Abstract
Cytochrome P450 monooxygenases (P450s) play important roles in the synthesis of diverse secondary compounds in Arabidopsis (Arabidopsis thaliana). Comparison of four data sets analyzing seedlings harvested over a 2-d period of constant conditions after growth with varying photoperiods and thermocycles recorded a total of 98 P450 loci as circadian regulated for at least one of the four conditions. Here, we further describe the circadian-regulated pathways using, as reporters, individual P450 loci that are likely to be rate limiting in secondary metabolic pathways. Reverse transcription-polymerase chain reaction gel blot analyses have confirmed circadian regulation of P450s in phenylpropanoid, carotenoid, oxylipin, glucosinolate, and brassinosteroid biosyntheses and have shown that both P450 and non-P450 genes in the many branches of the phenylpropanoid pathway have similar circadian patterns of expression. In silico analyses of the subsets of coregulated promoters have identified overrepresented promoter elements in various biosynthetic pathway genes, including MYB and MYB4 elements that are significantly more abundant in promoters for the core and lignin sections of phenylpropanoid metabolism. Interactions with these elements important for circadian regulation do not involve the MYB transcription factor PAP1, as previously proposed, since the expression patterns of circadian-regulated P450s are the same in pap1-D mutant seedlings as in wild-type seedlings. Further analysis of circadian-regulated promoters in other biochemical pathways provides us with the opportunity to identify novel promoter motifs that might be important in P450 circadian regulation.
The biological clock controls many processes in organisms as diverse as cyanobacteria and humans. In higher plants, circadian rhythms regulate physiological events including growth and development, photosynthesis, metabolic adaptation, protein synthesis, carbohydrate transport and storage, leaf and cotyledon movements, and hormone signaling responses (Harmer et al., 2000; Covington and Harmer, 2007; Michael et al., 2008). Microarray and enhancer-trapping experiments have estimated that between 15% and 36% of the Arabidopsis (Arabidopsis thaliana) genome is under circadian regulation at the transcriptional level (Michael and McClung, 2003; Edwards et al., 2006; Michael et al., 2008). Promoter analysis using these large microarray data sets has identified several promoter elements involved in phase-specific light and circadian regulation of expression, including the Morning Element (ME), CCA1-Binding Site (CBS), GATA, Evening Element (EE), and Midnight Module (PBX/TBX/SBX; Wang et al., 1997; Harmer et al., 2000; Hudson and Quail, 2003; Harmer and Kay, 2005; Michael et al., 2008). However, it remains unclear how specific pathways downstream of the core circadian clock are regulated at specific times of the day.
Cytochrome P450 monooxygenases (P450s) play critical roles in the synthesis of lignin, pigments, defense compounds, fatty acids, hormones, and signaling molecules in all plant species (Schuler, 1996; Werck-Reichhart et al., 2002; Nielsen and Moller, 2005). Because of their wide distribution in diverse metabolic processes, P450s can serve as downstream reporters for many biochemical pathways in Arabidopsis, where 246 P450 full-length genes and 26 P450 pseudogenes have been annotated (Paquette et al., 2000; Werck-Reichhart et al., 2002; Schuler et al., 2006). With many studies detailing the responses of this highly diverse gene family to biotic and abiotic stresses, the extent to which its members are regulated by circadian cues is unclear at present.
Carotenoids are the pigments responsible for many fruit and flower colors and some components of the light-harvesting complexes in photosynthesis (Bartley and Scolnick, 1995; DellaPenna and Pogson, 2006). Carotenoids also serve as precursors in the synthesis of abscisic acid. The carotenoid pathway contains two P450s in its downstream lutein branch mediating β-ring hydroxylations on α-carotenes (CYP97A3; LUT5) and subsequent ɛ-ring hydroxylations (CYP97C1; LUT1; Tian et al., 2004; Kim and DellaPenna, 2006).
Oxylipins are acyclic or cyclic oxidation products derived from polyunsaturated fatty acids that regulate many defense and developmental pathways in plants (Creelman and Mullet, 1997). The oxylipin pathway in Arabidopsis contains allene oxide synthase (CYP74A1; AOS), mediating the synthesis of 12-oxo-phytodienoic acid, jasmonate (JA), and methyl jasmonate (MeJ) from their linolenic acid precursor (Laudert et al., 1996), and hydroperoxide lyase (CYP74B2; HPL), mediating the breakdown of this precursor into C6-volatiles and octadecanoic acid (Bate et al., 1998; Matsui et al., 1999).
Glucosinolates are a class of naturally occurring thioglucosides responsible for some of the unique tastes of many condiments. Many P450s exist in the pathways branching to the production of these compounds, with CYP79F1 and CYP79F2 mediating distinct functions in the conversion of short- and long-chain Met derivatives to oximes (Hansen et al., 2001; Reintanz et al., 2001; Chen et al., 2003) prior to their modification by CYP83A1 to produce aliphatic glucosinolates (Bak and Feyereisen, 2001; Hemm et al., 2003; Naur et al., 2003). In a parallel pathway, CYP79B2 and CYP79B3 mediate steps in the conversion of Trp derivatives to indole-3-acetyldoxime (Hull et al., 2000; Mikkelsen et al., 2000) prior to its oxidation by CYP83B1 to produce indole glucosinolates (Bak and Feyereisen, 2001; Bak et al., 2001; Naur et al., 2003).
Brassinosteroids are steroidal plant hormones essential for many plant processes, including cell expansion and elongation, xylem differentiation, and pollen tube growth (Müssig et al., 2002; Asami et al., 2005; Bajguz, 2007). They have also been reported to improve resistance to chilling and drought stress (Clouse and Sasse, 1998). The brassinosteroid synthetic pathway, whose central CPD component is circadian regulated (Bancos et al., 2006), consists of a complex grid of metabolic reactions that includes CYP90A1 (CPD), CYP90B1 (DWF4), CYP90C1 (ROT3), CYP90D1, CYP85A1, and CYP85A2 (Bishop and Koncz, 2002; Fujioka and Yokota, 2003). Brassinosteroid degradation is mediated by P450s in two other families, CYP72C1 (SOB7) and CYP734A1 (BAS1; Neff et al., 1999; Turk et al., 2003, 2005; Nakamura et al., 2005; Takahashi et al., 2005).
Phenylpropanoid synthesis represents one of the best-characterized pathways because it generates a wide variety of products found in most plants, including flavonoids that act as signaling molecules, protectants against UV light damage and microorganisms, lignins that are structural components of cell walls, and anthocyanins that act as floral pigments and attractants to insect pollinators (Dixon and Paiva, 1995; Whetten and Sederoff, 1995; Winkel-Shirley, 2001). Harmer et al. (2000) first reported that the circadian clock regulated a large number of genes in this pathway, resulting in the daily cycling of its transcripts. Within this collection, four P450s mediate the hydroxylation of t-cinnamic acid (CYP73A5; C4H), p-coumaroylshikimic/quinic acids (CYP98A3; REF8), coniferaldehyde/ferulic acid (CYP84A1; FAH1), and narigenin/dihydrokaempferol (CYP75B1; TT7). These are widely distributed in the core pathway and the lignin and flavonoid/anthocyanin branches, which are postulated to provide scaffolds for the assembly of multienzyme channeling complexes (Meyer et al., 1996; Mizutani et al., 1997; Urban et al., 1997; Ruegger et al., 1999; Schoenbohm et al., 2000; Schoch et al., 2001; Winkel, 2004). From this work, it was also proposed that phenylpropanoid metabolism is regulated by PAP1, a MYB transcription factor (Harmer et al., 2000). More recent analyses have shown that PAP1 itself cycles and also that it regulates late flavonoid and anthocyanin gene expression (Borevitz et al., 2000; Tohge et al., 2005; Gonzalez et al., 2008). To date, there has been no evidence directly tying PAP1 to circadian regulation of the phenylpropanoid pathway.
The analyses presented here of P450 expression patterns in four data sets, varying with respect to the thermal and photoperiod cycles used for entrainment, indicate that different combinations of these P450s display coordinated in-phase expression in the different entrainment conditions. The characterization of P450 responses to circadian regulation has potential to identify nodes that globally coordinate transcript abundance of many pathways to specific times of the day. Until now, the activities of specific pathways have, for the most part, only been inferred from analysis of genome-wide patterns. To better understand the coordination of the downstream synthetic and catabolic pathways conferring time-of-day-specific activities, we have utilized P450s as reporters for different nodes in the network emerging from the central circadian clock.
RESULTS
Circadian Variations in P450 Transcripts
Given the importance of P450s in many metabolic pathways, it is clear that they can serve as global reporters for cellular responses to internal and external cues. To analyze the extent to which P450s might be regulated by the circadian clock, four previously published circadian time courses (Michael et al., 2008) were compared for their patterns in P450 expression. The four circadian conditions represent plants grown under different conditions of light and temperature (Table I). They are named to reflect how they were sampled: DD_DDHC, LL_LLHC, LL_LDHC, and LL_LDHH. The abbreviation DD (continuous dark, 22°C) or LL (continuous light, 22°C), before the underscore, represents the continuous circadian conditions under which the plants were harvested. Abbreviations after the underscore indicate the conditions under which plants were grown (entrained) before being released into circadian conditions; these include light/dark cycles (LD), continuous light (LL), continuous dark (DD), continuous temperature (HH, 22°C), and/or thermocycles (HC, 22°C/12°C) as detailed by Michael et al. (2008). Samples were harvested at 4-h intervals over a 2-d period and compared on Affymetrix ATH1 Genechips. Expression for all genes on the ATH1 Genechips across these circadian conditions as well as other diurnal conditions can be accessed at http://diurnal.cgrb.oregonstate.edu/. Identification of cycling genes, the time of their peak expression over the day (phase, in hours from subjective dawn), and statistical analysis of the entire data set have been described by Michael et al. (2008). Briefly, all four time courses were gcRMA (see “Materials and Methods”) normalized together, and cycling genes were called using the pattern-matching program HAYSTACK (http://haystack.cgrb.oregonstate.edu/) using a 5% false discovery rate (FDR; Michael et al., 2008). All of the cycling genes described are statistically significant using these criteria. Expression for all genes on the ATH1 Genechips across these circadian conditions as well as other diurnal conditions can be accessed at DIURNAL (http://diurnal.cgrb.oregonstate.edu/).
Table I.
Plant growth and harvest conditions
| Name | Entrainment Conditions | Harvest Conditions | Researcher/Laboratory |
|---|---|---|---|
| DD_DDHC | DD (continuous dark) | DD (continuous dark, 22°C) | Hazen, Kay |
| HC (thermocycles of 22°C/12°C) | |||
| LL_LLHC | LL (continuous light) | LL (continuous light, 22°C) | Michael, Chory |
| HC (thermocycles of 22°C/12°C) | |||
| LL_LDHC | LD (12-h-light/12-h-dark cycles) | LL (continuous light, 22°C) | Michael, Chory |
| HC (thermocycles of 22°C/12°C) | |||
| LL_LDHH | LD (12-h-light/12-h-dark cycles) | LL (continuous light, 22°C) | Edwards, Millar; Pan, Schuler |
| HH (continuous temperature of 22°C) |
Across the four circadian conditions, 233 of the 246 full-length P450 genes and 26 P450 pseudogenes in Arabidopsis were detected on Affymetrix ATH1 arrays, with 11 of these array elements representing closely related P450 genes. Using a 0.8 correlation cutoff for predicting cycling loci (all four time courses analyzed together; P = 0.05, FDR = 5%; Michael et al., 2008), 98 P450 loci listed in Table II showed statistically significant circadian phasing for at least one of the four array conditions. Between 4% and 22% of the 250 P450 transcripts were circadian regulated under any one of the four conditions, with 39% of the gene list overlapping with one of the other three conditions (Fig. 1). Between 33% and 42% of the genes were specifically circadian regulated under only one condition. The fewest genes displaying circadian rhythms were detected under DD_DDHC conditions, consistent with the fact that generally fewer genes cycle under this condition (Michael et al., 2008). The greatest numbers of genes showing circadian rhythms were detected under LL_LDHH and LL_LDHC conditions rather than LL_LLHC conditions, suggesting that entrainment by light/dark cycles plays a role in P450 expression.
Table II.
P450s showing circadian regulation
The 98 P450 loci shown to have statistically significant circadian phasing for at least one of the four array conditions (DD_DDHC, LL_LDHH, LL_LDHC, and LL_LLHC) are listed, with the numbers in columns 5 to 8 indicating the phasing time relative to subjective dawn. The first 27 P450s that have designated functions are sorted by biochemical pathway; the remaining 71 P450s (nos. 28 to 98) currently have no biochemically defined function.
| No. | Affymetrix Identifier | Arabidopsis Genome Initiative No. | Locus | DD_DDHC | LL_LDHH | LL_LDHC | LL_LLHC | Pathway |
|---|---|---|---|---|---|---|---|---|
| 1 | 267470_at | At2g30490 | CYP73A5 | 1 | 20 | 22 | Phenylpropanoid core | |
| 2 | 250558_at | At5g07990 | CYP75B1 | 20 | Phenylpropanoid/flavonol | |||
| 3 | 253088_at | At4g36220 | CYP84A1 | 1 | Phenylpropanoid/lignin | |||
| 4 | 245101_at | At2g40890 | CYP98A3 | 21 | 23 | 22 | Phenylpropanoid/lignin | |
| 5 | 267380_at | At2g26170 | CYP711A1 | 20 | 23 | 22 | Flavonoid | |
| 6 | 246268_at | At1g31800 | CYP97A3 | 14 | 19 | Carotenoid | ||
| 7 | 251969_at 251979_at | At3g53130 | CYP97C1 | 13 | 20 | Carotenoid | ||
| 8 | 245532_at | At4g15110 | CYP97B3 | 16 | Carotenoid | |||
| 9 | 249208_at | At5g42650 | CYP74A1 | 19 | 22 | Oxylipin | ||
| 10 | 245253_at | At4g15440 | CYP74B2 | 15 | 17 | Oxylipin | ||
| 11 | 262717_s_at | At1g16410 | CYP79F1 | 1 | Aliphatic glucosinolate | |||
| 12 | 262717_s_at | At1g16400 | CYP79F2 | 1 | Aliphatic glucosinolate | |||
| 13 | 254687_at | At4g13770 | CYP83A1 | 22 | Aliphatic glucosinolate | |||
| 14 | 252827_at | At4g39950 | CYP79B2 | 23 | Indole glucosinolate | |||
| 15 | 264052_at | At2g22330 | CYP79B3 | 0 | 23 | Indole glucosinolate | ||
| 16 | 256598_at | At3g30180 | CYP85A2 | 1 | Brassinolide | |||
| 17 | 250752_at | At5g05690 | CYP90A1 | 5 | 8 | 9 | Brassinolide | |
| 18 | 252184_at | At3g50660 | CYP90B1 | 12 | 8 | Brassinolide | ||
| 19 | 246216_at | At4g36380 | CYP90C1 | 3 | 6 | Brassinolide | ||
| 20 | 267614_at | At2g26710 | CYP734A1 | 5 | 6 | Degradation of brassinosteroids | ||
| 21 | 257035_at | At3g19270 | CYP707A4 | 7 | Degradation of abscisic acid | |||
| 22 | 246864_at | At5g25900 | CYP701A3 | 3 | 7 | GAs | ||
| 23 | 260241_at | At1g63710 | CYP86A7 | 3 | Fatty acid | |||
| 24 | 258973_at | At3g01900 | CYP94B2 | 1 | Fatty acid | |||
| 25 | 262820_at | At1g11680 | CYP51G1 | 1 | 1 | Sterols/steroids | ||
| 26 | 264877_at | At2g17330 | CYP51G2 | 5 | 5 | Sterols/steroids | ||
| 27 | 266996_at | At2g34490 | CYP710A2 | 20 | 19 | 23 | Sterols | |
| 28 | 267567_at | At2g30770 | CYP71A13 | 2 | ||||
| 29 | 254767_s_at | At4g13290 | CYP71A19 | 3 | ||||
| 30 | 254767_s_at | At4g13310 | CYP71A20 | 3 | ||||
| 31 | 262826_at | At1g13080 | CYP71B2 | 22 | 0 | 3 | ||
| 32 | 257635_at | At3g26280 | CYP71B4 | 4 | 7 | 7 | ||
| 33 | 262793_at | At1g13110 | CYP71B7 | 8 | ||||
| 34 | 246947_at | At5g25120 | CYP71B11 | 19 | ||||
| 35 | 246949_at | At5g25140 | CYP71B13 | 4 | ||||
| 36 | 257636_at | At3g26200 | CYP71B22 | 3 | ||||
| 37 | 257623_at | At3g26210 | CYP71B23 | 13 | ||||
| 38 | 257625_at | At3g26230 | CYP71B24 | 21 | 4 | |||
| 39 | 257628_at | At3g26290 | CYP71B26 | 5 | 7 | 9 | ||
| 40 | 251988_at | At3g53300 | CYP71B31 | 23 | ||||
| 41 | 256870_at | At3g26300 | CYP71B34 | 1 | ||||
| 42 | 256873_at | At3g26310 | CYP71B35 | 7 | ||||
| 43 | 252674_at | At3g44250 | CYP71B38 | 2 | 1 | |||
| 44 | 258063_at | At3g14620 | CYP72A8 | 15 | 17 | 20 | ||
| 45 | 258112_at | At3g14640 | CYP72A10 | 6 | ||||
| 46 | 267505_at | At2g45560 | CYP76C1 | 14 | 6 | 9 | 14 | |
| 47 | 267559_at | At2g45570 | CYP76C2 | 8 | ||||
| 48 | 267560_at | At2g45580 | CYP76C3 | 15 | ||||
| 49 | 250859_at | At5g04660 | CYP77A4 | 0 | ||||
| 50 | 258962_at | At3g10570 | CYP77A6 | 19 | ||||
| 51 | 250838_at | At5g04630 | CYP77A9 | 19 | ||||
| 52 | 262819_at | At1g11600 | CYP77B1 | 13 | ||||
| 53 | 266321_at | At2g46660 | CYP78A6 | 1 | ||||
| 54 | 250509_at | At5g09970 | CYP78A7 | 4 | ||||
| 55 | 249673_at | At5g35920 | CYP79A4P | 7 | ||||
| 56 | 246620_at | At5g36220 | CYP81D1 | 22 | 1 | |||
| 57 | 253096_at | At4g37330 | CYP81D4 | 20 | 21 | |||
| 58 | 253097_at | At4g37320 | CYP81D5 | 17 | 17 | 20 | ||
| 59 | 256386_at | At1g66540 | CYP81D10 | 18 | ||||
| 60 | 256589_at | At3g28740 | CYP81D11 | 3 | ||||
| 61 | 253100_at | At4g37400 | CYP81F3 | 0 | ||||
| 62 | 253052_at | At4g37310 | CYP81H1 | 22 | 22 | |||
| 63 | 253503_at | At4g31950 | CYP82C3 | 14 | ||||
| 64 | 253502_at | At4g31940 | CYP82C4 | 4 | ||||
| 65 | 249881_at | At5g23190 | CYP86B1 | 4 | ||||
| 66 | 250576_at | At5g08250 | CYP86B2 | 0 | 5 | 0 | ||
| 67 | 265020_at | At1g24540 | CYP86C1 | 4 | ||||
| 68 | 262882_at | At1g64900 | CYP89A2 | 0 | 5 | |||
| 69 | 247579_at | At5g61320 | CYP89A3 | 0 | ||||
| 70 | 266155_at | At1g64950 | CYP89A5 | 20 | ||||
| 71 | 262866_at | At1g64940 | CYP89A6 | 4 | ||||
| 72 | 250651_at | At5g06900 | CYP93D1 | 23 | ||||
| 73 | 248353_at | At5g52320 | CYP96A4 | 16 | 17 | 21 | ||
| 74 | 263894_at | At2g21910 | CYP96A5 | 4 | ||||
| 75 | 262435_at | At1g47620 | CYP96A8 | 2 | ||||
| 76 | 252896_at | At4g39480 | CYP96A9 | 23 | 0 | |||
| 77 | 252911_at | At4g39510 | CYP96A12 | 23 | 0 | 0 | ||
| 78 | 245550_at | At4g15330 | CYP705A1 | 4 | ||||
| 79 | 248727_at | At5g47990 | CYP705A5 | 16 | ||||
| 80 | 266308_at | At2g27010 | CYP705A9 | 11 | ||||
| 81 | 263276_at | At2g14100 | CYP705A13 | 12 | 12 | |||
| 82 | 257112_at | At3g20120 | CYP705A21 | 12 | ||||
| 83 | 261499_at | At1g28430 | CYP705A24 | 4 | ||||
| 84 | 256801_at | At3g20940 | CYP705A30 | 1 | ||||
| 85 | 256802_at | At3g20950 | CYP705A32 | 0 | ||||
| 86 | 256803_at | At3g20960 | CYP705A33 | 5 | ||||
| 87 | 254331_s_at | At4g22690 | CYP706A1 | 6 | ||||
| 88 | 254331_s_at | At4g22710 | CYP706A2 | 6 | ||||
| 89 | 254835_s_at | At4g12320 | CYP706A6 | 18 | 12 | 15 | 17 | |
| 90 | 254835_s_at | At4g12310 | CYP706A7 | 18 | 12 | 15 | 17 | |
| 91 | 248728_at 266251_s_at | At5g48000 | CYP708A2 | 17 | 17 | |||
| 92 | 252629_at | At3g44970 | CYP708A4 | 20 | 23 | |||
| 93 | 253886_at | At4g27710 | CYP709B3 | 1 | 6 | 7 | ||
| 94 | 267626_at | At2g42250 | CYP712A1 | 3 | ||||
| 95 | 246978_at | At5g24910 | CYP714A1 | 5 | ||||
| 96 | 249684_s_at | At5g36110 | CYP716A1 | 5 | ||||
| 97 | 245728_at | At1g73340 | CYP720A1 | 23 | ||||
| 98 | 261134_at | At1g19630 | CYP722A1 | 17 |
Figure 1.
P450 loci showing circadian phasing for at least one of the four array conditions. The Venn diagram shows the numbers of genes overlapping among the four circadian conditions.
Reverse transcription (RT)-PCR gel blot analyses of samples from the LL_LLHC and LL_LDHC time courses performed with gene-specific primers and probes confirmed the cycling of P450 transcripts distributed in many different pathways, including CYP73A5, CYP75B1, CYP98A3, and CYP84A1 in phenylpropanoid synthesis (nos. 1–4 in Table II; highlighted in red in Fig. 2A online), CYP97C1 in carotenoid synthesis (no. 7 in Table II), CYP74A1 in jasmonate synthesis (no. 9 in Table II), CYP79B3 in glucosinolate synthesis (no. 15 in Table II), and CYP90A1 in brassinosteroid synthesis (no. 17 in Table II). Comparison of the UBQ10-normalized RNA levels shown in the left panels of Figure 3 with the gcRMA-normalized ATH1 array data shown in the right panels of Figure 3 indicates strong correlations for most of the transcripts analyzed. Analysis using the model-based pattern-matching HAYSTACK algorithm (Fig. 3K) indicates that the four P450s in the phenylpropanoid pathway are phased with a maximum at 22 h under the LL_LLHC conditions. Complementary analysis using semiquantitative RT-PCR gel blots on these same RNA samples (Fig. 3, A and F) indicates that these four P450s are phased at around 24 h. Comparisons between these two analytic methods indicate that, in some instances, the RT-PCR gel blots show obvious cycling variations and the ATH1 array data show much lower levels of variation. This is the case for CYP75B1 and CYP84A1 in the LL_LLHC time course (Fig. 3, F and K) as well as for CYP98A3 in the LL_LLHC time course, where RT-PCR gel blot signals show as much as a 10-fold difference between the initial time point and each day's minimum and microarray data show only a 2.5-fold difference. This is also the case for CYP90A1 in the LL_LDHC time course, where RT-PCR gel blot signals decrease more dramatically than the microarray data to an extreme minimum at 16 to 20 h (Fig. 3, I and N). Differences in the magnitude of these variations likely arise from the greater sensitivity of the RT-PCR gel blots. It is well known that microarray data, especially oligonucleotide microarray data, suffer from poor dynamic range. Although the results from these two methods differ in magnitude, both demonstrate circadian regulation of the P450s analyzed, and within each method, the circadian expression pattern of each gene appears to be highly reproducible.
Figure 2.
Phenylpropanoid pathway. This map adapted from the AraCyc Pathway shows the genes responsible for steps in the phenylpropanoid pathway, with black boxes indicating genes that are circadian regulated and underlines indicating P450 loci. [See online article for color version of this figure.]
Figure 3.
Circadian-regulated transcripts in known pathways. A to E, RNAs from the LL_LLHC and LL_LDHC conditions were analyzed on RT-PCR gel blots for the transcripts listed above each panel or set of panels. The RT-PCR products for P450s in each sample were background corrected, normalized against RT-PCR products for constitutive UBQ10 in each sample, and recorded relative to the 0-h signal in each time course. The UBQ10 normalizations for B to E are shown in A. F to J, These P450 transcript levels were quantified and plotted relative to the 0-h signal for each time course. K to O, RNAs from the LL_LLHC and LL_LDHC conditions were analyzed on microarrays, normalized using gcRMA, and plotted relative to the 0-h signals. Values on the x axis indicate hours after each time-course sampling was initiated. White and gray bars on the x axis indicate subjective day and subjective night during the time course; blank and grid bars indicate high (22°C) and low (12°C) temperatures maintained during the time course. [See online article for color version of this figure.]
Circadian Regulation of P450 Transcripts in Different Secondary Pathways
With P450s occurring at important nodes in many secondary pathways displaying circadian cycling, variations in their activities can impact an array of downstream synthetic and catabolic pathways and alter physiological functions over the course of a day. Comparison of the circadian phasing of the four P450 transcripts in the core and various branches of the phenylpropanoid pathway (CYP73A5, CYP75B1, CYP98A3, and CYP84A1; Fig. 2) indicates that the LL_LDHH arrays show similar phasing just before subjective dawn (20–21 h maxima) for three of these P450s, with the most profound increase for CYP75B1 in anthocyanin synthesis and slightly later phasing for CYP84A1 (1 h maximum; Table II). Two of these also cycle with similar normalized profiles in the LL_LDHC arrays with peaks before subjective dawn (Fig. 3A). In addition, CYP711A1 (MAX1; no. 5 in Table II), which is reported to be a positive regulator of the flavonoid pathway (Lazar and Goodman, 2006) and to act downstream of carotenoid-derived hormones (Booker et al., 2005), shows circadian phasing (20–23 h maxima in LL_LDHH, LL_LDHC, and LL_LLHC arrays) similar to three of the four phenylpropanoid P450s.
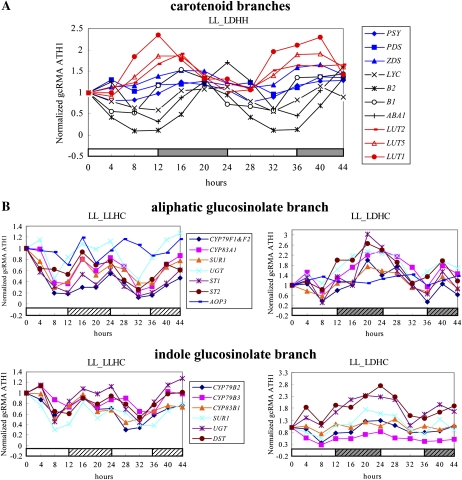
In carotenoid synthesis (Fig. 4A), CYP97A3 and CYP97C1 occur in the lutein branch. Both of these (nos. 6 and 7 in Table II) show circadian phasing in the LL_LLHC arrays (19–20 h maxima) and the LL_LDHH arrays (13–14 h maxima; Fig. 5A). CYP97B3 (no. 8 in Table II), whose protein shares 45% to 46% amino acid identity with CYP97A3 and CYP97C1, shows circadian phasing at 12 h in the LL_LDHH arrays (below the cutoff used for Table II) and at 16 h in the LL_LDHC arrays, which is similar to the phasing of CYP97A3 and CYP97C1 on the arrays. The similarity of this phasing suggests that CYP97B3 may be under the same transcriptional regulation as the other CYP97 family genes. CYP711A1, which was previously mentioned as acting downstream of carotenoid cleavage dioxygenases (Booker et al., 2005), shows circadian phasing at 20 to 22 h in three of the time courses (LL_LDHH, LL_LDHC, and LL_LLHC); this is significantly later than the phasing of the other P450s in this carotenoid pathway.
Figure 4.
Maps of four circadian-regulated pathways. These maps adapted from AraCyc Pathway correspond to the carotenoid (A), oxylipin (B), glucosinolate (C), and brassinosteroid (D) pathways. Black boxes indicate genes that are circadian regulated, and underlines indicate P450 loci. [See online article for color version of this figure.]
Figure 5.
Phasing in the carotenoid and glucosinolate pathways. A, Normalized microarray circadian gene expression data of LL_LDHH for genes in the carotenoid pathway include PSY (phytoene synthase; At5g17230), PDS (phytoene dehydrogenase; At4g14210), ZDS (ζ-carotene desaturase; At3g04870), LYC (lycopene cyclase; At3g10230), B2 (β-carotene hydroxylases; At5g52570), B1 (At4g25700), ABA1 (zeaxanthin epoxidase; At5g67030), NPQ1 (violaxanthin deepoxidase precursor; At1g08550), LUT2 (lutein-deficient 2; At5g57030), LUT5 (β-ring hydroxylase on carotenes; CYP97A3; At1g31800), and LUT1 (ɛ-ring hydroxylase on carotenes; CYP97C1; At3g53130). The carotenoid intermediate pathway is shown in blue, the zeaxanthin branch is shown in black, and the lutein branch is shown in red. B, Normalized microarray time-course data plotted every 4 h, with light and temperature conditions indicated below the data, are shown for genes in the aliphatic branch of glucosinolate synthesis and include three P450s, CYP79F1 (At1g16400), CYP79F2 (At1g16410), and CYP83A1 (At4g13770), as well as SUR1 (alkylthiohydroximate C-S lyase; At2g20610), UGT (UDP-glycosyltransferase; At1g24100), ST1 (sulfotransferases; At1g18590), ST2 (At1g74090), AOP2 (2-oxoglutarate-dependent dioxygenases; At4g03060), and AOP3 (At4g03050). Circadian data are also shown for genes in the indole branch of glucosinolate synthesis and include three P450s, CYP79B2 (At4g39950), CYP79B3 (At2g22330), and CYP83B1 (At4g31500), as well as SUR1, UGT, and DST (desulfoglucosinolate sulfotransferase; At1g74100). Values on the x axis indicate hours after each time-course sampling was initiated. White and gray bars on the x axis indicate subjective day and subjective night during the time course; blank and grid bars indicate high (22°C) and low (12°C) temperature maintained during the time course.
In oxylipin synthesis (Fig. 4B), CYP74A1 in JA synthesis and CYP74B2 in C6-volatile production are circadian regulated, with slightly different phasings in the LL_LLHC arrays (22 and 17 h maxima; nos. 9 and 10 in Table II). With many other loci mediating steps in JA synthesis, at least one locus at each step in the pathway is circadian regulated in the LL_LLHC and LL_LDHC arrays, with phasings between 13 and 18 h, at times slightly prior to the phasings seen for CYP74A1 and CYP74B2; among multiple loci coding for the same enzyme, those that are circadian regulated are indicated with boxes in Figure 4B. The only loci with noticeably different phasing from others in the JA synthetic pathway are one lipoxygenase (LOX1; no. 59 in Table III) mediating the synthesis of 9-hydroperoxides and not the 13-hydroperoxides needed for jasmonate production (Royo et al., 1996; Blee, 2002), one undefined 12-oxophytodienoate reductase (OPR; no. 66 in Table III), and S-adenosyl-l-Met:jasmonic acid carboxyl methyltransferase (JMT; no. 68 in Table III) catalyzing the last step in MeJ synthesis. As both LOX and OPR have many isoforms in Arabidopsis, it is likely that the individual members of these families are under different modes of transcriptional regulation. In the case of JMT, its phasing 2 to 11 h later than transcripts for previous steps in this pathway suggests that the proportions of JA and MeJ vary throughout these cycling periods.
Table III.
Elements overrepresented in different branches of biosynthetic pathways
The numbers in the columns for the four circadian array conditions (DD_DDHC, LL_LDHH, LL_LDHC, and LL_LLHC) are the phasing of corresponding genes. EE, CBS, and ME are given; the numbers indicate the positions (bp) where they present in the promoter of each gene. The statistical significance (e-value) and ratio of overrepresented element frequency shown in the branch and in the 27,457 promoters of the Arabidopsis genome are also given.
| Pathways and Subdivisions | Locus | Arabidopsis Genome Initiative No. | P450 | DD_DDHC | LL_LDHH | LL_LDHC | LL_LLHC | EE/CBS/ME | e-Value and Overrepresented Ratio in Element | |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenylpropanoid pathway | ||||||||||
| Core pathway | ||||||||||
| 1 | Phe ammonia-lyase | PAL1 | At2g37040 | 20 | 23 | CBS:363 | MYB4:2.44e-12(54/77609) | |||
| 2 | Phe ammonia-lyase | PAL2 | At3g53260 | 21 | 0 | 0 | MYB:1.84e-11(25/20163) | |||
| 3 | Phe ammonia-lyase | PAL3 | At5g04230 | 4 | GL-TRP5:4.26e-05(11/8446) | |||||
| 4 | Cinnamic acid 4-hydroxylase | C4H | At2g30490 | CYP73A5 | 1 | 20 | 22 | WLE1:1.10e-04(53/110661) | ||
| GL-MET3:6.16e-04(19/33391) | ||||||||||
| 5 | 4-Coumarate-CoA ligase | 4CL1 | At1g51680 | 19 | 22 | CBS:1444 | DPBF1&2:1.03e-03(29/60493) | |||
| CAROT-A2:1.39e-03(26/49795) | ||||||||||
| 6 | 4-Coumarate-CoA ligase | 4CL2 | At3g21240 | 22 | 22 | GL-MET4:1.64e-03(22/37935) | ||||
| GL-TRP2:9.71e-03(18/35624) | ||||||||||
| 7 | 4-Coumarate-CoA ligase | 4CL3 | At1g65060 | 20 | 18 | 20 | CBS:557 | |||
| 8 | 4-Coumarate-CoA ligase | 4CL4 | At3g21230 | 0 | ||||||
| 9 | 4-Coumarate-CoA ligase | At5g63380 | 0 | 1 | 2 | 3 | CBS:1497 | |||
| 10 | 4-Coumarate-CoA ligase | At1g20490 | 21 | 20 | CBS:356 | |||||
| Intermediate flavonoid branch | ||||||||||
| 11 | Naringenin-chalcone synthase | CHS | At5g13930 | 21 | 20 | 22 | SORLIP2:7.85e-04(12/22714) | |||
| Box II:1.30e-03(15/30853) | ||||||||||
| 12 | Chalcone isomerase | TT5 | At3g55120 | 21 | 20 | 22 | CBS:1619 | MOTIF8:2.86e-03(26/76487) | ||
| 13 | Chalcone isomerase | CHI | At5g05270 | 20 | 23 | ABRE-like:3.80e-03(8/121225) | ||||
| 14 | Naringenin 3-dioxygenase | F3H | At3g51240 | 23 | 20 | 22 | CBS:544 | G-box[LRE]:4.91e-03(6/8723) | ||
| JA2:6.37e-03(13/32554) | ||||||||||
| 15 | Flavanone 3-hydroxylase | At5g24530 | 1 | 3 | CBS:601 | PBX:7.66e-03(5/6251) | ||||
| GL-TRP3:8.50e-03(17/44906) | ||||||||||
| 16 | Flavanone 3-hydroxylase | F3H′ | At3g19000 | 19 | CBS:815 | |||||
| 17 | Flavanone 3-hydroxylase | At4g16330 | 7 | 12 | ||||||
| Flavonol branch | ||||||||||
| 18 | Flavonoid 3′-monooxygenase | TT7 | At5g07990 | CYP75B1 | 20 | EE:6.15e-04(6/3608) | ||||
| GL-MET2:8.25e-04(17/26923) | ||||||||||
| 19 | Flavonol synthase | FS | At3g50210 | 9 | 13 | 10 | EE:1037 | SORLIP1:9.63e-04(24/42489) | ||
| 20 | Flavonol synthase | At3g19010 | 9 | EE:692 | ||||||
| 21 | Flavonol synthase | At5g63590 | 20 | |||||||
| 22 | Flavonol synthase | FLS | At5g08640 | 21 | 19 | 21 | EE:270 | |||
| 23 | Flavonol 3-O-glucosyltransferase | At1g01390 | 15 | EE:934,34 | ||||||
| 24 | Flavonol 3-O-glucosyltransferase | F3OG1 | At1g01420 | 23 | ||||||
| 25 | Flavonol 3-O-glucosyltransferase | At2g18560 | 22 | EE:881 | ||||||
| 26 | Flavonol 3-O-glucosyltransferase | F3OG2 | At4g01070 | 17 | 18 | 21 | ||||
| 27 | Flavonol 3-O-glucosyltransferase | F3OG3 | At5g54060 | 22 | 21 | |||||
| Anthocyanin branch | ||||||||||
| 28 | Flavonoid 3′-monooxygenase | TT7 | At5g07990 | CYP75B1 | 20 | CBS:1847 | G-box[LRE]:5.00e-06(8/8723) | |||
| G-BOX EXTENDED: 2.44e-04(5/4921) | ||||||||||
| 29 | Dihydroflavonol 4-reductase | DFR | At5g42800 | 21 | 21 | ME:1090,868,4 | ||||
| ABRE-LIKE:1.97e-03(6/12125) | ||||||||||
| 30 | Leucoanthocyanidin dioxygenase | At4g22870 | 20 | 21 | ME:1701 | SORLREP4:2.99e-03(2/627) | ||||
| EE:1069 | BS2:9.98e-03(6/16468) | |||||||||
| 31 | Leucoanthocyanidin dioxygenase | ANS | At4g22880 | 20 | 21 | ME:1447,876,326,101 | ||||
| Lignin branch | ||||||||||
| 32 | Hydroxycinnamoyl-CoA:shikimate/quinatehydroxycinnamoyltransferase | CST/CQT | At5g48930 | 20 | 23 | ME:1225,364 | MYB:1.14e-10(28/20163) | |||
| MYB4:2.95e-07(63/77609) | ||||||||||
| WLE1:2.78e-04(75/110661) | ||||||||||
| 33 | Coumarate-3-hydroxylase | REF8 | At2g40890 | CYP98A3 | 21 | 23 | 22 | ME:1331,221 | GL-MET3:5.23e-03(25/33391) | |
| 34 | Caffeoyl-CoA o-methyltransferase | At4g34050 | 18 | 21 | CBS:1912 | |||||
| ME:234 | ||||||||||
| 35 | Caffeoyl-CoA o-methyltransferase | At1g24735 | 0 | 0 | 4 | ME:903,452,61 | ||||
| 36 | UDP-Glc 4-epimerase | At5g58490 | 14 | 18 | 18 | CBS:592 | ||||
| 37 | UDP-Glc 4-epimerase | At2g33590 | 20 | 2 | ME:1528 | |||||
| 38 | UDP-Glc 4-epimerase | At2g02400 | 2 | ME:1727,1434,772,294 | ||||||
| 39 | Cinnamoyl-CoA reductase | At5g14700 | 19 | CBS:1226 | ||||||
| 40 | Cinnamoyl-CoA reductase | At4g30470 | 20 | 21 | 0 | |||||
| 41 | Cinnamoyl-CoA reductase | At2g23910 | 19 | 16 | 18 | ME:1757 | ||||
| 42 | Cinnamoyl-CoA reductase | CCR1 | At1g15950 | 22 | 1 | 1 | CBS:1541 | |||
| ME:647 | ||||||||||
| 43 | Cinnamyl-alcohol dehydrogenase | CAD4 | At3g19450 | 19 | 22 | 23 | CBS:1628 | |||
| 44 | Cinnamyl-alcohol dehydrogenase | CAD5 | At4g34230 | 1 | CBS:1058 | |||||
| ME:1540,407 | ||||||||||
| 45 | Ferulate 5-hydroxylase | FAH1 | At4g36220 | CYP84A1 | 1 | CBS:1111,521,204 | ||||
| 46 | Flavonol 3′-O-methyltransferase | OMT1 | At5g54160 | 0 | 0 | |||||
| Carotenoid pathway | ||||||||||
| Copathway | ||||||||||
| 47 | Phytoene synthase | PSY | At5g17230 | 20 | 18 | 22 | 2 | CAROT-CO:7.50e-05(21/106974) | ||
| 48 | Phytoene dehydrogenase | PDS | At4g14210 | 23 | JA2:1.71e-04(10/32554) | |||||
| GL-TRP3:3.01e-03(10/44906) | ||||||||||
| 49 | ζ-Carotene desaturase | ZDS | At3g04870 | 16 | 19 | T-BOX:3.98e-03(10/46396) | ||||
| CAROT-A2:7.24e-03(10/49795) | ||||||||||
| ABFS:8.36e-03(2/1490) | ||||||||||
| Zeaxanthin branch | ||||||||||
| 50 | Lycopene cyclase | LYC | At3g10230 | 20 | 0 | 1 | CBS:401 | CAROT-A1:1.13e-08(35/96444) | ||
| 51 | β-Carotene hydroxylase | B2 | At5g52570 | 5 | 21 | 1 | 2 | CAROT-A3:1.42e-08(17/26316) | ||
| CAROT-A2:3.72e-05(18/49795) | ||||||||||
| 52 | β-Carotene hydroxylase | B1 | At4g25700 | 17 | 22 | 21 | CBS:692,348 | CAROT-CO:1.51e-03(27/106974) | ||
| 53 | Zeaxanthin epoxidase | ABA1 | At5g67030 | 23 | 4 | 3 | GL-MET1:5.93e-03(12/37568) | |||
| GL-MET2:8.34e-03(9/26923) | ||||||||||
| Feedback of 7 | ||||||||||
| 54 | Violaxanthin deepoxidase precursor | NPQ1 | At1g08550 | 19 | 10 | 12 | 14 | |||
| Lutein branch | ||||||||||
| 55 | Lutein-deficient 2 | LUT2 | At5g57030 | 14 | 22 | 22 | CAROT-B1:8.51e-08(12/15943) | |||
| 56 | Lycopene cyclase | LYC | At3g10230 | 20 | 0 | 1 | CBS:401 | DPBF1&2:2.33e-05(18/60493) | ||
| 57 | β-Ring hydroxylase on carotenes | LUT5 | At1g31800 | CYP97A3 | 14 | 19 | CAROT-B3:7.54e-05(13/35602) | |||
| CAROT-CO:4.09e-04(23/106974) | ||||||||||
| 58 | ɛ-Ring hydroxylase on carotenes | LUT1 | At3g53130 | CYP97C1 | 13 | 20 | CAROT-B2:8.28e-04(5/6397) | |||
| GL-TRP3:4.85e-03(12/44906) | ||||||||||
| CAROT-A1:7.26e-03(19/96444) | ||||||||||
| Oxylipin pathway | ||||||||||
| Jasmonate branch | ||||||||||
| 59 | Lipoxygenase | LOX1 | At1g55020 | 22 | JA3:1.64e-07(11/5533) | |||||
| 60 | Lipoxygenase | LOX2 | At3g45140 | 18 | JA1:4.06e-06(8/4520) | |||||
| GL-TRP 1:3.33e-04(14/19861) | ||||||||||
| 61 | Lipoxygenase | LOX3 | At1g17420 | 16 | EE:263 | MYB4:5.94e-04(36/77609) | ||||
| 62 | Allene oxide synthase | AOS | At5g42650 | CYP74A1 | 19 | 22 | EE:904 | JA2:1.01e-03(17/32554) | ||
| 63 | Allene oxide cyclase 1 | AOC1 | At3g25760 | 13 | MOTIF8:1.30e-03(33/76487) | |||||
| RAV1-A:1.35e-03(66/171358) | ||||||||||
| 64 | Allene oxide cyclase 2 | AOC2 | At3g25770 | 13 | EE:1573 | EE:2.81e-03(5/3608) | ||||
| 65 | Allene oxide cyclase 4 | AOC4 | At1g13280 | 7 | 13 | AG/AP1 BS IN SUP:4.08e-03(2/304) | ||||
| 66 | 12-Oxophytodienoate reductase | OPR | At1g18020 | 2 | 6 | 6 | MYB:7.16e-03(12/20163) | |||
| ATMYC2 BS IN RD22:8.52e-03 (14/26615) | ||||||||||
| 67 | 12-Oxophytodienoate reductase | OPR3 | At2g06050 | 13 | 15 | 17 | EE:993 | |||
| 68 | S-Adenosyl-l-Met:jasmonic acid carboxyl methyltransferase | JMT | At1g19640 | 20 | 0 | EE:1383 | ||||
| Hexenal branch | ||||||||||
| 69 | Lipoxygenase | LOX1 | At1g55020 | 22 | JA3:2.79e-08(11/5533) | |||||
| 70 | Lipoxygenase | LOX2 | At3g45140 | 18 | JA1:2.19e-05(7/4520) | |||||
| 71 | Lipoxygenase | LOX3 | At1g17420 | 16 | EE:263 | JA2:1.43e-03(17/32554) | ||||
| 72 | Allene oxide synthase | AOS | At5g42650 | CYP74A1 | 19 | 22 | EE:904 | MOTIF8:3.85e-03(29/76487) | ||
| 73 | Allene oxide cyclase 1 | AOC1 | At3g25760 | 13 | MYB4:5.30e-03(29/77609) | |||||
| 74 | Allene oxide cyclase 2 | AOC2 | At3g25770 | 13 | EE:1573 | GL-TRP1:6.41e-03(11/19861) | ||||
| 75 | Allene oxide cyclase 4 | AOC4 | At1g13280 | 7 | 13 | |||||
| 76 | Hydroperoxide lyase | HPL1 | At4g15440 | CYP74B2 | 15 | 17 | ME:1391,1348,1197,390, 376 | |||
| CBS:1573 | ||||||||||
| Glucosinolate pathway | ||||||||||
| Aliphatic glucosinolate branch | ||||||||||
| 77 | Monooxygenase | At1g16400 | CYP79F1 | 1 | CBS:445 | GL-MET3:2.88e-05(16/33391) | ||||
| 78 | Monooxygenase | At1g16410 | CYP79F2 | 1 | CBS:1331 | GL-TRP2:7.38e-05(16/35624) | ||||
| 79 | Monooxygenase | At4g13770 | CYP83A1 | 22 | GL-TRP5:7.95e-05(8/8446) | |||||
| 80 | Alkylthiohydroximate C-S lyase | SUR1 | At2g20610 | 19 | 22 | GL-TRP4:5.99e-04(9/14468) | ||||
| MYB:1.18e-03(10/20163) | ||||||||||
| 81 | UDP-glycosyltransferase | UGT | At1g24100 | 19 | 21 | CBS:1867,728,576 | MYB4:1.42e-03(25/77609) | |||
| GL-MET2:2.57e-03(12/26923) | ||||||||||
| 82 | Sulfotransferase | ST2 | At1g74090 | 17 | GL-MET4:5.00e-03(14/37935) | |||||
| IBOX:7.07e-03(12/30201) | ||||||||||
| Indole glucosinolate branch | ||||||||||
| 83 | Monooxygenase | At4g39950 | CYP79B2 | 23 | CBS:1829,810 EE:183 | GL-TRP2:1.89e-09(21/35624) | ||||
| 84 | Monooxygenase | At2g22330 | CYP79B3 | 0 | 23 | CBS:917,782 | GL-TRP5:1.01e-05(8/8446) | |||
| 85 | Alkylthiohydroximate C-S lyase | SUR1 | At2g20610 | 19 | 22 | GL-TRP4:3.61e-05(10/14468) | ||||
| GL-TRP1:7.25e-05(11/19861) | ||||||||||
| 86 | UDP-glycosyltransferase | UGT | At1g24100 | 19 | 21 | CBS:1867,728,576 | MOTIF3A:1.03e-03(5/5059) | |||
| CBS:1.53e-03(7/12234) | ||||||||||
| 87 | Desulfoglucosinolate sulfotransferase | DST | At1g74100 | 22 | STRE:6.16e-03(9/25932) | |||||
| GL-MET4:6.50e-03(12/37935) | ||||||||||
| Brassinosteroid pathway | ||||||||||
| Brassinosteroid biosynthesis | ||||||||||
| 88 | C-24 reduction | DWF1 | At3g19820 | 7 | 8 | EE:1577 | MOTIF1:4.26e-05(3/358) | |||
| 89 | 3-Oxo-5-α-steroid-4-dehydrogenase | At3g55360 | 22 | CBS:1085,177 | GATA[LRE]:1.46e-04(47/173280) | |||||
| BS2:1.48e-03(9/16468) | ||||||||||
| 90 | Steroid 22α-hydroxylase | DWF4 | At3g50660 | CYP90B1 | 12 | 8 | MOTIF8:1.77e-03(23/76487) | |||
| ATMYC2 BS IN RD22:2.14e-03 (11/26615) | ||||||||||
| 91 | 6-Deoxo-cathasterone 23α-hydroxylase | CPD | At5g05690 | CYP90A1 | 5 | 8 | 9 | |||
| BS1:7.13e-03(8/16242) | ||||||||||
| MOTIF1A:9.15e-03(3/2310) | ||||||||||
| 92 | C-2α-Hydroxylase | ROT3 | At4g36380 | CYP90C1 | 3 | 6 | CBS:280 | |||
| 93 | Brassinolide synthase | BR6ox2 | At3g30180 | CYP85A2 | 1 | EE:894,309 | ||||
| Degradation of brassinosteroid | ||||||||||
| 94 | Brassinosteroid breakdown | BAS1 | At2g26710 | CYP734A1 | 5 | 6 | EE:1902,514 | ATMYB2 BS IN RD22: 8.27e-03(3/7337) | ||
| CBS:1796,819 | ||||||||||
| 95 | Glucosyltransferase | UGT73C5 | At2g36800 | 16 | 21 | |||||
In the aliphatic glucosinolate branch (Fig. 4C), CYP79F1, CYP79F2, and CYP83A1 (nos. 11–13 in Table II) are circadian regulated, with similar phasings under LL_LLHC (21–1 h maxima) as a number of other enzymes in this branched pathway (Fig. 5B, top). Not surprisingly, CYP79B2 and CYP79B3 in the indole glucosinolate branch (nos. 14 and 15 in Table II) are circadian regulated, with exactly the same phasings as most other enzymes in its branch (Fig. 5B, bottom). While below the cutoff used for Table II, CYP83B1 in this branch also cycles with this same phasing (Fig. 5B, bottom). The glucosinolate pathway has not previously been reported to have circadian rhythms.
In brassinolide synthesis (Fig. 4D), four of the six synthetic P450s have different circadian phasings depending on the array conditions (nos. 16–19 in Table II). For example, the LL_LDHH arrays show similar phasing for CYP85A2 and CYP90C1 (ROT3; 1–3 h maxima) and a slightly later phasing for CYP90A1 (CPD; 5 h maximum), which is considered the initial rate-limiting enzyme in this pathway. The LL_LDHC arrays show significantly earlier phasing for CYP90A1 (8 h maximum) than for CYP90B1 (DWF4; 12 h maximum). The LL_LLHC arrays show slightly earlier phasing for CYP90C1 (6 h maximum) than for CYP90A1 and CYP90B1 (8–9 h maxima).
In the inactivation of plant hormones, the catabolism of brassinolide and other brassinosteroids is mediated by CYP734A1 (BAS1) and CYP72C1 (SOB1; Neff et al., 1999; Turk et al., 2003, 2005; Nakamura et al., 2005; Takahashi et al., 2005) and the catabolism of abscisic acid is mediated by four members of the CYP707A subfamily (Kushiro et al., 2004; Saito et al., 2004). At the resolution of the 4-h time points evaluated in these arrays, CYP734A1 (no. 20 in Table II) involved in inactivating brassinolide shows the same phasing as CYP90A1 involved in synthesizing brassinolide (Table II). Of the abscisic acid 8′-hydroxylases, only CYP707A4 (no. 21 in Table II) displays any distinct circadian regulation (7 h maximum). Of the three P450s in GA synthesis (CYP88A3, CYP88A4, and CYP701A3), only the second multifunctional CYP701A3 (no. 22 in Table II) in this pathway (Helliwell et al., 1998, 1999) is circadian regulated (3 h maximum LL_LDHC array, 7 h maximum in LL_LLHC array).
Identification of Circadian-Relevant Elements
To gain perspective on circadian controls over different pathways, circadian-regulated promoters in each branch of a pathway as well as in each overall pathway were searched for known elements that were overrepresented compared with their frequency in the 27,457 promoters of the Arabidopsis genome (annotated in the AGRIS sequence motif database [http://Arabidopsis.med.ohio-state.edu]; Davuluri et al., 2003; Palaniswamy et al., 2006). Promoters were also searched for novel elements using the Gibbs sampler program AlignACE (http://atlas.med.harvard.edu/; Hughes et al., 2000). These searches identified a number of five- to nine-nucleotide elements listed in Table III that are significantly overrepresented in these different pathways at a cutoff of P < 10−3; the sequences for elements identified in these searches are outlined in Supplemental Table S1.
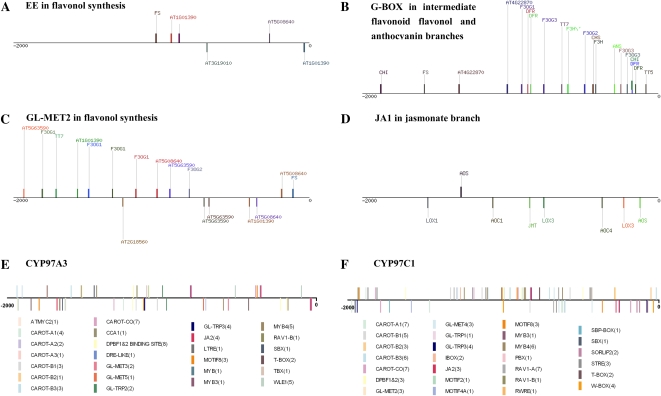
In promoters of genes for different branches of phenylpropanoid metabolism, many circadian-relevant elements are evident. The CBS (AAAAATCT) is overrepresented in the core pathway and the intermediate flavonoid branch. The ME (AACCAC) is frequent in the lignin and anthocyanin branches. The EE (AAAATATCT) reported as overrepresented in the phenylpropanoid pathway (Harmer et al., 2000) is only present in multiple promoters of the downstream flavonol branch and one promoter in the downstream anthocyanin branch but not in the core pathway, the intermediate flavonoid branch, or the lignin branch (Table III). The spacings of these EE relative to the translation start sites of genes in flavonol synthesis are shown in Figure 6A. Interestingly, even though different elements are overrepresented in the individual branches of the phenylpropanoid pathway, the phasing is quite consistent across the whole pathway and peaks before subjective dawn, as mentioned above. The SORLIP1 and/or SORLIP2 originally identified in phyA-induced (far-red light-regulated) promoters (Hudson and Quail, 2003) are overrepresented in the flavonol branch and intermediate flavonoid pathways, respectively, but not in the core pathway or either anthocyanin branch (pelargonidin versus cyanidin). Instead, both of the anthocyanin branches that depend on many of the same loci have overrepresented SORLREP4 elements identified in phyA-repressed promoters (Hudson and Quail, 2003). The intermediate pathway and anthocyanin branches contain overrepresented G-BOX [LRE] whose spacings in anthocyanin, flavonol, and intermediate pathway promoters are shown in Figure 6B.
Figure 6.
The positions of elements in the 2-kb promoter sequences of genes of different pathways. A, Positions of overrepresented EE in the promoters of genes in the flavonol branch. B, Positions of overrepresented G-BOX [LRE] in the promoters of genes in the intermediate flavonoid pathway and the downstream flavonol and anthocyanin branches. C, Positions of overrepresented GL-MET2 in the promoters of genes in the flavonol branch. D, Positions of overrepresented JA1 in the promoters of genes in the oxylipin pathway. E and F, Spacing of elements overrepresented in the CYP97A3 and CYP97C1 promoters in the lutein branch of the carotenoid pathway. The numbers of each element found in these promoters are shown in parentheses behind each motif name.
Analysis of genes associated with the CHS, TT5, CHI, F3H, TT7, FS, F3OG2, F3OG3, and DFR genes, whose promoters contain overrepresented G-BOX [LRE] motifs, was accomplished using a graphical Gaussian model to evaluate publicly available transcript profiling data (Ma et al., 2007). This analysis identified a network containing 97 nodes (genes) connected by 194 significant edges (interactions) with the gene pair Pearson correlation coefficient greater than 0.9 (Fig. 7A). MYB and MYB4 as well as the recently identified WLE1 (Hwang et al., 2008) are also significantly overrepresented in the core pathway and the lignin branch but not in the flavonol or anthocyanin branch. Among the novel elements identified in this pathway are GL-TRP2, GL-TRP5, GL-MET1, GL-MET3, and CAROT-A2 in the core pathway, GL-TRP3 and JA2 in the intermediate pathway, GL-MET3 in the lignin branch, and GL-MET2 in the flavonol branch (Fig. 6C); as their names indicate, most of these were first identified as overrepresented in the glucosinolate branched pathways utilizing Met and Trp. The promoter of CYP711A1, which has been reported to be a positive regulator of the flavonoid pathway (Lazar and Goodman, 2006) and shows the same phasing as phenylpropanoid P450s (20–22 h maxima in three of the array conditions), contains seven MYB4 and three additional MYB motifs (compared with 77,609 MYB4 and 20,163 MYB motifs in the 27,457 annotated Arabidopsis promoters) as well as G-BOX [LRE], GL-MET2, GL-MET3, GL-MET4, CAROT-A2, and MOTIF8. MYB4 (At4g38620), which negatively regulates this flavonoid pathway (Jin et al., 2000), is circadian regulated, with a 21 h maximum (LL_LLHC) that is the same as the phasing of CYP711A1, which positively regulates this pathway (data not shown).
Figure 7.
Phenylpropanoid subnetwork. Genes networked in their expression profiles are shown with nodes corresponding to CHS (chalcone synthase; At5g13930), TT5 (chalcone isomerase; At3g55120), CHI (chalcone isomerase; At5g05270), F3H (naringenin 3-dioxygenase; At3g51240), TT7 (flavonoid 3′-monooxygenase; CYP75B1; At5g07990), F3OG2 (flavonol 3-O-glucosyltransferases; At4g01070), F3OG3 (At5g54060), and DFR (dihydroflavonol 4-reductase; At5g42800). FS (flavonol synthase; At3g50210) is not connected to these. A, Genes at nodes in this network that contain G-BOX [LRE] have thick red circles; associated genes that also contain G-BOX [LRE] have thin red circles. B, Phasing of genes at nodes in this network. Values on the x axis indicate hours after each time-course sampling was initiated. White and gray bars on the x axis indicate subjective day and subjective night during the time course; blank and grid bars indicate high (22°C) and low (12°C) temperature maintained during the time course.
In the lutein branch of the carotenoid pathway, DPBF1&2 is the only previously described element that appears to be overrepresented; in the carotenoid intermediate pathway, ABFS and T-BOX are overrepresented. With few known elements overrepresented in these carotenoid intermediate and branched pathways, AlignACE algorithms identified a number of novel overrepresented elements in the promoters of the zeaxanthin/abscisic acid branch (designated A), lutein branch (designated B), and core pathway (designated CO). These elements are identified with alphabetic and numerical designations and correspond to CAROT-A1 (AGAGA[AG][AG]), CAROT-A2 (CCAAAN[CA]A), CAROT-A3 (GAGA[AT]GA[AG]), CAROT-B1 ([CT]TTG[AG]AAG), CAROT-B2 ([GA][AG]AGAAGCT), CAROT-B3 (GAAGCT), and CAROT-CO (AGAAGA). Of these, CAROT-CO is overrepresented in all three parts of this pathway, and the others are more specific for promoters in branches of this pathway. Spacings of these elements in the CYP97A3 and CYP97C1 promoters are shown in Figure 6D. The promoter of CYP97B3, which codes for a P450 closely related to CYP97A3 and CYP97C1 in the lutein branch and shows intermediate phasing, contains CAROT-B1, CAROT-B2, and CAROT-CO as well as GL-MET2, GL-TRP3, CAROT-A3, and T-BOX. Notably, no EE exist in any of the carotenoid pathway promoters, and CBS exist in only two promoters.
In the oxylipin pathway, circadian-regulated promoters in the AOS branch have overrepresented MYB4, MYB, RAV1-A, AG/AP1 BS, ATMYC2 in RD22, GL-TRP1, and MOTIF8 (ATTCANA), and five of the 10 promoters in this branch have EE. AlignACE analysis of this entire pathway identified three novel overrepresented elements, JA1 (ATGTGAAT), JA2 (AAGAA[GA]ANG), and JA3 (T[TC]GG[AG]CAA), that we had previously identified as overrepresented in MeJ-inducible promoters. Of these, JA2 is represented seven times in the AOC4 promoter, with six of these elements being present in short tandem direct repeats, indicating that its abundance is not uniform across promoters in the AOS branch. The circadian-regulated CYP74B2 promoter contains multiple ME, GATA [LRE], DPBF1&2, GL-TRP1, and MOTIF8, one each of the G-BOX [LRE], JA3, and CBS, and no EE; none of these, except possibly the ME, can be recorded as overrepresented, since this is the only locus in the HPL branch of oxylipin metabolism.
In the glucosinolate pathway, circadian-regulated promoters in the aliphatic glucosinolate branch contain MYB, MYB4, and I-BOX and the novel GL-MET3 (ANACCAAA), GL-TRP2 (ANNTTGAAA), GL-TRP4 (GTTGG[AT]G), and GL-TRP5 (ACCA[AG]CNA[AG]). Circadian-regulated promoters in the indole glucosinolate branch contain STRE (Marchler et al., 1993) and MOTIF3A (TNG[AT]N[AG][AT]GGAA[AG]) and the novel GL-TRP1 (ACATATT), GL-TRP2 (ANNTTGAAA), GL-TRP4 (GTTGG[AT]G), GL-TRP5 (ACCA[AG]CNA[AG]), and GL-MET4. Of these, MOTIF3A was previously identified as overrepresented in Arabidopsis P450 promoters induced by MeJ or salicylic acid (SA). CBS exist in five of eight promoters in these branched pathways.
Overrepresented elements in the collection of circadian-regulated brassinosteroid synthesis loci include the GATA [LRE] ([AT]GATA[GA]), ATMYB2 BS in RD22 (CTAACCA), MOTIF1 ([CT]GNTGATGTCA), MOTIF8, and novel BS1 (AAC[ACGT]CTTT) and BS2 (TATNTTAG). MOTIF1 was originally found to be overrepresented in the promoter sequences of Arabidopsis P450s induced by MeJ, SA, or BION and their combinations; MOTIF8 was originally found to be overrepresented in promoters of root-specific P450s. The only overrepresented element in the two circadian-regulated brassinosteroid degradation loci is ATMYB2 BS in RD22. EE are present once in the DWF1 promoter, twice in the CYP85A2 (BR6ox2) promoter, and twice in the CYP734A1 (BAS1) promoter. CBS are present in the 3-oxo-5α-steroid 4-dehydrogenase, CYP90C1, and CYP734A1 promoters. CYP72C1, another P450 involved in brassinosteroid degradation, is not circadian regulated but its promoter contains SORLREP3 and ATMYB2 BS in RD22, which are overrepresented in the CYP734A1 and UGT73C5 loci involved in brassinosteroid degradation as well as one CBS.
Analysis of a Hypothetical Node Controlling Circadian Cycling
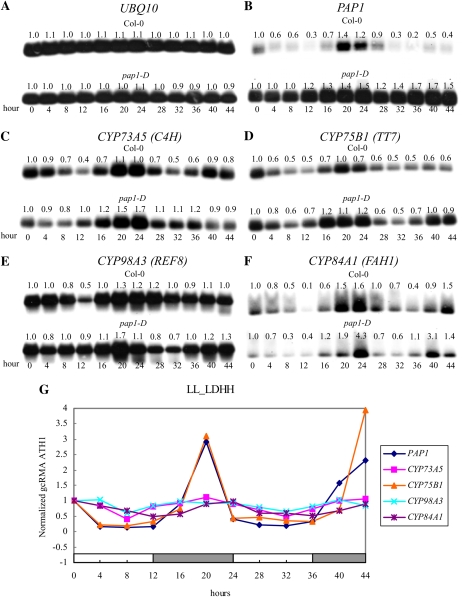
To better understand the relationships between these pathways and some of their predicted transcriptional regulators, we analyzed the expression patterns of PAP1, one MYB transcription factor that had been proposed to control the circadian regulation of the anthocyanin and lignin branches (Harmer et al., 2000), and examined the downstream effects of perturbing its expression. Analysis of PAP1 transcript abundance throughout these circadian cycles indicates that, consistent with previous hypotheses, the PAP1 locus is under circadian regulation (Fig. 8B). Its phasings on both LL_LDHH and LL_LDHC arrays (19 h maximum) are very similar to the four circadian-regulated P450s in the phenylpropanoid pathway (Fig. 8, C–E) and other associated circadian-regulated transcripts (Table III). To directly determine whether PAP1 has a role in circadian regulation of these branched pathways, the circadian regulation patterns of several phenylpropanoid transcripts were compared in wild-type and PAP1-overexpressing (pap1-D) seedlings over a 48-h period starting at 7 d of growth under the LL_LDHH conditions (top versus bottom panels of each blot in Fig. 8). Of the four P450 loci in the phenylpropanoid pathway, only CYP75B1 transcripts appear to accumulate at any higher level in pap1-D seedlings compared with wild-type seedlings. However, most importantly, CYP75B1 and three other P450 transcripts in this pathway maintain their normal circadian cycling despite the constant overexpression of the PAP1 protein (Fig. 8B).
Figure 8.
Circadian regulation of P450s in the phenylpropanoid pathway in wild-type and PAP1-overexpressing (pap1-D) mutant seedlings. RT-PCR gel blots for constitutive UBQ10 (A), PAP1 (B), CYP73A5 (C), CYP75B1 (D), CYP98A3 (E), and CYP84A1 (F) were compared in wild-type and pap1-D seedlings over a 48-h period starting at 7 d of growth under LL_LDHH conditions (collected and analyzed in the Schuler laboratory). UBQ10 expression levels (A) under LL_LDHH were used for normalization, and each normalized transcript level is shown above each lane. The gcRMA-normalized microarray data for PAP1 and these four phenylpropanoid P450s on the LL_LDHH arrays performed with wild-type seedling RNAs (collected and analyzed by the Millar laboratory) are shown in G. Values on the x axis indicate hours after each time-course sampling was initiated. White and gray bars on the x axis indicate subjective day and subjective night during the time course at continuous 22°C. [See online article for color version of this figure.]
DISCUSSION
Our profilings of the four P450 transcripts responsible for rate-limiting steps in phenylpropanoid metabolism emphasize the similar circadian phasing of all transcripts in this pathway, even those in diverse branches (intermediate flavonoid branch, lignin branch, flavonol branch, and pelargonidin and cyanidin branches). Nearly all, including those needed in flavonoid and anthocyanin production, are expressed at their maximal levels just before subjective dawn, at a time when there is little light. Under some of these circadian regimes (LL_LDHC), the cycling of the CYP75B1 transcript is especially prominent, suggesting that its normal circadian cycling pattern is enhanced by exposure to light at subjective dawn. In Arabidopsis, regulation of the flavonoid and anthocyanin pathway transcripts such as CYP75B1 is controlled by a MYB and TTG1 complex with basic helix-loop-helix proteins (Dubos et al., 2008; Gonzalez et al., 2008). The light-dependent regulation of these genes by complex sets of regulators as well as circadian cycles highlights the complex regulatory mechanisms modulating each of the individual branches in this pathway.
One element likely to be involved in light induction of this promoter and others in the flavonoid/anthocyanin branch is the G-BOX [LRE]. This has previously been identified as a light-responsive element (Menkens and Cashmore, 1994; Chattopadhyay et al., 1998; Michael et al., 2008), and in this study, it has been seen as overrepresented throughout the entire downstream flavonoid pathway, including the intermediate flavonoid, flavonol, and anthocyanin branches (Fig. 6B). In a scale-free network of genes coexpressed with the flavonoid pathway (Fig. 7A), eight out of the nine promoters in the intermediate flavonoid pathway, flavonol, and anthocyanin branches that contain G-BOX [LRE] (circled in red) occur at significant nodes. The expression patterns of seven of these genes are highly connected, with only the FS gene not connected to any of the other nodal genes and the F3OG2 gene isolated in a subnetwork. Analysis of the phasings of these last two genes indicates that F3OG2 is phased up to 4 h earlier than the other seven genes and FS is phased 8 to 12 h earlier. Expression of the F3OG2 transcript is correlated only with that of CHI in the nine genes. The coincidence of phasings for these seven other genes is very significant (Fig. 7B), making it likely that the multiple G-BOX [LRE] play a controlling role in the regulation of these promoters. Further comparisons between these simple G-BOX [LRE] motifs have indicated that a longer G-BOX EXTENDED element [CACGTG(G/T)(A/C)] exists within 900 bp of each of these seven similarly phased promoters. These seven promoters also all contain a minimum of one ABRE-like, two DPBF1&2, and two MYB4, suggesting that additional overrepresented elements are also important for coordinated regulation of these promoters. In contrast, the differently phased FS promoter contains only one G-BOX EXTENDED relatively far from its translation start site (−1,583 bp) and no additional simple G-BOX [LRE], making it likely that FS expression is under the control of another transcription factor. The very apparent shift in the phasing of the FS transcripts indicates that the flavonol branched pathway feeding off from the rest of the phenylpropanoid pathway is differentially regulated from the core and lignin and flavonoid branches.
Our promoter analyses have also indicated that MYB and MYB4 are significantly overrepresented in the core and lignin branch of this phenylpropanoid pathway. One potential MYB transcription factor, PAP1, which was proposed to control circadian regulation of the anthocyanin and lignin branches, is indeed circadian regulated, with the same phasing as these branched pathways. However, direct analyses of phenylpropanoid pathway loci potentially targeted by this transcription factor in overexpressing pap-1D seedlings have indicated that the circadian-regulated FAH1 (CYP84A1) and REF8 (CYP98A3) loci in lignin synthesis and C4H (CYP73A5) in the core pathway are not modulated by PAP1. In contrast, the TT7 (CYP75B1) locus, which is directly involved in flavonoid and anthocyanin syntheses, shows some degree of overall enhanced accumulation in pap1-D seedlings, suggesting that PAP1 can modestly enhance expression of the flavonoid branch of this pathway. And, contrary to the suggestion that PAP1 regulates circadian phasing of phenylpropanoid transcripts, these increases in CYP75B1 transcripts fluctuate, with a circadian rhythm that is unaffected by the high PAP1 levels in this mutant, providing further evidence that PAP1 does not control circadian fluctuations of these loci and indicating that other transcription factors modulate circadian cycles in this pathway. How PAP1 expression and these branched pathways are controlled certainly requires further investigation. These results also dramatically demonstrate that the relationships between cycling genes and the cycling network cannot be inferred from time-of-day information and that additional experiments are required to dissect cascades of regulation.
Since it has been demonstrated that the coordination of daily activities confers fitness for specific environments (Michael et al., 2003; Dodd et al., 2005), understanding how these branched pathways are coordinately as well as separately controlled can provide important information for optimizing plant growth and health. Interestingly, all of the genes involved in carotenoid synthesis exhibit circadian regulation, but with shifted phasings in the different branches of this pathway (nos. 47–58 in Table III; Fig. 5A). The phasings of the carotenoid intermediate pathway is 19 h on average, and that of the zeaxanthin branch is approximately 20 h relative to subjective dawn, except for NPQ1 (the violaxanthin deepoxidase precursor on the feedback loop), whose circadian phasing is opposite all other loci analyzed in carotenoid synthesis. The lutein branch shows phasing at 14 h prior to subjective dawn except for LYC, the last common component shared between the lutein and zeaxanthin branches. This phasing of the lutein branch is 5 to 6 h earlier (or 18–19 h later) than that of the zeaxanthin branch, potentially producing maximum expression of lutein derivatives such as α-carotene at the beginning of dark and maximum expression of zeaxanthin derivatives such as β-carotene in the middle of night. With it known that light-harvesting complex II (Kim and DellaPenna, 2006) and β-carotene-containing photosystems are produced almost exclusively under high light conditions and that α-carotene-containing photosystems are produced primarily in shade-grown leaves (Thayer and Bjorkman, 1990; Demmig-Adams and Adams, 1992; Dall'Osto et al., 2007), these results indicate that the circadian-regulated accumulation of transcripts for these branched pathways precedes accumulation of these carotene components by as much as 12 h.
Recent research has indicated that multiple hormone responses are intertwined with circadian cycling, including abscisic acid, 1-aminocyclopropane-1-carboxylic acid, brassinolide, cytokinin, indole-3-acetic acid, MeJ, and SA (S.L. Harmer, unpublished data). Auxin synthesis has been reported to be gated by the circadian clock, allowing a plant to respond to auxin at restricted times of day (Covington and Harmer, 2007). The key regulatory nodes, transcription factor/binding site relationships, and how time-of-day activities are maintained accurately in complex biochemical pathways remain to be established. Additional studies are also needed to determine whether these transcriptional variations manifest themselves in enzymatic and metabolic variations throughout the day. Initiated as an analysis of the factors affecting P450s in an array of synthetic and catabolic pathways, this study has provided, to our knowledge, the first glance at the varied range of biochemical pathways that are targeted by the circadian clock.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) Columbia ecotype and pap1-D mutant seeds were sterilized in 70% ethanol for 30 s and 15% bleach for 15 min and then rinsed in distilled water two to three times. One hundred to 200 seeds per time point were sown on half-strength Murashige and Skoog agar plates containing 0.8% agar without Suc and were kept in the dark at 4°C for 3 d before transfer to a growth chamber. Seedlings were entrained with 12-h-white-light/12-h-dark cycles at a continuous temperature (22°C) for 7 d prior to being released into continuous white light at 22°C (LL_LDHH conditions). After 1 d in continuous conditions, seedlings were harvested at subjective dawn and every 4 h over the course of the next 44 h.
Data Sources
The gene lists used for the analysis of different pathway promoters are derived from data available at The Arabidopsis Information Resource (http://www.arabidopsis.org). The circadian-regulated Arabidopsis P450 gene lists of four data sets are derived from analysis of Affymetrix Arabidopsis genome chips as described by Michael et al. (2008). Raw data and analyzed data for the entire gene set can be accessed at http://diurnal.cgrb.oregonstate.edu/. Growth conditions for DD_DDHC were constant dark and 22°C, those for LL_LLHC were constant light and 22°C/12°C, and those for LL_LDHC were 12 h of light/12 h of dark and 22°C/12°C. For LL_LLHC, growth conditions were constant light and 22°C/12°C and samples were collected on the second and third days after switching to these conditions.
Microarray Data Analysis and Normalizations
All microarray experiments were described previously (Mockler et al., 2007; Michael et al., 2008). Briefly, all techniques were as described in the manufacturer-supplied protocols, with RNAs extracted from frozen tissues and labeled probes were prepared and hybridized to Affymetrix Arabidopsis ATH1 Genechips. Array quality was checked using standard tools implemented in the Bioconductor packages simpleaffy and affyPLM. All microarrays described here were normalized together using gcRMA (robust multiarray average; Wu et al., 2003), and relative values are recorded as gcRNA/gcRMA in the microarray plots. Present/absent calls were made using the Affymetrix MAS5 program. The resulting gcRMA-normalized unlogged values were used to identify cycling genes with the HAYSTACK pattern-matching tool (http://haystack.cgrb.oregonstate.edu/). HAYSTACK, a model-based pattern-matching algorithm, compares a collection of diurnal/circadian models against microarray time-course data to identify cycling genes. HAYSTACK has been implemented in Perl and uses least-squares linear regression for each gene against all model cycling patterns with 24 possible phases. A series of statistical tests were used to identify the best-fit model and phase of expression and to estimate a P value and FDR for each gene. We selected cycling genes using a correlation cutoff of 0.8, which corresponds to a maximum FDR of 3.1% to 5.8% in different data sets. All microarray data can be accessed through the DIURNAL Web interface (http://diurnal.cgrb.oregonstate.edu/).
Autoradiographs of the RT-PCR gel blots were scanned using an Epson Perfection 1250 scanner and quantified using ImageJ 1.41 software (http://rsbweb.nih.gov/ij/). RT-PCR signals for each sample were then background corrected and normalized against the RT-PCR signals for UBQ10 and reported relative to the RT-PCR signal for the first sample in each time course.
RT-PCR Verifications
Approximately 100 seedlings per time point were frozen under liquid nitrogen and powdered, and total RNA was extracted using a beadbeater (Biospec Products), a Plant RNeasy kit, and on-column RNase-free DNase (Qiagen) according to the manufacturer's instructions. Quantitative RT-PCR gel-blot analysis of individual P450 transcripts was carried out by amplifying approximately 0.1 mg of total RNA from each sample in one-step RT-PCR containing 50 mm KCl, 10 mm Tris-HCl (pH 8.4), 200 mm each dNTP, 200 mg mL−1 gelatin, 40 pmol of a 5′ gene-specific primer, 80 pmol of a 3′ oligo(dT) primer, 2 units of AMV reverse transcriptase (Promega), 8 units of RNasin (Promega), and 1 unit of GoTaq polymerase (Promega). First-strand cDNAs were synthesized for 30 min at 42°C and subsequently PCR amplified for 18 to 26 cycles, with each cycle consisting of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min, followed by a final extension step of 72°C for 10 min. The numbers of PCR cycles used for each transcript (18 for CYP90A1 and UBQ10, 19 for CYP79B3, 20 for CYP73A5 and CYP84A1, 23 for CYP74A1, and 26 for CYP75B1 and CYP98A3) were determined to be within the linear PCR amplification range for each transcript. PCR products were fractionated on 1.5% agarose gels, transferred to Hybond-N (Amersham-Pharmacia Biotech), and probed with random hexamer 32P-labeled probes corresponding to approximately 150 nucleotides derived from the 3′ untranslated region of each P450 locus or the Arabidopsis UBQ10 cDNA. The gene-specific primers used in this analysis were as follows: 73A5-5′, 5′-TTGCACATCCTTAACCACTCC-3′; 75B1-5′, 5′-GACTCGGGTCGGGTTAAAAT-3′; 84A1-5′, 5′-GGGGTTTGGTATGGTGAAAA3′; 98A3-5′, 5′-TTGACCGGATCTTAACCGAG-3′; 74A1-5′, 5′-AGAAGAACCTCTCATCCATACATTTAGTC-3′; 90A1-5′, 5′-CAGTTGGGTTCCTGCAGAGC-3′; 79B3-5′, 5′-ACGTGTCGAGCTTATGGA-3′; PAP1-5′, 5′-GACAACAGAAAAGGGGGACA-3′; oligo(dT), 5′-CGGAATTCTTTTTTTTTTTTTTTTT-3′; UBQ10-5′, 5′-CTTGGTCCTGCGTCTTCGTGGTGGTTTC-3′; and UBQ10-3′, 5′-CGACTTGTCATTAGAAAGAAAGAGATAACAGG-3′. Probe sequences for each of the P450s transcripts are as follows: CYP73A5, 51 nucleotides upstream to 86 nucleotides downstream from the stop codon of the At2g30490 locus; CYP75B1, 14 nucleotides upstream to 112 nucleotides downstream from the stop codon of the At5g07990 locus; CYP84A1, 45 nucleotides downstream to 149 nucleotides downstream from the stop codon of the At4g36220 locus; CYP98A3, 521 nucleotides upstream to 376 nucleotides upstream from the stop codon of the At2g40890 locus; CYP74A1, 766 nucleotides upstream to 572 nucleotides downstream from the stop codon of the At5g42650 locus; CYP90A1, 103 nucleotides upstream to 147 nucleotides downstream from the stop codon of the At5g05690 locus; CYP79B3, 103 nucleotides upstream to 390 nucleotides downstream from the stop codon of the At2g22330 locus; and PAP1, 82 nucleotides upstream to 38 nucleotides downstream from the stop codon of the At1g56650 locus.
Promoter Analyses
Promoter searches for known cis-elements functional in Arabidopsis (annotated in the AGRIS sequence motif database; http://Arabidopsis.med.ohio-state.edu; Davuluri et al., 2003) have been initiated by evaluating the region 2 kb upstream from each gene's translation start site using an Arabidopsis promoter motif search program for matches to the degenerate sense and antisense forms of the motif (http://stan.cropsci.uiuc.edu/cgi-bin/elefinder/compare.cgi). Novel elements not present in the AGRIS database are being identified using the Gibbs sampling alignment algorithm, AlignACE3.0 (Hughes et al., 2000), as well as a novel degenerate promoter element search tool developed by M.E. Hudson, which is available for use via Web interface at http://stan.cropsci.uiuc.edu/cgi-bin/sift/sift.cgi.
For determination of overrepresented sequences, promoters within particular branched pathways listed in Table II (i.e. lignin and flavonol branches of phenylpropanoid synthesis) were analyzed for the frequency of each degenerate sequence motif compared with the frequency of each motif in the same size window upstream from the translation start sites of 27,457 genes annotated by the Arabidopsis Genome Initiative (ftp://ftp.arabidopsis.org/home/tair/Sequences/blast_datasets/). Pattern search methods and matching motifs are performed using scripts written in the programming language Perl 5.8.0 for i386-linux-thread-multi. P values for the probability of finding motifs in a subset of promoters are calculated by hypergeometric distribution, modeling sampling on a word-by-word basis. The significance cutoffs for P values are corrected for multiple testing according to the step-up procedure of Benjamini and Hochberg (1995) using a FDR of 5%.
Gene Network
The raw transcriptome data sets used for the analyses of circadian-regulated gene correlation were downloaded from Nottingham Arabidopsis Stock Centre arrays in the paper of Edwards et al. (2006) as a Microsoft Excel format. The genes chosen for network mapping in Figure 7 were those whose promoters contained G-BOX [LRE] elements and are listed in Supplemental Table S2. Methods for determining network layout and visualization are as described by Ma et al. (2007).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Sequences of overrepresented elements.
Supplemental Table S2. Transcript coefficients of the phenylpropanoid subnetwork shown in Figure 7.
Supplementary Material
Acknowledgments
We thank Dr. Hui Duan for his technical assistance and care of plants and Dr. Shisong Ma for instruction in network analysis.
This work was supported by the National Science Foundation (grant no. MCB 0115068 to M.A.S.), by a Physiological and Molecular Plant Biology Program Fellowship to Y.P., by Waksman Institute startup funds to T.P.M., and by the National Institutes of Health (grant nos. GM52413 and GM62932 to J.C. and grant nos. GM56006 and GM67837 to S.A.K.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Mary A. Schuler (maryschu@uiuc.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Asami T, Nakano T, Fujioka S (2005) Plant brassinosteroid hormones. Vitam Horm 72 479–504 [DOI] [PubMed] [Google Scholar]
- Bajguz A (2007) Metabolism of brassinosteroids in plants. Plant Physiol Biochem 45 95–107 [DOI] [PubMed] [Google Scholar]
- Bak S, Feyereisen R (2001) The involvement of two P450 enzymes, CYP83B1 and CYP83A1, in auxin homeostasis and glucosinolate biosynthesis. Plant Physiol 127 108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak S, Tax FE, Feldmann KA, Galbraith DW, Feyereisen R (2001) CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in Arabidopsis. Plant Cell 13 101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancos S, Szatmári AM, Castle J, Kozma-Bognár L, Shibata K, Yokota T, Bishop GJ, Nagy F, Szekeres M (2006) Diurnal regulation of the brassinosteroid-biosynthetic CPD gene in Arabidopsis. Plant Physiol 141 299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley GE, Scolnick PA (1995) Plant carotenoids: pigments for photoprotection, visual attraction, and human health. Plant Cell 7 1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate NJ, Sivasankar S, Moxon C, Riley JMC, Thompson JE, Rothstein SJ (1998) Molecular characterization of an Arabidopsis gene encoding hydroperoxide lyase, a cytochrome P-450 that is wound inducible. Plant Physiol 117 1393–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol 57 289–300
- Bishop GJ, Koncz C (2002) Brassinosteroids and plant steroid hormone signaling. Plant Cell (Suppl) 14 S97–S110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blee E (2002) Impact of phyto-oxylipins in plant defense. Trends Plant Sci 7 315–322 [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O (2005) MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell 8 443–449 [DOI] [PubMed] [Google Scholar]
- Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12 2383–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Ang LH, Puente P, Deng XW, Wei N (1998) Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 10 673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Glawischnig E, Jørgensen K, Naur P, Jørgensen B, Olsen CE, Hansen CH, Rasmussen H, Pickett JA, Halkier BA (2003) CYP79F1 and CYP79F2 have distinct functions in the biosynthesis of aliphatic glucosinolates in Arabidopsis. Plant J 33 923–937 [DOI] [PubMed] [Google Scholar]
- Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49 427–451 [DOI] [PubMed] [Google Scholar]
- Covington MF, Harmer SL (2007) The circadian clock regulates auxin signaling and responses in Arabidopsis. PLoS Biol 5: e222 [DOI] [PMC free article] [PubMed]
- Creelman RA, Mullet JE (1997) Oligosaccharins, brassinolides, and jasmonates: nontraditional regulators of plant growth, development and gene expression. Plant Cell 9 1211–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Osto L, Fiore A, Cazzaniga S, Giuliano G, Bassi R (2007) Different roles of α- and β-branch xanthophylls in photosystem assembly and photoprotection. J Biol Chem 282 35056–35068 [DOI] [PubMed] [Google Scholar]
- Davuluri RV, Sun H, Palaniswamy SK, Matthews N, Molina C, Kurtz M, Grotewold E (2003) AGRIS: Arabidopsis Gene Regulatory Information Server, an information resource of Arabidopsis cis-regulatory elements and transcription factors. BMC Bioinformatics 4 25. [DOI] [PMC free article] [PubMed]
- DellaPenna D, Pogson DJ (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol 57 711–738 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams W III (1992) Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ 15 411–419 [Google Scholar]
- Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AA (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309 630–633 [DOI] [PubMed] [Google Scholar]
- Dubos C, Le Gourrierec J, Baudry A, Huep G, Lanet E, Debeaujon I, Routaboul JM, Alboresi A, Weisshaar B, Lepiniec L (2008) MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J 55 940–953 [DOI] [PubMed] [Google Scholar]
- Edwards KD, Anderson PE, Hall A, Salathia NS, Locke JC, Lynn JR, Straume M, Smith JQ, Millar AJ (2006) FLOWERING LOCUS C mediates natural variation in the high-temperature response of the Arabidopsis circadian clock. Plant Cell 18 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Yokota T (2003) Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol 54 137–164 [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Zhao M, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53 814–827 [DOI] [PubMed] [Google Scholar]
- Hansen CH, Wittstock U, Olsen CE, Hick AJ, Pickett JA, Halkier BA (2001) Cytochrome P450 CYP79F1 from Arabidopsis catalyzes the conversion of dihomomethionine and trihomomethionine to the corresponding aldoximes in the biosynthesis of aliphatic glucosinolates. J Biol Chem 276 11078–11085 [DOI] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang H-S, Han B, Zhu T, Wang X, Kreps JA, Kay SA (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 5499 2110–2113 [DOI] [PubMed] [Google Scholar]
- Harmer SL, Kay SA (2005) Positive and negative factors confer phase-specific circadian regulation of transcription in Arabidopsis. Plant Cell 17 1926–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell CA, Poole A, Peacock WA, Dennis ES (1999) Arabidopsis ent-kaurene oxidase catalyzes three steps of gibberellin biosynthesis. Plant Physiol 119 507–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell CA, Sheldon CC, Olive MR, Walker AR, Zeevaart JA, Peacock WJ, Dennis ES (1998) Cloning of the Arabidopsis ent-kaurene oxidase gene GA3. Proc Natl Acad Sci USA 95 9019–9024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemm MR, Ruegger MO, Chapple C (2003) The Arabidopsis ref2 mutant is defective in the gene encoding CYP83A1 and shows both phenylpropanoid and glucosinolate phenotypes. Plant Cell 15 179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson ME, Quail PH (2003) Identification of promoter motifs involved in the network of phytochrome A-regulated gene expression by combined analysis of genomic sequence and microarray data. Plant Physiol 133 1605–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JD, Estep PW, Tavazoie S, Church GM (2000) Computational identification of cis-regulatory elements associated with groups of functionally related genes in Saccharomyces cerevisiae. J Mol Biol 296 1205–1214 [DOI] [PubMed] [Google Scholar]
- Hull AK, Vij R, Celenza JL (2000) Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc Natl Acad Sci USA 97 2379–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SH, Lee IA, Yie SW, Hwang DJ (2008) Identification of an OsPR10a promoter region responsive to salicylic acid. Planta 227 1141–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C (2000) Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J 19 6150–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, DellaPenna D (2006) Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3. Proc Natl Acad Sci USA 103 3474–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E (2004) The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J 23 1647–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudert D, Pfannschmidt U, Lottspeich F, Holländer-Czytko H, Weiler EW (1996) Cloning, molecular and functional characterization of Arabidopsis thaliana allene oxide synthase (CYP74), the first enzyme of the octadecanoid pathway to jasmonates. Plant Mol Biol 31 323–335 [DOI] [PubMed] [Google Scholar]
- Lazar G, Goodman HM (2006) MAX1, a regulator of the flavonoid pathway, controls vegetative axillary bud outgrowth in Arabidopsis. Proc Natl Acad Sci USA 103 472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Gong Q, Bohnert HJ (2007) An Arabidopsis gene network based on the graphical Gaussian model. Genome Res 17 1614–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler G, Schüller C, Adam G, Ruis HA (1993) Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J 12 1997–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Wilkinson J, Hiatt B, Knauf V, Kajiwara T (1999) Molecular cloning and expression of Arabidopsis fatty acid hydroperoxide lyase. Plant Cell Physiol 40 477–481 [DOI] [PubMed] [Google Scholar]
- Menkens AE, Cashmore AR (1994) Isolation and characterization of a fourth Arabidopsis thaliana G-box-binding factor, which has similarities to Fos oncoprotein. Proc Natl Acad Sci USA 91 2522–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Cusumano JC, Somerville C, Chapple CCS (1996) Ferulate-5-hydroxylase from Arabidopsis thaliana defines a new family of cytochrome P450-dependent monooxygenases. Proc Natl Acad Sci USA 93 6869–6874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, McClung CR (2003) Enhancer trapping reveals widespread circadian clock transcriptional control in Arabidopsis. Plant Physiol 132 629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, Mockler TC, Breton G, McEntee C, Byer A, Trout JD, Hazen SP, Shen R, Priest HD, Sullivan CM, et al (2008) Network discovery pipeline elucidates conserved time-of-day-specific cis-regulatory modules. PLoS Genet 4 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, Salomé PA, Yu HJ, Spencer TR, Sharp EL, McPeek MA, Alonso JM, Ecker JR, McClung CR (2003) Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 302 1049–1053 [DOI] [PubMed] [Google Scholar]
- Mikkelsen MD, Hansen CH, Wittstock U, Halkier BA (2000) Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J Biol Chem 275 33712–33717 [DOI] [PubMed] [Google Scholar]
- Mizutani M, Ohta D, Sato R (1997) Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in planta. Plant Physiol 113 755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler TC, Michael TP, Priest HD, Shen R, Sullivan CM, Givan SA, McEntee C, Kay SA, Chory J (2007) The DIURNAL project: diurnal and circadian expression profiling, model-based pattern matching, and promoter analysis. Cold Spring Harb Symp Quant Biol 72 353–363 [DOI] [PubMed] [Google Scholar]
- Müssig C, Fischer S, Altmann T (2002) Brassinosteroid-regulated gene expression. Plant Physiol 129 1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Satoh T, Tanaka S, Mochizuki N, Tokota T, Nagatani A (2005) Activation of the cytochrome P450 gene, CYP72C1, reduces the levels of active brassinosteroids in vivo. J Exp Bot 56 833–840 [DOI] [PubMed] [Google Scholar]
- Naur P, Petersen BL, Mikkelsen MD, Bak S, Rasmussen H, Olsen CE, Halkier BA (2003) CYP83A1 and CYP83B1, two nonredundant cytochrome P450 enzymes metabolizing oximes in the biosynthesis of glucosinolates in Arabidopsis. Plant Physiol 133 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Nguyen SM, Malancharuvil EJ, Fujioka S, Noguchi T, Seto H, Tsubuki M, Honda T, Takatsuto S, Yoshida S, et al (1999) BAS1: a gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc Natl Acad Sci USA 96 15316–15323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen KA, Moller BL (2005) Cytochrome P450s in plants. In PR Ortiz de Montellano, ed, Cytochrome P450: Structure, Mechanism, and Biochemistry, Ed 3. Kluwer Academic/Plenum Publishers, New York, pp 553–583
- Palaniswamy SK, James S, Sun H, Lamb RS, Davuluri RV, Grotewold E (2006) AGRIS and AtRegNet: a platform to link cis-regulatory elements and transcription factors into regulatory networks. Plant Physiol 140 818–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette SM, Bak S, Feyereisen R (2000) Intron-exon organization and phylogeny in a large superfamily, the paralogous cytochrome P450 genes of Arabidopsis thaliana. DNA Cell Biol 19 307–317 [DOI] [PubMed] [Google Scholar]
- Reintanz B, Lehnen M, Reichelt M, Gershenzon J, Kowalczyk M, Sandberg G, Godde M, Uhl R, Palme K (2001) bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. Plant Cell 13 351–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo J, Vancanneyt G, Perez AG, Sanz C, Stormann K, Rosahl S, Sanchez-Serrano JJ (1996) Characterization of three potato lipoxygenases with distinct enzymatic activities and different organ-specific and wound-regulated expression patterns. J Biol Chem 271 21012–21019 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Meyer K, Cusumano JC, Chapple C (1999) Regulation of ferulate-5-hydroxylase expression in Arabidopsis in the context of sinapate ester biosynthesis. Plant Physiol 119 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M (2004) Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol 134 1439–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch G, Goepfert S, Morant M, Hehn A, Meyer D, Ullmann P, Werck-Reichhart D (2001) CYP98A3 from Arabidopsis thaliana is a 3′-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. J Biol Chem 276 36566–36574 [DOI] [PubMed] [Google Scholar]
- Schoenbohm C, Martens S, Eder C, Forkmann G, Weisshaar B (2000) Identification of the Arabidopsis thaliana flavonoid 3′-hydroxylase gene and functional expression of the encoded P450 enzyme. Biol Chem 381 749–753 [DOI] [PubMed] [Google Scholar]
- Schuler MA (1996) The role of cytochrome P450 monooxygenases in plant-insect interactions. Plant Physiol 112 1411–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler MA, Duan H, Bilgin M, Ali S (2006) Arabidopsis P450s through the looking glass: a window on plant biochemistry. Phytochem Rev 5 205–237 [Google Scholar]
- Takahashi N, Nakazawa M, Shibata K, Yokota T, Ishikawa A, Suzuki K, Kawashima M, Ichikawa T, Shimada H, Matsui M (2005) shk1-D, a dwarf Arabidopsis mutant caused by activation of the CYP72C1 gene, has altered brassinosteroid levels. Plant J 42 13–22 [DOI] [PubMed] [Google Scholar]
- Thayer S, Bjorkman O (1990) Carotenoid distribution and deepoxidation in thylakoid pigment-protein complexes from cotton leaves and bundle-sheath cells of maize. Photosynth Res 23 331–343 [DOI] [PubMed] [Google Scholar]
- Tian L, Musetti V, Kim J, Magallanes-Lundback M, DellaPenna D (2004) The Arabidopsis LUT1 locus encodes a member of the cytochrome P450 family that is required for carotenoid ɛ-ring hydroxylation activity. Proc Natl Acad Sci USA 101 402–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohge T, Nishiyama Y, Hirai MY, Yano M, Nakajima J, Awazuhara M, Inoue E, Takahashi H, Goodenowe DB, Kitayama M, et al (2005) Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J 42 218–235 [DOI] [PubMed] [Google Scholar]
- Turk EM, Fujioka S, Seto H, Shimada Y, Takatsuto S, Yoshida S, Denzel MA, Torres QI, Neff MM (2003) CYP72B1 inactivates brassinosteroid hormones: an intersection between photomorphogenesis and plant steroid signal transduction. Plant Physiol 133 1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk EM, Fujioka S, Seto H, Shimada Y, Takatsuto S, Yoshida S, Wang H, Torres QI, Ward JM, Murthy G, et al (2005) BAS1 and SOB7 act redundantly to modulate Arabidopsis photomorphogenesis via unique brassinosteroid inactivation mechanisms. Plant J 42 23–34 [DOI] [PubMed] [Google Scholar]
- Urban P, Mignotte C, Kazmaier M, Delorme F, Pompon D (1997) Cloning, yeast expression, and characterization of the coupling of two distantly related Arabidopsis thaliana NADPH-cytochrome P450 reductases with P450 CYP73A5. J Biol Chem 272 19176–19186 [DOI] [PubMed] [Google Scholar]
- Wang ZY, Kenigsbuch D, Sun L, Harel E, Ong MS, Tobin EM (1997) A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhch gene. Plant Cell 9 491–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werck-Reichhart D, Bak S, Paquette S (2002) Cytochrome P450. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0028, http://www.aspb.org/publications/arabidopsis
- Whetten R, Sederoff R (1995) Lignin biosynthesis. Plant Cell 7 1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel BS (2004) Metabolic channeling in plants. Annu Rev Plant Biol 55 85–107 [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B (2001) Flavonoid biosynthesis: a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Irizarry R, Gentleman R, Murillo F, Spencer F (2003) A Model Based Background Adjustment for Oligonucleotide Expression Arrays. Technical Report. Johns Hopkins University, Department of Biostatistics Working Papers, Baltimore, MD
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.