Abstract
Phosphatidylinositol 4-OH kinase IIIβ (PI-4Kβ) is involved in the regulated local synthesis of phospholipids that are crucial for trans-Golgi network (TGN)-to-plasma membrane trafficking. In this study, we show that the calcium sensor proteins calneuron-1 and calneuron-2 physically associate with PI-4Kβ, inhibit the enzyme profoundly at resting and low calcium levels, and negatively interfere with Golgi-to-plasma membrane trafficking. At high calcium levels this inhibition is released and PI-4Kβ is activated via a preferential association with neuronal calcium sensor-1 (NCS-1). In accord to its supposed function as a filter for subthreshold Golgi calcium transients, neuronal overexpression of calneuron-1 enlarges the size of the TGN caused by a build-up of vesicle proteins and reduces the number of axonal Piccolo-Bassoon transport vesicles, large dense core vesicles that carry a set of essential proteins for the formation of the presynaptic active zone during development. A corresponding protein knockdown has the opposite effect. The opposing roles of calneurons and NCS-1 provide a molecular switch to decode local calcium transients at the Golgi and impose a calcium threshold for PI-4Kβ activity and vesicle trafficking.
Keywords: calcium binding protein 7, caldendrin, neuronal calcium sensor-1, phosphatidylinositol 4-OH kinase IIIβ, calcium binding protein 8
Phosphoinositides are low-abundant, negatively-charged phospholipids that are crucially implicated in the regulation of intracellular vesicle trafficking and exocytosis (1, 2). The levels of individual phosphoinositides are controlled by specific lipid kinases, whose activities and localization are in turn regulated by a variety of effectors (1). Phosphatidylinositol 4-OH kinase IIIβ (PI-4Kβ) is an enzyme that acts on phosphatidylinositol (PI) in the generation of phosphatidylinositol 4-phosphate (PIP), which is not only thought to be the rate-limiting step in the production of phosphatidylinositol 4,5-bisphosphate (PIP2), but seems to be a second messenger in its own right (3). A number of PIP and PIP2 binding proteins have been identified that are crucially involved in Golgi-to-membrane trafficking and in endo- and exocytosis (1–4). Accordingly, PI-4Kβ was shown to be essential for Golgi-to-plasma membrane transport (1–4).
The passage of proteins along the secretory pathway is also regulated by intracellular calcium (Ca2+) gradients (5–7), and the Golgi apparatus is an established Ca2+ microdomain containing Ca2+ release and sequestration apparatuses. Ca2+ signals within the Golgi microdomain are transduced into regulatory events via Ca2+-binding proteins that are either directly or indirectly attached to the Golgi membrane. Surprisingly little is known, however, about the underlying molecular mechanisms of Golgi Ca2+ signal transduction. It is therefore unclear why elevated Ca2+ levels are needed for the exit of vesicles from Golgi. Studies so far have showed that the neuronal calcium sensor-1 (NCS-1) via its N-terminal myristoylation associates in a Ca2+-independent manner with Golgi membranes (8, 9) where it interacts with PI-4Kβ (10, 11). This interaction appears to be an evolutionary highly-conserved mechanism that has evolved already in yeast (10, 12). Yeast null mutant strains of Frequenin, the Drosophila (13)/yeast orthologue (10) of NCS-1, and those of the yeast PI-4Kβ orthologue Pik1 are not viable, pointing to the essential role of both proteins in Golgi-to-plasma membrane trafficking (10, 14–16). This finding is, however, at variance with the situation in mammalia where NCS-1 seems to be more diffusely distributed in neurons with considerable amounts of the protein localized outside of the Golgi (17, 18). Moreover, its binding to PI-4Kβ seems to be of lower affinity as compared with the yeast proteins (11). It is therefore likely that the regulation of Pik1 and PI-4Kβ differs substantially with the latter being more susceptible to modulation via Ca2+ transients at the Golgi (19).
To date, NCS-1 is the only NCS protein known to interact with PI-4Kβ, whereas other members of this family, like Recoverin (10) or KChIP (16), apparently do not modulate PI-4Kβ activity. Based on their similarity to the synaptic Ca2+ sensor caldendrin (20) we have identified a subfamily of NCS proteins termed calneuron-1 and calneuron-2 (Fig. S1A and ref. 21). In contrast to classical NCS proteins, calneurons do not contain a N-terminal myristoylation site and their EF-hand organization differs substantially from that of other family members (Fig. S1A and refs. 21 and 22). In the present report we show that both calneurons are regulators of PI-4Kβ and trans-Golgi network (TGN)-to-plasma membrane trafficking in neurons.
Results
Calneurons Are Localized at the Golgi Apparatus and Associate with PI-4Kβ in Vivo.
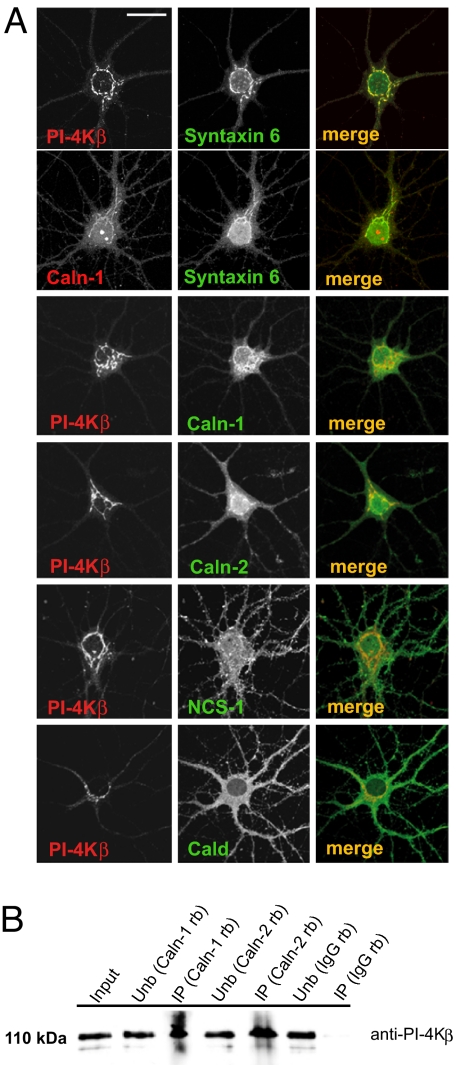
During their initial characterization we realized that, when expressed as GFP-fusion proteins in COS-7 cells, calneuron-1 and calneuron-2 consistently accumulated at cellular structures counterstained with the Golgi marker syntaxin-6 (Fig. S1B). This finding is in contrast to the localization of caldendrin expressed in COS-7 cells, and this highly-restricted localization was also not observed with a NCS-1–GFP construct (Fig. S1B). Of note, considerable overlap exists between the GFP–calneuron-1 and calneuron-2 fluorescence and the immunofluorescence of endogenous PI-4Kβ (Fig. S1B). Confocal laser scans also revealed overlap in the distribution of endogenous calneurons with PI-4Kβ and syntaxin 6 in neuronal cells (Fig. 1A) that was again stronger than those of caldendrin and NCS-1 (Fig. 1A). Further evidence that endogenous calneurons are in the same complex with PI-4Kβ in vivo came from coimmunoprecipitation experiments. PI-4Kβ immunoreactivity could be detected in precipitates obtained with calneuron-1 and calneuron-2 antibodies, but not after precipitation with control IgG (Fig. 1B). In addition, coimmunoprecipitation experiments from COS-7 cell extracts after overexpression of GFP–calneuron-1, calneuron-2, or -NCS-1 revealed the presence of PI-4Kβ in precipitates from the corresponding lysates of cells transfected with the different GFP–Ca2+-binding protein fusion constructs but not in GFP controls (Fig. S2A).
Fig. 1.
Distribution of endogenous calneurons compared with NCS-1 and caldendrin. (A) Note that in hippocampal primary neurons calneurons are much more restricted to the Golgi and show a much better overlap with PI-4Kβ than caldendrin and NCS-1. (Scale bar: 20 μm.) (B) Coimmunoprecipitation of calneuron-1 and calneuron-2 with PI-4Kβ from a rat brain extract. Rabbit polyclonal calneuron antibodies were used for immunoprecipitation, and the immunoblots were processed with a mouse PI-4Kβ antibody. Similar amounts of a rabbit IgG served as a control. Unb, unbound; IP, immunoprecipitate; rb, rabbit.
We next analyzed the association of endogenous calneurons and NCS-1 with PI-4Kβ by using gel filtration of extracts from Golgi-enriched microsomal fractions. We first simulated low Ca2+ conditions by adding EDTA to the extracts. Under these conditions PI-4Kβ was detected in complexes with molecular masses of 300–700 kDa and coeluted with the Golgi marker syntaxin-6 (Fig. S2B). Calneuron-1 and calneuron-2 are present in the higher molecular mass range of these PI-4Kβ positive fractions. In contrast, NCS-1 is associated with lower molecular mass complexes that show no overlap with calneuron-containing fractions (Fig. S2B). These data indicate that PI-4Kβ might exist in a calneuron- or NCS-1-bound form at the Golgi with no overlap of both complexes under low Ca2+ conditions. Elevating Ca2+ levels induced a shift of NCS-1 to higher molecular mass PI-4Kβ-containing complexes (Fig. S2C), whereas calneurons were excluded from these complexes or shifted to lower molecular mass fractions containing PI-4Kβ (Fig. S2C). This result suggests a dynamic regulation of the PI-4Kβ association for the 2 types of Ca2+ sensors.
Calneurons Physically Interact with PI-4Kβ and Compete with NCS-1 Binding in a Ca2+-Dependent Manner.
The limited overlap of elution profiles form molecular sieves and the possibility that NCS-1 and calneurons might be present in complexes with PI-4Kβ not purified with a microsomal protein preparation led us to ask under which Ca2+ conditions calneurons bind to PI-4Kβ and whether binding competes with that of NCS-1. We could confirm binding of both calneurons to GST–PI-4Kβ in GST-pull down assays (Fig. S2D). It has been shown that NCS-1 binds to PI-4Kβ in a Ca2+-independent manner (11, 18). Similarly, binding of calneurons to PI-4Kβ was found in the presence of either Ca2+ or the Ca2+ chelator EGTA in the pull-down buffer (Fig. S2D). It is therefore plausible that calneurons will associate with PI-4Kβ at resting cellular Ca2+ levels.
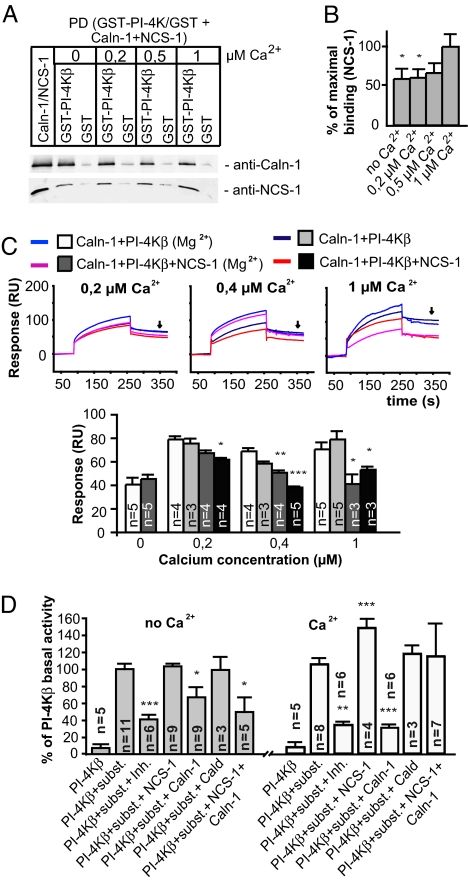
To test the hypothesis that this association will be competitive we performed competition pull-down assays with decalcified, bacterially-expressed calneuron-1, GST–PI-4Kβ, and myristoylated NCS-1. We observed direct binding of calneuron-1 and NCS-1 to GST–PI-4Kβ irrespective of the Ca2+ concentrations used. When equimolar amounts of calneuron-1 and NCS-1 were added to the pull-down buffer a significant reduction of NCS-1 binding to GST–PI-4Kβ was observed in the absence of Ca2+ (Fig. 2 A and B). Interestingly binding of NCS-1 appears to be stronger at higher Ca2+ levels (Fig. 2 A and B). The competition was Ca2+-sensitive with the most efficient NCS-1 binding to GST–PI-4Kβ in the presence of equimolar amounts of calneuron-1 at 1 μM Ca2+ and the most efficient competition by calneuron-1 at low or no Ca2+ in the buffer (Fig. 2 A and B). To confirm these data in a more quantitative manner we performed surface plasmon resonance measurements with His-tagged calneuron-1 coupled to the sensor chip. Even with recombinant PI-4Kβ–GST and NCS-1 in the running buffer, conditions that favor the initial formation of a PI-4Kβ–GST/NCS-1 complex, we found a prominent competition between calneuron-1 and NCS-1 for binding to PI-4Kβ (Fig. 2C). Moreover, the competition was Ca2+-sensitive with exclusive binding of PI-4Kβ to calneuron-1 under Ca2+-free conditions (Fig. 2C), whereas competitive binding of calneuron-1 was weaker in the presence of 0.4 μM Ca2+ as compared with 0.2 μM Ca2+ (Fig. 2C). Interestingly, the competition in binding to PI-4Kβ was also influenced by adding Mg2+ to the buffer. Calneuron relative to NCS-1 binding was stronger in the presence of Mg2+ (Fig. 2C) at 0.2 and 0.4 μM Ca2+, and Mg2+ reduced the molar binding activity of NCS-1 to PI-4Kβ but not that of calneuron-1 (Tables S1 and S2).
Fig. 2.
Calneuron-1 and NCS-1 compete for PI-4Kβ binding in a calcium- and magnesium-dependent manner and have the opposite effect on its enzymatic activity. (A) Competition pull-down with GST-PI-4Kβ coupled to the matrix. Equimolar amounts of myristoylated NCS-1 and calneuron-1 were used. Increased binding of NCS-1 with increasing Ca2+ concentrations is accompanied by decreased calneuron binding. All experiments were done in the presence of 1 mM Mg2+. The same amount of NCS-1 and calneuron-1 was used for the input and pull-downs. (B) Significantly higher amounts of NCS-1 are bound to GST–PI-4Kβ (binding to GST control was substructed for the each individual case) in the presence of 1 μM Ca2+ as compared with Ca2+-free conditions. Five independent experiments were used for each condition. Error bars represent the SEM. (C) Surface plasmon resonance competition assay. His-SUMO–calneuron-1 was directly immobilized on the sensor chip, and PI-4Kβ–GST alone or equimolar amounts of PI-4Kβ–GST and myr-NCS-1 were injected at different Ca2+ and Mg2+ concentrations. (Upper) Representative examples of obtained binding curves at 0,2, 0,4 and 1 μM Ca2+ are shown. (Lower) The response units (RU) at 350 s (dissociation phase) represent the amount of PI-4Kβ–GST bound to calneuron-1 on the sensor chip. Error bars represent the SEM. Note that under Mg2+-free conditions less GST-PI-4Kβ binds to calneuron-1 when coinjected with NCS-1 at low to moderate Ca2+ concentrations (0.2 and 0.4 μM). The minor unspecific binding of GST control alone or with NCS-1 to calneuron-1 was subtracted from the GST–PI-4Kβ values. (D) PI-4Kβ activity in the presence of recombinant calneuron-1, NCS-1, and caldendrin. Under Ca2+-free conditions (Left) calneuron-1 suppresses the activity of PI4-Kβ whereas NCS-1 increases enzyme activity. In the absence of Ca2+ the effect of calneuron-1 on PI-4Kβ activity was not changed by adding equimolar amounts of NCS-1. In the presence of 1 mM free Ca2+ (Right) NCS-1 increases PI-4Kβ activity, whereas calneuron-1 significantly decreases PI-4Kβ activity. Addition of equimolar amounts of NCS-1 and calneuron-1 led to a competition and NCS-1 reverses the suppressing effect of calneuron-1. Error bars represent the SEM. PD, pull-down. Inh., the PI-4Kß inhibitor Wortmannine (8 μM) was added to the assay buffer. ∗∗∗, P < 0.001; ∗∗, P < 0.01; ∗, P < 0.05.
The Calcium Binding Affinity of NCS-1 but Not Calneurons Is Regulated by Magnesium.
These results are puzzling because they suggest an association of calneurons at low to intermediate Ca2+ levels, which is counteracted by NCS-1 at higher Ca2+ levels. However, the Ca2+-binding affinities of calneuron-1 and NCS-1 are reportedly very similar (21, 23). In search of a mechanistic explanation for this apparent contradiction we used isothermal titration calorimetry (ITC) to investigate the influence of structural Mg2+ binding on Ca2+-binding isotherms. Previous work has shown that Mg2+ binding to EF-hand-2 and EF-hand-3 of NCS-1 reduces the Ca2+-binding affinity of NCS-1 from 90 to 440 nM (23). In sharp contrast to NCS-1, we found that Mg2+ does not bind at physiologically-relevant concentrations to calneuron-1 and calneuron-2 (Fig. S3 A–F). Moreover, in contrast to Ca2+, Mg2+ did not affect the conformation of apo-calneuron-1 as evidenced by fluorescence spectroscopy (Fig. S3 G and H). ITC data demonstrate the presence of 2 high-affinity Ca2+-binding sites (Table S3), with apparent global affinities of 180 nM for calneuron-1 and 230 nM for calneuron-2 (Fig. S3 and Table S3). In conclusion, we propose that calneurons, in contrast to NCS-1, have a very narrow dynamic range of Ca2+-induced unfolding with much less reversibility to the Ca2+-free state, which can explain their dominant role at low Ca2+ concentrations.
Calneuron-1 Regulates PI-4Kβ Activity in a Ca2+-Dependent Manner and Opposite of NCS-1.
To next address the question of which functional consequences calneuron-1 binding might have for PI-4Kβ's enzymatic activity we performed in vitro kinase assays with bacterially-expressed proteins. Conflicting evidence exists whether myristoylated NCS-1 activates PI-4Kβ in vitro in a Ca2+-dependent manner and whether the interaction by itself is Ca2+-independent (11, 19). We found that the basal activity of the kinase was unaltered in the presence of myristoylated NCS-1 when Ca2+ was omitted from the buffer (Fig. 2D). Addition of Ca2+ led to an increase in PI-4Kβ activity (Fig. 2D). Strikingly, calneuron-1 showed the opposite behavior with a strong inhibitory effect on kinase activity (≈66% of basal activity) already in Ca2+-free conditions. Addition of Ca2+ to the assay buffer further augmented the inhibitory effect of calneuron-1 on PI-4Kβ activity (≈28% of basal activity). Caldendrin, the closest homologue of calneurons in brain, had no effect on PI-4Kβ kinase activity under any of the conditions tested (Fig. 2D), suggesting, in conjunction with previous data (10, 16), that PI-4Kβ is specifically regulated by only a subset of calcium sensor proteins. To simulate an in vivo situation where NCS-1 and calneuron-1 might have competing influence on PI-4Kβ activity we performed the assay with equimolar amounts of both proteins. In support of the previous observations we found that under low Ca2+ conditions PI-4Kβ activity was inhibited to 60% if both NCS1 and calneuron-1 were present in equimolar amounts in the reaction mix. This effect was comparable to the effect of calneuron-1 alone (Fig. 2D). However, in the presence of high Ca2+ concentrations NCS-1 counteracted the inhibitory effect of calneuron-1 (Fig. 2D). To address the question of whether calneurons are also able to inhibit PI-4Kβ activity in vivo we transfected COS-7 cells, which do not endogenously express calneurons with GFP–calneuron-1 and calneuron-2 constructs. Quantification of PIP-levels revealed that overexpression of both calneurons significantly reduced PIP-production (Fig. S4), indicating that calneurons also inhibit PI-4Kβ activity in vivo.
Calneurons Regulate Vesicle Trafficking at Neuronal Golgi.
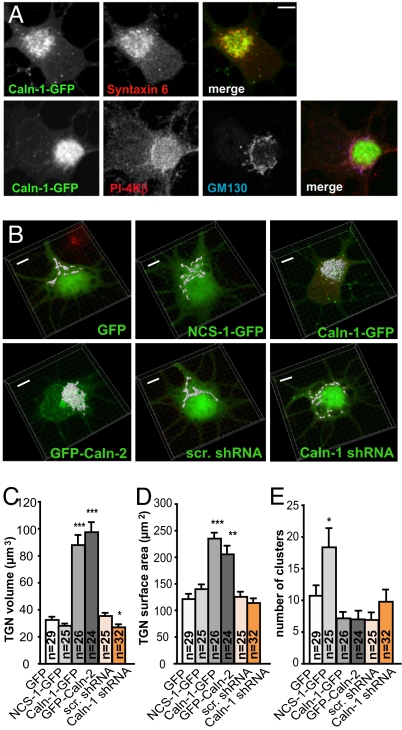
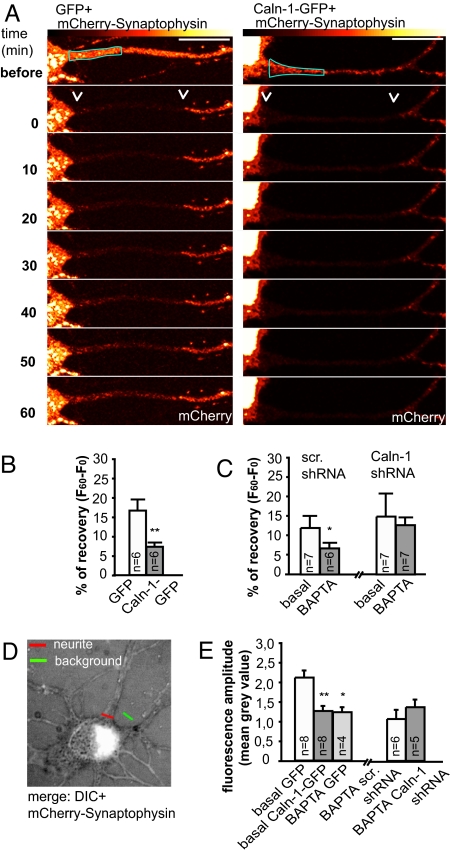
In the final set of experiments we more directly addressed the question of whether calneurons have a role in neuronal TGN-to-plasma membrane trafficking. Double-immunofluorescence stainings revealed no overlap of calneuron-1 with the endoplasmatic reticulum marker Calreticulin, and the cis-Golgi marker GM130 (Fig. S5A), and only limited overlap with the endosomal marker β-COP (Fig. S5E). Similarly, PI-4Kβ is highly abundant at the TGN and much less at the cis-Golgi (Fig. S5A). Moreover calneuron-1 when overexpressed in neurons accumulates only at syntaxin-6 and TGN-38-positive TGN but not at the GM-130-positive cis-Golgi (Fig. 3A and Fig. S5B). Interestingly, we found that overexpression of both calneurons at day in vitro (DIV) 5 cortical primary cultures led within 24 h to a significant enlargement of the TGN as evidenced by a 3D reconstruction of syntaxin-6 confocal laser scans with Imaris (Fig. 3 B and C). In addition, the Golgi surface area was increased (Fig. 3C). The enlarged TGN overlapped with the immunofluorescence for PI-4Kβ the synaptic vesicle marker synaptophysin (Fig. S5 C and D). Synaptophysin is a vesicular transmembrane protein that has to pass via the Golgi to enter the axon. We transfected cortical neurons with a mcherry-Synaptophysin fusion protein and found a prominent Golgi and axonal localization of this construct (Fig. 4A and Fig. S5E). Using time-lapse imaging of fluorescence recovery after photo-bleaching (FRAP) we could follow the exit of the mcherry fluorescence from the Golgi to the longest neurite as a read-out for trafficking of synaptophysin-containing vesicles (Fig. 4A). In these experiments cells transfected with calneuron-1–GFP showed significantly reduced FRAP as compared with GFP controls (Fig. 4 A and B). In a complementary set of experiments we quantified the intensity of mcherry fluorescence in proximal parts of axons by using line analysis without FRAP. Similar to the FRAP experiments we found reduced fluorescence in calneuron-1-transfected neurons compared with controls (Fig. 4 D and E and Fig. S6A), indicating a reduced frequency of entry of synaptophysin-containing vesicles into axons.
Fig. 3.
Overexpression of calneuron-1 and calneuron-2 in cortical neurons induces a prominent enlargement of the Golgi whereas RNAi knockdown of calneuron-1 has the opposite effect. (A) Overexpressed calneuron-1–GFP shows almost complete colocalization with PI-4Kβ and the trans-Golgi marker syntaxin 6 but only to a minor extent with the cis-Golgi marker GM130. (B) 3D reconstruction of the TGN using syntaxin-6 stainings and Imaris. Neurons were stained 24 h after transfection with GFP–calneuron-1, GFP–calneuron-2, NCS-1–GFP, and GFP or 72 h after transfection with calneuron-1 shRNA or scramble shRNA constructs. The syntaxin-6-positive area (depicted in gray) was reconstructed with Imaris and overlaid with the nonmodified GFP channel. For the maximal projection merged pictures see Fig. S4. (Scale bar: 5 μm.) (C–E) Quantification of different parameters of TGN size using Imaris 3D reconstruction of the syntaxin-6 staining. Error bars represent the SEM. ∗∗∗, P < 0.001; ∗∗, P < 0.01.
Fig. 4.
Calneuron-1 regulates the exit of synaptophysin from the TGN. (A) Examples of time-lapse imaging of FRAP for mcherry-synaptophysin. Arrows indicate the area that was photo-bleached. DIV 5–7 cortical neurons cotransfected with mcherry-synaptophysin and calneuron-1-GFP show much less recovery of axonal mCherry fluorescence after FRAP than neurons cotransfected with GFP. Representative pictures for the other experimental groups can be found in Fig. S6. (Scale bar: 10 μm.) (B and C) Quantification of FRAP 60 min after photo-bleaching. Initial fluorescence is taken as 100% and percentage of recovery is calculated as fluorescence at time point 60 (F60) minus fluorescence at time point 0 (F0) directly after photo-bleaching. Incubation for 1 h with 10 μM BAPTA-AM significantly reduced the basal recovery of mcherry-synaptophysin in scrambled shRNA but not mcherry-synaptophysin–calneuron-1 shRNA-cotransfected neurons. (D) Example of a GFP plus mcherry-synaptophysin-transfected neuron. The red line indicates the part of the neurite monitored during the experiment; the green line indicates the background used for normalization. (E) Line analysis of trafficking at the proximal part of the longest neurite of neurons cotransfected with mcherry-synaptophysin and calneuron-1–GFP shows decreased amplitude of mcherry fluorescence changes compared with GFP controls. This effect is similar to BAPTA-AM-preincubated GFP/mcherry-synaptophysin-cotransfected cells. Images were taken every 30 s for 30 min. The fluorescence change traces of each recorded cell are represented in Fig. S6. Error bars represent the SEM. ∗∗, P < 0.01; *, P < 0.05.
Cortical neurons express high levels of calneuron-1 whereas calneuron-2 transcripts are barely detectable (22). Using a calneuron-1 RNAi knockdown (Fig. S5 F and G) we could therefore investigate how the suppression of calneuron protein levels affects Golgi trafficking. First, we found that a knockdown of calneuron-1 was followed by a significant reduction in the size of the TGN, thus inducing the opposite effect from protein overexpression (Fig. 3 A and B). Moreover, similar to the NCS-1-overexpression phenotype a higher number of dispersed and small TGN fragments were found (Fig. 3D). Accordingly, reducing calneuron-1 protein levels increased FRAP of mcherry-synaptophysin in proximal axons if Ca2+ levels were lowered with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) (Fig. 4C and Fig. S6B), suggesting that lower calneuron-1 protein levels reduce the dependence on Ca2+ for mcherry-synaptophysin to exit from the Golgi. Finally mcherry-synaptophysin trafficking into proximal axons as quantified with line analysis was also decreased after application of BAPTA (Fig. 4E), whereas the calneuron-1 knockdown led only to a nonsignificant increase of the mcherry-synaptophysin fluorescence as compared with scrambled controls (Fig. 4E), probably because the method is less sensitive than FRAP.
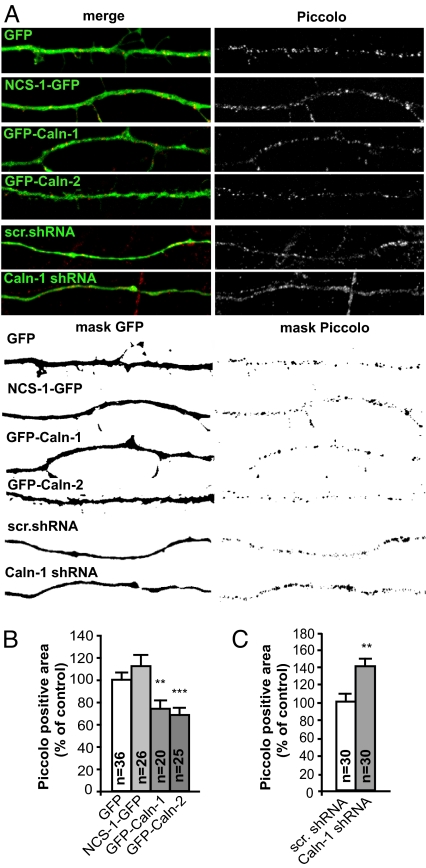
These data led us conclude that calneurons might constitutively inhibit various types of vesicle transport at the Golgi. To prove this hypothesis more directly we wanted to look at vesicle transport without interference by overexpression of vesicle proteins such as synaptophysin. At early stages of neuronal development components of the presynaptic cytomatrix are transported via the axon to nascent synaptic sites via so-called Piccolo-Bassoon transport vesicles (PTVs) (24). PTVs are large, dense core vesicles and can be easily identified because of their size, composition, and discrete localization in axons (24). We found that 2 protein components of these vesicles, Piccolo and SNAP-25, also accumulated at the TGN after calneuron-1 overexpression (Fig. S5 B and C). This accumulation was accompanied by a significantly reduced number of PTVs in the axon (Fig. 5 A and B), whereas the size of PTVs was not affected. Overexpression of NCS-1 had no effect on the size of the TGN (Fig. 3 B and C) although the number of PTVs in the axon was slightly, but not significantly, elevated (Fig. 5 A and B). Importantly, after calneuron-1 protein knockdown the number of axonal PTVs was clearly elevated as compared with scrambled control transfected neurons (Fig. 5 A and C), demonstrating that calneurons are involved in the control of vesicle trafficking endogenously and will play this role already during neuronal development.
Fig. 5.
The number of PTVs in axons of DIV5 cortical neurons is significantly reduced 24 h after transfection of GFP–calneuron-1 and calneuron-2 but not NCS-1–GFP. RNAi knockdown of calneuron-1 has the opposite effect. (A) (Upper) A 50-μm axonal segment from neurons transfected with different GFP-tagged constructs is shown. (Lower) Masks from images of the same segments from the GFP channel and the piccolo fluorescence channel are depicted. (B and C) The number of PTVs is represented as the ratio between the area covered by Piccolo immunoreactivity within a selected axonal segments and the total axonal area as defined by GFP fluorescence. The ratio in the case of GFP transfection was taken as 100% for the overexpression (B) and scrambled shRNA control for the knockdown experiments (C). Error bars represent the SEM. ∗∗∗, P < 0.001; ∗∗, P < 0.01.
Discussion
The present study demonstrates a molecular switch in the Ca2+ regulation of PI-4Kβ activity and an amazing example of the versatility of the same structural motif, the EF-hand, in the transduction of different Ca2+ conditions to a target interaction. Our data suggest that calneurons operate as a filter that suppresses PI-4Kβ activity at resting or submaximal amplitudes of Golgi Ca2+ transients and thereby provide a tonic inhibition that is released only under conditions of sustained Ca2+ release. The mechanism predicts that a Ca2+-dependent switch between inhibition and activation of PI-4Kβ might exist at Golgi membranes (Fig. S7). The opposing roles of calneurons and NCS-1 lead to a scenario with only 2 discrete states and little fine-tuning of enzyme activity between both states. Importantly, the switch from calneuron to NCS-1 binding can induce a locally restricted 3- to 4-fold increase in PIP production, which represents a major effect for the availability of this rare phospholipids. It is tempting to speculate that these interactions will be limited to discrete Golgi subdomains. It is known that Ca2+ chelation prevents the exit of vesicles from the Golgi (7), and the inhibition of PI-4Kβ provided by calneurons might contribute to the necessity to reach a certain Ca2+ level for overriding calneurons by NCS-1. NCS-1 had been the only Ca2+-binding protein known to interact with PI-4Kβ whereas Recoverin and KChiP apparently do not regulate the enzyme. That this mechanism appears to be highly specific for NCS-1 and calneurons is further underscored by the finding that caldendrin, the founding member of the neuronal CABP1–5 family (20, 25) and predominant isoform in brain (26), does not regulate PI-4Kβ activity. Calneurons are highly conserved between different species with 100% identity at the amino acid level between mouse, rat, monkey, and human orthologues, suggesting a tight structure–function relationship that is under considerable evolutionary pressure. The question that obviously arises is why is there a necessity at the neuronal Golgi for calneurons as antagonists for NCS-1.
The answer must come down to the not well-understood Ca2+ regulation of PI-4Kβ at the Golgi membrane. Although the existence of Golgi Ca2+ microdomains has been proposed (5) it is unclear how Ca2+ feeds back locally to PI-4Kβ. Thus, it is equally conceivable that calneurons and NCS-1 either associate with PI-4Kβ at different Golgi subdomains or transduce Ca2+ signals to PI-4Kβ in a competitive manner. At low Ca2+ levels both Ca2+-binding proteins seem to be segregated in different complexes, and calneurons dominate in the regulation of PI-4Kβ. Increasing Ca2+ seems to favor a complex consisting of NCS-1 and PI-4Kβ with the possibility of a complex consisting of all 3 proteins and a predicted competing and counteracting role of NCS-1 and calneurons at an intermediate state. This competition will be dynamically controlled by intracellular free Ca2+ levels in a manner that NCS-1 will be able to override the inhibition of PI-4Kβ activity via calneurons only at Ca2+ concentrations above ≈400 nM (Fig. S7). Sustained intracellular Ca2+ release in neurons usually requires high-frequency stimulation, a condition that is associated with an increased demand of membrane proteins, secretory vesicles, and TGN-to-plasma membrane trafficking (27, 28). PIP and PIP2 are essential for this latter process, so one can therefore speculate that calneurons add a further level of regulation, particularly in secretory cells like neurons that exhibit stimulus-dependent dynamics in TGN-to-plasma membrane trafficking. In the best available model, neuronal primary cultures, we could document a major role of calneuron-1 in this process. The data suggest that calneurons can interfere with the exit of PTVs from the Golgi in early postnatal development and potentially also other synaptic vesicles at later stages. We have chosen PTVs as a read-out of calneurons' function at the neuronal Golgi because they are the most accessible vesicle type for quantification, because of their size, small number, and segregation in axons (24, 29). Hence we found that not only Piccolo and SNAP25, which are specific PTV markers (24, 29), but also synaptophysin that is present on all synaptic vesicles accumulates at the Golgi after calneuron overexpression and is released from there after a corresponding calneuron protein knockdown. Taken together, the data provide evidence for a role of both calcium sensor proteins in the control of Golgi trafficking of exocytotic vesicles and PTVs. It will be an interesting question to determine why overriding the calneuron-induced inhibition of PI-4Kβ via local Ca2+ release is an advantageous regulatory mechanism for neurons.
The structural bases of the opposing actions of calneurons and NCS-1 with regard to PI-4Kβ activity are most plausibly related to their different EF-hand organization and structure. In addition, it was shown that the stimulatory effect of NCS-1 on PI-4Kβ activity requires N-terminal myristoylation (11), which might provide a Golgi membrane anchor. Calneurons do not harbor a N-myristoylation motif and therefore the question arises as to how they can be tethered to the Golgi. While this study was under review it was reported that calneurons contain in their C terminus a transmembrane domain that might be responsible for Golgi targeting of the overexpressed protein (30). It remains, however, elusive how this transmembrane domain can provide a Golgi membrane anchor. Interestingly, Golgi recruitment of PI-4Kβ in mammals is predominantly not regulated by NCS-1 but most likely involves NCS-1 binding to GTPase ADP-ribosylation factor 1 (ARF1) (19, 31). The interaction is Ca2+-dependent and ARF1 is instrumental for the recruitment of PI-4Kß to the TGN and subsequent modification of membrane trafficking (19, 31). It is therefore plausible that an interaction with another partner like ARF1 provides a structural link for calneurons to Golgi membranes.
Materials and Methods
Cell Culture, Immunocytochemistry, and Confocal Laser Scan Microscopy.
Transfection of cortical primary neurons was done at DIV2 for RNAi knockdown of calneuron-1 and at DIV4 for overexpression of calneuron-1, calneuron-2, NCS-1–GFP, and GFP (32). Details about cDNA constructs and antibodies are provided in SI Text. Transfection of COS-7 cells and subsequent immunofluorescence stainings were done as described (32).
Coimmunoprecipitation, Protein Purification, Pull-Down, and PI-4Kβ Activity Assays.
Immunoprecipitation experiments were done as described (32). Details about protein purification, PI-4Kβ activity assay, and all other assays are in SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. T. Balla (National Institutes of Health, Bethesda) for GST-PI-4Kβ and Dr. A. Fejtova (Leibniz Institute for Neurobiology) for mcherry-Synaptophysin plasmids, and O. Kobler for help with Imaris image analysis. This work was supported by grants from the Bundesministerium für Bildung und Forschung, Deutsche Forschungsgemeinschaft, and European Union. Y.S. and M.R.K. were supported by a Deutscher Akademischer Austauschdienst–Department of Science and Technology scholar exchange and a Bundesministerium für Bildung und Forschung–Department of Biotechnology international cooperation grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903001106/DCSupplemental.
References
- 1.Haucke V, Di Paolo G. Lipids and lipid modifications in the regulation of membrane traffic. Curr Opin Cell Biol. 2007;19:426–435. doi: 10.1016/j.ceb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michell RH. Inositol derivatives: Evolution and functions. Nat Rev Mol Cell Biol. 2008;9:151–161. doi: 10.1038/nrm2334. [DOI] [PubMed] [Google Scholar]
- 3.Balla A, Balla T. Phosphatidylinositol 4-kinases: Old enzymes with emerging functions. Trends Cell Biol. 2006;16:352–361. doi: 10.1016/j.tcb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- 5.Dolman NJ, Tepikin AV. Calcium gradients and the Golgi. Cell Calcium. 2006;40:505–512. doi: 10.1016/j.ceca.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Hay JC. Calcium: A fundamental regulator of intracellular membrane fusion? EMBO Rep. 2007;8:236–240. doi: 10.1038/sj.embor.7400921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JL, Ahluwalia JP, Stamnes M. Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. J Biol Chem. 2002;277:35682–35687. doi: 10.1074/jbc.M204157200. [DOI] [PubMed] [Google Scholar]
- 8.McFerran BW, Weiss JL, Burgoyne RD. Neuronal Ca2+ sensor 1. Characterization of the myristoylated protein, its cellular effects in permeabilized adrenal chromaffin cells, Ca2+-independent membrane association, and interaction with binding proteins, suggesting a role in rapid Ca2+ signal transduction. J Biol Chem. 1999;274:30258–30265. doi: 10.1074/jbc.274.42.30258. [DOI] [PubMed] [Google Scholar]
- 9.O'Callaghan DW, et al. Differential use of myristoyl groups on neuronal calcium sensor proteins as a determinant of spatio-temporal aspects of Ca2+ signal transduction. J Biol Chem. 2002;277:14227–14337. doi: 10.1074/jbc.M111750200. [DOI] [PubMed] [Google Scholar]
- 10.Hendricks KB, Wang BQ, Schnieders EA, Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, et al. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase β stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J Biol Chem. 2001;276:40183–40189. doi: 10.1074/jbc.M104048200. [DOI] [PubMed] [Google Scholar]
- 12.Huttner IG, et al. Molecular interactions of yeast frequenin (Frq1) with the phosphatidylinositol 4-kinase isoform, Pik1. J Biol Chem. 2003;278:4862–4874. doi: 10.1074/jbc.M207920200. [DOI] [PubMed] [Google Scholar]
- 13.Pongs O, et al. Frequenin: A novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron. 1993;11:15–28. doi: 10.1016/0896-6273(93)90267-u. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan CA, et al. Phosphatidylinositol 4-kinase: Gene structure and requirement for yeast cell viability. Science. 1993;262:1444–1448. doi: 10.1126/science.8248783. [DOI] [PubMed] [Google Scholar]
- 15.Strahl T, Hama H, DeWald DB, Thorner J. Yeast phosphatidylinositol 4-kinase, Pik1, has essential roles at the Golgi and in the nucleus. J Cell Biol. 2005;171:967–979. doi: 10.1083/jcb.200504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strahl T, Grafelmann B, Dannenberg J, Thorner J, Pongs O. Conservation of regulatory function in calcium-binding proteins: Human frequenin (neuronal calcium sensor-1) associates productively with yeast phosphatidylinositol 4-kinase isoform, Pik1. J Biol Chem. 2003;278:49589–49599. doi: 10.1074/jbc.M309017200. [DOI] [PubMed] [Google Scholar]
- 17.Bourne Y, Dannenberg J, Pollmann V, Marchot P, Pongs O. Immunocytochemical localization and crystal structure of human frequenin (neuronal calcium sensor 1) J Biol Chem. 2001;276:11949–11955. doi: 10.1074/jbc.M009373200. [DOI] [PubMed] [Google Scholar]
- 18.Taverna E, et al. Neuronal calcium sensor 1 and phosphatidylinositol 4-OH kinase β interact in neuronal cells and are translocated to membranes during nucleotide-evoked exocytosis. J Cell Sci. 2002;115:3909–3922. doi: 10.1242/jcs.00072. [DOI] [PubMed] [Google Scholar]
- 19.Haynes LP, Thomas GM, Burgoyne RD. Interaction of neuronal calcium sensor-1 and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol 4-kinase β trans-Golgi network-plasma membrane traffic. J Biol Chem. 2005;280:6047–6054. doi: 10.1074/jbc.M413090200. [DOI] [PubMed] [Google Scholar]
- 20.Seidenbecher CI, et al. Caldendrin, a novel neuronal calcium-binding protein confined to the somato-dendritic compartment. J Biol Chem. 1998;273:21324–21334. doi: 10.1074/jbc.273.33.21324. [DOI] [PubMed] [Google Scholar]
- 21.Mikhaylova M, et al. Neuronal Ca2+ signaling in the brain via caldendrin and calneurons. Biophys Biochem Acta Mol Cell Res. 2006;1763:1229–1237. doi: 10.1016/j.bbamcr.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 22.Wu YQ, Lin X, Liu CM, Jamrich M, Shaffer LG. Identification of a human brain-specific gene, calneuron 1, a new member of the calmodulin superfamily. Mol Genet Metab. 2001;72:343–350. doi: 10.1006/mgme.2001.3160. [DOI] [PubMed] [Google Scholar]
- 23.Aravind P, et al. Regulatory and structural EF-hand motifs of neuronal calcium sensor-1: Mg2+ modulates Ca2+ binding, Ca2+-induced conformational changes, and equilibrium unfolding transitions. J Mol Biol. 2008;376:1100–1115. doi: 10.1016/j.jmb.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 24.Zhai RG, et al. Assembling the presynaptic active zone: A characterization of an active zone precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 25.Burgoyne RD, O'Callaghan DW, Hasdemir B, Haynes LP, Tepikin AV. Neuronal Ca2+-sensor proteins: Multitalented regulators of neuronal function. Trends Neurosci. 2004;27:203–209. doi: 10.1016/j.tins.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Laube G, et al. The neuron-specific Ca2+-binding protein caldendrin: Gene structure, splice isoforms, and expression in the rat central nervous system. Mol Cell Neurosci. 2002;19:459–475. doi: 10.1006/mcne.2001.1078. [DOI] [PubMed] [Google Scholar]
- 27.Gogolla N, Galimberti I, Caroni P. Structural plasticity of axon terminals in the adult. Curr Opin Neurobiol. 2007;17:516–524. doi: 10.1016/j.conb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapira M, et al. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38:237–252. doi: 10.1016/s0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 30.McCue H, Burgoyne RD, Haynes LP. Membrane targeting of the EF-hand containing calcium-sensing proteins CaBP7 and CaBP8. Biochem Biophys Res Commun. 2009;380:825–831. doi: 10.1016/j.bbrc.2009.01.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haynes LP, Sherwood MW, Dolman NJ, Burgoyne RD. Specificity, promiscuity, and localization of ARF protein interactions with NCS-1 and phosphatidylinositol-4 kinase-IIIβ. Traffic. 2007;8:1080–1092. doi: 10.1111/j.1600-0854.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieterich DC, et al. Caldendrin–Jacob: A protein liaison that couples NMDA receptor signaling to the Nucleus. PLoS Biol. 2008;6e:286–306. doi: 10.1371/journal.pbio.0060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.