Abstract
Sacred lotus (Nelumbo nucifera) regulates temperature in its floral chamber to 32°C to 35°C across ambient temperatures of 8°C to 40°C with heating achieved through high alternative pathway fluxes. In most alternative oxidase (AOX) isoforms, two cysteine residues, Cys1 and Cys2, are highly conserved and play a role in posttranslational regulation of AOX. Further control occurs via interaction of reduced Cys1 with α-keto acids, such as pyruvate. Here, we report on the in vitro regulation of AOX isolated from thermogenic receptacle tissues of sacred lotus. AOX protein was mostly present in the reduced form, and only a small fraction could be oxidized with diamide. Cyanide-resistant respiration in isolated mitochondria was stimulated 4-fold by succinate but not pyruvate or glyoxylate. Insensitivity of the alternative pathway of respiration to pyruvate and the inability of AOX protein to be oxidized by diamide suggested that AOX in these tissues may lack Cys1. Subsequently, we isolated two novel cDNAs for AOX from thermogenic tissues of sacred lotus, designated as NnAOX1a and NnAOX1b. Deduced amino acid sequences of both confirmed that Cys1 had been replaced by serine; however, Cys2 was present. This contrasts with AOXs from thermogenic Aroids, which contain both Cys1 and Cys2. An additional cysteine was present at position 193 in NnAOX1b. The significance of the sequence data for regulation of the AOX protein in thermogenic sacred lotus is discussed and compared with AOXs from other thermogenic and nonthermogenic species.
Thermogenesis in Sacred Lotus
Sacred lotus (Nelumbo nucifera) is a thermogenic plant that regulates the temperature of its floral chamber between 32°C and 35°C for up to 4 d (Seymour and Schultze-Motel, 1996). Heating of plant tissues has been described as an adaptation to attract insect pollinators either by volatilization of scent compounds (Meeuse, 1975) or by providing a heat reward (Seymour et al., 1983), protect floral parts from low temperatures (Knutson, 1974), or provide the optimum temperature for floral development (Ervik and Barfod, 1999; Seymour et al., 2009). In sacred lotus, heat is produced by high rates of alternative pathway respiration (Watling et al., 2006; Grant et al., 2008); however, the mechanisms of heat regulation, which likely occur at a cellular level, remain unclear.
Alternative Oxidase
Alternative pathway respiration is catalyzed by the alternative oxidase protein (AOX), which acts as a terminal oxidase in the electron transport chain but, unlike the energy conserving cytochrome pathway (COX), complexes III and IV are bypassed and energy is released as heat. Traditionally, AOX activity was measured using oxygen consumption of tissue, cells, or isolated mitochondria in the presence or absence of AOX and COX inhibitors. However, this method does not accurately measure activity in vivo but does indicate the capacity of the alternative pathway (Ribas-Carbo et al., 1995; Day et al., 1996). The only method to date to accurately determine AOX activity, that is, flux of electrons through the AOX pathway in vivo, is to use oxygen isotope discrimination techniques (for review, see Robinson et al., 1995). Determining AOX activity in vivo is important because heat production in plants could be due to activity of either the AOX and/or plant uncoupling proteins. Using oxygen fractionation techniques, we have shown that flux through the AOX pathway is responsible for heating in sacred lotus (Watling et al., 2006; Grant et al., 2008). Furthermore, we were unable to detect any uncoupling protein in these tissues (Grant et al., 2008). AOX protein content within the sacred lotus receptacle increases markedly prior to thermogenesis, but it remains constant during heating (Grant et al., 2008), suggesting that regulation of heating occurs through posttranslational modification of the protein.
Posttranslational Regulation of AOX Protein
The plant AOX is a cyanide-insensitive dimeric protein located in the inner mitochondrial membrane (Day and Wiskich, 1995). The dimer subunits (monomers) can be linked via a noncovalent association (reduced protein) or covalently through the formation of a disulfide bridge (oxidized protein; Umbach and Siedow, 1993). The reduced protein when run on SDS-PAGE has a molecular mass of approximately 30 to 35 kD and the oxidized protein 60 to 71 kD; this holds true for AOX from a number of species, including soybean (Glycine max) roots and cotyledons (Umbach and Siedow, 1993), tobacco (Nicotiana tabacum) leaf (Day and Wiskich, 1995), and the thermogenic spadix of Arum maculatum (Hoefnagel and Wiskich, 1998).
Regulation of AOX has been well studied in nonthermogenic plant species, and two mechanisms have been identified. Most AOX isoforms have two highly conserved Cys residues, Cys1 and Cys2 (defined in Berthold et al., 2000 and Holtzapffel et al., 2003), located near the N-terminal hydrophilic domain of the protein. In these isoforms, Cys1 can either be reduced on both subunits of the AOX dimer, or the Cys1 sulfhydryl groups can be oxidized to form a disulfide bridge (Rhoads et al., 1998). Reduction/oxidation modulation of AOX in vitro can be achieved using the sulfhydryl reductant dithiothreitol (DTT) to reduce the protein or diamide to oxidize the Cys residues. The reduced dimer can be further activated via the interaction of Cys1 with α-keto acids, principally pyruvate (Rhoads et al., 1998; see McDonald [2008] for a model of posttranslational regulation of AOX). In addition, Cys2 may also be involved in regulating AOX activity through interaction with the α-keto acid glyoxylate (which can also stimulate activity at Cys1; Umbach et al., 2002).
Recently, however, AOX proteins with different regulatory properties have been reported. Naturally occurring AOX proteins without the two regulatory Cys residues have been identified and, along with site-directed mutagenesis studies, used to further elucidate the specific roles of Cys1 and Cys2. The LeAOX1b isoform from tomato (Lycopersicon esculentum), which has a Ser residue at the position of Cys1 and thus does not form disulfide linked dimers, is also activated by succinate rather than pyruvate when expressed in Saccharomyces cerevisiae (Holtzapffel et al., 2003). In Arabidopsis (Arabidopsis thaliana), uncharged or hydrophobic amino acid substitutions of either Cys result in an inactive enzyme, while positively charged substitutions produce an enzyme with higher than wild type basal activity but that is insensitive to pyruvate or succinate (Umbach et al., 2002). Single substitutions at Cys1 or Cys2 have revealed that glyoxylate can activate AOX via both Cys residues, but only one is needed for glyoxylate stimulation (Umbach et al., 2002, 2006). Double substitution mutants were not stimulated by either pyruvate or glyoxylate (Umbach et al., 2006).
Previously, we determined that thermogenesis via the AOX pathway in the sacred lotus receptacle is precisely regulated through changes in AOX flux rather than changes to protein content (Grant et al., 2008). In this study, we investigated the nature of this regulation in mitochondria isolated from heating receptacles. Our aim was to elucidate the reduction/oxidation behavior of the AOX protein and the mechanisms of activation of cyanide-resistant respiration in sacred lotus receptacles to provide insights into the mechanism(s) of heat regulation in this species. We further investigated AOX regulation by determining the amino acid sequence of two novel AOX genes isolated from thermogenic receptacle tissue of sacred lotus.
RESULTS
Activity of Sacred Lotus AOX Is Stimulated by Succinate But Not Pyruvate or Glyoxylate
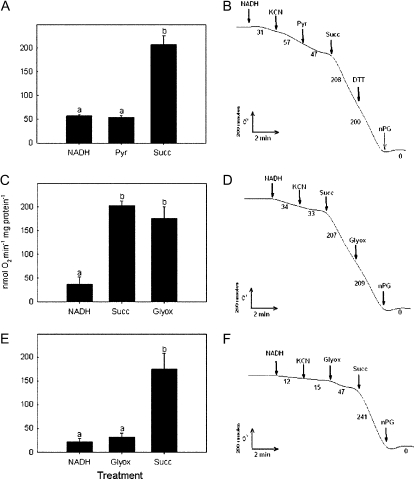
Residual mitochondrial respiration rates were quite low (<10 nmol O2 min−1 mg−1 protein). Addition of NADH and KCN stimulated activity to an average of 50 nmol O2 min−1 mg−1 protein, but this stimulation was not statistically significant. No stimulation was observed with subsequent addition of 5 mm pyruvate (Fig. 1) nor with concentrations of pyruvate up to 20 mm. Addition of succinate, however, produced a 4-fold increase in activity to a mean of 196 ± 20 nmol O2 min−1 mg−1 protein (F2,20 = 48.70, P < 0.0001; Fig. 1). Activation of respiration by succinate was similar in the presence or absence of malonate, which was used to inhibit complex II. Cyanide-resistant O2 uptake was not stimulated by the addition of glyoxylate, either before or after succinate stimulation (Fig. 1, C–F), and there was no increase in mitochondrial O2 uptake with the possible substrates: citrate, fumarate, oxalate, α-ketoglutarate, or malate (data not shown). Manipulation of AOX redox state by addition of the sulfhydryl redox reagents DTT (Fig. 1B) and diamide had no effect on O2 uptake. Cyanide-resistant oxygen uptake was almost completely inhibited by the AOX inhibitor n-propyl gallate (Fig. 1, B, D, and F).
Figure 1.
Rate of O2 uptake in mitochondria from thermogenic sacred lotus receptacles. Each column (means ± se, n = 3–4) represents a subsequent addition to the O2 electrode chamber of 2 mm NADH, 5 mm pyruvate (Pyr), or 20 mm succinate (Succ) and/or 5 mm glyoxylate (Glyox). These additions were made in the presence of 1 mm KCN. Columns with different letters are significantly different. Typical O2 uptake traces per milligram of mitochondrial protein are shown to the right of the graphs. Numbers below the traces are the respiration rates (nmoles O2 min−1 mg protein−1). nPG, n-Propyl gallate.
The Majority of AOX Protein Does Not Form Disulfide-Linked Dimers in the Presence of Diamide
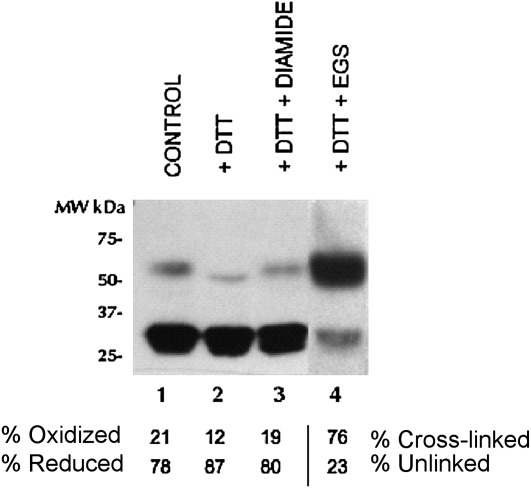
AOX protein isolated from thermogenic sacred lotus receptacles was predominantly in the reduced form (approximately 32 kD) with only 21% present in the oxidized state (approximately 64 kD; Fig. 2, lane 1). When treated with the reductant DTT (20 mm), almost all of the protein was present in the reduced state (Fig. 2, lane 2), although a small proportion (12%) remained oxidized. The reduced protein could be partially reoxidized with 10 mm diamide (19%; Fig. 2, lane 3); however, most of the protein was insensitive to diamide even at high concentrations (50–250 mm). In contrast, treatment with the Lys-Lys-specific cross-linker ethylene glycol bis(succinimidylsuccinate) (EGS; 1 mm) caused 76% dimerization of the AOX protein (Fig. 2, lane 4).
Figure 2.
Immunoblots of mitochondrial proteins from sacred lotus receptacles (as shown in Fig. 1) using antibodies raised against AOX proteins. Treatments are as follows: lane 1, untreated protein; lane 2, DTT (20 mm); lane 3, DTT (20 mm), wash, diamide (10 mm); and lane 4, DTT (20 mm), wash, EGS (5 mm). There was no reductant used in the stock sample preparation. Numbers to the left of the blots are approximate positions of molecular mass markers, with sizes in kilodaltons. Numbers below the blots show the percentage of the protein oxidized/reduced or cross-linked/unlinked. Lane 4 has been added from another experiment.
Two Novel AOX Isoforms Lacking Cys1 Occur in Thermogenic Sacred Lotus Tissue
Reverse transcription-PCR-based cloning of AOX transcripts was performed with total RNAs from thermogenic receptacles. Because two highly homologous partial fragments were detected during PCR analyses, full-length cDNAs of the corresponding transcripts were isolated and consequently named NnAOX1a and NnAOX1b (DNA data bank of Japan accession nos. AB491175 and AB491176, respectively). The deduced amino acid sequences of the encoded proteins indicate that NnAOX1a and NnAOX1b encode proteins of 39.0 and 39.3 kD, respectively, and 32.5 and 32.6 kD after cleavage of the mitochondrial targeting sequence. Both NnAOX1a and NnAOX1b contain some of the structural features typical of plant AOXs, such as four α-helical bundles and ligands for the two iron atoms of the active center (Moore and Albury, 2008). However, both NnAOX1a and NnAOX1b were found to contain a Ser residue at the site of the highly conserved Cys1 residue, which is necessary for the regulation of the plant AOX through both redox control and α-keto acid stimulation, although the second conserved Cys, Cys2, was present in both (Fig. 3). Additionally, in the case of NnAOX1b, a Leu residue at position 193 was substituted by Cys (Fig. 3).
Figure 3.
Deduced amino acid sequences of NnAOX1a and NnAOX1b aligned with those of previously reported AOXs expressed in thermogenic tissues. Bold characters highlight residues conserved across all of the AOX sequences in the alignment. The putative structural features are indicated as follows: asterisks for two highly conserved Cys residues, termed Cys1 and Cys2 (Berthold et al., 2000), double underline for ligands to iron atoms of the catalytic center, and gray bars for four α-helices. Abbreviations and data sources are as follows: DvAOX, D. vulgaris AOX (BAD51465); PbAOX, P. bipinnatifidum AOX (BAD51467); SgAOX, S. guttatum AOX (P22185); SrAOX, S. renifolius AOX (BAD83866).
NnAOX1a and NnAOX1b Are Similar to AOX Isoforms from Other Dicots
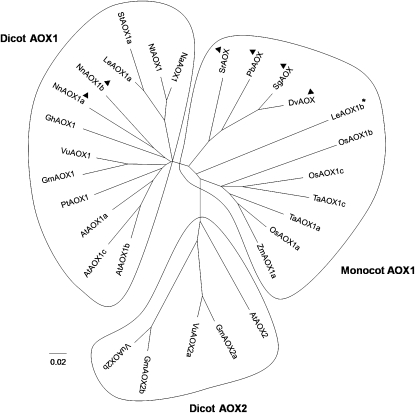
Sequence alignment indicated that NnAOX1a and NnAOX1b are distinct from AOX isoforms reported from other thermogenic species in that they lack Cys1, while Dracunculus vulgaris, Philodendron bipinnatifidum, Sauromatum guttatum, and Symplocarpus reinifolius all contain both Cys1 and Cys2 (Fig. 3). Further analysis indicated that NnAOX1a and NnAOX1b from thermogenic sacred lotus were more similar to AOXs from other dicots than they were to AOXs from other thermogenic plants (Fig. 4; Supplemental Fig. S1). NnAOX1b also contains an extra Cys residue at position 193; this is similar to AtAOX1a, LeAOX1a, LeAOX1b, and NtAOX1a, in which a Leu is replaced by Cys at the same position (Supplemental Fig. S1). Based on the AOX model for S. guttatum (Andersson and Nordlund, 1999), this Cys is located after the first α-helix, but we are unsure whether it sits within the membrane or matrix region of the protein.
Figure 4.
Unrooted dendrogram of a range of plant AOX proteins showing three distinct groups of AOX proteins: monocot AOX1, dicot AOX1, and dicot AOX2. The asterisk indicates LeAOX1b, which is unusual in that it is a dicot AOX1 that sits within the monocot AOX1 grouping, and arrowheads denote thermogenic species. Abbreviations and data sources are as follows: as in Figure 3 and AtAOX1a, A. thaliana AOX1a (NP_188876); AtAOX1b, A. thaliana AOX1b (NP_188875); AtAOX1c, A. thaliana AOX1c (NP_189399); AtAOX2, A. thaliana AOX2 (NP_201226); GhAOX1, G. hirsutum AOX1 (ABJ98721); GmAOX1, G. max AOX1 (AAC35354); GmAOX2a, G. max AOX2a (AAB97285); GmAOX2b, G. max AOX2b (AAB97286); LeAOX1a, L. esculentum AOX1a (AAK58482); LeAOX1b, L. esculentum AOX1b (AAK58483); NaAOX1, Nicotiana attenuata AOX1 (Q676U3); NtAOX1, N. tabacum AOX1 (AAC60576); OsAOX1a, Oryza sativa AOX1a (BAA28773); OsAOX1b, O. sativa AOX1b (BAA28771); OsAOX1c, O. sativa AOX1c (BAB71945); PtAOX1, Populus tremula × P. tremuloides AOX1 (Q9SC31); StAOX1a, Solanum tuberosum AOX1a (BAE92716); TaAOX1a, Triticum aestivum AOX1a (BAB88645); TaAOX1c, T. aestivum AOX1c (BAB88646); VuAOX1, Vigna unguiculata AOX1 (AAZ09196); VuAOX2a, V. unguiculata AOX2a (ABM66368); VuAOX2b, V. unguiculata AOX2b (AAZ09195); ZmAOX1a, Zea mays AOX1a (AAR36136).
DISCUSSION
In most plants studied to date, the α-keto acid pyruvate stimulates AOX activity (Day et al., 1994), and the specific site of this regulation is reduced Cys1 (Rhoads et al., 1998). Following pyruvate stimulation, glyoxylate can further increase AOX activity via Cys2 (Umbach et al., 2002) and can also initiate activity at either Cys alone (Umbach et al., 2006). Recent studies on AOX isoforms without the regulatory Cys residues have revealed stimulation by succinate, not pyruvate, when Cys1 is not present (Djajanegara et al., 1999; Holtzapffel et al., 2003), and the glyoxylate effect is absent when both Cys residues are missing (Umbach et al., 2006). Here, we report that AOX from thermogenic tissues of sacred lotus is stimulated by succinate rather than pyruvate (Fig. 1A), that there is no glyoxylate effect (Fig. 1, C–F), and that the majority of AOX could not be reversibly reduced and oxidized (Fig. 2). Our results thus suggested that the majority of AOX in these tissues lacked Cys1 and that Cys2 might also be missing. Subsequent sequencing of two cDNAs, NnAOX1a and NnAOX1b, isolated from thermogenic sacred lotus indicated that Cys1 is replaced by Ser but that Cys2 is present in both (Fig. 3). This confirmed our predictions, based on the in vitro studies of isolated mitochondria, that Cys1 was missing from the majority of AOX protein in these tissues. The situation with Cys2 is complicated, however, by the fact that glyoxylate stimulation of AOX containing this residue varies between naturally occurring and site-directed AOX substitutions. For example, similarly to our experiments, glyoxylate failed to stimulate tomato AOX (LeAOX1b) even though it contains Cys2 (Holtzapffel et al., 2003). LeAOX1b was also activated by succinate in a similar fashion to the thermogenic lotus AOX. In contrast, site-directed mutation of both Cys residues in Arabidopsis indicated that only one Cys was needed for glyoxylate stimulation (Umbach et al., 2002, 2006).
In the majority of plants, AOX can be reversibly reduced and oxidized (Umbach and Siedow, 1997). However, when extracted under nonreducing conditions, the sacred lotus receptacle AOX protein was predominantly in the reduced (i.e. nonlinked) state and could not be further oxidized with diamide across a range of concentrations up to 250 mm. This contrasts strongly with AOX proteins from soybean cotyledons and Arabidopsis leaves, where diamide concentrations of <5 mm were sufficient to oxidize AOX (Umbach and Siedow, 1993), while 200 mm diamide was able to oxidize AOX protein from chilled green tomato mitochondria (Holtzapffel et al., 2003). As formation of the oxidized dimer requires the presence of Cys1 (Rhoads et al., 1998; Djajanegara et al., 1999; Umbach et al., 2006), our results are consistent with this regulatory Cys being absent from the majority of AOX found in thermogenic sacred lotus. This results in an AOX that is permanently in the reduced state and ready for further activation by succinate. Thus, fine control of activity during heating may be modulated by succinate levels. Similarly, naturally occurring and mutated AOX proteins with Ser substitutions at Cys1 lack the ability to form oxidized dimers and, like the sacred lotus receptacle AOX, are poised for activation (Ito et al., 1997; Umbach et al., 2002; Holtzapffel et al., 2003; Umbach et al., 2006).
In contrast to the results with diamide, AOX from the sacred lotus receptacle was able to form dimers when exposed to the Lys-Lys cross-linker EGS. Monomeric AOX proteins such as those found in fungi (e.g. Neurospora crassa and Pichia stipitis) do not form dimers in the presence of EGS or diamide (Umbach and Siedow, 2000). Thus, while most of the thermogenic sacred lotus AOX protein is able to be covalently bound, only a small fraction (approximately 20%) can form disulfide bonds in the presence of diamide (Fig. 2). This suggests that there may be an additional isoform that unlike NnAOX1a and NnAOX1b contains Cys1. Alternatively, there is the possibility that the additional Cys at position 193 in NnAOX1b may be involved in disulfide bridge formation, although this Cys may not be close enough to Cys2 in the tertiary or quaternary structure of the protein to form disulfide bonds (Gilbert, 1990). Interestingly, a further isoform may be present in thermogenic sacred lotus, as we detected a small band around 60 kD that could not be reduced in the presence of DTT (Fig. 2, lane 2). This band represented around 12% of the total AOX protein present in our samples. Multiple AOX isoforms in the same tissue have been reported in a number of different species, including thermogenic S. guttatum, in which a 37-kD species is joined by a 35- and 36-kD species during thermogenesis (Rhoads and McIntosh, 1992). It is possible that these different isoforms could form heterodimers. A mixture of homodimers and heterodimers have been proposed to occur in soybean (Finnegan et al., 1997), while in tomato it was suggested that heterodimeric associations between LeAOX1a and LeAOX1b could explain why full oxidation of tomato AOX dimers did not occur (Holtzapffel et al., 2003). Whether AOX heterodimers occur in thermogenic sacred lotus and whether they have different catalytic properties from homodimers has yet to be investigated.
Crichton et al. (2005) suggested that changes to amino acids other than the regulatory Cys1 and Cys2 may influence AOX activity in thermogenic species. This suggestion is based on a constitutively active SgAOX, with both conserved Cys residues, which when expressed in yeast was insensitive to both pyruvate and succinate. However, the absence of Cys1 in both NnAOX1a and NnAOX1b, and the fact that succinate was required for full alternative pathway activity in mitochondria isolated from thermogenic sacred lotus, make it unlikely that these isoforms are regulated in a similar way to that hypothesized for S. guttatum (Crichton et al., 2005). Furthermore, AOX proteins that have been modified by amino acid substitutions or expressed in bacteria or yeasts may not reflect in vivo behavior; thus, comparisons with naturally occurring isoforms need to be approached with caution. Unlike previous studies, the AOX lacking Cys1 in sacred lotus is a naturally occurring isoform expressed in plant mitochondria.
Regulation of Heating via Posttranslational Regulation of AOX
Sacred lotus is, to our knowledge, the only thermoregulating dicot so far described. Thus, it is perhaps not surprising that NnAOX1a and NnAOX1b were more closely aligned with AOXs from other dicots than with those from other thermogenic plants, all of which are monocots (Fig. 4). Based on our phylogenetic analysis, the two deduced sacred lotus AOX sequences were more similar to GhAOX1 from cotton (Gossypium hirsutum) than to any other AOX. It was also interesting that the only dicot AOX that fell within the same group as the thermogenic monocots was LeAOX1b from tomato. These results suggest that there is no specific AOX sequence associated with thermogenic activity in plants, rather it may be the amount of AOX synthesized that allows these plants to generate heat. This is further supported by the fact that there appear to be only a few mechanisms of posttranslational regulation for AOX proteins from a wide variety of species and that the same mechanism may be shared by both nonthermogenic and thermogenic plants. For example, succinate activation of AOXs in which Cys1 has been replaced by Ser is found in both thermogenic sacred lotus and nonthermogenic tomato (Holtzapffel et al., 2003). Similarly, pyruvate activation via reduced Cys1 occurs in both thermogenic and nonthermogenic plants (Day et al., 1994; Onda et al., 2007). Modulation of AOX activity by either succinate or pyruvate could be important for those plants that thermoregulate, such as sacred lotus (Seymour and Schultze-Motel, 1996), S. renifolius (Knutson, 1974), and P. bipinnatifidum (Nagy et al., 1972). In contrast, S. guttatum, the only thermogenic plant in which a constitutively active AOX has been found, does not thermoregulate. Rather, this species has a single burst of heat production that lasts only a few hours (Meeuse, 1966).
Our observation that succinate stimulation of AOX occurs in thermogenic sacred lotus mitochondria even in the presence of malonate (a succinate dehydrogenase inhibitor) suggests a possible nonmetabolic interaction of succinate with the AOX protein. As succinate is a common TCA cycle intermediate, it is possible that upstream substrate availability could be a signal for AOX activation. Other thermogenic species that are poised in the reduced state and that use lipids instead of carbohydrates to fuel thermogenesis, for example P. bipinnatifidum (N. Grant and R. Miller, unpublished data), may use products from lipid metabolism to signal AOX activation. If substrate supply is the signal, succinate activation of sacred lotus AOX may play a larger role than previously thought; however, this requires further investigation. Ubiquinol reduction status (Wagner et al., 2008) as well as regions in the AOX sequence located near the C terminus of the protein unique to thermogenic species (Crichton et al., 2005; Onda et al., 2008) could also be involved in controlled thermogenesis in these species.
CONCLUSION
Through a combination of biochemical and molecular techniques, we have investigated the regulation of AOX activity in thermogenic tissues of sacred lotus. This has enabled us to expand our understanding of how heating may be regulated in this and other thermoregulating species. The major isoforms of AOX found in lotus, NnAOX1a and NnAOX1b, lack Cys1 and could therefore not form disulfide linked dimers. The lack of Cys1 also explains the pyruvate insensitivity of alternative pathway respiration in thermogenic lotus and also suggests that Cys-193, present in NnAOX1b, does not substitute for pyruvate activation via Cys1. Our sequence data indicated that AOXs from thermogenic plants do not form a functional grouping and that heating in these plants may thus be a function of the amount of AOX protein present rather than the structure of the protein. Fine control of AOX activity in thermoregulating species is yet to be elucidated but may involve modulation by the organic acids pyruvate or succinate, depending on which isoform of the protein is present.
MATERIALS AND METHODS
Plant Material
Lotus flowers (Nelumbo nucifera) were collected from an outdoor pond in the Adelaide Botanic Gardens, South Australia, in January and February 2007 to 2009. Flowers for mitochondrial measurements were collected early during the thermoregulatory period classified as stage 1 by Grant et al. (2008). Stage 2 flowers were used for isolation of total RNA.
Isolation of Mitochondria
Washed mitochondria were isolated from approximately 50 g of fresh sacred lotus receptacle tissue according to Day et al. (1985) with minor modifications (Grant et al., 2008). The mitochondria were purified using a three-step Percoll gradient (30 mL) made of equal amounts of 50% (v/v), 35% (v/v), and 20% (v/v) Percoll in a Suc wash buffer (250 mm Suc, 10 mm HEPES-KOH, pH 7.2, and 0.2% [w/v] fatty acid-free bovine serum albumin [BSA]). The gradients were centrifuged at 20,000g for 1 h at 4°C, and purified mitochondria were collected from the 20% to 35% interface. Mitochondria were then washed (0.4 m mannitol, 10 mm MOPS/KOH, pH 7.2, and 0.1% [w/v] fatty acid-free BSA) twice by centrifugation at 10,000g and the final pellet resuspended in 1 mL wash buffer. Mitochondrial protein was determined according to the method of Bradford (1976).
Treatment of Mitochondria with Diamide and DTT
Percoll purified mitochondria were left untreated or treated with either DTT, diamide, or EGS to final concentrations of 20, 10, and 5 mm, respectively. Higher concentrations of EGS completely cross-linked the AOX protein; however, the AOX signal was greatly reduced. A high dimethyl sulfoxide (DMSO)/protein ratio may have had a detrimental effect on the protein; therefore, lower concentrations of EGS were used. Following the addition of DTT, mitochondria were incubated on ice for 30 min. Mitochondria treated with EGS or diamide (30 min at room temperature) were incubated with DTT first, to ensure the AOX protein was in the reduced form, and then washed before addition of the aforementioned reagents. Reactions were quenched by adding excess Tris-HCl (1 m, pH 7.4). Stock solutions of diamide and EGS were prepared in DMSO. The DTT was prepared in purified water; however, DMSO was added to both DTT-treated and untreated mitochondria at the same final concentration as in the diamide treatment as a control. All solutions were prepared fresh on the day of use.
SDS-PAGE and Immunoblotting
Mitochondrial protein samples were separated by nonreducing SDS-PAGE gels and immunoblotted as previously described (Grant et al., 2008). Antibodies raised against Sauromatum guttatum AOX (Elthon et al., 1989) were used to detect the AOX protein. The proteins were visualized using SuperSignal west femto maximum sensitivity substrate (Pierce). All buffers were reductant free.
Mitochondrial Respiration Measurements
Oxygen uptake by purified mitochondria was measured at 25°C using a Clark-type oxygen electrode in 1.8 mL of reaction medium (0.2 m Suc, 10 mm KCl, 1 mm MgCl2, 5 mm KH2PO4, 20 mm MOPS/KOH, pH 7.2, and 0.1% [w/v] fatty acid-free BSA). The O2 concentration in air-saturated buffer at 25°C was estimated at 250 μm in each experiment. Mitochondrial O2 uptake was initiated with 2 mm NADH and 20 mm succinate (final cuvette concentration). Approximately 100 μg of mitochondrial protein was used in each assay. KCN at a final concentration of 1 mm was used to inhibit the COX pathway, and 100 μm n-propyl gallate was used to inhibit the AOX pathway. A steady state of O2 uptake was reached before addition of subsequent constituents. Depending on the experiment, the following were added to the reaction mix (shown as final cuvette concentration): 20 mm pyruvate, 5 mm glyoxylate, 10 mm citrate, 10 mm fumarate, 10 mm oxalate, 10 mm α-ketoglutarate, 10 mm malate, 5 mm DTT, and 5 mm diamide. To account for the effect of residual pyruvate, lactate dehydrogenase (5 units/mL) was added to the reaction medium to scavenge residual pyruvate. Malonate (1–10 mm) was used to determine whether succinate was acting as a substrate for succinate dehydrogenase (complex II) or an activator of AOX. Initial experiments showed no evidence of state 3 to state 4 transition following the addition of ADP, and the succinate-stimulated O2 uptake was not inhibited by KCN, suggesting that the bulk of respiration was occurring via the AOX pathway.
Isolation and Sequencing of the Full-Length NnAOX1a and NnAOX1b
For the isolation of transcripts encoding AOX proteins by reverse transcription-PCR, total RNA was first extracted from thermogenic receptacles using Fruit-mate (Takara Bio) and the FastPure RNA kit (Takara Bio). Quality of the isolated RNAs was checked using the FlashGel System (Lonza). First-strand cDNAs were generated with PrimeScript first-strand cDNA synthesis kit (TaKaRa Bio) using oligo(dT) primer. By aligning conserved cDNA sequences of AOX transcripts across several thermogenic plants, Dracunculus vulgaris AOX (Ito and Seymour, 2005), Philodendron bipinnatifidum AOX (Ito and Seymour, 2005), and S. guttatum AOX (Rhoads and McIntosh, 1991), primers were designed to amplify partial fragments: NnAOXF1 (5′-ACAGCGGCGGGTGGATCAAGGCCCTCCT-3′) and NnAOXR1 (5′-TCGCGGTGGTGGGCCTCGTCGG-3′). The obtained fragments were cloned into pCR 2.1 with TA cloning kit (Invitrogen) and then sequenced.
Based on the partial sequence data, 5′- and 3′-RACE reactions were performed using the SMART RACE cDNA amplification kit (CLONTECH Laboratories) with the primers indicated below: NnRV1(5′-AACTCGGTGTAGGAGTGGATGGCCTCCT-3′) and NnRV2 (5′-AAGGTCATCAGGTGCATCCGCTCGTTCT-3′) for 5′-fragments of the NnAOX1a and NnAOX1b, NnFW1 (5′-AGAACGAGCGGATGCACCTGATGACCTT-3′) and NnFW2 (5′-AGGAGGCCATCCACTCCTACACCGAGTT-3′) for 3′-fragment of the and NnAOX1b. RACE products were also cloned into pCR 2.1 and sequenced.
To obtain full-length cDNAs of NnAOX1a and NnAOX1b, PCR amplification was performed using KOD-Plus (TOYOBO). The final PCR products were subcloned into the HincII site of pUC118 (TaKaRa Bio) and their sequences determined. Nucleotide sequence data were analyzed with GENETYX software (Genetyx). Phylogenetic analyses of AOX sequence data were conducted using MEGA4 (Tamura et al., 2007). The phylogeny was deduced using the neighbor-joining method for 29 molecular species of AOX proteins and tested by bootstrap analysis with 500 replicates. Branches corresponding to partitions reproduced in <50% bootstrap replicates are collapsed. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. All positions containing gaps and missing data were eliminated from the data set.
Statistical Analysis
Changes in mitochondrial activity with respect to different substrates were compared using one-way ANOVA (JMP 5.1; SAS Institute). Tukey's honestly significant difference post hoc tests were used to identify significantly different means. Data sets were tested for normality and homogeneity of variances using Shapiro-Wilk W and Bartlett's tests, respectively. Significant differences between means were calculated at P = 0.05.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AB491175 (NnAOX1a) and AB491176 (NnAOX1b).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Sequence alignment of NnAOX1a, NnAOX1b, and AOX1 proteins from other dicot species.
Supplementary Material
Acknowledgments
We thank the Adelaide Botanical Gardens for access to their lotus pond, Laura Howie for technical assistance, Associate Professor Kathleen Soole, and Professor James Whelan for the kind donation of AOX antibodies. We also thank two anonymous reviewers for their constructive and insightful comments.
This work was supported by the Australian Research Council (grant no. DP0451617) and the 21st Century Centers of Excellence program from the Japan Society for the Promotion of Science. N.G. is a receipt of an Australian Postgraduate Award studentship, and Y.O. is supported by the Japan Society for the Promotion of Science Research Fellowships for Young Scientists.
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sharon Robinson (sharonr@uow.edu.au).
The online version of this article contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Andersson ME, Nordlund P (1999) A revised model of the active site of alternative oxidase. FEBS Lett 449 17–22 [DOI] [PubMed] [Google Scholar]
- Berthold DA, Andersson ME, Nordlund P (2000) New insight into the structure and function of the alternate oxidase. Biochim Biophys Acta 1460 241–254 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Crichton PG, Affourtit C, Albury MS, Carre JE, Moore AL (2005) Constitutive activity of Sauromatum guttatum alternative oxidase in Schizosaccharomyces pombe implicates residues in addition to conserved cysteines in α-keto acid activation. FEBS Lett 579 331–336 [DOI] [PubMed] [Google Scholar]
- Day DA, Krab K, Lambers H, Moore AL, Siedow JN, Wagner AM, Wiskich JT (1996) The cyanide-resistant oxidase: To inhibit or not to inhibit, that is the question. Plant Physiol 110 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Millar AH, Wiskich JT, Whelan J (1994) Regulation of alternative oxidase activity by pyruvate in soybean mitochondria. Plant Physiol 106 1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Neuburger M, Douce R (1985) Biochemical characterization of chlorophyll-free mitochondria from pea leaves. Aust J Plant Physiol 12 219–228 [Google Scholar]
- Day DA, Wiskich JT (1995) Regulation of alternative oxidase activity in higher plants. J Bioenerg Biomembr 27 379–385 [DOI] [PubMed] [Google Scholar]
- Djajanegara I, Holtzapffel RC, Finnegan PM, Hoefnagel MHN, Berthold DA, Wiskich JT, Day DA (1999) A single amino acid change in the plant alternative oxidase alters the specificity of organic acid activation. FEBS Lett 454 220–224 [DOI] [PubMed] [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L (1989) Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol 89 1311–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervik F, Barfod A (1999) Thermogenesis in palm inflorescences and its ecological significance. Acta Bot Venez 22 195–212 [Google Scholar]
- Finnegan PM, Whelan J, Millar AH, Zhang Q, Smith MK, Wiskich JT, Day DA (1997) Differential expression of the multigene family encoding the soybean mitochondrial alternative oxidase. Plant Physiol 114 455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert HF (1990) Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol 63 69–172 [DOI] [PubMed] [Google Scholar]
- Grant NM, Miller RE, Watling JR, Robinson SA (2008) Synchronicity of the thermogenic activity, alternative pathway respiratory flux, AOX protein content, and carbohydrates in receptacle tissues of sacred lotus during floral development. J Exp Bot 59 705–714 [DOI] [PubMed] [Google Scholar]
- Hoefnagel MHN, Wiskich JT (1998) Activation of the plant alternative oxidase by high reduction levels of the Q-pool and pyruvate. Arch Biochem Biophys 355 262–270 [DOI] [PubMed] [Google Scholar]
- Holtzapffel RC, Castelli J, Finnegan PM, Millar AH, Whelan J, Day DA (2003) A tomato alternative oxidase protein with altered regulatory properties. Biochim Biophys Acta 1606 153–162 [DOI] [PubMed] [Google Scholar]
- Ito K, Seymour R (2005) Expression of uncoupling protein and alternative oxidase depends on lipid or carbohydrate substrates in thermogenic plants. Biol Lett 1 427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Saisho D, Nakazono M, Tsutsumi N, Hirai A (1997) Transcript levels of tandem-arranged alternative oxidase genes in rice are increased by low temperature. Gene 203 121–129 [DOI] [PubMed] [Google Scholar]
- Knutson RM (1974) Heat production and temperature regulation in eastern skunk cabbage. Science 186 746–747 [DOI] [PubMed] [Google Scholar]
- McDonald AE (2008) Alternative oxidase: an inter-kingdom perspective on the function and regulation of this broadly distributed ‘cyanide-resistant’ terminal oxidase. Funct Plant Biol 35 535–552 [DOI] [PubMed] [Google Scholar]
- Meeuse BJ (1966) The voodoo lily. Sci Am 218 80–88 [Google Scholar]
- Meeuse BJD (1975) Thermogenic respiration in aroids. Annu Rev Plant Physiol Plant Mol Biol 26 117–126 [Google Scholar]
- Moore AL, Albury MS (2008) Further insights into the structure of the alternative oxidase: from plants to parasites. Biochem Soc Trans 36 1022–1026 [DOI] [PubMed] [Google Scholar]
- Nagy KA, Odell DK, Seymour RS (1972) Temperature regulation by the inflorescence of Philodendron. Science 178 1195–1197 [DOI] [PubMed] [Google Scholar]
- Onda Y, Kato Y, Abe Y, Ito T, Ito-Inaba Y, Morohashi M, Ito Y, Ichikawa M, Otsuka M, Koiwa H, Ito K (2007) Pyruvate sensitive AOX exists as a non-covalently associated dimer in the homeothermic spadix of the skunk cabbage, Symplocarpus renifolius. FEBS Lett 581 5852–5858 [DOI] [PubMed] [Google Scholar]
- Onda Y, Kato Y, Abe Y, Ito T, Morohashi M, Ito Y, Ichikawa M, Matsukawa K, Kakizaki Y, Kiowa H, Ito K (2008) Functional coexpression of the mitochondrial alternative oxidase and uncoupling protein underlies thermoregulation in the thermogenic florets of skunk cabbage. Plant Physiol 146 636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads DM, McIntosh L (1991) Isolation and characterization of a cDNA clone encoding an alternative oxidase protein of Sauromatum guttatum (Schott). Proc Natl Acad Sci USA 88 2122–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads DM, McIntosh L (1992) Salicylic acid regulation of respiration in higher plants: alternative oxidase expression. Plant Cell 4 1131–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads DM, Umbach AL, Sweet CR, Lennon AM, Rauch GS, Siedow JN (1998) Regulation of the cyanide-resistant alternative oxidase of plant mitochondria. The identification of the cysteine residue involved in α-keto acid stimulation and intersubunit disulfide bond formation. J Biol Chem 273 30750–30756 [DOI] [PubMed] [Google Scholar]
- Ribas-Carbo M, Berry JA, Yakir D, Giles L, Robinson SA, Lennon AM, Siedow JN (1995) Electron partitioning between the cytochrome and alternative pathways in plant mitochondria. Plant Physiol 109 829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SA, Ribas-Carbo M, Yakir D, Giles L, Reuveni Y, Berry JA (1995) Beyond SHAM and cyanide: opportunities for studying the alternative oxidase in plant respiration using oxygen-isotope discrimination. Aust J Plant Physiol 22 487–496 [Google Scholar]
- Seymour RS, Bartholomew GA, Barnhart MC (1983) Respiration and heat production by the inflorescence of Philodendron selloum Koch. Planta 157 336–343 [DOI] [PubMed] [Google Scholar]
- Seymour RS, Ito Y, Onda Y, Ito K (2009) Effects of floral thermogenesis on pollen function in Asian skunk cabbage Symplocarpus renifolius. Biol Lett (in press) [DOI] [PMC free article] [PubMed]
- Seymour RS, Schultze-Motel P (1996) Thermoregulating lotus flowers. Nature 383 305 [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596–1599 [DOI] [PubMed] [Google Scholar]
- Umbach AL, Gonzàlez-Meler MA, Sweet CR, Siedow JN (2002) Activation of the plant alternative oxidase: insights from site-directed mutagenesis. Biochim Biophys Acta 1554 118–128 [DOI] [PubMed] [Google Scholar]
- Umbach AL, Ng VS, Siedow JN (2006) Regulation of plant alternative oxidase activity: a tale of two cysteines. Biochim Biophys Acta 1757 135–142 [DOI] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN (1993) Covalent and noncovalent dimers of the cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol 103 845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN (1997) Changes in the redox state of the alternative oxidase regulatory sulfhydryl/disulfide system during mitochondrial isolation: implications for inferences of activity in vivo. Plant Sci 123 19–28 [Google Scholar]
- Umbach AL, Siedow JN (2000) The cyanide-resistant alternative oxidase from the fungi Pichia stipitis and Neurospora crassa are monomeric and lack regulatory features of the plant enzyme. Arch Biochem Biophys 2 234–245 [DOI] [PubMed] [Google Scholar]
- Wagner AM, Krab K, Wagner MJ, Moore AL (2008) Regulation of thermogenesis in flowering Araceae: the role of the alternative oxidase. Biochim Biophys Acta 1777 993–1000 [DOI] [PubMed] [Google Scholar]
- Watling JR, Robinson SA, Seymour RS (2006) Contribution of the alternative pathway to respiration during thermogenesis in flowers of the sacred lotus, Nelumbo nucifera. Plant Physiol 140 1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.