall metabolism and fruit softening: looking to the future. J Sci Food Agric 87 1435–1448 [Google Scholar]
- Villarreal NM, Rosli HG, Martínez GA, Civello PM (2008) Polygalacturonase activity and expression of related genes during ripening of strawberry cultivars with contrasting fruit firmness. Postharvest Biol Technol 47 141–150 [Google Scholar]
- Wade P (1964) Insoluble cell wall polysaccharides of strawberries. J Sci Food Agric 15 51–56 [Google Scholar]
- Woolley LC, James DJ, Manning K (2001) Purification and properties of an endo-β-1,4-glucanase from strawberry and down-regulation of the corresponding gene, cel1. Planta 214 11–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.