Abstract
Microorganisms employ a wealth of gene regulatory mechanisms to adjust their growth programs to variations in the environment. It was pointed out long ago [Savageau M (1977) Proc Natl Acad Sci USA 74: 5647–5651] that the particular mode of gene regulation employed may be correlated with the “demand” on the regulated gene, i.e., how frequently the gene product is needed in its natural habitat. An evolutionary “use-it-or-lose-it” principle was proposed to govern the choice of gene regulatory strategies. Here, we examine quantitatively the forces selecting for and against two opposing modes of gene regulation, in the context of an evolutionary model that takes genetic drift, mutation, and time-dependent selection into account. We consider the effect of time-dependent selection, with periods of strong selection alternating with periods of neutral evolution. Using a variety of analytical methods, we find the effective population size and the typical time scale of environmental variations to be key parameters determining the fitness advantage of the different modes of regulation. Our results support Savageau's use-it-or-lose-it principle for small populations with long time scales of environmental variations and support a complementary “wear-and-tear” principle for the opposite situation.
Keywords: transcription control, design principles, molecular evolution, time-dependent selection
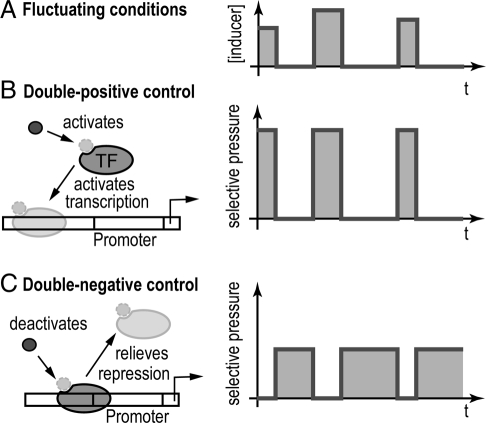
Much effort is currently devoted to the study of design principles for functional modules in molecular and cell biology (1). Typically, design principles are based on optimizing the functional performance of a module. However, different designs can be functionally equivalent. Already 30 years ago, Savageau raised the fundamental question of whether nature's choice between functionally equivalent module designs is random or whether there is an evolutionary selection criterion (2, 3). This question was posed in the context of a simple genetic switch, the elementary unit of gene regulatory modules. A basic function of a genetic switch is to assure that the expression of a specific gene is turned on when a signal, e.g., a nutrient, is present and turned off when the signal is absent. Importantly, the presence of the signal may vary on long time scales, e.g., a population of Escherichia coli cells can use lactose as the carbon source in a mammalian infant, but lactose may then become unavailable for a long time in the same host (2). The desired regulatory function can be implemented by a double-positive (++) mode of control, e.g., the signal activates a transcription factor that then activates the gene (Fig. 1B). The same function can also be obtained with a double-negative (−−) mode of control, e.g., the signal disables specific binding of a transcriptional repressor to its operator site, thereby relieving repression (Fig. 1C). Indeed, both of these control modes are ubiquitously used in bacterial gene regulation (4).
Fig. 1.
Two modes of gene regulation. In fluctuating conditions (A), a gene that is desired to be “ON” only when an inducer molecule is abundant, can be regulated by a (++) mode of control (B) or a (−−) mode of control (C). These 2 basic modes of control implement the same regulatory function, but lead to different time-dependent selection pressures on the transcription factor (TF) and its DNA-binding sequence.
Savageau empirically examined many bacterial genetic switches for correlations between the mode of gene regulation and temporal patterns in the input signal (2, 3). The latter were estimated, for instance, from physiological measurements of nutrient absorption rates in the mammalian intestine, which are an indication for the likelihood that a specific nutrient reaches the colon where E. coli colonizes. The study suggested a strong correlation between the “demand” for the product of the regulated gene and the mode of control: Genes whose protein products were needed most of the time (“high demand”) were found to be under (++) control, whereas genes whose products were rarely needed (“low demand”) were under (−−) control. To rationalize this correlation, Savageau proposed an intriguing “use-it-or-lose-it” principle, wherein the mode of gene regulation should be chosen to maximize the usage of the regulator, so as to avoid the loss of functionality during the periods when they are not used. Indeed, an activating transcription factor is only needed to be functional (e.g., bind to its functional DNA-binding site) when the target gene needs to be expressed, whereas a repressor is only needed to be functional when default expression of the target gene needs to be turned OFF. Hence, the use-it-or-lose-it principle is consistent with regulation by an activator for genes under high demand and regulation by a repressor for genes under low demand.
The proposed qualitative principle calls for a quantitative theoretical formulation and analysis, as recognized already in the original work of Savageau (2). Indeed, a more recent theoretical study by Savageau (5) yielded some support for an evolutionary choice of repressors at low demand and activators at high demand. However, that study did not explicitly consider stochastic fluctuations in the form of genetic drift, which had been suggested to play an important role for the use-it-or-lose-it principle (2). Moreover, a recent article (6) challenges the evolutionary basis of the empirical correlations and discusses some ideas for alternative, functional explanations. Thus, an explicit theoretical formulation of the use-it-or-lose-it principle is clearly needed, together with an assessment of the conditions under which the principle may be applicable.
Here, we use the framework of theoretical population genetics to provide a quantitative formulation of the problem. On the one hand, this framework allows us to assess the conditions under which the use-it-or-lose-it principle is borne out and show that significant genetic drift is indeed an essential requirement (with a detailed discussion of our findings in comparison with those of the previous theoretical study (5) below). On the other hand, our framework reveals another, more general aspect to the problem: The use-it-or-lose-it principle is contrary to the well-established population genetics concept of genetic robustness (7), which focuses on the “mutational load,” i.e., the average fitness reduction of individuals in a population incurred by mutations. One expects this load to be minimal when a transcriptional regulator is rarely used, because the fitness of a strain with a dysfunctional regulator is reduced only during the periods when the regulator is needed. We will loosely refer to the evolutionary design principle based on this argument as the “wear-and-tear” principle. We will show that, somewhat surprisingly, our quantitative formulation supports either of the two opposing principles, depending on the time scale of the nutrient fluctuations, the population size, and the mutation rate.
From a theoretical perspective, an important aspect of our study is that of time-dependent selection. Indeed, the selection pressure on transcriptional regulators must be explicitly time-dependent, e.g., genetic switches responding to the state of the cellular environment are useful only when the environmental conditions are variable (otherwise, the production could be kept at a constant optimal level) (8). Although various aspects of evolutionary dynamics under time-dependent selection have been studied, see, e.g., refs. 9–12, the problem at hand presents a new set of theoretical questions, due to the fact that the regulating transcription factor (and its binding site on the DNA) experience alternating intervals of neutral and strongly selective evolution. Our quantitative formulation is based on what we believe to be the simplest model of this class. Yet, we find that it displays surprisingly rich behavior, and it may provide a good starting point for more detailed evolutionary models of regulatory systems.
Model
We envisage an evolutionary scenario where, initially, a gene or operon is constitutively expressed at an intermediate level. This level may be a compromise between a benefit provided by the gene product under certain conditions and its detrimental (side) effects when these conditions are not present. It is then advantageous to evolve regulatory control for this gene (13). We envision that one population of bacterial cells picks up (++) control, whereas another population picks up (−−) control. What we seek to answer is which population has the higher fitness over long periods of time, if both are exposed to statistically similar environmental variations.
Quantitative Formulation.
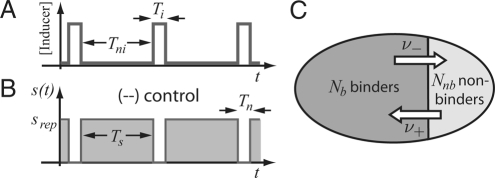
To address this question, we adopt the model of Savageau (5) and take the signal inducing the gene to vary periodically between a high and a low level; the inducing condition persists for a time Ti and the noninducing condition for a time Tni, with the total period T + Tni + Ti; see Fig. 2A. The “demand” D for the gene product is taken to be the fraction of time D ≡ Ti/T the inducing condition is operative. The periodic variation is chosen for mathematical convenience; we discuss the effect of stochastic variation qualitatively in the SI Appendix Section VI.
Fig. 2.
Schematic illustration of the evolution model. (A and B) Periodic induction (A) leads to a periodic selection function (B) with alternating intervals of selection and neutral evolution. (C) Two-allele model.
Since both the (++) and (−−) mode of control can achieve the same regulatory task of assuring that the gene product is made only when the inducer is present, we posit that the fitness of cells is independent of the control mode as long as the genetic switch is operational. However, for either mode of control, mutants with nonoperational genetic switches will be generated (e.g., nonsense mutations in the coding region of the transcription factor). We refer to those mutants as “nonbinders” and denote the rate at which a functional “binder” cell produces a “nonbinder” cell by ν−, whereas the reverse rate is ν+, see Fig. 2C. We invoke a population model with a constant total population size N and denote the number of binders and nonbinders at time t by Nb(t) and Nnb(t), respectively.
The selection pressure s(t) against nonbinders depends on the state of the environment and also on the mode of control. Fig. 2B displays the case of (−−) control, where selection against nonbinders occurs under noninducing conditions, whereas evolution is neutral under inducing conditions, as the repressor is anyway not binding DNA in the presence of inducer. We denote the fitness cost of derepressing the gene under noninducing conditions by srep > 0. Then s(t) consists of alternating intervals of s = srep with duration Ts = Tni for evolution under selection and s = 0 with duration Tn = Ti for neutral evolution. For (++) control, the situation is just reversed: Selection against nonbinders occurs only under inducing conditions, with a fitness cost sact > 0 for duration Ts = Ti, whereas this allele is neutral under noninducing conditions, i.e., s = 0 for duration Tn = Tni.
There is clearly a resemblance between the low-demand situation for (++) control and the high-demand situation for (−−) control and, similarly, the low-demand situation for (−−) control and the high-demand situation for (++) control, see Fig. S1 for full illustration. In fact, if the outcome of evolution is not sensitive to the values of sact and srep (see below), then the behavior for one mode of control at a demand D is exactly that of the other at a demand 1 − D (for a fixed period T). For this reason, we will often focus our discussion to the low-demand situation of Fig. 2B, where a fitness advantage for the (−−) control indicates the dominance of the use-it-or-lose-it principle, and a fitness advantage for the (++) control indicates the dominance of the wear-and-tear principle.
Parameter Regimes.
Generally, one expects the fitness cost generated by expressing the target gene under noninducing conditions to be less than the cost of not expressing it under inducing conditions, srep < sact. Here, we will not make any specific assumptions on these selection pressures other than that selection is strong in both cases, such that sactTi and srepTni are both significantly >1. In other words, the time scale of adaptation is short compared to the other time scales in the problem. Reverse mutations that recover the function are less likely than mutations that destroy the function, hence ν+ < ν−. Typical mutation rates in bacteria are ≈10−8 per base and generation. Loss-of-function mutations can arise in the TF coding sequence and in its target binding site. Assuming 10–100 sensitive nucleotide positions within this relevant DNA region, we estimate ν− ≈ 10−7 to 10−6 per generation. A reverse mutation can restore the function either by undoing the exact same mutation or by compensating its effect. With 1–10 positions for compensatory mutations, ν+ ≈ 10−8 to 10−7. We assume that the mutation rates are constant in time and do not depend on the mode of gene regulation.†
Results
Average Mutational Load.
The constant generation of nonbinders by mutation lowers the average fitness of the cells in each population, an effect generally referred to as genetic (or mutational) load (14). To quantitatively compare the alternative modes of gene regulation, we determine how this fitness cost depends on the model parameters. In large populations, where sampling fluctuations are negligible, mutation and selection lead to a deterministic dynamics for the fraction x = Nnb/N of nonbinders in the population,
with ν = ν− + ν+ (see SI Appendix Section I for the detailed analysis of the deterministic dynamics). At any given point in time, the average fitness in the population is below its maximal value by the load γ(t) = −s(t) x(t), because s(t) is the fitness cost of a nonbinder relative to a binder at that time. A meaningful comparison between the 2 modes of gene regulation can be made using the time average
over the time period T = Tn + Ts. Provided that selection during the interval Ts is much stronger than mutation (s≫ ν), and that mutation-selection-balance is rapidly reached (sTs ≫ 1), we can derive the result
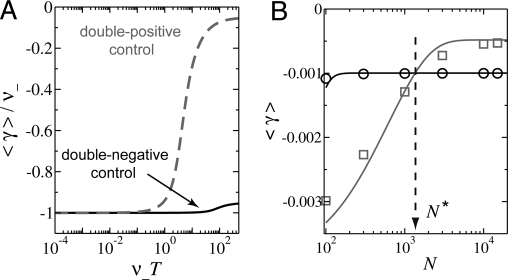
Remarkably, this average load does not depend on the strength of selection. Indeed, this fact is well known as “Haldane's principle” (15) for the special case of time-independent selection (Tn = 0), where it follows from the observation that the equilibrium fraction of deleterious mutations in the population is inversely proportional to their selective disadvantage. Eq. 3 shows that this compensation between severeness and prevalence of mutations remains true on average under time-dependent selection. However, the load in Eq. 3 does depend on the mode of gene regulation through its explicit dependence on the neutral period Tn, because for a given profile of the inducing signal (characterized by Ti and Tni), Tn = Tni for (++) control, whereas Tn = Ti for (−−) control. In Fig. 3A, we plot 〈γ〉 for the (++) mode (dashed line) and the (−−) mode (solid line) as a function of the period T at the same low demand D = 0.05, with ν+/ν− = 0.1. We see that when the probability of a deleterious mutation within a single period and individual is small (ν−T≪ 1), the average fitness cost approaches the deleterious mutation rate, i.e., 〈γ〉 = ν−, for both modes of control. However, when ν−T is large (≳1), a difference becomes evident: the (++) control (dashed line) suffers a significantly smaller load than the (−−) control (solid line). This provides an example of the wear-and-tear principle whereby the usage of the regulator should be minimized to reduce the detrimental effect of inevitable mutations.
Fig. 3.
Mutational load at low demand. (A) The mutational load at large population size, Eq. 3, is plotted for the (++) and (−−) modes of control as a function of the period T (in units of ν− and with ν+ = 0.1ν−). At small ν−T, the load for both modes is equal to ν−. However, for large ν−T, the load is much smaller for (++) control at low demand (here: D = 0.05), making this the preferred mode of control. (B) The mutational load at limited population sizes: The symbols (squares for (++) control and circles for (−−) control) are numerical results obtained for different population sizes N using one set of parameters corresponding to ν−T ≈ 5. The lines represent the analytical 2-state approximation (see Eq. 7). With increasing N, the loads approach the value of the large population limit, Eq. 3. The population size at which the relative fitness between the 2 modes changes sign is indicated by N*. The fixed parameters, ν− = 10−3, ν+ =10−4, Ti = 100, Tni = 5000, s = 0.2, were chosen such that the stochastic simulations are computationally feasible. However, the good agreement with the analytical theory validates the 2-state approximation, which is then applicable for a broad range of parameters (see also Fig. S2).
Rare Stochastic Events.
In a finite population, the fraction of nonbinders, x(t), fluctuates stochastically as subsequent generations are formed (genetic drift). To study the effect of these fluctuations, we adopt the Wright–Fisher model (14), a standard model for an idealized population with a constant size N. We first solve the model numerically as described in SI Appendix Sections II and III. The results are shown as the symbols for the 2 modes of control in Fig. 3B, where the average fitness cost 〈 γ 〉 is plotted as a function of N, at a fixed value of the period, with ν−T = 5. For the (−−) mode (circles), the cost is largely insensitive to the population size. In contrast, the (++) mode shows a marked dependence on N: for large populations where fluctuation effects are negligible, we recover the deterministic results discussed above as expected, i.e., (++) control leads to a higher average fitness. However, the advantage diminishes as the population size decreases and turns into a disadvantage at small N, where the (−−) mode becomes advantageous. Correspondingly, in a high-demand situation, the (++) mode becomes advantageous at small N. Hence, the use-it-or-lose-it principle is borne out by the model for sufficiently small population size. We find this important qualitative result to be independent of the values of the fitness costs, sact and srep, as long as selection is strong, see below.
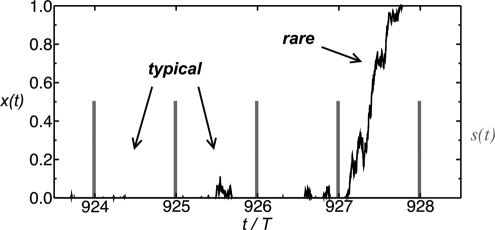
The above observations raise 2 immediate questions: What is the mechanism behind the sign reversal in the relative fitness? And how does the “critical” population size, where the average fitness of both designs is equal, depend on the other model parameters? To clarify the mechanism, we inspect stochastic evolutionary trajectories of x(t) for the (++) control, see Fig. 4 for an exemplary trajectory. The typical behavior is as follows: During the selection interval, practically all nonbinders are eliminated from the population, whereas during the neutral interval, a fluctuating fraction of nonbinders emerges, which typically remains small until being wiped out when selection sets in again. However, very rarely, fluctuations accumulate over a single neutral interval and lead to the complete loss of the binders. Such events, one of which is captured in Fig. 4, provide a vivid demonstration of the use-it-or-lose-it principle. Loss of the binders leads to a large fitness cost during the subsequent selection intervals until the binders are regenerated by mutation. This is the origin of the significant drop in fitness for the (++) control seen in Fig. 3B at small N. Given a low demand, these rare events are clearly much more likely for the (++) control than the (−−) control, because the neutral period for the latter is much shorter. Hence, the fitness of the (−−) control is not affected even at small N.
Fig. 4.
Rare events. A stochastic evolutionary trajectory x(t) for the (++) control, generated as described in SI Appendix Section II. The figure shows a small segment of a typical trajectory of x(t) (the jagged curve) over several cycles of environmental variations, with a short interval of induction (selection, indicated by the vertical lines) followed by a long neutral interval. In this low-demand environment (D = 0.02), typical fluctuations of the fraction of nonbinders for a population of n = 1,000 individuals are the small “blips” that appear during the neutral intervals. However, once in a while, a rare event occurs wherein the nonbinders completely take over the population during 1 neutral period (during cycle 927 in this case), providing a vivid demonstration of the use-it-or-lose-it principle. Loss of the binders leads to a large fitness cost during the subsequent selection intervals until the binders are regenerated by mutation.
To quantify the regime where the use-it-or-lose-it principle is operative, we need to determine the probability for a loss-of-function event to occur during a single cycle for the different model parameters. In population genetics, such fixation probabilities are pivotal (14), e.g., a classic result of Kimura and Ohta (16) is that a single mutation in a homogeneous asexual population of size N has a chance q(s) = (1 − e−2s)/(1− e−2Ns) to become fixed in the population (s is the fitness difference of the mutant to the wild type). Here, we need the fixation probability after a finite time, for neutral mutations produced at a constant rate ν−; see ref. 17 for a related problem in a different context. The functional form of this probability follows from a simple consideration: The average number of nonbinder mutants generated in a neutral interval is ν−NTn. The fixation probability for each mutant is q(0) = 1/N at large times, whereas at finite times it must take the form N−1f(Tn/N) with a scaling function f(τ), because N sets the time scale for the drift dynamics. Hence, the total fixation probability per cycle is
where we neglect reverse mutations. The function f(τ) can be calculated analytically, see SI Appendix Section IV. It approaches the simple form
when the neutral period (measured in generations) is shorter than the population size. Hence, the probability becomes exponentially small when the neutral interval is less than N generations.
Competing Design Principles.
The trajectory of Fig. 4 suggests a simplified view of the evolutionary dynamics over time scales of many periods T: The population of cells essentially switches stochastically between 2 states, a “b-state” where the population consists primarily of binders (x ≈ 0), and a “nb-state” where the entire population consists of nonbinders (x = 1). Above, we determined the transition rate R− from the b-state to the nb-state. Similarly, we can determine the transition rate R+ from nb-state to the b-state,
This form arises because the generation of a single functional mutant within the period under selection typically suffices to reestablish the b-state where most individuals are functional. In the b-state, we can approximate the average fitness cost γb by the expression of Eq. 3 for an infinite population. In contrast, in the rare nb-state, the cost is much larger, γnb = −sTs/T. Within this “2-state approximation,” the average cost is
The solid lines in Fig. 3B display the result of Eq. 7 as a function of the population size N for the 2 modes of control. We see that the simple analytical approximation captures the behavior of the numerical solution (symbols) reasonably well. In particular, it predicts rather accurately the position of the crossing point in Fig. 3B, i.e., the population size N* where the fitnesses associated with the 2 modes of control are equal.
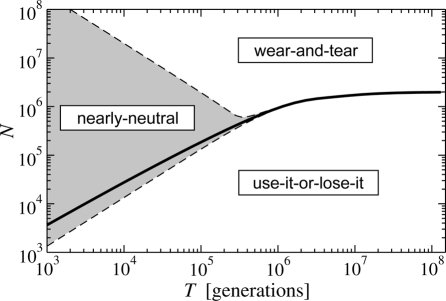
The existence of this “critical” population size N* is a key result of this article. As we have seen above, the use-it-or-lose-it principle applies for population sizes below N*, whereas the wear-and-tear principle applies for populations larger than N*. What parameters control the value of N*? Using the 2-state approximation (Eq. 7) and assuming that the demand is either very low (Ti ≪ T) or high (Tni ≪ T), we find that N* is determined by the equation
Remarkably, Eq. 8 shows that the critical population size N* is independent of the precise value of the demand as long as it is either very low or very high. Fig. 5 shows the “phase diagram” where the thick solid line is the predicted N*(T), for fixed mutation rates ν = 10−6 and ν+ = 10−7, and a selection pressure‡ s = 0.2. For short time scales, N*(T) rises approximately linearly, N* ≈ cT, with a slope c that depends only logarithmically on the selection pressure and the mutation rates (typically c is on the order of 1). For large time scales, N*(T) levels off, confirming that the wear-and-tear principle always dominates as we approach the deterministic limit of very large populations. Within our 2-state approximation§, N* reaches a plateau of approximately s/ν+ at large time scales.
Fig. 5.
Phase diagram for the evolutionary design principles in the (N,T)-parameter space (effective population size and time scale of the environmental variation). Below the solid line, the average fitness is maximized when the mode of regulation is chosen according to the use-it-or-lose-it principle, whereas the wear-and-tear principle holds above. Within the “grey zone,” the choice of the design is nearly neutral. The diagram is obtained within the 2-state approximation (see main text) for fixed mutation rates and selection pressure, and is valid both in the limit of very high and very low demand.
For parameter combinations that are right on the solid line in Fig. 5, our evolutionary scenario does not yield a preference between the 2 modes of control. Close to the line, the selection is expected to be very weak. In order to delineate the approximate boundaries of the nearly-neutral grey zone where no evolutionary design principle is effective, we plotted the dashed contour lines in Fig. 5. These lines indicate where the product of N and the fitness difference between the 2 regulation modes is equal to the mutation rate ν+. The observation that the grey zone takes away from the wear-and-tear parameter space more than it does from the use-it-or-lose-it parameter space indicates that the latter principle is more “robust” than the former. This tendency is indeed confirmed also when we brake the symmetry of the deleterious mutation rates in our model, e.g., allowing for a slightly larger ν− in activators than repressors: Whereas the general behavior of the model is conserved, the use-it-or-lose-it principle is more robust.
Extinction Catastrophes
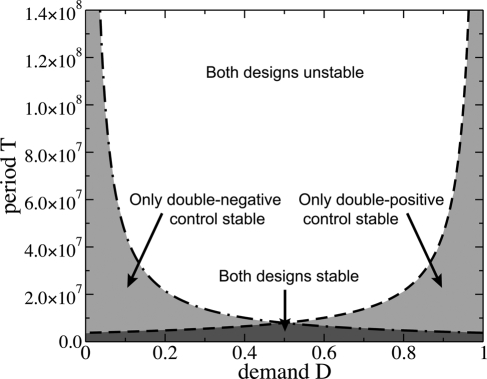
So far we have implicitly assumed that upon loss of the functional allele in a population, it will be regenerated by mutation before the population becomes extinct. For E. coli, the adaptation of regulatory mechanisms may be rapid enough to be observed even in laboratory evolution experiments 18, and we indeed expect a large regeneration rate (Eq. 6) due to its large population size. More generally, however, there are certainly biological contexts where (i) loss-of-function entails a severe reduction of viability and (ii) adaptation is slow, such that the population will face extinction before the function is regenerated. Under such conditions, the question becomes which design is evolutionarily more stable, and thus less prone to extinction 17.
Within our model and analysis, the effective “extinction rate” kext is obtained directly from the fixation probability per cycle in Eq. 4, as kext = R−/T. The associated time scale for extinction, 1/kext, must be compared with another biological time scale, such as the typical lifetime of a bacterial species or the time until a change of host (where the regulation of the gene under study may no longer be needed). If 1/kext is larger than this time scale, we can consider a mode of gene regulation to be stable. As shown in Fig. 6 and SI Appendix Section V, we find that for short periods T, both modes of gene regulation are stable, independent of the demand. However, for longer periods, only (++) control is stable at high demand, whereas only (−−) control is stable at low demand. Hence, the use-it-or-lose-it principle explicitly emerges here as an evolutionary design criterion that minimizes the probability of extinction due to fixation of a nonfunctional allele. Note that the opposite wear-and-tear principle, which is based on a quasi equilibrium, average fitness, cannot emerge in this extreme case where fixation is coupled to extinction. This observation explains why Savageau's previous quantitative analysis (5) yielded only the use-it-or-lose-it principle, but not the wear-and-tear principle: Effectively, ref. 5 also analyzes the stability against extinction, however within a deterministic framework. Although true extinction events occur only in finite populations with genetic drift, ref. 5 obtained a qualitatively similar effect by introducing a stability criterion whereby the fraction of nonbinders in the population should never exceed a threshold θ. Our stochastic analysis of the problem sheds light on the possible interpretation of the ad hoc parameter θ, as an indirect measure for the lifetime of a regulation strategy. At a more quantitative level, our stochastic treatment leads to rather different shapes for the stability regions in the parameter space spanned by the period T and the demand D. In particular, it is not possible within our model to have extinction occurring at small period T due to the low probability of having a rare event at small T; however, ref. 5 allows for extinction at arbitrarily small T. The difference is again attributed to the ad hoc parameter θ as elaborated in SI Appendix Section V.
Fig. 6.
Stability against extinction catastrophes. For a given combination of the demand D and the period of the environmental fluctuations, the stochastic model predicts whether both the (++) control and the (−−) control are evolutionarily stable or whether only 1 or none of the regulatory designs are evolutionarily stable.
Discussion
Our theoretical analysis has resolved an apparent contradiction between the use-it-or-lose it principle and the concept of genetic robustness (7): We found that with a time-dependent selection function, where intervals of neutral evolution alternate with intervals of selection, there are 2 opposing effects, (i) a rare large fitness cost incurred when the functionality of the regulator is lost from an entire population due to genetic drift during 1 neutral period, and (ii) a continuous small load produced by nonfunctional mutants during the periods when the regulator is required to set the expression of the target gene to the desired level. The 2 fitness costs depend oppositely on the demand for the target gene. Hence they lead to the 2 opposing evolutionary design principles, which either posit that gene regulation mechanisms should be designed to maximize the usage of the regulators (the use-it-or-lose-it principle) or to minimize the usage (the wear-and-tear principle). We characterized the interplay of the 2 effects, and demonstrated that depending on the evolutionary parameters, one or the other design principle dominates. The most critical parameters are the effective population size N and the time scale T of the environmental variations, see Fig. Fig. 5. Below, we discuss the meaning and expected ranges of these parameters in some detail, because the interpretation of Savageau's empirical study hinges on them.
Estimates of the effective size of the E. coli population (19,20) span at least a range from 2 × 108 down to 105. These numbers are notable for 2 reasons: First, they are both clearly much smaller than the census size (total number of E. coli cells). Such estimates are obtained by relating observed sequence variabilities to the idealized Wright–Fisher model, which predicts the variability as a function of the effective population size. An effective size smaller than the census size then accounts for effects ignored in the model, e.g., population bottlenecks and substructure. For bacteria, where the rate of recombination is very low, an important mechanism leading to small effective population sizes is the periodic action of selective sweeps, which can quickly drive any allele to fixation (“genetic draft”), even in a large population (21).
Second, the wide range of literature values indicates that the estimates depend strongly on the type of sequence data used. A plausible explanation of this fact was proposed by Berg (22) on the basis of the pronounced population structure of E. coli: The low value of ref. 20 is attributed to nearly neutral genetic drift within single bacterial lines, consisting, e.g., of colonies in the guts of individual animals (or family groups). In contrast, the higher value of ref. 20 is attributed to the dynamics of spreading throughout the species, involving many invasion or recolonization events between the different lines.
The environmental variations, which modulate the selection pressure on metabolic genetic switches of E. coli, act on individual colonies. Thus, taking N ≈ 105 as the appropriate population size following Berg (22), the phase diagram of Fig. 5 predicts the use-it-or-lose-it principle to be effective for time scales exceeding T ≈ 8 × 104 generations. Assuming an average generation time of a few hours, this value corresponds to a few decades in real time, which is comparable with the lifespan of host animals for E. coli. (It is interesting to note that this time scale could be reduced, if there were a weak positive selection favoring the loss of the regulator during the periods when it is not needed.) In summary, a possible evolutionary scenario supporting Savageau's observations is as follows: Suppose an ancestral bacterial strain produced a certain metabolic enzyme constitutively, i.e., the associated gene was unregulated. Then, a divergence occurred, where one strain picked up the (++) control for this gene, whereas another strain picked up the (−−) control. Individual colonies of these 2 strains would then have competed with each other over many invasion and recolonization events, whereas each of them experienced average fitness costs as calculated within our model. Ultimately one of the strains would have taken over the population, more likely the one with the smaller fitness cost.
For our quantitative formulation of the use-it-or-lose-it and wear-and-tear principles, we discussed the steady-state average fitness cost for the cells in a population and the evolutionary stability of the control modes. Alternatively, evolutionary design principles can be formulated based on the concept of evolvability (23), i.e., the likelihood that a given design arises given the mechanisms of molecular evolution. However, evolvability appears not to be a constraint in the present context, since both (++) and (−−) control is widely observed in microorganisms. In contrast, it is important to keep in mind that evolutionary design principles of the type discussed here provide weak discrimination and are relevant only when the alternative modes of gene regulation are nearly functionally equivalent. An interpretation of Savageau's observations based on function, i.e., the molecular biology of gene regulation, was recently discussed by Shinar et al. (6). This study ignored genetic drift effects in the analysis, obtaining the effect of the use-it-or-lose-it principle as a direct consequence of an assumption that usage of regulators is associated with a positive fitness contribution, and pointing out also the opposite effect if one makes the opposite assumption. The authors compiled an interesting list of possible effects that could support one or the other assumption. However, at present it is not clear how the fitness cost/gain of these effects can be estimated to see whether they overwhelm the mechanisms of evolution we discussed here, nor is it clear whether any of these effects cannot be circumvented, e.g., through appropriate choices and spacings of protein–DNA-binding sequences.
Here, we have discussed the use-it-or-lose-it principle in the context of regulatory design and evolution. Indeed, the evolution of regulatory systems remains far less understood than the molecular evolution of proteins (24,25). Time-dependent selection may turn out to play an important role in regulatory evolution, as mentioned above. However, recent experimental studies have mainly documented examples where the regulatory mechanisms changed considerably, whereas the biological function remains conserved (26–28). On the theoretical side, there are few explicit theoretical models of regulatory evolution, see, e.g., refs. 13 and 29. However, we stress that the use-it-or-lose-it principle is potentially relevant in a broader context than regulatory evolution for any functional module responding to a variable input. Clearly, much is to be learned, and the study of functional design principles and regulatory evolution must go hand in hand.
Supplementary Material
Acknowledgments.
We thank L. Chao, J. Hermisson, J. Krug, and M. Savageau for useful discussions. U.G. thanks the Center for Theoretical Biological Physics (CTBP) at the University of California at San Diego for hospitality and support during an extended visit. This work was supported in part by Deutsche Forschungsgemeinschaft Grant GE1098 (to U.G.), National Institutes of Health Grant R01GM77298 (to T.H.), and by National Science Foundation Grants PHY-0216576 and PHY-0225630 through the Physics Frontier Center-sponsored CTBP.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808500106/DCSupplemental.
Activating and repressing TFs in bacteria do not differ in size, length of binding sequence, or other obvious properties that would significantly affect the mutation rates. The robustness of the model behavior against small differences in the mutation rates is addressed below.
For a low-demand situation, this corresponds to the selection sact on the activator, whereas it corresponds to the selection srep on the repressor in a high-demand situation
Note that in a strict sense, the validity of the 2-state approximation breaks down when the distribution of x is no longer peaked at the boundaries, i.e., as we approach the deterministic limit. However, it nevertheless yields a useful estimate for the phase boundary.
References
- 1.Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402:C47–C50. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- 2.Savageau MA. Genetic regulatory mechanisms and the ecological niche of Escherichia coli. Proc Natl Acad Sci USA. 1974;71:2453–2455. doi: 10.1073/pnas.71.6.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savageau MA. Design of molecular control mechanisms and the demand for gene expression. Proc Natl Acad Sci USA. 1977;74:5647–5651. doi: 10.1073/pnas.74.12.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neidhardt FC, editor. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Washington DC: Am Soc Microbiol Press; 1996. [Google Scholar]
- 5.Savageau MA. Demand theory of gene regulation. Genetics. 1998;149:1665–1691. doi: 10.1093/genetics/149.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinar G, Dekel E, Tlusty T, Alon U. Rules for biological regulation based on error minimization. Proc Natl Acad Sci USA. 2006;103:3999–4004. doi: 10.1073/pnas.0506610103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Visser JA, et al. Perspective: Evolution and detection of genetic robustness. Evolution (Lawrence, Kans) 2003;57:1959–1972. doi: 10.1111/j.0014-3820.2003.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 8.Kussell E, Leibler S. Phenotypic diversity, population growth, and information in fluctuating environments. Science. 2005;309:2075–2078. doi: 10.1126/science.1114383. [DOI] [PubMed] [Google Scholar]
- 9.Takahata N, Ishii K, Matsuda H. Effect of temporal fluctuation of selection coefficient on gene frequency in a population. Proc Natl Acad Sci USA. 1975;72:4541–4545. doi: 10.1073/pnas.72.11.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson M, Snoad N. Error thresholds for quasispecies on dynamic fitness landscapes. Phys Rev Lett. 2000;84:191–194. doi: 10.1103/PhysRevLett.84.191. [DOI] [PubMed] [Google Scholar]
- 11.Wilke CO, Ronnewinkel C, Martinetz T. Dynamic fitness landscapes in molecular evolution. Phys Rep. 2001;349:395–446. [Google Scholar]
- 12.Mustonen V, Lässig M. From fitness landscapes to seascapes: Non-equilibrium dynamics of selection and adaptation. Trends Genet. 2009 doi: 10.1016/j.tig.2009.01.002. in press. [DOI] [PubMed] [Google Scholar]
- 13.Gerland U, Hwa T. On the selection and evolution of regulatory DNA motifs. J Mol Evol. 2002;55:386–400. doi: 10.1007/s00239-002-2335-z. [DOI] [PubMed] [Google Scholar]
- 14.Ewens WJ, editor. Mathematical Population Genetics. New York: Springer; 2004. [Google Scholar]
- 15.Haldane JB. The effect of variation of fitness. Am Nat. 1937;71:337–349. [Google Scholar]
- 16.Kimura M, Ohta T. The average number of generations until fixation of a mutant gene in a finite population. Genetics. 1969;61:763–771. doi: 10.1093/genetics/61.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masel J, King OD, Maughan H. The loss of adaptive plasticity during long periods of environmental stasis. Am Nat. 2007;169:38–46. doi: 10.1086/510212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philippe N, Crozat E, Lenski RE, Schneider D. Evolution of global regulatory networks during a long-term experiment with Escherichia coli. BioEssays. 2007;29:846–860. doi: 10.1002/bies.20629. [DOI] [PubMed] [Google Scholar]
- 19.Hartl DL, Moriyama EN, Sawyer SA. Selection intensity for codon bias. Genetics. 1994;138:227–234. doi: 10.1093/genetics/138.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulmer M. The selection-mutation-drift theory of synonymous codon usage. Genetics. 1991;129:897–907. doi: 10.1093/genetics/129.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majewski J, Cohan FM. Adapt globally, act locally: The effect of selective sweeps on bacterial sequence diversity. Genetics. 1999;152:1459–1474. doi: 10.1093/genetics/152.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg O. Selection intensity for codon bias and the effective population size of Escherichia coli. Genetics. 1996;142:1379–1382. doi: 10.1093/genetics/142.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirschner M, Gerhart J. Evolvability. Proc Natl Acad Sci USA. 1998;95:8420–8427. doi: 10.1073/pnas.95.15.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig MZ. Functional evolution of noncoding DNA. Curr Opin Genet Dev. 2002;12:634–639. doi: 10.1016/s0959-437x(02)00355-6. [DOI] [PubMed] [Google Scholar]
- 25.Gelfand MS. Evolution of transcriptional regulatory networks in microbial genomes. Curr Opin Struct Biol. 2006;16:1–10. doi: 10.1016/j.sbi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig MZ, Bergman C, Patel NH, Kreitman M. Evidence for stabilizing selection in a eukaryotic enhancer element. Nature. 2000;403:564–567. doi: 10.1038/35000615. [DOI] [PubMed] [Google Scholar]
- 27.Tanay A, Regev A, Shamir R. Conservation and evolvability in regulatory networks: The evolution of ribosomal regulation in yeast. Proc Natl Acad Sci USA. 2005;102:7203–7208. doi: 10.1073/pnas.0502521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsong AE, Tuch BB, Li H, Johnson AD. Evolution of alternative transcriptional circuits with identical logic. Nature. 2006;443:415–420. doi: 10.1038/nature05099. [DOI] [PubMed] [Google Scholar]
- 29.Berg J, Willmann S, Lässig M. Adaptive evolution of transcription factor binding sites. BMC Evol Biol. 2004;4:42. doi: 10.1186/1471-2148-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.