Abstract
Immune dysfunction develops in patients with many cancer types and may contribute to tumor progression and failure of immunotherapy. Mechanisms underlying cancer-associated immune dysfunction are not fully understood. Efficient IFN signaling is critical to lymphocyte function; animals rendered deficient in IFN signaling develop cancer at higher rates. We hypothesized that altered IFN signaling may be a key mechanism of immune dysfunction common to cancer. To address this, we assessed the functional responses to IFN in peripheral blood lymphocytes from patients with 3 major cancers: breast cancer, melanoma, and gastrointestinal cancer. Type-I IFN (IFN-α)-induced signaling was reduced in T cells and B cells from all 3 cancer-patient groups compared to healthy controls. Type-II IFN (IFN-γ)-induced signaling was reduced in B cells from all 3 cancer patient groups, but not in T cells or natural killer cells. Impaired-IFN signaling was equally evident in stage II, III, and IV breast cancer patients, and downstream functional defects in T cell activation were identified. Taken together, these findings indicate that defects in lymphocyte IFN signaling arise in patients with breast cancer, melanoma, and gastrointestinal cancer, and these defects may represent a common cancer-associated mechanism of immune dysfunction.
Immune dysfunction is an early event in cancer development and expands with progression to metastatic disease (1). Tumor-associated antigen (TAA)-specific CD8 T lymphocytes are often present in the blood of cancer patients and accumulate at tumor sites and in tumor-draining lymph nodes (2). While TAA-specific CD8 T cells can be elicited by current peptide vaccines and other immunotherapies, the presence or magnitude of these responses does not reliably correlate with clinical outcome or response to vaccination (3–5). Such cells may be specifically driven into apoptosis or rendered nonresponsive in vivo, preventing appropriate activation and cytolytic responses against tumor cells (6, 7). Current immunotherapeutic strategies are subject to the immunosuppressive effects of cancer and of regulatory T cells, which likely contribute to their lack of success thus far (4, 5). The nature and molecular mechanisms underlying immune dysfunction in cancer are not clearly defined. Elucidation of the mechanisms will allow rational design of strategies to reverse immune dysfunction and normalize lymphocyte populations to improve the endogenous immune response to cancer and to enhance the efficacy of cancer immunotherapy.
Potential mechanisms of immune dysfunction in cancer include defects in antigen recognition (first signal), costimulation (second signal), and cytokines, (e.g., IFNs; third signal). Efficient IFN signaling is critical to provide the third signal to enable full activation, clonal expansion, and memory development rather than tolerance (8), and for efficient natural killer (NK)-cell-mediated cytotoxicity (9). We hypothesized that impaired IFN signaling may be a common immune defect in cancer patients (10). This study aimed to determine whether altered IFN signaling is a general mechanism of immune function in patients with cancer of different types and stages. A further aim of this study was to assess which subsets of peripheral blood leukocytes exhibit defects in IFN signaling, and which downstream IFN-stimulated functional responses are affected. We assessed IFN signaling in peripheral blood lymphocytes from patients with 3 major types of cancer via real-time quantitative PCR to measure expression of IFN-stimulated genes (ISGs), extensive Phosflow analyses, and functional analyses to measure downstream responses to IFNs. STAT1 tyrosine-701-phosphorylation (pSTAT1) is critical for both type-I and -II IFN signaling via the JAK-STAT pathway (11); therefore, we assessed pSTAT1 in response to IFN-α or -γ in T, B, and NK cells from cohorts of breast cancer patients, melanoma patients, gastrointestinal (GI) cancer patients (including colon, rectum, stomach, and pancreatic cancer), and compared against age-matched healthy controls. Our analyses included the main peripheral blood lymphocyte populations that are involved in tumor immunity and may be negatively impacted or promoted by tumors, specifically T cells, including naive, effector and memory subsets, B cells, and NK cells. IFN signaling is critical in the proper activation and homeostatic control of these cell types, and impaired IFN signaling in these cell types may aid tumor progression and confound immunotherapeutic approaches.
Results
ISG Expression Is Down-Regulated in Lymphocytes from Breast Cancer Patients.
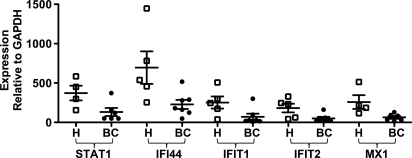
To assess the integrity of the IFN-signaling pathway in lymphocytes from cancer patients, expression levels of 5 major ISGs (STAT1, IFI44, IFIT1, IFIT2, and MX1) was measured by real-time quantitative PCR in peripheral blood lymphocytes from breast cancer patients and age-matched healthy controls. The normalized expression levels of each of the ISGs were statistically significantly reduced in lymphocytes from breast cancer patients compared to the controls (Fig. 1), suggesting dysregulation of the IFN signaling pathway.
Fig. 1.
Real-time quantitative PCR analysis of ISG expression in lymphocytes from breast cancer patients and healthy controls. The expression levels of ISGs STAT1, IFI44, IFIT1, IFIT2, and MX1 were measured in unstimulated lymphocytes from breast cancer patients (BC filled circles) and age-matched healthy controls (H open squares) by real-time quantitative PCR. Expression of each gene was normalized to GAPDH. Medians are indicated by the bar in each data set. Two-sided Wilcoxon-Mann-Whitney tests were performed to compare ISG expression values from breast cancer patients with healthy controls; STAT1 P = 0.0381, IFI44 P = 0.0303, IFIT1 P = 0.0480, IFIT2 P = 0.0177, MX1 P = 0.019.
IFN Signaling Is Impaired in Lymphocytes from Cancer Patients.
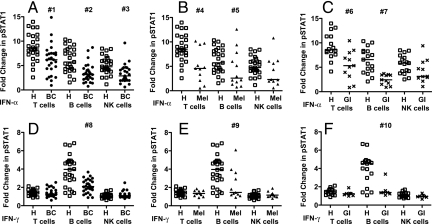
To demonstrate impaired IFN signaling in lymphocytes from cancer patients, the induction of pSTAT1 (Y701) in response to IFN-α and IFN-γ stimulation was measured by Phosflow in peripheral blood lymphocytes from 27 breast cancer patients (stages II, III, and IV), 12 melanoma patients (stage III and IV), 11 GI cancer patients (stages II, III, and IV), and 28 healthy controls (Fig. S1). The fold-change in pSTAT1 induced by IFN-α was reduced in T, B, and NK cells from breast cancer, melanoma and GI cancer patients versus healthy controls (Fig. 2 A–C), demonstrating a defect in IFN-α signaling in the main lymphocyte populations in these cancer patients. The reduction was statistically significant in T and B cells from all 3 cancer patient groups, and in NK cells from breast cancer patients versus healthy controls [see Fig. 2, Table S1]. NK cells from melanoma and GI cancer patients showed the same trend of reduced response to IFN-α. The impairment in response to IFN-α was greatest in B cells, intermediate in T cells, and lowest in NK cells in breast cancer and GI cancer patients, whereas in melanoma the impairment was greatest in T cells, intermediate in B cells and least in NK cells, as measured by the lower end point of the 95% CI, which reflects the magnitude and significance of the difference between patients and controls (see Table S1). In healthy controls, T cells showed the highest IFN-α-induced pSTAT1 levels, B cells were intermediate, and NK cells had the lowest levels (Fig. S2A), which provides an explanation for our increased ability to detect differences in IFN-α-induced pSTAT1 in T and B cells from cancer patients versus healthy controls.
Fig. 2.
IFN-α- and IFN-γ-stimulated fold-change in pSTAT1 (Y701) in peripheral blood mononuclear cells (PBMC) subsets from breast cancer patients, melanoma patients and GI cancer patients versus healthy controls. PBMCs were stimulated with 1,000 IU/ml IFN-α, IFN-γ, or unstimulated and pSTAT1 was measured by Phosflow. The IFN-induced fold-change in pSTAT1 was measured in T, B, and NK cells from healthy controls (H open squares), patients with breast cancer (BC filled circles), melanoma (Mel filled triangles), and GI cancer (GI x). (A) IFN-α- BC, (B) IFN-α- Mel, (C) IFN-α- GI, (D) IFN-γ- BC, (E) IFN-γ- Mel, (F) IFN-γ- GI. Medians are indicated by the bar in each data set. Two-sided Wilcoxon-Mann-Whitney tests were used to compare values from cancer patients with age-matched healthy controls; P-values less than 0.05 are denoted; #1 P = 0.00077, #2 P = 0.00012, #3 P = 0.00040, #4 P = 0.00420, #5 P = 0.04408, #6 P = 0.00270, #7 P = 0.00003, #8 P = 0.00012, #9 P = 0.00570, #10 P = 0.00190. P-values and estimated differences with 95% confidence intervals (CI) for each comparison are shown in Table S1.
In response to IFN-γ stimulation, pSTAT1 induction was significantly reduced in B cells, but not T or NK cells, from all 3 cancer patient groups compared to healthy controls (see Fig. 2 D–F). In healthy controls, B cells exhibited high levels of IFN-γ-induced pSTAT1, whereas T cells and NK cells showed very low levels of IFN-γ-induced pSTAT1 (Fig. S2B). This may underlie our ability to detect differences in IFN-γ-induced pSTAT1 in B cells but not T and NK cells from cancer patients versus healthy controls.
Impaired IFN Signaling Is Equally Evident in Early- and Late-Stage Breast Cancer.
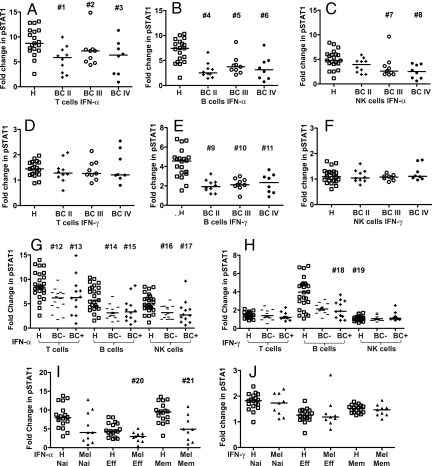
To determine whether impaired IFN signaling is a function of cancer stage, IFN-α- and IFN-γ-stimulated pSTAT1 was compared between stage II, III, and IV breast cancer subgroups. IFN-α-stimulated pSTAT1 was equally reduced in T, B, and NK cells from breast cancer patients with each disease stage (Fig. 3 A–C). In response to IFN-γ, the fold-change in pSTAT1 was equally reduced in B cells, but not T or NK cells, from breast cancer patients at each stage (Fig. 3 D–F). There was no significant difference between IFN-induced pSTAT1 from patients of different stages (Table S2). These data indicate that down-regulation of IFN signaling in lymphocytes is an early event in immune modulation in cancer that persists during progression to late stage disease.
Fig. 3.
Effect of cancer stage, chemotherapy, and T-cell phenotype on IFN-α- and IFN-γ-stimulated fold-changes in pSTAT1 in cancer patients and healthy controls. PBMCs were stimulated with IFN-α, IFN-γ, or unstimulated and pSTAT1 was measured by Phosflow in T, B, and NK cells from healthy controls (H open squares), BC patients with stage II (BC II filled diamonds), stage III (BC III open circles), stage IV (BC IV filled circles) disease, BC patients not treated (BC –), and BC patients treated with chemotherapy (BC +) and stage III–IV melanoma patients (Mel filled triangles). Naive, effector, and memory T-cell subsets were gated based on CD27 and CD45RA expression. (A) IFN-α- T cells; (B) IFN-α- B cells; (C) IFN-α- NK cells; (D) IFN-γ-T cells; (E) IFN-γ- B cells; (F) IFN-γ- NK cells; (G) IFN-α- T, B, and NK cells from BC patients not treated (BC–) or treated (BC+) with chemotherapy; (H) IFN-γ- T, B, and NK cells from BC– or BC+ patients; (I) IFN-α- naive, effector, and memory T cells; (J) IFN-γ- naive, effector, and memory T cells [(A–H) BC patients, (I–J) Mel patients). The median is indicated by the bar in each data set. Two-sided Wilcoxon-Mann-Whitney tests were performed to compare values from cancer patients with age-matched healthy controls; P-values less than 0.05 are denoted; #1 P = 0.00447, #2 P = 0.03327, #3 P = 0.03985, #4 P = 0.00077, #5 P = 0.02690, #6 P = 0.01180, #7 P = 0.01107, #8 P = 0.00108, #9 P = 0.00286, #10 P = 0.01019, #11 P = 0.01349, #12 P = 0.00069, #13 P = 0.04490, #14 P = 0.00037, #15 P = 0.00764, #16 P = 0.00339, #17 P = 0.00527, #18 P = 0.00233, #19 P = 0.00131, #20 P = 0.01806, #21 P = 0.03107. There was no significant difference in the frequency of T-cell phenotypes between melanoma patients and healthy controls. P-values and estimated differences with 95% CI for each comparison are shown in Table S1A.
Defects in IFN Signaling Are Not Caused or Influenced by Chemotherapy.
To determine whether the impairment in IFN signaling is caused or affected by chemotherapy, breast cancer patients were divided into chemotherapy-treated (neoadjuvant/adjuvant chemotherapy at least 3 weeks before analysis) and those that had not received any systemic therapy. There was no difference in IFN-α- or IFN-γ-induced pSTAT1 between treated and untreated subgroups, and both subgroups showed significant reductions in IFN-induced pSTAT1 versus healthy controls (Fig. 3 G–H, and see Table S1A and S2). All but 1 of the stage IV patients received chemotherapy, while only 1 stage II patient received chemotherapy; yet these 2 subgroups showed no significant difference in pSTAT1 induction (see Table S2). To assess the effects of chemotherapy in patients with the same disease stage, stage III patients were divided into neoadjuvant-treated and untreated subgroups. IFN-α or IFN-γ-induced pSTAT1 levels were not different between these subgroups (Fig. S3). Taken together, these data demonstrate that impaired IFN signaling in peripheral blood lymphocytes from breast cancer patients is not because of or influenced by chemotherapy.
IFN Signaling Defect Is Present in Memory, Effector, and Naive T Cells.
To determine whether the defect in IFN-α signaling in T cells is global or specific to effector/memory cells that could have experienced TAAs, IFN-induced pSTAT1 levels were measured in naive, effector, and memory T cells from melanoma patients and healthy controls (the gating strategy is shown in Fig. S1). IFN-α-induced pSTAT1 was reduced in naive, effector, and memory T cells. The reduction was statistically significant in memory and effector T cells (Fig. 3I and see Table S1B) from melanoma patients, while naive T cells showed the same trend.
Defects in Downstream Functional Responses.
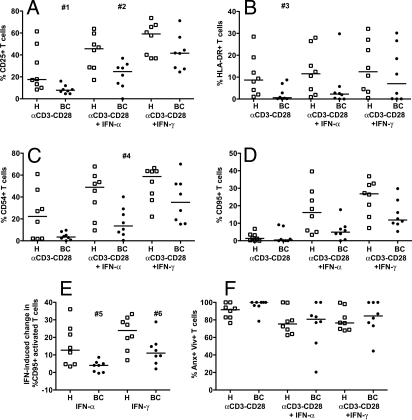
To investigate the downstream effects of impaired IFN-signaling, activation responses of T cells from breast cancer patients were compared to healthy controls. Lymphocytes were stimulated with anti-CD3/anti-CD28 antibody-coated beads alone or in combination with IFN-α or -γ. Expression of CD25 as a general activation marker, HLA-DR, CD95, and CD54 as activation markers that are further induced by IFNs, and activation-induced cell death (AICD) were measured by flow cytometry. T cells from breast cancer patients showed reduced expression of CD25, HLA-DR, CD54, and CD95 in response to anti-CD3/CD28 stimulation alone and in combination with IFN-α or -γ (Fig. 4 A–D, Table S3). The additional induction of CD95 by IFNs in anti-CD3/CD28-activated T cells was reduced in T cells from breast cancer patients versus healthy controls (P = 0.02 for IFN-α, P = 0.05 for IFN-γ) (Fig. 4E). When considered collectively by multivariate analysis, the expression levels of these activation markers were highly correlated and significantly reduced in T cells stimulated with anti-CD3/CD28 alone and in combination with IFN-α in breast cancer patients versus healthy controls (see Table S3). These results suggest that impaired IFN signaling in T cells from breast cancer patients results in poor transduction of the third signal required to initiate appropriate full activation.
Fig. 4.
Expression of activation markers and apoptosis of T cells stimulated with anti-CD3/CD28 antibodies and IFN-α or IFN-γ in breast cancer patients and healthy controls. Lymphocytes from BC patients (filled circles) and healthy controls (open squares) were stimulated with anti-CD3 and anti-CD28 antibody-coated beads alone or with IFN-α or -γ, or unstimulated. The % T cells positive for CD25, HLA-DR, CD54, CD95, Annexin V (Anx), and ViViD (Viv) were measured by FACS at 48 h. (A) % CD25+; (B) % HLA-DR+; (C) % CD54+; (D) % CD95+; (E) IFN-induced change in % CD95+ activated T cells (% CD95+ in conditions stimulated with anti-CD3 anti-CD28 plus IFN-α or -γ minus the % CD95+ in conditions stimulated only with anti-CD3 anti-CD28); (F) % Anx+ Viv+ (all T cells that were positive for either or both Anx and Viv). The median is indicated by the bar in each data set. Two-sided Wilcoxon-Mann-Whitney tests were used to compare data from breast cancer patients and healthy controls; P-values ≤0.05 are denoted; #1 P = 0.004, #2, P = 0.020, #3 P = 0.018, #4 P = 0.016, #5 P = 0.018, #6 P = 0.053. Multivariate analyses are shown in Table S3.
AICD because of anti-CD3/CD28 stimulation was higher in T cells from breast cancer patients versus healthy controls (Fig. 4F, see Table S3), while stimulation with anti-CD3/CD28 plus IFN-α or -γ resulted in less apoptosis in breast cancer patient T cells. In healthy T cells, there was no significant difference between percent apoptotic cells because of anti-CD3/CD28 plus IFN-α versus anti-CD3/CD28 stimulation alone (P = 0.078), whereas in breast cancer, there was a significant reduction in percent apoptotic T cells because of anti-CD3/CD28 plus IFN-α versus anti-CD3/CD28 alone (P = 0.036). These results suggest that activation induces apoptosis to a greater degree in lymphocytes from breast cancer patients (than healthy controls) because of impaired IFN signaling, and this could be rescued with IFN-α.
Discussion
Immune dysfunction has been documented in patients with many cancer types at early stages and throughout development to metastatic disease; the cellular and molecular mechanisms are not well understood. For development of full adaptive immune responses, 3 signals are required: antigen (first signal), costimulation (second signal), and a cytokine signal that can be provided by type-I IFNs (third signal). We hypothesized that altered IFN signaling may be a key mechanism of immune dysfunction common to cancer. In this study, we investigated the function of the IFN signaling pathway in lymphocytes from stage II, III, and IV breast cancer patients, stage III and IV melanoma patients, and stage II, III, and IV GI cancer patients. Using real-time quantitative PCR, we showed that the expression levels of 5 major ISGs were down-regulated in peripheral blood lymphocytes from breast cancer patients versus healthy controls in direct ex vivo experiments, indicating a defect in IFN signaling in lymphocytes in vivo from breast cancer patients. By extensive Phosflow analyses, we demonstrated that the induction of pSTAT1 in response to IFN-α was significantly reduced in T cells and B cells from all 3 cancer patient groups and in NK cells from breast cancer patients. NK cells from melanoma and GI cancer patients showed the same trend of impaired IFN-α signaling. In response to IFN-γ, the induction of pSTAT1 was significantly down-regulated in B cells from all 3 cancer patient groups, but not in T cells or NK cells. These results show that impaired type-I and type-II IFN signaling in lymphocyte populations is a defect common to multiple major cancer types.
Each lymphocyte subset showed different levels of response to IFN-α and IFN-γ. The difference between cell types was most striking in IFN-γ-stimulated cells: B cells exhibited high levels of IFN-γ-induced pSTAT1, whereas T cells and NK cells showed only minimal induction of pSTAT1 in response to IFN-γ. This may provide an explanation for why a difference in IFN-γ-induced pSTAT1 could not be detected in T cells and NK cells from cancer patients. The observed differences in IFN-induced activation of pSTAT1 between lymphocyte subtypes (T cells, B cells, and NK cells) are likely the result of differences in densities of the IFN-binding and signal-transducing components of the type-I and -II IFN receptors on the surface of each cell type, or variations in the control of the STAT1 signaling cascade between cell types (12, 13).
Impaired IFN signaling was evident in breast cancer patients with stage II, III, and IV disease, indicating that it is an early mechanism of immune dysfunction in cancer that persists throughout tumor progression to metastatic disease. In addition, this defect was equally observed in breast cancer patients who had received neoadjuvant or adjuvant chemotherapy and patients who had not yet received any systemic therapy, demonstrating that chemotherapy does not underlie or affect the observed impairment in IFN signaling in cancer patients. The reduced activation of STAT1 in response to IFN-α was most pronounced in memory and effector subsets of T cells from melanoma patients, but naive T cells showed the same trend, indicating that defective IFN signaling in T cells is likely to be global, affecting both antigen-experienced memory cells and inexperienced naive cells.
T cells from breast cancer patients showed reduced expression of activation markers CD25, HLA-DR, CD54, and CD95 [IFN-inducible death receptor (FAS)] in response to anti-CD3 and anti-CD28 stimulation alone or in combination with IFNs, demonstrating that down-regulated IFN signaling is associated with downstream functional impairments in T cell activation. These findings represent a mechanism contributing to the nonresponsiveness and inability to mount effective antitumor immunity that have been observed in cancer patients, and present a barrier to cancer immunotherapies. T cells from breast cancer patients also exhibited deregulated survival responses when stimulated with anti-CD3 and anti-CD28 antibodies alone or in combination with IFNs, further demonstrating that impaired IFN signaling in T cells from cancer patients results in downstream functional consequences that affect appropriate homeostasis. The lower IFN-induced T-cell apoptosis in breast cancer patients may be explained by our observed reduction in CD95 expression in activated T cells stimulated with IFNs from breast cancer patients. In these conditions, activated T cells from breast cancer patients are less susceptible to CD95-mediated apoptosis and thus are partially protected from apoptotic effects of cellular activation. Normal responses to IFNs are required for T-cell homeostasis by regulating AICD and survival; impaired IFN signaling and the resulting deregulation of activation responses, as we have shown, may be involved in the altered homeostasis of lymphocyte populations in cancer patients (14, 15).
IFNs support the activation and clonal expansion of both T and B lymphocytes (16–18). In NK cells, the precise mechanism of IFN action in NK cell activation is not elucidated, but it has been shown that IFN signals are critical for optimal NK function (19). Nonresponsiveness or anergy of lymphocytes in cancer have been shown to be partly caused by degradation of the CD3ζ chain and other signaling components of the T-cell receptor signaling cascade (20, 21). Additional peripheral blood immune processes, such as B-cell receptor signaling and inflammatory responses, have also been shown to be impacted in the cancer state (22) and may contribute to immune dysfunction. Impaired IFN signaling, as shown in our study, represents a further insight into the mechanisms underlying altered regulation of apoptosis in the cancer state and to general immune nonresponsiveness, both of which are critical aspects of tumor-induced immune dysfunction. Low activation of STAT1 in response to IFNs would result in poor transduction of the third signal required to initiate appropriate activation and survival responses for lymphocyte effector and memory function. The low response to IFN-α may also impair the cytotoxic function of CD8 T cells and NK cells, as IFN-α induces the expression of perforin and granzyme, which are low in these effector cells in the blood of cancer patients (23).
The impaired activation of STAT1 in response to IFNs in lymphocytes from cancer patients may be a result of functional defects of components of the IFN signaling pathway, including the IFN receptors, JAK1, Tyk2, and negative regulators. The gene expression levels of the IFN-receptor subunits, STAT2, JAK1, JAK2, and Tyk2 are not significantly different in lymphocytes from melanoma patients versus healthy controls (10); however, functional alterations of these components may be involved in the impaired phosphorylation of STAT1 in response to IFNs. Negative regulation of IFN signaling occurs via protein tyrosine phosphatases, such as Src homology 2-containing phosphatase-1 and -2 and CD45, protein inhibitors of activated STATS that are expressed constitutively and are early regulators of cytokine signaling (24) and the suppressors of cytokine signaling that form a rapid classical negative feedback loop to regulate JAK-STAT signaling (25). Altered peripheral blood cytokine profiles in cancer patients may be involved in the mechanism via which IFN signaling is impaired in lymphocytes in cancer patients. Multiple cytokines, such as IL-4, IL-10, and TGF-β1, produced by tumor cells, tumor-associated leukocytes, regulatory or suppressive peripheral blood leukocytes, such as Tregs and myeloid-derived suppressor cells, are able to induce expression of the negative regulators of IFN signaling (26–30), and may be part of the mechanism underlying impaired IFN signaling in lymphocytes in cancer patients. Immunosuppressive growth factors, such as VEGF that is elevated in the blood of cancer patients, may also be involved in deregulation of IFN signaling indirectly via inhibition of dendritic cell maturation or by promotion of myeloid-derived suppressor cells (31) that in turn produce cytokines that may interfere with IFN signaling in lymphocytes.
Impaired IFN signaling may hinder therapeutic approaches designed to stimulate antitumor immunity, as immunotherapeutic strategies require functional immune activation (32, 33). Assessing IFN signaling in lymphocytes may provide a method for selecting cancer patients for IFN versus alternate therapy. Longitudinal studies of IFN signaling in cancer patients during therapy, disease-free periods, and upon recurrence will be important in determining the kinetics of IFN signaling dysfunction and assessing the utility of IFN responses in predicting presence of disease and responses to IFN-based therapies. Our results suggest that strategies to correct this IFN signaling defect will enhance the efficacy of cancer immunotherapy. Such strategies may extend to other conditions in which immune dysfunction is an underlying factor, because deregulated IFN signaling has been observed in both multiple sclerosis and chronic hepatitis C infection (34, 35), for which IFN therapy is commonly used. In chronic hepatitis C infection, African American patients show lower responses to IFN therapy compared to patients of other races, indicating that race may influence the function of IFN signaling (35). Patients in this study were of diverse races, including Caucasians, Hispanics, African Americans, Asians, and others, suggesting that the defect in IFN signaling was associated with cancer and not race in this study.
In summary, we have identified an immune defect common to 3 major types of cancer. We have demonstrated that IFN-α signaling is reduced in T and B cells, and IFN-γ signaling is reduced in B cells, from patients with breast cancer, melanoma, and GI cancer. We have further shown that the defect is equally evident in patients with stage II, III, and IV breast cancer, and is not influenced by chemotherapy. Our findings demonstrate that impaired IFN signaling is an early, persistent, and global mechanism of immune dysfunction in cancer patients. These findings represent an important insight into immune dysfunction in cancer, and may lead to novel strategies to correct this dysfunction in cancer patients and enhance the success of cancer immunotherapeutic strategies.
Methods
Patient and Healthy Donor Samples.
PBMCs were obtained from 32 patients with American Joint Committee on Cancer (AJCC) stage II, III, and IV breast cancer, 12 patients with AJCC stage III and IV melanoma [a new cohort independent of a previously studied melanoma cohort (10)], and 11 patients with stage II to IV GI cancer (Stanford University Medical Center) with informed consent. PBMCs were also obtained from 28 age-matched healthy donors (Stanford Blood Center). PBMCs were cryopreserved in 90% NCS 10% DMSO. The experiments were approved by the Institutional Review Board of Stanford University. Age, gender, disease, stage, and therapy data are shown in Table S4, Table S5, Table S6, and Table S7.
Real-Time Quantitative PCR Measurement of ISG Expression.
Cyropreserved PBMCs from breast cancer patients and age-matched healthy controls were thawed and rested overnight in IMDM 10% FBS. Lymphocytes were negatively enriched using RosetteSep granulocyte and monocyte depletion cocktails (Stem Cell Technologies). Cells were washed, and replated (≈106 cells/well) in IMDM 10% FBS for 2 h. A small aliquot was stained with mouse anti-human CD3-FITC, CD19 APC, and CD14-PE [Invitrogen (Caltag)] to check for enrichment of lymphocytes. RNA was isolated using TRIzol (Invitrogen), quantified using RiboGreen RNA Quantitation Reagent (Invitrogen), and cDNA was synthesized using the Omniscript RT Kit (Qiagen) according to the manufacturer's instructions. Quantitative RT-PCR analysis was performed using the Quantitect SYBR Green PCR Kit (Qiagen) and the iCycler thermocycler (Bio-Rad). Primer sequences for STAT1, IFI44, IFIT1, IFIT2, and MX1 were previously described (10). All gene-expression data presented were normalized to GAPDH levels for each sample.
IFN Stimulation and Detection of STAT1-pY701.
Cryopreserved PBMCs were thawed, extensively washed, and rested overnight in IMDM containing 10% FBS. PBMCs were ficolled, washed, resuspended in IMDM 5% human AB serum (HS) to 3 × 106 cells/test in 50 μl and stained with combinations of the following mouse anti-human mAbs CD16 PE, CD3 PE-Cy7, (BD Biosciences), CD19 PE-TR, CD27 FITC and CD45RA PE (Invitrogen) for 30 min. Next, 1.45 ml IMDM 5% HS was added to each test and the cells were divided 500 μl per tube and rested at 37 °C 7% CO2 for 15 min. IFN-α or -γ (NIAID Reference Reagent Repository, and R&D Systems) were added to 1,000 IU/ml and cells were incubated at 37 °C for 15 min. The cells were fixed with formalin at 3% for 10 min at 37 °C. The fixed cells were washed twice in 5 ml PBS, permeabilized in 1 ml 1× Custom Perm Buffer - PBMC (Number 643435, BD Biosciences) for 30 min at room temperature and washed twice in stain buffer (1× PBS, 2% FBS, 0.09% sodium azide). The cells were resuspended to 50 μl in stain buffer and stained with 7 μl of anti-STAT1-pY701 Alexa Fluor 647 (BD Biosciences), CD45RA PE at room temperature for 60 min, washed twice in stain buffer, and analyzed using the FACS Aria (BD Biosciences). The Phosflow method enables sensitive, quantitative detection of phosphorylation events in multiple cell populations in small clinical samples. Custom Perm Buffer − PBMC (BD Biosciences) is a new formulation of Phosflow permeabilization buffer that enables optimal staining of pSTAT1 with a high signal-to-noise ratio, whilst preserving the fluorescence of the cell surface antibodies used to detect cell subsets (see Fig. S1).
Measurement of Activation, Proliferation, and Apoptosis in Response to IFN Stimulation in Resting and Anti-CD3/CD28-Stimulated PBMCs.
Cryopreserved PBMCs from breast cancer patients and healthy controls were thawed, extensively washed, and rested overnight in IMDM 10% FBS. Lymphocytes were purified using RosetteSep Granulocyte and Monocyte Depletion Cocktails (StemCell Technologies) and washed in PBS. A small aliquot of the cells was stained with CD3-FITC, CD19 APC, and CD14 PE [Invitrogen (Caltag)] mAbs and measured by FACS to determine the percentage of CD3+ lymphocytes. Cells were plated at 2 to 5 × 105 cells/well in IMDM 10% FBS 2% HS in 96-well plates and stimulated with Dynabeads CD3/CD28 T Cell Expander Beads (Invitrogen) at a bead:T cell ratio of 1:10, or IFN-α or -γ at 1,000 IU/ml, or cells were left unstimulated. Cells were incubated at 37 °C 7% CO2 for 48 h. Cells were then stained with CD3 PE-AF700, HLA-DR Pacific Orange (Invitrogen), CD25 PE-Cy7, CD95 PE, CD54 APC mAbs (BD Biosciences), and Live/Dead Fixable Violet Dead Cell Stain Kit (Invitrogen) at room temperature for 30 min. Cells were washed in Annexin V Binding Buffer (BD Biosciences) and stained with Annexin V Cy5.5 (BD Biosciences) at room temperature for 15 min. Cells were analyzed using the FACSAria.
Analysis of Flow Cytometry Data.
FCS files were analyzed using Flowjo 8.2 (Treestar). The mean fluorescence intensity (MFI) of STAT1 pY701-Alexa Fluor 647 staining was calculated for IFN-α- and IFN-γ-stimulated cells and unstimulated cells. The fold-change in STAT1 pY701 staining was calculated by dividing the MFI of stimulated cells by the MFI of unstimulated cells. The percentages of CD54+, CD25+, CD95+, and HLA-DR+ cells were calculated using the Population Comparison platform in Flowjo 8.2.
Statistical Analyses.
Data were analyzed using the R statistical package(R Foundation for Statistical Computing) and Prism (GraphPad). All P-values are from 2-sided Wilcoxon-Mann-Whitney tests. The Wilcox_test function in R was used to calculate exact P-values, estimated differences and 95% CI. Comparisons were made between cancer patients and age-matched healthy controls. The mean age of the breast cancer patients (52.4, SD 11 y) and melanoma patients (50.0, SD 14.8 y) were not statistically different and the Phosflow data from these patients was compared to a group of age-matched healthy controls (mean 54.0, SD 12.4 y). There was no significant difference in IFN-induced pSTAT1 levels between males and females in the healthy controls or cancer patients (see Fig. S2); therefore, the breast cancer patients (all female) were compared to the mixed-gender healthy controls. The mean age of the GI cancer patients (66, SD 12.5 y) was higher than the total healthy control group; therefore, the GI patient Phosflow data were compared to an age-matched healthy control subgroup (mean age 60.8, SD 9.4 y). For multivariate analyses, standard principal component analysis was applied using the princomp() function in R; then, the usual Mann-Whitney test along the first principal component was performed (36).
Supplementary Material
Acknowledgments.
We thank Dr. David Parks and the Stanford FACS Facility for help with flow cytometry using the BD FACSAria, and BD Biosciences for the Custom Perm Buffer for Phosflow. We thank Dr. Jeffery A. Norton, Dr. Robin M. Cisco, and Laurie Ann Columbo for obtaining PBMC samples from gastrointestinal cancer patients and Gerald Lee for processing the samples. This work was funded by Department of Defense Era of Hope Scholar Award BC0516650 (to P.P.L.) and National Institutes of Health Grant R01 GM086884-2 (to S.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901329106/DCSupplemental.
References
- 1.Staveley-O'Carroll K, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 3.Anichini A, et al. An expanded peripheral T cell population to a cytotoxic T lymphocyte (CTL)-defined, melanocyte-specific antigen in metastatic melanoma patients impacts on generation of peptide-specific CTLs but does not overcome tumor escape from immune surveillance in metastatic lesions. J Exp Med. 1999;190:651–667. doi: 10.1084/jem.190.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KH, et al. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J Immunol. 1999;163:6292–6300. [PubMed] [Google Scholar]
- 5.Weber J, et al. Granulocyte-macrophage-colony-stimulating factor added to a multipeptide vaccine for resected stage II melanoma. Cancer. 2003;97:186–200. doi: 10.1002/cncr.11045. [DOI] [PubMed] [Google Scholar]
- 6.Saito T, Dworacki G, Gooding W, Lotze MT, Whiteside TL. Spontaneous apoptosis of CD8+ T lymphocytes in peripheral blood of patients with advanced melanoma. Clin Cancer Res. 2000;6:1351–1364. [PubMed] [Google Scholar]
- 7.Zippelius A, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 8.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 9.Reiter Z. Interferon–a major regulator of natural killer cell-mediated cytotoxicity. J Interferon Res. 1993;13:247–257. doi: 10.1089/jir.1993.13.247. [DOI] [PubMed] [Google Scholar]
- 10.Critchley-Thorne RJ, et al. Down-regulation of the interferon signaling pathway in T lymphocytes from patients with metastatic melanoma. PLoS Med. 2007;4:e176. doi: 10.1371/journal.pmed.0040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 12.Bernabei P, et al. IGF-1 down-regulates IFN-gamma R2 chain surface expression and desensitizes IFN-gamma/STAT-1 signaling in human T lymphocytes. Blood. 2003;102:2933–2939. doi: 10.1182/blood-2003-01-0100. [DOI] [PubMed] [Google Scholar]
- 13.Haring JS, Corbin GA, Harty JT. Dynamic regulation of IFN-gamma signaling in antigen-specific CD8+ T cells responding to infection. J Immunol. 2005;174:6791–6802. doi: 10.4049/jimmunol.174.11.6791. [DOI] [PubMed] [Google Scholar]
- 14.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2004;10:3755–3762. doi: 10.1158/1078-0432.CCR-04-0054. [DOI] [PubMed] [Google Scholar]
- 16.Dondi E, Roue G, Yuste VJ, Susin SA, Pellegrini S. A dual role of IFN-alpha in the balance between proliferation and death of human CD4+ T lymphocytes during primary response. J Immunol. 2004;173:3740–3747. doi: 10.4049/jimmunol.173.6.3740. [DOI] [PubMed] [Google Scholar]
- 17.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006;176:3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- 18.Le Bon A, et al. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006;176:2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- 19.Jewett A, Bonavida B. Interferon-alpha activates cytotoxic function but inhibits interleukin-2-mediated proliferation and tumor necrosis factor-alpha secretion by immature human natural killer cells. J Clin Immunol. 1995;15:35–44. doi: 10.1007/BF01489488. [DOI] [PubMed] [Google Scholar]
- 20.Lai P, et al. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2:161–173. [PubMed] [Google Scholar]
- 21.Dworacki G, et al. Decreased zeta chain expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma. Clin Cancer Res. 2001;7:947s–957s. [PubMed] [Google Scholar]
- 22.Chaussabel D, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29:150–164. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillot B, et al. The expression of cytotoxic mediators is altered in mononuclear cells of patients with melanoma and increased by interferon-alpha treatment. Br J Dermatol. 2005;152:690–696. doi: 10.1111/j.1365-2133.2005.06512.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu B, et al. Inhibition of Stat1-mediated gene activation by PIAS1. Proc Natl Acad Sci USA. 1998;95:10626–10631. doi: 10.1073/pnas.95.18.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starr R, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 26.Ito S, et al. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma- induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93:1456–1463. [PubMed] [Google Scholar]
- 27.Larner AC, Petricoin EF, Nakagawa Y, Finbloom DS. IL-4 attenuates the transcriptional activation of both IFN-alpha and IFN-gamma-induced cellular gene expression in monocytes and monocytic cell lines. J Immunol. 1993;150:1944–1950. [PubMed] [Google Scholar]
- 28.Park IK, Shultz LD, Letterio JJ, Gorham JD. TGF-beta1 inhibits T-bet induction by IFN-gamma in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1. J Immunol. 2005;175:5666–5674. doi: 10.4049/jimmunol.175.9.5666. [DOI] [PubMed] [Google Scholar]
- 29.Viguier M, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 30.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Gabrilovich D, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 32.Moschos SJ, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 33.Kirkwood JM, et al. Immunomodulatory effects of high-dose and low-dose interferon alpha2b in patients with high-risk resected melanoma: the E2690 laboratory corollary of intergroup adjuvant trial E1690. Cancer. 2002;95:1101–1112. doi: 10.1002/cncr.10775. [DOI] [PubMed] [Google Scholar]
- 34.Feng X, et al. Low expression of interferon-stimulated genes in active multiple sclerosis is linked to subnormal phosphorylation of STAT1. J Neuroimmunol. 2002;129:205–215. doi: 10.1016/s0165-5728(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 35.He XS, et al. Global transcriptional response to interferon is a determinant of HCV treatment outcome and is modified by race. Hepatology. 2006;44:352–359. doi: 10.1002/hep.21267. [DOI] [PubMed] [Google Scholar]
- 36.Rousson V. On distribution-free tests for the multivariate two-sample location-scale model. J Multivariate Anal. 2002;80:42–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.