Abstract
The mitochondrial flavoenzyme l-galactono-γ-lactone dehydrogenase (GALDH) catalyzes the ultimate step of vitamin C biosynthesis in plants. We found that recombinant GALDH from Arabidopsis (Arabidopsis thaliana) is inactivated by hydrogen peroxide due to selective oxidation of cysteine (Cys)-340, located in the cap domain. Electrospray ionization mass spectrometry revealed that the partial reversible oxidative modification of Cys-340 involves the sequential formation of sulfenic, sulfinic, and sulfonic acid states. S-Glutathionylation of the sulfenic acid switches off GALDH activity and protects the enzyme against oxidative damage in vitro. C340A and C340S GALDH variants are insensitive toward thiol oxidation, but exhibit a poor affinity for l-galactono-1,4-lactone. Cys-340 is buried beneath the protein surface and its estimated pKa of 6.5 suggests the involvement of the thiolate anion in substrate recognition. The indispensability of a redox-sensitive thiol provides a rationale why GALDH was designed as a dehydrogenase and not, like related aldonolactone oxidoreductases, as an oxidase.
l-Ascorbate (vitamin C) is the most consumed vitamin on earth. It is a multifunctional antioxidant that is particularly abundant in plants where it can reach millimolar concentrations, representing over 10% of the soluble carbohydrate content. l-Ascorbate is a cofactor for a number of enzymes and it is a major constituent of the intracellular redox buffer. Its main function in plants is to scavenge reducing equivalents originating from respiration and photosynthetic activity, protecting proteins, unsaturated fatty acids, and DNA from irreversible oxidative damage (Smirnoff and Wheeler, 2000).
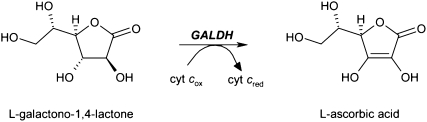
The terminal step of l-ascorbate biosynthesis in plants is catalyzed by the mitochondrial flavoenzyme l-galactono-γ-lactone dehydrogenase (GALDH; l-galactono-1,4-lactone:ferricytochrome c oxidoreductase; EC 1.3.2.3). GALDH mediates the two-electron oxidation of l-galactono-1,4-lactone into l-ascorbic acid with the concomitant reduction of cytochrome c (Scheme 1):
Scheme 1.
Besides from producing l-ascorbate, the exploitation of the electron transport chain by GALDH is important for the proper functioning of plant mitochondria (Alhagdow et al., 2007). Furthermore, it has been reported that GALDH is required for the correct assembly of respiratory complex I (Pineau et al., 2008).
GALDH and other aldonolactone oxidoreductases are two-domain proteins with a conserved vanillyl-alcohol oxidase (VAO)-type FAD domain (Fraaije et al., 1998; Leferink et al., 2008a). Most aldonolactone oxidoreductases are hydrogen peroxide-producing oxidases containing covalently bound FAD, while GALDH reacts poorly with molecular oxygen and contains noncovalently bound FAD (Leferink et al., 2008b). Aldonolactone oxidoreductases have been isolated from various sources, but they are not well characterized. No crystal structure is available, and little is known about the nature of the active site and the catalytic mechanism. Several aldonolactone oxidoreductases, including GALDH from plants (Mapson and Breslow, 1958; Ôba et al., 1995; Østergaard et al., 1997; Imai et al., 1998; Yabuta et al., 2000), l-gulono-1,4-lactone oxidase from animals (Nishikimi, 1979), d-arabinono-1,4-lactone oxidase from yeast (Huh et al., 1994), and trypanosomal aldonolactone oxidoreductases (Logan et al., 2007), are sensitive toward inactivation by thiol-modifying agents. In line with this, we previously found that recombinant GALDH from Arabidopsis (Arabidopsis thaliana) is slowly inactivated during storage and that the activity can be completely restored by treatment with the reducing agent dithiothreitol (DTT; Leferink et al., 2008b).
So far nothing is known about the nature of the thiol inactivation, and to our knowledge the effect of oxidants on the activity of aldonolactone oxidoreductases has not been studied before. Here we investigated the susceptibility of GALDH to oxidative stress and identified a critical Cys in the substrate binding site that makes the enzyme vulnerable toward irreversible inactivation.
RESULTS
Assignment of the Critical Cys
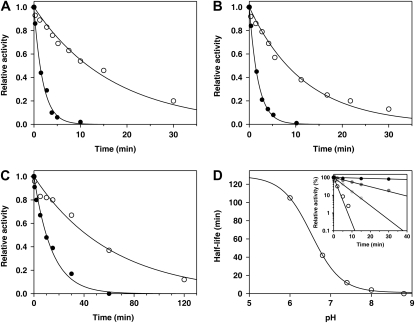
Previously we established that recombinant GALDH from Arabidopsis is slowly inactivated during storage and that DTT restores the activity (Leferink et al., 2008b). In line with this observation, GALDH was readily inactivated by the thiol-modifying agents 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB; Fig. 1A) and N-ethylmaleimide (NEM; Fig. 1B). Preincubation of the enzyme with l-galactono-1,4-lactone protected the enzyme from thiol modification, increasing the half-life of inactivation by 1 order of magnitude (Fig. 1, A and B).
Figure 1.
Time-dependent inactivation of GALDH with thiol-modifying agents. GALDH (2 μm) was incubated with 50 μm DTNB (A), 50 μm NEM (B), or 5 mm hydrogen peroxide (C) in 50 mm sodium phosphate, pH 7.4, in the presence (white circles) or absence (black circles) of 1 mm l-galactono-1,4-lactone. Data were fitted according to pseudo first-order kinetics. D, Half-life of inactivation of GALDH (2 μm) by hydrogen peroxide (5 mm) as a function of pH. The inset shows the time-dependent inactivation of GALDH (2 μm) by hydrogen peroxide (5 mm) in 50 mm BisTris-Cl, pH 6.0 (black circles), 50 mm Tris-Cl, pH 7.4 (dark-gray circles), 50 mm Tris-Cl, pH 8.0 (light-gray circles), and 50 mm Tris-Cl, pH 8.8 (white circles). For clarity, only selected traces are shown.
The number of reactive sulfhydryl groups in GALDH was estimated with the method of Ellman (1959). DTNB analysis of unfolded GALDH yielded a total number of five Cys per polypeptide chain, consistent with the amino acid sequence of mature GALDH (Table I). DTNB analysis of native GALDH revealed only one reactive Cys (Table I). DTNB analysis of NEM-inactivated GALDH yielded no response, indicating that both thiol-modifying reagents react with the same accessible Cys.
Table I.
Thiol content of native and unfolded GALDH variants as determined by DTNB analysis
Data are presented as the mean ± sd of two experiments.
| GALDH | Thiol Groups
|
|
|---|---|---|
| Native | Unfolded | |
| mol/mol FAD | ||
| Wild type | 0.8 ± 0.1 | 4.9 ± 0.5 |
| NEM-GALDH | 0.2 ± 0.1 | 3.8 ± 0.4 |
| C340S | 0.2 ± 0.1 | 3.8 ± 0.1 |
| C340A | 0.1 ± 0.05 | 3.6 ± 0.1 |
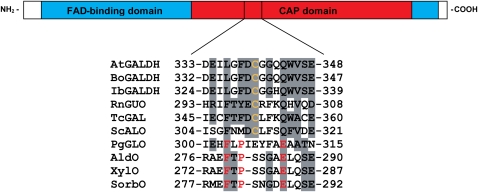
To identify the critical Cys residue, the amino acid sequence of GALDH was compared with other aldonolactone oxidoreductases. Interestingly, only one Cys residue, Cys-340 in recombinant GALDH, is conserved among the aldonolactone oxidoreductases that are sensitive toward inactivation by thiol-modifying agents. d-Gluconolactone oxidase from Penicillium griseoroseum (PgGLO), which is resistant to inactivation by these compounds (Takahashi et al., 1976), lacks this Cys, as well as the related VAO family members alditol oxidase, xylitol oxidase, and sorbitol oxidase (Fig. 2). The conserved Cys is located in the C-terminal cap domain, which determines the substrate specificity in VAO-type flavoproteins (Fraaije et al., 1998).
Figure 2.
Sequence comparison of GALDH and related VAO members. Top section, Schematic representation of the primary structure of mature GALDH with the FAD-binding domain in blue and the cap domain in red. Bottom section, Sequence alignment around the conserved Cys. The accession numbers used are as follows: Arabidopsis GALDH (AtGALDH), Q8GY16; Brassica oleracea GALDH (BoGALDH), O47881; Ipomoea batatas GALDH (IbGALDH), Q9ZWJ1; Rattus norvegicus l-gulono-1,4-lactone oxidase (RnGUO), P10867; Trypanosoma cruzi galactonolactone oxidase (TcGAL), Q4DPZ5; Saccharomyces cerevisiae d-arabinono-1,4-lactone oxidase (ScALO), P54783; P. griseoroseum d-gluconolactone oxidase (PgGLO), Q671X8; alditol oxidase (AldO), Q9ZBU1; xylitol oxidase (XylO), Q9KX73; and sorbitol oxidase (SorbO), P7011. Similar residues are shaded in gray. The conserved Cys is indicated in orange, and conserved residues lining the active site entrance in AldO are marked in red.
To confirm Cys-340 as the target of modification, two GALDH variants were created by site-directed mutagenesis in which Cys-340 was replaced by a Ser residue (C340S) or an Ala residue (C340A). Both mutant proteins were expressed as holoproteins and could be purified in similar quantities as the wild-type protein (Leferink et al., 2008b). DTNB analysis showed that native C340S and C340A contained no surface-accessible Cys residues (Table I). Both C340S and C340A are also insensitive toward inactivation by DTNB. These data establish that Cys-340 is the target reactive Cys in wild-type GALDH.
Cys-340 Is Located in or Near the Active Site
To investigate the role of Cys-340 in GALDH catalysis, the biochemical properties of the C340S and C340A variants were studied in more detail. Both mutant proteins have flavin absorption characteristics similar to wild-type GALDH (data not shown). The molar absorption coefficient (ɛ450) of the protein-bound flavin was determined to be 13.2 and 13.0 mm−1 cm−1 for C340S and C340A, respectively, similar to the value of 12.9 mm−1 cm−1 reported for wild-type GALDH (Leferink et al., 2008b). Steady-state kinetic analysis revealed that the Michaelis constant for l-galactono-1,4-lactone is 30- to 50-fold higher for C340S and C340A than for wild-type GALDH (Table II). However, the turnover rate is only slightly lower in the Cys-340 variants (Table II). The high Km and kcat of the mutant proteins suggest that Cys-340 is involved in substrate binding rather than in catalysis.
Table II.
Kinetic parameters of GALDH variants with l-galactono-1,4-lactone
Data are presented as the mean ± sd of at least two experiments.
| GALDH | Km | kcat | kcat/Km |
|---|---|---|---|
| mm | s−1 | mm−1 s−1 | |
| Wild typea | 0.17 ± 0.01 | 134 ± 5 | 770 |
| C340S | 5.0 ± 0.5 | 59 ± 2 | 12 |
| C340A | 9.0 ± 0.6 | 79 ± 3 | 9 |
Data obtained from Leferink et al. (2008b).
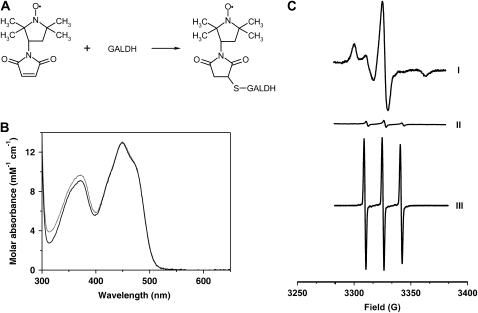
To probe the microenvironment around Cys-340, wild-type GALDH was modified with the maleimide spin label 3-maleimido-2,2,5,5-tetramethyl-1-pyrrolidinyloxy (3-maleimido-PROXYL) via Cys-340 (Fig. 3A). The flavin absorption properties of the spin-labeled protein were identical to the unlabeled protein (Fig. 3B), suggesting that the spin label is not located in close vicinity of the isoalloxazine moiety of FAD. Electron paramagnetic resonance (EPR) analysis of the modified enzyme revealed a highly immobile spin label (Fig. 3C), indicating that Cys-340 has a buried location and is not surface exposed. Maleimide spin labeling of the C340A variant revealed the covalent incorporation of a minor amount of highly mobile spin label (Fig. 3C). The spin labeling of C340A most likely originates from a slow reaction with surface Lys or other less accessible Cys and does not result in any loss of activity. This confirms that the reaction of GALDH with 3-maleimido-PROXYL is highly specific for Cys-340.
Figure 3.
Spin labeling in EPR of wild-type GALDH and C340A. A, Spin-labeling reaction of wild-type GALDH with 3-maleimido-PROXYL via Cys-340. B, Normalized absorption spectra of wild-type GALDH (solid line) and C340A (dotted line) labeled with 3-maleimido-PROXYL recorded in 50 mm sodium phosphate, pH 7.4. C, EPR spectra of wild-type GALDH modified with the 3-maleimido-PROXYL spin label (I), GALDH C340A modified with the 3-maleimido-PROXYL spin label (II), and free spin label (III). A sample of 30 μm spin-labeled enzyme or free spin label in 50 mm sodium phosphate, pH 7.4, was used.
Susceptibility of GALDH to Oxidative Stress
The requirement of Cys-340 for optimal catalysis might explain the sensitivity of GALDH toward (reversible) inactivation during long-term storage at low temperature (Leferink et al., 2008b). Because this inactivation is likely caused by Cys oxidation, the effect of oxidative stress on the enzyme activity was examined in further detail. Figure 1C shows that at room temperature, pH 7.4, GALDH is completely inactivated within 1 h by incubation with 5 mm hydrogen peroxide. In agreement with the thiol modifications reported above, the time-dependent inactivation of GALDH by hydrogen peroxide is retarded in the presence of l-galactono-1,4-lactone (Fig. 1C). l-Ascorbate protects the enzyme from fast inactivation in a similar fashion as l-galactono-1,4-lactone, implying that the product can bind to the oxidized enzyme. The peroxide inactivation of wild-type GALDH is strongly pH dependent (Fig. 1D). From the pH dependence of enzyme inactivation a pKa value of 6.5 was estimated for Cys-340, which is significantly lower than the pKa of 8.3 for free Cys in solution.
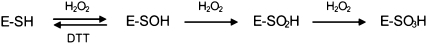
Treatment of the peroxide-inactivated enzyme with DTT restores only part of the initial enzyme activity, indicating that irreversible oxidative damage has occurred. The partial reversible inactivation of GALDH with hydrogen peroxide is consistent with the following reaction sequence (van Berkel and Müller, 1987; Poole et al., 2004; Scheme 2),where E-SH, E-SOH, E-SO2H, and E-SO3H represent the sulfhydryl, sulfenic, sulfinic, and sulfonic acid forms of Cys-340, respectively. The sulfenic acid is generally unstable and readily reacts with other thiols to form (mixed) disulfides or it can undergo further oxidation to the sulfinic or sulfonic acid (Poole et al., 2004; Jacob et al., 2006).
Scheme 2.
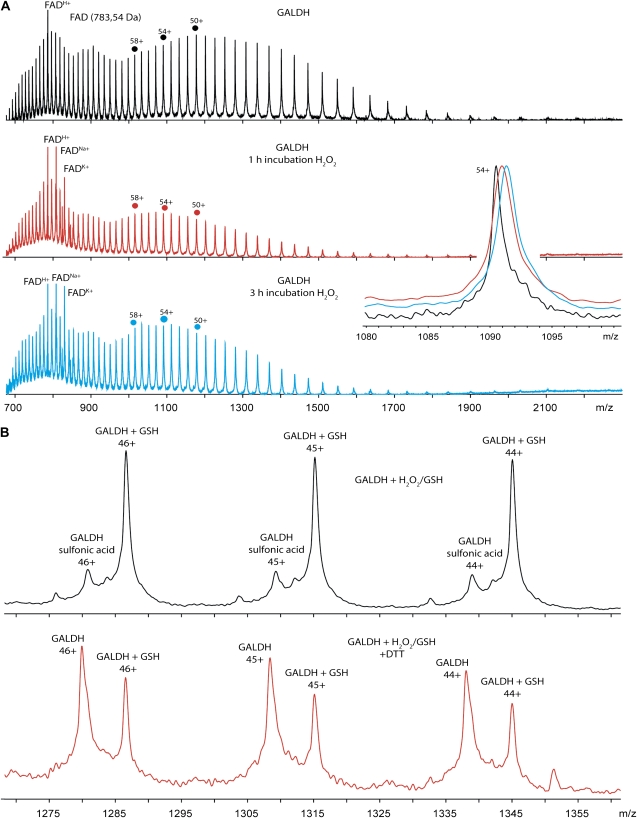
The oxidation state of Cys-340 resulting from the reaction of GALDH with hydrogen peroxide was determined by electrospray ionization mass spectrometry (ESI-MS; Table III). For this purpose, wild-type GALDH and the C340A variant were treated with 5 mm hydrogen peroxide for 1 h (partial inactivation) or 3 h (complete inactivation). The measured mass of denatured wild-type GALDH (Fig. 4A, top section) is 58,832.64 ± 8.69 D. The mass spectrometric data show that after 1 h of incubation with hydrogen peroxide a species with a mass increase of 32 D is observed (Fig. 4A, middle section). After 3 h of incubation with peroxide, only a species with a mass increase of 48 D is present (Fig. 4A, bottom section). The inset shows the separation of the three different GALDH species by ESI-MS, zoomed in on charge state 54+. The measured mass of denatured C340A is 58,800.78 ± 7.21 D; as expected, the C340A mutant did not show any mass increase upon treatment with peroxide (Table III), confirming that Cys-340 is the only site of oxidation.
Table III.
Determination of oxidative modifications of GALDH by ESI-MS
H2O2, Hydrogen peroxide. nd, Not determined.
| Treatment | Relative Activity | Average Mass | Δ Mass | Intensity | Modification |
|---|---|---|---|---|---|
| % | D | D | % | ||
| Wild-type GALDH | |||||
| Controla | 100 | 58,832.64 ± 8.69 | – | 100 | – |
| H2O2 (1 h) | 24 | 58,863.29 ± 10.48 | +30.65 | 100 | Sulfinic acid |
| H2O2 (1 h) + DTT | 53 | 58,866.72 ± 13.06 | +34.10 | 100 | Sulfinic acid |
| H2O2 (3 h) | 1.4 | 58,879.06 ± 10.81 | +46.42 | 100 | Sulfonic acid |
| H2O2 (3 h) + DTT | 6.9 | nd | nd | nd | nd |
| H2O2/GSH | 0.3 | 59,137.68 ± 6.88 | +305.04 | 76 | Glutathionylation |
| 58,876.88 ± 9.41 | +44.24 | 24 | Sulfonic acid | ||
| H2O2/GSH + DTT | 68 | 59,134.11 ± 10.81 | +301.95 | 46 | Glutathionylation |
| 58,831.60 ± 12.18 | – | 54 | None | ||
| GALDH C340A | |||||
| Controlb | 100 | 58,800.78 ± 7.21 | – | 100 | – |
| H2O2 (1 h) | 109 | 58,805.22 ± 6.35 | – | 100 | None |
| H2O2 (3 h) | 100 | 58,810.82 ± 6.58 | – | 100 | None |
Calculated mass apo his-tagged GALDH is 58,821.95 D.
Calculated mass apo his-tagged GALDH C340A is 58,789.89 D.
Figure 4.
ESI-MS spectra of oxidized and S-glutathionylated GALDH. A, ESI-MS spectra of oxidized GALDH. Shown are mass spectra of untreated wild-type GALDH (black), GALDH incubated with hydrogen peroxide for 1 h (red), and GALDH incubated with hydrogen peroxide for 3 h (blue). Spectra were recorded under denaturing conditions in 50 mm ammonium acetate solution with 5% formic acid. The inset shows a zoom in of the overlayed mass spectra at charge state 54+. Under denaturing conditions FAD dissociates from GALDH, resulting in apo-GALDH and FAD in the mass spectra. Singly charged FAD is observed via protonation, and cationization by Na+ and K+. B, ESI-MS spectra of S-glutathionylated GALDH. Shown are mass spectrum of GALDH incubated with hydrogen peroxide and GSH (black) and mass spectrum of S-glutathionylated GALDH treated with DTT (red). Both spectra only show a zoom in on the charge states 44+ to 46+. Spectra were recorded under denaturing conditions in 50 mm ammonium acetate solution with 5% formic acid.
Wild-type GALDH treated with peroxide for 1 h regained nearly 50% of its activity after DTT treatment. However, ESI-MS of the DTT-treated wild-type sample did not show any reversibility of the +32 D species (Table III). The inability to detect a +16 D species indicates that the sulfenic acid is indeed highly reactive, and not stable. In line with the abovementioned scheme (Scheme 2), the obtained results show that Cys-340 in GALDH is readily oxidized to the sulfinic and sulfonic acid stage, and that the oxidation is irreversible.
S-Glutathionylation of GALDH
Reduced glutathione (GSH; γ-l-glutamyl-l-cysteinyl-Gly) is the most prevalent low-Mr thiol present in cells (millimolar range). GSH is often associated with the protection of surface-exposed protein thiols against overoxidation. Glutathionylated proteins are of clinical relevance, glutathionyl hemoglobin is, for example, used as a marker for oxidative stress in human blood, underlining the growing relevance of oxidative stress as a pathological factor in many (chronic) diseases (Pastore et al., 2003; Mandal et al., 2007). Mixed disulfides between protein Cys residues and GSH can be formed upon thiol disulfide exchange with oxidized glutathione or by the reaction of GSH with Cys sulfenic acid (Dalle-Donne et al., 2007). Not all protein thiols readily form mixed disulfides with GSH, the accessibility and reactivity of the Cys residues involved are the major determinants for their susceptibility to S-glutathionylation (Dalle-Donne et al., 2007).
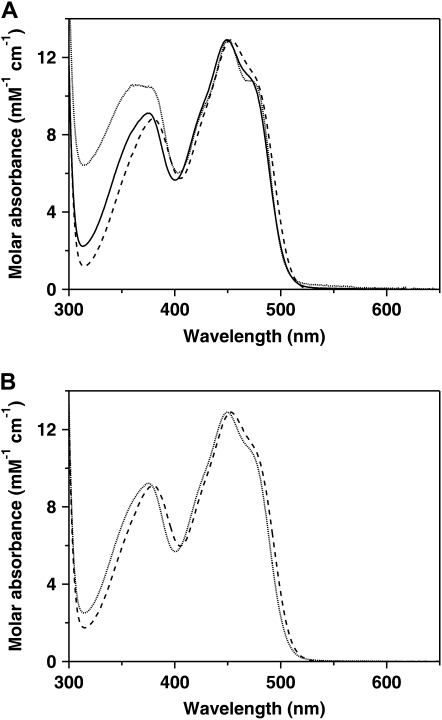
To investigate the ability of GALDH to form a mixed disulfide with GSH via Cys-340, DTNB-inactivated GALDH (GALDH-TNB) was incubated with excess GSH. GALDH-TNB has near-UV spectral properties that are clearly distinct from wild-type GALDH (Fig. 5A). Upon incubation with GSH the spectral perturbations caused by 5-thio-2-nitrobenzoate (TNB) anion binding disappeared and another species appeared, which shows a red shift compared to the spectrum of unmodified GALDH (Fig. 5A). DTT readily reactivated GALDH-TNB with the concomitant release of the TNB anion. GSH, however, did slowly release the TNB anion and could not restore the activity, suggesting that a mixed disulfide has formed between Cys-340 and GSH.
Figure 5.
Absorption spectra of thiol-modified GALDH. A, DTNB-treated GALDH (dotted line) and GALDH-TNB treated with GSH (dashed line) compared to untreated GALDH (solid line). B, Hydrogen peroxide-inactivated GALDH (dotted line) and hydrogen peroxide- and GSH-inactivated GALDH (dashed line). Excess reagents were removed by gel filtration prior to recording of the absorption spectra.
Wild-type GALDH incubated with hydrogen peroxide for 3 h is completely inactive and has spectral properties identical to the untreated enzyme. Incubation of wild-type GALDH with hydrogen peroxide in the presence of GSH yielded also inactive GALDH, but the spectral properties of this species are identical to the species obtained after incubation of GALDH-TNB with GSH (Fig. 5B). The activity of the hydrogen peroxide/GSH-treated GALDH could be restored upon incubation with excess DTT, indicating that the modification is reversible, in contrast to the modification with hydrogen peroxide alone. To confirm that indeed S-glutathionylation had occurred, the hydrogen peroxide/GSH-treated GALDH sample was analyzed with ESI-MS (Table III). The observed mass was 59,137.68 ± 6.88 D, an increase of 305 D compared with unmodified GALDH, which matches with the attachment of one molecule of GSH (Fig. 4B, top section). Upon treatment with DTT, the mass of native GALDH was obtained, showing the reversibility of the S-glutathionylation reaction (Fig. 4B, bottom section). Incubation with oxidized glutathione did not inactivate GALDH, suggesting that S-glutathionylation of GALDH occurs via the sulfenic acid of Cys-340.
DISCUSSION
GALDH is the terminal enzyme of vitamin C biosynthesis in plants (Leferink et al., 2008b). It is already known for a long time that GALDH and related aldonolactone oxidoreductases are inactivated by thiol-modifying agents (Mapson and Breslow, 1958; Nishikimi, 1979; Huh et al., 1994; Ôba et al., 1995; Østergaard et al., 1997; Imai et al., 1998; Yabuta et al., 2000; Logan et al., 2007). However, information concerning the site(s) of modification and implications for catalysis are lacking. Here we showed that GALDH from Arabidopsis is readily inactivated by DTNB, NEM, and hydrogen peroxide and that the loss of activity is retarded in the presence of the l-galactono-1,4-lactone substrate or l-ascorbate product. Cys-340 was found to be the target of thiol modification. The critical involvement of this residue in substrate binding makes GALDH sensitive toward oxidative stress. Cys-340 is the only conserved Cys among the aldonolactone oxidoreductases, suggesting a similar critical function for this residue in the corresponding enzymes. Intriguingly, oxidation inactivates the enzyme, while replacement of Cys-340 by Ala or Ser merely reduces the catalytic efficiency. Presumably, the bulky sulfonic acid group prevents substrate binding, while replacement with Ala and Ser weakens substrate binding. From pH-dependent inactivation experiments, we estimated an apparent pKa of 6.5 for the Cys-340 side chain. This relatively low pKa suggests that during GALDH catalysis, which is optimal at pH 8.8 (Leferink et al., 2008b), Cys-340 participates as the thiolate anion. Interestingly, a recently discovered lactonase that catalyzes the interconversion of l-galactonate to l-galactono-1,4-lactone in the ascorbate biosynthetic pathway of the photosynthetic algae Euglenia gracilis also seems to use a Cys for recognition of the galactonolactone (Ishikawa et al., 2008).
Due to the lack of three-dimensional structural information of the aldonolactone oxidoreductase family, the exact role and location of Cys-340 in GALDH remains elusive. Maleimide spin labeling of GALDH indicated that Cys-340 has a buried location, and is not surface exposed. The local protein environment around Cys-340 reduces the rotational mobility of the spin label, resulting in an EPR spectrum that shows severe line broadening and decreased amplitudes. Either Cys-340 is located on the surface, and the spin label is oriented inwards, or Cys-340 is located inside the substrate entrance tunnel. The latter option seems more likely, because the maleimide group is directly attached to the nitroxide spin label in the absence of a linker, restricting the ability of the spin label to make large movements relative to the maleimide group.
Due to its buried location away from the flavin, Cys-340 is not directly involved in catalysis. This is supported by the fact that bound l-galactono-1,4-lactone protects the enzyme from fast inactivation by thiol-modifying agents and that replacement of Cys-340 by Ser or Ala strongly weakens substrate binding while retaining the catalytic activity. The location of Cys-340 in or near the substrate entrance pocket is further supported by the crystal structure of alditol oxidase (Protein Data Bank accession no. 2VFS), a related VAO family member. Residues lining the entrance of the catalytic pocket of alditol oxidase, including Phe-279, Pro-281, and Glu-286 (Forneris et al., 2008), align around Cys-340 of GALDH and seem to be conserved in PgGLO (Fig. 2). Apparently, the nature of the entrance to the catalytic pocket is different in aldonolactone oxidoreductases than in other related VAO family members.
ESI-MS showed that the hydrogen peroxide-induced oxidative damage of GALDH is solely due to the modification of Cys-340 and involves the sequential formation of sulfenic, sulfinic, and sulfonic acid states. Protein sulfinic and sulfonic acids are generally stable and not reduced by low-Mr thiols. One exception is the active site Cys of peroxiredoxin that can be overoxidized to the sulfinic acid and reduced back to the sulfenic acid via the action of sulfiredoxin (Jacob et al., 2006). Protein sulfonic acids are resistant to any type of cellular repair, and their formation leads to the proteasomal degradation of the protein (Dalle-Donne et al., 2007). Oxidation of GALDH by air results in the sulfenic acid form of Cys-340 causing enzyme inactivation. This mild oxidation is fully reversed by DTT.
Reactive protein thiols can be protected against overoxidation through site-specific S-glutathionylation. The glutathionylated protein remains stable under oxidative stress conditions, and can be deglutathionylated once the redox balance is restored. Glutaredoxins are implicated in the regulation of protein activity via (de)glutathionylation in vivo. Plants contain a high number of glutaredoxin genes of which the protein products have different predicted subcellular localizations (Rouhier et al., 2008). Our results show that GSH protects GALDH from overoxidation in vitro via reversible S-glutathionylation of Cys-340. Evidence for posttranslational modification of the GALDH activity in vivo was found in bean (Phaseolus vulgaris) nodules under oxidative stress conditions, in which the mRNA levels did not correspond to the GALDH activity and ascorbate content (Loscos et al., 2008). GALDH is inhibited during the early stages of programmed cell death, which is characterized by a burst of reactive oxygen species (Valenti et al., 2007). Furthermore, GALDH activity in plants quickly changes in response to light (Smirnoff, 2000). All together these studies support the proposal that GALDH can be regulated in vivo via modification of Cys-340, presumably via S-glutathionylation.
l-Ascorbate is one of the most abundant soluble carbohydrates in plant leaves (Noctor and Foyer, 1998). It acts, for example, as a photoprotectant during photosynthesis and confers resistance to abiotic stresses by scavenging reactive oxygen species (Noctor and Foyer, 1998). Hence, the l-ascorbate content and GALDH transcript levels are increased during high light conditions (Tamaoki et al., 2003; Yabuta et al., 2007). The production of high levels of l-ascorbate in plants (up to 5 mm in green leaves) might be an important reason why GALDH was designed by nature as a dehydrogenase and not, like related aldonolactone oxidoreductases (Leferink et al., 2008a), as an oxidase. Oxidase activity would generate high amounts of hydrogen peroxide and stimulate the irreversible inactivation of GALDH. Furthermore, high levels of hydrogen peroxide will deregulate the expression and functioning of ascorbate peroxidases and other thiol-modulated enzymes and stimulate ageing, senescence, and cell death (Giorgio et al., 2007; Navrot et al., 2007; Ishikawa and Shigeoka, 2008). Galactonolactone oxidase activity in the mitochondrial intermembrane space will also consume high amounts of oxygen, which might affect respiration. Recently we showed that Arabidopsis GALDH can be converted into an efficient galactonolactone oxidase by a single Ala to Gly substitution. The mutation creates space in the active site, allowing oxygen to access the reduced flavin (Leferink et al., 2009). This subtle gatekeeper mechanism seems to be a general strategy for regulating the oxygen reactivity of flavoenzymes and allows the easy generation of dehydrogenases that do not form reactive oxidants with a long lifetime such as hydrogen peroxide.
In conclusion, we have shown that GALDH, the ultimate vitamin C producer in plants, is susceptible to oxidative stress. The sensitivity toward oxidative damage is due to a reactive Cys located in or near the substrate binding site. Reversible oxidative modification (Brandes et al., 2009) of this conserved Cys might regulate the in vivo activity of GALDH.
MATERIALS AND METHODS
Mutagenesis, Expression, and Purification of GALDH Variants
The GALDH variants C340S and C340A were constructed using pET-GALDH-His6 (Leferink et al., 2008b) as template with the QuikChange II method (Stratagene). The following oligonucleotides (Eurogentec) were used (only sense primers are shown, changed nucleotides are underlined): 5′-GAAATTCTGGGCTTTGACTCTGGTGGTCAGCAGTG-3′ (C340S) and 5′-GAAATTCTGGGCTTTGACGCTGGTGGTCAGCAGTG-3′ (C340A). For enzyme production Escherichia coli BL21(DE3) cells, harboring a pET-GALDH plasmid, were grown in Luria-Bertani medium supplemented with 100 μg mL−1 ampicillin until an OD600 of 0.7 was reached. Expression was induced by addition of 0.4 mm isopropyl-thio-β-d-galactopyranoside and the incubation was continued for 16 h at 37°C. The recombinant proteins were purified essentially as described before (Leferink et al., 2008b).
Spectral Analysis
Absorption spectra were recorded at 25°C on a Hewlett-Packard 8453 diode array spectrophotometer in 50 mm sodium phosphate, pH 7.4. Enzyme concentrations were routinely determined by measuring the A450 using a molar absorption coefficient of 12.9 mm−1 cm−1 for wild-type GALDH (Leferink et al., 2008b). The molar absorption coefficients of C340S and C340A were determined by recording absorption spectra in the presence and absence of 0.1% (w/v) SDS, assuming a molar absorption coefficient for free FAD of 11.3 mm−1 cm−1 at 450 nm.
Activity Measurements
GALDH activity was routinely assayed by following the reduction of cytochrome c at 550 nm (Leferink et al., 2008b). Initial velocities were calculated using a molar difference absorption coefficient (Δɛ550) of 21 mm−1 cm−1 for reduced minus oxidized cytochrome c. One unit of enzyme activity is defined as the amount of enzyme that oxidizes 1 μmol of l-galactono-1,4-lactone per min, which is equivalent to the reduction of 2 μmol of cytochrome c (Ôba et al., 1995).
Cys Modification
All thiol modifications were carried out with purified enzyme preparations freshly incubated with 1 mm DTT and 200 μm FAD for 15 min at room temperature. Excess reagents were removed by Bio-Gel P-6DG (Bio-Rad) gel filtration in 50 mm sodium phosphate, pH 7.4. The estimation of sulfhydryl groups of native and unfolded GALDH was performed according to the method of Ellman (1959) with the modifications of Habeeb (1972). The assay was performed on 2 μm GALDH in 100 mm sodium phosphate, 0.5 mm EDTA, pH 8.0, with a 25-times molar excess of DTNB (Merck). The time-dependent release of TNB was measured at 412 nm (ɛ412 TNB = 13.6 mm−1 cm−1). Recombinant GALDH was labeled with NEM (Sigma-Aldrich) or 3-maleimido-PROXYL (Syva) by incubating 100 μm enzyme with 1 mm maleimide for 15 min at room temperature. The reaction was stopped by the addition of 5 mm DTT; excess reagents were removed by Bio-Gel P-6DG gel filtration in 50 mm sodium phosphate, pH 7.4. The time-dependent inactivation of GALDH by the different thiol-modifying agents was performed at room temperature. The incubation mixtures contained 2 μm enzyme, and 50 μm DTNB, 50 μm NEM, or 5 mm hydrogen peroxide. The pH dependence of the hydrogen peroxide inactivation was performed at 25°C in 50 mm BisTris-Cl or Tris-Cl, pH 6.0 to 8.8. Aliquots were withdrawn at intervals and assayed for residual enzyme activity using the standard assay procedure. The pKa of Cys-340 was calculated by fitting the inactivation data to Equation 1:
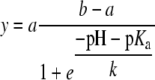 |
(1) |
where y is the observed half-life of inactivation, a the calculated half-life at high pH, b the calculated half-life at low pH, and k the rate of inactivation.
EPR
EPR spectra of wild-type GALDH and the C340A mutant, labeled with the 3-maleimido-PROXYL spin label, were recorded on an X-band Bruker Elexsys E-500 ESR system equipped with a super-high-Q cavity ER 4122SHQE in combination with a SuperX X-Band microwave bridge, type ER 049X. The concentration of spin-labeled enzyme was about 30 μm. Spectra were obtained at room temperature using 50-μL glass capillaries inserted in a quartz EPR tube. Spectra were recorded with a scan range of 10 mT, a modulation amplitude of 0.2 mT, a time constant of 10.24 ms, a scan time of 42 s, and a microwave power of 5 mW. Up to 50 spectra were accumulated to improve the signal-to-noise ratio. All spectra were analyzed using the Xepr software (Bruker).
MS
For the identification of hydrogen peroxide-induced modifications, 100 μm GALDH or C340A in 50 mm sodium phosphate, pH 7.4, was incubated with 5 mm hydrogen peroxide for 1 or 3 h at room temperature. S-Glutathionylated GALDH was prepared by incubation of GALDH-TNB with 5 mm GSH, or by incubation of wild-type GALDH with 5 mm hydrogen peroxide and 5 mm GSH in 50 mm sodium phosphate, pH 7.4, for 3 h at room temperature. Excess reagents were removed by Bio-Gel P-6DG gel filtration in 50 mm ammonium acetate, pH 6.8. The protein samples were analyzed by MS. As control, untreated wild-type and C340A GALDH in 50 mm ammonium acetate, pH 6.8, were also analyzed. All mass spectrometric analyses were performed at protein concentrations of 2 μm under denaturing conditions to allow accurate molecular mass determinations. A solution of 5% (v/v) formic acid was used to denature the protein samples.
MS measurements were performed in positive ion mode using an ESI time-of-flight instrument (LCT; Waters) equipped with a Z-spray nano-ESI source. Needles were made from borosilicate glass capillaries (Kwik-Fil; World Precision Instruments) on a P-97 puller (Sutter Instruments), coated with a thin gold layer using an Edwards Scancoat (Edwards Laboratories) six Pirani 501 sputter coater. Mass spectra were recorded with a capillary voltage of 1.2 kV and cone voltage of 70 V. The source pressure was raised to 6.8 mbar (Tahallah et al., 2001), and the pressure in the time of flight was 1·10−6 mbar. All spectra were mass calibrated by using an aqueous solution of cesium iodide (25 mg mL−1).
Acknowledgments
We are grateful to Cor Wolfs (Laboratory of Biophysics, Wageningen University) for assistance with the EPR experiments.
This work was supported by a grant from the Carbohydrate Research Centre Wageningen and the Netherlands Proteomics Centre, which is part of the Netherlands Genomics Initiative.
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Willem J.H. van Berkel (willem.vanberkel@wur.nl).
References
- Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, et al (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase (L-GalLDH) affects plant and fruit development in tomato. Plant Physiol 145 1408–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes N, Schmitt S, Jakob U (2009) Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal 11 997–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A (2007) S-glutathionylation in protein redox regulation. Free Radic Biol Med 43 883–898 [DOI] [PubMed] [Google Scholar]
- Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82 70–77 [DOI] [PubMed] [Google Scholar]
- Forneris F, Heuts DPHM, Delvecchio M, Rovida S, Fraaije MW, Mattevi A (2008) Structural analysis of the catalytic mechanism and stereoselectivity in Streptomyces coelicolor alditol oxidase. Biochemistry 47 978–985 [DOI] [PubMed] [Google Scholar]
- Fraaije MW, van Berkel WJH, Benen JAE, Visser J, Mattevi A (1998) A novel oxidoreductase family sharing a conserved FAD-binding domain. Trends Biochem Sci 23 206–207 [DOI] [PubMed] [Google Scholar]
- Giorgio M, Trinei M, Migliaccio E, Pelicci PG (2007) Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 8 722–728 [DOI] [PubMed] [Google Scholar]
- Habeeb AFSA (1972) Reaction of protein sulfhydryl groups with Ellman's reagent. Methods Enzymol 25 457–464 [DOI] [PubMed] [Google Scholar]
- Huh WK, Kim ST, Yang KS, Seok YJ, Hah YC, Kang SO (1994) Characterisation of D-arabinono-1,4-lactone oxidase from Candida albicans ATCC 10231. Eur J Biochem 225 1073–1079 [DOI] [PubMed] [Google Scholar]
- Imai T, Karita S, Shiratori G, Hattori M, Nunome T, Ôba K, Hirai M (1998) L-galactono-γ-lactone dehydrogenase from sweet potato: purification and cDNA sequence analysis. Plant Cell Physiol 39 1350–1358 [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Nishikawa H, Gao Y, Sawa Y, Shibata H, Yabuta Y, Maruta T, Shigeoka S (2008) The pathway via D-galacturonate/L-galactonate is significant for ascorbate biosynthesis in Euglena gracilis: identification and functional characterization of aldonolactonase. J Biol Chem 283 31133–31141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Shigeoka S (2008) Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci Biotechnol Biochem 72 1143–1154 [DOI] [PubMed] [Google Scholar]
- Jacob C, Knight I, Winyard PG (2006) Aspects of the biological redox chemistry of cysteine: from simple redox responses to sophisticated signalling pathways. Biol Chem 387 1385–1397 [DOI] [PubMed] [Google Scholar]
- Leferink NGH, Fraaije MW, Joosten HJ, Schaap PJ, Mattevi A, van Berkel WJH (2009) Identification of a gatekeeper residue that prevents dehydrogenases from acting as oxidases. J Biol Chem 284 4392–4397 [DOI] [PubMed] [Google Scholar]
- Leferink NGH, Heuts DPHM, Fraaije MW, van Berkel WJH (2008. a) The growing VAO flavoprotein family. Arch Biochem Biophys 474 292–301 [DOI] [PubMed] [Google Scholar]
- Leferink NGH, van den Berg WAM, van Berkel WJH (2008. b) L-Galactono-γ-lactone dehydrogenase from Arabidopsis thaliana, a flavoprotein involved in vitamin C biosynthesis. FEBS J 275 713–726 [DOI] [PubMed] [Google Scholar]
- Logan FJ, Taylor MC, Wilkinson SR, Kaur H, Kelly JM (2007) The terminal step in vitamin C biosynthesis in Trypanosoma cruzi is mediated by a FMN-dependent galactonolactone oxidase. Biochem J 407 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscos J, Matamoros MA, Becana M (2008) Ascorbate and homoglutathione metabolism in common bean nodules under stress conditions and during natural senescence. Plant Physiol 146 1282–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal AK, Woodi M, Sood V, Krishnaswamy PR, Rao A, Ballal S, Balaram P (2007) Quantitation and characterization of glutathionyl haemoglobin as an oxidative stress marker in chronic renal failure by mass spectrometry. Clin Biochem 40 986–994 [DOI] [PubMed] [Google Scholar]
- Mapson LW, Breslow E (1958) Biological synthesis of ascorbic acid: L-galactono-γ-lactone dehydrogenase. Biochem J 68 395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navrot N, Rouhier N, Gelhaye E, Jacquot JP (2007) Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol Plant 129 185–195 [Google Scholar]
- Nishikimi M (1979) L-Gulono-γ-lactone oxidase (rat and goat liver). Methods Enzymol 62 24–30 [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49 249–279 [DOI] [PubMed] [Google Scholar]
- Ôba K, Ishikawa S, Nishikawa M, Mizuno H, Yamamoto T (1995) Purification and properties of L-galactono-γ-lactone dehydrogenase, a key enzyme for ascorbic acid biosynthesis, from sweet potato roots. J Biochem 117 120–124 [DOI] [PubMed] [Google Scholar]
- Østergaard J, Persiau G, Davey MW, Bauw G, Van Montagu M (1997) Isolation of a cDNA coding for L-galactono-γ-lactone dehydrogenase, an enzyme involved in the biosynthesis of ascorbic acid in plants: purification, characterization, cDNA cloning, and expression in yeast. J Biol Chem 272 30009–30016 [DOI] [PubMed] [Google Scholar]
- Pastore A, Mozzi AF, Tozzi G, Gaeta LM, Federici G, Bertini E, Lo Russo A, Mannucci L, Piemonte F (2003) Determination of glutathionyl-hemoglobin in human erythrocytes by cation-exchange high-performance liquid chromatography. Anal Biochem 312 85–90 [DOI] [PubMed] [Google Scholar]
- Pineau B, Layoune O, Danon A, De Paepe R (2008) L-galactono-1,4-lactone dehydrogenase is required for the accumulation of plant respiratory complex I. J Biol Chem 283 32500–32505 [DOI] [PubMed] [Google Scholar]
- Poole LB, Karplus PA, Claiborne A (2004) Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol 44 325–347 [DOI] [PubMed] [Google Scholar]
- Rouhier N, Lemaire SD, Jacquot JP (2008) The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol 59 143–166 [DOI] [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B Biol Sci 355 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol 35 291–314 [DOI] [PubMed] [Google Scholar]
- Tahallah N, Pinkse M, Maier CS, Heck AJR (2001) The effect of the source pressure on the abundance of ions of noncovalent protein assemblies in an electrospray ionization orthogonal time-of-flight instrument. Rapid Commun Mass Spectrom 15 596–601 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Yamashita H, Kato E, Mitsumoto M, Murakawa S (1976) Purification and some properties of D-glucono-γ-lactone dehydrogenase: D-erythorbic acid producing enzyme of Penicillium cyaneo-fulvum. Agric Biol Chem 40 121–129 [Google Scholar]
- Tamaoki M, Mukai F, Asai N, Nakajima N, Kubo A, Aono M, Saji H (2003) Light-controlled expression of a gene encoding L-galactono-γ-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Sci 164 1111–1117 [Google Scholar]
- Valenti D, Vacca RA, de Pinto MC, De Gara L, Marra E, Passarella S (2007) In the early phase of programmed cell death in Tobacco Bright Yellow 2 cells the mitochondrial adenine nucleotide translocator, adenylate kinase and nucleoside diphosphate kinase are impaired in a reactive oxygen species-dependent manner. Biochim Biophys Acta 1767 66–78 [DOI] [PubMed] [Google Scholar]
- van Berkel WJH, Müller F (1987) The elucidation of the microheterogeneity of highly purified p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens by various biochemical techniques. Eur J Biochem 167 35–46 [DOI] [PubMed] [Google Scholar]
- Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S (2007) Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot 58 2661–2671 [DOI] [PubMed] [Google Scholar]
- Yabuta Y, Yoshimura K, Takeda T, Shigeoka S (2000) Molecular characterization of tobacco mitochondrial L-galactono-γ-lactone dehydrogenase and its expression in Escherichia coli. Plant Cell Physiol 41 666–675 [DOI] [PubMed] [Google Scholar]