Abstract
Practically all animals are affected by humans, especially in urban areas. Although most species respond negatively to urbanization, some thrive in human-dominated settings. A central question in urban ecology is why some species adapt well to the presence of humans and others do not. We show that Northern Mockingbirds (Mimus polyglottos) nesting on the campus of a large university rapidly learn to assess the level of threat posed by different humans, and to respond accordingly. In a controlled experiment, we found that as the same human approached and threatened a nest on 4 successive days, mockingbirds flushed from their nest at increasingly greater distances from that human. A different human approaching and threatening the nest identically on the fifth day elicited the same response as the first human on the first day. Likewise, alarm calls and attack flights increased from days 1–4 with the first human, and decreased on day 5 with the second human. These results demonstrate a remarkable ability of a passerine bird to distinguish one human from thousands of others. Also, mockingbirds learned to identify individual humans extraordinarily quickly: after only 2 30-s exposures of the human at the nest. More generally, the varying responses of mockingbirds to intruders suggests behavioral flexibility and a keen awareness of different levels of threat posed by individuals of another species: traits that may predispose mockingbirds and other species of urban wildlife to successful exploitation of human-dominated environments.
Keywords: individual recognition, nest defense, nest predation, urban ecology, urban wildlife
More than half of the world's human population lives in urban environments (1). Both the proportion and total number of humans in cities are expected to grow, increasingly impacting wildlife (2–6). It is often obvious why some nondomesticated species are negatively affected by urbanization (e.g., habitat destruction) (5, 7). Much more puzzling is how other species have been able to adjust to the presence of humans and become “urban exploiters” (3, 5, 8). Among birds, behavioral flexibility and innovation are hypothesized predictors of success in urban environments (5, 7, 9). Such hypotheses are notoriously difficult to test with controlled experiments.
Nesting behavior provides an opportunity to test reactions of birds to humans in an unusually standardized manner. Nest predation is especially important, because eggs and nestlings are highly vulnerable; for passerine species, most mortality occurs in the nest (10, 11). Also, defense behavior is costly, because it entails risks to the parents, detracts from their foraging time, and draws attention to the nest (12). Therefore, the ability of a species to recognize and respond appropriately to different types of nest predators in a new environment may help explain why some species are able to successfully reproduce among humans and others are not. We experimentally tested the hypothesis that Northern Mockingbirds, an abundant species in urban settings of eastern North America, can quickly learn to distinguish individual humans who approach their nest, and that they respond sooner and more aggressively as perceived risk by humans to their nest increases.
One human (hereafter “intruder”) approached an incubating mockingbird on the University of Florida campus (Gainesville, FL) on 4 consecutive days, standing within 1 m of the nest for 30 s each day, and placing his/her hand on the rim of the nest for half of that time. On the fifth day, a different intruder (a control) approached and stood by the nest exactly the same way. We recorded the distance the intruder was from the nest when the bird flushed, the number of alarm calls, and the number of attack flights at the intruder. We repeated this set of trials for 24 nests, involving a total of 10 intruders who varied their appearance from day to day (i.e., wore different clothes). To quantify each bird's typical exposure to humans, we also recorded the number of pedestrians that passed within 5 m of the nest during 1 5-min period on each of 5 days.
Results
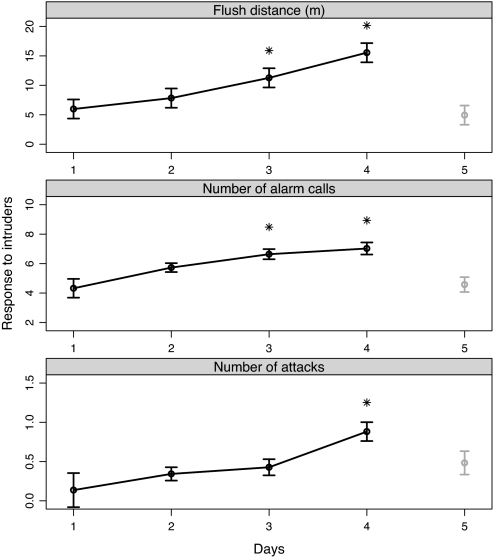
Mockingbirds flushed from the nest at progressively greater distances when approached by the same intruder on consecutive days (Fig. 1A). Flush distance was significantly greater on days 3 and 4 than on day 1, increasing on average 320% over 4 days (t92 = 3.2 and 5.7, respectively; P < 0.002). Importantly, flush distance for the control intruder on day 5 did not differ from flush distance for the first intruder on day 1, when he or she was also unknown to the incubating bird (t92 = −0.6; P = 0.53). Number of alarm calls showed an identical pattern (Fig. 1B); mockingbirds reacted more strongly to repeated visits by the first intruder, and showed relatively little response to the second intruder (t89 = 2.0 and 2.3 for days 3 vs. 1, and 4 vs. 1, respectively; P < 0.04; and t89 = −0.10 for days 5 vs. 1; P = 0.91). Last, mockingbirds attacked significantly more frequently on day 4 than day 1 (t38 = 3.6; P = 0.001), but no more frequently on day 5 (control intruder) than day 1 (t38 = 0.5; P = 0.61).
Fig. 1.
Responses (means ± SEM) of incubating mockingbirds to intruders approaching the nest on 5 consecutive days. Black points and lines show responses to the same intruder over the first 4 days; gray points and lines show responses to a novel intruder (control) on the fifth day. Asterisks indicate significant differences (P < 0.05) between indicated day and first day.
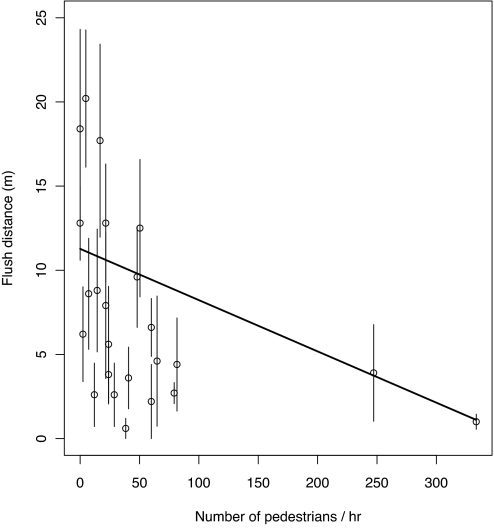
Mockingbirds nesting in busier areas of campus, for example, by sidewalks, showed greater overall tolerance to humans. Averaging across days, the number of pedestrians walking within 5 m of a nest was negatively correlated with flush distance (Fig. 2) (t22 = −2.2; P = 0.036). Alarm calls and attacks showed no relationship with number of pedestrians.
Fig. 2.
Distance from approaching intruder at which incubating mockingbirds flushed from their nest as a function of number of pedestrians per hour that pass within 5 m of the nest (slope = −0.4; t22 = −2.2; P = 0.036). Points show average flush distance (± SEM) for each incubating bird over the 5 days of the experiment. The fitted line represents the effect of pedestrians on the flush distance on the third day of the experiment. The negative relationship remains significant if the 2 right-most points are removed (slope = −0.1; t20 = −2.7; P = 0.014). Birds nesting near busy sidewalks are more tolerant of humans, yet still respond strongly to repeated visits by the same human (Fig. 1).
Discussion
These results support our hypothesis; mockingbirds quickly learned to recognize humans who approached their nest, increasing their response intensity as the same human visited on sequential days. Individual recognition is common within species, and has been extensively studied through controlled experiments (13). However, reports of one species recognizing different individuals of another species are much rarer and generally restricted to social mammals (14–16) and livestock (17).
In birds, previous studies of interspecific recognition of different individuals are anecdotal and/or restricted to laboratory settings that lack ecological relevance (e.g., pigeons in boxes, pecking at projected images) (18–20). Likewise, honey bees can learn to forage preferentially under photographs of particular human faces that are associated with rewards (21), demonstrating the ability of bees to distinguish between two-dimensional, stationary images of faces, but not resolving whether they can learn to distinguish between actual humans. The primary strengths of our study are that it was done on wild birds and used an experimental approach to measure a behavior directly linked to fitness (12). Also, it used a far more challenging task than typical of recognition studies. Birds were not required to distinguish among a few individuals or images, but rather between one individual and thousands of others, all of whom were potential threats and varied in daily appearance.
Interpreting results of experiments on individual recognition is controversial because of semantic disagreements, ecologically or evolutionarily irrelevant methodology, and the difficulty of knowing how animals process stimuli (14, 22–24). Although cognition is often inferred, it remains unclear how, for example, a honey bee's preference for nectar under a photograph of a human face reflects underlying intelligence (25, 26). Assuming that mockingbirds' ability to quickly recognize individual humans in a complex and novel environment somehow reflects cognition, it is surprising to find such an ability in a small passerine; it would be most likely predicted in parrots and corvids (crows and ravens) because of their “cognitive superiority [over] other birds” (27).
More surprising, mockingbirds learned to recognize intruders extraordinarily quickly, displaying a significant change in response after only 2 trials, representing a total of 60 s of intruder exposure at the nest. In contrast, practically all studies in which animals learn in an experimental context to identify and classify other individuals of the same or other species require training periods that are longer by 2–4 orders of magnitude.
We note that mockingbirds showed a graded response to the first intruder, gradually increasing flush distance, alarm calls, and attack frequency over 4 days. The lack of difference in response to intruders on days 1 and 5 rules out the possibility that this change in response over the first 4 days was caused by sensitization to intruders in general. These patterns suggest that mockingbirds not only learned to recognize the first intruder, but weighed costs and benefits of nest defense differently as that intruder became an apparently greater threat (28). Likewise, because mockingbirds show absolutely no reaction to the vast majority of humans who approach within 5 m of their nest, their response to the first intruder indicates an ability to make fine-scale assessments about perceived risk, and to exhibit the strongest antipredator response during brief and infrequent episodes of high risk, as predicted by theory (29). Alternatively, their increased response to known intruders may simply reflect reinforcement; success in driving away a potential predator on one day subsequently increased the behavior that presumably caused that success (30).
Last, our results address a paradox of urban ecology. Urban birds enjoy generally high nesting success, even though urban environments are characterized by large populations of nest predators (31). We hypothesize that urban species may be especially perceptive about the behavior of potential nest predators and be unusually effective in responding to their presence. However, we do not believe that mockingbirds evolved a specific ability to distinguish among humans. Rather, we suggest that mockingbirds' perceptive ability and rapid learning predispose them to success in novel environments. For example, transitioning from a natural habitat to an urban habitat entails learning to recognize and properly respond to a different set of nest predators, some immensely threatening and others (like humans) relatively unthreatening (3). Given that mockingbirds can quickly learn to recognize the same human, dressed differently and approaching from different directions, it is likely that they can learn to distinguish among, for example, different cats or other urban-associated predators. Such an ability would be ecologically relevant because individual predators likely vary in their degree of threat, and it would be advantageous to vary one's response in proportion to that degree of threat.
In conclusion, urban species commonly habituate to the presence of humans. During a 23-day nesting period, a typical mockingbird at our study site experiences ≈15,000 instances of a human walking within 5 m of its nest; the vast majority elicit no response (Movie S1). Mockingbirds' ability to rapidly learn and respond to different levels of threat posed by one particular human and to maintain habituation to all other humans demonstrates a previously unsuspected keen level of awareness about the human element of urban environments.
Materials and Methods
Description of Trials.
We located mockingbird nests with newly completed clutches, and identified 2 or 3 lines of approach to each nest that provided the incubating bird a clear view of the intruder. Nests and approaches were usually along sidewalks, paths, small roads, or parking lots. Because only females incubate (32), we could be assured that the same bird viewed and responded to the intruder each day. Ten humans worked in teams of 2, which varied from nest to nest. On the first day of a trial, one approach path was chosen at random. Likewise, a coin flip determined which human would be the intruder and which would record the trial with a video camera. The person recording the trial stayed >30 m from the nest and hidden as much as possible. The intruder started 30 m from the nest and walked directly toward it at a rate of 1 m/s. Once at the nest, he/she stood still for 15 s, placed a hand on the rim of the nest or reached upwards toward the nest for 15 s, and then walked away on the same path and at the same rate used for the approach. Thus, the total time of a trial was 90 s. Trials were repeated once per day for the next 4 days, with approach paths alternating when there were 2 paths and chosen at random from those least used on previous trials when there were 3. The intruder on the fifth day was the person who had recorded the previous trials or someone else; in either case, he/she had not previously approached the nest.
When the intruder observed the female leaving the nest, he/she signaled with a subtle wave of the hand, allowing us to accurately determine flush distance by reviewing the video recording of the trial. Because we were unable to assign alarm calls and attacks to males and females, we tallied the total number of each per trial. Alarm calls were loud and of short duration, with a wide bandwidth (32). An attack was defined as a swooping flight within 1 m of the intruder; attacks often resulted in direct contact with the intruder.
To estimate the number of humans who walk near mockingbird nests, we counted the number of pedestrians passing within 5 m of each nest during 1 5-min period on each of 5 days. Days and times were haphazardly chosen, but were always during daylight, occurred during the nesting season, and included weekends.
Physical Appearance of Intruders.
We did not use masks or standardize appearance of intruders in any other way, because we wanted to mimic natural conditions and were more interested in documenting individual recognition than in deciphering potential cues used by the birds. We emphasize that the 10 intruders in the study were representative of the full range of humans on university campuses, including males and females of widely varying age, physique, skin color, and hair amount, type, and style. Also, because all intruders wore what they would have normally worn on a given day, colors and styles of clothes varied widely, spanning the spectrum typically seen by mockingbirds in urban environments.
Statistical Methods.
We analyzed bird response to predators with a mixed-model approach, treating day and number of pedestrians as fixed effects, and individual bird and day as random effects. The aim of our analysis was to examine the magnitude of each response (alarm calls, attacks, and flush distance) over the length of the experiment, and to statistically compare the first day of the experiment, when the first intruder was unknown, with the following 4 days. Estimating the random variation among individual birds allows us to extrapolate our results to other unmeasured populations; whereas including day as a random effect accounts for temporal autocorrelation by removing any general trend in response across the 5 days of experimentation. Our response variables included both count (number of alarm calls and attacks) and continuous data (flush distance), and therefore, we analyzed them as generalized linear mixed models (GLMMs) or linear mixed models (LMMs), following recommendations by Bolker et al. (33). All statistical analyses were performed in R 2.7.2 (34).
We first modeled the number of alarm calls with a Poisson model (log-link) by using Laplace approximation to estimate parameters (glmer in lme4 package). The residuals indicated overdispersion (the variance of the counts were significantly greater than the mean) so we refitted the data with a quasi-Poisson model (log-link) with Laplace approximation. Because the glmer function does not calculate P values on fixed effects, we assessed statistical significance of the fixed effects by comparing the frequency with which t statistics from simulated data were greater than the observed t statistic. We did this comparison by fitting the null model (e.g., model with no fixed effects) to the data, simulating new response values from the null model 1,000 times, each time fitting the full model to the simulated data and extracting the t statistic for each parameter. We then calculated P values for each parameter from the null distribution of the simulated statistics (e.g., the frequency with which |tobs| < | tsim|).
We modeled flush distance by using maximum likelihood estimation (lme function in nlme package) to derive precise estimates of the fixed effects. Examination of residuals indicated that a normal error distribution was appropriate.
We used a conditional analysis for attacks, because the data were zero-inflated. We modeled the number of attacks, given an attack occurred, by using a quasi-Poisson distribution and log-link.
Animal Use.
Our experimental protocol was reviewed and approved by University of Florida Institutional Animal Care and Use Committee (protocol D884-2007).
Supplementary Material
Acknowledgments.
We thank A. Hoover, T. Hackenberg, S. Phelps, E. Tibbetts, and C. Wynne for helpful discussions and/or comments on the manuscript. B. Bolker and C. Clark provided help with statistical analyses. M. Martin, M. Phillips, A. Spalding, and C. Vaughan assisted with trials and searching for nests.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811422106/DCSupplemental.
References
- 1.Grimm NB, et al. Global change and the ecology of cities. Science. 2008;319:756–760. doi: 10.1126/science.1150195. [DOI] [PubMed] [Google Scholar]
- 2.Miller JR, Hobbs RJ. Conservation where people live and work. Conserv Biol. 2002;16:330–337. [Google Scholar]
- 3.Marzluff JM, Bowman R, Donnelly R, editors. Avian Ecology and Conservation in an Urbanizing World. Boston: Kluwer; 2001. [Google Scholar]
- 4.Brown LR, Gardner G, Halweil B. Worldwatch Paper 143. Washington, DC: Worldwatch Institute; 1998. [Google Scholar]
- 5.Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol. 2006;21:186–191. doi: 10.1016/j.tree.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Markovchick-Nicholls L, et al. Relationships between human disturbance and wildlife land use in urban habitat fragments. Conserv Biol. 2008;22:99–109. doi: 10.1111/j.1523-1739.2007.00846.x. [DOI] [PubMed] [Google Scholar]
- 7.Chace JF, Walsh JJ. Urban effects on native avifauna: A review. Landscape Urban Plan. 2006;74:46–69. [Google Scholar]
- 8.Kark S, Iwaniuk A, Schalimtzek A, Banker E. Living in the city: Can anyone become an ‘urban exploiter’? J Biogeogr. 2007;34:638–651. [Google Scholar]
- 9.Bonier F, Martin PR, Wingfield JC. Urban birds have broader environmental tolerance. Biol Lett. 2007;3:670–673. doi: 10.1098/rsbl.2007.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricklefs RE. An analysis of nesting mortality in birds. Smithson Contrib Zool. 1969;9:1–48. [Google Scholar]
- 11.Martin TE. Nest predation and nest sites - New perspectives on old patterns. Bioscience. 1993;43:523–532. [Google Scholar]
- 12.Montgomerie RD, Weatherhead PJ. Risks and rewards of nest defense by parent birds. Q Rev Biol. 1988;63:167–187. [Google Scholar]
- 13.Tibbetts EA, Dale J. Individual recognition: It is good to be different. Trends Ecol Evol. 2007;22:529–537. doi: 10.1016/j.tree.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Kazial KA, Kenny TL, Burnett SC. Little brown bats (Myotis lucifugus) recognize individual identity of conspecifics using sonar calls. Ethology. 2008;114:469–478. [Google Scholar]
- 15.Boysen ST, Berntson GG. Cardiac correlates of individual recognition in the chimpanzee (Pan troglodytes) J Comp Psychol. 1986;100:321–324. [PubMed] [Google Scholar]
- 16.Slobodchikoff CN, Kiriazis J, Fischer C, Creef E. Semantic information distinguishing individual predators in the alarm calls of Gunnison prairie dogs. Anim Behav. 1991;42:713–719. [Google Scholar]
- 17.Taylor AA, Davis H. Individual humans as discriminative stimuli for cattle (Bos taurus) Appl Anim Behav Sci. 1998;58:13–21. [Google Scholar]
- 18.Heinrich B. Mind of the Raven. New York: Cliff Street Books; 1999. [Google Scholar]
- 19.Herrnstein RJ, Loveland DH, Cable C. Natural concepts in pigeons. J Exp Psychol Anim B. 1976;2:285–302. doi: 10.1037//0097-7403.2.4.285. [DOI] [PubMed] [Google Scholar]
- 20.Merritt PG. Observer recognition by the Northern Mockingbird. J Field Ornithol. 1984;55:252–253. [Google Scholar]
- 21.Dyer AG, Neumeyer C, Chittka L. Honeybee (Apis mellifera) vision can discriminate between and recognise images of human faces. J Exp Biol. 2005;208:4709–4714. doi: 10.1242/jeb.01929. [DOI] [PubMed] [Google Scholar]
- 22.Steiger S, Muller JK. ‘True’ and ‘untrue’ individual recognition: Suggestion of a less restrictive definition. Trends Ecol Evol. 2008;23:355. doi: 10.1016/j.tree.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Tibbetts EA, Sheehan MJ, Dale J. A testable definition of individual recognition. Trends Ecol Evol. 2008;23:356. doi: 10.1016/j.tree.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Johnston RE, Jernigan P. Golden-hamsters recognize individuals, not just individual scents. Anim Behav. 1994;48:129–136. [Google Scholar]
- 25.Dyer AG. Response to ‘What can bees really tell us about the face processing system in humans?’. J Exp Biol. 2006;209:3267. doi: 10.1242/jeb.02411. [DOI] [PubMed] [Google Scholar]
- 26.Pascalis O, Kelly DJ, Caldara R. What can bees really tell us about the face processing system in humans? J Exp Biol. 2006;209:3266. doi: 10.1242/jeb.02411. [DOI] [PubMed] [Google Scholar]
- 27.Emery NJ. Cognitive ornithology: The evolution of avian intelligence. Philos Trans R Soc London Ser B. 2006;361:23–43. doi: 10.1098/rstb.2005.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eaton SB, Eaton SBI. In: Evolution in health and disease. Stearns S, editor. Oxford: Oxford Univ Press; 1999. pp. 251–266. [Google Scholar]
- 29.Lima SL, Dill LM. Behavioral decisions made under the risk of predation - a review and prospectus. Can J Zoolog. 1990;68:619–640. [Google Scholar]
- 30.Knight RL, Temple SA. Why does intensity of avian nest defense increase during the nesting cycle? Auk. 1986;103:318–327. [Google Scholar]
- 31.Lima SL, Bednekoff PA. Temporal variation in danger drives antipredator behavior: The predation risk allocation hypothesis. Am Nat. 1999;153:649–659. doi: 10.1086/303202. [DOI] [PubMed] [Google Scholar]
- 32.Derrickson KC, Breitwisch R. In: The Birds of North America. Poole A, Stettenheim P, Gill F, editors. Philadelphia: Academy of Natural Sciences and American Ornithologists' Union; 1992. [Google Scholar]
- 33.Bolker BM, et al. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol Evol. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 34.R Development Core Team. R Foundation for Statistical Consulting. Austria: Vienna; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.